Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

glucagon receptors and to -adrenergic receptors. In such
cases, the amount of cAMP produced is the sum of that in-
duced by the individual hormones. G proteins may also act
in other ways than by activating AC: They are known, for
example, to stimulate the opening of K
channels in heart
cells and to participate in the phosphoinositide signaling
system (Section 19-4A).
Some ligand–GPCR complexes inhibit rather than acti-
vate AC (Fig. 19-17, right). These include the
2
-adrenergic
receptor and receptors for somatostatin and opioids. The
inhibitory effect is mediated by “inhibitory” G protein, G
i
,
which may have the same and subunits as does “stimula-
tory”G protein,G
s
, but has a different subunit, G
i
(41 kD).
G
i
acts analogously to G
s
in that on binding to its corre-
sponding ligand–GPCR complex, its G
i
subunit exchanges
bound GDP for GTP and dissociates from G
. However,
G
i
inhibits rather than activates AC, through direct inter-
actions and possibly because the liberated G
binds to and
sequesters G
s
. The latter mechanism is supported by the
observation that liver cell membranes contain far more G
i
than G
s
. The activation of G
i
in such cells would therefore
release enough G
to bind the available G
s
.
G
s
and G
i
are members of a family of related proteins,
many of which have downstream effectors other than AC.
This family also includes:
1. G
q
, which forms a link in the phosphoinositide sig-
naling system (Section 19-4Ba).
2. Transducin (G
t
), a variant of G
i
, which transduces
visual stimuli by coupling the light-induced conformational
Section 19-2. Heterotrimeric G Proteins 691
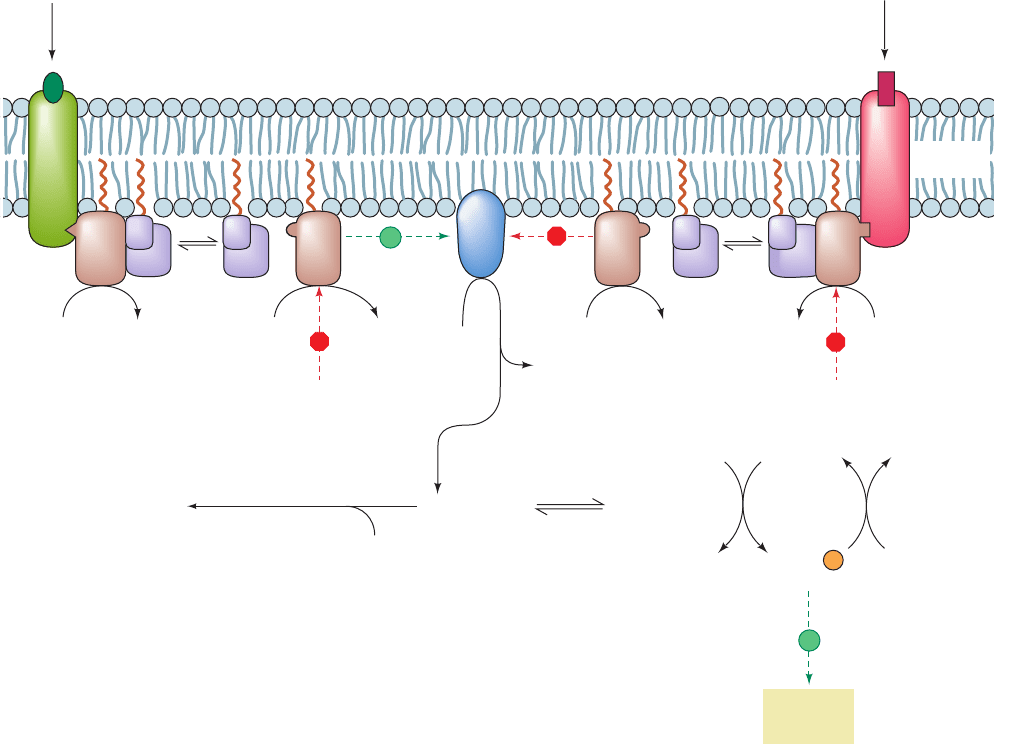
Figure 19-17 Mechanism of receptor-mediated activation/
inhibition of AC. The binding of hormone to a stimulatory
receptor, R
s
(left), induces it to bind G
s
protein, which, in turn,
stimulates the G
s
subunit of this G
s
G
heterotrimer to
exchange its bound GDP for GTP. The G
s
GTP complex then
dissociates from G
and, until it catalyzes the hydrolysis of its
bound GTP to GDP, stimulates adenylate cyclase (AC) to
convert ATP to cAMP. The binding of hormone to the inhibitory
++
Stimulatory
external
signal
Inhibitory
external
signal
Cholera
toxin
4ATP
Pertussis
toxin
phosphodiesterase
4cAMP
+ R
2
C
2
R
2
⋅ cAMP
4
+ 2C
Protein-
Cellular
response
phosphoprotein
phosphatase
AC
4PP
i
P
i
ADP
ATP
GDP
GDP
GTP GTP
GTP GTP
γ
ββ
γ
ββ
4H
2
O
GDP
GDP
Plasma
membrane
Cytosol
4AMP
γ
γ
H
2
OH
2
O
G
sα
⋅ GDP
+ P
i
G
iα
⋅ GDP
+ P
i
R
S
R
i
sα sα iα iα
H
2
O
Protein
inactive( )
active ( )
P
receptor, R
i
(right), triggers an almost identical chain of events
except that the presence of G
i
GTP complex inhibits AC from
synthesizing cAMP. R
2
C
2
represents protein kinase A (PKA),
whose catalytic subunit, C, when activated by the dissociation of
the regulatory dimer as R
2
cAMP
4
(Section 18-3Cb), activates
its target cellular proteins by catalyzing their phosphorylation.
The sites of action of cholera and pertussis toxins are indicated.
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 691
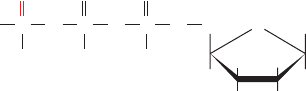
change of rhodopsin to the activation of a specific phos-
phodiesterase, which then hydrolyzes cGMP to GMP. This
cGMP-phosphodiesterase (cGMP-PDE) is an ␣␥
2
het-
erotetramer that is activated by the displacement of its
inhibitory ␥ subunits (PDE␥) by their tighter binding to
G
t␣
ⴢ GTP. A cation-specific transmembrane channel (Sec-
tion 20-3A) that is held open by the binding of cGMP
closes on the resulting reduction in [cGMP], thereby trig-
gering a nerve impulse (Section 20-5B) indicating that light
has been detected.
3. G
olf
, a variant of G
s␣
, which is expressed only in olfac-
tory sensory neurons and participates in odorant signal
transduction.
4. G
12␣
and G
13␣
, which participate in the regulation of
the cytoskeleton.
This heterogeneity in G proteins occurs in the  and ␥ sub-
units as well as in the ␣ subunits. In fact, 21 different ␣ sub-
units, 6 different  subunits, and 12 different ␥ subunits
have been identified in humans, some of which appear to
be ubiquitously expressed whereas others are expressed
only in specific cells.Thus, a cell may contain several closely
related G proteins of a given type that interact with varying
specificities with receptors and effectors. This complex sig-
naling system presumably permits cells to respond in a
graded manner to a variety of stimuli.
a. G Proteins Often Require Accessory Proteins
to Function
The proper physiological functioning of a G protein
often requires the participation of several other types of
proteins:
1. A GTPase-activating protein (GAP), which as its
name implies, stimulates its corresponding G protein to hy-
drolyze its bound GTP. This rate enhancement can be
⬎2000-fold. The downstream effectors of G
t␣
and G
q␣
,
cGMP-PDE (Section 19-3E) and PLC- (Section 19-4Ba),
respectively, exhibit GAP activities toward G
t␣
and G
q␣
(which otherwise would hydrolyze GTP at physiologically
insignificant rates), but AC does not exhibit GAP activity
toward either G
s␣
or G
i␣
. However, in humans, a diverse
family of 37 RGS proteins (for regulators of G protein sig-
naling) function as GAPs for G
␣
subunits by binding most
avidly to them when they are in the transition state confor-
mation for hydrolyzing GTP.
