Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

domain, as we have seen for PLC (Section 19-4Ba), also
does so via Ca
2⫹
-mediated binding to the membrane’s
phosphatidylserine head groups. These interactions are
synergistic in that the greater the Ca
2⫹
concentration, the
lower the concentration of phorbol ester or DAG neces-
sary to activate PKC and vice versa. Nevertheless, both the
C1 and C2 domains must be membrane anchored in order
to activate the protein kinase. This is because the confor-
mation required to do so extracts the N-terminal pseudo-
substrate from the protein kinase active site.
b. PKC Is Primed by Phosphorylation
The activation of all mammalian PKCs but the atypical
PKCs is accompanied by their phosphorylation at three
conserved Ser or Thr residues. One of these residues (Thr
500 in PKCII) is in the protein kinase’s activation loop,
whereas the remaining two are in its C-terminal segment
(Thr 641 and Ser 660 of the 673-residue PKCII). In the
atypical PKCs, the latter Ser/Thr residue is replaced by a
phosphate-mimicking Glu residue. The sequence of events
that activate PKCs, which was largely elucidated by
Alexandra Newton, occurs as follows (Fig. 19-60):
1. Newly synthesized PKC binds to the membrane
(or possibly to the underlying cytoskeleton), where
phosphoinositide-dependent protein kinase-1 (PDK1)
phosphorylates its activation loop (at Thr 500 in PKCII).
The resulting negative charge on the activation loop is pos-
tulated to properly align the active site residues of PKC for
catalysis, much as we have seen for PKA (Section 18-3Cb;
Section 19-4. The Phosphoinositide Cascade 731
500
641
660
500
500
641
660
Ca
2+
off
1
PH
C
C
C
N
N
N
Membrane
PDK1
Activation
loop
PKC
C1
C2
2
on
C
N
3
DAG
PS
Figure 19-60 Activation of PKC. (1) Newly synthesized PKC
is phosphorylated on its activation loop (here represented by Thr
500 of PKCII; yellow ball) by phosphoinositide-dependent
protein kinase-1 (PDK1), which is tethered to the membrane via
its C-terminal pleckstrin homology domain (PH). (2) The now
catalytically competent PKC autophosphorylates 2 sites on its
C-terminal segment (here represented by Thr 641 and Ser 660 of
PKCII). However, the N-terminal pseudosubstrate segment
now binds to PKC’s active site, so that the enzyme remains
inactive. (3) On the binding of PKC’s C1 domain to
membrane-bound DAG (the product of extracellular signals
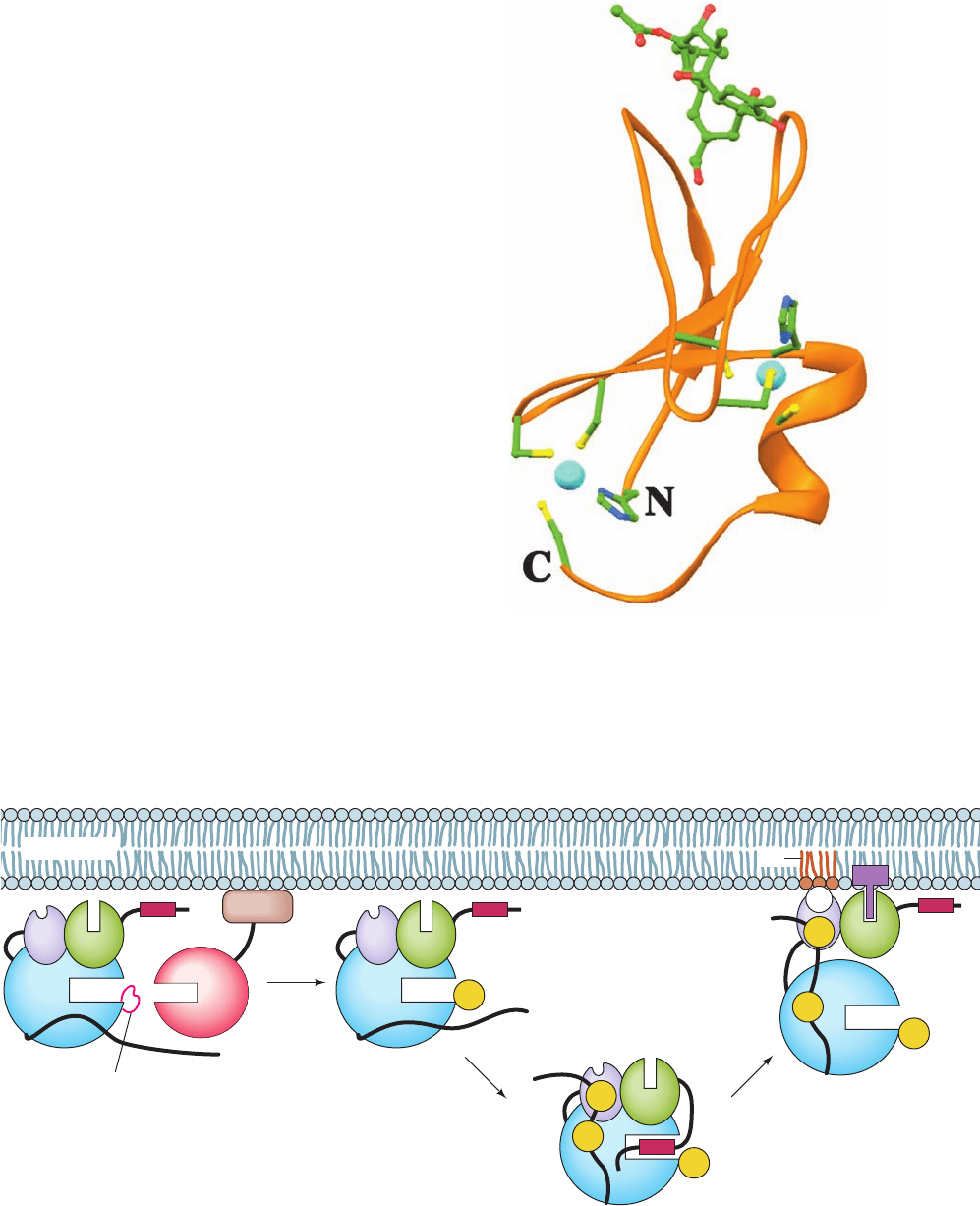
Figure 19-59 X-ray structure of the C1B motif of PKC␦ in complex with
12-O-myristoylphorbol-13-acetate. The protein tetrahedrally ligands two Zn
2⫹
ions (cyan spheres), each via a His side chain and three Cys side chains. These
side chains are shown in ball-and-stick form with C green, N blue, O red, and S
yellow, as is the bound 12-O-myristoylphorbol-13-actetate. The C1B domains
of PKC␣ and ␥ have similar structures. [Based on an X-ray structure by James
Hurley, NIH. PDBid 1PTR.]
inducing phosphoinositide hydrolysis) together with the
Ca
2⫹
-mediated binding of the C2 domain to phosphatidylserine
(PS) in the membrane, the pseudosubstrate is ejected from the
PKC active site, thereby yielding active enzyme. [After a drawing
by Toker,A. and Newton,A.C., Cell 103, 187 (2000).]
JWCL281_c19_671-743.qxd 7/1/10 1:13 PM Page 731
PKA’s activation loop is also phosphorylated by PDK1). In
fact, the mutagenic replacement of PKC␣’s activation loop
Thr with a neutral nonphosphorylatable residue yields an in-
activatable enzyme, whereas its replacement with Glu yields
an enzyme that requires only DAG and Ca
2⫹
for activation.
2. The now catalytically competent PKC rapidly au-
tophosphorylates its other two phosphorylation sites. The
autophosphorylation of Thr 641 appears to lock PKC into
its active conformation, as suggested by the observation
that in PKCII, which has been phosphorylated at only Thr
500 and Thr 641, the selective dephosphorylation of Thr 500
yields active enzyme. The autophosphorylation of the third
phosphorylation site correlates with the release of PKC into
the cytosol, where PKC is maintained in its inactive state by
the binding of its pseudosubstrate to its active site.
3. This autoinhibition is relieved, as described above,
when PKC again binds to the membrane via DAG binding
to its C1 domain and Ca
2⫹
-mediated binding of its C2 do-
main to phosphatidylserine (PS).
