Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

F. Protein Phosphatases
As we previously discussed (Section 19-2E), to prevent an
intracellular signaling pathway from being stuck in the
“on” position, its signals must be rapidly eliminated once
the message has been delivered. For proteins with phos-
phoTyr or phospho-Ser/Thr residues, this task is carried out
by a variety of protein phosphatases, of which ⬃500 are en-
coded by the human genome (around the same number as
species of protein kinases, which suggests that these fami-
lies have similar levels of complexity). The protein phos-
phatases, as we shall see, are not just simple housekeeping
enzymes but are signal transducers in their own right.Thus,
whereas kinases control the amplitude of a signaling re-
sponse, protein phosphatases control its rate and duration.
a. Protein Tyrosine Phosphatases Also Mediate
Signal Transduction
The enzymes that dephosphorylate Tyr residues, the pro-
tein tyrosine phosphatases (PTPs), which were discovered
by Nicholas Tonks, form a large family of diverse proteins
that are present in all eukaryotes (humans have 107 PTP
genes vs 90 PTK genes). Each PTP contains at least one
conserved ⬃280-residue phosphatase domain that has the
11-residue signature sequence (I/V)HCXAGXGR(S/T)G,
the so-called HCX
5
R motif, which contains the enzyme’s
catalytically essential Cys and Arg residues. The reaction
proceeds via the nucleophilic attack of the Cys thiolate
group on the P atom of the bound phosphoTyr to yield Tyr
and a cysteinyl–phosphate intermediate that is subse-
quently hydrolyzed. The Arg side chain participates in sub-
strate binding and stabilizes the cysteinyl–phosphate inter-
mediate.
The PTPs have been classified into three groups: (1) recep-
torlike PTPs, (2) intracellular PTPs, and (3) dual-specificity
PTPs, which can also dephosphorylate phospho-Ser/Thr
residues. The receptorlike PTPs are constructed much
like the RTKs (Fig. 19-25); that is, they have, from N- to
C-terminus, an ectodomain consisting of often multiple
repeating modules that occur in other proteins, a single
transmembrane helix, and a cytosolic domain consisting of
a catalytically active PTP domain that, in most cases, is fol-
lowed by a second PTP domain with little or no catalytic
activity. Nevertheless, these inactive PTP domains, which
are highly conserved, are important for the activity, speci-
ficity, and stability of the PTP as a whole.
Biochemical and structural analyses indicate that ligand-
induced dimerization of a receptorlike PTP reduces its
catalytic activity, probably by blocking its active sites. Intra-
cellular PTPs contain only one PTP domain, which is
flanked by regions containing motifs, such as SH2 domains,
that participate in protein–protein interactions. Structural
studies reveal that the active sites of receptorlike and intra-
cellular PTPs are too deep to bind phospho-Ser/Thr side
chains—as we also saw to be the case for both PTK and
SH2 domains (Sections 19-3Ac and 19-3Cb). However, the
active site pockets of dual-specificity PTPs are sufficiently
shallow to bind both phosphoTyr and phospho-Ser/Thr
residues.
b. SHP-2 Is Inactivated by Binding Its Unliganded
N-Terminal SH2 Domain
The cytoplasmic PTP SHP-2 (for SH2 domain-containing
phosphatase 2), which is expressed in all mammalian cells,
binds to PTKs that are activated by a variety of ligands,
including cytokines, growth factors, and hormones. The
591-residue SHP-2 consists of two tandem SH2 domains,
followed by a PTP domain and a 66-residue C-terminal tail
that contains Tyr-phosphorylation sites as well as a Pro-rich
segment that may bind SH3- or WW-containing proteins.
SHP-2’s PTP activity is increased ⬃10-fold on binding pep-
tides with a single phosphoTyr residue and ⬃100-fold with
those having two phosphoTyr residues and at much lower
peptide concentrations. SHP-2 binds to both growth factors
and certain cytokine receptors via its SH2 domains and,
when its C-terminal tail is phosphorylated, also functions
as an adaptor to recruit Grb2 so as to activate MAP kinase
pathways (Section 19-3D). Mutations in the gene encoding
SHP-2 are responsible for ⬃50% of the cases of Noonan
syndrome, a relatively common (1 in ⬃2000 live births) dis-
order principally characterized by cardiac abnormalities,
short stature, learning disabilities, and distinctive facial
features.
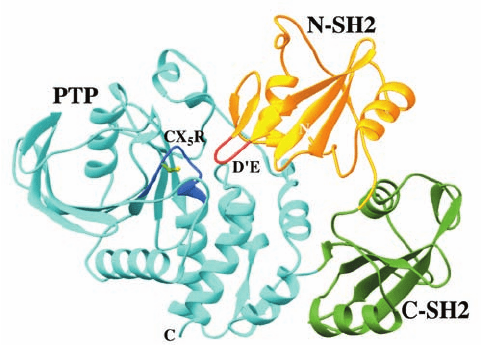
The X-ray structure of SHP-2 lacking its C-terminal tail
(Fig. 19-50), determined by Eck and Steven Shoelson, re-
veals that the N-terminal SH2 domain (N-SH2) interacts
extensively with the PTP domain. N-SH2 inhibits the PTP
by inserting its D¿E loop far into the PTP’s 9-Å-deep cat-
alytic cleft, where the loop interacts with the PTP’s cat-
alytic Arg and Cys residues and prevents the active site clo-
sure observed in the X-ray structure of a PTP in complex
with a phosphopeptide. In contrast, the more C-terminal
SH2 domain (C-SH2) does not have a significant interface
with either the N-SH2 or the PTP domains.
Section 19-3. Tyrosine Kinase–Based Signaling 721
Figure 19-50 X-ray structure of the protein tyrosine phosphatase
SHP-2. In this structure, its N-SH2 domain is gold with its D¿E
loop red, its C-SH2 domain is green, and its PTP domain is cyan,
with its 11-residue signature sequence, its CX
5
R motif, blue and
the side chain of its catalytically essential Cys residue shown in
ball-and-stick form with C green and S yellow. [Based on an
X-ray structure by Michael Eck and Steven Shoelson, Harvard
Medical School. PDBid 2SHP.]
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 721
The phosphopeptide-binding sites on both SH2 do-
mains face away from the PTP domain and are therefore
fully exposed on the protein surface. However,the compar-
ison of the structure of N-SH2 complexed to a phospho-
peptide with that in the above autoinhibited form of SHP-
2 indicates that, in the autoinhibited form, N-SH2 adopts a
conformation in which it is unable to bind phosphoTyr. Ev-
idently, the conformations of N-SH2’s PTP-binding surface
and phosphopeptide binding site are allosterically linked
such that its binding of PTP and phosphopeptide are mutu-
ally exclusive. The C-SH2 domain does not participate in
PTP activation, although it almost certainly contributes
binding energy and specificity to the binding of a phospho-
peptide.
c. Bubonic Plague Virulence Requires a PTP
Bacteria lack PTKs and hence do not synthesize phos-
phoTyr residues. Nevertheless, PTPs are expressed by bac-
teria of the genus Yersinia, most notably Yersinia pestis, the
pathogen that causes bubonic plague (the flea-transmitted
“Black Death,” which, since the sixth century, has been re-
sponsible for an estimated ⬃200 million human deaths in-
cluding about one-third of the European population in the
years 1347–1350). The Y. pestis PTP, YopH, which is re-
quired for bacterial virulence, is far more active than other
known PTPs. Hence,when Yersinia injects YopH into a cell,
the cell’s phosphoTyr-containing proteins are catastrophi-
cally dephosphorylated. Although YopH is only ⬃15%
identical in sequence to mammalian PTPs, it contains all of
their invariant residues and their X-ray structures are
closely similar. This suggests that an ancestral Yersinia ac-
quired a PTP gene from a eukaryote. However, the discov-
ery of a dual-specificity protein phosphatase in a free-living
cyanobacterium raises the possibility that PTPs arose be-
fore the divergence of eukaryotes and prokaryotes.