2. A guanine nucleotide exchange factor [GEF; alter-
natively guanine nucleotide releasing factor (GRF)], which
induces its corresponding G protein to release its bound
GDP. The G protein subsequently binds another guanine
nucleotide (GTP or GDP, which most G proteins bind with
approximately equal affinities), but since cells maintain a
GTP concentration that is 10-fold higher than that of GDP,
this, in effect, exchanges the bound GDP for GTP. For het-
erotrimeric G proteins, the agonist–GPCR complexes
function as GEFs.
3. A guanine nucleotide-dissociation inhibitor (GDI).
A G
␥
may be regarded as its associated G
␣
’s GDI because
GDP dissociates slowly from isolated G
␣
subunits but is es-
sentially irreversibly bound by heterotrimers.
b. The X-Ray Structures of G
␣
Proteins Rationalize
Their Functions
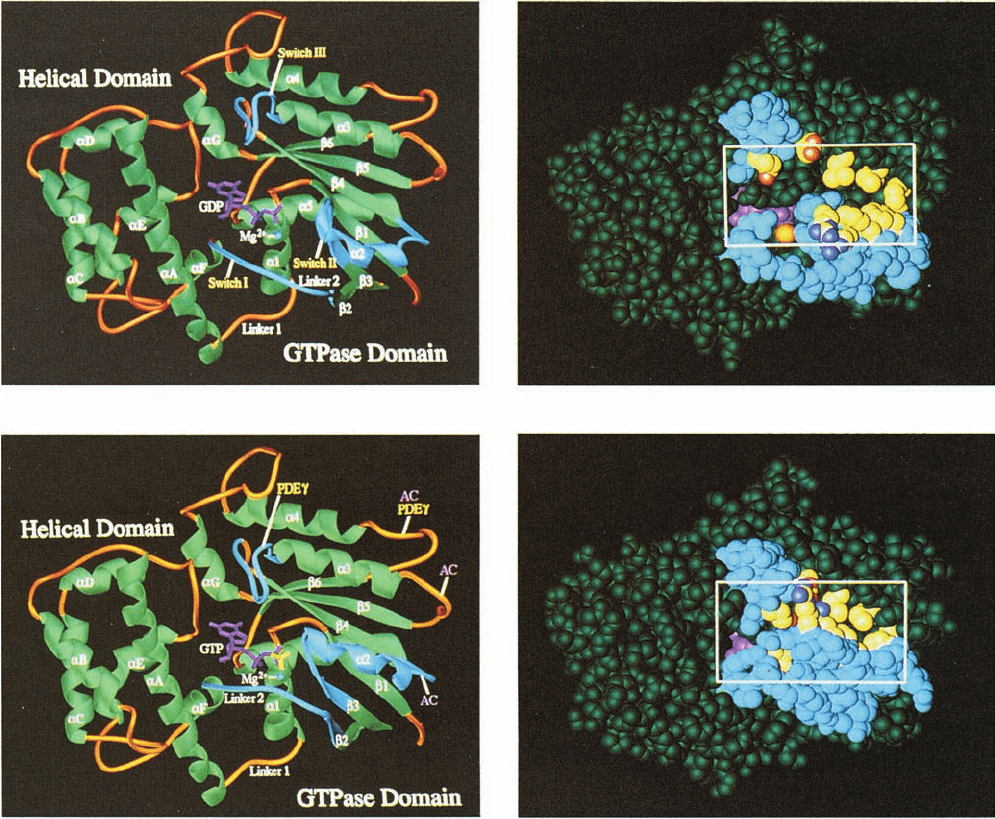
The X-ray structures of the C-terminal 325 residues of
the 350-residue bovine transducin-␣ (G
t␣
) in its complexes
with GDP (Fig. 19-18a,b) and with the poorly hydrolyzable
GTP analog GTP␥S
(Fig. 19-18c,d) were determined by Heidi Hamm and Paul
Sigler. G
t␣
consists of two clearly delineated domains con-
nected by two polypeptide linkers: (1) a highly conserved
GTPase domain that is structurally similar to those in other
G proteins of known structure (and hence is often de-
scribed as a Ras-like domain), and (2) a helical domain
that is unique to heterotrimeric G proteins. Guanine
nucleotides bind to G
t␣
in a deep cleft that is flanked by
these domains. The X-ray structures of G
i␣
ⴢ GTP␥S and
G
s␣
ⴢ GTP␥S, both determined by Gilman and Stephen
Sprang, closely resemble that of the G
t␣
ⴢ GTP␥S.
Comparison of the structures of the G
t␣
ⴢ GDP and
G
t␣
ⴢ GTP␥S complexes reveals that the presence of GTP’s
␥ phosphate group promotes significant conformational
shifts in three loops known as switch regions, all of which
are located on the facing side of G
t␣
in Fig. 19-18. The ␥
phosphate hydrogen bonds to side chains on Switches I and
II, thereby pulling these polypeptide segments in toward it
and causing Switch II to contact Switch III in a way that
pulls it to the right in Fig. 19-18.These concerted conforma-
tional shifts cause an extensive cavity over the GDP-
binding site to largely fill in the GTP␥S complex.
Switches I and II have counterparts in other G proteins
of known structure. Portions of these polypeptide segments
have been implicated in the interactions of G
t␣
with the
cGMP-PDE it activates and in the interactions between
the closely related G
s␣
with its target AC (Section 19-2D).
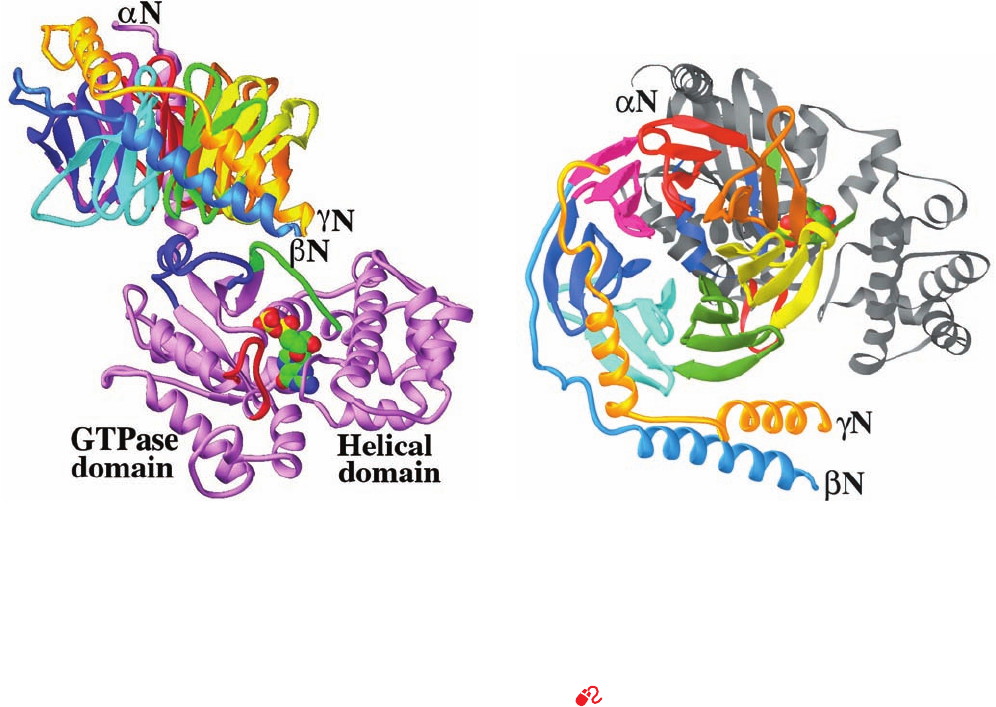
c. The X-Ray Structures of Heterotrimeric G Proteins
The X-ray structures of heterotrimeric G proteins were
determined by Gilman and Sprang (G
i
ⴢ GDP; Fig. 19-19)
and by Hamm and Sigler (G
t
ⴢ GDP). These structures
reveal that the G

subunit (Fig. 19-19b) consists of an
N-terminal helical domain followed by a C-terminal domain
comprising seven 4-stranded antiparallel  sheets arranged
like the blades of a propeller¬a  propeller whose blades
are each formed by a WD40 sequence motif (Section
12-4Cb)¬that surround a water-filled central channel. The
WD40 motif occurs in a functionally diverse group of 4- to
8-bladed -propeller proteins, including the 7-bladed
N-terminal domain of the clathrin heavy chain (Section
12-4Cb). The G
␥
subunit consists mainly of two helical
GTP␥S
O
O
O
P
⫺
O
⫺
O
HH
HH
OH OH
CH
2
G
OP PO
⫺
O
⫺
O
OS
692 Chapter 19. Signal Transduction
JWCL281_c19_671-743.qxd 6/4/10 10:52 AM Page 692
segments joined by a polypeptide link (Fig. 19-19b). It is
closely associated with G
along its entire extended length
through mainly hydrophobic interactions and hence has no
tertiary structure. The X-ray structure of isolated G
is
essentially identical to that in the G
GDP–G
complex,
thereby indicating that the structure of G
is unchanged
by its association with G
GDP.