The activity of PKC is regulated by the protein phosphatase
PHLPP (for PH domain leucine-rich repeat protein phos-
phatase), which specifically dephosphorylates its Thr 641.
D. The Phosphoinositide 3-Kinases
The inositol head group of phosphatidylinositol has 5 free
hydroxyl groups that can be phosphorylated (Fig. 19-53).
However, only its 3-, 4-, and 5-positions are known to be
phosphorylated in vivo, and these occur in all seven possible
combinations (Fig. 19-61), each of which participates in sig-
naling. In addition to the plasma membrane, they occur in
ER, Golgi, and endosome membranes, although with dif-
ferent distributions in each of these several subcellular
compartments.
The phosphorylations of these various phosphoinosi-
tides are catalyzed by ATP-dependent enzymes known as
phosphoinositide 3-kinases (PI3Ks), phosphoinositide 4-
kinases (PIP4Ks), and phosphoinositide 5-kinases (PIP5Ks).
Their various products function as second messengers by
recruiting the proteins that bind them to the cytosolic sur-
face of the plasma membrane (see below). The resulting
colocalization of enzymes and substrates results in further
signaling activity that controls such vital functions as cell
survival, proliferation, cytoskeletal rearrangement, endo-
cytosis, and vesicle trafficking.
The PI3Ks are the presently best understood phospho-
inositide kinases. Consequently, in this subsection, we dis-
cuss the PI3Ks and their products as a paradigm of all
phosphoinositide kinases and the signals they produce.
a. PI3Ks Have Three Classes
Mammalian PI3Ks are divided into three classes ac-
cording to their structures (Fig. 19-62), substrate specifici-
ties, and modes of regulation:
1. Class I PI3Ks are heterodimeric receptor-regulated
enzymes that preferentially phosphorylate PIP
2
(alterna-
tively, PtdIns-4,5-P
2
). Their ⬃1070-residue catalytic sub-
units interact with Ras ⴢ GTP via a Ras-binding domain
(RBD) near their N-termini. Their regulatory subunits are
adaptor proteins that link the catalytic subunits to up-
stream signaling events and hence form two subclasses ac-
cording to the type of upstream effectors with which they
interact:
(a) The class IA PI3Ks (PI3K␣, , and ␦) are activated
by RTKs via the mediation of the adaptor subunit p85 (of
which there are seven isoforms), which contains SH2 and
SH3 domains and may be phosphorylated on specific Tyr
side chains. The gene encoding PI3K␣ is one of the two
most frequently mutated oncogenes in human cancers, thus
indicating this enzyme’s central regulatory role. Several of
its most common oncogenic mutations result in enhanced
enzymatic activity by altering the interactions between its
kinase domain and its regulatory domains or p85.
732 Chapter 19. Signal Transduction
PtdIns
PtdIns-4-PPtdIns-3-P PtdIns-5-P
PtdIns-3,5-P
2
PtdIns-4,5-P
2
PtdIns-3,4-P
2
PtdIns-3,4,5-P
3
IP3
DAG
PIP5K
PtdIns4K
Class III (or I or II)
PI3K
Class I (or II)
PI3K
PIP4K
PIP5K
PIP5K
PIP5K
PI3K
Class I or II
PI3K
PIP4K
PLC
Class I
RBD C2
domain
Helical
domain
Kinase-
domain
C2
Class II
Class III
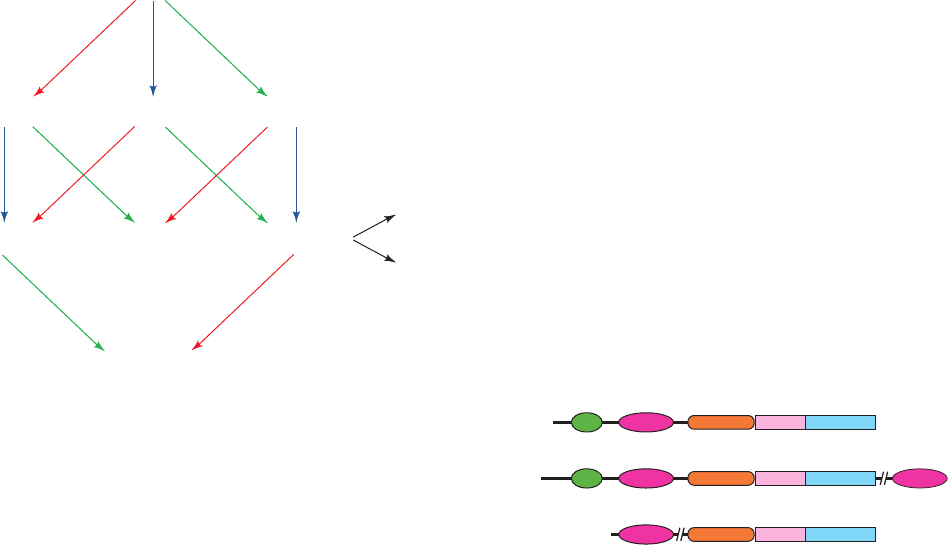
Figure 19-61 Flowchart of reactions in the synthesis of
phosphoinositides in mammalian cells. PtdIns, PtdIns-4-P, and
PtdIns-4,5-P
2
(PIP
2
) are written in bold type to indicate their
abundance:Together they comprise ⬃90% of the cell’s total
phosphoinositides. PtdIns-3-P and PtdIns-5-P each comprise
2–5% of the total, whereas the levels of PtdIns-3,4-P
2
and
PtdIns-3,4,5-P
3
(PIP
3
) are barely detectable in quiescent cells but
rise to 1 to 3% of the total in stimulated cells. PtdIns-3,5-P
2
comprises ⬃2% of the phosphoinositides in fibroblasts. [After
Fruman, D.A., Meyers, R.E., and Cantley, L.C., Annu. Rev.
Biochem. 67, 501 (1998).]
Figure 19-62 Domain organization of the three classes of
PI3Ks. [After Walker, E.H., Persic, O., Ried, C., Stephens, L., and
Williams, R.L., Nature 402, 314 (1999).]
JWCL281_c19_671-743.qxd 6/11/10 6:59 AM Page 732
(b) The class IB PI3K, of which PI3K is its only mem-
ber, is activated by the G
dimers of heterotrimeric G pro-
teins, with its adaptor subunit p101 rendering it far more
sensitive to G
.
2. Class II PI3Ks (PI3K-C2, , and ) are ⬃1650-
residue monomers that are characterized by a C-terminal
C2 domain that does not bind Ca
2
. They preferentially
phosphorylate PtdIns and PtdIns-4-P. Since they lack
adaptors, the way in which class II PI3Ks are controlled is
unknown.
3. Class III PI3K, which has one known isoform, phos-
phorylates only PtdIns. It is a heterodimer with an 887-
residue catalytic subunit and an adaptor subunit known as
p150. Class III PI3K is constitutively active, that is, it is un-
regulated and hence is thought to be the cell’s main
provider of PtdIns-3-P, whose level is essentially unaltered
by cellular stimulation. It is thought to be the evolutionary
predecessor of the other classes because it is the only class
of PI3K present in yeast.
In addition to their lipid kinase activities, all PI3Ks have
Ser/Thr protein kinase activity, although the physiological
significance of this dual specificity is unclear.
b. PI3K Is a Multidomain Protein
The X-ray structure of PI3K ATP, in which the PI3K
lacks its N-terminal 143 residues (which are important for
interaction with the p101 adaptor; the analogous portion of
PI3K interacts with its p85 adaptor), was determined by
Roger Williams. It reveals that its RBD, C2, and helical do-
mains form a relatively compact layer that packs against the
“back” of the kinase domain (Fig. 19-63). As expected, the
kinase domain is grossly similar to those of protein kinases
in that it is bilobal, with its N-lobe consisting largely of a
5-stranded sheet and its C-lobe being predominantly helical.
However, there are also major differences between these
kinase domains, as can be seen by comparing the catalytic
domain in Fig. 19-63 with that in, for example, Fig. 19-28a.
