Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

self-limiting: Individuals with fructose intolerance rapidly
develop a strong distaste for anything sweet.
B. Galactose
Galactose comprises half of the milk sugar lactose and is
thus a major fuel constituent of dairy products. Galactose
and glucose are epimers that differ only in their configura-
tion about C4:
CH
2
OH
H
OH H
H OH
HO
H H
OH
O
α-D-Glucose
H
OH H
H OH
H
HO H
OH
O
α-D-Galactose
CH
2
OH
The enzymes of glycolysis are specific; they do not recognize
the galactose configuration.An epimerization reaction must
therefore be carried out before galactose enters the gly-
colytic pathway. This reaction takes place after the conver-
sion of galactose to its uridine diphosphate derivative. The
role of UDP–sugars and other nucleotidyl–sugars is dis-
cussed in more detail in Sections 18-2 and 23-3. The entire
pathway converting galactose to a glycolytic intermediate,
which was elucidated by Luis Leloir and is therefore known
as the Leloir pathway, involves four reactions (Fig. 17-36):
1. Galactose is phosphorylated at C1 by ATP in a reac-
tion catalyzed by galactokinase.
2. Galactose-1-phosphate uridylyltransferase transfers
UDP–glucose’s uridylyl group to galactose-1-phosphate to
yield glucose-1-phosphate (G1P) and UDP–galactose by the
reversible cleavage of UDP–glucose’s pyrophosphoryl bond.
Section 17-5. Metabolism of Hexoses Other Than Glucose 631
Figure 17-35 Metabolism of fructose. In muscle (left), the
conversion of fructose to the glycolytic intermediate F6P involves
only one enzyme, hexokinase. In liver (right), seven enzymes
participate in the conversion of fructose to glycolytic
–2
O
3
POCH
2
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
H OH
H HO
O
alcohol
dehydrogenase
O
H OH
H HO
HO H
HOCH
2
HOCH
2
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
CH
2
OH
Fructose
Fructose-6-
phosphate
ATP
ADP
fructo-
kinase
1
H OH
H HO
HO H
O
Fructose-1-
phosphate
OHCH
OHCH
HCHO
C
O
Fructose-1-phosphate
(open chain)
fructose-1-phosphate
aldolase
2
+
C O
Dihydroxy-
acetone
phosphate
C OH
OHCH
Glyceraldehyde
OHCH
Glycerol
ATP
ADP
glycerol
kinase
5
OHCH
Glycerol-3-phosphate
4
NADH
glycerol phosphate
dehydrogenase
6
7
glycer-
aldehyde
kinase
3
C OH
OHCH
Glyceraldehyde-3-
phosphate
triose
phosphate
isomerase
ATP
hexokinase
ADP
Glycolysis
NADH NAD
+
NAD
+
HO H
Muscle
ATP
ADP
Liver
intermediates: (1) fructokinase, (2) fructose-1-phosphate
aldolase, (3) glyceraldehyde kinase, (4) alcohol dehydrogenase,
(5) glycerol kinase, (6) glycerol phosphate dehydrogenase, and
(7) triose phosphate isomerase.
JWCL281_c17_593-637.qxd 6/3/10 8:38 AM Page 631
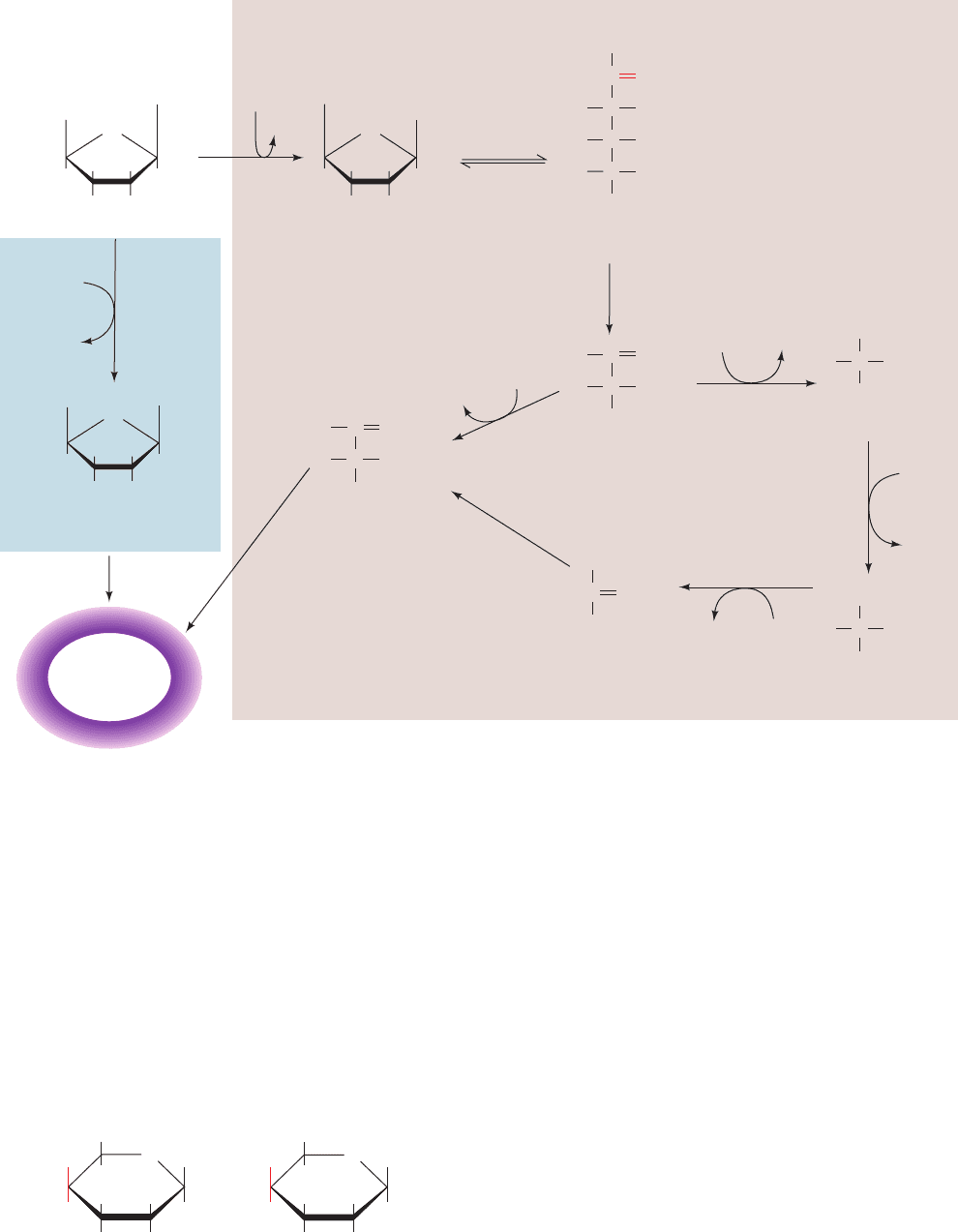
3. UDP–galactose-4-epimerase converts UDP–galactose
to UDP–glucose. This enzyme has an associated NAD
,
which suggests that the reaction involves the sequential
oxidation and reduction of the hexose C4 atom:
4. G1P is converted to the glycolytic intermediate G6P
by the action of phosphoglucomutase (Section 18-1B).