d. Cells Contain Several Types of Protein
Ser/Thr Phosphatases
The protein Ser/Thr phosphatases were first character-
ized by Earl Sutherland (who also discovered the role of
cAMP as a second messenger; Section 18-3Eb) and by
Edmond Fischer and Edwin Krebs (who discovered the
role of protein phosphorylation in controlling glycogen
metabolism; Section 18-3C).The majority of these enzymes
are members of two protein families:the PPP family, which
consists of PP1, PP2A, and PP2B (PP for phosphoprotein
phosphatase); and the PPM family, which consists of
PP2C. The PPP and PPM families are unrelated to each
other or to the PTKs. We have already considered PP1 in
connection with the role of its catalytic subunit,PP1c,in de-
phosphorylating the proteins that regulate glycogen me-
tabolism as well as the roles of its targeting subunits, G
M
and G
L
, in binding PP1c to glycogen in muscle and liver
(Section 18-3Cg). Indeed, all PP1c’s are associated with
one or two regulatory (R) subunits that function to modu-
late the activity of their bound PP1c’s, target them to sub-
strates in specific subcellular locations, or modify their sub-
strate specificities. It is the large variety of these mostly
unrelated R subunits that permits the limited number
(1–8) of genetically distinct but closely similar (⬃90% se-
quence identity) PP1c’s in a eukaryotic cell to carry out
their diverse functions.
X-ray structures have shown that PPP catalytic centers
each contain an Fe
2
(or possibly an Fe
3
) ion and a Zn
2
(or possibly an Mn
2
) ion, whereas PPM catalytic centers
each contain two Mn
2
ions.These binuclear metal ion cen-
ters nucleophilically activate water molecules to dephos-
phorylate substrates in a single reaction step.
e. PP2A Is Structurally Variable and
Functionally Diverse
PP2A participates in a wide variety of regulatory
processes including those governing metabolism, DNA
replication, transcription, and development. It consists of
three different subunits:
1. An ⬃36 kD catalytic subunit (C), whose N-terminal
catalytic domain contains the ⬃280-residue catalytic core
common to all PPP family members. Its C-terminal re-
gulatory domain contains an activating binding site for
Ca
2
–calmodulin, an inactivating Tyr phosphorylation site
that is targeted by a variety of PTKs including the EGF and
insulin receptors, and a C-terminal autoinhibitory tail. The
C subunit is highly conserved from yeast to mammals.
2. An ⬃65 kD scaffold subunit (A; also called PR65),
with which the C subunit is tightly associated in the cell.
3. One of four dissimilar types of regulatory subunits
(B, B¿,B–, and B9–¿) that bind to both the A and C subunits
and, to a large extent, control the substrate specificity of
PP2A.
All of PP2A’s subunits have multiple isoforms and
splice variants that are expressed in a tissue-specific and
developmentally specific manner, thereby generating an
enormous panoply of enzymes that are targeted to differ-
ent phosphoproteins in distinct subcellular sites. This com-
plexity is a major cause of our limited understanding of
how PP2A carries out its diverse cellular functions, even
though it comprises between 0.3 and 1% of cellular pro-
teins and, together with PP1, accounts for 90% of the
Ser/Thr phosphatase activity in most cells.
The X-ray structure of PP2A’s A subunit (Fig. 19-51a),
determined by David Barford, reveals a remarkable sole-
noidal protein that consists of 15 imperfect tandem repeats
of a 39-residue sequence termed HEAT (because it occurs
in proteins named Huntingtin, EF3, A subunit of PP2A,
and TOR1). Successive HEAT repeats, which each consist
of two antiparallel helices joined by a short linker, stack on
one another with their corresponding helices nearly paral-
lel so as to form an ⬃100-Å-long right-handed superhelix
(helix of helices) with a hooklike shape.
The X-ray structure of a PP2A holoenzyme (complete
enzyme; Fig. 19-51b), determined independently by Yigong
Shi and Wenqing Xu, reveals, unexpectedly, that its regu-
latory subunit consists of 8 tandem HEAT-like repeats
arranged like those of the A subunit, despite their lack of
722 Chapter 19. Signal Transduction
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 722
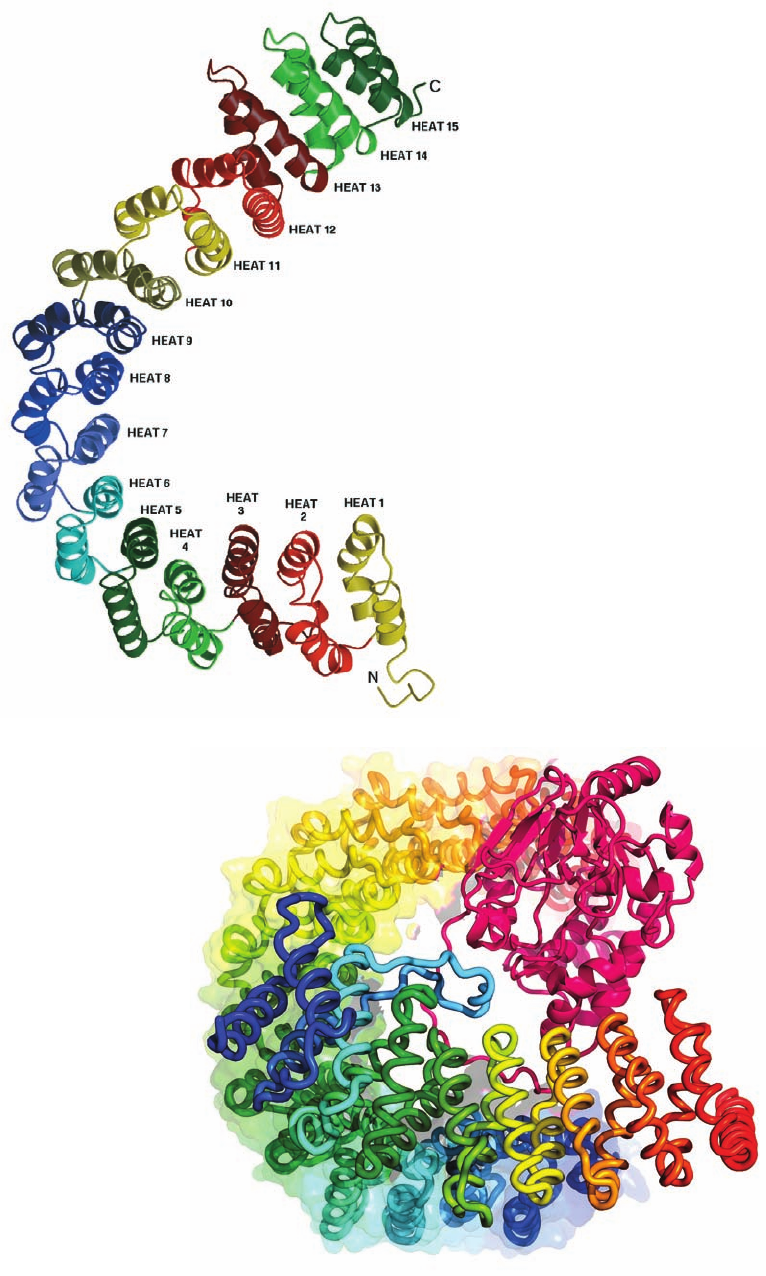
sequence similarity. The C subunit binds to the A subunit’s
concave surface along a ridge of conserved hydrophobic
side chains spanning HEAT repeats 11 to 15. The regula-
tory subunit similarly interacts with the A subunit’s HEAT
repeats 2 to 8 and also binds to the C subunit via a ridge
spanning its own HEAT-like repeats 6 to 8. The highly
acidic, convex side of the regulatory subunit (lower part of
Fig. 19-51b) is thereby left unoccupied, which suggests that
it interacts with substrate proteins. The C subunit is struc-
turally similar to the catalytic subunits of PP1 and PP2B.
