Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

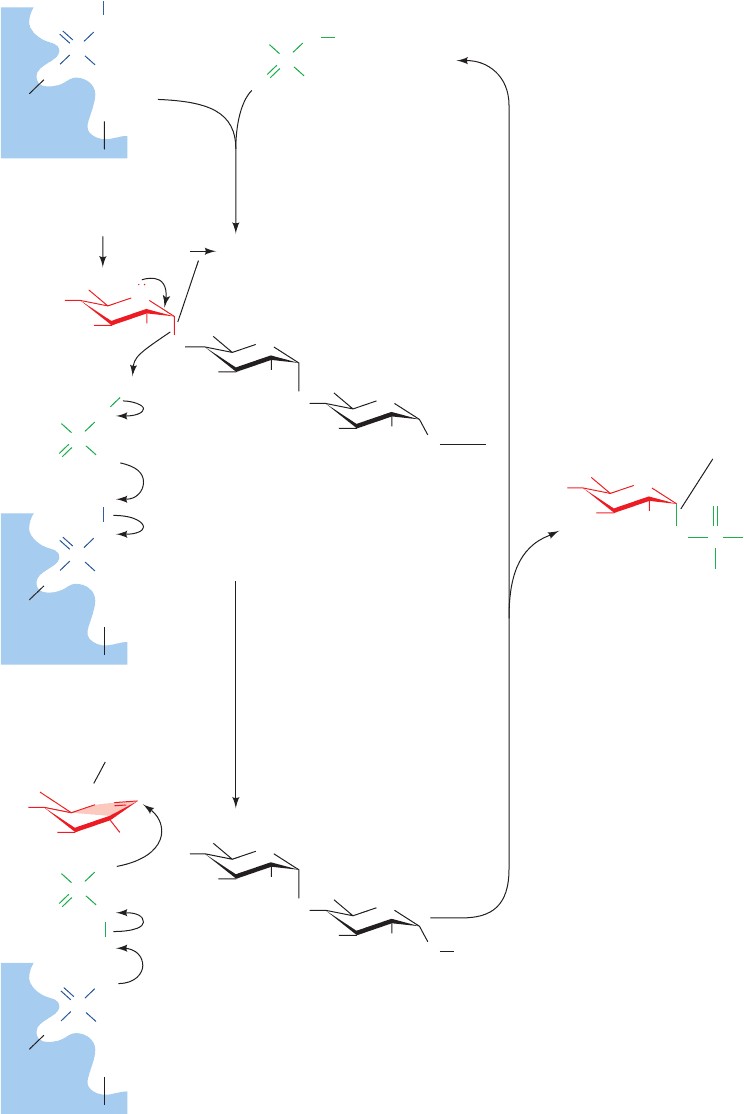
mechanism of PLP participation in the phosphorylase re-
action must differ from that in these other enzymes be-
cause, for example, reduction of the Schiff base with
NaBH
4
(HCN
S
H
2
C NH ) has no effect on
the activity of phosphorylase, whereas it inactivates the
PLP-requiring enzymes of amino acid metabolism. This is
¬¬¬¬“¬
an intriguing example of nature’s opportunism in using the
same cofactor to perform different chemistries.
Extensive studies on phosphorylase using PLP analogs
in which various parts of this molecule are missing or mod-
ified indicate that only its phosphate group participates in
the catalytic process. Indeed, the X-ray structures of phos-
phorylase reveal that only PLP’s phosphate group is near
this enzyme’s active site. This phosphoryl group most prob-
ably functions as an acid–base catalyst.
c. Kinetics and Reaction Mechanism
The phosphorylase reaction results in the cleavage of
the C1 O1 bond from a nonreducing terminal glucosyl
unit of glycogen, yielding G1P. This reaction proceeds with
retention of configuration, which suggests that the phos-
phorolysis occurs via a double displacement mechanism
(two sequential nucleophilic substitutions, each occurring
with inversion of configuration; Fig. 16-6c) involving a
covalent glucosyl–enzyme intermediate. Yet, phosphory-
lase exhibits Rapid Equilibrium Random Bi Bi kinetics
(Section 14-5), not Ping Pong kinetics, as would be
expected for a double displacement mechanism. Further-
more, all attempts to establish the existence of the putative
covalent intermediate have been unsuccessful.
An alternative mechanism (Fig. 18-3), which is compat-
ible with the available kinetic, chemical, and structural
data, commences with the formation of a ternary enzyme ⴢ
P
i
ⴢ glycogen complex, followed by the generation of an
intermediate shielded oxonium ion similar to the transi-
tion state in the lysozyme reaction (which also involves
¬
Section 18-1. Glycogen Breakdown 641
Figure 18-3 The reaction mechanism of glycogen
phosphorylase. Here PL is an enzyme-bound pyridoxal group
and BH
is a positively charged amino acid side chain, probably
that of Lys 568, necessary for the maintenance of PLP electrical
neutrality. (1) Formation of an E ⴢ P
i
ⴢ glycogen ternary complex.
(2) Formation of a shielded oxonium ion intermediate from the
-linked terminal glucosyl residue involving acid catalysis by P
i
as facilitated by proton transfer from PLP. The oxonium ion has
the half-chair conformation. (3) Reaction of P
i
with the oxonium
ion with overall retention of configuration about C1 to form
-
D-glucose-1-phosphate. The glycogen, which has one less
residue than before, cycles back to Step 1.
O
O
E
PL
Nonreducing end
1
Glycogen
(n glucosyl units)
Glycogen
(n – 1 glucosyl units)
Half-chair oxonium ion
intermediate
+ Glycogen
H
O
–
O
P
–
O
OH
O
–
O
P
CH
2
OH
HO
HO
O
OH
CH
2
OH
O
HO
O
OH
CH
2
OH
O
HO
O
OH
α(1 4) linkage
O
…
CH
2
OH
HO
HO
O
+
OH
CH
2
OH
HO
HO
O
OH
CH
2
OH
O
HO
O
OH
O
…
O
O
E
BH
+
PL
H
O
–
O
P
O
O
–
O
–
O
P
BH
+
E
BH
+
PL
O
H
O
–
O
–
O
P
O
H
O
–
–
O
O
P
2
3
O
P
O
–
O
–
O
CH
2
OH
HO
HO
O
OH
α linkage
to phosphate
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 641
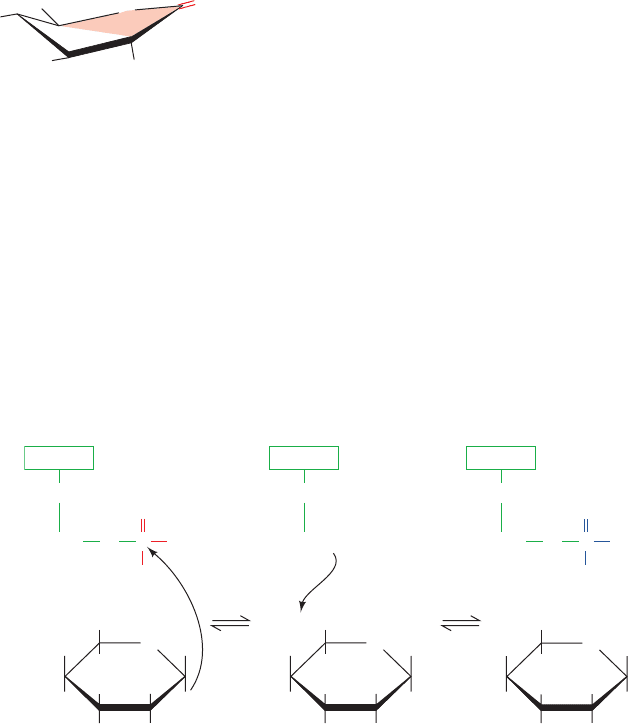
glycosidic bond cleavage in a polysaccharide; Section 15-2B).
