Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

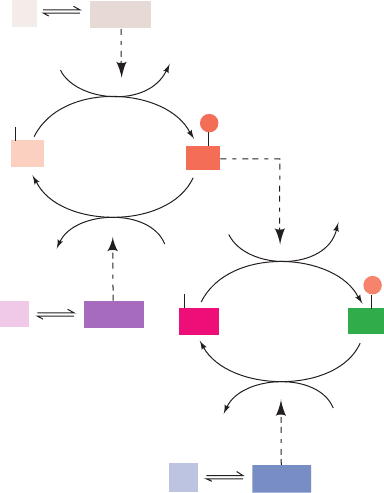
The activities of both glycogen phosphorylase and glyco-
gen synthase are controlled by bicyclic cascades. Let us now
examine the enzymatic interconversions involved in these bi-
cyclic cascades. We shall specifically focus on the covalent
modifications of glycogen phosphorylase and glycogen syn-
thase, the structural effects of these covalent modifications,
and how these structural changes affect the interactions of
their allosteric effectors.We shall then consider the cyclic cas-
cades as a whole, studying the various modification enzymes
involved and their “ultimate” allosteric effectors. Finally, we
shall see how the various cyclic cascades of glycogen metab-
olism function in different physiological situations.
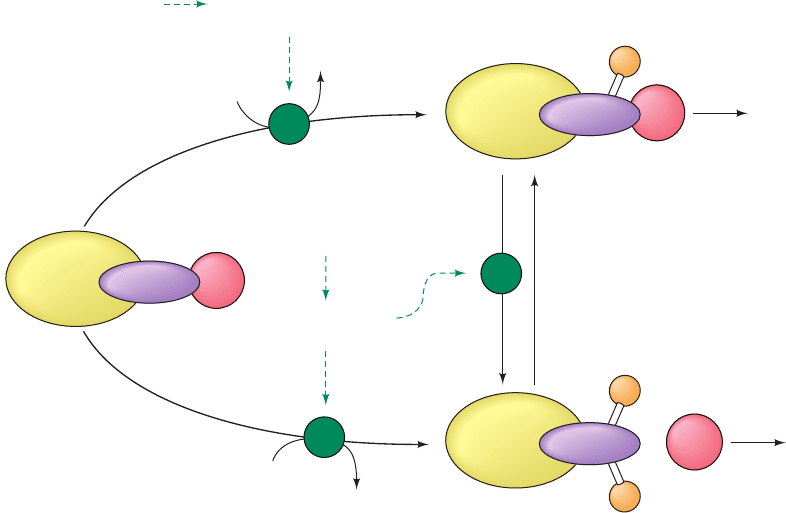
C. Glycogen Phosphorylase Bicyclic Cascade
In 1938, Carl and Gerty Cori found that glycogen phospho-
rylase exists in two forms, the b form that requires AMP for
activity, and the a form that is active without AMP. It nev-
ertheless took 20 years for the development of the protein
chemistry techniques through which Edwin Krebs and
Edmond Fischer demonstrated, in 1959, that phosphory-
lases a and b correspond to forms of the protein in which a
specific residue, Ser 14, is enzymatically phosphorylated or
dephosphorylated, respectively.
a. Glycogen Phosphorylase: The Cascade’s
Target Enzyme
The activity of glycogen phosphorylase is allosterically
controlled, as we saw, through AMP activation and ATP,
G6P, and glucose inhibition (Section 18-3A). Superim-
posed on this allosteric control is control by enzymatic in-
terconversion through a bicyclic cascade involving the ac-
tions of three enzymes (Figs. 18-13 and 18-14, left):
1. Phosphorylase kinase, which specifically phosphory-
lates Ser 14 of glycogen phosphorylase b (Fig. 18-13,
enzyme F
2
).
2. Protein kinase A, which phosphorylates and thereby
activates phosphorylase kinase (Fig. 18-13, enzyme F
1
).
3. Phosphoprotein phosphatase-1, which dephosphory-
lates and thereby deactivates both glycogen phosphorylase a
and phosphorylase kinase (Fig. 18-13, enzymes R
1
and R
2
).
In an interconvertible enzyme system, the “modified” form
of the enzyme bears the prefix m and the “original” (unmod-
ified) form bears the prefix o, whereas the enzyme’s most ac-
tive and least active forms are identified by the suffixes a and
b, respectively. In this case, o-phosphorylase b (unmodified,
least active) is the form under allosteric control by AMP,
ATP, and G6P (Fig. 18-10, left). Phosphorylation to yield m-
phosphorylase a (modified, most active) all but removes the
effects of these allosteric modulators. In terms of the sym-
metry model of allosterism (Section 10-4B), the phosphory-
lation of Ser 14 shifts the enzyme’s T (inactive) 34 R (active)
equilibrium in favor of the R state (Fig. 18-10, right). Indeed,
phosphorylase a’s Ser 14-phosphoryl group is analogous to
an allosteric activator: It forms ion pairs with two Arg side
chains on the opposite subunit, thereby knitting the subunits
together in much the same way as does AMP when it binds
tightly to a site between the subunits (Fig. 18-11b).
Section 18-3. Control of Glycogen Metabolism 651
Figure 18-13 A bicyclic enzyme cascade. See the legend of
Fig. 18-12 for symbol definitions. In a bicyclic cascade, one of
the modifying enzymes (F
2
) is also subject to covalent
F
2b
F
2a
K
1
F
1
K
2
P
i
P
i
R
1
e
2
e
1
ADP
ATP
ADP
ATP
H
2
O
H
2
O
R
1
• e
2
F
1
• e
1
E
a
E
b
P
P
K
3
R
2
e
3
R
2
• e
3
modification. It is active in the modified state (F
2a
) and inactive
in the unmodified state (F
2b
).
