Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

CO
...
Zn
2
+
H
C
–
HO
CH
2
OPO
2
3
–
Enzyme
CO
–
...
Zn
2
+
H
C
HO
CH
2
OPO
2
3
–
Enzyme
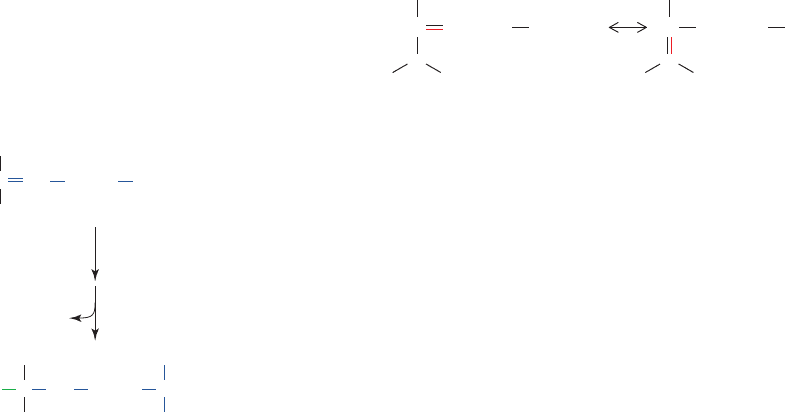
Are the two classes of aldolases related? Although both
classes exhibit the Uni Bi kinetics implicit in their mecha-
nisms, they exhibit only ⬃15% sequence identity, which
places them in the lower end of the twilight zone for estab-
lishing homology (Section 7-4Ba). Nevertheless, their X-ray
structures reveal that they have the same fold, the /
barrel. The evolution of this particularly common fold is
discussed in Section 8-3Bh.
b. Why Two Classes of Aldolase?
Since glycolysis presumably arose very early in evolu-
tionary history, the existence of two classes of aldolase is
unexpected. It had originally been postulated that, since
Class I aldolases occur in higher organisms, Class II al-
dolases must be the more primitive enzyme form, that is,
less metabolically capable than are the Class I enzymes.
However, the discovery that some organisms simultane-
ously express both classes of aldolase suggests that both
enzyme classes are evolutionarily ancient and equally
interconvertible C3 compounds that can therefore enter a
common degradative pathway.The enolate intermediate in
the aldol cleavage reaction is stabilized by resonance, as
shown, as a result of the electron-withdrawing character of
the carbonyl oxygen atom.
Note that at this point in the pathway the atom number-
ing system changes. Atoms 1, 2, and 3 of glucose become