Bond cleavage, with its consequent oxonium ion formation,
is assisted by protonation of the glycosidic oxygen by the P
i
substrate (acid catalysis). Phosphorylase has no protein
nucleophilic or carboxylate groups in the vicinity of the
scissile glycosidic bond and hence would be unable to
form a covalent intermediate as does lysozyme. However,
since the PLP phosphoryl group is within hydrogen bond-
ing distance of the P
i
, it appears that bond cleavage is facil-
itated by the simultaneous protonation of the reacting P
i
by
the PLP phosphoryl group in a kind of proton relay.The re-
sulting oxonium ion (Fig. 18-3) is stabilized through its for-
mation of an ion pair with the anionic P
i
(electrostatic
catalysis), which subsequently collapses to yield product,
G1P, in a reaction step that is facilitated by the abstraction
of a proton from P
i
by the PLP phosphoryl group (base
catalysis).
Support for the oxonium ion mechanism comes from
the observation that 1,5-gluconolactone
is a potent inhibitor of phosphorylase. 1,5-Gluconolactone
has the same half-chair conformation as the proposed oxo-
nium ion, suggesting that it is a transition state analog that
mimics the oxonium ion at the active site of phosphorylase
(Section 15-1F).
B. Phosphoglucomutase
Phosphorylase converts the glucosyl units of glycogen to
G1P, which, in turn, is converted by phosphoglucomutase
to G6P either for entry into glycolysis in muscle or hydrol-
O
HO
CH
2
OH
HO
OH
1,5-Gluconolactone
O
ysis to glucose in liver. The X-ray structure of rabbit mus-
cle phosphoglucomutase indicates that the active site of
this 561-residue monomeric enzyme is largely buried at
the bottom of a particularly deep crevice in the protein.
The phosphoglucomutase reaction is similar to that cat-
alyzed by phosphoglycerate mutase (Section 17-2Ha). A
phosphoryl group is transferred from the active phospho-
enzyme to G6P, forming glucose 1,6-bisphosphate
(G1,6P), which then rephosphorylates the enzyme to yield
G1P (Fig. 18-4). An important difference between this en-
zyme and phosphoglycerate mutase is that the phosphoryl
group in phosphoglucomutase is covalently bound to a Ser
hydroxyl group rather than to a His imidazole nitrogen.
G1,6P occasionally dissociates from phosphoglucomu-
tase, resulting in the inactivation of this enzyme. The pres-
ence of small amounts of G1,6P is therefore necessary to
keep phosphoglucomutase fully active.This intermediate is
provided by phosphoglucokinase, which catalyzes the
phosphorylation of the C6 OH group of G1P by ATP.
C. Glycogen Debranching Enzyme
Glycogen debranching enzyme, an ⬃1540-residue monomer,
acts as an (1 S 4) transglycosylase (glycosyl transferase) by
transferring an (1 S 4)-linked trisaccharide unit from a
“limit branch”of glycogen to the nonreducing end of another
branch (Fig. 18-5). This reaction forms a new (1 S 4) link-
age with three more units available for phosphorylase-
catalyzed phosphorolysis. The (1 S 6) bond linking the
remaining glycosyl residue in the branch to the main chain
is hydrolyzed (not phosphorylized) by the same debranching
enzyme to yield glucose and debranched glycogen. Thus
debranching enzyme has different active sites for the trans-
ferase reaction and the (1 S 6)-glucosidase reaction. The
presence of two independent catalytic activities on the
same enzyme no doubt improves the efficiency of the de-
branching process.
¬
642 Chapter 18. Glycogen Metabolism
Figure 18-4 The mechanism of action of phosphoglucomutase.
(1) The OH group at C1 of G6P attacks the phosphoenzyme to
form a dephosphoenzyme–G1,6P intermediate. (2) The Ser OH¬
–2
O
3
POCH
2
–2
O
3
POCH
2
H
OH H
HOH
HO
HH
OH
O
O
O
–
PO
–
CH
2
O
Ser
Enzyme
Glucose-6-
phosphate
(G6P)
H
OH H
HOH
HO
HH
O
2
CH OH
Ser
Enzyme
Glucose-1,6-
bisphosphate
(G1,6P)
12
2
HOCH
H
OH H
HOH
HO
HH
O
O
O
–
PO
–
CH
2
O
Ser
Enzyme
Glucose-1-
phosphate
(G1P)
OPO
2
3
–
OPO
2
3
–
group on the dephosphoenzyme attacks the phosphoryl group
at C6 to regenerate the phosphoenzyme with the formation of
G1P.
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 642
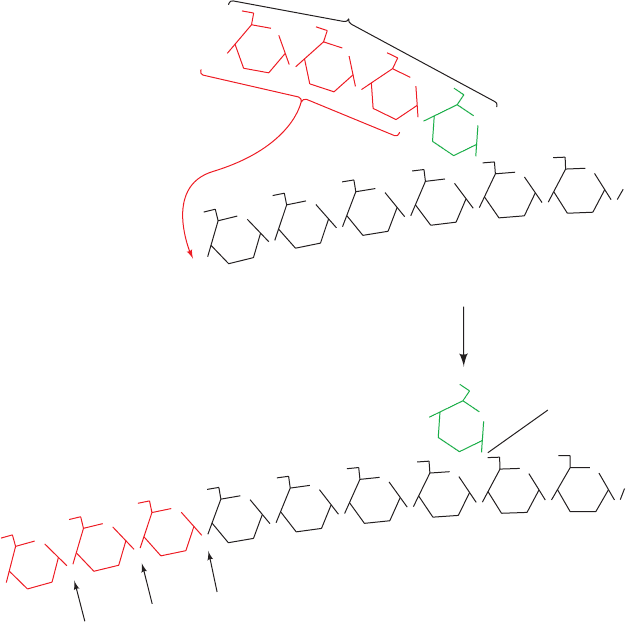
The maximal rate of the glycogen phosphorylase reac-
tion is much greater than that of the glycogen debranching
reaction. Consequently, the outermost branches of gly-
cogen, which comprise nearly half of its residues, are
degraded in muscle in a few seconds under conditions of
high metabolic demand. Glycogen degradation beyond this
point requires debranching and hence occurs more slowly.