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 651
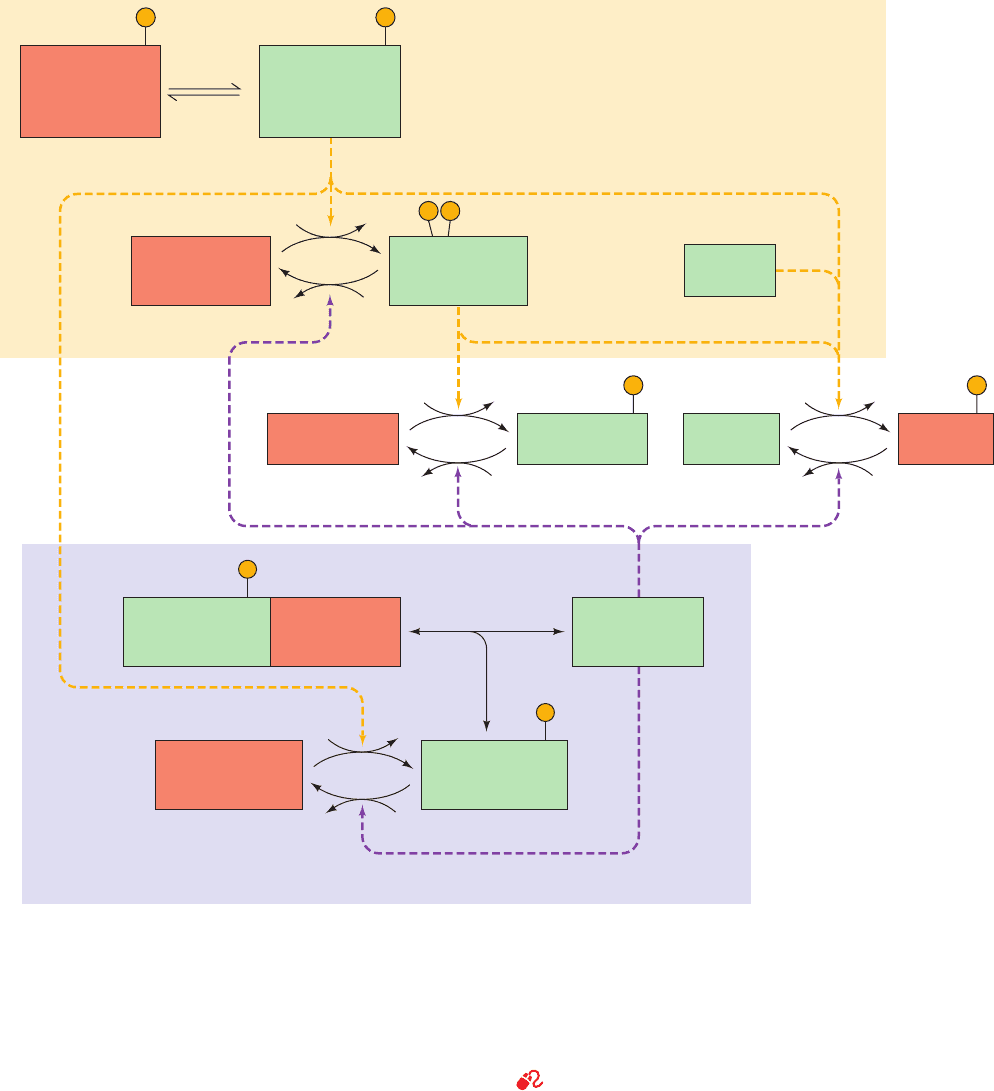
In the resting cell, the concentrations of ATP and G6P
are high enough to inhibit phosphorylase b. The level of
phosphorylase activity is therefore largely determined by the
fraction of the enzyme present as phosphorylase a. The
steady-state fraction of phosphorylated enzyme (E
a
) de-
pends on the relative activities of phosphorylase kinase
(F
2
), protein kinase A (F
1
), and phosphoprotein phos-
phatase-1 (R
1
and R
2
).This interrelationship is remarkably
elaborate for glycogen phosphorylase. Let us consider the
actions of these enzymes.
b. Protein Kinase A: A Crucial Regulatory Link
Phosphorylase kinase, which converts phosphorylase b
to phosphorylase a, is itself subject to covalent modification
652 Chapter 18. Glycogen Metabolism
R
2
C
2
Protein kinase
A (PKA)
(inactive)
m-Phosphoprotein
phosphatase
inhibitor-1 a
Phosphoprotein
phosphatase-1
(inactive)
(α β γ δ)
4
o-Phosphorylase
kinase b
o-Glycogen
phosphorylase b
m-Glycogen
phosphorylase a
ATP ADP
P
i
H
2
O
ATP ADP
P
i
H
2
O
m-Glycogen
synthase b
o-Glycogen
synthase a
ATP ADP
P
i
H
2
O
4cAMP
ATP ADP
P
i
H
2
O
m-Phosphoprotein
phosphatase
inhibitor-1 a
o-Phosphoprotein
phosphatase
inhibitor-1 b
DEPHOSPHORYLATION
SYSTEM
PHOSPHORYLATION
SYSTEM
+ R
2
(cAMP)
4
2
P
P
P
PP
P
PP
(α β γ δ)
4
m-Phosphorylase
kinase a
Other
kinases
C
Protein kinase
A (PKA)
(active)
Phosphoprotein
phosphatase-1
(active)
Figure 18-14 Schematic diagram of the major enzymatic
modification/demodification systems involved in the control of
glycogen metabolism in muscle. Modification (phosphorylation)
systems are shaded in yellow, demodification (dephosphorylation)
systems are shaded in lavender, active enzymes/inhibitors are
shaded in green, and inactive enzymes/inhibitors are shaded in
orange. Dashed yellow and purple arrows indicate facilitation of
a modification and demodification reaction. Note that glycogen
phosphorylase activity is controlled by a bicyclic enzyme cascade
(left) and glycogen synthase activity is controlled by both a
bicyclic and a monocyclic enzyme cascade (right). By convention,
the modified form of the enzyme bears the prefix m and the
“original” (unmodified) form bears the prefix o. The most active
and least active forms of the enzymes are identified by the
suffixes a and b, respectively. Further control of phosphoprotein
phosphatase-1 covalent modification is diagrammed in Fig. 18-22.
See Guided Exploration 15: Control of Glycogen Breakdown, and
the Animated Figures
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 652
(Fig. 18-14). For phosphorylase kinase to be fully active,
Ca
2
must be present (see below) and the protein must be
phosphorylated.
In both the glycogen phosphorylase and glycogen syn-
thase cascades, the primary intracellular signal, e
1
,is
adenosine-3ⴕ,5ⴕ-cyclic monophosphate (3ⴕ,5ⴕ-cyclic
AMP or cAMP). The cAMP concentration in a cell is a
function of the ratio of its rate of synthesis from ATP by
adenylate cyclase (AC; also called adenylyl cyclase)
and its rate of breakdown to AMP by a enzymes known
as cAMP-phosphodiesterases (cAMP-PDEs; Section
19-2E):
N
N
N
N
H
2
C
HH
HH
HO OH
O
NH
2
–
OP
O
O
–
PPO
O
O
–
O
O
O
–
H
H
ATP
N
N
N
N
H
2
C
H
HH
O
OH
O
NH
2
H
H
3ⴕ,5ⴕ-Cyclic AMP
(cAMP)
–
O
O
O
H
adenylate cyclase
PP
i
phosphodiesterase
H
2
O
N
N
N
N
H
2
C
HH
HH
HO OH
O
NH
2
P
–
O
O
O
–
H
H
O
O
P
AMP
AC is, in turn, activated by certain hormones (Sections
18-3Ea and 19-2D).
cAMP is absolutely required for the activity of protein
kinase A [PKA; also called cAMP-dependent protein
kinase (cAPK)], an enzyme that phosphorylates specific
Ser and/or Thr residues of numerous cellular proteins,
including phosphorylase kinase and glycogen synthase.
These proteins all contain PKA’s consensus recognition
sequence, Arg-Arg-X-Ser/Thr-Y, where Ser/Thr is the
phosphorylation site, X is any small residue, and Y is a large
hydrophobic residue. In the absence of cAMP, PKA is an
inactive heterotetramer consisting of two regulatory (R)
and two catalytic (C) subunits, R
2
C
2
. The cAMP binds to
the regulatory subunits so as to cause the dissociation of
active catalytic monomers (Fig. 18-14; top). The intracellu-
lar concentration of cAMP therefore determines the fraction
of PKA in its active form and thus the rate at which it phos-
phorylates its substrates. In fact, in all known eukaryotic
cases, the physiological effects of cAMP are exerted
through the activation of specific protein kinases.