The RBD domain of PI3K has the same fold as that of
RafRBD (Fig. 19-41). Indeed, in the X-ray structure of
PI3K–Ras GMPPNP (Fig. 19-64), also determined by
Williams, the PI3K RBD interacts with Ras in a similar
manner as we have seen that RafRBD interacts with the
Ras homolog Rap1A (Fig. 19-41) in that they continue each
other’s central sheets. However, Ras bound to PI3K is
rotated by 35° relative to Rap1A bound to RafRBD. Con-
tacts between the Switch I region of Ras and the PI3K sta-
bilize this interaction and ensure its dependence on
Ras GTP. This complex also contains intermolecular con-
tacts involving the Switch II region of Ras. Such an interac-
tion had previously only been observed between Ras and its
upstream effectors. Comparison of the structure of the
PI3K–Ras complex with that of PI3K ATP (Fig. 19-63)
indicates that Ras binding induces the C-lobe of PI3K’s
catalytic domain to pivot relative to its N-lobe in a way that
substantially alters the putative binding pocket for the
phosphoinositide head group. This, presumably, accounts
for the ⬃15-fold activation of PI3K on binding Ras GTP.
Section 19-4. The Phosphoinositide Cascade 733
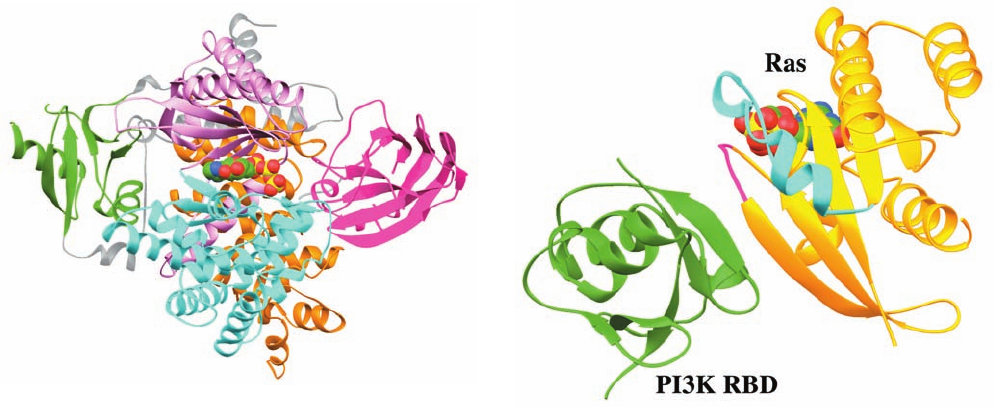
Figure 19-64 X-ray structure of PI3K–Ras GMPPNP. Here
only the PI3K RBD (green) and the Ras GMPPNP (gold) are
drawn, with the Switch I and Switch II regions of Ras magenta
and cyan and its bound GMPPNP shown in space-filling form
(C green, N blue, O red, and P yellow).The view, which is similar
to that of Fig. 19-41, is related to that in Fig. 19-63 by rotating it
clockwise by ⬃40° about its vertical axis and then turning it 180°
about the axis perpendicular to the page. [Based on an X-ray
structure by Roger Williams, MRC Laboratory of Molecular
Biology, Cambridge, U.K. PDBid 1HE8.]
Figure 19-63 X-ray structure of PI3K ATP. The protein is
shown in ribbon form with its Ras-binding domain (RBD) green,
its C2 domain magenta, its helical domain orange, the N- and
C-lobes of its kinase domain pink and cyan, and interdomain
segments gray. The ATP is shown in space-filling form with
C green, N blue, O red, and P yellow. The protein is oriented
such that its kinase domain is seen in “standard” view. The protein
appears fragmented because several of its segments are
disordered, including much of the kinase’s activation loop.
[Based on an X-ray structure by Roger Williams, MRC
Laboratory of Molecular Biology, Cambridge, U.K. PDBid 1E8X.]
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 733
The C2 domain of PI3K forms the same sandwich of
two 4-stranded antiparallel sheets seen in the C2 domain
of PLC-1 (Section 19-4Ba). However, in contrast to the
C2 domain of PLC-1, that of PI3K does not bind Ca
2
ions. Nevertheless, the PI3K C2 domain appears to partic-
ipate in membrane association, as indicated by the obser-
vation that this isolated C2 domain binds to phospholipid
vesicles with an affinity similar to that of the intact enzyme.
This interaction is presumably mediated by patches of ba-
sic residues on the surface of the C2 domain.
The PI3K helical domain consists of five repeating
pairs of antiparallel helices that form a superhelix, which
closely resembles that formed by the HEAT repeats in the
A subunit of protein phosphatase 2A (PP2A; Fig. 19-51a),
even though PI3K does not contain a HEAT sequence
motif. In analogy with the function of the A subunit of
PP2A to bind other proteins (Section 19-3Fe), it is pro-
posed that the largely solvent-exposed helical domain of
PI3K functions to interact with the proteins that bind
PI3K, such as its p101 adaptor and G
.
c. Akt Activation Requires Its PH Domain–Mediated
Binding to 3-Phosphoinositides
The PtdIns-3,4-P
2
and PtdIns-3,4,5-P
3
products of
PI3Ks (Fig. 19-61) bind to their downstream effectors
mainly via pleckstrin homology (PH) domains that prefer-
entially bind the head groups of these 3-phosphoinositides
rather than that of PIP
2
(as does the PH domain of PLC-;
Fig. 19-58). Another example of a PH domain-containing
protein that does so is the 556-residue phosphoinositide-
dependent protein kinase-1 (PDK1), which, as we have
seen, phosphorylates the activation loops of PKA and
PKC (Section 19-4Cb).
PDK1 also phosphorylates the Ser/Thr protein kinase
Akt [also called protein kinase B (PKB)], a proto-
oncogene product that is implicated in regulating multiple
biological processes including gene expression, apoptosis,
glucose uptake, and cellular proliferation, and hence
phosphorylates many target proteins. The ⬃480-residue
Akt consists of an N-terminal PH domain that binds
3-phosphoinositides and a C-terminal kinase domain that is
homologous to those of PKA and PKC (and is thus a mem-
ber of the AGC family of protein kinases).Akt is present in
multicellular organisms in three isoforms (Akt1/PKB,
Akt2/PKB, and Akt3/PKB) but is absent in yeast, which
suggests that it evolved from another AGC family member
coincidentally with multicellular organisms.
The full activation of Akt requires its phosphorylation
at both its Ser 473 and Thr 308. Ser 473 is phosphorylated
by mTORC2 [for mammalian target of rapomycin complex
2; rapamycin is an immunosuppressant similar to FK506
(Section 9-2B)]. This stimulates PDK1 to phosphorylate
Thr 308, which is located in Akt’s activation loop. Muta-
tions of the residues in Akt’s PH domain responsible for
lipid binding block its phosphorylation in vitro by PDK1.
However, the deletion of Akt’s PH domain overcomes this
enzyme’s need for binding to 3-phosphoinositides. This
suggests that the binding of Akt to these membrane-bound
lipids induces a conformational change that permits PDK1
to phosphorylate and hence activate Akt. It therefore ap-
pears that it is the 3-phosphoinositide-mediated colocaliza-
tion of Akt and PDK1 that leads to Akt activation and
hence that it is the action of PI3K that is functionally re-
sponsible for this process. In contrast, the PDK1-mediated
phosphorylation of PKA and PKC,which lack PH domains,
occurs in the absence of 3-phosphoinositides and is there-
fore constitutive. The protein phosphatase PHLPP regu-
lates Akt activity by dephosphorylating its Ser 473, in much
the same way as we have seen that PHLPP dephosphory-
lates PKC (Section 19-4Cb).
d. The FYVE Domain Binds the PtdIns-3-P
Head Group
The singly phosphorylated PtdIns-3-P is rarely bound
by PH domains. Rather, its direct effects are mediated by
FYVE domains [named after the four proteins in which it
was first identified: Fab1p, YOTB, Vac1p, and early endo-
some antigen 1 (EEA1)], which have been identified in
⬃60 proteins. For instance, the 1410-residue eukaryotic
protein EEA1, which has a 65-residue, C-terminal FYVE
domain, initiates endosome fusion in eukaryotic cells (Fig.
12-91) by recruiting the membrane-anchored small G pro-
tein Rab5 and the transmembrane SNARE protein syn-
taxin (Section 12-4Db).