G
and G
associate mainly via highly conserved
contacts between the Switch I and II regions of G
and
the loops and turns at the bottom of G
’s propeller
(Fig. 19-19). In addition, there is a less extensive interaction
between the N-terminal helix of G
(which is disordered
in G
alone) and the first blade of the G
propeller
(back side of Fig. 19-19a). Comparison of the structure
Section 19-2. Heterotrimeric G Proteins 693
Figure 19-18 Structural differences between the inactive and
active forms of G
t
(transducin). The change in structure is
indicated by the comparison of the X-ray structure of G
t
GDP
in its (a) ribbon and (b) space-filling forms with that of G
t
GTPS in its (c) ribbon and (d) space-filling forms, all viewed
from the same direction. In the ribbon drawings, helices and
sheets are green; the segments linking them are gold; the guanine
nucleotides are magenta, except for the phosphate of GTPS,
which is yellow; and the bound Mg
2
ion is represented by a
blue ball.The protein’s three switch regions (I, II, and III)
are highlighted in cyan. In Part c, the two loop regions of
the protein that are implicated in its interaction with the
(a) (b)
(c) (d)
cGMP-phosphodiesterase subunit to which it binds (PDE) are
pointed out with yellow labels, whereas the three loop regions
that are implicated in the interaction of the homologous G
s
with
adenylate cyclase (AC) are indicated with pink labels. The space-
filling models are colored similarly to the ribbon diagrams except
for the yellow residues, which here represent those that appear
to propagate or stabilize the structural transitions induced by the
binding of the phosphate group. The box in the space-filling
models outlines the cavity in G
t
GDP that closes when the GDP
is replaced by GTPS and that has been implicated in modulating
the affinity of G
t
for G
and for the receptor. [Courtesy of Paul
Sigler,Yale University. PDBids 1TAG and 1TND.]
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 693
of G
␣
ⴢ GDP–G
␥
with that of G
␣
ⴢ GTP␥S reveals why
G
␣
cannot simultaneously bind GTP and G
␥
: In
G
a
ⴢ GDP–G
bg
, the Switch II segment of G
a
contacts G
b
in a
way that prevents Switch II from assuming the conforma-
tion it requires to bind GTP’s g phosphate. Moreover, the
conformational changes in Switch II are coordinated with
those in Switch I so that, together, they close over the GDP
bound to G
␣
–G
␥
, thereby accounting for its tight binding
relative to that in G
␣
ⴢ GDP.
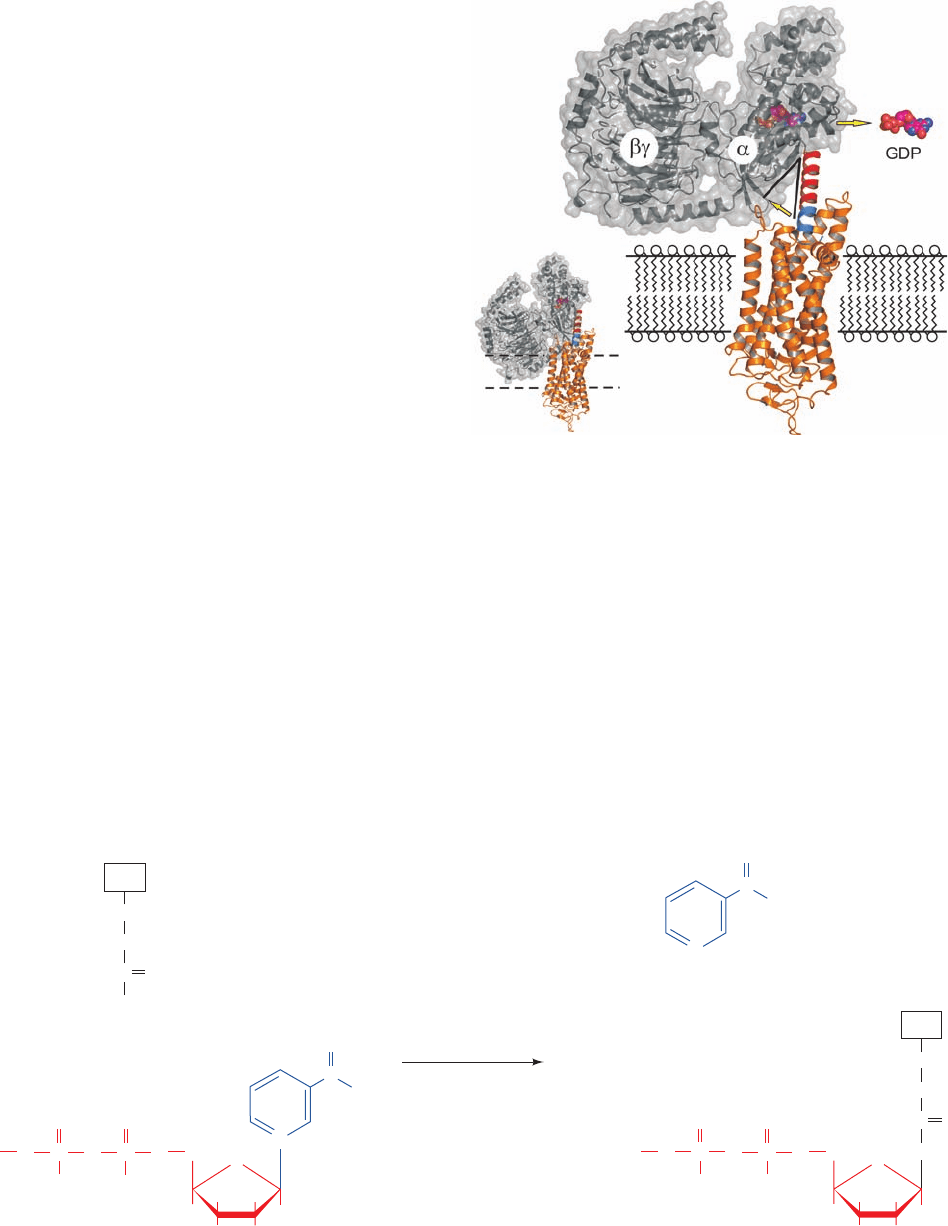
The question remains, how does a liganded GCPR in-
duce its target G
␣
subunit to exchange its bound GDP for
GTP? The X-ray structure of opsin in an activated confor-
mation in complex with the 11-residue C-terminal segment
of G
t␣
, determined by Oliver Ernst, suggests a partial an-
swer to this question.This helical segment (the right end of
helix ␣5 in Fig. 19-18a and the left end of the nearly hori-
zontal gray helix at the top of Fig. 19-19b), which has been
shown to be a major site of interaction with activated
opsin, binds at the cytosolic face of opsin. This prompted
the construction of the model in which the remainder
of G
t␣
G
␥
is appended onto the G
t␣
C-terminal segment
(Fig. 19-20).To avoid a steric clash between G
t␣
G
␥
and the
membrane, G
t␣
G
␥
must be tilted upward by 40°. A simple
mechanism for this process is a reorientation of the loop
connecting helix ␣5 and strand 6 (Fig. 19-18a). Since this
loop participates in binding the guanine ring of GDP, a rea-
sonable assumption is that this conformational adjustment
expels GDP from its binding pocket, which when opsin
subsequently releases G
t␣
G
␥
, allows GTP to bind in its
place.