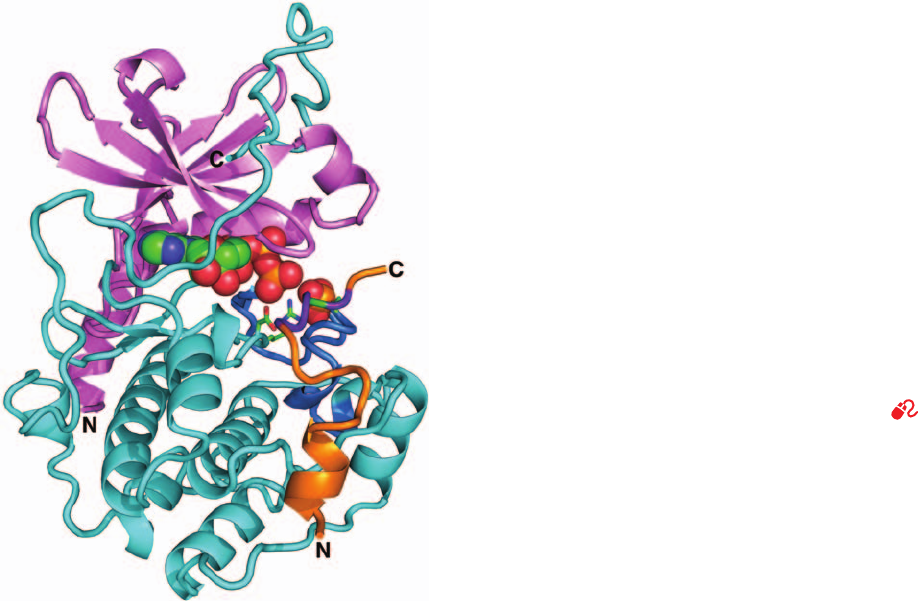
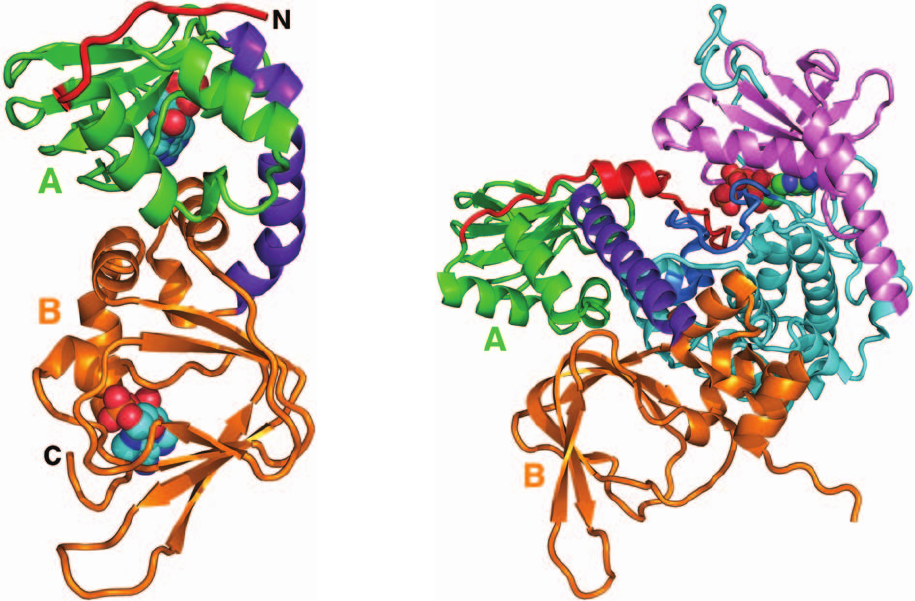
The X-ray structure of the 350-residue C subunit of
mouse PKA in complex with Mg
2
–ATP and a 20-residue
inhibitor peptide was determined by Susan Taylor and
Janusz Sowadski (Fig. 18-15), and that of a similar complex
of the porcine heart enzyme was determined by Robert
Huber.The C subunit, as are other kinases of known struc-
ture (e.g., Figs. 17-5 and 17-15), is bilobal. It has an N-
terminal domain that consists of a 5-stranded sheet and
an helix, and a larger C-terminal domain that is mainly
helical. A deep cleft between the lobes is occupied by the
Mg
2
–ATP and the segment of the inhibitor peptide that
includes the above 5-residue consensus sequence.This cleft
therefore contains PKA’s catalytic site, with the small do-
main contributing the nucleotide-binding site and the large
subunit supplying the substrate-binding and catalytic
residues.
The C subunit of PKA must be phosphorylated at Thr
197 for activity. Thr 197 is part of the so-called activation
loop (comprising residues 184–208), which is located at the
“mouth” of the cleft between PKA’s N- and C-terminal do-
mains. The phosphoryl group at Thr 197 knits together the
various components of PKA into its active conformation
via extensive interactions with the protein. Most notably,
the phosphoryl group interacts with Arg 165, a conserved
residue that is adjacent to Asp 166, the catalytic base that
activates the substrate protein’s target Ser/Thr hydroxyl
group for phosphorylation, thereby properly orienting
PKA’s active site residues.
Protein kinases play key roles in the signaling pathways
by which many hormones, growth factors, neurotransmit-
ters, and toxins affect the functions of their target cells
(Chapter 19), as well as in controlling metabolic pathways.
Indeed, 518 human proteins constituting ⬃2.3% of human
genes are predicted to be protein kinases, which accounts
for the observation that ⬃30% of the proteins in mam-
mallian cells are phosphorylated.The 1000 different pro-
tein kinases that have been sequenced share a conserved
catalytic core corresponding to residues 40 to 280 of PKA’s
Section 18-3. Control of Glycogen Metabolism 653
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 653
C subunit. In addition to phosphorylating other proteins,
many protein kinases are themselves phosphoproteins
whose activities are controlled by phosphorylation,often at
their activation loops. However, since PKA is normally
fully phosphorylated at Thr 197 and resistant to dephos-
phorylation, it is unclear whether its activity is regulated in
vivo by phosphorylation/dephosphorylation.
c. PKA’s R Subunit Competitively Inhibits
Its C Subunit
The R subunit of PKA has a well-defined domain struc-
ture that was first characterized by limited proteolysis. It
consists of, from N- to C-terminus, a dimerization domain,
an autoinhibitor segment, and two tandem homologous
cAMP-binding domains,A and B. In the R
2
C
2
complex, the
autoinhibitor segment, which resembles the C subunit’s
substrate peptide, binds in the C subunit’s active site (as
does the inhibitory peptide in Fig. 18-15) so as to block sub-
strate binding. Thus, the R subunit is a competitive
inhibitor of PKA’s substrate proteins.
Each R subunit cooperatively binds two cAMPs. When
the B domain lacks bound cAMP, it masks the A domain so
as to prevent it from binding cAMP. However, the binding
of cAMP to the B domain triggers a conformational
change that permits the A domain to bind cAMP, which, in
turn, releases the C subunits from the complex (see below).