632 Chapter 17. Glycolysis
Figure 17-36 Metabolism of galactose. Four enzymes
participate in the conversion of galactose to the glycolytic
intermediate G6P: (1) galactokinase, (2) galactose-1-phosphate
H
OH H
HOH
HO
H
H
OH
O
Galactose
ATP
galactokinase
ADP
H
OH H
HOH
HO
H
H
OPO
O
Galactose-1-phosphate
3
2
–
1
2
3
H
OH H
HOH
HO
H
H
OPO
O
Glucose-1-phosphate (G1P)
phosphoglucomutase
H
OH H
HOH
HO
HH
OH
O
Glucose-6-phosphate
(G6P)
3
2
–
3
2
–
4
CH
2
OHCH
2
OHCH
2
OH
CH
2
OH
CH
2
OPO
CH
2
OH
H
OH H
HOH
HO
H
H
O
O
UDP–glucose
P
P
O
O
–
O
O
O
–
O Uridine
H
OH H
HOH
HO
H
H
O
O
UDP–galactose
P
P
O
O
–
O
O
O
–
O Uridine
galactose-1-phosphate
uridylyl transferase
UDP–galactose-
4-epimerase
NAD
+
Glycolysis
uridylyltransferase, (3) UDP–galactose-4-epimerase, and
(4) phosphoglucomutase.
H
OH H
H OH
H
HO H
O
O
UDP
H
O
O
UDP–Galactose
H
OH H
H OH
HO
H
O
UDP–Glucose
CH
2
OHCH
2
OH
NAD
+
NAD
+
NADHNADH
H
OH H
H OH
H
O UDP
O
CH
2
OH
UDP
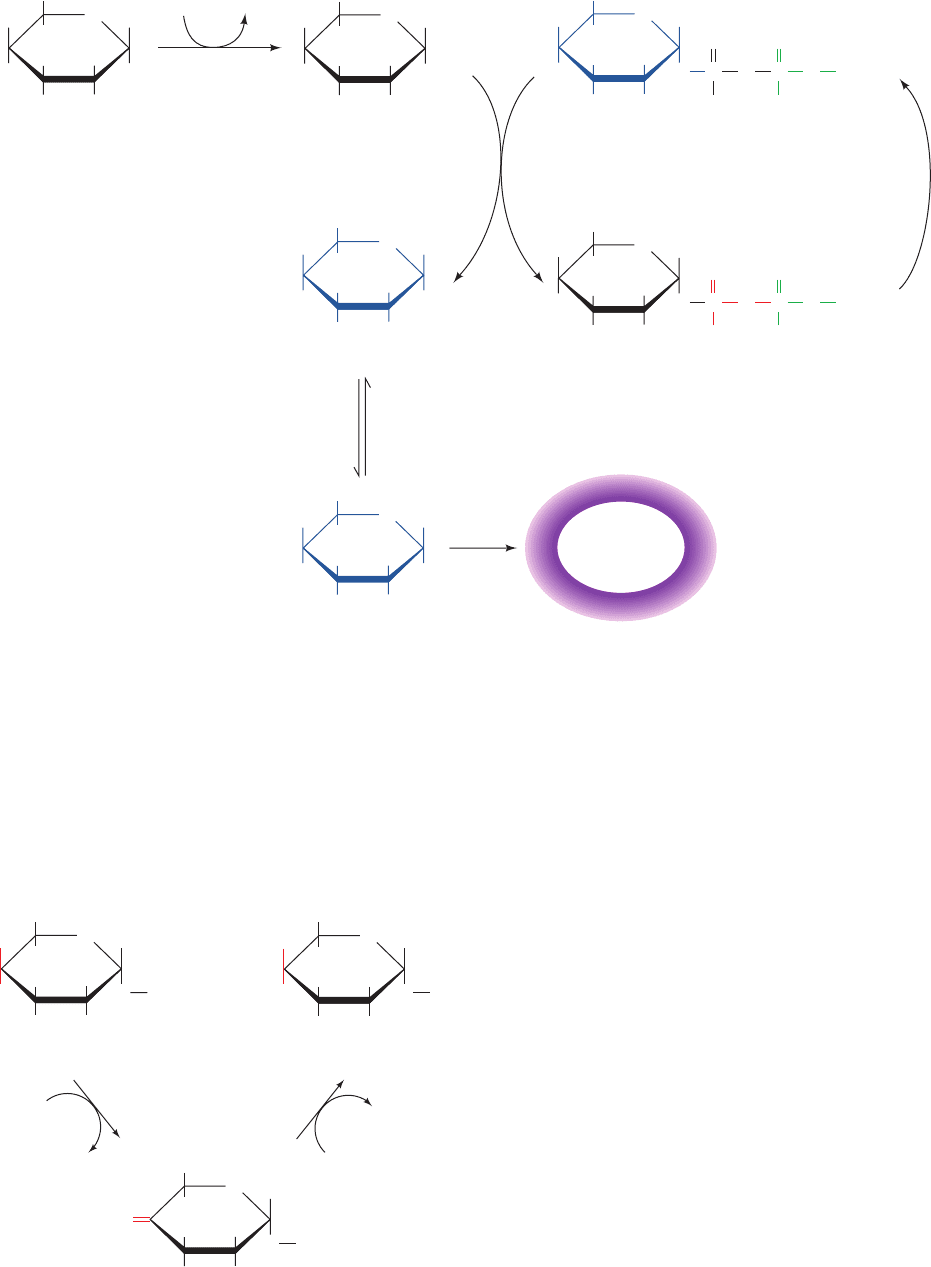
a. Galactosemia
Galactosemia is a genetic disease characterized by the
inability to convert galactose to glucose. Its symptoms in-
clude failure to thrive, mental retardation, and in some in-
stances death from liver damage. Most cases of galac-
tosemia involve a mutation in the enzyme catalyzing
Reaction 2 of the interconversion, galactose-1-phosphate
uridylyltransferase. This reaction is a double displacement
in which an enzyme His side chain first nucleophilically at-
tacks the -phosphoryl group of UDP–glucose, displacing
G1P and forming a uridylyl–His intermediate:
Galactose-1-phosphate then displaces the uridylyl group
from the enzyme’s His to form UDP–galactose:
A Gln residue forms hydrogen bonds to the uridylyl group’s
phosphoryl oxygens to stabilize the intermediate uridy-
lyl–His. Mutation of this Gln to Arg inactivates the enzyme.