This, in part, accounts for the fact that a muscle can sustain
its maximum exertion for only a few seconds.
D. Thermodynamics of Glycogen Metabolism:
The Need for Separate Pathways of Synthesis
and Breakdown
The G°¿ (G under standard biochemical conditions) for
the phosphorylase reaction is 3.1 kJ ⴢ mol
1
, so, as Eq.
[3.15] indicates, this reaction is at equilibrium (G 0) at
25°C when [P
i
]冫[G1P] 3.5. In the cell, however, this
concentration ratio varies between 30 and 100, which
places G in the range 5 to 8 kJ ⴢ mol
1
; that is, under
physiological conditions, glycogen breakdown is exer-
gonic. The synthesis of glycogen from G1P under physio-
logical conditions is therefore thermodynamically unfa-
vorable without free energy input. Consequently, glyco-
gen biosynthesis and breakdown must occur by separate
pathways. Thus we encounter a recurrent metabolic strat-
egy: Biosynthetic and degradative pathways of metabolism
are almost always different (Section 16-1). There are two
important reasons for this. The first, as we have seen, is
that both pathways may be required under similar in vivo
metabolite concentrations. This situation is thermody-
namically impossible if one pathway is just the reverse of
the other. The second reason is equally important: Reac-
tions catalyzed by different enzymes can be independ-
ently regulated, which permits very fine flux control. We
have seen this principle in operation in the glycolytic con-
version of fructose-6-phosphate (F6P) to fructose-1,6-
bisphosphate (F1,6P) by phosphofructokinase (PFK;
Section 17-4F). The reverse process in that case (hydroly-
sis of F1,6P) is catalyzed by fructose bisphosphatase
(FBPase). Independent control of those two enzymes
provides precise regulation of glycolytic flux.
Glycogen metabolism, like glycolysis, is exquisitely reg-
ulated by the independent control of its synthetic and
Section 18-1. Glycogen Breakdown 643
glycogen debranching
enzyme
Limit branch
Available for
hydrolysis
Available for
further phosphorolysis
Outer glycogen chains
(after phosphorylase action)
HO
O
O
O
O
O
O
O
O
HO
O
O
O
O
O
O
O
O
O
O
O
O
O
O
HO
O
OO
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
HO
...
...
Figure 18-5 Reactions catalyzed by debranching enzyme. The
enzyme transfers the terminal three (1 S 4)-linked glucose
residues from a “limit branch” of glycogen to the nonreducing
end of another branch.The (1 S 6) bond of the residue
remaining at the branch point is hydrolyzed by further action of
debranching enzyme to yield free glucose. The newly elongated
branch is subject to degradation by glycogen phosphorylase.
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 643
degradative pathways. In the next section we examine the
pathway of glycogen synthesis and, in Section 18-3, we ex-
plore the regulatory process.
2 GLYCOGEN SYNTHESIS
Although the thermodynamic arguments presented in Sec-
tion 18-1D demonstrate that glycogen synthesis and break-
down must occur by separate pathways, it was not thermo-
dynamic arguments that led to the general acceptance of
this idea. Rather, it was the elucidation of the cause of
McArdle’s disease, a rare inherited glycogen storage dis-
ease that results in painful muscle cramps on strenuous
exertion (Section 18-4).The muscle tissue from individuals
with McArdle’s disease exhibits no glycogen phosphory-
lase activity and is therefore incapable of glycogen break-
down.Their muscles, nevertheless, contain moderately high
quantities of normal glycogen. Clearly, there must be sepa-
rate pathways for glycogen synthesis and breakdown.
Since the direct conversion of G1P to glycogen and P
i
is
thermodynamically unfavorable (positive G) under all
physiological P
i
concentrations, glycogen biosynthesis re-
quires an additional exergonic step. This is accomplished,
as Luis Leloir discovered in 1957, by combining G1P with
uridine triphosphate (UTP) to form uridine diphosphate
glucose (UDP–glucose or UDPG):
UDPG’s “high-energy” status permits it to spontaneously
donate glucosyl units to the growing glycogen chain.
The enzymes catalyzing the three steps involved in the
glycogen synthesis pathway are UDP–glucose pyrophos-
phorylase, glycogen synthase, and glycogen branching
enzyme. In this section, we examine the reactions catalyzed
by these enzymes. Discussion of how these enzymes are
controlled is reserved for Section 18-3.
A. UDP–Glucose Pyrophosphorylase
UDP–glucose pyrophosphorylase catalyzes the reaction of
UTP and G1P (Fig. 18-6). In this reaction, the phosphoryl
oxygen of G1P attacks the phosphorus atom of UTP to
form UDPG and release PP
i
. The G°¿ of this phosphoan-
hydride exchange is, as expected, nearly zero. However,
the PP
i
formed is hydrolyzed in an exergonic reaction by
the omnipresent enzyme inorganic pyrophosphatase. The
overall reaction for the formation of UDPG is therefore
also exergonic:
⬃0
Overall:
The cleavage of a nucleoside triphosphate to form PP
i
is a
common biosynthetic strategy. The free energy of PP
i
hy-
drolysis can then be utilized together with the free energy of
nucleoside triphosphate hydrolysis to drive an otherwise
endergonic reaction to completion (Section 16-4Ca).
19.2G1P UTP Δ UDPG 2P
i
19.2H
2
O PP
i
Δ 2P
i
G1P UTP Δ UDPG PP
i
¢G°¿ (kJ ⴢ mol
1
)
CH
2
HO
OH
OH
H
HH
H
H
OH
O
O
H
H
H
H
HO
OH
O
O
N
HN
H
H
O
PO
O
–
O
P
O
–
O
CH
2
O
Uridine diphosphate glucose
(UDPG)
644 Chapter 18. Glycogen Metabolism
Figure 18-6 Reaction catalyzed by UDP–glucose
pyrophosphorylase. The reaction is a phosphoanhydride
exchange in which the phosphoryl oxygen of G1P attacks the
phosphorus atom of UTP to form UDPG and release PP
i
.The
PP
i
is rapidly hydrolyzed by inorganic pyrophosphatase.