Taylor determined the X-ray structure of the R subunit
lacking its N-terminal 91 residues and in complex with two
cAMPs (Fig. 18-16a). This truncated protein is unable to
dimerize but, in the absence of cAMP, forms a tight inactive
complex with the C subunit, and on binding cAMP releases
active C subunits as do intact R
2
dimers.As previously pre-
dicted by sequence alignments, the A and B domains are
structurally similar to each other and to the prokaryotic
cAMP-binding transcriptional regulator named catabolite
gene activator protein (CAP; Section 31-3Cb). The autoin-
hibitory segment, which in the free R subunit is extremely
sensitive to proteolysis, has its first 21 residues disordered
in the X-ray structure.
The X-ray structure of the truncated R subunit in com-
plex with the C subunit binding AMPPNP (Fig. 18-16b),
also determined by Taylor, reveals that the R subunit has
undergone a massive conformational reorganization rela-
tive to its cAMP-binding structure.The most striking such
change is that the 25-residue, bent 2-helix segment linking
domains A and B (purple in Fig. 18-16a) has coalesced to
form a single straight helix such that the  sandwich in the
R subunit’s B domain has rotated by ⬃180° relative to
that in the A domain and separated from it. As a conse-
quence of this ⬃60-Å screwlike shift of the B domain, the
elongated globular structure of the cAMP complex
changes to a dumbbell-like shape in which the interface
between the A and B domains in the cAMP complex is re-
placed by extensive interactions with the large domain of
the C subunit. In addition, the helical regions of the A and
B domains undergo extensive conformational changes
that eliminate their cAMP binding sites by separating
their phosphate-binding pockets from their adenine-bind-
ing pockets. All of this positions the R subunit’s autoin-
hibitor segment in the C subunit’s active site cleft, thereby
inactivating it.
654 Chapter 18. Glycogen Metabolism
Figure 18-15 X-ray structure of the catalytic (C) subunit of
mouse protein kinase A (PKA). The protein is in complex with
ATP and a 20-residue peptide segment of a naturally occurring
protein kinase inhibitor.The N-terminal domain is pink and its
C-terminal domain is cyan with its activation loop blue.The
polypeptide inhibitor is orange and its pseudo–target sequence,
Arg-Arg-Asn-Ala-Ile, is purple, with the Ala replacing the Ser to
be phosphorylated green (note that the enzyme’s true target
sequence is Arg-Arg-X-Ser/Thr-Y, where X is a small residue,Y
is a large hydrophobic residue, and Ser/Thr, which is replaced by
Ala in the polypeptide inhibitor, is the residue that the enzyme
phosphorylates).The ATP and the phosphoryl group of
phosphoThr 197 are shown in space-filling form and the side
chains of the catalytically essential Arg 165,Asp 166, and Thr 197
are shown in stick form, all colored according to atom type
(C green, N blue, O red, and P orange). Note that the inhibitor’s
pseudo–target sequence is in close proximity to the ATP’s ␥
phosphate group, the group that the enzyme transfers. [Based on
an X-ray structure by Susan Taylor and Janusz Sowadski,
University of California at San Diego. PDBid 1ATP.]
See
Interactive Exercise 10 and Kinemage Exercise 15-1
JWCL281_c18_638-670.qxd 10/19/10 7:29 AM Page 654
d. Phosphorylase Kinase: Coordination of Enzyme
Activation with [Ca
2ⴙ
]
Phosphorylase kinase (PhK) is activated by Ca
2⫹
con-
centrations as low as 10
⫺7
M as well as by covalent modifica-
tion. This 1300-kD enzyme consists of four nonidentical
subunits that form the active oligomer (␣␥␦)
4
. The iso-
lated ␥ subunit is capable of full catalytic activity (ability to
convert phosphorylase b to phosphorylase a), whereas the
␣, , and ␦ subunits are inhibitors of the catalytic reaction.
The ␦ subunit, which is known as calmodulin (CaM),
confers Ca
2⫹
sensitivity on the complex. When Ca
2⫹
binds
to calmodulin’s four Ca
2⫹
-binding sites, this ubiquitous
eukaryotic regulatory protein undergoes an extensive
conformational change (see below) that activates phospho-
rylase kinase. Glycogen phosphorylase therefore becomes
phosphorylated and the rate of glycogen breakdown
increases. The physiological significance of this Ca
2⫹
activa-
tion process is that nerve impulses trigger muscle con-
traction through the release of Ca
2⫹
from intracellular
reservoirs (Section 35-3C). This transient increase in cytoso-
lic [Ca
2⫹
] induces both muscle contraction and the increase
in glycogen breakdown that supplies glycolysis, which in
turn, generates the ATP required for muscle contraction.
e. Calmodulin: A Ca
2ⴙ
-Activated Switch
Calmodulin is a ubiquitous eukaryotic Ca
2⫹
-binding
protein that participates in numerous cellular regulatory
processes. In some of these, CaM functions as a monomeric
protein, whereas in others (e.g., PhK) it is a subunit of a
larger protein.The X-ray structure of this highly conserved
Section 18-3. Control of Glycogen Metabolism 655
Figure 18-16 X-ray structures of the regulatory (R) subunit of
bovine protein kinase A (PKA). (a) The R subunit lacking its
N-terminal 91 residues (which form its dimerization domain) in
complex with cAMP. The N-terminal region, which includes its
autoinhibitor segment, is red, domain A is green, domain B is
orange, and the 2-helix segment linking domains A and B is
purple. The cAMPs, which are drawn in space-filling form
colored according to atom type (C cyan, N blue, O red, and P
orange), each bind at the opening of an 8-stranded  sandwich
with their phosphate group abutting the N-terminal end of a
short helical segment. (b) The truncated R subunit in complex
with the C subunit that is binding AMPPNP. The R subunit is
colored as in Part a and the C subunit is colored as in Fig. 18-15.
The C subunit is rotated 180° about the vertical axis relative to
Fig. 18-15 and the  sandwich of domain A is oriented similarly
to that in Part a. Note the dramatic conformational differences
between the R subunit in Parts a and b, which in large part is
caused by the coalescence and straightening of the two helices
linking domains A and B in the cAMP complex.Also note that
the now ordered N-terminal portion of the R subunit’s
autoinhibitor segment occupies the C subunit’s active site cleft.
[Based on X-ray structures by Susan Taylor, University of
California at San Diego. PDBids 1RGS and 2QCS.]
