Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

Steroids, being water insoluble, are transported in the
blood in complex with the glycoprotein transcortin and, to
a lesser extent, by albumin. The steroids (including vitamin
D) spontaneously pass through the membranes of their
target cells to the cytosol, where they bind to their cognate
receptors. The steroid–receptor complexes then migrate to
the cell nucleus, where they function as transcription fac-
tors to induce, or in some cases repress, the transcription of
specific genes (a process that is discussed in Section 34-3Bn).
In this way, the glucocorticoids and the mineralocorticoids
influence the expression of numerous metabolic enzymes
in their respective target tissues. Thyroid hormones, which
are also nonpolar, function similarly. However, as we shall
see in the following sections, all other hormones act less
directly in that they bind to their cognate cell-surface re-
ceptors and thereby trigger complex cascades of events
within cells that ultimately influence transcription as well
as other cellular processes.
Impaired adrenocortical function, either through disease
or trauma,results in a condition known as Addison’s disease,
which is characterized by hypoglycemia, muscle weakness,
Na
loss, K
retention, impaired cardiac function, loss of ap-
petite, and a greatly increased susceptibility to stress. The
victim, unless treated by the administration of glucocorti-
coids and mineralocorticoids, slowly languishes and dies
without any particular pain or distress. The opposite prob-
lem, adrenocortical hyperfunction, which is usually caused
by a tumor of the adrenal cortex or the pituitary gland (Sec-
tion 19-1H), results in Cushing’s syndrome, which is charac-
terized by fatigue, hyperglycemia, edema (water retention),
and a redistribution of body fat to yield a characteristic
“moon face.” Long-term treatments of various diseases with
synthetic glucocorticoids result in similar symptoms.
b.
Gonadal Steroids Mediate Sexual
Development and Function
The gonads (testes in males, ovaries in females), in addi-
tion to producing sperm or ova, secrete steroid hormones
(androgens and estrogens) that regulate sexual differentiation,
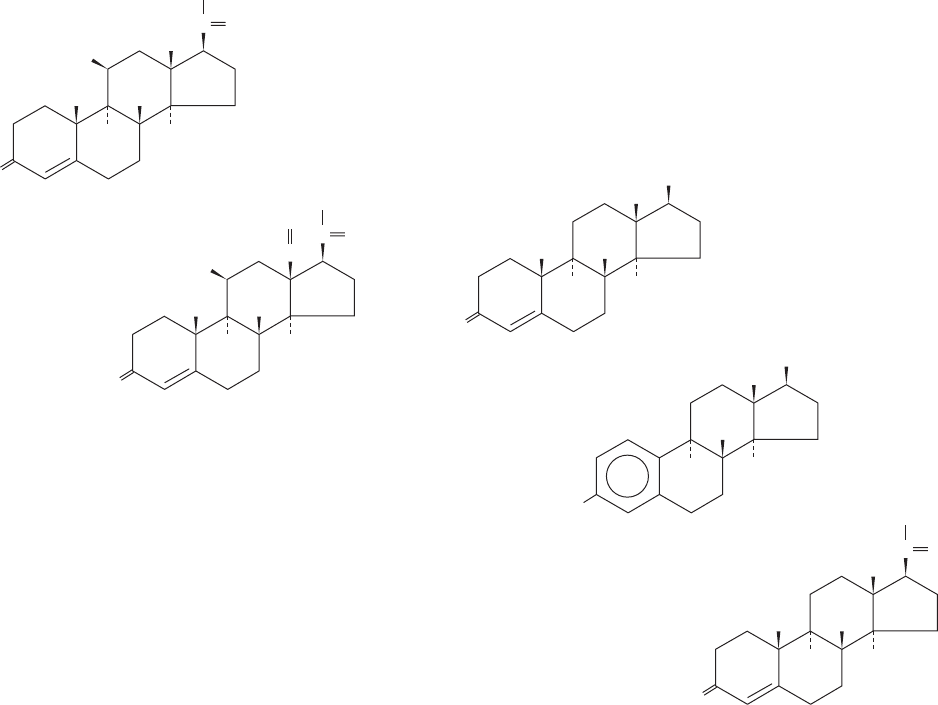
Corticosterone
Aldosterone
H
3
C
H
3
C
O
HO
CO
CH
2
OH
H
H
H
HC
O
H
3
C
O
CO
CH
2
OH
H
H
H
HO
the expression of secondary sex characteristics, and sexual
behavior patterns. Although testes and ovaries both synthe-
size androgens and estrogens, the testes predominantly se-
crete androgens, which are therefore known as male sex
hormones, whereas ovaries produce mostly estrogens,
which are consequently termed female sex hormones.
Androgens, of which testosterone is prototypic,
Section 19-1. Hormones 681
Testosterone
-Estradiol
Progesterone
OH
H
3
C
HO
H
H
H
H
3
C
H
3
C
O
CO
CH
3
H
H
H
OH
H
3
C
O
H
3
C
H
H
H
lack the C
2
substituent at C17 present in glucocorticoids
and are therefore C
19
compounds. Estrogens, such as
-estradiol, resemble androgens but lack a C10 methyl group
because they have an aromatic A ring and are therefore
C
18
compounds. Interestingly, testosterone is an intermedi-
ate in estrogen biosynthesis (Section 25-6C). A second
class of ovarian steroids, C
21
compounds called progestins,
help mediate the menstrual cycle and pregnancy (Section
19-1I). Progesterone, the most abundant progestin, is, in
fact, a precursor of glucocorticoids, mineralocorticoids, and
testosterone (Section 25-6C).
c. Sexual Differentiation Is Both Hormonally
and Genetically Controlled
What factors control sexual differentiation? If the go-
nads of an embryonic male mammal are surgically re-
moved, that individual will become a phenotypic female.
Evidently, mammals are programmed to develop as females
unless embryonically subjected to the influence of testicular
hormones. Indeed, genetic males with absent or nonfunc-
tional cytosolic androgen receptors are phenotypic fe-
males, a condition named testicular feminization. Curi-
ously, estrogens appear to play no part in embryonic
female sexual development, although they are essential for
female sexual maturation and function.
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 681
Normal individuals have either the XY (male) or the
XX (female) genotypes (Section 1-4C). However, those
with the abnormal genotypes XXY (Klinefelter’s syn-
drome) and X0 (only one sex chromosome; Turner’s syn-
drome) are, respectively, phenotypic males and phenotypic
females, although both are sterile.Apparently, the normal Y
chromosome confers the male phenotype, whereas its ab-
sence results in the female phenotype. There are, however,
rare (1 in 20,000) XX males and XY females. These XX
males (who are sterile and have therefore been identified
through infertility clinics) have a small segment of a nor-
mal Y chromosome translocated onto one of their X chro-
mosomes, whereas XY females are missing this segment.