d. Cholera Toxin Stimulates Adenylate Cyclase by
Permanently Activating G
s␣
The major symptom of cholera, an intestinal disorder
caused by the bacterium Vibrio cholerae, is massive diar-
rhea that, if untreated, frequently results in death from de-
hydration. This dreaded disease is not an infection in the
usual sense since the vibrio neither invades nor damages
tissues but merely colonizes the intestine, much like E. coli.
The catastrophic fluid loss that cholera induces (often over
6 liters per hour!) occurs in response to a bacterial toxin.
Indeed, merely replacing cholera victims’ lost water and
salts enables them to survive the few days necessary to im-
munologically eliminate the bacterial infestation.
Cholera toxin (CT; also known as choleragen) is an
87-kD protein of subunit composition AB
5
in which the B
subunits (103 residues each) form a pentagonal ring to which
the A subunit (240 residues) is bound. Previous to CT’s se-
cretion, its A subunit is cleaved at a single site by a bacter-
ial protease to yield two fragments, A1 (the N-terminal
694 Chapter 19. Signal Transduction
(a)
(b)
Figure 19-19 X-ray structure of the heterotrimeric G protein
G
i
. (a) The G
␣
subunit is violet with its Switch I, II, and III
segments green, blue, and red, respectively, and with its bound
GDP shown in space-filling form with C green, N blue, O red,
and P yellow. The G

subunit’s N-terminal segment is blue and
each blade of its  propeller has a different color.The G
␥
subunit
is gold.The view is perpendicular to the axis of the G

subunit’s
 propeller.The plasma membrane would be at the top of the
drawing as inferred from the positions of the N terminus of G
␣
and the neighboring C terminus of G
␥
, which, in vivo, are
lipid-linked to the plasma membrane. However, the orientation
of the protein relative to the plasma membrane is unknown (but
see Fig. 19-20). (b) View related to that in Part a by a 90° rotation
about its horizontal axis and thus looking from the general
direction of the plasma membrane. The protein is colored as in
Part a except that the G
␣
subunit is mainly gray. [Based on an
X-ray structure by Alfred Gilman and Stephan Sprang,
University of Texas Southwestern Medical Center. PDBid
1GP2.]
See Interactive Exercise 12
JWCL281_c19_671-743.qxd 10/19/10 7:36 AM Page 694
⬃195 residues) and A2 (the C-terminal ⬃45 residues), that
remain joined by a disulfide bond. On the binding of CT to
its cell surface receptor, ganglioside G
M1
(Sections 12-1D
and 25-8Cd), the nicked A subunit (but not the B subunits)
is taken into the cell via receptor-mediated endocytosis and
travels backward through the secretory pathway (Section
12-4B) to the Golgi apparatus. From there, it is conducted
into the endoplasmic reticulum (ER) via the binding of
A2’s C-terminal KDEL sequence to a KDEL receptor
(which normally functions to retrieve ER-resident proteins
that have escaped the ER; Section 12-4Ch). The A1 frag-
ment is then released from A2 and enters the cytoplasm
through the translocon (which normally conducts grow-
ing and still unfolded polypeptides into the ER; Section
12-4Bd) via a process in which A1 is unfolded through the
chaperonelike action of protein disulfide isomerase (PDI;
Section 9-2A).
In the cytoplasm, A1 catalyzes the irreversible transfer
of the ADP–ribose unit from NAD
⫹
to a specific Arg side
chain of G
s␣
(Fig. 19-21). This reaction is greatly acceler-
ated by the interaction of A1 with the small Ras-like G
protein ADP-ribosylation factor (ARF) in complex with
GTP, which normally functions to prime the formation of
clathrin-coated vesicles (Section 12-4Cd).
Section 19-2. Heterotrimeric G Proteins 695
Figure 19-21 Mechanism of action of cholera toxin. The
cholera toxin’s A1 fragment in complex with ARF GTP
catalyzes the ADP-ribosylation of a specific Arg residue on G
s␣
Figure 19-20 Model for signal transmission from an activated
GPCR to its target heterotrimeric G protein. (a) The X-ray
structure of activated bovine opsin (orange, oriented relative to
that in Fig. 19-16 by an ⬃90° rotation about the vertical axis) in a
complex with the 11-residue C-terminal helical segment from G
t␣
(blue).The X-ray structure of G
t␣
G
␥
(gray) is positioned over
the opsin structure such that this portion of the G
t␣
’s C-terminal
helix (the remainder of which is red) is superimposed on the
helical segment bound to opsin.The dashed lines delineate the
membrane in which opsin is embedded. Note that this model
results in a steric clash between the G protein and the membrane.
(b) To alleviate this steric clash, the G protein has been rotated
by 40° (lower yellow arrow) through a conformational change in
the loop preceding the C-terminal helix of G
t␣
.This loop
participates in binding guanine nucleotides (GDP and GTP) and
hence it is postulated that its reorganization expels GDP (drawn
in space-filling form with C magenta, N blue, and O and P
orange) from its binding pocket (upper yellow arrow). On the
subsequent dissociation of opsin, the G
t␣
binds GTP (which the
cell maintains at an ⬃10-fold higher concentration than GDP),
and consequently dissociates from G
␥
. [Courtesy of Oliver
Ernst, Charité–Universitätsmedizin Berlin, Germany. PDBids
3DQB and 1GOT.]
(a)
(b)
OP
O
C
O
O
–
OP
O
O
–
O
C
NH
NH
2
NH
2
(CH
2
)
3
O
HH
H
H
OHOH
CH
2
A
denosine
N
+
NH
2
C
O
N
H
+
NH
2
NAD
+
Nicotinamide
Arg
+
G
s␣
+
OP
O
O
–
OP
O
O
–
O
C
NH
NH
NH
2
(CH
2
)
3
O
HH
H
H
OHOH
CH
2
Adenosine
ADP-ribosylated G
s
G
s␣
+
A1 subunit of
cholera toxin
ARF GTP
+
by NAD
⫹
, thereby rendering this subunit incapable of hydrolyz-
ing GTP.