Section 19-3. Tyrosine Kinase–Based Signaling 723
(a)
(b)
Figure 19-51 X-ray structure of protein phosphatase PP2A.
(a) The structure of an isolated scaffold (A) subunit. HEAT
repeats, which are drawn here in different colors, each consist of
two antiparallel helices joined by a short linker.These stack on
one another with their corresponding helices nearly parallel to
form an ⬃100-Å-long right-handed superhelix (helix of helices)
with a hooklike shape. Compare this structure to that of a
portion of human ankyrin (Fig. 12-39), which also forms a
right-handed solenoid, although consisting of ankyrin repeats.
[Courtesy of Bostjan Kobe, St. Vincent’s Institute of Medical
Research, Fitzroy,Victoria, Australia. X-ray structure by David
Barford, University of Oxford, U.K. PDBid 1B3U.] (b) The
structure of a PP2A heterotrimer viewed with the scaffold
subunit oriented approximately as in Part a. Here the scaffold
(A; 589 residues) and regulatory (B¿; 449 residues) subunits are
drawn in worm form, each colored in rainbow order from its
N-terminus (blue) to its C-terminus (red). In addition, the A
subunit is embedded in its transparent molecular surface. The
catalytic (C; 309 residues) subunit (magenta) is drawn in ribbon
form. Note the close structural resemblance of the A and B¿
subunits. [Based on an X-ray structure by Yigong Shi, Princeton
University. PDBid 2NPP.]
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 723
f. PP2B Is the Target of Immunosuppressant Drugs
PP2B, which is also known calcineurin (CaN), is unique
among protein Ser/Thr phosphatases in that it is activated
by Ca
2⫹
. CaN is a heterodimer composed of a catalytic A
subunit (CaNA) and a regulatory B subunit (CaNB).
CaNA contains an N-terminal catalytic domain followed
by a CaNB-binding domain, a calmodulin (CaM)-binding
domain, and a C-terminal autoinhibitory segment. CaNB,
which has 35% sequence identity with CaM, binds four
Ca
2⫹
ions via its four EF hand motifs (Section 18-3Ce).
CaN is activated by the binding of Ca
2⫹
to CaNB and
Ca
2⫹
–CaM to CaNA.
Calcineurin plays an essential role in the antigen in-
duced proliferation of T cells. As we discuss in Section
35-2D, the binding of an antigenic peptide to a T cell recep-
tor, a tyrosine kinase–associated receptor, initiates a com-
plex series of signaling events involving the Src-like PTKs
Lck and Fyn, a MAP kinase cascade,and the phosphoinosi-
tide cascade (Section 19-4), which, among other things,
releases Ca
2⫹
into the cytosol. The Ca
2⫹
, in turn, activates
CaN to dephosphorylate the transcription factor NFAT
p
(for nuclear factor of activated T cells). NFAT
p
in complex
with CaN is then translocated to the nucleus where, in
concert with other transcription factors, it induces some of
the early steps in T cell proliferation.
As we discussed Section 9-2B, the fungal products cy-
closporin A (CsA) and FK506 are highly effective im-
munosuppressants that are in clinical use for the preven-
tion of organ-transplant rejection and for the treatment of
autoimmune disorders (processes that are mediated by
T cells). CsA and FK506, respectively, bind to the peptidyl
prolyl cis–trans isomerases (rotamases) cyclophilin and
FK506 binding protein (FKBP12), which are therefore col-
lectively known as immunophilins. However, the observa-
tion that both CsA and FK506 (also known as tacrolimus)
are effective immunosuppressants at concentrations far be-
low those of the immunophilins suggests that it is the pres-
ence of the cyclophilin ⴢ CsA and FKBP12 ⴢ FK506 com-
plexes themselves, rather than the inhibition of their
rotamase activity, that interferes with T cell proliferation. It
is, in fact, the binding of either complex to CaN that pre-
vents it from dephosphorylating NFAT
p
and thereby sup-
presses T cell proliferation.
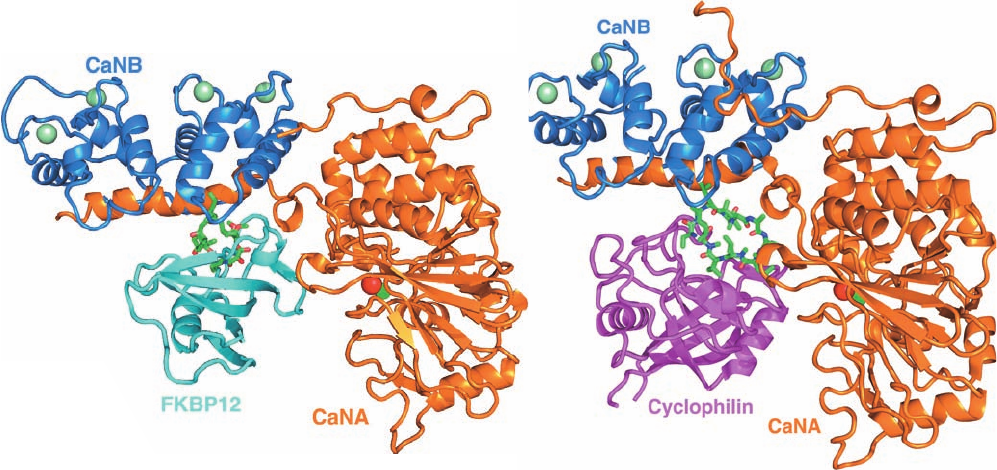
The X-ray structures of the bovine complex FKBP12 ⴢ
FK506–CaN, by Manuel Navia, and the corresponding hu-
man complex, by Ernest Villafranca, reveal how the
FKBP12 ⴢ FK506 complex binds to CaN (Fig. 19-52a). The
catalytic domain of CaNA, with its binuclear Fe
2⫹
–Zn
2⫹
center marking its active site, resembles those of other
protein Ser/Thr phosphatases of known structure. A
22-residue ␣ helix at the C-terminal end of this phosphatase
domain, which extends out from the phosphatase domain
by up to 40 Å, provides much of the CaNB-binding site.Be-
yond this helix, the C-terminal portion of CaNA, which
contains the CaM-binding site and the autoinhibitory seg-
ment, is not visible due to disorder. However, in the X-ray
structure of CaN alone, the autoinhibitory segment is seen
to bind in the CaNA active site so as to block the access of
substrate phosphoproteins. The structure of CaNB, which
724 Chapter 19. Signal Transduction
Figure 19-52 Calcineurin. (a) The X-ray structure of bovine
FKBP12 ⴢ FK506–CaN. The CaNA subunit is orange, the CaNB
subunit is blue, and the FKBP12 is cyan.The FK506 is drawn in
stick form with C green, N blue, and O red; the Fe
2⫹
and Zn
2⫹
ions in the CaNA active site are, respectively, represented by red
and green spheres; and the four Ca
2⫹
ions bound to CaNB are
represented by pale green spheres. [Based on an X-ray structure
by Manual Navia,Vertex Pharmaceuticals, Cambridge,
(b)
Massachusetts. PDBid 1TCO.] (b) The X-ray structure of human
cyclophilin ⴢ CsA–CaN. The CaN and its bound metal ions are
represented and oriented as in Part a, the cyclophilin is magenta,
and the CsA is drawn in stick form with C green, N blue, and O
red. Note the close resemblance between the two structures.