The NMR structure of the EEA1 FYVE domain, deter-
mined by Michael Overduin, reveals that it assumes similar
conformations in the free state, when binding dibutanoyl-
PtdIns-3-P (Fig. 19-65), and when bound to dodecylphos-
phocholine (DPC) micelles enriched with this PtdIns-3-P.
The protein is largely held together by two bound Zn
2
ions, each of which is tetrahedrally liganded by four con-
served Cys side chains.The PtdIns-3-P head group is held in
its binding pocket by a network of electrostatic, hydrogen
bonding, and hydrophobic interactions involving a highly
conserved (R/K)(R/K)HHCR motif (RRHHCR in EEA1).
The NMR evidence indicates that, on the addition of
DPC micelles, the FYVE domain PtdIns-3-P complex
inserts a hydrophobic 5-residue loop (FSVTV; orange in
Fig. 19-65), which is flanked by basic residues (blue in Fig.
19-65), into the lipid layer. This also occurs in the absence
of PtdIns-3-P but to a much lesser extent. Conversely,
membrane insertion increases the binding affinity of the
FYVE domain for PtdIns-3-P 20-fold (from 1 M to 50 nM).
The origin of this latter effect appears to be that the 10-
residue segment preceding the membrane insertion loop,
the unliganded protein’s most disordered region, becomes
more ordered and moves toward the binding pocket on
binding PtdIns-3-P. This has led to the proposal that the
FYVE domain is recruited to membranes via the insertion
of its hydrophobic loop into the lipid bilayer. This, in turn,
primes the protein for the recognition of PtdIns-3-P, whose
binding induces the protein’s otherwise mobile N-terminal
segment to clamp down over the PtdIns-3-P head group.
E. Inositol Polyphosphate Phosphatases
Signaling via the phosphoinositide cascade is terminated
through the actions of a variety of inositol phosphatases that
734 Chapter 19. Signal Transduction
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 734
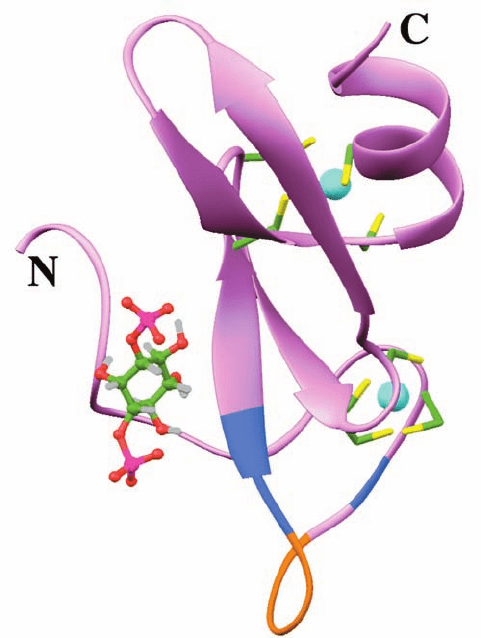
are functionally classified as 1-, 3-, 4-, and 5-phosphatases.
We end our consideration of the phosphoinositide cascade
by discussing the characteristics of these essential enzymes.
a. The Inositol Polyphosphate 5-Phosphatases
Act In Numerous Signaling Pathways
The first inositol polyphosphate 5-phosphatases that
were studied hydrolyze IP
3
(Ins-1,4,5-P
3
) to IP
2
(Ins-1,4-P
2
)
and thereby terminate cellular Ca
2
mobilization (Fig.
19-54, bottom). Mammals express 10 isozymes that
have 5-phosphatase activity. These enzymes share a com-
mon catalytic core and have been classified according to
their substrate specificities into two groups: Type I
enzymes dephosphorylate inositol phosphates, whereas
type II enzymes, in addition, hydrolyze the corresponding
phosphoinositides.
Type I 5-phosphatases, which hydrolyze only IP
3
and
Ins-1,3,4,5-P
4
, are membrane-anchored via prenylation.
That expressed in blood platelets (a type of blood cell
that participates in blood clotting; Section 35-1), which is
representative of this group, forms a stoichiometric complex
with pleckstrin, a 350-residue protein that consists largely
of two PH domains. When platelets are stimulated by the
proteolytic clotting enzyme thrombin (Section 35-1B),
pleckstrin is phosphorylated on Ser and Thr residues by
PKC, which in turn activates its associated 5-phosphatase.
Note that PKC is activated by DAG, a product of PLC,
which simultaneously generates the type I 5-phosphatase
substrate IP
3
(Fig. 19-54). Hence, the PLC product IP
3
activates Ca
2
ion release, whereas its coproduct DAG
activates type I 5-phosphatase through pleckstrin phospho-
rylation to terminate the Ca
2
signal. This termination is
apparently important for normal cell growth as a decrease
in the expression of type I 5-phosphatase causes increased
and even uncontrolled (malignant) cell growth.
Type II 5-phosphatases share increased similarities in
their catalytic cores relative to type I enzymes and, in addi-
tion,have a so-called type II domain on the N-terminal side
of their catalytic cores. They occur in three main subtypes:
GIPs, SHIPs, and SCIPs. GIPs are so called because they
have a C-terminal GAP domain (GAP-containing inositol
phosphatase), although they have no demonstrated GAP
activity. GIPs hydrolyze IP
3
and Ins-1,3,4,5-P
4
and their
corresponding lipids, PtdIns-4,5-P
2
and PtdIns-3,4,5-P
3
,al-
though with varying catalytic efficiencies.
There are only two known GIPs, 5-phosphatase II and
OCRL. OCRL is so called because its mutation causes the
X-linked hereditary disease oculocerebrorenal dystrophy
(also called Lowe syndrome), which is characterized by con-
genital cataracts, progressive retinal degeneration, mental
retardation, and renal tubule defects leading to kidney fail-
ure in early adulthood. The 901-residue OCRL occurs
mainly on the surface of lysosomes, where it is anchored
through prenylation. Renal tubule cells from Lowe syn-
drome patients are deficient in PtdIns-4,5-P
2
and PtdIns-
3,4,5-P
3
hydrolytic activity, whereas the corresponding inos-
itol phosphates are hydrolyzed normally, thereby indicating
that OCRL is a lipid phosphatase. PtdIns-4,5-P
2
stimulates
the budding of membrane vesicles from lysosomes, so that
the accumulation of this lipid probably leads to abnormally
increased trafficking of enzymes from the lysosome to the
extracellular space. Indeed, the lysosomal enzymes in these
cells appear to be missorted (as are various lysosomal hy-
drolases in I-cell disease; Section 12-4Cg). It is therefore
proposed that this lifelong leakage of enzymes from the
lysosomes in Lowe syndrome patients causes tissue damage
that eventually results in kidney failure and blindness.
SHIPs only hydrolyze substrates that also have a phos-
phate in their 3-positions. The two known members of this
group, SHIP (for SH2-containing inositol-5-phosphatase)
and SHIP2, are ⬃1200-residue proteins that have an
N-terminal SH2 domain. Thus, these proteins can bind to
PTKs and, in fact, are phosphorylated by them to yield a
consensus binding sequence for PTB domains (NPXpY;
Section 19-3Cc). Moreover, they also contain a C-terminal
Pro-rich domain that may bind to SH3-containing proteins.
Thus, it appears that SHIP activity may be under the con-
trol of several systems. Indeed, SHIP, which is expressed
only in hematopoietic (blood-forming) cells, associates
Section 19-4. The Phosphoinositide Cascade 735
Figure 19-65 NMR structure of the EEA1 FYVE domain in
complex with PtdIns-3-P. The head group of PtdIns-3-P is drawn in
ball-and-stick form (C green, O red, P magenta, H gray).The
protein binds two Zn
2
ions (cyan spheres) that are each
tetrahedrally liganded by four Cys side chains that are drawn in
stick form (C green and S yellow).The 5-residue loop that inserts
into DPC micelles is orange and its flanking basic residues are
blue. [Based on an NMR structure by Michael Overduin,
University of Colorado Health Sciences Center. PDBid 1HYI.]
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 735
with the adaptor proteins Grb2 and Shc (Section 19-3Cf).
It functions to hydrolyze PtdIns-3,4,5-P
3
, which is impli-
cated in activating Akt and PLC. SHIP2 functions similarly
in nonhematopoietic cells, where it limits cellular responses
to insulin, EGF, and PDGF.