Early male and female embryos—through the sixth
week of development in humans—have identical undiffer-
entiated genitalia. Evidently, the Y chromosome contains a
gene, testes-determining factor (TDF), that induces the
differentiation of testes, whose hormonal secretions, in
turn, promote male development. The misplaced chromo-
somal segments in XY females and XX males have a com-
mon 140-kb sequence that contains a structural gene
dubbed SRY (for sex-determining region of Y) that en-
codes an 80-residue DNA-binding motif. Several sex-
reversed XY women have a mutation in the region of their
SRY gene encoding this DNA-binding domain that elimi-
nates its ability to bind DNA, a mutation that is not present
in their father’s gene. SRY is expressed in embryonic go-
nadal cells previously shown to be responsible for testis de-
termination. Moreover, of eleven XX mice that were made
transgenic for Sry (the mouse analog of SRY), three were
males. Thus, TDF/SRY is the first clear example of a mam-
malian gene that controls the development of an entire or-
gan system (development is discussed in Section 34-4B).
H. Control of Endocrine Function: The
Hypothalamus and Pituitary Gland
The anterior lobe of the pituitary gland (the adenohypo-
physis) and the hypothalamus, a nearby portion of the brain,
constitute a functional unit that hormonally controls much
of the endocrine system. The neurons of the hypothalamus
synthesize a series of polypeptide hormones known as re-
leasing factors and release-inhibiting factors which, on de-
livery to the adenohypophysis via a direct circulatory con-
nection (their half-lives are on the order of a few minutes),
stimulate or inhibit the release of the corresponding trophic
hormones into the bloodstream. Trophic hormones, by defi-
nition, stimulate their target endocrine tissues to secrete
the hormones they synthesize. Since releasing and release-
inhibiting factors, trophic hormones, and endocrine hor-
mones are largely secreted in nanogram, microgram, and
milligram quantities per day, respectively, and tend to have
progressively longer half-lives, these hormonal systems can
be said to form amplifying cascades. Four such systems are
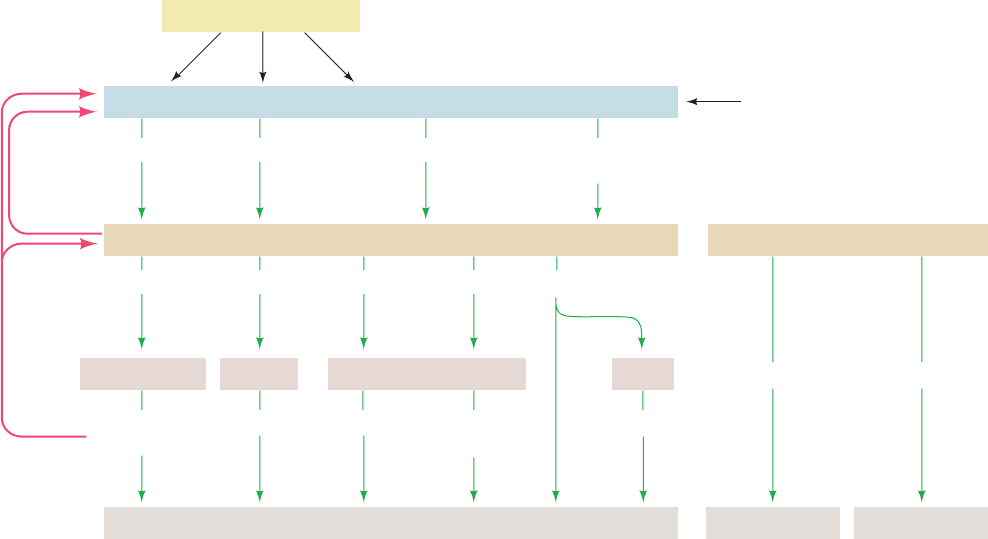
prominent in humans (Fig. 19-8; left):
1. Corticotropin-releasing factor (CRF; 41 residues)
causes the adenohypophysis to release adrenocorticotropic
682 Chapter 19. Signal Transduction
Figure 19-8 Hormonal control circuits, indicating the
relationships between the hypothalamus, pituitary, and target
tissues. Releasing factors and release-inhibiting factors secreted
by the hypothalamus signal the adenohypophysis to secrete or
stop secreting the corresponding trophic hormones, which, for
the most part, stimulate the corresponding endocrine gland(s) to
Hypothalamus
Higher brain centers
Adenohypophysis (anterior pituitary)
CRF TRF GnRF
GRF
Somatostatin (GRIF)
ACTH TSH FSH Growth hormone
LH
Adrenocortical
hormones
T
3
& T
4
Androgens SomatomedinsEstrogens,
progestins
Adrenal cortex Thyroid Testes/Ovaries Liver
Muscles, liver, and other tissues + sex accessory tissues + bones
Central nervous system
Neurohypophysis (posterior pituitary)
Water resorption Uterine contraction
Vasopressin Oxytocin
Feedback
inhibition
Feedback
inhibition
secrete their respective endocrine hormones.The endocrine
hormones, in addition to controlling the growth, differentiation,
and metabolism of their corresponding target tissues, influence
the secretion of releasing factors and trophic hormones through
feedback inhibition.The levels of trophic hormones likewise
influence the levels of their corresponding releasing factors.
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 682
hormone (ACTH; 39 residues), which stimulates the re-
lease of adrenocortical steroids. The entire system is under
feedback control:ACTH inhibits the release of CRF and the
adrenocortical steroids inhibit the release of both CRF and
ACTH. Moreover, the hypothalamus, being part of the
brain, is also subject to neuronal control, so the hypothala-
mus forms the interface between the nervous system and the
endocrine system.
2. Thyrotropin-releasing factor (TRF), a tripeptide
with an N-terminal pyroGlu residue (a Glu derivative in
which the side chain carboxyl group forms an amide bond
with its amino group),
stimulates the adenohypophysis to release the trophic hor-
mone thyrotropin (thyroid-stimulating hormone; TSH)
which, in turn, stimulates the thyroid to synthesize and re-
lease T
3
and T
4
. TRF, as are other releasing factors, is pres-
ent in the hypothalamus in only vanishingly small quanti-
ties. It was independently characterized in 1969 by Roger
Guillemin and Andrew Schally using extracts of the hypo-
thalami from over 2 million sheep and 1 million pigs.
3. Gonadotropin-releasing factor (GnRF; 10 residues)
stimulates the adenohypophysis to release luteinizing hor-
mone (LH) and follicle-stimulating hormone (FSH), which
are collectively known as gonadotropins. In males, LH
stimulates the testes to secrete androgens, whereas FSH
promotes spermatogenesis. In females, FSH stimulates the
development of ovarian follicles (which contain the imma-
ture ova), whereas LH triggers ovulation.
4. Growth hormone-releasing factor (GRF; 44 residues)
and somatostatin [14 residues; also known as growth hor-
mone release-inhibiting factor (GRIF)], stimulate/inhibit
the release of growth hormone (GH) from the adenohy-
pophysis. GH (also called somatotropin), in turn, stimu-
lates generalized growth (see Fig. 5-5 for a striking example
of its effect). GH directly accelerates the growth of a vari-
ety of tissues (in contrast to TSH, LH, and FSH, which act
only indirectly by activating endocrine glands) and induces
the liver to synthesize a series of polypeptide growth fac-
tors termed somatomedins that stimulate cartilage growth
and have insulinlike activities.
TSH, LH, and FSH are heterodimeric glycoproteins, which
in a given species, all have the same subunit (92 residues)
and a homologous subunit (114, 114, and 118 residues,
respectively, in humans). Human GH consists of a single
191-residue polypeptide chain, which is unrelated to TSH,
LH, or FSH.