[Based on an X-ray structure by Hengming Ke, University of
North Carolina, Chapel Hill, North Carolina. PDBid 1M63.]
(a)
(a)
JWCL281_c19_671-743.qxd 6/11/10 6:58 AM Page 724

has four bound Ca
2⫹
ions, resembles that of Ca
2⫹
–CaM in
complex with a helical target peptide (Fig. 18-19) except
that CaNB’s two globular domains are on the same side of
its bound peptide rather than on opposite sides as are those
of Ca
2⫹
–CaM. CaNB thereby forms a continuous groove in
which the CaNA helix binds.
FKBP12 ⴢ FK506, a mixed inhibitor of CaN (Section
14-3C), binds to it so as to contact both CaNA and CaNB,
with the portion of FK506 that extends out from its
FKBP12-binding site forming a significant part of this in-
terface. The structures of FKBP12 and CaN in this com-
plex closely resemble those in the X-ray structures of
these proteins alone. It therefore appears that FK506 pro-
vides a critical component of this contact. Nevertheless, no
part of the FKBP12 ⴢ FK506 complex is within 10 Å of
CaN’s phosphatase site (although the CaN autoinhibitory
segment has been displaced). This accounts for the obser-
vations that FKBP12 ⴢ FK506 strongly inhibits CaN from
dephosphorylating a 20-residue phosphopeptide but actu-
ally increases the rate at which CaN dephosphorylates the
much smaller p-nitrophenylphosphate by a factor of 3.
The X-ray structure of human cyclophilin ⴢ CsA–CaN
(Fig. 19-52b), independently determined by Hengming Ke
and Harrison, is strikingly similar to that of FKBP12 ⴢ
FK506–CaN despite the fact that there is no structural re-
semblance of cyclophilin ⴢ CsA to FKBP12 ⴢ FK506. Cy-
clophilin binds to essentially the same region of CaNA as
does FKBP12 with CsA forming an essential component of
this contact, thereby limiting access to the CaN active site
in the same way as does FKBP12 ⴢ FK506. However, the
characteristics of rotamases that apparently uniquely suit
them for their roles in CaN inhibition remain enigmatic.
4 THE PHOSPHOINOSITIDE CASCADE
Extracellular signals often cause a transient rise in the cytoso-
lic [Ca
2⫹
], which, in turn, activates a great variety of enzymes
through the intermediacy of calmodulin and its homologs.
An increase in cytosolic [Ca
2⫹
] triggers such diverse cellular
processes as glycogenolysis (Section 18-3Ce) and muscle
contraction (Section 35-3C).What is the source of this Ca
2⫹
and how does it enter the cytosol? In certain types of cells,
neurons (nerve cells; Fig. 1-10d), for example, the Ca
2⫹
orig-
inates in the extracellular fluid. However, the observation
that the absence of extracellular Ca
2⫹
does not inhibit
certain Ca
2⫹
-mediated processes led to the discovery that,
in these cases, cytosolic Ca
2⫹
is obtained from intracellular
reservoirs, mostly the endoplasmic reticulum (and its equiv-
alent in muscle, the sarcoplasmic reticulum). Extracellular
stimuli leading to Ca
2⫹
release must therefore be mediated
by an intracellular signal.
The first clue as to the nature of this signal came from
observations that the intracellular mobilization of Ca
2⫹
and the turnover of phosphatidylinositol-4,5-bisphosphate
(PIP
2
or PtdIns-4,5-P
2
; Fig. 19-53), which occurs mainly in
the plasma membrane as a minor (⬍1%) component of its
cytosolic leaflet, are strongly correlated. This information
led Robert Michell to propose, in 1975, that PIP
2
hydrolysis
is somehow associated with Ca
2⫹
release.
A. Ca
2ⴙ
, Inositol Trisphosphate, and Diacylglycerol
Are Second Messengers
Investigations, notably by Mabel and Lowell Hokin,
Michael Berridge, and Michell, eventually revealed that
PIP
2
is part of an important second messenger system, the
phosphoinositide cascade, that mediates the transmission
of numerous hormonal signals including those of vaso-
pressin, CRF, TRF (Section 19-1H), acetylcholine (a neuro-
transmitter;Section 20-5Cb), epinephrine (with ␣
1
-adrenergic
receptors; Section 19-1F), EGF, and PDGF. Remarkably,
this system yields up to three separate types of second
messengers through the following sequence of events
(Fig. 19-54):
1–3. The ligand–receptor interactions described below
activate a phosphoinositide-specific phospholipase C
(PLC; Section 19-4B) to hydrolyze PIP
2
to inositol-1,4,5-
trisphosphate (IP
3
or Ins-1,4,5-P
3
) and sn-1,2-diacylglycerol
(DAG or DG)
Section 19-4. The Phosphoinositide Cascade 725
Figure 19-53 Molecular formula of the phosphatidylinositides.
The head group of these glycerophospholipids is myo-inositol
that may be phosphorylated at its 3-, 4-, and/or 5-positions. R
1
is
predominantly the hydrocarbon tail of stearic acid (an 18:0 fatty
acid;Table 12-1) and R
2
is predominantly the hydrocarbon tail of
arachidonic acid (a 20:4 fatty acid).
P
CH
2
CH
2
CH
OH
H
H
H
O
OR
2
O
⫺
O
OPO
3
2
⫺
OPO
3
2
⫺
HHO
HO
OH H
OR
1
CH
2
CH
2
CH
⫺
2
O
3
PO H
H
H
H
O
OR
2
OPO
3
2
⫺
OPO
3
2
⫺
HHO
HO
OH
OH
H
OR
1
PLC
H
2
O
⫹
DAG
IP
3
PIP
2
PCH
2
CH
2
OCR
1
CHCR
2
O
H
H
H
H
O
O
O
OO
⫺
O
OY
OY
HHO
HO
OY H
Y ⫽ H or PO
3
2
⫺
16
25
34
JWCL281_c19_671-743.qxd 6/30/10 1:49 PM Page 725
[the stereospecific numbering (sn) system is described in
the legend of Fig. 12-3]. PLCs catalyze the hydrolysis of the
bond linking a glycerophospholipid to its phosphoryl group
as indicated in Fig. 19-55 (which also shows the actions of
other types of phospholipases). Note that this reaction oc-
curs at the interface between the aqueous phase and the
membrane in such a way that both PIP
2
and its amphi-
pathic hydrolysis product DAG remain associated with the
membrane during the catalytic reaction.
4. The water-soluble IP
3
, acting as a second messenger,
diffuses through the cytoplasm to the ER, from which it
stimulates the release of Ca
2
into the cytoplasm by bind-
ing to and thereby opening an ER-bound transmembrane
Ca
2
-specific ion channel known as the IP
3
receptor (ion
channels are discussed in Chapter 20).