Formation of UDP–galactose from galactose-1-phosphate
UDP–glactose E–His
Galactose-1-phosphate E–His-UMP Δ
glucose-1-phosphate E–His-UMP
UDP–glucose E–His166 Δ
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 632
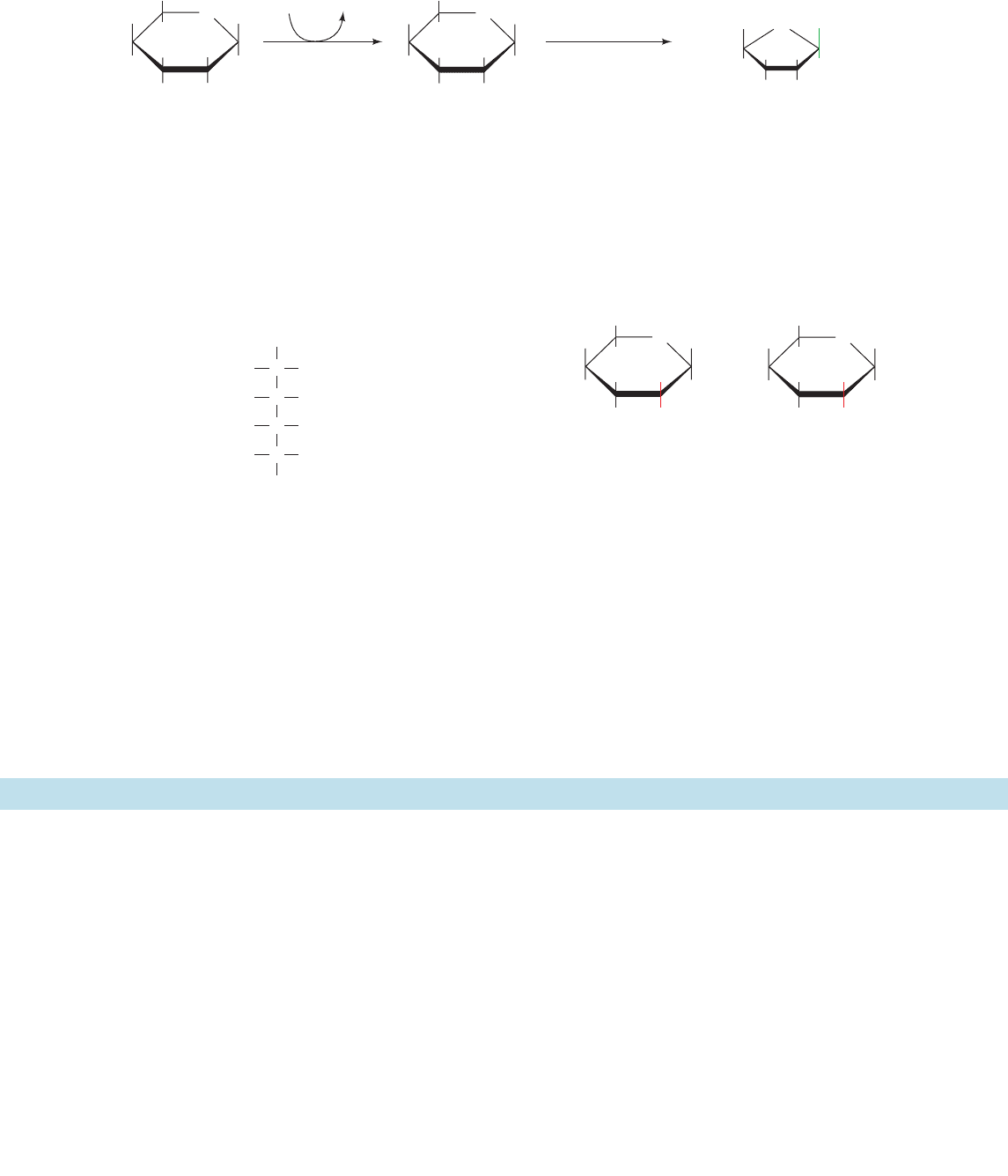
is thus prevented, leading to a buildup of toxic metabolic
by-products. For example, the increased galactose concen-
tration in the blood results in a higher galactose concentra-
tion in the lens of the eye where this sugar is reduced to
galactitol.
The presence of this sugar alcohol in the lens eventually
causes cataract formation (clouding of the lens).
Galactosemia is treated by a galactose-free diet. Except
for the mental retardation, this reverses all symptoms of
the disease. The galactosyl units that are essential for the
synthesis of glycoproteins (Section 11-3C) and glycolipids
(Section 12-1D) may be synthesized from glucose by a re-
versal of the epimerase reaction.These syntheses therefore
do not require dietary galactose.
CH
2
OH
CH
2
OH
OHCH
HCHO
HCHO
C
OHH
Galactitol
C. Mannose
Mannose, a common component of glycoproteins (Section
11-3C), and glucose are C2 epimers:
Mannose enters the glycolytic pathway after its conversion
to F6P via a two-reaction pathway (Fig. 17-37):
1. Hexokinase (Section 17-2A) converts mannose to
mannose-6-phosphate.
2. Phosphomannose isomerase then converts this al-
dose to the ketose F6P. The mechanism of the phospho-
mannose isomerase reaction resembles that catalyzed by
phosphoglucose isomerase (Section 17-2B); it involves an
enediolate intermediate.
CH
2
OH
H
OH H
H OH
HO
HH
OH
O
α-D-Glucose
H
OH HO
H H
HO
HH
OH
O
α-D-Mannose
CH
2
OH
Chapter Summary 633
1 The Glycolytic Pathway Glycolysis is the metabolic
pathway by which most life-forms degrade glucose to two mol-
ecules of pyruvate with the concomitant net generation of two
ATPs. The overall reaction:
occurs in 10 enzymatically catalyzed reactions.
2 The Reactions of Glycolysis In the preparatory stage
of glycolysis, which encompasses its first five reactions, glucose
reacts with two ATPs, in an “energy investment,” to form
fructose-1,6-bisphosphate, which is subsequently converted to
two molecules of glyceraldehyde-3-phosphate. In the second
stage of glycolysis, the “payoff” stage, which comprises its last
five reactions, glyceraldehyde-3-phosphate reacts with NAD
⫹
and P
i
to form the “high-energy” compound 1,3-bisphospho-
glycerate. This compound reacts in the last four reactions of
2NADH ⫹ 2pyruvate ⫹ 2ATP ⫹ 2H
2
O ⫹ 4H
⫹
Glucose ⫹ 2NAD
⫹
⫹ 2ADP ⫹ 2P
i
¡
the pathway with two ADPs to form pyruvate and two ATPs
per molecule. The mechanisms of the 10 glycolytic enzymes
have been elucidated through chemical and kinetic measure-
ments combined with X-ray structural studies. The glycolytic
enzymes exhibit stereospecificity in the reactions that they cat-
alyze. In at least two kinases,phosphoryl transfer from substrate
to water is prevented by substrate-induced conformational
changes that form the active site and exclude water from it.
3 Fermentation: The Anaerobic Fate of Pyruvate The
NAD
⫹
consumed in the formation of 1,3-BPG must be regener-
ated if glycolysis is to continue. In the presence of O
2
,NAD
⫹
is
regenerated by oxidative phosphorylation in the mitochondria.
Under anaerobic conditions in muscle, pyruvate is reduced by
NADH, yielding lactate and NAD
⫹
in a reaction catalyzed by
lactate dehydrogenase. In many muscles, particularly during
strenuous activity, the process of homolactic fermentation is a
major free energy source. In anaerobic yeast, NAD
⫹
is regener-
ated by alcoholic fermentation in two reactions.First pyruvate is
CHAPTER SUMMARY
Figure 17-37 Metabolism of mannose. Two enzymes are required to convert mannose to the
glycolytic intermediate F6P: (1) hexokinase and (2) phosphomannose isomerase.
CH
2
OH
H
OH HO
H H
HO
HH
OH
O
Mannose
ATP
hexokinase
ADP
H
OH HO
H H
HO
HH
OH
O
Mannose-6-phosphate
1
phosphomannose
isomerase
2
CH
2
OPO
3
2–
H OH
H HO
HO H
O
–2
O
3
POCH
2
CH
2
OH
Fructose-6-phosphate (F6P)
JWCL281_c17_593-637.qxd 6/3/10 8:39 AM Page 633

634 Chapter 17. Glycolysis
decarboxylated to acetaldehyde by pyruvate decarboxylase, an
enzyme that requires thiamine pyrophosphate as a cofactor.
The acetaldehyde is then reduced by NADH to form ethanol
and NAD
in a reaction catalyzed by alcohol dehydrogenase.
4 Metabolic Regulation and Control Metabolic regula-
tion is the process by which the steady-state flow of metabolites
through a pathway is maintained. Metabolic control is the force
exerted on the enzymes of the pathway in response to an exter-
nal signal in order to increase or decrease the flow while main-
taining the steady state to the extent possible. Homeostasis is
the regulation of the steady state. Metabolic flow must be con-
trolled to balance supply with demand and also to maintain
homeostasis. It is possible for more than one enzyme to be rate-
limiting in a metabolic pathway. Metabolic control analysis pro-
vides a framework for the study of metabolic systems in vivo
that share control among more than one enzyme, and it quanti-
tatively describes flux control and homeostasis.The flux control
coefficient measures the sensitivity of the flux to a change in en-
zyme concentration.The elasticity coefficient measures the sen-
sitivity of an enzymatic rate to the change in substrate concen-
tration. Both supply and demand are involved in flux control
and homeostasis. The response of the steady-state concentra-
tion of intermediates to changes in the supply or demand blocks
depends entirely on the elasticity coefficients of the two blocks
at the steady state. When the supply elasticity coefficient is
greater than the demand elasticity coefficient, flux control lies
in the demand portion of the pathway, and vice versa. Home-
ostasis control depends on large elasticity coefficients, whereas
flux control requires a low elasticity coefficient and a high flux
control coefficient. If the demand block controls the flux, the
function of the supply block is to control homeostasis. Feedback
inhibition determines the range of concentration of intermedi-
ates at which there is a steady state. It maintains homeostasis at
physiologically reasonable metabolite concentrations, some-
times far from their equilibrium values.