JWCL281_c19_671-743.qxd 6/30/10 2:22 PM Page 695
ADP-ribosylated G
s
GTP can activate AC but is in-
capable of hydrolyzing its bound GTP. As a consequence,
the AC remains “locked” in its active state. The epithelial
cells of the small intestine normally secrete digestive fluid
(an -rich salt solution) in response to small increases
in [cAMP] that activate intestinal Na
pumps through their
phosphorylation by PKA (ion pumps are discussed in Sec-
tions 20-3 and 20-4). The ⬃100-fold rise in intracellular
[cAMP] induced by CT causes these epithelial cells to pour
out enormous quantities of digestive fluid, thereby produc-
ing the symptoms of cholera. CT also affects other tissues
in vitro but does not do so in vivo because CT is not ab-
sorbed from the gut into the bloodstream.
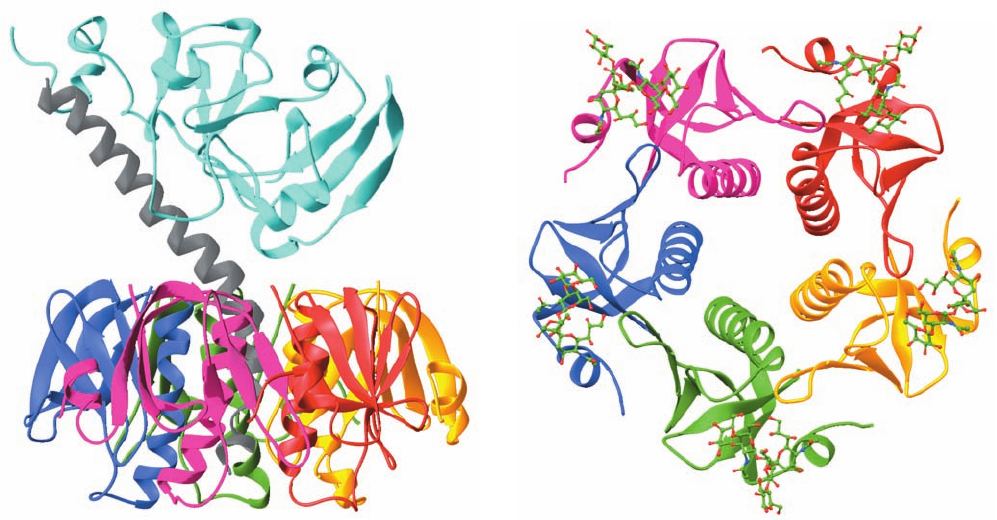
The remarkable X-ray structure of CT (Fig. 19-22a), de-
termined by Graham Shipley and Edwin Westbrook, re-
veals that its A2 segment forms an unusual extended helix
whose C-terminal end inserts into the B
5
pentamer’s solvent-
filled central pore, where it is noncovalently anchored.
The N-terminal segment of A2 extends beyond the B
5
pen-
tamer so as to tether the wedge-shaped A1 segment to B
5
,
much like a balloon on a string.The X-ray structure of only
B
5
in complex with the pentasaccharide from its G
M1
re-
ceptor (Fig. 19-22b), determined by Wim Hol, indicates that
this pentasaccharide binds through an extensive hydrogen
bonded network to each B subunit on the face of B
5
opposite
HCO
3
that which binds A.The binding of the A subunit or the re-
ceptor pentasaccharide to B
5
causes only modest structural
changes at their respective binding sites without altering
B
5
’s subunit interfaces. A1 contains an elongated crevice in
the vicinity of a catalytically implicated residue, Glu 112,
that presumably forms its active site.
Certain strains of E. coli cause a diarrheal disease (trav-
elers’ diarrhea) similar to, although considerably less se-
vere than, cholera through their production of heat-labile
enterotoxin (LT), a protein that closely resembles CT
(their A and B subunits are 80% identical and form AB
5
toxins that have closely similar X-ray structures) and has
the same mechanism of action. The reasons for the differ-
ence in severity of these infections are unclear (cholera can
be fatal within hours, whereas enterotoxic strains of E. coli
usually only temporarily incapacitate adults, although they
are responsible for the deaths of hundreds of thousands of
children annually). It might be due to the modest structural
differences between the toxins, differences in the amounts
of toxin secreted, and/or variations in microbial ecology.
The foregoing results provide a structural basis for the
design of ligands that interfere with the binding of CT and
LT to their receptors. Since these receptors occur on the
surface of the intestinal epithelium, ligands that compete
with them need not pass through any membrane. This
696 Chapter 19. Signal Transduction
Figure 19-22 X-ray structure of cholera toxin. (a) The entire
AB
5
complex as viewed parallel to the presumed direction of the
plane of the plasma membrane to which it binds, extracellular
side up. The A1 segment is cyan, the A2 segment is gray, and each
B subunit has a different color.Although the A1 and A2
segments in this structure form a continuous polypeptide chain,
residues 193–195, which immediately precede the peptide bond
that is cleaved on toxin activation, are disordered and hence not
visible here (upper left). The C-terminal end of the A2 helix
binds in the pentamer’s central pore. [Based on an X-ray
(a)
(b)
structure by Graham Shipley, Boston University School of
Medicine, and Edwin Westbrook, Northwestern University.
PDBid 1XTC.] (b) The structure of only the B
5
pentamer in
which each subunit is binding CT’s G
M1
receptor pentasaccharide.
The structure is viewed as from the bottom of Part a. The
subunits of the B
5
pentamer are colored as in Part a and the
pentasaccharides are shown in ball-and-stick form with C green,
N blue, and O red. Note the pentamer’s large central pore.
[Based on an X-ray structure by Wim Hol, University of
Washington. PDBid 2CHB.]
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 696
greatly increases the usual ⬃500-D size limit for an effec-
tive drug candidate (Section 15-4Ba). Moreover,a large lig-
and is unlikely to enter the bloodstream and hence would
have minimal side effects. Consequently, the synthesis of
multivalent ligands that simultaneously bind with high
affinity to all five receptor binding sites on an AB
5
mole-
cule has yielded promising lead compounds against CT
and LT.
e. Pertussis Toxin ADP-Ribosylates G
i
Bordetella pertussis, the bacterium that causes pertussis
(whooping cough; a disease that is still responsible for
⬃300,000 infant deaths per year worldwide), produces an
AB
5
protein, pertussis toxin (PT), that ADP-ribosylates a
specific Cys residue in G
i
. In doing so, it prevents G
i
from
exchanging its bound GDP for GTP and therefore from in-
hibiting AC. PT’s X-ray structure, determined by Randy
Read, reveals that its A and B subunits are structurally ho-
mologous to those of CT and LT, although the A subunit of
PT extends from the opposite face of its B pentamer rela-
tive to that in CT. Moreover, PT’s B pentamer consists of
four different subunits (one in two copies), each of which is
only ⬃15% identical to the B subunits of CT and LT.
f. Receptors Are Subject to Desensitization
One of the hallmarks of biological signaling systems is
that they adapt to long-term stimuli by reducing their re-
sponse to them, a process named desensitization. These sig-
naling systems therefore respond to changes in stimulation
levels rather than to their absolute values. What is the mech-
anism of desensitization? In the case of -adrenergic re-
ceptors, an epinephrine–receptor complex, but not recep-
tor alone, is phosphorylated at one or more of the Ser or
Thr residues on its C-terminal tail by -adrenergic recep-
tor kinase (ARK). This cytosolic protein is recruited to
the plasma membrane through its interaction with G
when it is not bound to G
s
, which is also a consequence of
receptor activation. The phosphorylation of the receptor
decreases the ability of epinephrine to influence it, at least
in part by reducing the receptor’s epinephrine-binding
affinity. The phosphorylated receptor, in turn, is bound by
either of two 78% identical proteins known as -arrestins
in a way that blocks the activated receptor from activating
its target G protein. Moreover, the -arrestin binds to the
adaptor protein AP2 in clathrin-coated pits (Section 12-4Cd),
whereupon the -adrenergic receptor–-arrestin complex
is endocytotically sequestered (Section 12-5Bc) in spe-
cialized vesicles. The receptor’s otherwise extracellular,
epinephrine-binding surface then faces the interior of the
vesicle and the -arrestin is located on its outside, from
which it is subsequently released. The vesicles are devoid
of both heterotrimeric G protein and AC,thus further attenu-
ating the cell’s response to epinephrine. If, however, the ep-
inephrine level is reduced, the receptor is slowly dephos-
phorylated by a phosphatase and exocytotically returned
to the cell surface, thus restoring the cell’s epinephrine sen-
sitivity.Alternatively, the vesicles may fuse with endosomes
for the delivery of their contents to lysosomes, where the
receptor is proteolytically degraded (Fig. 12-91). In this latter
case, the restoration of epinephrine sensitivity requires the
synthesis of additional receptor.