SCIPs (Sac1-containing inositol phosphatases) are so
named because they contain an N-terminal domain that is
homologous to the yeast phosphatidylinositol phosphatase
Sac1. The first SCIP to be characterized is named synap-
tojanin1 because it was purified from synaptic vesicles
and because the presence of two phosphatase domains is
reminiscent of the two kinase domains in Janus kinases
(JAKs; Section 19-3Eb).The 1575-residue synaptojanin1’s
5-phosphatase domain hydrolyzes PIP
3
and PtdIns-4,5-P
2
and its Sac1 phosphatase domain hydrolyzes PtdIns-3-P
and PtdIns-4-P. Synaptojanin1 is expressed only in neurons,
where it forms complexes with the G protein dynamin
(Section 12-4Cd) and thereby participates in synaptic vesi-
cle recycling. The closely similar synaptojanin2 is ubiqui-
tously expressed but its functions are largely unknown.
b. Inositol Polyphosphate 1-Phosphatase Is
Implicated in Bipolar Disorder
Mammals express only one type of inositol polyphos-
phate 1-phosphatase, a 399-residue enzyme that hydrolyzes
Ins-1,4-P
2
and Ins-1,3,4-P
3
(IP
3
) but does not act on lipid
substrates. This enzyme is inhibited by Li
⫹
ion. The thera-
peutic efficacy of Li
⫹
in controlling the incapacitating mood
swings of manic-depressive individuals (those with bipolar
disorder) therefore suggests that this mental illness is
caused by an aberration of 1-phosphatase in the brain, pos-
sibly resulting in abnormal activation of Ca
2⫹
-mobilizing
receptors (Fig. 19-54, bottom). Indeed, Drosophila in which
this 1-phosphatase has been deleted exhibit neurological
deficits (the so-called “shaker” phenotype) that appear
identical to those of wild-type Drosophila treated with Li
⫹
.
c. The Inositol Polyphosphate 3-Phosphatase PTEN
Is a Tumor Suppressor
The inositol polyphosphate 3-phosphatases undo the
actions of the PI3Ks. The best characterized of these en-
zymes is the 403-residue PTEN (for phosphatase and
tensin homolog; tensin is a cytoskeletal actin-binding pro-
tein), which in vitro dephosphorylates all 3-phosphorylated
phosphoinositides and Ins-1,3,4,5-P
4
. PTEN is a tumor sup-
pressor (a protein whose loss of function is a cause of in
cancer), presumably because its 3-phosphatase activity
functions to downregulate the PtdIns-3,4,5-P
3
-activated
Akt. In fact, PTEN mutation or loss commonly occur in
many types of cancers. PTEN can also dephosphorylate
Ser-, Thr-, and Tyr-phosphorylated peptides, although this
activity requires the peptides to be highly acidic.
The X-ray structure of PTEN, determined by Jack Dixon
and Nikola Pavletich, reveals the protein to consist of an N-
terminal phosphatase domain and a C-terminal C2 domain
(Fig. 19-66). The structure of its phosphatase domain resem-
bles that common to protein tyrosine phosphatase (PTP)
domains (e.g.,Fig. 19-50),but with a larger active site pocket,
presumably to accommodate the large size of its PtdIns-
3,4,5-P
3
substrate. The C2 domain lacks bound Ca
2⫹
ion as
well as the ligands to bind it but, nevertheless, binds to phos-
pholipid membranes, as does the C2 domain of PI3K␥ (Fig.
19-63).The phosphatase and C2 domains associate across an
extensive interface, whose residues are frequently mutated
in cancer. A similar tight interface between the C2 and ki-
nase domain occurs in PLC-II (Fig. 19-57). This suggests
that PTEN’s C2 domain functions to productively position
its attached phosphatase domain at the membrane.
d. The Inositol Polyphosphate 4-Phosphatases
Control the Level of PtdIns-3,4-P
2
There are two isoforms of inositol 4-phosphatases,
4-phosphatases I and II, which catalyze the hydrolysis of Ins-
1,3,4-P
3
, Ins-2,4-P
2
, and PtdIns-3,4-P
2
. In fact, these ⬃940-
residue proteins account for ⬎95% of the observed PtdIns-
3,4-P
2
phosphatase activity in many human tissues, thereby
suggesting that they play an important role in the metabolism
of this second messenger.This is supported by the observation
that stimulating human platelets by thrombin or Ca
2⫹
ion re-
sults in the inactivation of 4-phosphatase I through its prote-
olytic cleavage by the Ca
2⫹
-dependent protease calpain. This
inactivation of 4-phosphatase I correlates with the Ca
2⫹
-
and/or aggregation-dependent accumulation of PtdIns-3,4-P
2
characteristic of human platelets (which aggregate in the ini-
tial stages of blood clot formation; Section 35-1).
F. Epilog: Complex Systems and
Emergent Properties
Complex systems are, by definition, difficult to understand
and substantiate. Familiar examples include Earth’s
weather system, the economies of large countries, the
ecologies of even small areas, and the human brain. Biolog-
ical signal transduction systems, as is amply evident from a
reading of this chapter, are complex systems. Thus, as we
736 Chapter 19. Signal Transduction
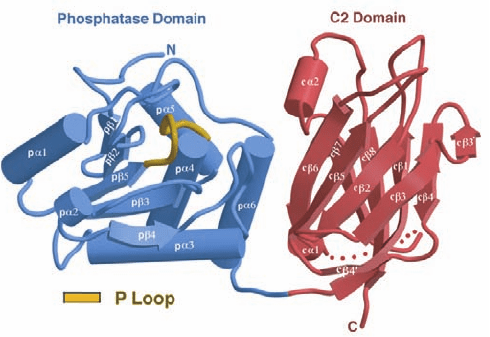
Figure 19-66 X-ray structure of PTEN. The protein is shown
with its phosphatase domain blue, its C2 domain red, and the
P loop, which interacts with the substrate, tan. The dotted line
represents a 24-residue segment that was deleted from the
protein to facilitate its crystallization. [Courtesy of Nikola
Pavletich, Memorial Sloan-Kettering Cancer Center, New, York,
New York. PDBid 1D5R.]
JWCL281_c19_671-743.qxd 7/20/10 5:49 PM Page 736
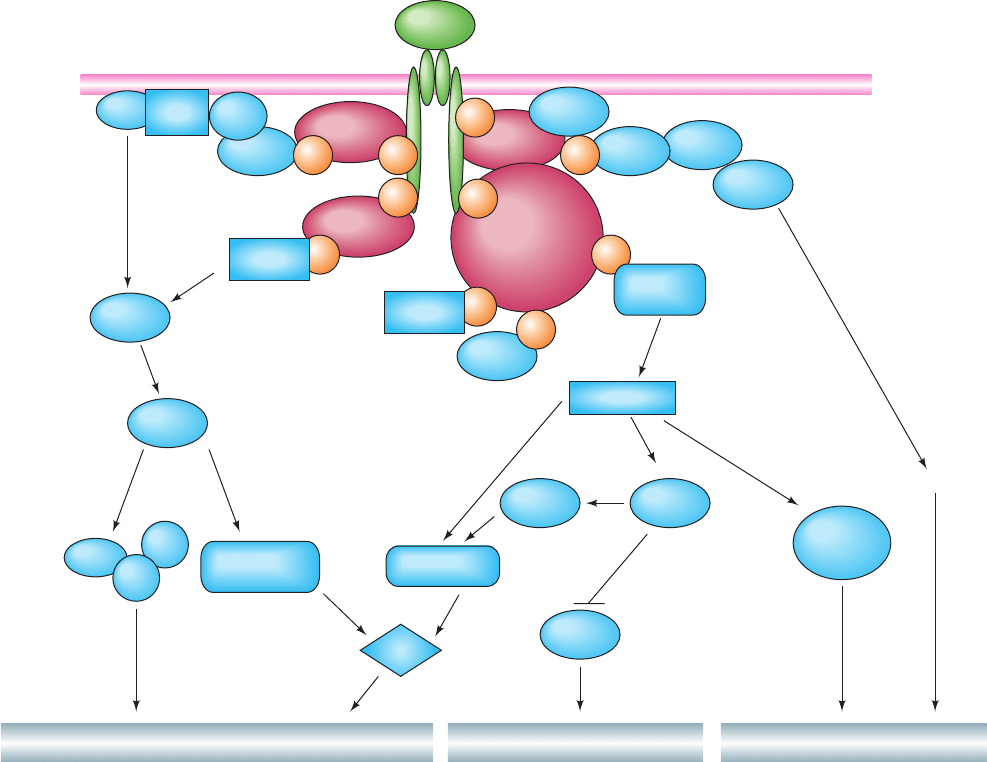
have seen, a hormonal signal is typically transduced
through several intracellular signaling pathways, each of
which consists of numerous components, many of which in-
teract with components of other signaling pathways. For
example, the insulin signaling system (Fig. 19-67), although
not yet fully elucidated, is clearly highly complex. On bind-
ing insulin, the insulin receptor autophosphorylates itself
at several Tyr residues (Section 19-3Ac) and then Tyr-
phosphorylates its target proteins, thereby activating several
signaling pathways that control a diverse array of effects:
1. Phosphorylation of Shc (Section 19-3Cc) results in
stimulation of a MAP kinase cascade (Section 19-3D), ulti-
mately affecting growth and differentiation.