The flux through a reaction that is close to equilibrium is
very sensitive to changes in substrate concentration. Hence,
the steady-state flux through a metabolic pathway can only be
regulated by a nonequilibrium reaction. Nonequilibrium reac-
tions are controlled by allosteric interactions, substrate cycles,
covalent modification, and genetic (long-term) control mecha-
nisms. In muscle glycolysis, phosphofructokinase (PFK) cat-
alyzes one of the flux-generating steps.Although PFK is inhib-
ited by high concentrations of one of its substrates, ATP, the
10% variation of [ATP] over the range of metabolic activity
has insufficient influence on PFK activity to account for the
observed 100-fold range in glycolytic flux. [AMP] has a 4-fold
variation in response to the 10% variation of [ATP] through
the action of adenylate kinase. Although AMP relieves the
ATP inhibition of PFK, its concentration variation is also in-
sufficient to account for the observed glycolytic flux range.
However, the product of the PFK reaction, fructose-1,6-
bisphosphate, is hydrolyzed to F6P by FBPase, which is inhib-
ited by AMP. The substrate cycle catalyzed by these two en-
zymes confers, at least in principle, the necessary sensitivity of
the glycolytic flux to variations in [AMP]. Substrate cycling is
an important source of nonshivering thermogenesis.
5 Metabolism of Hexoses Other than Glucose Diges-
tion of carbohydrates yields glucose as the primary product.
Other prominent products are fructose, galactose, and man-
nose. These monosaccharides are metabolized through their
conversion to glycolytic intermediates.
General
Cornish-Bowden, A. (Ed.), New Beer in an Old Bottle: Eduard
Buchner and the Growth of Biochemical Knowledge, Universi-
tat de València (1997). [The 1897 paper by Eduard Buchner re-
porting the discovery of cell-free fermentation (in the original
German as well as its English and Spanish translations) to-
gether with a series of essays discussing the historical context of
this discovery and the modern study of multienzyme systems.]
Fersht, A., Structure and Mechanism in Protein Science, Freeman
(1999).
Frey, P.A. and Hegeman, A.D., Enzymatic Reaction Mechanisms,
Oxford University Press (2007).
Fruton, J.S., Molecules and Life: Historical Essays on the Interplay
of Chemistry and Biology, Wiley–Interscience (1974). [In-
cludes a detailed historical account of the elucidation of fer-
mentation.]
Kim, J. and Dang, C.V., Multifaceted roles of glycolytic enzymes,
Trends Biochem. Sci. 30, 142–150 (2005). [Discusses the vari-
ous nonglycolytic functions, mainly regulatory, that glycolytic
enzymes have acquired.]
Saier, M.H., Jr., Enzymes in Metabolic Pathways, Chapter 5,
Harper & Row (1987).
Enzymes of Glycolysis
The Enzymes of Glycolysis: Structure, Activity and Evolution,
Philos. Trans. R. Soc. London Ser. B 293, 1–214 (1981). [A
collection of authoritative discussions on the enzymes of gly-
colysis.]
Allen, S.C. and Muirhead, H., Refined three-dimensional struc-
ture of cat-muscle (M1) pyruvate kinase at a resolution of
2.6 Å, Acta Cryst. D52, 499–504 (1996).
Bennett, W.S., Jr. and Steitz, T.A., Glucose-induced conforma-
tional change in yeast hexokinase, Proc. Natl. Acad. Sci. 75,
4848–4852 (1978).
Bernstein, B.E., Michels, P.A.M., and Hol, W.G.J., Synergistic ef-
fects of substrate-induced conformational changes in phospho-
glycerate activation, Nature 385, 275–278 (1997).
Biesecker, G., Harris, J.I.,Thierry, J.C.,Walker, J.E., and Wonacott,
A.J., Sequence and structure of D-glyceraldehyde-3-phosphate
dehydrogenase from Bacillus stearothermophilus, Nature 266,
328–333 (1977).
Cleland, W.W. and Kreevoy, M.M., Low-barrier hydrogen bonds
and enzymic catalysis, Science 264, 1887–1890 (1994); and
Gerlt, J.A. and Gassman, P.G., Understanding the rates of cer-
tain enzyme-catalyzed reactions: Proton abstraction from car-
bon acids, acyl-transfer reactions, and displacement of phos-
phodiesters, Biochemistry 32, 11943–11952 (1993).
Dalby,A., Dauter, Z., and Littlechild,J.A., Crystal structure of hu-
man muscle aldolase complexed with fructose 1,6-bisphos-
phate: Mechanistic implications, Protein Sci. 8, 291–297 (1999).
Davenport, R.C., Bash, P.A., Seaton, B.A., Karplus, M., Petsko,
G.A., and Ringe,D., Structure of the triosephosphate isomerase–
REFERENCES
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 634
References 635
phosphoglycohydroxamate complex:An analogue of the inter-
mediate on the reaction pathway, Biochemistry 30, 5821–5826
(1991); and Lolis, E. and Petsko, G.A., Crystallographic analy-
sis of the complex between triosephosphate isomerase and
2-phosphoglycolate at 2.5 Å resolution: Implications for catal-
ysis, Biochemistry 29, 6619–6625 (1990).
Evans, P.R. and Hudson, P.J., Structure and control of phospho-
fructokinase from Bacillus stearothermophilus, Nature 279,
500–504 (1979).
Gefflaut,T., Blonski, C.,Perie, J., and Willson,M., Class I aldolases:
Substrate specificity, mechanism, inhibitors and structural
aspects, Prog. Biophys. Mol. Biol. 63, 301–340 (1995).
Hall, D.R., Leonard, G.A., Reed, C.D., Watt, C.I., Berry, A., and
Hunter, W.N., The crystal structure of Escherichia coli class II
fructose-1,6-bisphosphate aldolase in complex with phospho-
glycohydroxamate reveals details of mechanism and speci-
ficity, J. Mol. Biol. 287, 383–394 (1999).
Harlos, K., Vas, M., and Blake, C.C.F., Crystal structure of the bi-
nary complex of pig muscle phosphoglycerate kinase and its
substrate 3-phospho-
D-glycerate, Proteins 12, 133–144 (1992).
Jedrzejas, M.J., Structure, function, and evolution of phosphoglyc-
erate mutase: Comparison with fructose-2,6-bisphosphatase,
acid phosphatase, and alkaline phosphatase, Prog. Biophys.
Mol. Biol. 73, 263–287 (2000).
Jeffrey, C.J., Bahnson, B.J., Chien, W., Ringe, D., and Petsko, G.A.,
Crystal structure of rabbit phosphoglucose isomerase, a glycolytic
enzyme that moonlights as neuroleukin, autocrine motility factor,
and differentiation mediator, Biochemistry 39, 955–964 (2000).
Jogl, G., Rozovsky, S., McDermott, A.E., and Tong, L., Optimal
alignment for enzymatic proton transfer: Structure of the
Michaelis complex of triosphosphate isomerase at 1.2-Å reso-
lution, Proc. Natl.Acad. Sci. 100, 50–55 (2003).
Joseph, D., Petsko, G.A., and Karplus, M., Anatomy of a confor-
mational change: Hinged “lid” motion of the triosephosphate
isomerase loop, Science 249, 1425–1428 (1990).
Knowles, J.R., Enzyme catalysis: Not different, just better, Nature
350, 121–124 (1991). [A lucid discussion of TIM’s catalytic
mechanism.]