ARK is a member of a family of seven proteins known
as GCPR kinases (GRKs) that,together with several other
types of protein kinases including PKA, phosphorylate the
C-terminal tails and/or the cytosolic loops of most agonist-
occupied GPCRs. As above, the phosphorylated GPCRs
are bound by a -arrestin and sequestered into endocytotic
vesicles, thereby isolating them from their corresponding
heterotrimeric G proteins. Moreover, the sites of a given
GCPR that are phosphorylated vary in a tissue-specific
way that provides further regulatory flexibility.
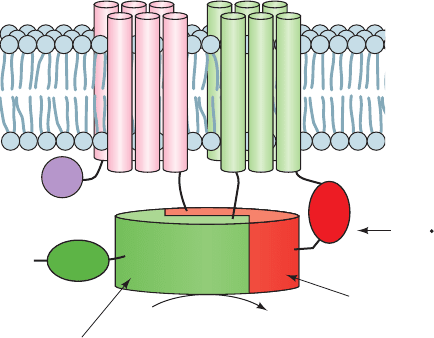
D. Adenylate Cyclases
The heterotrimeric G proteins G
s
and G
i
function to con-
trol the activities of adenylate cyclase (AC). In fact, mam-
mals have 9 known membrane-bound isoforms of AC,AC1
through AC9 (alternatively, AC-I through AC-IX), which
are each expressed in a tissue-specific manner and differ
in their regulatory properties. These ⬃120-kD transmem-
brane glycoproteins each consist of a small N-terminal do-
main (N), followed by two repeats of a unit consisting of a
transmembrane domain (M) followed by two consecutive
cytoplasmic domains (C), thus forming the sequence
NM
1
C
1a
C
1b
M
2
C
2a
C
2b
(Fig. 19-23). The ⬃30% identical C
1a
and C
2a
domains associate to form the AC’s catalytic core,
whereas C
1b
, as well as C
1a
and C
2a
, bind regulatory mole-
cules. Thus, G
i
inhibits AC1, 5, and 6 by binding to C
1a
;G
s
activates all AC isoforms but AC9 by binding to C
2a
;G
in-
hibits AC1, 3, and 8 but activates AC2, 4, and 7 by binding
Section 19-2. Heterotrimeric G Proteins 697
Figure 19-23 Schematic diagram of a typical mammalian AC.
The M
1
and M
2
domains are each predicted to contain six
transmembrane helices. C
1a
and C
2a
form the enzyme’s
pseudosymmetric catalytic core. The domains with which various
regulatory proteins are known to interact are indicated. [After
Tesmer, J.J.G. and Sprang, S.R., Curr. Opin. Struct. Biol. 8, 713
(1998).]
N
C
C
1a
C
2a
G
iα
G
sα,
PKC, G
βγ
M
1
M
2
ATP
2Mg
2+
cAMP + PP
i
C
2b
C
1b
Ca
2+
,
Ca
2+
CaM,
PKA
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 697
to C
2a
; and Ca
2⫹
–calmodulin (Ca
2⫹
–CaM; Section 18-3Ce)
activates AC1, 3, and 8 by binding to C
1b
. Moreover, the C
2a
of AC2, 5, and 7 are activated by the phosphorylation of
specific Ser/Thr control sites, for example, by protein
kinase C (PKC; Section 19-4C), whereas the C
1b
of AC5
and 6 are similarly inhibited by PKA, for example. Clearly,
cells can respond to a great variety of stimuli in determin-
ing their cAMP levels.
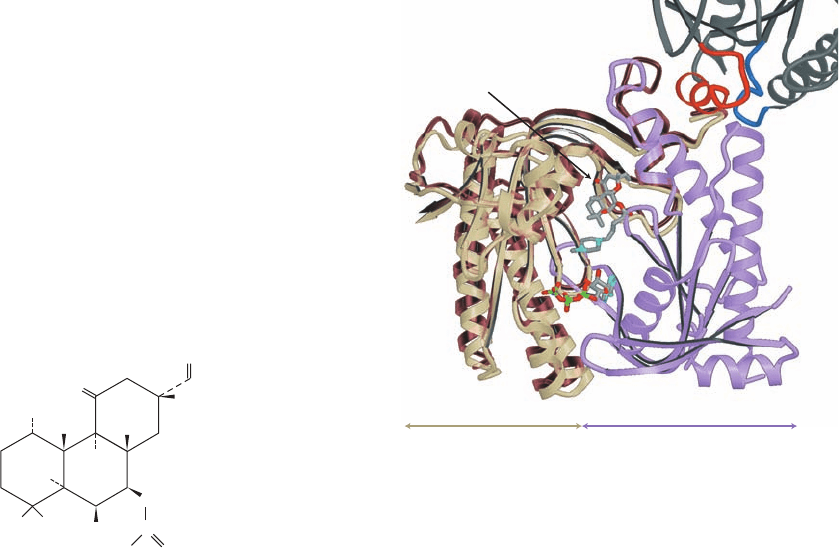
No X-ray structure of an intact AC isoform has yet been
reported. However, Sprang has determined the X-ray
structure of a hybrid catalytic core consisting of the C
1a
domain of AC5 (VC
1
) and the C
2a
domain of AC2 (IIC
2
) in
complex with G
s␣
ⴢ GTP␥S, ATP␣S (an isomer of ATP␥S
with the S atom on the ␣ phosphate), and forskolin
(a product of the plant Coleus forskohlii that activates all
ACs but AC9 and functions to lower blood pressure). The
VC
1
ⴢ IIC
2
catalytic core is enzymatically active and is sen-
sitive to both G
s␣
ⴢ GTP and forskolin. Its X-ray structure
(Fig. 19-24) reveals that VC
1
and IIC
2
form a pseudosym-
metric heterodimer that binds ATP␥S and forskolin at
pseudosymmetrically related sites of their interface.
G
s␣
ⴢ GTP␥S interacts with IIC
2
mainly via its Switch II
helix, which binds in a cleft on IIC
2
.
The X-ray structure of the catalytically inactive C
2a
homodimer in complex with two symmetrically arranged
forskolin molecules, by James Hurley, provides a perhaps
crude model for the inactivated heterodimer. Comparison
of these structures (Fig. 19-24) suggests that the binding of
G
s␣
ⴢ GTP to the C
1a
ⴢ C
2a
catalytic core pries open the
Switch II binding cleft on C
2a
in a way that mechanically
forces C
1a
to rotate ⬃10° with respect to C
2a
. This is postu-
lated to reorient the complex’s active site residues such
that they can efficiently catalyze the conversion of ATP to
cAMP. The conformational change that G
s␣
undergoes on
hydrolyzing its bound GTP to GDP (Fig. 19-18) apparently
reorients its Switch II region such that it no longer can bind
to C
2a
, thereby causing AC to revert to its inactive confor-
mation.