(a) (b)
JWCL281_c18_638-670.qxd 6/30/10 11:59 AM Page 655
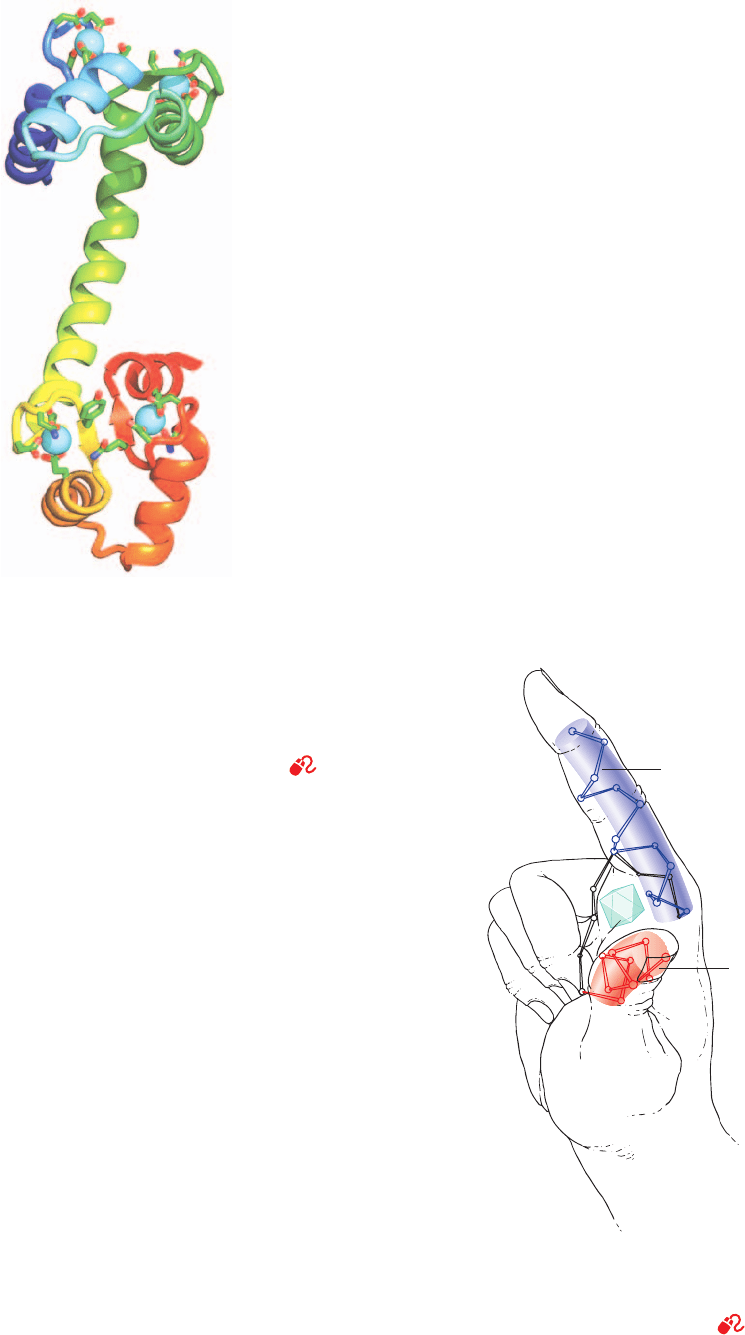
148-residue protein, determined by Charles Bugg, has a cu-
rious dumbbell-like shape in which CaM’s two globular do-
mains are connected by a seven-turn helix (Fig. 18-17).
CaM has two high-affinity Ca
2
-binding sites on each of its
globular domains, both of which are formed by nearly su-
perimposable helix–loop–helix motifs known as EF hands
(Fig. 18-18) that also occur in numerous other Ca
2
-sensing
proteins of known structure. The Ca
2
ion in each of these
sites is octahedrally coordinated by oxygen atoms from the
backbone and side chains of the loop as well as from a
protein-associated water molecule.
The binding of Ca
2
to either domain of CaM induces a
conformational change in that domain, which exposes an
otherwise buried Met-rich hydrophobic patch. This patch,
in turn, binds with high affinity to the CaM-binding domain
of the phosphorylase kinase subunit, as well as to the
CaM-binding domains of numerous other Ca
2
-regulated
proteins (many of which interact with CaM that is free in
solution), and in doing so modulates the activities of these
proteins. These CaM-binding domains have little mutual
sequence similarity but are all basic amphiphilic helices.
In fact, ⬃20-residue segments of these helices, as well as
synthetic amphiphilic helices composed of only Leu, Lys,
and Trp residues, are bound by Ca
2
–CaM as tightly as the
target proteins themselves.
Despite uncomplexed CaM’s extended appearance in
its X-ray structure (Fig. 18-17), a variety of studies indicate
that both of its globular domains can simultaneously bind
to a single target helix. Evidently, CaM’s central helix
serves as a flexible linker rather than as a rigid spacer, a
property that probably further increases the range of tar-
get sequences to which CaM can bind. This idea is con-
firmed by the NMR structure (Fig. 18-19) of (Ca
2
)
4
–CaM
in complex with its 26-residue CaM-binding target
polypeptide of skeletal muscle myosin light chain kinase
(MLCK; a homolog of the PKA C subunit, which phospho-
rylates and thereby activates the light chains of the muscle
protein myosin; Section 35-3Da), which was determined by
Marius Clore, Angela Gronenborn, and Ad Bax. Indeed,
the extended conformation of CaM’s central helix in Fig.
18-17 is probably an artifact arising from crystal packing
forces, considering that this helix’s central two turns con-
tact no other portion of the protein and hence are maxi-
mally solvent-exposed (almost all other known helices
are at least partially buried in a protein). Moreover, a
polypeptide with the sequence of this helix assumes a ran-
dom coil conformation in aqueous solution. Nevertheless,
the flexible linker is essential to the function of CaM: In the
presence of Ca
2
, CaM’s individual domains (obtained by
656 Chapter 18. Glycogen Metabolism
Figure 18-17 X-ray structure of rat testis calmodulin. This
monomeric 148-residue protein, which is colored in rainbow
order from N-terminus (blue) to C-terminus (red), contains two
remarkably similar globular domains separated by a seven-turn
helix.The two Ca
2
ions bound to each domain are represented
by cyan spheres. The side chains liganding the Ca
2
ions are
drawn in stick form colored according to atom type (C green, N
blue, and O red). [Based on an X-ray structure by Charles Bugg,
University of Alabama at Birmingham. PDBid 3CLN.]
See
Kinemage Exercise 16-1
Figure 18-18 EF hand. The Ca
2
-binding sites in many
proteins that function to sense the level of Ca
2
are formed by
helix–loop–helix motifs named EF hands. [After Kretsinger,
R.H., Annu. Rev. Biochem. 45, 241 (1976).]