O
H
H
HO OH
O
O
N
HN
HH
UTP
O
PO
–
O
O
P
–
O
O
OH
2
C
O
P
–
O
–
O
UDP–glucose
PP
i
2 P
i
inorganic
pyrophos-
phatase
O
H
H
HO OH
O
O
N
HN
HH
O
P
O
–
O
–
O OH
2
C
H
2
O
O
P
G1P
O
OH
HO
HO
CH
2
OH
O
P
O
–
O
–
O
O
OH
HO
HO
CH
2
OH
O
JWCL281_c18_638-670.qxd 6/3/10 1:48 PM Page 644
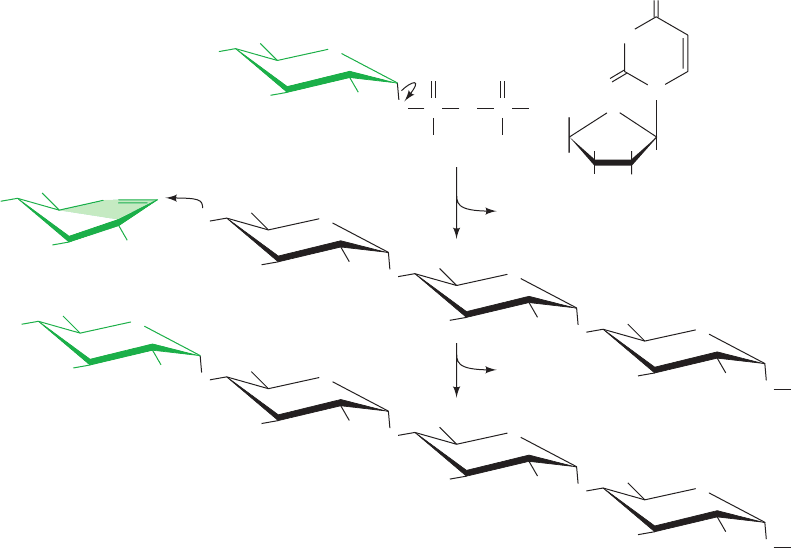
B. Glycogen Synthase
In the next step of glycogen synthesis, the glycogen syn-
thase reaction, the glucosyl unit of UDPG is transferred to
the C4 OH group on one of glycogen’s nonreducing ends
to form an (1 S 4)-glycosidic bond (Fig. 18-7). The glyco-
gen synthase reaction, like those of glycogen phosphory-
lase and lysozyme, is thought to involve a glucosyl oxonium
ion intermediate or transition state since it is also inhibited
by 1,5-gluconolactone, an analog that mimics the oxonium
ion’s half-chair geometry.
The G°¿ for the glycogen synthase reaction is –13.4
kJ ⴢ mol
1
, making the overall reaction spontaneous under
the same conditions that glycogen breakdown by glycogen
phosphorylase is also spontaneous. The rates of both reac-
tions may then be independently controlled.There is, how-
ever, an energetic price for doing so. In this case, for each
molecule of G1P that is converted to glycogen and then re-
generated, one molecule of UTP is hydrolyzed to UDP and
P
i
.The cyclic synthesis and breakdown of glycogen is there-
fore not a perpetual motion “machine” but, rather, is an “en-
gine” that is powered by UTP hydrolysis. The UTP is
replenished through a phosphate-transfer reaction medi-
ated by nucleoside diphosphate kinase (Section 28-1Ba):
so that UTP hydrolysis is energetically equivalent to ATP
UDP ATP Δ UTP ADP
¬
hydrolysis.This reaction occurs via a Ping Pong mechanism
in which an active site His residue is transiently phosphory-
lated at its N
position much as occurs in the phosphoglyc-
erate mutase reaction of glycolysis (Section 17-2Ha).
Mammals express two ⬃70% identical isoforms of
glycogen synthase, one mainly in muscle, and the other in
liver, much as is the case with glycogen phosphorylase.
Plants and bacteria express starch/glycogen synthases but
these employ ADP–glucose as glucose donors rather than
UDP–glucose and exhibit very little sequence similarity to
animal glycogen synthases.
a. Glycogenin Initiates Glycogen Synthesis
Glycogen synthase cannot simply link together two glu-
cose residues; it can only extend an already existing
(1 S 4)-linked glucan chain. How, then, is glycogen syn-
thesis initiated? The answer is that the first step in glycogen
synthesis is the self-catalyzed attachment of a glucose
residue to the Tyr 194 OH group of a 332-residue homo-
dimeric protein named glycogenin. Glycogenin, which was
discovered by William Whelan, further extends the glucan
chain by up to ⬃9 additional UDP–glucose-supplied
residues, forming a “primer” for the initiation of glycogen
synthesis. Only at this point does glycogen synthase com-
mence glycogen synthesis, which it initiates on the
“primer” while tightly complexed to glycogenin. However,
these proteins dissociate after the growing glycogen
Section 18-2. Glycogen Synthesis 645
Figure 18-7 Reaction catalyzed by glycogen synthase. The reaction involves a glucosyl
oxonium ion intermediate.
UDP
UDP–glucose
Glycogen (n residues)
Oxonium
ion intermediate
Glycogen (n + 1 residues)
O
H
H
HO OH
O
O
N
HN
HH
O
P
O
–
O
–
O OH
2
C
O
PO
OH
HO
HO
CH
2
OH
O
O
OH
HO
HO
CH
2
OH
O
O
OH
HO
O
CH
2
OH
O
OH
HO
CH
2
OH
O
O
OH
HO
HO
CH
2
OH
O
OH
HO
HO
CH
2
OH
+
O
...
H
+
O
OH
HO
CH
2
OH
O
O
OH
HO
O
CH
2
OH
O
OH
HO
CH
2
OH
O
...
O
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 645
granule has reached some minimum size.Analysis of glyco-
gen granules for glycogenin and glycogen synthase shows
that they are present in a 1:1 ratio. Evidently, each glycogen
molecule (Fig. 18-1c) is associated with one molecule each
of glycogenin and glycogen synthase.
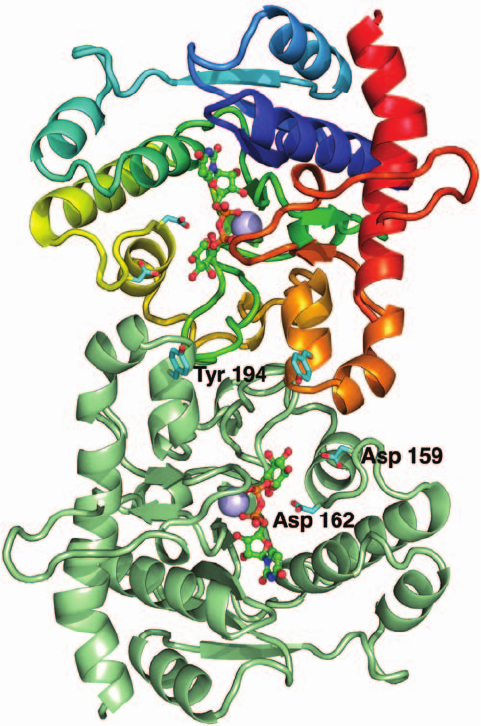
The X-ray structure of rabbit muscle glycogenin in com-
plex with UDPG (Fig. 18-8), determined by Thomas Hurley
and Peter Roach, reveals that the enzyme’s N-terminal por-
tion consists of a Rossmann-like fold (Section 8-3Bi) that is
common to the nucleotide-binding domains of most
glycosyltransferases.The OH group of its Tyr 194 is 21 and 16
Å distant from C1 of the UDPG’s glucosyl group on the
same and on the opposite subunit. Moreover, the glucosyl-
transferase reaction occurs with retention of configuration
about the glucosyl C1 atom. This suggests that the glucosyl
group is first transferred to an intermediate nucleophile and
then transferred to Tyr 194 or to a glucose group that is
linked to Tyr 194 (a double displacement reaction). Site-
directed mutagenesis has identified Asp 162 as the probable
nucleophile, whereas Asp 159 appears to have a role in
binding and activating the acceptor molecule. Evidently,
glycogenin undergoes large conformational changes during
its catalytic cycle, although the nature of these changes is
unknown. Indeed, it is unclear whether a given subunit
transfers glucosyl groups to itself or to the opposite subunit.