atoms 3, 2, and 1 of DHAP, thus reversing order. Atoms 4,
5, and 6 become atoms 1, 2, and 3 of GAP (Fig. 17-3).
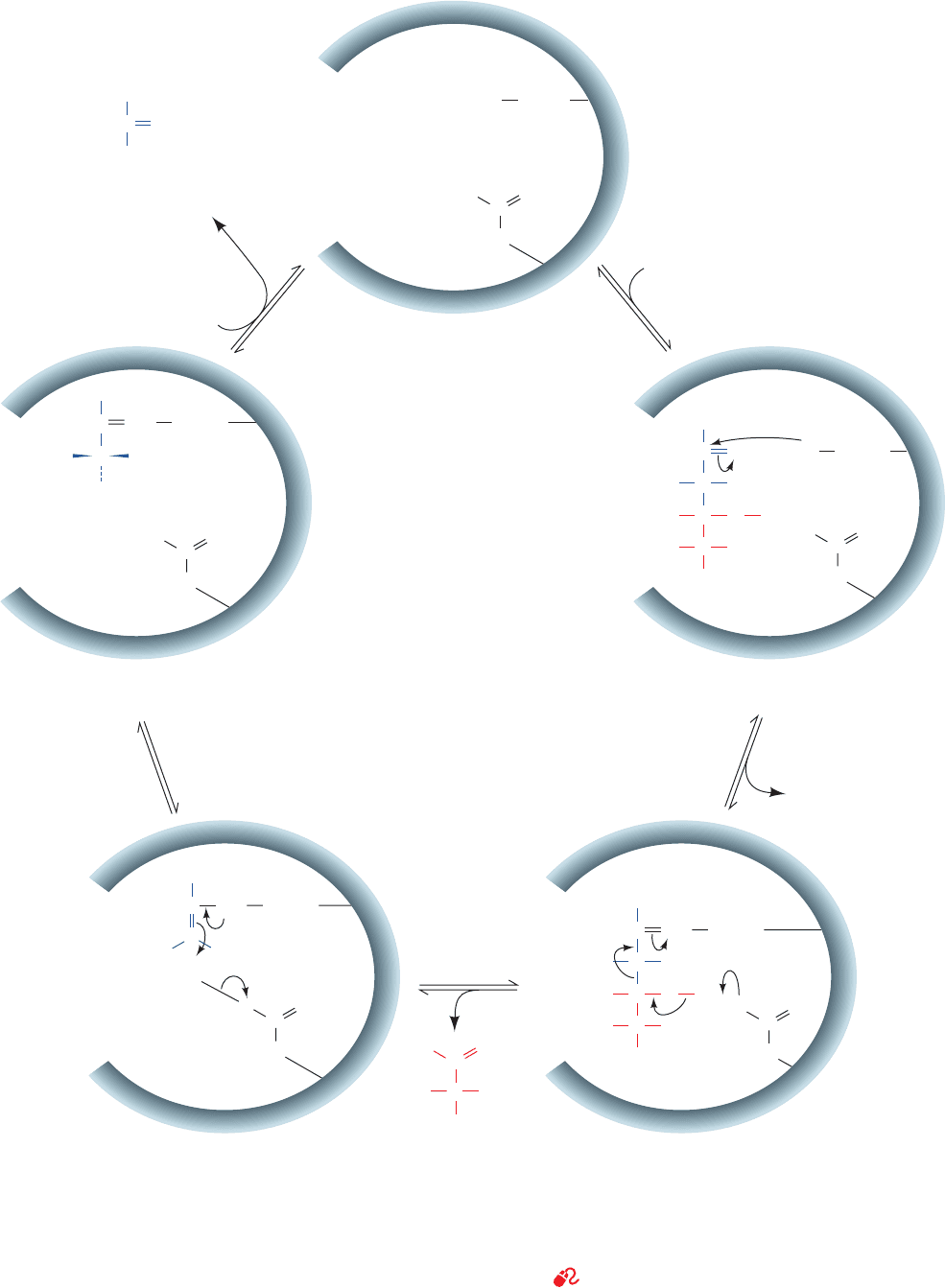
a. There Are Two Mechanistic Classes of Aldolases
Aldol cleavage is catalyzed by stabilizing its enolate in-
termediate through increased electron delocalization.
There are two types of aldolases that are classified accord-
ing to the chemistry they employ to stabilize the enolate. In
Class I aldolases, which occur in animals and plants, the re-
action occurs as follows (Fig. 17-9):
Step 1 Substrate binding.
Step 2 Reaction of the FBP carbonyl group with the
e-amino group of the active site Lys 229 to form an
iminium cation, that is, a protonated Schiff base.
Step 3 bond cleavage resulting in enamine for-
mation and the release of GAP. The iminium ion, as we saw
in Section 16-2E, is a better electron-withdrawing group
than is the oxygen atom of the precursor carbonyl group.
Thus, catalysis occurs because the enamine intermediate
(Fig. 17-9, Step 3) is more stable than the corresponding
enolate intermediate of the base-catalyzed aldol cleavage
reaction (Fig. 17-8, Step 2).
Step 4 Protonation of the enamine to an iminium cation.
Step 5 Hydrolysis of this iminium cation to release
DHAP, with regeneration of the free enzyme.
Proof for the formation of the Schiff base in Step 2 was
provided by “trapping”
14
C-labeled DHAP on the enzyme
by reacting it with NaBH
4
, which reduces imines to
amines:
The radioactive product was hydrolyzed and identified as
N
6
--glyceryl lysine.