See Kinemage
Exercise 16-1
Ca
2+
EF hand
E helix
F helix
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 656
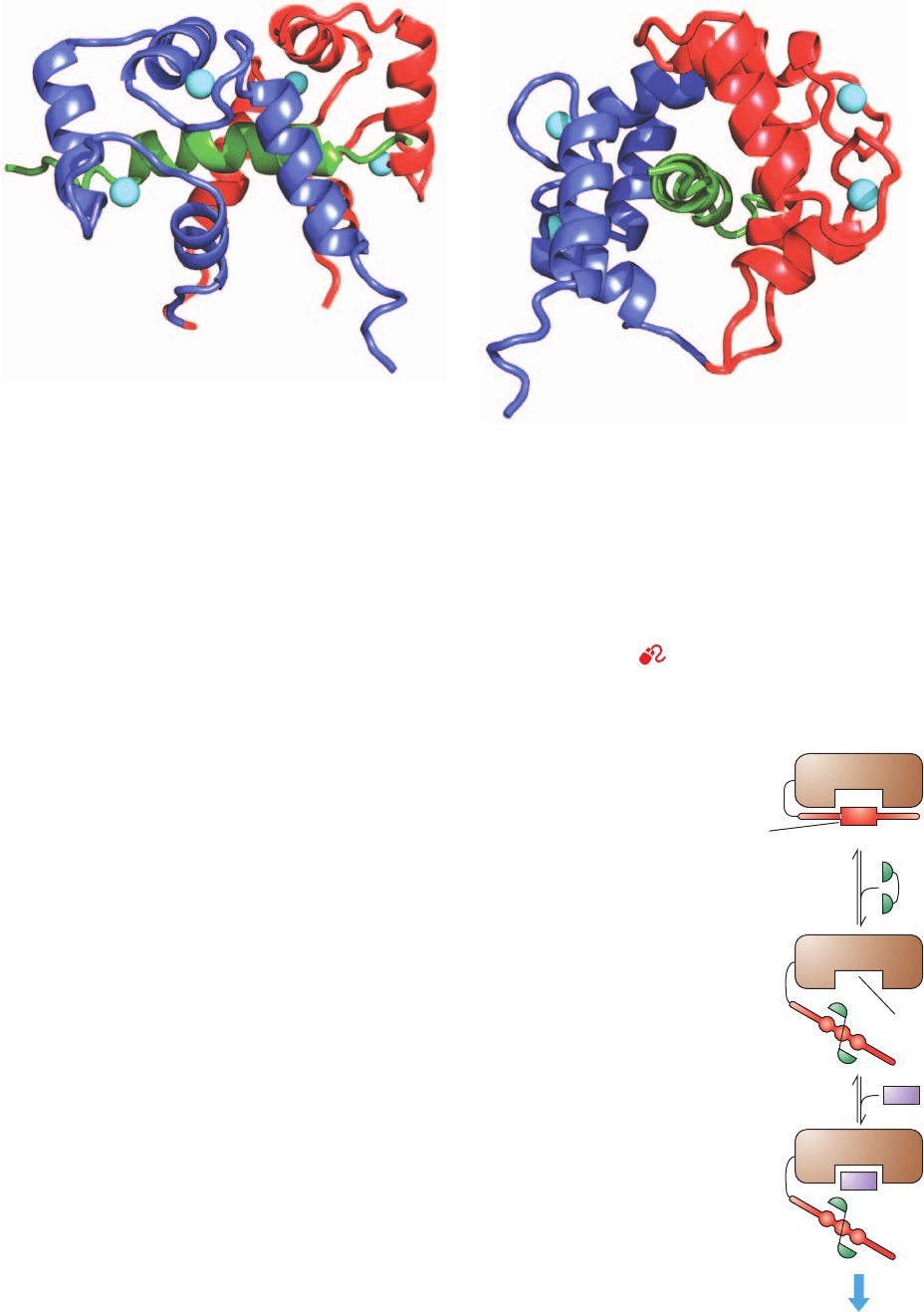
tryptic cleavage), when in high concentration, are able to
bind their target proteins but fail to even marginally acti-
vate them unless present in several hundred-fold excess.
How does Ca
2
–CaM activate its target protein ki-
nases? MLCK contains a C-terminal segment whose se-
quence resembles that of MLCK’s target polypeptide on
the light chain of myosin but lacks a phosphorylation site.
A model of MLCK, based on the X-ray structure of the
30% identical C subunit of PKA, strongly suggests that this
autoinhibitor peptide inactivates MLCK by binding in its
active site. Indeed, the excision of MLCK’s autoinhibitor
peptide by limited proteolysis permanently activates this
enzyme. MLCK’s CaM-binding segment overlaps this au-
toinhibitor peptide. Evidently, the binding of Ca
2
–CaM to
this peptide segment extracts the autoinhibitor from MLCK’s
active site, thereby activating this enzyme (Fig. 18-20).
Ca
2
–CaM’s other target proteins, including the phos-
phorylase kinase subunit, are presumably activated in the
Section 18-3. Control of Glycogen Metabolism 657
(a)
(b)
(inactive)
(active)
regulatory domain
Catalytic domain
Cellular response
Ca
2+
– CaM
CaM-binding
protein kinase
Substrate
protein
Active site
CaM binding
site
Figure 18-20 Schematic diagram of the Ca
2
–CaM-dependent activation of protein kinases.
Autoinhibited kinases have an N- or C-terminal “pseudosubstrate” sequence (red ) that binds at
or near the enzyme’s active site (brown) so as to inhibit its function. This autoinhibitory segment
is in close proximity with or overlaps a Ca
2
–CaM-binding sequence. Consequently, Ca
2
–CaM
(green) binds to this sequence so as to extract it from the enzyme’s active site, thereby activating
the enzyme to phosphorylate other proteins (purple). [After Crivici, A. and Ikura, M., Annu. Rev.
Biophys. Biomol. Struct. 24, 88 (1995).]
Figure 18-19 NMR structure of (Ca
2ⴙ
)
4
–CaM from
Drosophila melanogaster in complex with its 26-residue target
polypeptide from rabbit skeletal muscle myosin light chain
kinase (MLCK). The N terminal domain of CaM is blue, its
C-terminal domain is red, the target polypeptide is green, and the
Ca
2
ions are represented by cyan spheres. (a) A view of the
complex in which the N-terminus of the target polypeptide is on
the right, and (b) the perpendicular view as seen from the right
side of Part a. In both views, the pseudo-2-fold axis relating the
N- and C-terminal domains of CaM is approximately vertical.
Note how the middle segment of the long central helix in
uncomplexed CaM (Fig. 18-17) has unwound and bent (bottom
loop in Part b) such that CaM forms a globular protein that
largely encloses the helical target polypeptide within a
hydrophobic tunnel in a manner resembling two hands holding a
rope (the target polypeptide assumes the random coil
conformation in solution). However, the conformations of CaM’s
two globular domains are essentially unchanged by the
complexation. Evidently, CaM’s bound Ca
2
ions serve to
organize and stabilize the target binding conformations of its
globular domains. [Based on an NMR structure by Marius Clore,
Angela Gronenborn, and Ad Bax, National Institutes of Health.
PDBid 2BBM.]
See Kinemage Exercise 16-2
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 657
same way.The X-ray structures of two homologous protein
kinases support this so-called intrasteric mechanism, those
of calmodulin-dependent protein kinase I (CaMKI) and
twitchin kinase. Although the details of binding of the au-
toinhibitory sequence differ for each of these protein ki-
nases, the general mode of autoinhibition and activation by
Ca
2⫹
–CaM is the same.
PKA’s R subunit, as we have seen, contains a similar au-
toinhibitory sequence adjacent to its two tandem cAMP-
binding domains. In this case, however, the autoinhibitory
peptide is allosterically ejected from the C subunit’s active
site by the binding of cAMP to the R subunit (which lacks
a Ca
2⫹
–CaM binding site).
f. Phosphorylase Kinase’s ␥ Subunit Is Controlled
by Multiple Autoinhibitors
Phosphorylase kinase’s 386-residue ␥ subunit consists of
an N-terminal kinase domain, which is 36% identical in
sequence to the C subunit of PKA, and a C-terminal regula-
tory domain, which contains a CaM-binding peptide and an
overlapping autoinhibitor segment. Evidently, Ca
2⫹
–CaM
relieves this inhibition, as is diagrammed in Fig. 18-20.This
explains why the N-terminal 298-residue segment of the
PhK ␥ subunit, termed PhK␥
t
(t for truncated), displays
catalytic activity comparable to that of fully activated PhK
but is unaffected by Ca
2⫹
or phosphorylation signals.