VC
1
has a cleft that corresponds to the G
s␣
-binding cleft
on IIC
2
. This suggests that this cleft on VC
1
provides the
binding site for G
i␣
. Indeed, mutagenesis studies on VC
1
are consistent with this hypothesis. However, the cleft on
VC
1
is too narrow to accommodate the binding of a Switch
II helix. This further suggests that the binding of
G
i␣
ⴢ GTP to C
1a
pries open this cleft in a way that reorients
Forskolin
OH
OH
OH
OH
H
O
O
O
C
CH
2
CH
3
CH
3
CH
3
H
3
C
CH
3
the complex’s catalytic residues so as to reduce its cat-
alytic activity.
E. Phosphodiesterases
In any chemically based signaling system, the signal molecule
must eventually be eliminated in order to control the ampli-
tude and duration of the signal and to prevent interference
with the reception of subsequent signals. In the case of cAMP,
this second messenger is hydrolyzed to AMP by enzymes
known as cAMP-phosphodiesterases (cAMP-PDEs).
The PDE superfamily, which includes both cAMP-
PDEs and cGMP-PDEs, is encoded in mammals by at least
20 different genes grouped into 12 families (PDE1 through
PDE12). Moreover, many of the mRNAs transcribed from
these genes have alternative initiation sites and alternative
splice sites (Section 34-3C), so that mammals express ⬃50
PDE isoforms. These are functionally distinguished by
their substrate specificities (for cAMP, cGMP, or both) and
kinetic properties, their responses (or lack of them) to var-
ious activators and inhibitors (see below), and their tissue,
698 Chapter 19. Signal Transduction
Figure 19-24 X-ray structure of an AC catalytic core. This core
consists of dog VC
1
and rat IIC
2
in complex with bovine G
s␣
ⴢ
GTP␥S and forskolin, and is shown with a model of ATP. VC
1
is
tan, IIC
2
is violet, and G
s␣
, which is only shown in part, is dark
gray, with its IIC
2
-contacting segments, Switch II and the ␣3–5
loop, highlighted in red and blue. The forskolin and the ATP are
shown in stick form with C gray, N cyan, O red, and P green.The
brown ribbon shows the nonoverlapping portions of the
catalytically inactive rat IIC
2
homodimer in which one of its
subunits is superimposed on IIC
2
in the VC
1
–IIC
2
complex.
[Courtesy of Heidi Hamm, Northwestern University Medical
School.The X-ray structures of the VC
1
–IIC
2
–Gs
s␣
complex and
the IIC
2
homodimer were determined by John Tesmer and
Stephen Sprang, University of Texas Southwestern Medical
Center, and by James Hurley, NIH. PDBids 1AZS and 1AB8.]
␣1⬘–␣2⬘
␣2
Forskolin
Gs␣
␣3–4
␣1–␣2
ATP
IIC
2
VC
1
␣3–5
␣3⬘
Adenylyl Cyclase
JWCL281_c19_671-743.qxd 6/30/10 1:14 PM Page 698
cellular, and subcellular distributions. The PDEs have
characteristic modular architectures with a conserved
⬃270-residue catalytic domain near their C-termini and
widely divergent regulatory domains or motifs, usually in
their N-terminal portions. Some PDEs are membrane-
anchored, whereas others are cytosolic.
PDE activity, as might be expected, is elaborately con-
trolled. Depending on its isoform, a PDE may be activated
by one or more of a variety of agents including Ca
2
–CaM;
phosphorylation by PKA, insulin-stimulated protein ki-
nase (Section 18-3Cg), and calmodulin-dependent protein
kinase II; and the binding of cGMP to a noncatalytic site.
However, for some PDEs, cGMP is inhibitory. Phosphory-
lated PDEs are dephosphorylated by a variety of protein
phosphatases including Ca
2
–CaM-dependent phosphatase
and protein phosphatase-2A. Thus, the PDEs provide a
means for cross talk between cAMP-based signaling sys-
tems and those using other types of signals.
PDEs are inhibited by a variety of drug agents that influ-
ence such widely divergent disorders as asthma, congestive
heart failure, depression, erectile dysfunction, inflammation,
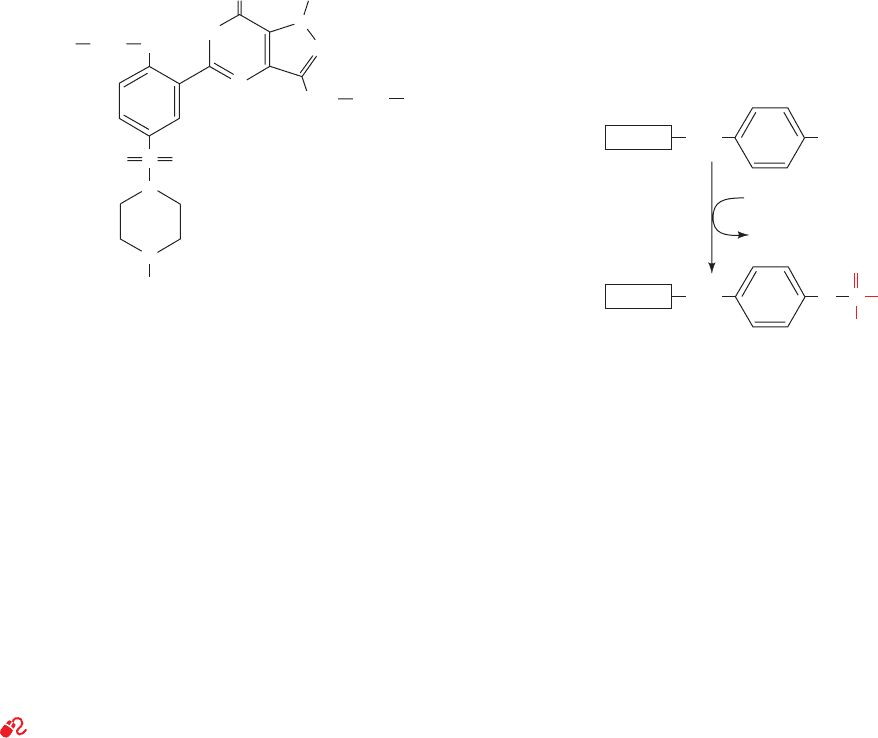
and retinal degeneration. Sildenafil (trade name Viagra),
a compound used to treat erectile dysfunction, specifically
inhibits PDE5, which hydrolyzes only cGMP. Sexual stimu-
lation in males causes penile nerves to release NO, which
activates guanylate cyclase to produce cGMP. This induces
vascular smooth muscle relaxation in the penis, thereby in-
creasing the inflow of blood, which results in an erection.
This cGMP is eventually hydrolyzed by PDE5. Sildenafil is
therefore an effective treatment in men who produce in-
sufficient NO and hence cGMP to otherwise generate a
satisfactory erection.
3 TYROSINE KINASE–BASED
SIGNALING
See Guided Exploration 17: Mechanisms of hormone signaling
involving the receptor tyrosine kinase system
We have seen that
glycogen synthesis and breakdown are regulated by the
phosphorylation/dephosphorylation of the enzymes that
Sildenafil (Viagra™
™
)
O
SOO
O
CH
3
CH
2
CH
2
N
N
N
N
CH
3
CH
3
CH
3
CH
2
N
HN
catalyze these metabolic processes as well as of many of the
enzymes that catalyze these modification/demodification
processes (Section 18-3). Numerous other processes in
multicellular organisms are similarly regulated. In fact, over
one-third of the proteins in vertebrates are subject to re-
versible phosphorylation, and the human genome contains
518 protein kinase genes (the so-called kinome; the Protein
Kinase Resource at http://pkr.genomics.purdue.edu/ and
kinase.com at http://kinase.com/ are databases for the
kinome).The vast majority of the phosphorylated amino acid
residues are Ser or Thr; only about 1 in 2000 is Tyr. Never-
theless, as we discuss in this section, Tyr phosphorylation is
of central importance in regulating a variety of essential
cellular processes.