CH
2
OPO
3
2
CH
2
OH
NaBH
4
reduction
(CH
2
)
4
NH Enzyme
P
i
hydrolysis
CH
2
OH
CH
2
OH
14
C
14
C
(CH
2
)
4
NH
H
CH
COO
NH
3
N
6
--Glyceryl lysine
C3¬O4
Cys and His residues were initially thought to act as the
acid and base catalysts that facilitate the proton transfers in
the aldolase reaction because the appropriate group-
specific reagents inactivate the enzyme by reacting with
these residues. For example, the reaction of a specific Cys
residue of aldolase with iodoacetic acid inactivates the en-
zyme and results in the buildup of the FBP observed in the
early glycolysis inhibition studies (Section 17-1A). How-
ever, site-directed mutagenesis to Ala of the Cys residue
thought to be involved in the catalytic activity results in no
loss of enzymatic function. Modification of this Cys residue
apparently prevents the conformational changes required
for productive substrate binding.
An early X-ray structure of aldolase suggested that a
Tyr side chain was positioned to act as the active site
acid–base catalyst and that the His was instead necessary
for the maintenance of the Tyr’s catalytically active orien-
tation. A re-examination of the X-ray data caused yet an-
other modification of the mechanism. The Tyr originally
seen at the active site has changed position in this new
analysis, so that it is out of reach of the active site. Asp 33
and Lys 229 now appear to be acting as acid–base catalysts.
These residues are evolutionarily conserved and their mu-
tagenesis eliminates enzyme activity. This is an excellent
example of the caution that must be exercised in the inter-
pretation of chemical modification and structural data, and
the power of site-directed mutagenesis in the study of en-
zyme mechanisms (although see Section 15-3Ba).
Class II aldolases, which occur in fungi, algae, and some
bacteria, do not form a Schiff base with the substrate.
Rather, a divalent cation, usually Zn
2
or Fe
2
, polarizes
the carbonyl oxygen of the substrate to stabilize the eno-
late intermediate of the reaction (Fig. 16-12d):
Section 17-2. The Reactions of Glycolysis 601
JWCL281_c17_593-637.qxd 6/30/10 10:53 AM Page 601
602 Chapter 17. Glycolysis
C
O
O
CH
2
–
C
O
O
CH
2
–
C
O
O
CH
2
C
O
O
CH
2
–
C
O
O
CH
2
–
CH
2
OPO
3
2
–
(CH
2
)
4
OCH
CH
Fructose-1,6-bisphosphate
O
C
H
H
C
HO
OH
H
2
N
..
Lys 229
(CH
2
)
4
OCH
CH
C
H
H
C
HO
OH
NH
. . .
+
substrate
binding
(CH
2
)
4
Enamine
intermediate
C
H
R
C
HO
NH
..
. . .
H
C
C
H OH
OH
Glyceraldehyde-
3-phosphate
(product 1)
(CH
2
)
4
Enzyme –product
protonated Schiff base
C
H
pro–R
C
HO
NH
H
pro–S
(CH
2
)
4
Dihydroxyacetone
phosphate
(product 2)
C
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
NH
2
–
. . .
–
–
+
1
2
3
4
Schiff base
hydrolysis
H
2
O
CH
2
OH
O
Free enzyme
Asp 33
Enzyme –substrate
complex
protonated Schiff
base formation
H
2
O
Enzyme–substrate
protonated Schiff base
5
tautomerization
+
protonation
aldol cleavage
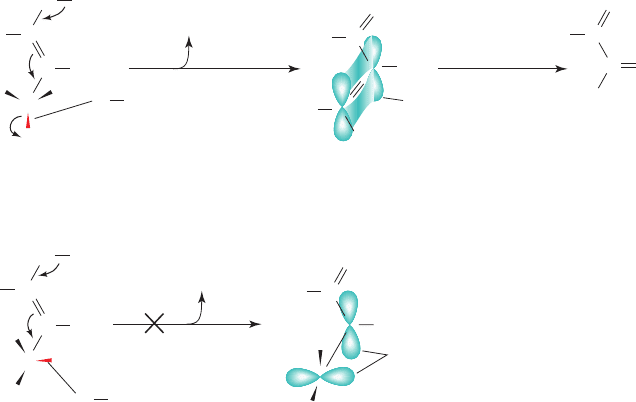
Figure 17-9 Enzymatic mechanism of Class I aldolase. The
reaction involves (1) substrate binding; (2) Schiff base formation
between the enzyme’s active site Lys residue and FBP; (3) aldol
cleavage to form an enamine intermediate of the enzyme and
DHAP with release of GAP (shown with its re face up);
(4) tautomerization and protonation to the iminium form of the
Schiff base; and (5) hydrolysis of the Schiff base with release of
DHAP.