Kuby, S.A. (Ed.), A Study of Enzymes,Vol. II, CRC Press (1991).
[Chapters 17, 18, 19, and 20 discuss the mechanisms of adeny-
late kinase, PFK, PGI and TIM, and aldolase, respectively.
Chapter 4 discusses thiamine-dependent reaction mechanisms.]
Kuser, P., Cupri, F., Bleicher, L., and Polikarpov, I., Crystal struc-
ture of yeast hexokinase PI in complex with glucose: A classi-
cal “induced fit” example revised, Proteins 72, 731–740 (2008).
Marsh, J.J. and Lebherz, H.G., Fructose-bisphosphate aldolases:An
evolutionary history, Trends Biochem. Sci. 17, 110–113 (1992).
Maurer, P.J. and Nowak,T., Fluoride inhibition of yeast enolase. 1.
Formation of ligand complexes, Biochemistry 20, 6894–6900
(1981); and Nowak, T. and Maurer, P.J., Fluoride inhibition of
yeast enolase. 2. Structural and kinetic properties of ligand
complexes determined by nuclear relaxation rate studies, Bio-
chemistry 20, 6901–6911 (1981).
Morris,A.J. and Tolan,D.R., Lysine-146 of rabbit muscle aldolase is
essential for cleavage and condensation of the C3-C4 bond of
fructose 1,6-bis(phosphate), Biochemistry 33, 12291–12297
(1994); and Site-directed mutagenesis identifies aspartate 33 as
a previously unidentified critical residue in the catalytic mecha-
nism of rabbit aldolase A, J. Biol. Chem. 268, 1095–1100 (1993).
Muirhead, H. and Watson, H. Glycolytic enzymes: From hexose to
pyruvate, Curr. Opin. Struct. Biol. 2, 870–876 (1992).
Reed, G.H., Poyner, R.R., Larsen, T.M., Wedekind, J.E., and
Rayment, I., Structural and mechanistic studies on enolase,
Curr. Opin. Struct. Biol. 6, 736–743 (1996).
Seeholzer, S.H., Phosphoglucose isomerase: A ketol isomerase
with aldol C2-epimerase activity, Proc. Natl. Acad. Sci. 90,
1237–1241 (1993).
Williams, J.C.and McDermott,A.E., Dynamics of the flexible loop
of triosephosphate isomerase: The loop motion is not ligand-
gated, Biochemistry 34, 8309–8319 (1995).
Enzymes of Anaerobic Fermentation
Boyer, P.D. (Ed.), The Enzymes (3rd ed.),Vol. 11,Academic Press
(1975). [Contains authoritative reviews on alcohol dehydroge-
nase, lactate dehydrogenase, and the evolutionary and struc-
tural relationships among the dehydrogenases.]
Dyda, F., Furey, W., Swaminathan, S., Sax, M., Farrenkopf, B., and
Jordan, F., Catalytic centers in the thiamin diphosphate de-
pendent enzyme pyruvate decarboxylase at 2.4-Å resolution,
Biochemistry 32, 6165–6170 (1993).
Golbik, R., Neef, H., Hubner, G., Konig, S., Seliger, B., Meshalkina,
L., Kochetov, G.A., and Schellenberger, A., Function of the
aminopyridine part in thiamine pyrophosphate enzymes,
Bioinorg. Chem. 19, 10–17 (1991).
Park, J.H., Brown, R.L., Park, C.R., Cohn, M., and Chance, B., En-
ergy metabolism in the untrained muscle of elite runners as
observed by
31
P magnetic resonance spectroscopy: Evidence
suggesting a genetic endowment for endurance exercise. Proc.
Natl.Acad. Sci. 85, 8780–8785 (1988).
Control of Metabolic Flux
Crabtree, B. and Newsholme, E.A., A systematic approach to de-
scribing and analyzing metabolic control systems, Trends
Biochem. Sci. 12, 4–12 (1987).
Fell, D.A., Metabolic control analysis:A survey of its theoretical and
experimental development, Biochem. J. 286, 313–330 (1992).
Fell, D., Understanding the Control of Metabolism, Portland Press
(1997).
Hofmeyr, J.-H.S. and Cornish-Bowden,A., Regulating the cellular
economy of supply and demand, FEBS Lett. 476, 47–51 (2000).
Kacser, H. and Burns, J.A. (with additional comments by Kacser,
H. and Fell, D.A.),The control of flux, Biochem. Soc.Trans. 23,
341–366 (1995).
Kacser, H. and Porteous, J.W., Control of metabolism:What do we
have to measure? Trends Biochem. Sci. 12, 5–14 (1987).
Lardy, H. and Schrago, E., Biochemical aspects of obesity, Annu.
Rev. Biochem. 59, 689–710 (1990).
Newsholme, E.A., Challiss, R.A.J., and Crabtree, B., Substrate cy-
cles: their role in improving sensitivity in metabolic control,
Trends Biochem. Sci. 9, 277–280 (1984).
Perutz, M.F., Mechanism of cooperativity and allosteric regulation
in proteins, Q. Rev. Biophys. 22, 139–236 (1989). [Section 6 dis-
cusses PFK.]
Schaaf, I., Heinisch, J., and Zimmermann, K., Overproduction of
glycolytic enzymes in yeast, Yeast 5, 285–290 (1989).
Schirmer, T. and Evans, P.R., Structural basis of the allosteric
behaviour of phosphofructokinase, Nature 343, 140–145 (1990).
Walsh, K. and Koshland, D.E., Jr., Characterization of rate-
controlling steps in vivo by use of an adjustable expression
vector, Proc. Natl. Acad. Sci. 82, 3577–3581 (1985).
Metabolism of Hexoses Other Than Glucose
Frey, P.A.,The Leloir pathway:A mechanistic imperative for three
enzymes to change the stereochemical configuration of a sin-
gle carbon in galactose, FASEB J. 10, 461–470 (1996).
Valle, D. (Ed.), The Metabolic & Molecular Bases of Inherited Dis-
ease, http://www.ommid.com. [Chapters 70 and 72 discuss
fructose and galactose metabolism and their genetic disorders.]
JWCL281_c17_593-637.qxd 6/30/10 11:02 AM Page 635
636 Chapter 17. Glycolysis
1. Write out the reactions of the glycolytic pathway from glu-
cose to lactate using structural formulas for all intermediates.
Learn the names of these intermediates and the enzymes that cat-
alyze the reactions.
2. ⌬G°¿ for the aldolase reaction is ⫹22.8 kJ ⴢ mol
⫺1
. In the cell,
at 37°C, the mass action ratio [DHAP]/[GAP] ⫽ 5.5. Calculate the
equilibrium ratio of [FBP]/[GAP] when [GAP] is (a) 2 ⫻ 10
⫺5
M
and (b) 10
⫺3
M.
3. The pH dependence of the rate of the triose phosphate iso-
merase (TIM) reaction has characteristic pK’s of 6.5 and 9.5.His 95,
a catalytically essential residue, has been shown to have a pK of 4.5.
Why doesn’t the pH rate curve indicate the existence of this pK?
4. Arsenate , a structural analog of phosphate, can
act as a substrate for any reaction in which phosphate is a sub-
strate. Arsenate esters, unlike phosphate esters, are kinetically as
well as thermodynamically unstable and hydrolyze almost instan-
taneously. Write a balanced overall equation for conversion of
glucose to pyruvate in the presence of ATP, ADP, NAD
⫹
, and ei-
ther (a) phosphate or (b) arsenate. (c) Why is arsenate a poison?
5. When glucose is degraded anaerobically via glycolysis there is
no overall oxidation or reduction of the substrate.The fermentation
reaction is therefore said to be “balanced.”The free energy required
for ATP formation is nevertheless obtained from favorable electron-
transfer reactions. Which metabolic intermediate is the electron
donor and which is the electron acceptor when glucose is degraded
by a balanced glycolytic fermentation:(a) in muscle and (b) in yeast?