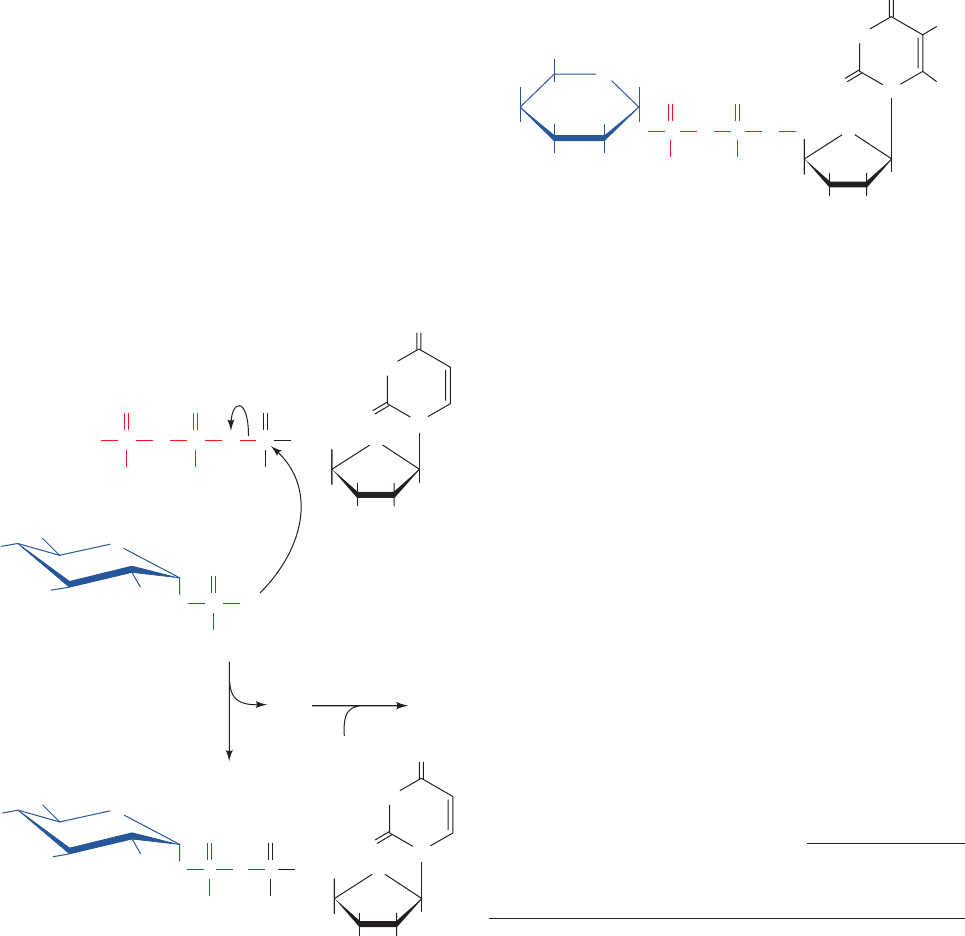
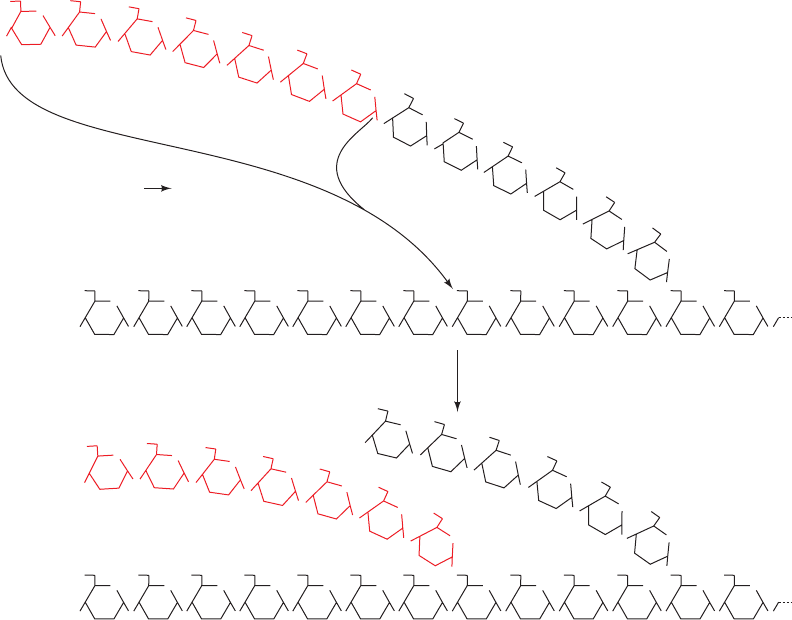
C. Glycogen Branching Enzyme
Glycogen synthase catalyzes only (1 S 4)-linkage forma-
tion to yield -amylose. Branching to form glycogen is
accomplished by an ⬃700-residue monomeric enzyme,
amylo-(1,4 S 1,6)-transglycosylase (glycogen branching
enzyme), which is distinct from glycogen debranching en-
zyme. Branches are created by the transfer of terminal
chain segments consisting of ⬃7 glucosyl residues to the
C6 OH groups of glucose residues on the same or an-
other glycogen chain (Fig. 18-9). Each transferred segment
must come from a chain of at least 11 residues, and the new
branch point must be at least 4 residues away from other
branch points.