Gonadotropin-releasing factor (GnRF)
pyroGlu-His-Trp-Ser-Tyr-Gly-Leu-Arg-Pro-Gly-NH
2
110
CH C
C
O
O
His NH
2
Pro
3
N
H
pyroGlu
Thyrotropin-releasing factor (TRF)
a. The Neurohypophysis Secretes Oxytocin
and Vasopressin
The posterior lobe of the pituitary, the neurohypophy-
sis, which is anatomically distinct from the adenohypophy-
sis, secretes two homologous nonapeptide hormones (Fig.
19-8, right): vasopressin [also known as antidiuretic hor-
mone (ADH)], which increases blood pressure and stimu-
lates the kidneys to retain water; and oxytocin, which
causes contraction of uterine smooth muscle and therefore
induces labor:
The rate of vasopressin release is largely controlled by os-
moreceptors, which monitor the osmotic pressure of the
blood.
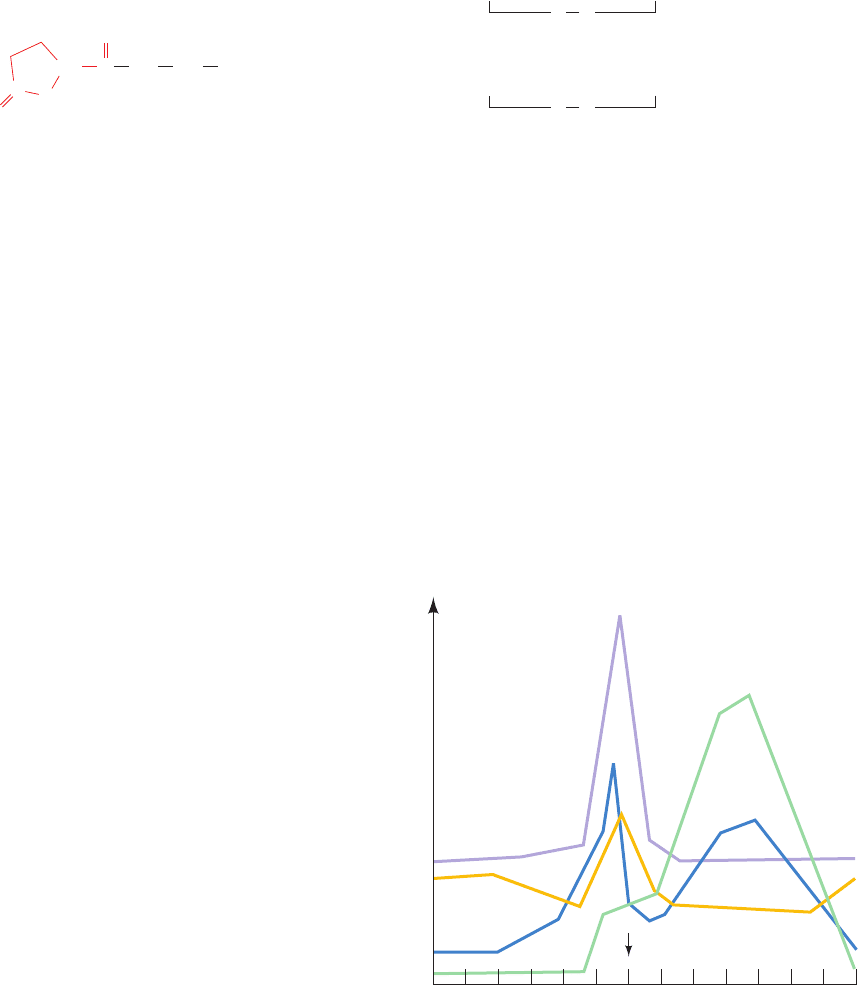
I. Control of the Menstrual Cycle
The menstrual cycle and pregnancy are particularly illus-
trative of the interactions among hormonal systems. The
⬃28-day human menstrual cycle (Fig. 19-9) begins during
menstruation with a slight increase in the FSH level that
initiates the development of a new ovarian follicle. As the
follicle matures, it secretes estrogens that act to sensitize
the adenohypophysis to GnRF. This process culminates in
a surge of LH and FSH, which triggers ovulation. The rup-
tured ovarian follicle, the corpus luteum, secretes proges-
terone and estrogens, which inhibit further gonadotropin
Human vasopressin
1
9
Cys-Tyr-Phe-Gln-Asn-Cys-Pro-Arg-Gly-NH
2
SS
Human oxytocin
19
Cys-Tyr- Ile - Gln-Asn-Cys-Pro-Leu-Gly-NH
2
SS
Section 19-1. Hormones 683
Figure 19-9 Patterns of hormone secretion during the
menstrual cycle in the human female.
2 6 10 14128418222016 24 26 28
Hormone level
Menstrual cycle (days)
OvulationEstradiol
FSH
LH
Progesterone
LH surge
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 683
secretion by the adenohypophysis and stimulate the uter-
ine lining to prepare for the implantation of a fertilized
ovum. If fertilization does not occur, the corpus luteum re-
gresses, progesterone and estrogen levels fall, and menstru-
ation (the sloughing off of the uterine lining) ensues. The
reduced steroid levels also permit a slight increase in the
FSH level, which initiates a new menstrual cycle.
A fertilized ovum that has implanted into the hormon-
ally prepared uterine lining soon commences synthesizing
chorionic gonadotropin (CG). This heterodimeric glyco-
protein hormone contains a 145-residue  subunit that has
a high degree of sequence identity with those of LH (85%),
FSH (45%), and TSH (36%) over their N-terminal 114
residues and the same ␣ subunit. CG stimulates the corpus
luteum to continue secreting progesterone rather than re-
gressing and thus prevents menstruation. Pregnancy tests
utilize immunoassays that can detect CG in blood or urine
within a few days after embryo implantation. Most female
oral contraceptives (birth control pills) contain proges-
terone derivatives, whose ingestion induces a state of
pseudopregnancy in that they inhibit the midcycle surge of
FSH and LH so as to prevent ovulation.
J. Growth Hormone and Its Receptor
The binding of growth hormone activates its receptor to
stimulate growth and metabolism in muscle, bone, and car-
tilage cells.This 620-residue receptor is a member of a large
family of structurally related protein growth factor recep-
tors, which includes those for various interleukins (Section
19-3Eb).All of these receptors consist of an N-terminal ex-
tracellular ligand-binding domain, a single transmembrane
segment that is almost certainly helical, and a C-terminal
cytoplasmic domain that is not homologous within the su-
perfamily but in many cases contains a tyrosine kinase func-
tion (Section 19-3A).