See the Animated Figures.
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 602
adept at carrying out their metabolic functions. Thus, the
expression of both classes of aldolase in some organisms
probably represents an ancient metabolic redundancy that
evolution has eliminated in most contemporary organisms.
Whatever the reason for the occurrence of two classes of
aldolase, the fact that Class II aldolases do not occur in
mammals makes them an attractive target in the develop-
ment of antibacterial drugs.
c. Aldolase Is Stereospecific
The aldolase reaction provides another example of the
extraordinary stereospecificity of enzymes. In the nonenzy-
matic aldol condensation to form hexose-1,6-bisphosphate
from DHAP and GAP, there are four possible products de-
pending on whether the pro-R or pro-S hydrogen at C3 of
DHAP is removed and whether the resulting carbanion
attacks GAP on its re or its si face:
In the enzymatic aldol condensation (Fig. 17-9 in reverse),
carbanion formation from the enzyme–DHAP iminium ion
(Fig.17-9, Step 4 in reverse) occurs with removal of only the
pro-S hydrogen. Attack of this carbanion occurs only on
the si face of the enzyme-bound GAP carbonyl group, so
that only FBP is formed (Fig. 17-9, Step 3 in reverse).
E. Triose Phosphate Isomerase
Only one of the products of the aldol cleavage reaction,
GAP, continues along the glycolytic pathway (Fig. 17-3).
However, DHAP and GAP are ketose–aldose isomers just
as are F6P and G6P. Interconversion of GAP and DHAP
therefore probably occurs via an enediol or enediolate in-
termediate in analogy with the phosphoglucose isomerase
reaction (Fig. 17-6). Triose phosphate isomerase (TIM or
CH
2
OPO
3
2
CH
2
OPO
3
2
CO
C
C
CH
HO H
HOH
OH
CH
2
OPO
3
2
CH
2
OPO
3
2
CO
C
C
CH
H
HOH
OH
OH
D-Fructose
1,6-bisphosphate
D-Psicose
1,6-bisphosphate
CH
2
OPO
3
2
CH
2
OPO
3
2
CO
C
C
CH
HO
HO H
H
OH
CH
2
OPO
3
2
CH
2
OPO
3
2
CO
C
C
CH
H
HO H
OH
OH
D-Tagatose
1,6-bisphosphate
D-Sorbose
1,6-bisphosphate
TPI; Figs. 8-19b and 8-52) catalyzes this process in Reac-
tion 5 of glycolysis, the final reaction of Stage I:
Support for this reaction scheme comes from the use of
the transition state analogs phosphoglycohydroxamate and
2-phosphoglycolate, stable compounds whose geometric
structures resemble that of the proposed enediol or enedi-
olate intermediate:
Since enzymes catalyze reactions by binding the transition
state complex more tightly than the substrate (Section
15-1F), phosphoglycohydroxamate and 2-phosphoglycolate
should bind more tightly to TIM than does substrate. In
fact, phosphoglycohydroxamate and 2-phosphoglycolate
bind 155- and 100-fold more tightly to TIM than do either
GAP or DHAP.
a. Glu 165 Functions as a General Base
The pH dependence of the TIM reaction is a bell-
shaped curve with pK’s of 6.5 and 9.5. The similarity of
these pK’s to the corresponding quantities of the phospho-
glucose isomerase reaction suggests the participation of
both an acid and a base in the TIM reaction as well. How-
ever, pH studies alone, as we have seen, are difficult to
C
N
O
–
Phosphoglyco-
hydroxamate
OH
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
C
O
O
–
2-Phosphoglycolate
C
CH
O
–
Proposed enediolate
intermediate
OH
C
CO
1
2
3
1
2
3
OH
C
Glyceraldehyde-
3-phosphate
(an aldose)
Enediol
intermediate
Dihydroxyacetone
phosphate
(a ketose)
OHH
C
C
H
H
COH
OH
OHH
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
Section 17-2. The Reactions of Glycolysis 603
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 603

interpret in terms of specific amino acid residues since the
active site environment may alter the pK of an acidic or ba-
sic group.