Debranching (Section 18-1C) involves breaking and
reforming (1 S 4)-glycosidic bonds and only the hydroly-
sis of (1 S 6)-glycosidic bonds; branching, on the other
hand, involves breaking (1 S 4)-glycosidic bonds and
reforming (1 S 6) linkages. The need to hydrolyze
glycogen’s (1 S 6)-glycosidic bonds rather than to convert
them to (1 S 4) linkages is explained by the energetics of
these reactions. The free energy of hydrolysis of an
(1 S 4)-glycosidic bond is 15.5 kJ ⴢ mol
1
, whereas that
of an (1 S 6)-glycosidic bond is only 7.1 kJ ⴢ mol
1
.Con-
sequently, the hydrolysis of an (1 S 4)-glycosidic bond
drives the synthesis of an (1 S 6)-glycosidic bond, but the
reverse reaction is endergonic.
a. Glycogen Particles Are Fabricated to Optimize
Glucose Mobilization
The biological function of glycogen is to maximize the
density of stored glucose units consistent with the need to
rapidly mobilize it under conditions of high metabolic
demand. To do so, three related parameters must be opti-
mized: the number of tiers of branches in a glycogen mole-
cule, the number of branches per tier, and the average
chain length per tier. For a glycogen molecule with a fixed
¬
646 Chapter 18. Glycogen Metabolism
Figure 18-8 X-ray structure of rabbit muscle glycogenin. The
upper subunit of this homodimeric enzyme is colored in rainbow
order from its N-terminus (blue) to its C-terminus (red) and the
lower subunit is pale green. UDPG is drawn in ball-and-stick
form with C green, N blue, O red, and P orange. The side chains
of Tyr 194, to which the glucose residue at glycogen’s reducing
end is linked, and Asp 159 and Asp 162, are drawn in stick form
with C cyan and O red. Mn
2
ions, which are thought to
electrostatically stabilize the leaving group, UDP, are represented
by lavender spheres. Residues 262 to 332, which are implicated in
binding glycogen synthase, are not visible in the X-ray structure
and hence disordered. [Based on an X-ray structure by Thomas
Hurley and Peter Roach, Indiana University School of Medicine.
PDBid 1LL2.]
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 646
number of residues, the number of outer branches from
which glucose can be mobilized before debranching is
required decreases as the average chain length increases
(recall that debranching is a slower process than phospho-
rolysis). However, molecules with longer chains have a
greater number of glucose residues that can be phospho-
rylized between branch points. Since the density of outer-
most branches is sterically limited, the maximum size of a
glycogen molecule decreases as the average number of
branches per tier increases. Mature glycogen particles from
a variety of animals have ⬃12 tiers of branches, with ⬃2
branches per tier and branch lengths averaging ⬃13
residues. Mathematical analysis suggests that these values
are close to optimal for mobilizing the greatest amount of
glucose in the shortest possible time.
3 CONTROL OF GLYCOGEN
METABOLISM
We have just seen that both glycogen synthesis and break-
down are exergonic under the same physiological condi-
tions. If both pathways operate simultaneously, however,
all that is achieved is wasteful hydrolysis of UTP. This
situation is similar to that of the phosphofructokinase–
fructose bisphosphatase substrate cycle (Section 17-4Ff).
Glycogen phosphorylase and glycogen synthase therefore
must be under stringent control such that glycogen is ei-
ther synthesized or utilized according to cellular needs.
The astonishing mechanism of this control is our next
topic of discussion. It involves not only allosteric control
and substrate cycles but enzyme-catalyzed covalent modi-
fication of both glycogen synthase and glycogen phospho-
rylase.The covalent modification reactions are themselves
ultimately under hormonal control through an enzymatic
cascade.
A. Direct Allosteric Control of Glycogen
Phosphorylase and Glycogen Synthase
As we saw in Section 17-4B, the net flux of reactants, J,
through a step in a metabolic pathway is the difference be-
tween the forward and reverse reaction velocities, v
f
and v
r
.
The fractional variation in the flux through any step in a path-
way with a change in substrate concentration approaches
infinity as that reaction step approaches equilibrium (v
f
⬇ v
r
;
Eq. [17.4]). The flux through a near-equilibrium reaction
Section 18-3. Control of Glycogen Metabolism 647
Figure 18-9 The branching of glycogen. Branches are formed by transferring a 7-residue
terminal segment from an ␣(1 S 4)-linked glucan chain to the C6 OH group of a glucose
residue on the same or another chain.
¬
glycogen branching enzyme
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
HO
13
12
11
10
9
8
7
6
5
4
3
2
1
O
HO
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
HO
13
12
11
10
9
8
7
6
5
4
3
2
1
O
α(1 4)-terminal
chains of glycogen
O
HO
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
HO
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
O
JWCL281_c18_638-670.qxd 6/3/10 1:48 PM Page 647
is therefore all but uncontrollable. As we have seen for the
case of PFK and FBPase, however, precise flux control of a
pathway is possible when an enzyme functioning far from
equilibrium is opposed by a separately controlled enzyme.
Then, v
f
and v
r
vary independently. In fact, under these cir-
cumstances, even the flux direction is controllable if v
r
can be
made larger than v
f
. Exactly this situation occurs in glyco-
gen metabolism through the opposition of the glycogen
phosphorylase and glycogen synthase reactions. The rates
of both of these reactions are under allosteric control by
effectors that include ATP, G6P, and AMP. In muscle, glyco-
gen phosphorylase is activated by AMP and inhibited by
ATP and G6P (Fig. 18-10, left). Glycogen synthase, on the
other hand, is activated by G6P.When there is high demand
for ATP (low [ATP], low [G6P], and high [AMP]), glycogen
phosphorylase is stimulated and glycogen synthase is inhib-
ited, so flux through this pathway favors glycogen break-
down. When [ATP] and [G6P] are high, the reverse is true
and glycogen synthesis is favored.