726 Chapter 19. Signal Transduction
Protein kinase
C (PKC)
inositol
polyphosphate
5-phosphatase
Protein
(inactive)
Protein
(inactive)
Protein
(active)
External
signal
1
2
3 3 6
5
5
4
+
R
qα
GDP
GTP GDP
qα
GTP
H
2
O
ATP
ADP
ADP
Ca
2+
– CaMCa
2+
Ca
2+
IP
3
IP
3
IP
3
-gated Ca
2+
transport channel
IP
2
ATP
GDP
+ P
i
H
2
OP
i
S
PIP
2
DAGDAG PS
P
Cellular
response
Plasma membrane
Cytosol
Endoplasmic reticulum
membrane
kinase
γ
β
γ
β
Phospho-
lipase C
(PLC)
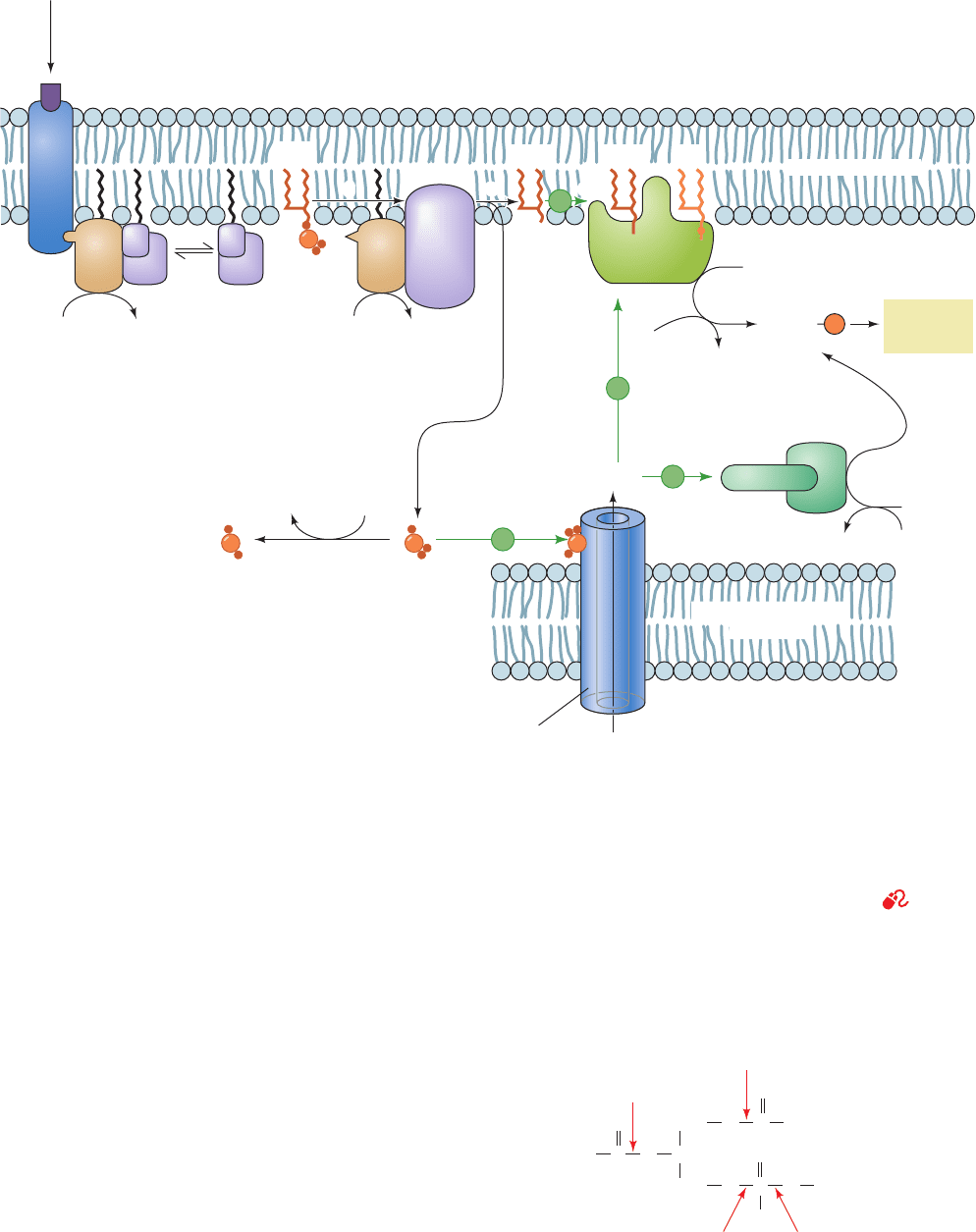
Figure 19-54 Role of PIP
2
in intracellular signaling. (1) The
binding of a ligand to a cell-surface receptor, R, activates a
phosphoinositide-specific phospholipase C through the
intermediacy of what is shown here as (2) a G protein (G
q
;Fig.
19-17) but in many cases is an RTK, an NRTK, or, possibly, Ca
2
.
Phospholipase C catalyzes the hydrolysis of PIP
2
to IP
3
and DAG
(3). The water-soluble IP
3
stimulates the release of Ca
2
sequestered in the endoplasmic reticulum (4), which in turn
Figure 19-55 A phospholipase is named according to the bond
that it cleaves on a glycerophospholipid. X is a phosphoinositol
group for this discussion.
activates numerous cellular processes through the intermediacy
of calmodulin and its homologs (5). The nonpolar DAG remains
associated with the inner leaflet of the membrane, where it
activates protein kinase C to phosphorylate and thereby
modulate the activities of a number of cellular proteins (6). This
latter activation process also requires the presence of the
membrane lipid phosphatidylserine (PS) and Ca
2
. See the
Animated Figures
PCH
2
CH
2
OCR
1
CHCR
2
O
O
O
O
O
O
OX
1
2
3
phospholipase A
1
phospholipase A
2
phospholipase C phospholipase D
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 726
5. The Ca
2
, in turn, stimulates a variety of cellular
processes, mainly through the intermediacy of calmodulin
and its homologs.
6. The amphipathic DAG is constrained to remain in
the inner leaflet of the plasma membrane, where it never-
theless also acts as a second messenger by activating pro-
tein kinase C (PKC; Section 19-4C) in the presence of Ca
2
and phosphatidylserine (PS; which is located exclusively on
the cytosolic face of the plasma membrane).This membrane-
bound enzyme (actually a family of enzymes; Section 19-
4C), in turn, Ser/Thr-phosphorylates and thereby modu-
lates the activities of several different proteins including
glycogen synthase (Sections 18-3D). DAG, which predomi-
nantly has a stearoyl group at its 1-position and an arachi-
donoyl group at its 2-position, is further degraded in some
cells by cytosolic phospholipase A
2
(cPLA
2
) to yield
arachidonate, the major substrate for the biosynthesis of
prostaglandins, prostacyclins, thromboxanes, leukotrienes,
and lipoxins. These paracrine hormones, as we discuss in
Section 25-7, mediate or modulate a wide variety of physi-
ological functions.
B. The Phospholipases C
The phosphoinositide-specific PLCs in mammals are clas-
sified in six families comprising 13 isozymes according to
their sequences and modes of regulation: (1–4), (1–2),
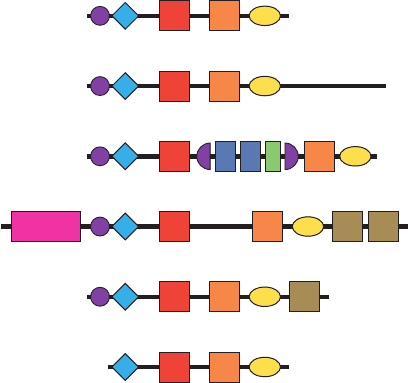
(1, 3, 4), ε, (1–2), and (Fig. 19-56; the isozyme origi-
nally named PLC- is actually a proteolytic fragment of
PLC-1), several of which have splice variants.All of these
PLCs require the presence of Ca
2
ion for enzymatic
activity. The PLC- isozymes (⬃760 residues) consist of,
from N- to C-terminus, an ⬃120-residue pleckstrin homol-
ogy (PH) domain (Section 19-3Ce); an ⬃130-residue EF
hand domain that contains four EF hand motifs (Fig.