Section 19-4. The Phosphoinositide Cascade 737
pY
pY
pY
pYpY
pY
IR
Plasma membrane
Glucose transportGlycogen synthesisDNA/RNA/Protein synthesis
MetabolismCellular growth and differentiation
Lipid rafts
and
caveolae
Fyn
PKB
PKCζ
PKCλ
GSK3β
MAPK
MEK
Gab-1
Shc
APS/Cbl
Insulin
IRS
proteins
Grb2
CAP
CrkII
C3G
TC10
mTOR
Myc
Raf1
SHP-2
SHP-2
PI3K
p90
rsk
S6 kinase
PDK1
Jun
Fos
Sos
S6
Ras
pY
pY
pY
pY
Figure 19-67 Insulin signal transduction. The binding of
insulin to the insulin receptor (IR) induces its autophosphorylation
at several Tyr residues on its subunits. Several proteins,
including Shc, Gab-1, the APS/Cbl complex, and IRS proteins,
bind to these pY residues where they are Tyr-phosphorylated by
the activated insulin receptor, thereby activating MAPK and
PI3K phosphorylation cascades as well as a lipid raft and
caveolae-associated regulation process.The MAPK cascade
regulates the expression of genes involved in cellular growth and
differentiation.The PI3K cascade leads to changes in the
phosphorylation states of several enzymes, so as to stimulate
glycogen synthesis, as well as other pathways. The PI3K cascade
also participates in the control of vesicle trafficking, leading to
the translocation of the GLUT4 glucose transporter to the cell
surface and thus increasing the rate of glucose transport into the
cell (Section 20-2Ec). Glucose transport control is also exerted
by the APS/Cbl system in a PI3K-independent manner involving
lipid rafts and caveolae. Other symbols: Myc, Fos, and Jun
(transcription factors; Section 19-3D), SHP-2 (an SH2-containing
PTP; Section 19-3Fb), CAP (Cbl-associated protein), C3G [a
guanine nucleotide exchange factor (GEF)], CrkII [an SH2/SH3-
containing adaptor protein), PDK1 (phosphoinositide-dependent
protein kinase-1; Section 19-4Cb), PKB (protein kinase B, also
named Akt; Section 19-4Dc), mTOR [for mammalian target of
rapamycin, a PI3K-related protein kinase (Section 9-2B); mTOR
is also known as FKBP12-rapamycin-associated protein
(FRAP)], S6 [a protein subunit of the eukaryotic ribosome’s
small subunit (Section 32-3Ab); its phosphorylation stimulates
translation], and PKC and PKC (atypical isoforms of protein
kinase C; Section 19-4C). [After Zick, Y., Trends Cell Biol. 11,
437 (2001)].
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 737

2. Phosphorylation of Gab-1 (Grb2-associated binder-1)
similarly activates this MAP kinase cascade.
3. Phosphorylation of insulin receptor substrate (IRS)
proteins (Section 19-3Cg) activates a phosphoinositide cas-
cade via a PI3K (Section 19-4Da), ultimately stimulating a
variety of metabolic processes including glycogen synthesis
(Section 18-3E) and glucose transport (Section 20-2E), as
well as cell growth and differentiation.
4. Phosphorylation of the APS/Cbl complex (APS for
adaptor protein containing pleckstrin homology and Src
homology-2 domains; Cbl is an SH2/SH3-binding docking
protein that is a proto-oncogene product) leads to the stim-
ulation of TC10 [a G protein in the Rho family (Section 35-
3E)], and to the PI3K-independent regulation of glucose
transport involving the participation of lipid rafts and
caveolae (Section 12-3Cb).
The predominant approach in science is reductionist: the
effort to understand a system in terms of its component
parts. Thus chemists and biochemists explain the properties of
molecules in terms of the properties of their component
atoms, cell biologists explain the nature of cells in terms of
the properties of their component macromolecules, and biol-
ogists explain the characteristics of multicellular organisms in
terms of the properties of their component cells. However,
complex systems have emergent properties, properties that
are not readily predicted from an understanding of their
component parts (i.e., the whole is greater than the sum of its
parts). Indeed, life itself is an emergent property that arises
from the numerous chemical reactions that occur in a cell.
In order to elucidate the emergent properties of a com-
plex system, an integrative approach is required. For signal
transduction systems, such an approach would entail deter-
mining how each of the components of each signaling path-
way in a cell interacts with all of the other such components
under the conditions that each of these components experi-
ences within its local environment. Yet techniques for doing
so are not often available. Moreover, these systems are by no
means static but vary,over multiple time scales,in response to
cellular and organismal programs. Consequently, the means
for understanding the holistic performance of cellular signal
transduction systems are only in their earliest stages of devel-
opment. Such an understanding is likely to have important
biomedical consequences since many diseases, including can-
cer, diabetes, and a variety of neurological disorders, are
caused by malfunctions of signal transduction systems.
Finally, you should note that we have only outlined the
major signal transduction pathways that occur in eukary-
otic cells. Moreover, we have not considered numerous
other such pathways that control cellular functions (al-
though many of them are discussed in later chapters). Nev-
ertheless, it is clear that a full understanding of a cell’s sig-
nal transduction pathways and how they interact is the key
to understanding the molecular basis of life.
738 Chapter 19. Signal Transduction
1 Hormones Chemical messengers are classified as au-
tocrine, paracrine, or endocrine hormones if they act on the
same cell, cells that are nearby, or cells that are distant from
the cell that secreted them, respectively. The body contains a
complex endocrine system that controls many aspects of its
metabolism. Hormone levels may be determined through ra-
dioimmunoassays. Receptors are membrane-bound proteins
that bind their ligands according to the laws of mass action.
The parameters describing the binding of a radiolabeled lig-
and to its receptor can be determined from Scatchard plots.
The dissociation constants of additional ligands for the same
receptor-binding site can then be determined through compet-
itive binding studies.
The pancreatic islet cells secrete insulin and glucagon,
polypeptide hormones that induce liver and adipose tissue to
store or release glucose and fat, respectively. Gastrointestinal
polypeptide hormones coordinate various aspects of digestion.
The thyroid hormones, T
3
and T
4
, are iodinated amino acid de-
rivatives that generally stimulate metabolism by activating cel-
lular transcription factors. Ca
2⫹
metabolism is regulated by the
levels of PTH, vitamin D, and calcitonin. PTH and vitamin D in-
duce an increase in blood [Ca
2⫹
] by stimulating Ca
2⫹
release
from bone and its absorption from kidney and intestine,
whereas calcitonin has the opposite effects. Vitamin D is a
steroid derivative that must be obtained in the diet or by expo-
sure to UV radiation. Vitamin D, after being sequentially
processed in the liver and kidney to 1,25(OH)
2
D, stimulates the
synthesis of a Ca
2⫹
-binding protein in the intestinal epithelium.