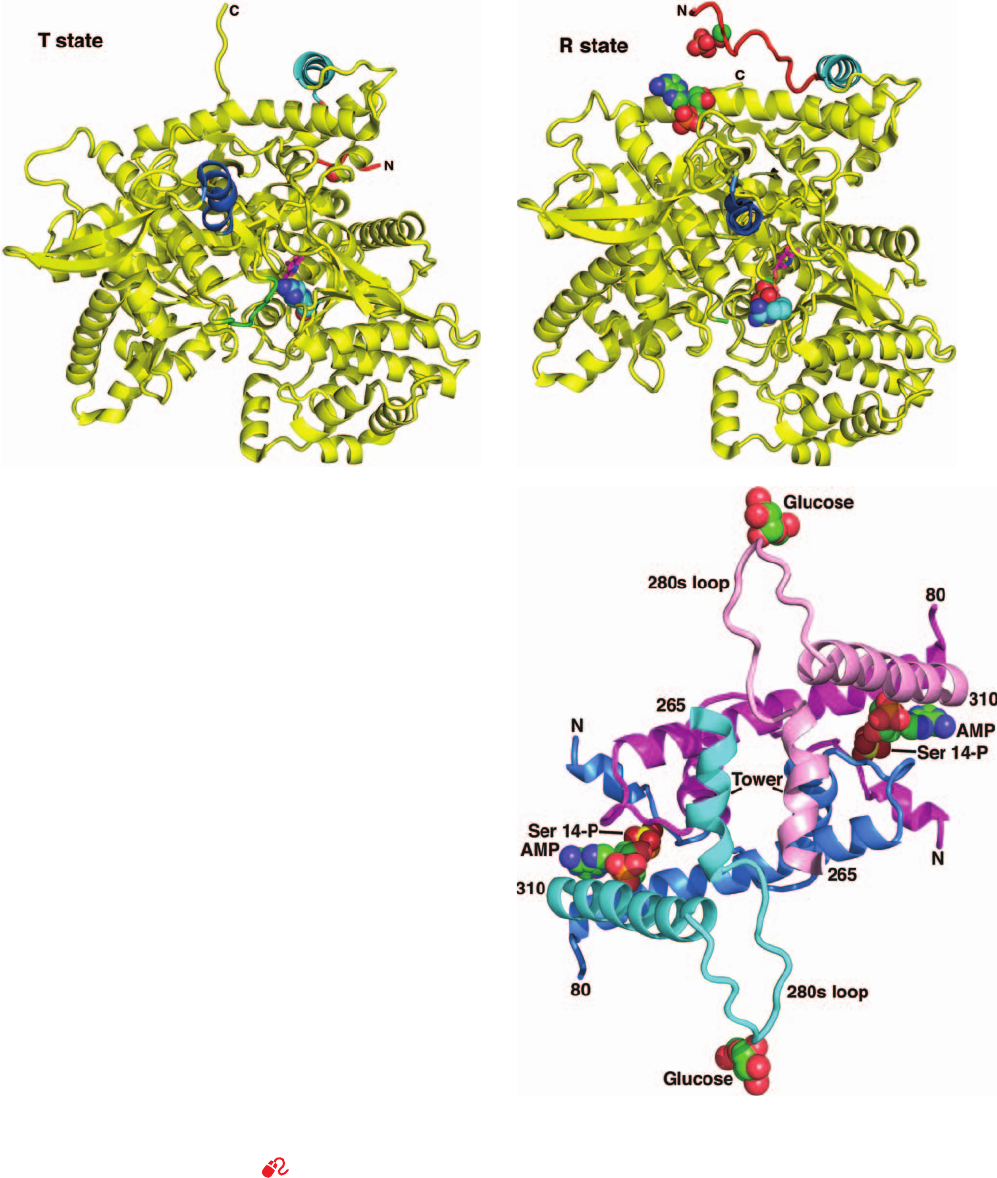
The structural differences between the active (R) and
inactive (T) conformations of glycogen phosphorylase
(Figs. 18-11a and 18-11b) can be understood in terms of
the symmetry model of allosterism (Section 10-4B). The
T-state enzyme has a buried active site and hence a low
affinity for its substrates, whereas the R-state enzyme has
an accessible catalytic site and a high-affinity phosphate
binding site.
AMP promotes phosphorylase’s T (inactive) S R (ac-
tive) conformational shift by binding to the R state of the
enzyme at its allosteric effector site (Fig. 18-10, left). In do-
ing so, AMP’s adenine, ribose, and phosphate groups bind
to separate segments of the polypeptide chain so as to link
the active site, the subunit interface, and the N-terminal re-
gion (Fig. 18-11c), the latter having undergone a large con-
formational shift (36 Å for Ser 14) from its position in the
T-state enzyme (Figs. 18-11a and 18-11b).AMP binding also
causes glycogen phosphorylase’s tower helices (Figs. 18-2
and 18-11) to tilt and pull apart so as to achieve a more fa-
vorable packing. These tertiary movements trigger a con-
certed T S R transition, which largely consists of an ⬃10°
relative rotation of the two subunits about an axis at the
subunit interface that is perpendicular to the dimer’s 2-fold
axis of symmetry. The enzyme’s 2-fold symmetry is thereby
preserved in accordance with the symmetry model of al-
losterism (Section 10-4B). The movement of the tower he-
lices also displaces and disorders a loop (the 280s loop,
residues 282–286), which covers the T-state active site so as
to prevent substrate access. It also causes the Arg 569 side
chain, which is located in the active site near the PLP phos-
phoryl group and the P
i
-binding site, to rotate in a way that
increases the enzyme’s binding affinity for its anionic P
i
substrate (Figs. 18-11a and 18-11b).
Curiously, ATP also binds to the allosteric effector site,
but in the T state, so that it inhibits rather than promotes
648 Chapter 18. Glycogen Metabolism
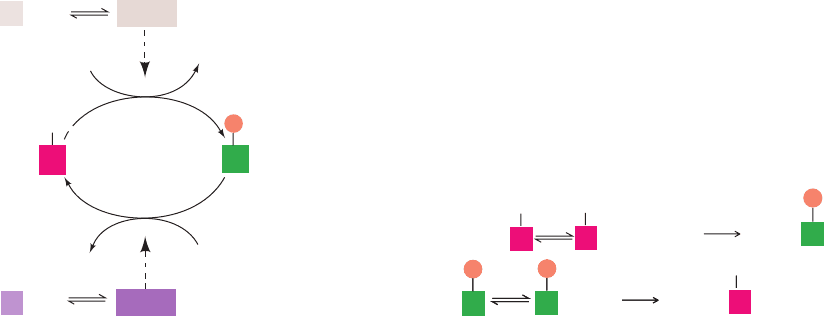
Figure 18-10 The control of glycogen phosphorylase activity.
The enzyme may assume the enzymatically inactive T
conformation (above) or the catalytically active R form (below).
The conformation of phosphorylase b is allosterically controlled
by effectors such as AMP,ATP, and G6P and is mostly in the T
state under physiological conditions. In contrast, the modified
form of the enzyme, phosphorylase a, is largely unresponsive to
2H
2
O
2ADP2ATP
2P
i
phosphorylase
kinase
T form
(inactive)
P
P
phosphoprotein
phosphatase
AMP
Glucose
ATP
and/or
G6P
Phosphorylase b Phosphorylase a
P
P
R form
(active)
these effectors and is mostly in the R state unless there is a high
level of glucose. Under usual physiological conditions, the
enzymatic activity of glycogen phosphorylase is essentially
determined by its rates of modification and demodification. Note
that only the T form enzyme is subject to phosphorylation and
dephosphorylation, so effector binding influences the rates of
these modification/demodification events.
JWCL281_c18_638-670.qxd 6/3/10 1:48 PM Page 648
the T S R conformational shift.This is because, as structural
analysis indicates, the and phosphate groups of ATP
bind to the enzyme such that its ribose and phosphate
groups are displaced relative to those of AMP, thus desta-
bilizing the R state. The inhibitory action of ATP on phos-
phorylase is therefore simply understood: It competes with
Section 18-3. Control of Glycogen Metabolism 649
Figure 18-11 Conformational changes in glycogen
phosphorylase. Ribbon diagrams of one subunit of the dimeric
enzyme glycogen phosphorylase b (a) in the T state in the
absence of allosteric effectors and (b) in the R state with bound
AMP. The view is of the lower (orange) subunit in Fig. 18-2
rotated by
⬃45° about a horizontal axis.The tower helix is blue;
the N-terminal helix is cyan; the N-terminal residues that change
conformation on AMP binding are red; and residues 281 to 288,
the 280s loop, which in the R state are mostly disordered and
hence not seen, are green. Of the groups that are shown in
space-filling representation, the side chain of Ser 14, the
phosphorylation site, and AMP, which binds only to the R state
enzyme, have C green, N blue, O red, and P orange; the side
chain of Arg 569, which reorients in the T S R transition so as to
interact with the substrate phosphate, has C cyan and N blue; and
sulfate ions bound near Ser 14 and at the active site of the R
state enzyme, which mimic the sterically similar phosphate ions,
have O red and S orange. The PLP at the active site is drawn in
stick form with C magenta, N blue, O red, and P orange. [Based
on X-ray structures by Louise Johnson, Oxford University, U.K.