18-18); two conserved regions known as X and Y that
together form the ⬃250-residue PLC catalytic domain and
which are separated by an ⬃60-residue linker; and an
⬃120-residue C2 domain, a domain that in many cases
binds Ca
2
and which occurs in ⬃650 human proteins that
mainly participate in signal transduction and membrane
interactions. The PLC- isozymes (⬃1200 residues) have
an additional ⬃420-residue C-terminal tail that has been
implicated in both membrane association and regulation
by G proteins (see below). In contrast, the PLC-
isozymes (⬃1270 residues) contain an ⬃420-residue insert
between X and Y that consists of an additional PH domain
that is split by two SH2 domains that are implicated in
binding to activated PTKs (see below) as well as an SH3
domain. PLC-ε (⬃2300 residues), which is activated by
several Ras-related G-proteins, has an N-terminal RasGEF
domain and two C-terminal Ras-binding (RA) domains.
PLC- (⬃1000 residues), which occurs only in nerve
tissue, contains a C-terminal Ser/Pro (S/P)-rich region of
unknown function. PLC- (⬃650 residues), the smallest of
the PLCs and the only one that lacks a PH
domain, occurs only in sperm. The observation that the
PLCs in plants and lower eukaryotes such as yeast are
-like suggests that the various PLC isozymes in mammals
evolved from a primordial PLC-.
a. G
q
GTP, G
, and Rac GTP Activate PLC-s by
Bringing Them Into Contact with the Membrane
The PLC-s are hormonally regulated by certain G
protein-coupled receptors (e.g., those for histamine, vaso-
pressin, TSH, thromboxane A
2
, and angiotensin II) via
their associated heterotrimeric G proteins, as indicated in
Fig. 19-54. In particular,they are activated through their in-
teractions with the subunits of the G
q
subfamily (Section
19-2C) in complex with GTP. G
q
GTPS activates the
PLC- isoforms in order of potency 1 3 2, with the
position of 4 in this hierarchy indeterminate because it is
inhibited by GTPS. Moreover, an important aspect of the
regulation of PLC-s by G
q
GTP is that the PLC-s
function as GAPs to increase the GTPase activity of G
q
by
50-fold, thereby limiting the activating function of G
q
.
The PLC- isoforms are independently activated by G
complexes, which may be supplied by the dissociation of
heterotrimeric G proteins other than G
q
G
. Moreover,
their order of efficacy with G
differs from that with
G
q
GTP: 3 2 1, with 4 insensitive to the pres-
ence of G
. Although the concentration of G
required
for maximal activation of the PLC-s is much greater than
that of G
q
GTP, their final extents of activation are simi-
lar. The sites on PLC-2 that interact with G
are its PH
domain and a 10-residue segment near the N-terminus of
its Y region. The region of G
that interacts with PLC-s
overlaps the region through which it binds G
subunits,
thereby explaining why a G
cannot simultaneously bind a
PLC- and a G
GDP.
The Rho family small G protein named Rac1, in complex
with GTP, activates PLC-2,-3,and -2.The X-ray structure
of a complex of PLC-2 lacking its C-terminal tail (but
still catalytically active in vitro) with Rac1 GTPS was
Section 19-4. The Phosphoinositide Cascade 727
Figure 19-56 Domain organization of the six classes of
phosphoinositide-specific PLCs.
Y
Y
SH2 SH2 SH3
RasGEF X
X
X
X
Y
Y
RA
SP
RA
Y
Y
PLC-δ (1,3,4)
PLC-β (1–4)
PLC-γ (1–2)
PLC-ε
PH EF-hand C2
Y
X
Y
PLC-η (1–2)
Y
X
Y
PLC-ζ
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 727
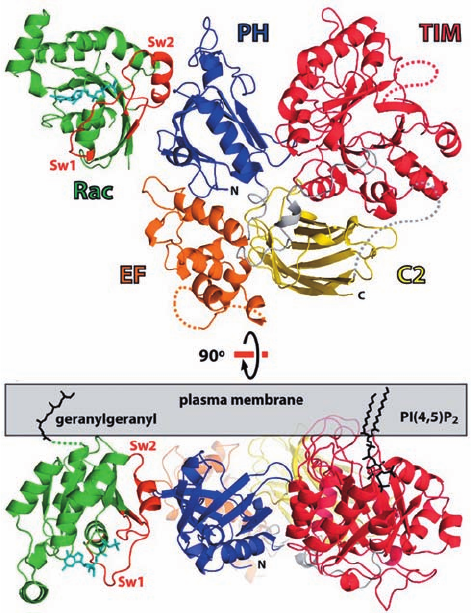
determined by John Sondek. It reveals (Fig. 19-57) that PLC-
2’s remaining four domains are linked by extended
polypeptide segments that form tight interactions with these
domains. EF hand motifs 1 and 2 form a lobe that is closely
superimposable on the N- and C-terminal lobes of calmod-
ulin (Figs. 18-17 and 18-19),as do EF hands 3 and 4.Although
EF hands are usually associated with Ca
2⫹
ions, the residues
necessary to do so are poorly conserved in the PLCs. Thus,
although mutations to this domain are generally deleterious
to a PLC’s catalytic activity, its function is unclear.
The X and Y regions of PLC-2’s catalytic domain form
a TIM (or ␣/) barrel (Section 8-3B). The X region con-
tributes a typical ␣␣␣ motif to form nearly half the
barrel with the Y region contributing its remaining portion.
The visible parts of the partially disordered ⬃70-residue
peptide linking X and Y occlude the enzyme’s active site,
which as is the case for all TIM barrel enzymes, is located in
the mouth of the barrel at the C-terminal ends of its
 strands. Deletion of this apparently autoinhibitory X–Y
linker increases the enzyme’s phospholipase activity by
⬃10-fold, although it can still be further activated by vari-
ous G proteins. Although the X–Y linkers of the several
PLCs share little sequence identity, all of them contain
clusters of negatively charged residues. This suggests that
when a PLC approaches the negatively charged cytosolic
face of a membrane, its X–Y linker is electrostatically re-
pelled from the PLC’s active site, thereby permitting these
enzymes access to their membrane-bound PIP
2
substrates.
All PLCs require a bound Ca
2⫹
ion for catalytic activity.
The constellation of active site residues in the PLC-2
structure closely resemble that in the X-ray structure of
PLC-␦1 in complex with IP
3
and Ca
2⫹
ion. In this latter
structure, a 6-coordinate Ca
2⫹
is bound at the bottom of
the active site, with one of its ligands contributed by the
2-hydroxyl group of IP
3
and the remainder formed by highly
conserved Asp,Glu,and Asn side chains.The catalytic reac-
tion is therefore postulated to occur via a mechanism anal-
ogous to that of the RNase A–catalyzed hydrolysis of RNA
(Fig. 15-3) in which PIP
2
’s 2-hydroxyl group nucleophili-
cally attacks the neighboring 1-phosphate group to form
DAG and a cyclic phosphodiester intermediate that is sub-
sequently hydrolyzed to yield IP
3
. The Ca
2⫹
ion (rather
than a His side chain as in RNase A) is properly positioned
to promote the deprotonation of the 2-hydroxyl group so
as to enhance its nucleophilicity and to subsequently help
stabilize the developing negative charge on the pentava-
lent phosphorus in the catalytic reaction’s transition state
(Fig. 16-6b). This explains why 2-deoxy-PIP
2
is not hy-
drolyzed by mammalian phosphinositide-specific PLCs.