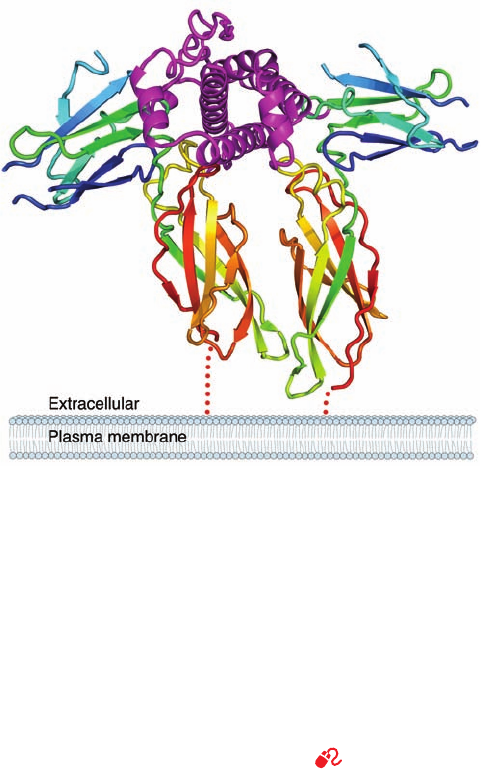
The X-ray structure of the 191-residue human growth hor-
mone (hGH) in complex with the 238-residue ectodomain
(extracellular portion; Greek: ectos, outside) of its binding
protein (hGHbp), determined by Abraham de Vos and
Anthony Kossiakoff, revealed that this complex consists of
two molecules of hGHbp bound to a single hGH molecule
(Fig. 19-10). hGH consists largely of an up–up–down–down
four-helix bundle, which closely resembles that in the
previously determined X-ray structure of porcine GH,
although with significant differences that may be caused by
the binding of hGH to its receptor.A variety of other pro-
tein growth factors with known structures, including many
interleukins (Section 19-3Eb), contain similar four-helix
bundles. Each hGHbp molecule consists of two structurally
similar ⬃100-residue fibronectin type III domains, each of
which forms a topologically identical sandwich of a three-
and a four-stranded antiparallel  sheet that resembles the
immunoglobulin fold (Fig. 8-48). Fibronectin type III do-
mains, so called because they were first observed in the
multidomain extracellular matrix glycoprotein fibronectin,
are among the most common structural modules in recep-
tor ectodomains.
The two hGHbp molecules bind to hGH with near 2-fold
symmetry about an axis that is roughly perpendicular to
the helical axes of the hGH four-helix bundle and, pre-
sumably, to the plane of the cell membrane to which the in-
tact hGH receptor is anchored (Fig. 19-10).The C-terminal
domains of the two hGHbp molecules are almost parallel
and in contact with one another. Intriguingly, the two
hGHbp molecules use essentially the same residues to bind
to sites that are on opposite sides of hGH’s four-helix bun-
dle and that have no structural similarity. The X-ray struc-
ture is largely consistent with the results of mutational
studies designed to identify the hGH and hGHbp residues
important for receptor binding.
The ligand-induced dimerization of hGHbp has im-
portant implications for the mechanism of signal trans-
duction. The dimerization, which does not occur in the
absence of hGH, apparently brings together the intact re-
ceptors’ intracellular domains in a way that activates an
effector protein such as a tyrosine kinase (Section 19-3A).
Indeed, hGH mutants that cannot induce receptor
684 Chapter 19. Signal Transduction
Figure 19-10 X-ray structure of human growth hormone
(hGH) in complex with two molecules of its receptor’s
extracellular domain (hGHbp). The proteins are drawn in ribbon
form, with the hGH magenta and the two hGHbp molecules,
which together bind one molecule of hGH, each colored in
rainbow order from N-terminus (blue) to C-terminus (red).The
view is along the axis of the hGH’s four-helix bundle with the
approximate 2-fold axis relating the two hGHbp molecules
vertical.The dotted red lines represent the pathways that the
hGHbp chains take in penetrating the membrane. Note that the
width of a membrane is actually nearly the ⬃75-Å height of the
hGHbp–hGH–hGHbp complex. [Based on an X-ray structure by
Abraham de Vos and Anthony Kossiakoff, Genentech Inc., South
San Francisco, California. PDBid 3HHR.]
See Interactive
Exercise 11
JWCL281_c19_671-743.qxd 10/19/10 7:35 AM Page 684

dimerization are biologically inactive. Numerous other
protein growth factors also induce the dimerization of
their receptors.
a. Abnormal GH Production Causes
Abnormal Growth
Overproduction of GH, usually a consequence of a pi-
tuitary tumor, results in excessive growth. If this condition
commences while the skeleton is still growing, that is, be-
fore its growth plates have ossified, then this excessive
growth is of normal proportions over the entire body, re-
sulting in gigantism. Moreover, since excessive GH in-
hibits the testosterone production necessary for growth
plate ossification, such “giants” continue growing through-
out their abnormally short lives. If, however, the skeleton
has already matured, GH stimulates only the growth of
soft tissues, resulting in enlarged hands and feet and thick-
ened facial features, a condition named acromegaly (Fig.
19-11). The opposite problem, GH deficiency, which re-
sults in insufficient growth (dwarfism), can be treated be-
fore skeletal maturity by regular injections of hGH (ani-
mal GH is ineffective in humans). hGH was, at first,
available only from the pituitaries of cadavers and there-
fore was in extremely short supply. Since the early 1980s,
however, hGH has been synthesized in virtually unlimited
amounts via recombinant DNA techniques. Indeed, hGH
has been illicitly taken by individuals wishing to increase
their athletic prowess (the ban on using hGH could not be
enforced until the early 2000s, when blood tests were
developed that could distinguish between natural and
recombinant hGH).
K. Opioid Peptides
Among the most intriguing hormones secreted by the
adenohypophysis are polypeptides that have opiatelike ef-
fects on the central nervous system. These include the
31-residue -endorphin, its N-terminal pentapeptide, termed
methionine-enkephalin, and the closely similar leucine-
enkephalin (although the enkaphalins are independently
expressed).
These substances bind to opiate receptors in the brain and
have been shown to be their physiological agonists. The
role of these so-called opioid peptides has yet to be defini-
tively established, but it appears they are important in the
control of pain and emotional states. Pain relief through
the use of acupuncture and placebos as well as such phe-
nomena as “runner’s high” may be mediated by opioid
peptides.
L. The Hormonal Function of Nitric Oxide
Nitric oxide (NO) is a reactive and toxic free radical gas.
Thus, it came as a great surprise that this molecule functions
as paracrine signal in regulating blood vessel dilation and
serves as a neurotransmitter. It also functions in the immune
response. The role of NO in vasodilation was discovered
Tyr - Gly - Gly-Phe-Met-Thr - Ser - Glu - Lys-Ser
10
Gln-Thr-Pro -Leu-Val - Thr -Leu-Phe-Lys-Asn
20
Ala - Ile - Val-Lys -Asn -Ala -His -Lys - Lys -Gly
30
Gln-
Tyr - Gly - Gly-Phe-Leu
15
11 15
21
31
25
-Endorphin
Leucine-enkephalin (Leu-enkephalin)
Tyr - Gly - Gly-Phe-Met
Methionine-enkephalin (Met-enkephalin)
Morphine (an opiate)
HO
OH
CH
3
O
N
H
Section 19-1. Hormones 685
Figure 19-11 Acromegaly. The characteristic enlarged features
of Akhenaten, the Pharaoh who ruled Egypt in the years
1379–1362
B.C., strongly suggest that he suffered from
acromegaly. [Bildarchiv Preussischer Kulturbesitz/Art Resource.]