A. Receptor Tyrosine Kinases
Many protein growth factors variously control the differ-
entiation, proliferation, migration, metabolic state, and sur-
vival of their target cells by binding to their cognate recep-
tor tyrosine kinases (RTKs). The RTKs form a diverse
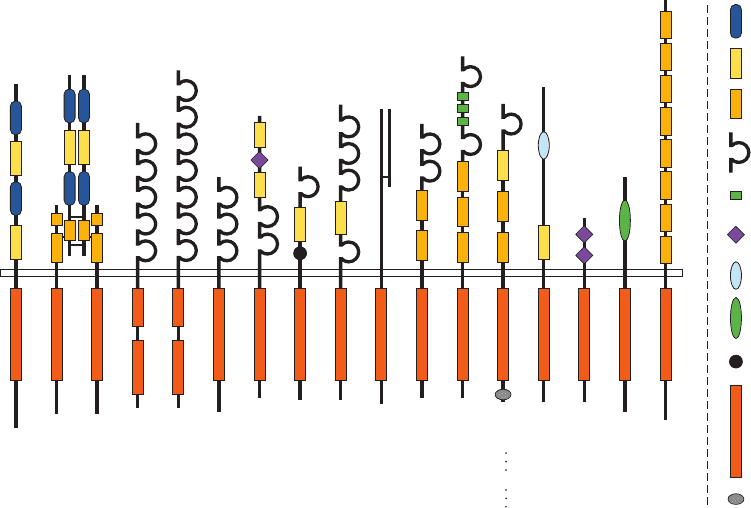
family of 58 transmembrane glycoproteins (Fig. 19-25) that
each have a C-terminal cytoplasmic protein tyrosine kinase
(PTK) domain and a single-pass transmembrane segment
that is presumably an helix. As their name indicates,
PTKs catalyze the ATP-dependent phosphorylation of
their target proteins at specific Tyr residues:
The PTK domains of RTKs are homologous to and, as we
shall see, structurally resemble the far more abundant
Ser/Thr-specific protein kinases such as PKA (Fig. 18-15).
RTKs are activated by the binding of a cognate protein
growth factor to their ectodomains. It seems unlikely that
the single transmembrane helix of monomeric RTKs such
as platelet-derived growth factor receptor (PDGFR) and
epidermal growth factor receptor (EGFR; Fig. 19-25) has
the structural complexity to transmit the ligand-binding
state of its ectodomain to its cytoplasmic tyrosine kinase do-
main. Rather, as we have seen for human growth hormone
receptor (which is not an RTK), ligand binding induces
receptor dimerization (Fig.19-10).This, in turn,activates the
RTK’s PTK activity, as we discuss below. For RTKs that are
permanent dimers, such as the insulin receptor (InsR;
Fig. 19-25), the PTK is thought to be activated by a ligand-
induced structural change (probably a counter-rotation of
the two protomers that preserves the dimer’s 2-fold axis of
symmetry) that is transmitted across the membrane.
OHCH
2
Protein
O O
O
O
PCH
2
Protein
ATP
ADP
Section 19-3. Tyrosine Kinase–Based Signaling 699
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 699
a. FGF and Heparin Sulfate Are Required to
Activate FGF Receptor
The mammalian fibroblast growth factors (FGFs) form
a family of at least 21 structurally related proteins
(FGF1–21) that regulate a variety of critical biological
processes including cell growth, differentiation, and migra-
tion and that are expressed in specific spatial and temporal
patterns in embryos and adults. FGF-stimulated processes
are mediated by four FGF receptors (FGFR1–4), which
each bind a unique subset of the FGFs, thereby accounting
for the diversity and tight regulation of the foregoing
processes. FGFR dimerization in solution requires the
presence of heparan sulfate proteoglycans (Section 11-3A)
in addition to that of FGF.
FGF receptors each consist of, from N- to C-terminus
(Fig. 19-25), three extracellular immunoglobulin-like do-
mains (D1–D3 for domains 1–3), a single transmembrane
helix, and a cytoplasmic domain with PTK activity. Of
these, only the D2 and D3 domains are involved in FGF
binding (in general, only a few of the domains in the extra-
cellular portions of RTKs participate in ligand binding).
Moosa Mohammadi determined the X-ray structure of the
2:2:2 complex of FGF2, the D2–D3 segment of FGFR1,
and a heparin decasaccharide (Fig. 11-21). It reveals
(Fig. 19-26) that each FGF monomer binds to the D2 and
D3 domain on one FGFR subunit and, more tenuously, to
the D2 domain on the other subunit, whereas the heparin
cross-links each FGF monomer to both D2 domains
(whose contacts in the absence of FGF and heparin are in-
sufficient to support appreciable FGFR dimerization).
b. RTK Dimers Are Activated by
Autophosphorylation
The dimerization of an RTK (or its conformational
change in the case of the insulin receptor subfamily) brings
its cytoplasmically located PTK domains into apposition,
such that they cross-phosphorylate each other on specific
Tyr residues on their activation loops (Fig. 19-27a). This
autophosphorylation activates the PTK in much the same
way as we saw that activation loop phosphorylation in-
duces PKA to phosphorylate its target proteins (Section
18-3Cb). In many cases, the activated PTK further phos-
phorylates the opposing RTK subunit at specific Tyr
residues outside of the PTK domain (Fig. 19-27).This, as we
shall see in Sections 19-3Cb and 19-3Cc, provides binding
sites for certain cytoplasmic proteins. The activated PTK
may also phosphorylate specific Tyr residues on a variety of
cytoplasmic proteins. In both cases, as we discuss in Section
700 Chapter 19. Signal Transduction
EGFR
ErbB2
ErbB3
ErbB4
InsR
IGF1R
IRR
PDGFR
α
PDGFR
β
CSF1R
Kit
Flk2
Flt1
KDR
Flt4
FGFR1
FGFR2
FGFR3
FGFR4
TrkA
TrkB
TrkC
Ror1
Ror2
MuSK Met
Ron
Sea
Axl
Eyk
Tyro3
Nyk
Tie
Tek
EphA1
EphB1
Ret Ryk DDR1
DDR2
Ros
SAM
tyrosine
kinase
kringle
discoidin
cadherin
leucine-rich
EGF
lg
(immunoglobulinlike)
fibronectin
type III
cysteine-rich
L
Figure 19-25 Domain organization in a variety of receptor
tyrosine kinase (RTK) subfamilies. One to five members of each
subfamily are depicted.The narrow rectangle that extends
horizontally across the diagram represents the plasma
membrane, with the extracellular region above it and the cytosol
below. The polypeptides are shown only approximately to scale,
with their N-termini above. EGFR, InsR, PDGFR, and FGFR
refer to epidermal growth factor receptor, insulin receptor,
platelet-derived growth factor receptor, and fibroblast growth
factor receptor, respectively. The RTKs’ extracellular portions
are modularly constructed from a variety of often repeating
domains that are identified at the right of the diagram. Note that
the tyrosine kinase domains of PDGFR and Flt1 subfamilies are
interrupted by ⬃100-residue kinase inserts and that the members
of the InsR subfamily are ␣
2

2
heterotetramers, whose subunits
are disulfide-linked (short horizontal lines). [Courtesy of Stevan
Hubbard, New York University School of Medicine.]
JWCL281_c19_671-743.qxd 6/30/10 1:15 PM Page 700