6. In which carbon atoms of pyruvate would radioactivity be
found if glucose metabolized by the glycolytic pathway were la-
beled with
14
C at: (a) C1 and (b) C4? (Note: Assume that triose
phosphate isomerase is able to equilibrate dihydroxyacetone
phosphate and glyceraldehyde-3-phosphate.)
*7. The following reaction is catalyzed by an enzyme very sim-
ilar to Class I aldolases:
C
C
C
HO H
CO
CH
2
OH
CH
2
OPO
3
2⫺
CH
2
OPO
3
2⫺
Fructose-6-
phosphate
C
C
OH
C
OH
H
H
Erythrose-4-
phosphate
CH
2
OPO
3
2⫺
CHOH
C
C
C
HO
H
H
H
H
OH
OH
OH
CO
CH
2
OH
CH
2
OPO
3
2⫺
Sedoheptulose-7-
phosphate
H
H
OH
OH
C
Glyceraldehyde-3-
phosphate
H
O
C
H
O
⫹
⫹
transaldolase
(AsO
3⫺
4
)
Write a plausible mechanism for this reaction using curved arrows
to indicate the electron flow.
8. The half-reactions involved in the LDH reaction and their
standard reduction potentials are
Calculate ⌬G for the reaction at the biochemical standard state
under the following conditions:
(a) [lactate]/[pyruvate] ⫽ 1; [NAD
⫹
]/[NADH] ⫽ 1
(b) [lactate]/[pyruvate] ⫽ 160; [NAD
⫹
]/[NADH] ⫽ 160
(c) [lactate]/[pyruvate] ⫽ 1000; [NAD
⫹
]/[NADH] ⫽ 1000
(d) Under what conditions will the reaction spontaneously
favor NADH oxidation?
(e) In order for the free energy change of the glyceraldehyde-
3-phosphate dehydrogenase reaction to favor glycolysis,
the [NAD
⫹
]/[NADH] ratio must be maintained close to
10
3
. Under anaerobic conditions in mammalian muscle,
lactate dehydrogenase performs this function. How high
can the [lactate]/[pyruvate] ratio become in muscle cells
before the LDH-catalyzed reaction ceases to be favorable
in the direction of NAD
⫹
production while maintaining
the foregoing [NAD
⫹
]/[NADH] ratio constant?
9. Although it is not the primary flux-control point for glycol-
ysis, pyruvate kinase is subject to allosteric regulation. (a) What is
the metabolic importance of regulating flux through the pyruvate
kinase reaction? (b) What is the advantage of activating pyruvate
kinase with fructose-1,6-bisphosphate?
*10. Based on the involvement of thiamine pyrophosphate
(TPP) in the pyruvate decarboxylase reaction, which of the fol-
lowing reactions, if any, might be expected to utilize TPP as a
cofactor?
Write hypothetical mechanisms for each reaction showing where
TPP is involved or why it is unnecessary.
11. The glycolytic pathway for glucose breakdown is almost
universal. Some bacteria, however, utilize an alternate route
called the Entner–Doudoroff pathway (Fig. 17-38). Like the gly-
colytic pathway in yeast, the final product is ethanol. (a) Write bal-
anced equations for the conversion of glucose to ethanol and CO
2
via the Entner–Doudoroff pathway and the yeast alcoholic fer-
mentation. (b) Infer from your stoichiometries why the glycolytic
pathway rather than the Entner–Doudoroff pathway is almost
universal.
CH
2
CC
O
⫺
O
O
O
O
⫺
C H
3
CCO
2
C
O
O
O
⫺
O
⫺
O
O
C
⫹
HOCO
2
C
C
CH
3
CH
3
C
O
⫺
O
O
C
CH
3
C
⫹
2
(a)
(b)
NAD
⫹
⫹ 2H
⫹
⫹ 2e
⫺
¡
NADH ⫹ H
⫹
e°¿ ⫽⫺0.315 V
Pyruvate ⫹ 2H
⫹
⫹ 2e
⫺
¡
lactate
e°¿ ⫽⫺0.185 V
PROBLEMS
JWCL281_c17_593-637.qxd 6/30/10 2:19 PM Page 636
*12. The hydrolysis of ATP to ADP in the cell results in a con-
comitant change in [AMP] as mediated by adenylate kinase. (a)
Assuming that [ATP] [AMP] and that the total adenine nu-
cleotide concentration in the cell, A
T
[AMP] [ADP] [ATP],
Problems 637
is constant, derive an expression for [AMP] in terms of [ATP] and
A
T
. (b) Assuming an initial [ATP]/[ADP] of 10 and A
T
5 mM,
calculate the ratio of the final to initial values of [AMP] on a 10%
decrease of [ATP].
Figure 17-38 Entner–Doudoroff pathway for glucose breakdown.
C
C
C
C
CHOH
PO
3
2
Mg
2
OH
OH
OH
H
H
H
O
H
HO
C
C
C
C
CH
2
O P
OH
OH
OH
H
H
H
O
H
HO
C
C
C
C
CH
2
O P
OH
OH
OH
H
H
H
O
O
H
HO
C
C
C
C
CH
2
O P
CH
3
O
OH
OH
H
H
H
O
C
C OHH
H
C
H
C
H
C
O
C
2ATP
O
C
C O
OO
H
CH
2
O P
P
glucokinase
ATP ADP
glucose-6-
phosphate
dehydrogenase
NADH
6-phospho-
gluconate
dehydrase
(analogous
to enolase)
H
2
O
H
2
O
Glucose Glucose-6-
phosphate
(G6P)
6-Phospho-
gluconate
2-Keto-3-deoxy-
6-phosphogluconate
(KDPG)
321
NAD
NAD
NADH
KDPG-
aldolase
4
Pyruvate
Glyceraldehyde-3-
phosphate (GAP)
same reactions as
in glycolysis and
alcoholic fermentation
CO
2
CH
3
CH
2
OH
Ethanol
H
2
O
2ADP P
i
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 637
638
CHAPTER 18
Glycogen Metabolism
1 Glycogen Breakdown
A. Glycogen Phosphorylase
B. Phosphoglucomutase
C. Glycogen Debranching Enzyme
D. Thermodynamics of Glycogen Metabolism: The Need for
Separate Pathways of Synthesis and Breakdown
2 Glycogen Synthesis
A. UDP–Glucose Pyrophosphorylase
B. Glycogen Synthase
C. Glycogen Branching Enzyme
3 Control of Glycogen Metabolism
A. Direct Allosteric Control of Glycogen Phosphorylase and
Glycogen Synthase
B. Covalent Modification of Enzymes by Cyclic Cascades:
Effector “Signal” Amplification
C. Glycogen Phosphorylase Bicyclic Cascade
D. Glycogen Synthase Bicyclic Cascade
E. Integration of Glycogen Metabolism Control Mechanisms
F. Maintenance of Blood Glucose Levels
G. Response to Stress
4 Glycogen Storage Diseases
Everything should be made as simple as possible, but not
simpler.
Albert Einstein
Glucose, a major metabolic fuel source,is degraded via gly-
colysis to produce ATP (Chapter 17). Higher organisms
protect themselves from potential fuel shortage by poly-
merizing excess glucose for storage as high molecular mass
glucans (glucose polysaccharides) that may be readily mo-
bilized in times of metabolic need. In plants, this glucose
storage substance is starch, a mixture of the (1 S 4)-linked
glucan -amylose (Fig. 11-18) and amylopectin, which dif-
fers from -amylose by the presence of (1 S 6) branches
every 24 to 30 residues (Fig. 11-19). In animals, the storage
glucan is glycogen (Fig. 18-1), which differs from amy-
lopectin only in that its branches occur every 8 to 14
residues. Glycogen occurs in 100- to 400-Å-diameter cyto-
plasmic granules (Figs. 11-20 and 18-1c), which contain up
to 120,000 glucose units. They are especially prominent in
the cells that make the greatest use of glycogen, muscle
(maximally 1–2% glycogen by weight) and liver cells (max-
imally 10% glycogen by weight, an ⬃12-h energy supply
for the body). Glycogen granules also contain the enzymes
that catalyze glycogen synthesis and degradation as well as
some of the enzymes that regulate these processes.