JWCL281_c19_671-743.qxd 6/30/10 1:48 PM Page 685
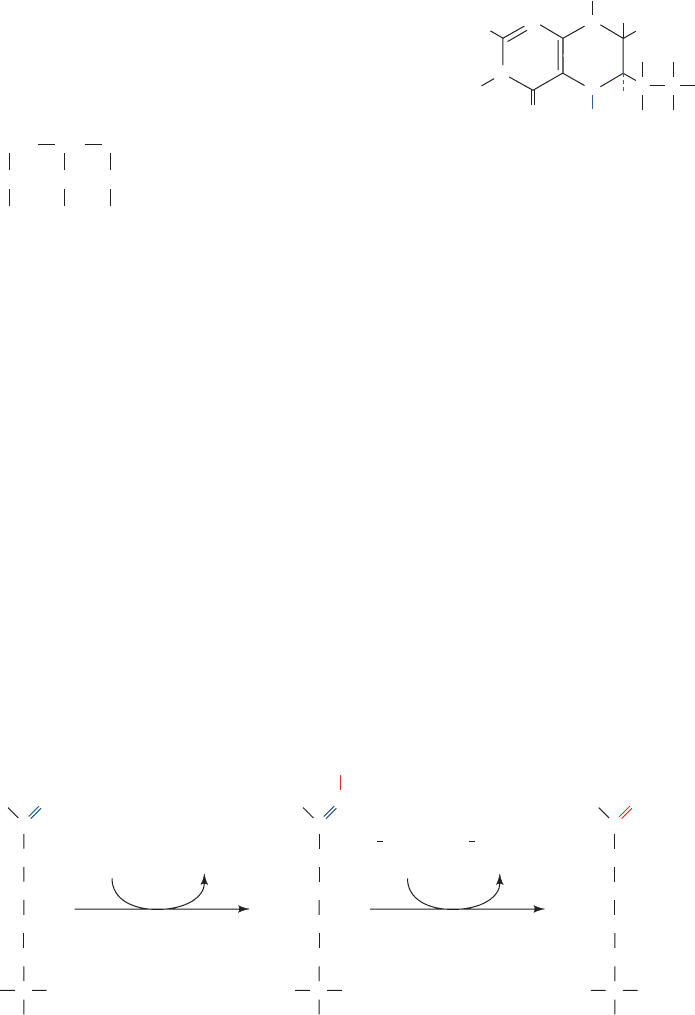
through the observation that substances such as acetyl-
choline (Section 20-5Cb) and bradykinin (Section 7-5B),
which act through the phosphoinositide signaling system
(Section 19-4) to increase the flow through blood vessels
by eliciting smooth muscle relaxation, require an intact en-
dothelium overlying the smooth muscle (the endothelium is
a layer of cells that lines the inside of certain body cavities
such as blood vessels). Evidently, endothelial cells respond
to the presence of these vasodilation agents by releasing
a diffusible and highly labile substance (half-life ⬃5 s) that
induces the relaxation of smooth muscle cells. This sub-
stance was identified as NO, in part, through parallel stud-
ies identifying NO as the active metabolite that mediates
the well-known vasodilating effects of antianginal organic
nitrates such as nitroglycerin
(angina pectoris is a condition caused by insufficient blood
flow to the heart muscle, leading to severe chest pain; nitro-
glycerin’s vasodilating properties were discovered in the
nineteenth century through the observation that workers
with angina pectoris in a factory that produced nitroglycerin
experienced greater pain on weekends).
a. Nitric Oxide Synthase Requires Five
Redox-Active Cofactors
NO is synthesized by NO synthase (NOS), which cat-
alyzes the NADPH-dependent 5-electron oxidation of
L-arginine by O
2
to yield NO and the amino acid L-citrulline
with the intermediate formation of N
-hydroxy-L-arginine
(NOHA; Fig. 19-12). Three isozymes of NOS have been
identified in mammals, neuronal NOS (nNOS), in-
ducible NOS (iNOS), and endothelial NOS (eNOS), which
are also known as NOS-1, -2, and -3, respectively. These
isozymes, which have 50 to 60% sequence identity, are all
CH
2
NO
2
O
CH
2
CH
NO
2
O
NO
2
O
Nitroglycerin
homodimeric proteins of 125- to 160-kD subunits that each
consist of two domains:
1. An N-terminal, ⬃500-residue, oxygenase or heme
domain that catalyzes both reaction steps of Fig. 19-12
and contains the dimer interface. This domain binds the
substrates O
2
and L-arginine and two redox-active pros-
thetic groups, Fe(III)-heme and 5,6,7,8-tetrahydrobiopterin
(H
4
B),
a compound that also functions in the hydroxylation of
phenylalanine to tyrosine (Section 26-3Ha). The X-ray
structures of the oxygenase domains of nNOS (Fig. 19-13a),
iNOS, and eNOS are closely similar.
2. A C-terminal, ⬃600-residue, reductase domain that
supplies the electrons for the NOS reaction. It binds
NADPH and two redox-active prosthetic groups, an FAD
(Fig. 16-8) and a flavin mononucleotide (FMN; FAD lack-
ing its AMP residue; Fig. 22-17a) via three nucleotide bind-
ing modules. This domain is homologous to cytochrome
P450 reductase, an enzyme that participates in detoxifica-
tion processes (Section 15-4Bc).The X-ray structure of the
reductase domain of nNOS, determined by John Tainer and
Elizabeth Getzoff, is shown in Fig. 19-13b.
NADPH bound to the reductase domain transmits its
electrons via the FAD and then the FMN to the heme in
the oxygenase domain. Interestingly, the reductase do-
main transmits its electrons to the oxygenase domain on
the opposite subunit. This was shown by Dennis Stuehr
through his construction of an NOS heterodimer in which
one subunit was full length and the other consisted of only a
oxygenase domain. If a mutation that disrupts the
L-arginine
5,6,7,8-Tetrahydrobiopterin
N
N
N
N
H
2
N
CH
3
H
H
H
O
H
HO
H
H
OH
H
CC
8
7
6
5
H
686 Chapter 19. Signal Transduction
Figure 19-12 The NO synthase (NOS) reaction. The N
-hydroxy-L-arginine intermediate is
tightly bound to the enzyme.
H
2
N
H
3
N
COO
C
C
H
NH
CH
2
CH
2
NH
2
L
-Arginine
H
2
N
H
3
N
COO
C
C
H
OH
NH
CH
2
CH
2
NH
N
-Hydroxy-
L
-arginine (NOHA)
H
2
N
H
3
N
COO
C
C
H
O
NH
CH
2
CH
2
CH
2
CH
2
CH
2
L
-Citrulline
NO
NADPH
O
2
NADP
H
2
O
1
2
NADPH
O
2
1
2
NADP
H
2
O
+
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 686
binding site was in the full-length subunit, the enzymatic
activity of this heterodimer was unaffected, but if it
was in the oxygenase domain–only subunit, activity was
abolished.
The heme Fe atom is 5-coordinated with its axial ligand
supplied by a specific Cys S atom.The
L-arginine substrate
binds on the opposite side of the heme from this Cys with
the N atom to be hydroxylated ⬃4.0 Å distant from the Fe
atom, a distance too large for covalent bond formation.