The adrenal medulla secretes the catecholamines epinephrine
and norepinephrine, which bind to ␣- and -adrenergic recep-
tors on a great variety of cells so as to prepare the body for
“fight or flight.”The adrenal cortex secretes glucocorticoid and
mineralocorticoid steroids. Glucocorticoids affect metabolism
in a manner opposite to that of insulin as well as mediating a
wide variety of other vital functions. Mineralocorticoids regu-
late the excretion of salt and water by the kidney.The gonads se-
crete steroid sex hormones, the androgens (male hormones)
and estrogens (female hormones), which regulate sexual differ-
entiation, the development of secondary sex characteristics, and
sexual behavior patterns. Ovaries, in addition, secrete pro-
gestins that help mediate the menstrual cycle and pregnancy.
Mammalian embryos develop as females unless subjected to
the influence of the androgen testosterone. SRY, a gene that en-
codes a DNA-binding protein and that is normally located on
the Y chromosome, induces the development of testes, which in
turn secrete testosterone.The hypothalamus secretes a series of
polypeptide releasing factors and release-inhibiting factors such
as CRF,TRF, GnRF, and somatostatin that control the secretion
of the corresponding trophic hormones from the pituitary
gland’s adenohypophysis. Most of these trophic hormones, such
as ACTH, TSH, LH, and FSH, stimulate their target endocrine
glands to secrete the corresponding hormones. However,
growth hormone acts directly on tissues as well as stimulating
liver to synthesize growth factors known as somatomedins.
The pituitary gland’s neurohypophysis secretes the polypep-
tides vasopressin, which stimulates the kidneys to retain water,
and oxytocin, which stimulates uterine contraction. The men-
strual cycle results from a complex interplay of hypothalamic,
CHAPTER SUMMARY
JWCL281_c19_671-743.qxd 6/30/10 1:18 PM Page 738
adenohypophyseal, and steroid sex hormones.A fertilized and
implanted ovum secretes CG, which binds to the same recep-
tor and has similar effects as LH, thus preventing menstrua-
tion.The binding of hGH to its receptor causes the receptor to
dimerize, thereby providing the intracellular signal that the re-
ceptor has bound hCG. Many other hormonal signals are sim-
ilarly mediated. The adenohypophysis also secretes opioid
peptides that have opiatelike effects on the central nervous
system. Nitric oxide (NO), a highly reactive radical gas, func-
tions as a local mediator that regulates vasodilation, serves as
neurotransmitter, and functions in the immune response. In
mammals, it is synthesized by three isozymes of nitric oxide
synthase (NOS), an enzyme that contains 5 redox-active pros-
thetic groups. eNOS and nNOS are activated by Ca
2⫹
through
their binding of Ca
2⫹
–calmodulin; iNOS is transcriptionally
controlled. NO activates guanylate cyclase to produce cGMP,
which in turn activates cGMP-dependent protein kinase.
2 Heterotrimeric G Proteins Ligand (hormone) binding
to G protein-coupled receptors (GPCRs) activates the G
s␣
subunit of a stimulatory G protein to replace its bound GDP
with GTP, release its associated G
␥
subunits, and activate
adenylate cyclase (AC) to synthesize cAMP. Activation con-
tinues until G
s␣
hydrolyzes its bound GTP to GDP and recom-
bines with G
␥
. Several types of activated hormone receptors
in a cell may stimulate the same G
s
protein. There are also in-
hibitory G proteins, which may have the same G

and G
␥
sub-
units as does G
s
, but which have an inhibitory G
i␣
subunit that
deactivates adenylate cyclase. Cholera toxin (CT) and heat-
labile enterotoxin (LT), related bacterial AB
5
proteins, induce
uncontrolled cAMP production by ADP-ribosylating G
s␣
so as
to render it incapable of hydrolyzing GTP. Pertussis toxin, also
an AB
5
protein, similarly ADP-ribosylates G
i␣
. Biological sig-
naling systems are subject to desensitization through the phos-
phorylation and endocytotic sequestering of the cell-surface
receptors. The catalytic core of the numerous isoforms of AC
are pseudosymmetric heterodimers that are activated, in most
cases, by the binding of the Switch II region of G
s␣
ⴢ GTP to a
cleft in an AC’s C
1a
domain. cAMP and cGMP are eliminated
through the actions of numerous phosphodiesterases (PDEs),
whose activities are controlled by a variety of agents, thereby
providing for cross talk between signaling systems.
3 Tyrosine Kinase–Based Signaling The binding of lig-
ands such as hormones and protein growth factors activates
receptor tyrosine kinases (RTKs) by inducing them to dimer-
ize and then autophosphorylate specific Tyr residues in the ac-
tivation loops of their tyrosine kinase domains. This is usually
followed by the autophosphorylation of Tyr residues on other
cytoplasmic domains. Cancer cells’ immortality and their
uncontrolled proliferation endow them with the capacity to
form invasive and metastatic tumors. Rous sarcoma virus, a
retrovirus causing sarcomas in chickens, carries an oncogene,
v-src, that is homologous to the normal cellular gene c-src.
Both genes encode a protein tyrosine kinase (PTK) that stim-
ulates cell division. Oncogene products include analogs of
growth factors, growth factor receptors, nuclear proteins that
stimulate transcription and/or cell division, and G proteins.
Two-hybrid systems are used to identify interacting proteins.
An autophosphorylated RTK may activate other proteins by
phosphorylating them on specific Tyr side chains. It can also
modulate the activities of specific proteins through the binding of
an RTK’s phosphoTyr-containing peptide segment to SH2 and
PTB domains on these proteins or on adaptors that bind to these
proteins. Grb2, an adaptor protein, binds to certain activated
RTKs in this way and simultaneously, via its SH3 domains, to Sos
protein.The bound Sos, in turn, functions as a guanine nucleotide
exchange factor (GEF) to induce the small G protein Ras to ex-
change its bound GDP for GTP. Ras is a poor GTPase but it is
aided in eventually hydrolyzing its bound GTP to GDP by the
GTPase activating protein (GAP) RasGAP, which insinuates a
catalytically important Arg side chain into Ras’s otherwise ineffi-
cient active site. Mutations that interfere with the ability of
Ras–RasGAP to hydrolyze Ras’s bound GTP are oncogenic.
The binding of Ras
ⴢ GTP to Raf, a protein Ser/Thr kinase,
activates Raf to phosphorylate MEK, a MAP kinase kinase
(MKK), which in turn phosphorylates MAP kinase (MAPK).
The activated MAPK phosphorylates various cytoplasmic and
membrane-associated proteins and, in addition, is translocated
to the nucleus where it phosphorylates certain transcription
factors, which then induce the transcription of their target
genes. The proteins of such MAP kinase cascades are organ-
ized by their binding to scaffold proteins, which also prevents
the members of different MAP kinase cascades in a cell from
inappropriately phosphorylating one another. However, acti-
vated members of a MAP kinase cascade may phosphorylate
other regulatory proteins, thereby eliciting cross talk between
different signal transduction pathways.
Tyrosine kinase–associated receptors, such as cytokine re-
ceptors, transduce the signal that they have bound effector by
activating associated nonreceptor tyrosine kinases (NRTKs),
many of which are members of the Src or JAK families. Acti-
vated JAK proteins phosphorylate STAT proteins, which then
dimerize and are translocated to the nucleus, where they func-
tion as transcription factors. Gleevec is a highly selective Abl
inhibitor that is clinically effective in the treatment of chronic
myelogenous leukemia (CML). Many cancers require elevated
levels of Hsp90 activity for viability because their oncogenic
proteins tend to be relatively unstable. Phosphorylated proteins
are deactivated by protein phosphatases. Some protein tyro-
sine phosphatases (PTPs) are transmembrane receptors that
are deactivated by ligand-induced dimerization. Other PTPs
are cytoplasmic and are activated by their binding to activated
PTKs, for example, via SH2 domains, as does SHP-2.
Cells contain several types of Ser/Thr protein phos-
phatases: PP1 participates in the regulation of glycogen metab-
olism; PP2A, which participates in a wide variety of regulatory
processes, is a heterotrimer with numerous variants and hence
specificities and cellular locations; and calcineurin (CaN; also
called PP2B) is a Ca
2⫹
-activated heterodimeric phosphatase
that is the target of the immunosuppressive drugs cyclosporin
A and FK506 via the binding of their complexes with the rota-
mases cyclophilin and FKBP12 to CaN so as to prevent the
binding of CaN’s target phosphopeptides.