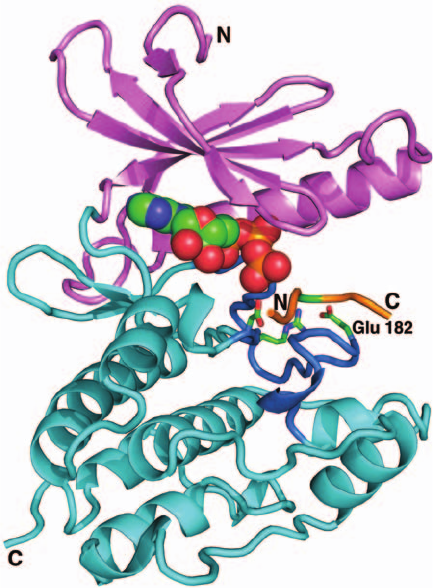
The X-ray structure of PhK␥
t
in complex with ATP and
a heptapeptide related to the natural substrate was deter-
mined by Johnson (Fig. 18-21). It reveals, as expected, that
PhK␥
t
structurally resembles PKA (Fig. 18-15) as well as
other protein kinases of known structure including CaMKI
and twitchin kinase. Comparisons of these various struc-
tures shed light on how the catalytic activity of PhK is
regulated. Numerous protein kinases, including PKA, are
activated by the phosphorylation of Ser, Thr, and/or
Tyr residues in their activation loops, which, as we saw in
Fig. 18-15, interacts with a conserved Arg residue that
thereby correctly positions the adjacent catalytically im-
portant Asp residue. However, the PhK ␥ subunit is not
subject to phosphorylation. Rather, its activation loop
residue that might otherwise be phosphorylated is Glu 182,
whose negative charge mimics the presence of a phosphate
group by interacting with Arg 148 so as to correctly posi-
tion Asp 149 (Fig. 18-21).Thus, the PhK catalytic site main-
tains an active conformation but, in the absence of Ca
2⫹
,is
inactivated by the binding of its C-terminal autoinhibitor
segment.
Sites on both the ␣ and  subunits of PhK are subject to
phosphorylation by PKA (Fig. 18-14).This activates PhK at
much lower Ca
2⫹
concentrations than otherwise, and full
enzyme activity is obtained in the presence of Ca
2⫹
only
when both these subunits are phosphorylated. The  sub-
unit does, in fact, have an autoinhibitor sequence, suggest-
ing that phosphorylation changes its conformation so as to
make it unavailable for inhibiting the ␥ subunit’s active
site.This would explain the synergistic effect of phosphory-
lation and Ca
2⫹
on the activity of PhK: Ca
2⫹
–CaM se-
questers the ␥ subunit’s autoinhibitory segment, whereas
phosphorylation of the  subunit removes yet another au-
toinhibitor.The way in which the phosphorylation of the ␣
subunit modulates the activity of PhK is, as yet, unknown.
g. Phosphoprotein Phosphatase-1
The steady-state phosphorylation levels of most en-
zymes involved in cyclic cascades are maintained by the
opposition of kinase-catalyzed phosphorylations and the
hydrolytic dephosphorylations catalyzed by phosphopro-
tein phosphatases. The phosphatase involved in the cyclic
cascades controlling glycogen metabolism is phosphoprotein
phosphatase-1. This enzyme, as is indicated in Fig. 18-14,
hydrolyzes the phosphoryl groups from m-glycogen phos-
phorylase a, both the ␣ and  subunits of phosphorylase
kinase, and two other proteins involved in glycogen meta-
bolism, as discussed below.
The catalytic subunit of phosphoprotein phosphatase-1
(PP1), which is designated PP1c, hydrolyzes phosphoryl
groups on Ser/Thr residues via a single step mechanism.
658 Chapter 18. Glycogen Metabolism
Figure 18-21 X-ray structure of rabbit muscle PhK␥
t
in
complex with ATP and a heptapeptide (RQMSFRL). This
heptapeptide is related in sequence to the enzyme’s natural
substrate (KQISVRG).The protein is shown in the “standard”
protein kinase orientation with its N terminal domain pink, its
C-terminal domain cyan, and its activation loop blue. The
heptapeptide is orange, with its residue to be phosphorylated
(Ser) green.The ATP is shown in space-filling form and the side
chains of the catalytically essential Arg 148,Asp 149, and Glu 182
are shown in stick form, all colored according to atom type
(C green, N blue, O red, and P orange). Note the structural simi-
larities and differences between this protein and the homologous
C subunit of PKA (Fig. 18-15). [After an X-ray structure by
Louise Johnson, Oxford University, Oxford, U.K. PDBid 2PHK.]
JWCL281_c18_638-670.qxd 6/3/10 1:49 PM Page 658
The X-ray structure of PP1c indicates that it contains a bin-
uclear metal ion center (both metals are Mn
2
in the re-
combinant enzyme) which, it is proposed, activates a water
molecule (promotes its ionization to OH
, Section 15-1Cb)
for nucleophilic attack on the phosphoryl group.
PP1c binds to glycogen through the intermediacy of
regulatory proteins in both muscle and liver. In muscle,
PP1c is only active when it is bound to glycogen through
this glycogen-binding G
M
subunit. The activity of PP1c
and its affinity for the G
M
subunit are regulated by phos-
phorylation of the G
M
subunit at two separate sites (Fig.
18-22). Phosphorylation of site 1 by insulin-stimulated
protein kinase activates phosphoprotein phosphatase-1,
whereas phosphorylation of site 2 by PKA (which can
also phosphorylate site 1) causes the enzyme to be
released into the cytoplasm, where it cannot dephos-
phorylate the glycogen-bound enzymes of glycogen
metabolism.
In the cytosol, PP1c is also inhibited by its binding
to the protein phosphoprotein phosphatase inhibitor 1
(inhibitor-1). This latter protein provides yet another ex-
ample of control by enzymatic interconversion: It too is
modified by PKA and demodified by PP1c (Fig. 18-14,
bottom), although, in this case, a Thr, not a Ser, is phospho-
rylated/dephosphorylated. The protein is a functional in-
hibitor only when it is phosphorylated. The concentration
of cAMP therefore controls the fraction of an enzyme in its
phosphorylated form, not only by increasing the rate at
which it is phosphorylated, but also by decreasing the rate at
which it is dephosphorylated. In the case of glycogen phos-
phorylase, an increase in [cAMP] results not only in an
increase in this enzyme’s rate of activation, but also in a de-
crease in its rate of deactivation.
The activity of phosphoprotein phosphatase-1 in liver is
also controlled by its binding to glycogen through the inter-
mediacy of a glycogen-binding subunit, here named G
L
.
When bound to G
L
, PP1c is activated toward dephosphory-
lation of the glycogen-bound enzymes of glycogen metabo-
lism. However, G
L
is not subject to control via phosphory-
lation as is G
M
in the muscle. Rather, the binding of
m-phosphorylase a to G
L
strongly inhibits the activity of
PP1c by an allosteric mechanism.