Since O
2
is known to react with the heme Fe atom, it pre-
sumably binds between it and this N atom.
NOS requires bound H
4
B to produce NO. In the ab-
sence of this prosthetic group, NOS efficiently catalyzes the
NADPH-mediated oxidation of O
2
to H
2
O
2
. Investigations
by Steuhr have established that H
4
B functions as an inter-
nal redox agent in that, during the reactions forming both
NOHA and NO, it is oxidized to its radical form (H
4
B
ⴢ
⫹
)
and then re-reduced to its reduced form (H
4
B). Thus, H
4
B
does not undergo net oxidation in the NOS reaction (as it
does in the reaction hydroxylating phenylalanine to tyro-
sine; Section 26-3Ha).
NO rapidly diffuses across cell membranes, although its
high reactivity prevents it from traveling ⬎1 mm from its
site of synthesis (in particular, it efficiently reacts with both
oxyhemoglobin and deoxyhemoglobin: NO ⫹ HbO
2
S
NO
3
⫺
⫹ metHb; and NO ⫹ Hb S HbNO; Section 10-1A).
The physiological target of NO in smooth muscle cells is
guanylate cyclase (GC), which catalyzes the reaction of
GTP to yield 3ⴕ,5ⴕ-cyclic GMP (cGMP),
an intracellular second messenger that resembles 3¿,5¿-cyclic
AMP (cAMP; GC is a homolog of adenylate cyclase;
Section 19-2D). cGMP causes smooth muscle relaxation
through its stimulation of protein phosphorylation by
cGMP-dependent protein kinase. NO reacts with GC’s
heme prosthetic group to yield nitrosoheme, whose pres-
ence increases GC’s activity by up to 200-fold, presumably
via a conformation change resembling that in hemoglobin
on binding O
2
(Section 10-2Ba; although GC binds O
2
quite poorly).
P
3⬘,5ⴕ-Cyclic GMP
(cGMP)
N
O
N
H
H
2
N
N
N
H
O
O
O
⫺
O
HH
H
H
O OH
CH
2
Section 19-1. Hormones 687
(a)
(b)
Figure 19-13 X-ray structure of rat nNOS. (a) Its N-terminal
oxygenase domain in complex with heme, L-arginine, and H
4
B.
(b) Its C-terminal reductase domain in complex with FMN, FAD,
and NADP
⫹
. In both structures the homodimeric protein is
viewed in semitransparent ribbon form with its 2-fold axis
vertical and with one subunit colored in rainbow order from
N-terminus (blue) to C-terminus (red) and the other subunit
blue-gray. The various bound groups are drawn in space-filling
form colored according to atom type (heme and FMN C green,
L-arginine and FAD C cyan, H
4
B and NADP
⫹
C magenta, N
blue, O red, P orange, and Fe red-brown). [Part a based on an
X-ray structure by Thomas Poulos, University of California at
Irvine; and Part b based on an X-ray structure by John Tainer
and Elizabeth Getzoff, The Scripps Research Institute, La Jolla,
California. PDBids 1OM4 and 1TLL.]
JWCL281_c19_671-743.qxd 6/4/10 10:52 AM Page 687

b. eNOS and nNOS but Not iNOS Are
Regulated by [Ca
2ⴙ
]
Ca
2⫹
–calmodulin activates eNOS and nNOS by binding
to the ⬃30-residue segments linking their oxygenase and
reductase domains. Thus, for example, the stimulatory ac-
tion of vasodilatory agents on the phosphoinositide signal-
ing system (Section 19-4A) in endothelial cells to produce
an influx of Ca
2⫹
results in the synthesis of NO. Hence, NO
functions to transduce hormonally induced increases in in-
tracellular [Ca
2⫹
] in endothelial cells to increased rates of
production of cGMP in neighboring smooth muscle cells.
NO produced by nNOS mediates vasodilation through
endothelium-independent neural stimulation of smooth
muscle. In this signal transduction pathway, which is re-
sponsible for the dilation of cerebral and other arteries as
well as penile erection (see Section 19-2E), nerve impulses
cause an increased [Ca
2⫹
] in nerve terminals, thereby stim-
ulating neuronal NOS. The resultant NO diffuses to nearby
smooth muscle cells, where it binds to guanylate cyclase
and activates it to synthesize cGMP as described above.
Inducible NOS (iNOS) is unresponsive to Ca
2⫹
even
though it has two tightly bound calmodulin subunits. How-
ever, it is transcriptionally induced in macrophages and
neutrophils (white blood cells that function to ingest and
kill bacteria), as well as in endothelial and smooth muscle
cells (in contrast, eNOS and nNOS are expressed constitu-
tively, that is, at a constant rate). Several hours after expo-
sure to cytokines (protein growth factors that regulate the
differentiation, proliferation, and activities of many types
of cells; Section 19-3Eb) and/or endotoxins (bacterial cell
wall lipopolysaccharides that elicit inflammatory re-
sponses; Section 35-2Fb), these cells begin to produce large
quantities of NO and continue to do so for many hours.Ac-
tivated macrophages and neutrophils also produce super-
oxide ion (O
⫺
2
), which chemically combines with NO to
form the even more toxic peroxynitrite (OONO
⫺
, which
rapidly reacts with H
2
O to yield the highly reactive hydrox-
ide radical, OHⴢ, and NO
2
) that they use to kill ingested
bacteria. Indeed, NOS inhibitors block the cytotoxic ac-
tions of macrophages.
Cytokines and endotoxins induce a long-lasting and
profound vasodilation and a poor response to vasocon-
strictors such as epinephrine. The sustained release of NO
has been implicated in septic shock (an often fatal immune
system overreaction to bacterial infection that results in a
catastrophic reduction in blood pressure), in inflamma-
tion-related tissue damage as occurs in autoimmune dis-
eases such as rheumatoid arthritis, and in the damage to
neurons in the vicinity of but not directly killed by a stroke
(reperfusion injury; Section 10-1Aa). Many of these condi-
tions might be alleviated if drugs can be developed that se-
lectively inhibit iNOS and/or nNOS, while permitting
eNOS to carry out its essential function of maintaining
vascular tone. Moreover, the administration of NO itself
appears to be medically useful. For example, the inhala-
tion of low levels NO has been used to reduce pulmonary
hypertension (high blood pressure in the lung, an often fa-
tal condition caused by constriction of its arteries) in new-
born infants.
2 HETEROTRIMERIC G PROTEINS
We have seen (Section 18-3) that hormones such as
glucagon and epinephrine regulate glycogen metabolism
by stimulating adenylate cyclase (AC) to synthesize the
second messenger cAMP from ATP. The cAMP then
binds to protein kinase A (PKA) so as to activate this en-
zyme to initiate cascades of phosphorylation/dephospho-
rylation events that ultimately control the activities of
glycogen phosphorylase and glycogen synthase. Numer-
ous other extracellular signaling molecules (known ago-
nists, ligands, or effectors) also activate the intracellular
synthesis of cAMP, thereby eliciting a cellular response.