Rac1 ⴢ GTP␥S binds to the PH domain of PLC-2 exclu-
sively via Rac1’s switch regions (Fig. 19-57), which explains
why Rac1 ⴢ GDP does not bind to PLC-2. However, compar-
ison of the X-ray structure of PLC-2 alone with that of its
Rac1 ⴢ GTP␥S complex indicates that the binding of
Rac1 ⴢ GTP␥S does not alter the conformation of PLC-2.
How, then, does Rac1 ⴢ GTP␥S activate PLC-2? Most PH
domains bind membrane-associated phosphoinositides (see
below) but those of PLC-2 and -3 lack the residues neces-
sary to do so. Instead, Rac1’s C-terminal geranygeranyl mem-
brane anchor (Section 12-3Ba) functions to localize PLC-2
and probably PLC-3 to the cytosolic face of the plasma mem-
brane (Fig. 19-57b), where they can efficiently hydrolyze phos-
phinositides. Similarly, the membrane anchored G
q␣
ⴢ GTP
and G
␥
serve to localize PLC-2 to the membrane.
The C2 domain consists of a sandwich of two 4-stranded
antiparallel  sheets with variable interstrand loops. The
X-ray structure of PLC-␦1 complexed with the calcium
analog lanthanum revealed that the C2 domain contains
three Ca
2⫹
ions sites in close proximity to one another.All
of these metal ions lie in a crevice at one end of the  sand-
wich, where they are exposed on the surface of the enzyme.
It therefore seems likely that, in vivo, they associate with
anionic head groups such as that of phosphatidylserine on
the surface of a membrane. Since the extensive interface
between the C2 and catalytic domain appears to be rigid,
this interaction probably helps bind the catalytic domain to
the membrane such that it can productively interact with
728 Chapter 19. Signal Transduction
(a)
(b)
Figure 19-57 X-ray structure of PLC-2 lacking its
C-terminal tail in complex with Rac1 ⴢ GTP␥S. The pleckstrin
homology (PH) domain of PCL-2 is blue, its EF hand (EF)
domain is orange, its TIM (␣/) barrel is red, and it’s C2 domain
is yellow. The Rac1 is green with its switch regions (Sw1 and
Sw2) red and its bound GTP␥S drawn in stick form in cyan. (a)
View toward the cytosolic surface of the membrane. (b) View
rotated 90° about the horizontal axis relative to Part a.The
geranylgeranyl group at the C-terminus of Rac1 and the PIP
2
substrate (black sticks) are modeled as sites of membrane
attachment. Note that the three loops of the TIM barrel, which
form a hydrophobic ridge, are inserted into the membrane.
[Courtesy of John Sondek, University of North Carolina School
of Medicine. PDBid 2FJU.]
JWCL281_c19_671-743.qxd 6/4/10 10:55 AM Page 728
PIP
2
molecules. This association appears to be supple-
mented through the interactions of a hydrophobic ridge
comprised of three loops from one side of the active site
opening that is postulated to penetrate into the mem-
brane’s nonpolar region during catalysis (Fig. 19-57b). This
would explain how the enzyme can catalyze the hydrolysis
of PIP
2
to DAG and IP
3
while the former two compounds
remain associated with the membrane.
The C-terminal tail that is unique to PLC- isoforms
(Fig. 19-56) has been implicated, via studies of truncated
enzymes, in binding to G
q␣
ⴢ GTP. The X-ray structure of
this ⬃420-residue segment reveals that it consists almost
entirely of three long helices which form a coiled coil that
dimerizes along its long axis. It contains a large number of
basic residues that are clustered on one face of the dimer
and whose mutation results in reduced responses to
G
q␣
ⴢ GTP. This positively charged surface is likely to inter-
act with acidic phospholipids so as to recruit its attached
PLC- to the membrane. It therefore appears that the acti-
vation of PLC-bs is almost entirely the result of their close
association with the negatively charged membrane.
b. The Pleckstrin Homology Domain Tethers
PLC-␦1 to the Membrane
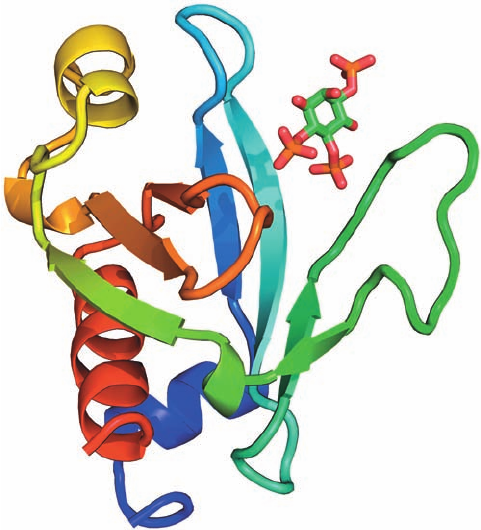
The X-ray structure of PLC-␦1’s N-terminal PH domain
in complex with IP
3
, determined by Joseph Schlessinger
and Sigler,reveals that the IP
3
binds to a positively charged
surface of the protein (Fig. 19-58). This is consistent with
the PH domain’s proposed role as a membrane anchor, as
are the observations that this PH domain binds PIP
2
with
much greater affinity (K
D
⫽ 1.7 M) than does PLC-␦1’s
catalytic domain (K
D
⬎ 0.1 mM). Since the peptide seg-
ment linking the PH domain to the rest of the enzyme is
probably flexible, it appears that the PH domain in PLC-␦1
functions to tether the enzyme to the membrane. This ac-
counts for kinetic measurements indicating that the en-
zyme catalyzes multiple cycles of PIP
2
hydrolysis without
releasing the membrane.
Despite the foregoing, the way in which the PLC-␦
isozymes are regulated is poorly understood. The higher
sensitivities of the PLC-␦ isozymes to Ca
2⫹
ion compared
to those of the other PLCs suggests that the PLC-␦
isozymes are regulated by changes in intracellular [Ca
2⫹
].
Thus, the activation of PLC-␦ isozymes may occur second-
arily to the receptor-mediated activation of other PLC
isozymes through their induction of the opening of Ca
2⫹
channels (Fig. 19-54).
c. Protein Tyrosine Kinases Recruit PLC-␥
Isozymes to the Membrane
The PLC-␥ isozymes in a wide variety of cells are acti-
vated by certain protein growth factors including EGF,
PDGF, FGF, and NGF. These growth factors cause their
corresponding receptors, which are RTKs (Section 19-3A), to
autophosphorylate at particular Tyr residues. Some of
these phosphoTyr sites are specifically bound,as mutational
studies indicate, by the more N-terminal SH2 (N-SH2) do-
main on PLC-␥1 (Figs. 19-56 and 19-27a) but not its C-SH2
domain. The N-SH2 domain binds peptides containing a
phosphoTyr residue followed by at least 5 predominantly
hydrophobic residues, in contrast to the SH2 domain of
Src, which preferentially binds pYEEI containing peptides
(Section 19-3Cb).