As we shall see in this chapter, glycogen’s glucose units
are mobilized by their sequential removal from the glucan
chains’ nonreducing ends (ends lacking a C1 OH group).
Glycogen’s highly branched structure is therefore physio-
logically significant: It permits glycogen’s rapid degradation
through the simultaneous release of the glucose units at the
end of every branch.
Why does the body go to such metabolic effort to use
glycogen for energy storage when fat, which is far more
abundant in the body, seemingly serves the same purpose?
The answer is threefold:
1. Muscles cannot mobilize fat as rapidly as they can
glycogen.
2. The fatty acid residues of fat cannot be metabolized
anaerobically (Section 25-2).
3. Animals cannot convert fatty acids to glucose (Sec-
tion 23-1), so fat metabolism alone cannot adequately
maintain essential blood glucose levels (Section 18-3F).
As with all metabolic processes, there are several levels on
which glycogen metabolism may be understood.We shall ex-
amine this process in order to understand the pathway’s ther-
modynamics and the reaction mechanisms of its individual
steps but will emphasize the mechanisms by which glycogen
synthesis and breakdown rates are controlled.We began our
consideration of metabolic control mechanisms in Section
17-4 with a discussion of the role of allosteric interactions and
substrate cycles in the regulation of glycolysis. Glycogen me-
tabolism’s more complex control systems provide us with ex-
amples of several additional control processes:covalent mod-
ification of enzymes and enzyme cascades. In addition, we
shall consider glycogen metabolism as a model for the role of
hormones in the overall regulatory process.We end the chap-
ter by discussing the consequences of genetic defects in vari-
ous enzymes of glycogen metabolism.
1 GLYCOGEN BREAKDOWN
Liver and muscle are the two major storage tissues for
glycogen. In muscle,the need for ATP results in the conver-
sion of glycogen to glucose-6-phosphate (G6P) for entry
into glycolysis. In liver, low blood glucose concentration
triggers glycogen breakdown to G6P, which in this case is
¬
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 638
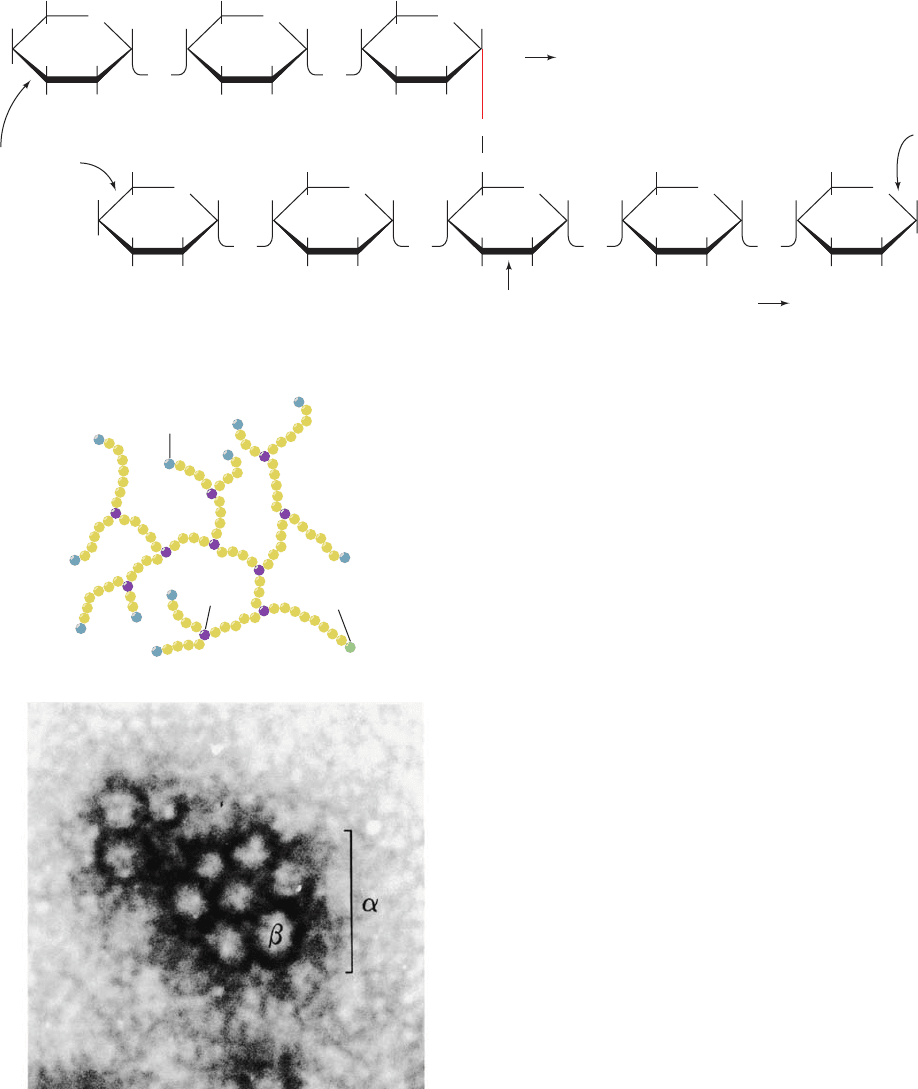
hydrolyzed to glucose and released into the bloodstream to
reverse this situation.
Glycogen breakdown requires the actions of three
enzymes:
1. Glycogen phosphorylase (or simply phosphorylase)
catalyzes glycogen phosphorolysis (bond cleavage by the
substitution of a phosphate group) to yield glucose-1-
phosphate (G1P).
(n residues) (n 1 residues)
This enzyme will only release a glucose unit that is at least
five units from a branch point.
2. Glycogen debranching enzyme removes glycogen’s
branches, thereby permitting the glycogen phosphorylase re-
action to go to completion. It also hydrolyzes (1 S 6)-linked
glucosyl units to yield glucose. Consequently, ⬃92% of glyco-
gen’s glucose residues are converted to G1P. The remaining
⬃8%, those at the branch points, are converted to glucose.
3. Phosphoglucomutase converts G1P to G6P, which, as
we have seen (Section 17-2A),is also formed in the first step
of glycolysis through the action of either hexokinase or glu-
cokinase. G6P can either continue along the glycolytic path-
way (as in muscle) or be hydrolyzed to glucose (as in liver).
In this section, we discuss the structures and mechanisms of
action of these three enzymes.
A. Glycogen Phosphorylase
Glycogen phosphorylase is a dimer of identical 842-residue
(97-kD) subunits that catalyzes the controlling step in glyco-
gen breakdown.It is regulated both by allosteric interactions
and by covalent modification. The enzyme-catalyzed modifi-
cation/demodification process yields two forms of phospho-
rylase: phosphorylase a, which has a phosphoryl group ester-
ified to Ser 14 in each of its subunits, and phosphorylase b,
which lacks these phosphoryl groups. Phosphorylase’s al-
losteric inhibitors,ATP,G6P, and glucose,and its allosteric ac-
tivator, AMP (to name only the enzyme’s most prominent
Glycogen P
i
Δ
glycogen
G1P
Section 18-1. Glycogen Breakdown 639
Nonreducing
ends
Branch
point
O
(a)
α(1 6) linkage
linkage
α(1 4)
Reducing
end
CH
2
HO
O
OH
OH H
HHH
H
HOH
O
CH
2
O
OH
OH H
HH
H
HOH
O
CH
2
OH
OH H
H
H
HOH
O
CH
2
HO
O
OH
OH H
HHH
H
HOH
O
CH
2
O
OH
OH H
HH
O
HH
H
HOH
O
CH
2
O
OH
OH H
HH
H
HOH
O
CH
2
OH
OH H
H
H
HOH
OH
O
CH
2
OH H
H
HOH
O
Branch
point
Reducing
end
Nonreducing
end
(b)
Figure 18-1 Structure of glycogen. (a) Molecular formula. In
the actual molecule the chains are much longer than shown.