But what is the mechanism through which the binding of
an agonist to a receptor induces AC to synthesize cAMP
in the cytosol? In answering this question we shall see
that the systems that link receptors to AC as well as other
effectors have a surprising complexity that endows them
with immense capacity for both signal amplification and
regulatory flexibility.
A. Overview
See Guided Exploration 16: Mechanisms of hormone signaling in-
volving the adenylate cyclase system Adenylate cyclase, which is
located on the plasma membrane’s cytosolic surface, and
the receptors that activate it, whose agonist-binding sites
are exposed to the extracellular space, are separate pro-
teins that do not physically interact. Rather, they are func-
tionally coupled by heterotrimeric G proteins (Fig. 19-14),
so called because they specifically bind the guanine nu-
cleotides GTP and GDP.
AC is activated by a heterotrimeric G protein (often
called just a G protein) but only when the G protein is
complexed with GTP. However, G protein slowly hy-
drolyzes GTP to GDP ⫹ P
i
(at the leisurely rate of 2–3
min
⫺1
) and thereby deactivates itself (if G proteins were
efficient enzymes, they would be unable to effectively acti-
vate AC). G protein is reactivated by the exchange of its
bound GDP for GTP, a process that is mediated by the
agonist–receptor complex but not by unoccupied receptor.
Heterotrimeric G protein therefore mediates the transduc-
tion of an extracellular signal to an intracellular signal (the
cAMP). Moreover, the receptor–G protein–AC system am-
plifies the extracellular signal because each agonist–receptor
complex activates many G proteins before it is inactivated
by the spontaneous dissociation of the agonist and, during
its lifetime,each G protein ⴢ GTP–AC complex catalyzes the
formation of many cAMP molecules. In this section, we dis-
cuss how this process occurs.
Heterotrimeric G proteins are members of the super-
family of regulatory GTPases that are collectively known
as G proteins (whether one is referring to a heterotrimeric
or some other species of G protein is usually clear from
context). G proteins other than heterotrimeric G proteins
have a wide variety of essential functions including signal
transduction (e.g., Ras; Section 19-3Cf), vesicle trafficking
(e.g.,Arf, dynamin, and Rab; Sections 12-4Cd and 12-4Db),
translation (as ribosomal accessory factors; Section 32-3),
and targeting [as components of the signal recognition
688 Chapter 19. Signal Transduction
JWCL281_c19_671-743.qxd 6/4/10 10:52 AM Page 688
particle (SRP) and the SRP receptor; Section 12-4Ba].The
many G proteins share common structural motifs that bind
guanine nucleotides (GDP and GTP) and catalyze the hy-
drolysis of GTP to GDP P
i
(see below).
B. G Protein-Coupled Receptors
The receptors responsible for activating AC and other tar-
gets of heterotrimeric G proteins are all integral proteins
with 7 transmembrane helices (Fig. 19-15) that have their
N-termini in the extracellular space and their C-termini in
the cytosol. These G protein-coupled receptors (GPCRs;
also called heptahelical, 7TM, and serpentine receptors)
constitute one of the largest known protein families (800
species in humans, which constitutes 3.5% of the ⬃23,000
genes in the human genome). They include receptors for
nucleosides, nucleotides, Ca
2
, catecholamines [epineph-
rine and norepinephrine as well as dopamine (Section
26-4B)] and other biogenic amines (e.g., histamine and
serotonin; Section 26-4B), eicosanoids (prostaglandins,
prostacyclins, thromboxanes, leukotrienes, and lipoxins,
derivatives of the C
20
fatty acid arachidonic acid, which are
potent local mediators of numerous important physiologi-
cal processes; Section 25-7), and for most of the large vari-
ety of peptide and protein hormones discussed in Section
19-1. In addition, GPCRs have important sensory func-
tions: They constitute the olfactory (odorant) and gusta-
tory (taste) receptors (of which there are estimated to be
460 different types in humans), as well as the several light-
sensing proteins in the retina, which are known as
rhodopsins. In addition, the GPCRs constitute the most
important class of drug targets in the pharmaceutical arse-
nal (Section 15-4): ⬃50% of approved drugs elicit their
therapeutic effects by selectively interacting with specific
GPCRs.
a. GPCRs Have Similar Structures
Despite the foregoing, the structures of only few species
of GPCRs have yet been elucidated, mainly due to the ap-
parent flexibilities of their extramembranous segments and
the general difficulty of crystallizing transmembrane
proteins (Section 12-3Aa). The first GPCR whose X-ray
Section 19-2. Heterotrimeric G Proteins 689
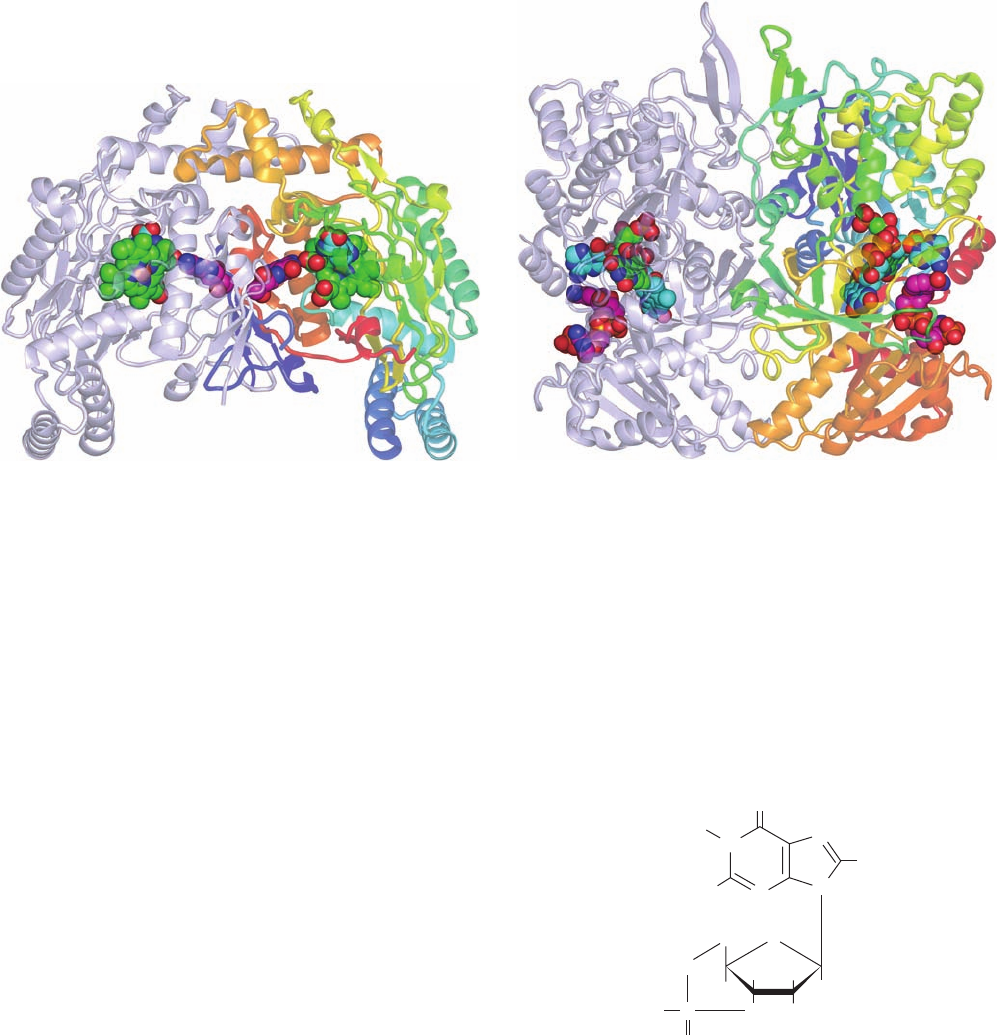
Figure 19-14 Activation/deactivation cycle for hormonally
stimulated AC. (a) In the absence of hormone, heterotrimeric G
protein binds GDP and AC is catalytically inactive. (b) The
hormone–receptor complex stimulates the G protein to
exchange its bound GDP for GTP. (c) The G protein GTP
(a)
(b)(d)
(c)
Receptor
G protein
Hormone
Adenylate
cyclase
GDP
GDP
GTP
ATP
GDP
GTP
cAMP + PP
i
P
i
Cellular responses
Extracellular space
Cytoplasm
N
C
1735246
Plasma
membrane
Figure 19-15 General structure of a G protein-coupled
receptor (GPCR).