Among the major conformational changes that glycogen
phosphorylase undergoes in converting from the T to the R
state is the movement of the Ser 14-phosphoryl group from
the surface of the T-state (inactive) enzyme to a position
buried a few angstroms beneath the protein’s surface at the
dimer interface in the R-state (active) enzyme (Figs. 18-11b).
Both the R and T forms of phosphorylase a strongly bind the
G
L
ⴢ PP1c complex, but only in the T-state enzyme is the Ser
14-phosphoryl group accessible for hydrolysis by PP1c. Con-
sequently, under the conditions that phosphorylase a con-
verts to the T state (Section 18-3G), PP1c hydrolyzes its now
exposed Ser 14-phosphoryl group.This converts m-phospho-
rylase a to o-phosphorylase b, which has only a low affinity
for binding the G
L
ⴢ PP1c complex and hence does not in-
hibit PP1c. One effect of phosphorylase a demodification,
therefore, is to relieve the inhibition of PP1c and thus allow
it to excise the phosphoryl groups of other susceptible phos-
phoproteins. Since phosphorylase a has a high affinity for
the G
L
ⴢ PP1c complex and is in ⬃10-fold greater concentra-
tion, relief of PP1c inhibition only occurs when more than
Section 18-3. Control of Glycogen Metabolism 659
P1
Glycogen
Decreased phosphorylation
leading to increased
glycogen synthesis
Increased phosphorylation
leading to increased
glycogen breakdown
G
M
subunit PP1c
PP1c
PP1c
P1
P2
Glycogen G
M
subunit
+
Protein kinase A
(PKA)
Epinephrine
Insulin-stimulated
protein kinase
ATP
ATP
ADP
ADP
(less active)
(more active)
(inactive)
Glycogen G
M
subunit
Insulin
Figure 18-22 The antagonistic effects of insulin and
epinephrine on glycogen metabolism in muscle. This occurs
through their effects on the phosphoprotein phosphatase-1
catalytic subunit, PP1c, via its glycogen-bound G
M
subunit. Green
discs and dashed arrows indicate activation.
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 659
⬃90% of the glycogen phosphorylase is in the o-phosphory-
lase b form. Glycogen synthase is among the proteins that
are dephosphorylated by the G
L
ⴢ PP1c complex when it is
no longer inhibited by phosphorylase. However, in contrast
to phosphorylase, dephosphorylation activates glycogen
synthase. This enzyme is involved in its own bicyclic cascade
whose properties we shall now examine.
D. Glycogen Synthase Bicyclic Cascade
Like glycogen phosphorylase, glycogen synthase exists in
two enzymatically interconvertible forms:
1. The modified (m; phosphorylated) form that is inac-
tive under physiological conditions (the b form).
2. The original (o; dephosphorylated) form that is
active (the a form).
m-Glycogen synthase b is under allosteric control; it is
strongly inhibited by physiological concentrations of ATP,
ADP, and P
i
and hence the modified enzyme is almost
totally inactive in vivo. The activity of the unmodified en-
zyme is essentially independent of these effectors, so a
cell’s glycogen synthase activity varies with the fraction of
the enzyme in its unmodified form.
The mechanistic details of the interconversion of modi-
fied and unmodified forms of glycogen synthase are partic-
ularly complex and are therefore not as well understood as
those of glycogen phosphorylase. It has been clearly estab-
lished that the fraction of unmodified glycogen synthase is,
in part, controlled by a bicyclic cascade involving phospho-
rylase kinase (PhK) and phosphoprotein phosphatase-1,
enzymes that are also involved in the glycogen phosphory-
lase bicyclic cascade (Fig. 18-14, right). This demodification
process is facilitated by G6P, whose binding to m-glycogen
synthase b induces it to undergo a conformational change
that exposes its phosphoryl groups to the surface of the
protein, thereby making them available for dephosphory-
lation by phosphoprotein phosphatase-1.
Glycogen synthase is phosphorylated at several sites.
Several protein kinases are known to at least partially de-
activate human muscle glycogen synthase by phosphory-
lating this homotetramer at 1 or more of 9 Ser residues in
the N- and C-terminal segments on its 737-residue sub-
units. These enzymes include PhK, PKA (so glycogen syn-
thase deactivation may also be considered to occur via a
monocyclic cascade), CaMKI (which is activated by the
presence of Ca
2⫹
), protein kinase C (PKC; which responds
to the extracellular presence of certain hormones via a
mechanism described in Sections 18-3G and 19-4Cb),
AMP-dependent protein kinase (AMPK; which responds
to ATP availability and hence acts as a fuel gauge; Sections
25-5a and 27-1), glycogen synthase kinase-3 [GSK3; which
is inhibited by insulin (Sections 18-3Ea and 18-3F), whose
presence therefore results in the dephosphorylation and
hence activation of glycogen synthase], and casein kinases
1 and 2 (which participate in a variety of cellular control
processes).Why glycogen synthase deactivation is so elab-
orately controlled compared to its activation or the activa-
tion/deactivation of glycogen phosphorylase is unclear, al-
though, whatever the reasons, it closely monitors the or-
ganism’s metabolic state.
E. Integration of Glycogen Metabolism
Control Mechanisms
Whether there is net synthesis or degradation of glycogen
and at what rate depends on the relative balance of the ac-
tive forms of glycogen synthase and glycogen phosphory-
lase. This, in turn, largely depends on the rates of the phos-
phorylation and dephosphorylation reactions of the two
bicyclic cascades.These cascades, one controlling the rate of
glycogen breakdown and the other controlling the rate of
glycogen synthesis, are intimately related. They are linked
by protein kinase A and phosphorylase kinase, which,
through phosphorylation, activate glycogen phosphorylase
as they inactivate glycogen synthase (Fig. 18-14). The cas-
cades are also linked by phosphoprotein phosphatase-1,
which in liver is inhibited by phosphorylase a and therefore
is unable to activate (dephosphorylate) glycogen synthase
unless it first inactivates (also by dephosphorylation) phos-
phorylase a.
a. Hormones Are Important Regulators
of Glycogen Metabolism
Glycogen metabolism is largely regulated by the pep-
tide hormone insulin (Fig. 7-2) acting in opposition to
glucagon, another peptide hormone,
⫹
H
3
N - His - Ser - Glu- Gly - Thr - Phe - Thr - Ser - Asp- Tyr - 10
Ser - Lys - Tyr - Leu - Asp- Ser - Arg - Arg - Ala - Gln- 20
Asp- Phe- Val - Gln - Trp - Leu - Met - Asn - Thr - COO
⫺
29
Glucagon
together with the adrenal hormones epinephrine (adrena-
line) and norepinephrine (noradrenaline):
Hormonal stimulation of cells at their plasma mem-
branes occurs through the mediation of transmembrane
proteins called receptors. Different cell types have different
complements of receptors and thus respond to different sets
of hormones. For example, both muscle and liver cells have
abundant insulin and adrenergic receptors (receptors re-
sponsive to epinephrine and norepinephrine), whereas
glucagon receptors are more prevalent in liver than in ske-
latal muscle.
CH
2
C
H
+
NH
2
X
HO
OH
OH
X = CH
3
Epinephrine
X = H Norepinephrine
660 Chapter 18. Glycogen Metabolism
JWCL281_c18_638-670.qxd 6/30/10 12:00 PM Page 660