Affinity labeling reagents have been employed in an ef-
fort to identify the base at the active site of TIM. Both bro-
mohydroxyacetone phosphate and glycidol phosphate
inactivate TIM by forming esters of Glu 165, whose car-
boxylate group, X-ray studies indicate, is ideally situated to
abstract the C2 proton from the substrate (general base
catalysis). In fact, the mutagenic replacement of Glu 165 by
Asp, which X-ray studies show withdraws the carboxylate
group only ⬃1 Å farther away from the substrate than its
position in the wild-type enzyme, reduces TIM’s catalytic
power ⬃1000-fold. Note that Glu 165’s pK is drastically al-
tered from the 4.1 value of the free amino acid to the ob-
served 6.5 value. This provides yet another striking exam-
ple of the effect of environment on the properties of amino
acid side chains.
b. The TIM Reaction Probably Occurs via Concerted
General Acid–Base Catalysis Involving Low-Barrier
Hydrogen Bonds
The X-ray structure of yeast TIM in complex with
phosphoglycohydroxamate indicates that His 95 is hydro-
gen bonded to and hence is properly positioned to proto-
nate the carbonyl oxygen atom of GAP (general acid
catalysis):
However, NMR studies indicate that His 95 is in its neutral
imidazole form rather than its protonated imidazolium
form. How can an imidazole group, which has a
highly basic pK of ⬃14, protonate a carbonyl oxygen atom
N3¬H
Glu 165
Asn 10
His 95
Ser 96
Glu 97
H
H
H
H
H
H
H
N
H
O
O
O
O
O
O
N
N
H
H
H
H
H
N
N
N
O
Lys 12
O
O
O
O
O
P
2
CH
2
Br
CH
2
OPO
3
2
CO
H
2
C
CH
2
OPO
3
2
CH
O
Bromohydroxyacetone
phosphate
Glycidol phosphate
that, when protonated, has a very acidic pK of 0? Like-
wise, how can the Glu 165 carboxylate group (pK 6.5) ab-
stract the C2 proton from GAP (pK ⬃17)? A plausible an-
swer is that these proton shifts are facilitated by the
formation of low-barrier hydrogen bonds (LBHBs). These
unusually strong associations (40 to 80 kJ ⴢ mol
1
vs
12 to 30 kJ ⴢ mol
1
for normal hydrogen bonds), as we
have seen in the case of the serine protease catalytic triad
(Section 15-3Dd), form when the pK’s of the hydrogen
bonding donor and acceptor groups are nearly equal.They
can be important contributors to rate enhancement if they
form only in the transition state of an enzymatically cat-
alyzed reaction.
In converting GAP to the enediol (or enediolate) inter-
mediate (Fig. 17-10, left), the pK of the protonated form of
its carbonyl oxygen, which becomes a hydroxyl group, in-
creases to ⬃14, which closely matches that of neutral His
95. The resulting LBHB between this hydroxyl group and
His 95 permits the neutral imidazole side chain to proto-
nate the oxygen atom. Likewise, as the carbonyl oxygen is
protonated, the pK of the proton decreases to ⬃7,
close to the pK of the Glu 165 carboxylate. It therefore ap-
pears that the reaction occurs via simultaneous proton ab-
straction by Glu 165 and protonation by His 95 (concerted
general acid–base catalysis). The LBHBs postulated to
form in the transition state, but not in the Michaelis com-
plex, between Glu 165 and C2¬H and between His 95 and
the carbonyl oxygen atom are thought to provide some of
the transition state stabilization necessary to catalyze the
reaction.The positively charged side chain of Lys 12, which
is probably responsible for the 9.5 pK observed in TIM’s
pH rate profile, is thought to electrostatically stabilize the
negatively charged transition state. The conversion of the
enediol(ate) intermediate to DHAP is likewise facilitated
by the formation of transition state LBHBs (Fig. 17-10,
right). In fact, in the high resolution (1.2 Å) X-ray structure
of TIM in complex with DHAP, determined by Ann
McDermott and Liang Tong, the hydrogen bond between
His 95 and O2 of DHAP has an unusually short distance of
2.6 Å. In addition, a carboxylate oxygen atom of Glu 165
forms extraordinarily close contacts of ⬃3.0 Å with both
C1 and C2 of DHAP.
c. A Flexible Loop Both Preferentially Binds
and Protects the Enediol Intermediate
The comparison of the X-ray structure of the TIM ⴢ
phosphoglycohydroxamate complex with that of TIM
alone reveals that a 10-residue loop, which is closed over
the active site in the enzyme–substrate complex, is flipped
up in the unoccupied active site like a hinged lid, a move-
ment that involves main chain shifts of 7 Å (Fig. 17-11).A
four-residue segment of this loop makes a hydrogen bond
with the phosphate group of the substrate. Mutagenic exci-
sion of these four residues neither significantly distorts the
protein nor greatly impairs substrate binding.The catalytic
power of the mutant enzyme is, nevertheless, reduced 10
5
-
fold and it only weakly binds phosphoglycohydroxamate.