The activated receptors for all four of the above growth
factors phosphorylate PLC-␥1 at the same three Tyr
residues, 771, 783 (located between the C-SH2 and SH3 do-
mains), and 1254 (located in the C-terminal tail). In fact,
mutating Tyr 783 to Phe completely blocks the activation of
PLC-␥1 by PDGF, although this mutant PLC-␥1 still asso-
ciates with the PDGF receptor. Conversely, mutating
certain RTK autophosphorylation sites (e.g., the PDGF
receptor’s Tyr 1021) disrupts their binding of PLC-␥1 and
hence its activation, even though these mutant receptors
catalyze detectable levels of growth factor–dependent Tyr
phosphorylation on PLC-␥1. Evidently, growth factor–
induced activation of PLC-␥1 requires both the activating
Tyr phosphorylation of PLC-␥1 and its association with the
growth factor receptor, the latter presumably bringing
PLC-␥1 into contact with its PIP
2
substrate in the cytosolic
leaflet of the plasma membrane. The PLC-␥ isozymes may
also be activated by NRTKs, such as members of the Src
and JAK families (all of which are membrane associated),
that have been activated by tyrosine kinase–associated re-
ceptors (Section 19-3E). The function of the PLC-␥ SH3
domain is unclear.
Section 19-4. The Phosphoinositide Cascade 729
Figure 19-58 X-ray structure of the pleckstrin homology domain
of PLC-␦1 in complex with PIP
3
. The peptide chain is shown in
ribbon form colored in rainbow order from its N-terminus (blue)
to its C-terminus (red).The PIP
3
is drawn in stick form with
C green, O red, and P orange.The PH domain consists largely of
the  barrel/ sandwich of 7 antiparallel strands and C-terminal
␣ helix common to the numerous PH domains of known structure.
[Based on an X-ray structure by Joseph Schlessinger, New York
University Medical Center, and Paul Sigler,Yale University.
PDBid 1MAI.]
JWCL281_c19_671-743.qxd 6/11/10 6:58 AM Page 729
d. PLC-d Is Activated by Ras GTP
The presence of RasGEF and Ras-binding (RA) do-
mains on PLC-
ε suggests that PLC-ε is activated by
Ras GTP. This is, in fact, the case, as indicated by the ob-
servation that PLC-
ε binds Ras GTP with high affinity
but does not bind Ras GDP. Since Ras is membrane-
anchored, this interaction brings PLC-
ε into proximity with
the membrane, much as we saw with the Rac1 GTP acti-
vation of PLC-2 (Section 19-4Ba). Although the growth
factor–induced activation of Ras is terminated by the hy-
drolysis of its bound GTP to GDP, the resulting Ras GDP
may be rapidly converted to Ras GTP by the RasGEF do-
main of PLC-
ε, thereby prolonging the receptor-mediated
activation of PLC-
ε. PLC-ε may also be activated by G
12
.
C. The Protein Kinases C
Protein kinase C (PKC), a member of the AGC family of
protein kinases (AGC for PKA,PKG, and PKC), is the
Ser/Thr protein kinase that transduces the numerous signals
mediated by the release of DAG (Fig. 19-54). In mammals, it
comprises a family of ten ⬃700-residue monomeric isozymes
classified in three subfamilies: the “conventional” PKCs (,
I, II, and , of which I and II are splice variants of the
same gene), the “novel” PKCs (,
ε, , and ), and the “atypi-
cal” PKCs [ and ι(human)/(mouse)]. The conventional
PKCs, which are activated by both DAG and Ca
2
, each con-
sist of an N-terminal autoinhibitory pseudosubstrate (which
resembles the enzyme’s target peptide but with the Ser/Thr
phosphorylation site replaced by Ala) followed by four con-
served domains, C1 through C4 (C1 for conserved region 1 of
PKC, etc.). The DAG-binding C1 domain, which occurs in
50 other proteins including Raf (in which it does not bind
DAG), consists of two tandemly repeated ⬃50-residue Cys-
rich motifs, C1A and C1B. However, only C1B binds DAG.
C2, which often binds Ca
2
ion, is also a component of PLC
(Fig. 19-57) as well as numerous other signaling proteins. C3
and C4 form the N- and C-terminal lobes of the protein ki-
nase, which is similar in sequence and structure to the cat-
alytic subunit of PKA (Fig. 18-15). The protein kinase is
maintained in its inactive state through its binding of the
pseudosubstrate (as with MLCK; Section 18-3Ce).The novel
PKCs, which are activated by DAG but not by Ca
2
, resem-
ble the conventional PKCs except that their C2 domains do
not bind Ca
2
.The atypical PKCs, which are unresponsive to
both DAG and Ca
2
,have only one Cys-rich motif in their C1
domains and lack C2 domains.
In addition to their different regulatory properties, the
various PKCs are localized to different subcellular com-
partments (e.g., plasma membrane, nuclear membrane, en-
doplasmic reticulum, Golgi apparatus, mitochondria) in
ways that vary with the cell type and external stimuli. This
specificity is provided both by targeting sequences on the
PKC as well as by scaffolding proteins that localize individ-
ual PKCs to specific membrane microdomains in close
proximity to their substrate and regulatory proteins. For
example, the members of a family of membrane-associated
proteins known as RACKs (for receptors of activated
C-kinases) each anchor a specific activated PKC to a par-
ticular subcellular location.
a. The C1 and C2 Domains Anchor PKC to the
Plasma Membrane
Phorbol esters such as 12-O-myristoylphorbol-13-acetate
(which occurs in croton seed oil and was used as a drastic
purgative in folk medicine) are potent activators of protein
kinase C; they structurally resemble DAG but bind to PKC
with ⬃250-fold greater affinity. Consequently, phorbol
esters are the most effective known tumor promoters,
substances that are not in themselves carcinogenic but
increase the potency of known carcinogens. They do so by
inhibiting the apoptosis (programmed cell death; Section
34-4E) and stimulating the proliferation of precancerous
cells (cells with only some of the mutations that make them
cancerous but which nevertheless reduce the cell’s sensitiv-
ity to apoptotic signals and increase it for proliferative
signals) as well as stimulating metabolic processes that
generate carcinogenic agents (e.g., free radicals). This in-
creases the probability that such a cell will complete its ma-
lignant transformation. Not surprisingly, therefore, altered
PKC activity is associated with various types of cancers.
The X-ray structure of the C1B motif of PKC in com-
plex with 12-O-myristoylphorbol-13-acetate, determined by
Hurley, reveals that this 50-residue motif is largely knit to-
gether by two Zn
2
ions, each of which is tetrahedrally lig-
anded by one His and three Cys side chains (Fig. 19-59).The
phorbol ester binds in a narrow groove between two loops
that consist mainly of nonpolar residues. Since phorbol es-
ters are also nonpolar,the entire top third of the complex, as
shown in Fig. 19-59, forms a highly conserved hydrophobic
surface. Very few soluble proteins have such a large fraction
of their surface formed by a continuous nonpolar region.
Moreover,the middle third of the protein surface,that below
the nonpolar region, forms a positively charged belt about
the protein.This suggests that, in vivo, the hydrophobic por-
tion of the complex is inserted into the nonpolar region of its
associated membrane such that the motif’s positively
charged belt interacts with the membrane’s negatively
charged head groups. This hypothesis is supported by NMR
measurements indicating that residues on the ligand-binding
portion of C1B interact with lipid.The fatty acyl group that
is esterified to phorbol’s 12-position in effective tumor
promoters presumably extends into the membrane so as to
help anchor the C1 domain to the membrane.
The comparison of this structure with that of C1B alone
indicates that C1B does not undergo significant structural
change on binding phorbol ester. Evidently, phorbol esters,
and presumably DAG, activate PKC by anchoring it to the
membrane rather than by an allosteric mechanism.The C2
H
3
C
H
3
C
O
OH
CH
2
OH
CH
3
CH
3
CH
3
OH
H
O
O
CC
13
H
27
O C
O
12-O-Myristoylphorbol-13-acetate
1
2
3
4
5
6
7
8
9
10
11
18
12
13
14
15
16
17
730 Chapter 19. Signal Transduction
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 730