PDBids 8GPB and 7GPB.] (c) The portion of the glycogen
phosphorylase a dimer in the vicinity of the dimer interface
showing the position of the Ser 14 phosphate group, the AMP
bound in the allosteric effector site, and the active site–bound
glucose molecule. The view is along the molecular 2-fold axis and
hence is similar to that in Fig. 18-2. Residues 5 to 80 and 265 to
310 are, respectively, cyan and blue in one subunit and pink and
magenta in the other.The AMP and glucose are shown in
space-filling representation with C green, N dark blue, O red,
and P orange. The Ser 14 phosphate group is also shown in
space-filling representation with O dark red and P yellow. [X-ray
structure coordinates courtesy of Stephen Sprang, University of
Texas Southwest Medical Center.]
See Kinemage Exercises
14-2 and 14-3
(a) (b)
(c)
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 649
AMP for binding to phosphorylase and, in doing so, pre-
vents the relative motions of the three polypeptide seg-
ments required for phosphorylase activation.
The above allosteric interactions are superimposed on
an even more sophisticated control system involving cova-
lent modifications (phosphorylation/dephosphorylation) of
glycogen phosphorylase and glycogen synthase.These mod-
ifications alter the structures of the enzymes so as to change
their responses to allosteric regulators. We shall therefore
discuss the general concept of covalent modification and
how it increases the sensitivity of a metabolic system to ef-
fector concentration changes.We subsequently consider the
functions of such modifications in glycogen metabolism.
Only then will we be in a position to take up the detailed
consideration of allosteric control in glycogen metabolism.
B. Covalent Modification of Enzymes by Cyclic
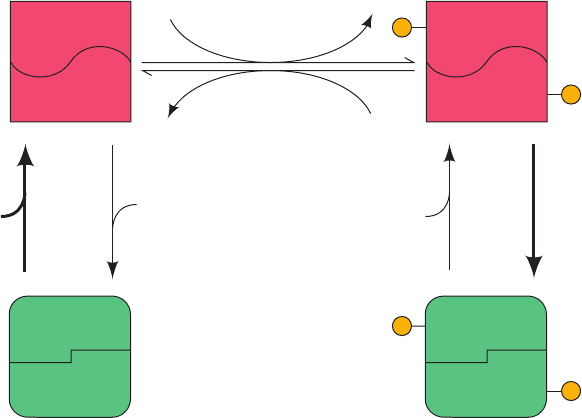
Cascades: Effector “Signal” Amplification
Glycogen synthase and glycogen phosphorylase can each be
enzymatically interconverted between two forms with differ-
ent kinetic and allosteric properties through a complex
series of reactions known as a cyclic cascade. The intercon-
version of these different enzyme forms involves distinct,
enzyme-catalyzed covalent modification and demodifica-
tion reactions.
Compared with other regulatory enzymes, enzymati-
cally interconvertible enzyme systems:
1. Can respond to a greater number of allosteric stimuli.
2. Exhibit greater flexibility in their control patterns.
3. Possess enormous amplification potential in their re-
sponses to variations in effector concentrations.
This is because the enzymes that modify and demodify a tar-
get enzyme are themselves under allosteric control. It is
therefore possible for a small change in concentration of an
allosteric effector of a modifying enzyme to cause a large
change in the concentration of an active, modified target en-
zyme. Such a cyclic cascade is diagrammed in Fig. 18-12.
a. Description of a General Cyclic Cascade
Figure 18-12a shows a general scheme for a cyclic cascade
where, by convention, the more active target enzyme form
has the subscript a and the less active form has the subscript
b. Here, modification, in this case, phosphorylation, activates
the enzyme. Note that the modifying enzymes, F and R, are
active only when they have bound their respective allosteric
effectors e
1
and e
2
.The kinetic mechanisms for the intercon-
version of the unmodified and modified forms of the target
enzyme, E
b
and E
a
, are indicated in Fig. 18-12b.
In the steady state, the fraction of E in the active form,
[E
a
]冫[E]
T
(where [E]
T
[E
a
] [E
b
] is the total enzyme con-
centration),determines the rate of the reaction catalyzed by
E. This fraction is a function of the total concentrations of
the modifying enzymes, [F]
T
and [R]
T
, the concentrations of
their allosteric effectors, e
1
and e
2
, the dissociation constants
of these effectors, K
1
and K
2
, and the substrate dissociation
constants, K
f
and K
r
, of the target enzymes, as well as the
rate constants, k
f
and k
r
,for the interconversions themselves
(Fig. 18-12). This relationship is obviously quite complex.
Nevertheless, it can be shown that, in a cyclic cascade, a rel-
atively small change in the concentration of e
1
, the allosteric
effector of the modifying enzyme F, can result in a much
larger change in [E
a
]冫[E]
T
, the fraction of E in the active
form. In other words, the cascade functions to amplify the
sensitivity of the system to an allosteric effector.
We have so far considered the covalent modification of
only one enzyme, a monocyclic cascade. Imagine a bicyclic
cascade involving the covalent modification of one of the
modifying enzymes (F), as well as the metabolic target en-
zyme (E) (Fig. 18-13). As you might expect, the amplifica-
tion potential of a “signal,” e
1
, as well as the control flexibil-
ity of such a system, is enormous.
650 Chapter 18. Glycogen Metabolism
Figure 18-12 A monocyclic enzyme cascade. (a) General
scheme, where F and R are, respectively, the modifying and
demodifying enzymes.These are allosterically converted from
their inactive to their active conformations on binding their
respective effectors, e
1
and e
2
.The target enzyme, E, is more
E
b
E
b
E
b
ATP F • e
1
F
• e
1
• ATP • F • e
1
K
f
P
i
k
f
k
r
R
R
R
e
2
e
2
e
2
(b)
ADP
E
a
E
a
E
a
K
r
P
P
P
•
•
•
•
E
b
P
i
k
r
R
R
R
e
2
e
2
e
2
E
a
E
a
K
r
P
P
•
•
•
•
E
b
E
a
K
1
K
2
P
i
R
F
e
2
e
1
(a)
ADP
ATP
H
2
O
R • e
2
F • e
1
P
More
active
More
active
More
active
Less
active
Less
active
Less
active
active in the modified form (E
a
) and less active in the unmodified
form (E
b
). Dashed arrows symbolize catalysis of the indicated
reactions. (b) Chemical equations for the interconversion of the
target enzyme’s unmodified and modified forms E
b
and E
a
.
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 650