4 The Phosphoinositide Cascade PIP
2
, a minor phos-
pholipid component of the plasma membrane’s inner leaflet,
can yield up to three types of second messengers. Hormone–
receptor interactions, through the intermediacy of a G protein
or an RTK, stimulate the corresponding phospholipase C
(PLC) to hydrolyze PIP
2
to the water-soluble IP
3
and the
membrane-bound DAG.The IP
3
stimulates the release of Ca
2⫹
from the endoplasmic reticulum through ligand-gated chan-
nels. The Ca
2⫹
binds to calmodulin, which in turn activates a
variety of cellular processes. The DAG activates protein ki-
nase C (PKC) to phosphorylate and thereby modulate the ac-
tivities of numerous cellular proteins. DAG may also be
Chapter Summary 739
JWCL281_c19_671-743.qxd 6/30/10 1:18 PM Page 739

740 Chapter 19. Signal Transduction
General
Gomperts, B.D., Tatham, P.E.R., and Kramer, I.M., Signal Trans-
duction, Academic Press (2002).
Helmreich, E.J.M., The Biochemistry of Cell Signaling, Oxford
(2001).
Krauss, G., Biochemistry of Signal Transduction and Regulation
(4th ed.),Wiley-VCH (2008).
Marks, F., Klingmüller, U., and Müller-Decker, K., Cellular Signal
Processing. An Introduction to the Molecular Mechanisms of
Signal Transduction, Garland Science (2009).
Nelson, J., Structure and Function in Cell Signaling, Wiley (2008).
Science’s Signal Transduction Knowledge Environment (STKE).
http://stke.sciencemag.org/cm/. [A database on signaling mole-
cules and their relationships to each other.This database is in-
troduced in a series of authoritative articles in Science 296,
1632–1657 (2002). Full access to the database requires an indi-
vidual or institutional subscription.]
Hormones
Alderton, W.K., Cooper, C.E., and Knowles, R.G., Nitric oxide
synthases: structure, function, and inhibition, Biochem. J. 357,
593–615 (2002).
Capel, B., Sex in the 90s: SRY and the switch to the male pathway,
Annu. Rev. Physiol. 60, 497–523 (1998).
Cary, S.P.L., Winger, J.A., Derbyshire, E.R., and Marletta, M.A.,
Nitric oxide signaling: no longer simply on or off, Trends
Biochem. Sci. 31, 231–239 (2006).
DeGroot, L.J. and Jameson, J.L. (Eds.), Endocrinology (5th ed.),
Saunders (2006). [A 3-volume compendium.]
Garcin, E.D., Bruns, C.M., Lloyd, S.J., Hosfield, D.J., Tiso, M.,
Gachhui, R., Stuehr, D.J., Tainer, J.A., and Getzoff, E.D., Struc-
tural basis for isozyme-specific regulation of electron transfer
in nitric-oxide synthase, J. Biol. Chem. 36, 37918–37927 (2004).
[The X-ray structure of the nNOS reductase domain.]
Greenstein, B. and Wood, D., The Endocrine System at a Glance
(2nd ed.), Blackwell Publishing (2006).
Hadley, M.E. and Levine, J.E., Endocrinology (6th ed.), Benjamin
Cummings (2007).
Ignarro, L.J. (Ed.), Nitric Oxide. Biology and Pathobiology, Aca-
demic Press (2000).
Kossiakoff, A.A. and de Vos, A.M., Structural basis for cytokine
hormone–receptor recognition and receptor activation, Adv.
Protein Chem. 52, 67–108 (1999).
Li, H. and Poulos, T.L., Structure–function studies on nitric oxide
synthases, J. Inorg. Biochem. 99, 293–305 (2005).
Ma, Y.-A., Sih, C.J., and Harms, A., Enzymatic mechanism of thy-
roxine biosynthesis. Identification of the “lost three-carbon
fragment,” J. Am. Chem. Soc. 121, 8967–8968 (1999).
Murphy, K.G. and Bloom, S.R., Gut hormones and the regulation
of energy homeostasis, Nature 444, 854–859 (2006).
Prosser, D.E. and Jones, G., Enzymes involved in the activation
and inactivation of vitamin D, Trends Biochem. Sci. 29,
664–673 (2004).
Schafer, A.J. and Goodfellow, P.N., Sex determination in humans,
Bio Essays 18, 955–963 (1996).
Wei, C.-C., Wang, Z.-Q., Tejero, J., Yang, Y.-P., Hemann, C., Hille,
R., and Steuhr, D.J., Catalytic reduction of a tetrahydro-
biopterin radical with nitric-oxide synthatase, J. Biol. Chem.
283, 11734–11742 (2008).
Heterotrimeric G Proteins
Cooper, D.M.F. and Crossthwaite,A.J., Higher order organization
and regulation of adenylyl cyclases, Trends Pharmacol. Sci. 27,
426–431 (2006).
Corbin, J.D. and Francis, S.H., Cyclic GMP phosphodiesterase-5:
Target of sildenafil, J. Biol. Chem. 274, 13729–13732 (1999).
Fan, E., Merritt, E.A., Verlinde, C.L.M.J., and Hol, W.G.J., AB
5
toxins: Structures and inhibitor design, Curr. Opin. Struct. Biol.
10, 680–686 (2000).
Hanson, M.A. and Stevens, R.C., Discovery of new GPCR biol-
ogy: one receptor structure at a time, Structure 17, 8–17 (2009).
[Compares the known structures of GPCRs.]
REFERENCES
degraded to yield arachidonate, an obligate intermediate in
the biosynthesis of prostaglandins and related compounds.
The various classes of PLCs are activated in different ways,
all of which bring the PLC into contact with its PIP
2
substrate
in the membrane: PLC-’s by binding G
q␣
ⴢ GTP, G
␥
, and the
membrane-anchored Rac1
ⴢ GTP; PLC-␥’s by binding to
phosphorylated PTKs via SH2 domains followed by phospho-
rylation of the PLC by the PTK; PLC-␦’s by Ca
2⫹
; and PLC-ε
by binding Ras
ⴢ GTP. “Conventional” PKCs are activated by
both Ca
2⫹
and DAG. Phorbol esters, which are DAG mimics
that activate PKC, are the most potent known tumor promot-
ers. DAG and Ca
2⫹
synergistically bind PKC to the membrane
via its C1 and C2 domains, which conformationally extracts
PKC’s N-terminal pseudosubstrate from the kinase’s active
site. The kinase is catalytically activated by phosphorylation
on its activation loop by PDK1 followed by autophosphoryla-
tion at two more sites.
Phosphoinositides may be phosphorylated at their inositol
head group’s 3-, 4-, and 5-positions in all seven combinations,
yielding membrane-bound second messengers that function by
recruiting the proteins that bind them to the membrane surface.
Mammalian phosphoinositide 3-kinases (PI3Ks) form three
classes that differ according to their structures, substrate speci-
ficities, and modes of regulation. The PtdIns-3,4-P
2
and PtdIns-
3,4,5-P
3
products of PI3Ks bind to the PH domain of the proto-
oncogene product Akt (PKB), thereby colocalizing Akt with
PDK1, which is also tethered to the membrane via its PH do-
main, so that PDK1 phosphorylates and thereby activates Akt.
PtdIns-3-P is bound by FYVE domains, which, like PH domains,
are held together by two tetrahedrally liganded Zn
2⫹
ions.
The various types of inositide polyphosphate phosphatases
function to terminate signaling by the phosphoinositide cascade.
OCRL, a type II 5-phosphatase that participates in controlling
vesicle budding from the lysosome, is mutated in oculocere-
brorenal disease (Lowe syndrome). The only 1-phosphatase
expressed by mammals, which hydrolyzes Ins-1,4-P
2
and PIP
3
,is
inhibited by Li
⫹
ion and is thereby implicated in bipolar disorder.
The 3-phosphatase PTEN, a tumor suppressor whose mutant
forms are common to many cancers, undoes the actions of
PI3Ks. Type I 4-phosphatase in blood platelets is inactivated
through proteolytic cleavage by the Ca
2⫹
-activated protease
calpain. Cellular signal transduction systems, such as the insulin
signaling system, are complex systems with emergent properties
that are, as yet, poorly understood.
JWCL281_c19_671-743.qxd 6/4/10 10:56 AM Page 740