Evidently, the closed loop preferentially stabilizes the en-
zymatic reaction’s enediol-like transition state.
C2¬H
604 Chapter 17. Glycolysis
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 604
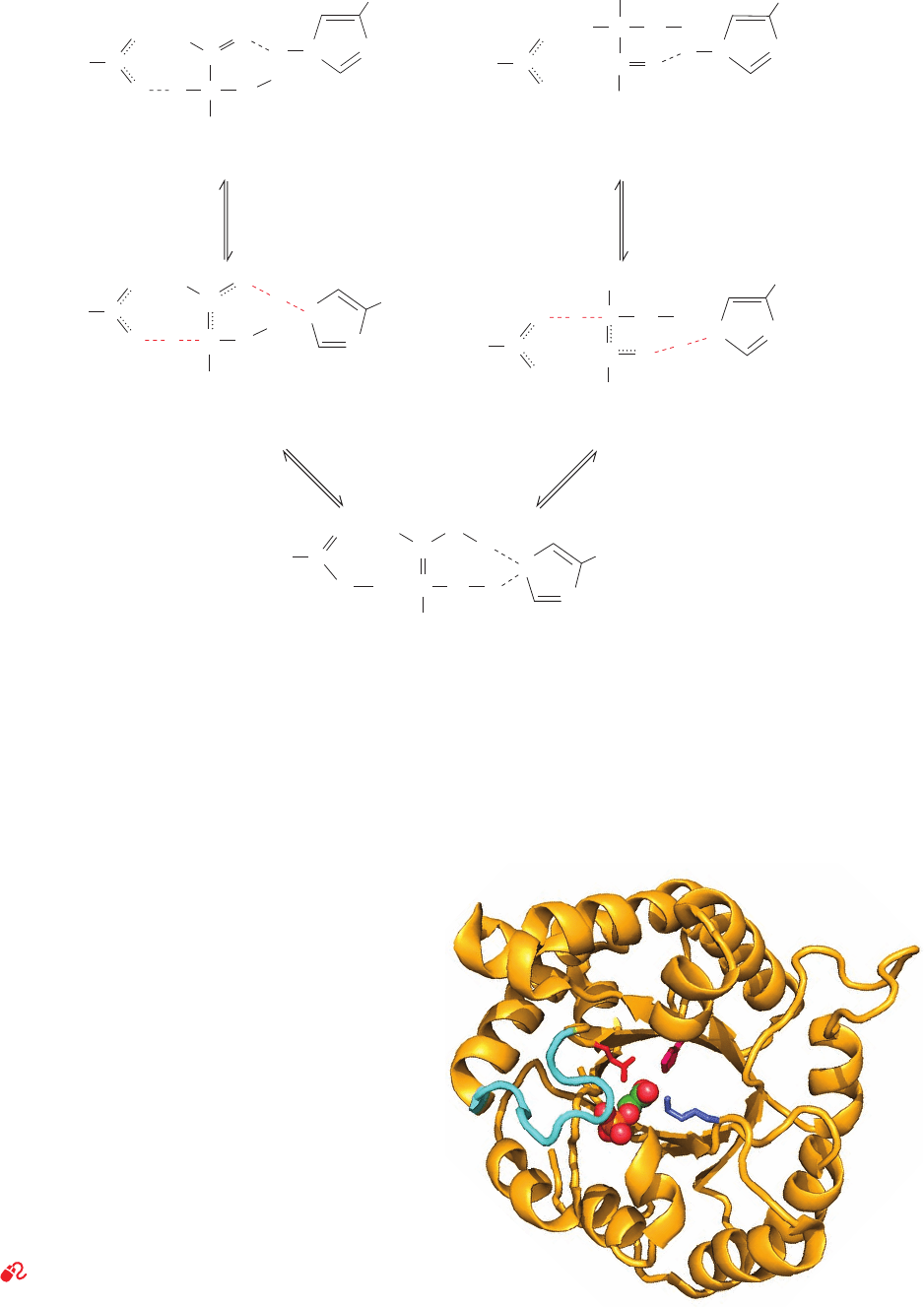
Section 17-2. The Reactions of Glycolysis 605
Figure 17-10 Proposed enzymatic mechanism of the TIM
reaction. The reaction proceeds via the concerted abstraction of
the proton of GAP by the carboxylate group of Glu 165
and the protonation of the GAP carbonyl oxygen atom by the
imidazole group of His 95.The pK’s of the corresponding donor
and acceptor groups participating in each proton transfer process
become nearly equal in the transition state and hence are
C2¬H
Figure 17-11 Ribbon diagram of yeast TIM in complex with its
transition state analog 2-phosphoglycolate. A single 248-residue
subunit of this homodimeric enzyme is viewed roughly along the
axis of its ␣/ barrel.The enzyme’s flexible loop, residues 168
through 177, is cyan and the side chains of Lys 12, His 95, and
Glu 165 are purple, magenta, and red, respectively. The
2-phosphoglycolate is shown in space-filling form colored
according to atom type (C green, O red, P yellow). [Based on an
X-ray structure by Gregory Petsko, Brandeis University. PDBid
2YPI.]
See Interactive Exercise 2 and Kinemage Exercises
12-1 and 12-2
CH
2
OPO
3
2
⫺
GAP
•
TIM Michaelis complex
DHAP
•
TIM Michaelis complex
Transition state
Transition state
C
⫺
O
O
H
Glu
165
C
C
O
O
C
H
H
H
N
N
His
95
C
C
O
CH
2
OPO
3
2
⫺
C
O
O
H
Glu
165
O
H
H
H
N
N
His
195
1
2
3
CH
2
OPO
3
2
⫺
C
⫺
O
O
Glu
165
C
C
O
H
O
H
H
H
NN
His
95
1
2
3
CH
2
OPO
3
2
⫺
C
⫺
O
O
Glu
165
C
O
H
O
H
H
H
NN
His
95
Enediol (or enendiolate) intermediate
⫺
CH
2
OPO
3
2
⫺
C
⫺
O
O
H
Glu
165
C
C
O
O
H
H
H
N
N
His
95
3
proposed to form low-barrier hydrogen bonds (red dashed lines),
which act to stabilize the transition state. The resulting enediol