complex, in turn, binds to and thereby activates AC to produce
cAMP. (d) The eventual G protein-catalyzed hydrolysis of its
bound GTP to GDP causes G protein to dissociate from and
hence deactivate AC.
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 689
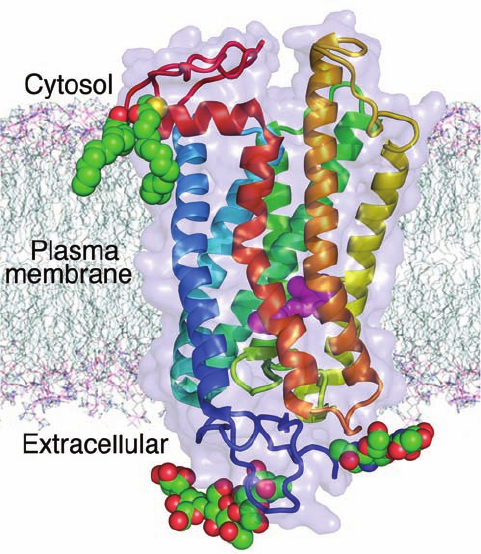
structure was reported was bovine rhodopsin (Fig. 19-16).
Rhodopsin consists of the 348-residue protein opsin that is
covalently linked to the chromophore retinal (Fig. 12-24)
via a Schiff base to Lys 296, much as occurs in the homolo-
gous bacteriorhodopsin (Section 12-3Ab),which is a hepta-
helical light-driven proton pump (Section 22-3Bh). The
absorption of a photon causes the rhodopsin-bound retinal
to isomerize from its ground state 11-cis form to its all-trans
form. This isomerization is accompanied by a transient con-
formational change in opsin before the all-trans-retinal is
hydrolyzed and dissociated from the opsin (which is
subsequently regenerated by the addition of 11-cis-retinal
delivered from adjacent epithelial cells in the retina). It
is this conformational change, which occurs mainly on
rhodopsin’s cytosolic surface, that activates its cognate G
protein.
Rhodopsins are unique among GPCRs in that their 11-cis
retinal “agonist” is covalently bound to the protein. All
other GPCRs bind diffusible ligands. The X-ray structures
of
1
- and
2
-adrenergic receptors and the human A
2A
adenosine receptor, the only other species of GPCRs
whose structures have yet been determined, reveal that
their transmembrane portions closely resemble each other
and that of rhodopsin, and that their bound ligands occupy
positions similar to that of retinal in rhodopsin.Note that the
transmembrane helices of the GPCRs are more or less uni-
form in size (20–27 residues), but their extramembranous
segments, which largely form their ligand- and G protein-
binding sites, vary widely in length with the identity of the
GPCR (7–595 residues for the N- and C-termini and 5–230
residues for the loops connecting their TM helices).
C. Heterotrimeric G Proteins: Structure
and Function
Heterotrimeric G proteins, which were first characterized
by Alfred Gilman and Martin Rodbell, are more complex
than Fig. 19-14 implies: They consist, as their name indi-
cates, of three different subunits, ,,and (45, 37,and 9 kD,
respectively), of which it is G
that binds GDP and GTP
(Fig. 19-17) and hence is a member of the G protein
superfamily. The binding of G
GDP–G
G
to its cognate
ligand–GPCR complex induces the G
to exchange its
bound GDP for GTP and, in so doing, to dissociate from
G
G
. In contrast, G
and G
bind one another with such
high affinity that they only dissociate under denaturing
conditions. Consequently, we shall henceforth refer to their
complex as G
.
Both G
and G
are membrane-anchored proteins: G
through its myristoylation or palmitoylation or both at or
near its N-terminus (Section 12-3Bb), and G
through the
prenylation of G
at its C-terminus (Section 12-3Ba).These
lipid modifications stabilize the interactions of G
with
G
, as they localize both to the inner surface of the plasma
membrane.
GTP binding, in addition to decreasing G
’s affinity for
its cognate ligand–GPCR complex, increases its affinity for
its effector,AC.Thus, it is the binding of G
GTP that acti-
vates AC (Fig. 19-17, left).
G
can also directly participate in signal transduction:
It activates a wide variety of signaling proteins including
several isoforms of AC (Section 19-2D), certain Na
,K
,
and Ca
2
-specific ion channels, various protein tyrosine ki-
nases (Section 19-3A), and phospholipase C- (PLC-; a
component of the phosphoinositide signaling system; Sec-
tion 19-4Ba). G
thereby provides an important source of
cross talk between signaling systems.
On the eventual G
-catalyzed hydrolysis of GTP, the
resulting G
GDP complex dissociates from AC and reas-
sociates with G
to reform inactive G protein. Since G
hydrolyzes its bound GTP at a characteristic rate, it func-
tions as a molecular clock that limits the length of time that
both G
GTP and G
can interact with their effectors.
Several types of ligand–GPCR complexes may activate
the same G protein. This occurs, for example, in liver cells in
response to the binding of the corresponding hormones to
690 Chapter 19. Signal Transduction
Figure 19-16 X-ray structure of bovine rhodopsin. The protein
is viewed parallel to the plane of the plasma membrane with its
approximate position therein indicated.The protein is represented
by its transparent molecular surface with its polypeptide chain in
ribbon form colored in rainbow order from its N-terminus (blue)
to its C-terminus (red). Note its bundle of seven nearly parallel
transmembrane helices.The protein’s retinal prosthetic group
(magenta) is drawn in space-filling form as are its two Cys-linked
palmitoyl groups and its two N-linked oligosaccharide groups
(colored according to atom type with C green, N blue, O red, and
S yellow). [Based on an X-ray structure by Tetsuji Okada, National
Institute of Advanced Industrial Science and Technology, Kyoto,
Japan; and Volker Buss, University of Duisberg-Essen, Duisberg,
Germany. PDBid 1U19.]
JWCL281_c19_671-743.qxd 3/16/10 7:16 PM Page 690