(b) Schematic diagram illustrating its branched structure. Branch
points in the actual molecule are separated by 8 to 14 glucosyl
units. Note that the molecule, no matter how big, has but one
reducing end. (c) Electron micrograph of a glycogen granule
from rat skeletal muscle. Each granule () consists of several
spherical glycogen molecules () and its associated proteins.
[From Calder, P.C., Int. J. Biochem. 23, 1339 (1991). Copyright
Elsevier Science. Used with permission.]
(c)
JWCL281_c18_638-670.qxd 6/3/10 1:47 PM Page 639
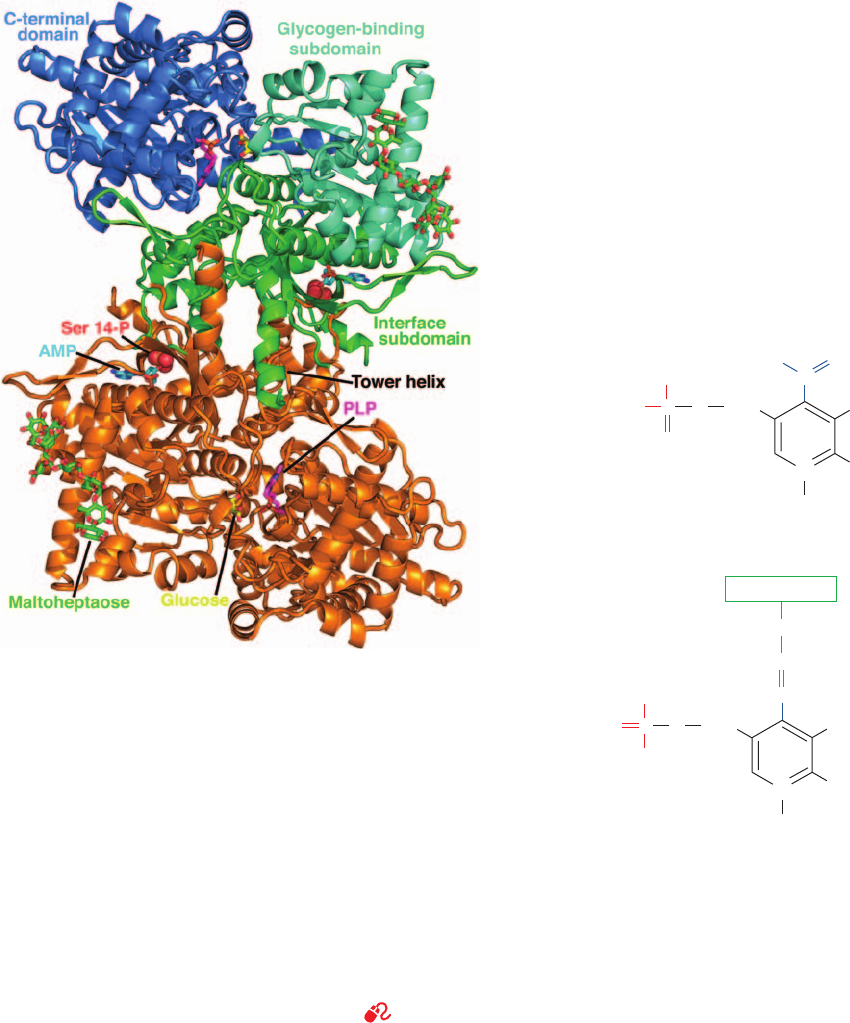
effectors), interact differently with the phospho- and dephos-
phoenzymes, resulting in an extremely sensitive control
process. We study this control process in Section 18-3C.
a. Structural Domains and Binding Sites
The high-resolution X-ray structures of phosphorylase a
and phosphorylase b were determined by Robert Fletterick
and Louise Johnson, respectively.The structure of phospho-
rylase b, despite its lack of a Ser-linked phosphate, is very
similar to that of phosphorylase a (Fig. 18-2). Both struc-
tures have two domains, an N-terminal domain (residues
1–484; among the largest known domains), and a C-termi-
nal domain (residues 485–842). The N-terminal domain is
further divided into an interface subdomain (residues
1–315), which includes the covalent modification site (Ser
14), the allosteric effector site, and all the intersubunit con-
tacts in the dimer; and a glycogen-binding subdomain
(residues 316–484), which contains the “glycogen storage
site”(see below).The catalytic site is located at the center of
the subunit where these two subdomains come together
with the C-terminal domain. In Section 18-3 we discuss the
allosteric behavior of glycogen phosphorylase and the con-
formational differences between phosphorylases a and b.
Glycogen forms a left-handed helix with 6.5 glucose
residues per turn, similar to ␣-amylose (Fig. 11-18b). An
⬃30-Å-long crevice on the surface of the phosphorylase
monomer that has the same radius of curvature as glycogen
connects the glycogen storage site, which binds glycogen, to
the active site, which phosphorylizes it. Since this crevice
can accommodate four or five sugar residues in a chain but
is too narrow to admit branched oligosaccharides, it pro-
vides a clear physical rationale for the inability of phospho-
rylase to cleave glycosyl residues closer than five units from
a branch point. Presumably the glycogen storage site in-
creases the catalytic efficiency of phosphorylase by permit-
ting it to phosphorylize many glucose residues on the same
glycogen particle without having to dissociate and reassoci-
ate completely between catalytic cycles.
b. Pyridoxal Phosphate Is an Essential Cofactor
for Phosphorylase
Phosphorylase contains pyridoxal-5¿¿ -phosphate (PLP)
and requires it for activity. This vitamin B
6
derivative is co-
valently linked to phosphorylase via a Schiff base to Lys
679. PLP is similarly linked to a variety of enzymes in-
volved in amino acid metabolism, where it is an essential
cofactor in transamination reactions (Section 26-1Aa).The
O
–
PLP covalently bound to
phosphorylase via a
Schiff base to Lys 679
O
OH
CH
N
(CH
2
)
4
CH
3
H
2
C
H
N
P
O
O
–
+
Phosphorylase
Lys 679
Pyridoxal-5ⴕ-phosphate (PLP)
O
OH
C
O
H
CH
3
H
2
C
H
N
P
–
O
O
O
–
+
640 Chapter 18. Glycogen Metabolism
Figure 18-2 X-ray structure of rabbit muscle glycogen
phosphorylase a. The homodimeric enzyme is drawn in ribbon
form and viewed along its 2-fold axis. Each subunit consists of an
N-terminal domain, which is subdivided into an interface
subdomain (residues 1–315) and a glycogen-binding subdomain
(residues 316–484), and a C-terminal domain (residues 485–842).
The enzyme’s several ligands are drawn in stick form colored
according to type with N blue, O red, P orange, and C atoms as
indicated.The active site is marked by a bound glucose molecule
(C yellow). Pyridoxal phosphate (PLP) is covalently linked to the
side chain of Lys 678 in the C-terminal domain (C magenta). In
addition, the enzyme binds its allosteric effector AMP (C cyan)
and maltoheptaose (C green), an ␣(1 S 4)-linked glucose
heptamer, which is bound in the enzyme’s glycogen storage site.
Ser 14-P, the phosphoryl group on Ser 14, is drawn in space-filling
form. [X-ray structure coordinates courtesy of Stephen Sprang,
University of Texas Southwest Medical Center.]
See
Kinemage Exercise 14-1
JWCL281_c18_638-670.qxd 6/3/10 1:47 PM Page 640