(or possibly the electrostatically stabilized enediolate) intermedi-
ate then reacts in a similar fashion with the carboxyl group of
Glu 165 protonating C1, while the deprotonated N3 atom of His
95 abstracts the proton on the 2-hydroxyl group to yield DHAP.
JWCL281_c17_593-637.qxd 10/19/10 7:26 AM Page 605
The closed loop conformation in the TIM reaction pro-
vides a striking example of the stereoelectronic control that
enzymes can exert on a reaction (Section 15-1Eb). In solu-
tion, the enediol intermediate readily breaks down with the
elimination of the phosphate at C3 to form the toxic com-
pound methylglyoxal (Fig. 17-12a). On the enzyme’s surface,
however, this reaction is prevented because the phosphate
group is held by the flexible loop in the plane of the enediol,
a position that disfavors phosphate elimination. In order for
this elimination to occur, the bond to the phosphate
group must lie, as shown in Fig. 17-12a, in the plane perpen-
dicular to that of the enediol. This is because, if the phos-
phate group were to be eliminated while this bond
was in the plane of the enediol as diagrammed in Fig. 17-
12b, the CH
2
group of the resulting enol product would be
twisted 90° out of the plane of the rest of the molecule. Such
a conformation is energetically prohibitive because it pre-
vents the formation of the enol’s double bond by eliminat-
ing the overlap between its component p orbitals. In the
mutant enzyme lacking the flexible loop, the enediol is able
to escape: ⬃85% of the enediol intermediate is released
into solution, where it rapidly decomposes to methylglyoxal
and P
i
.Thus, flexible loop closure also ensures that substrate
is efficiently transformed to product.
On the basis of the foregoing X-ray structures, it had
been widely assumed that the binding of substrate to TIM is
ligand-gated, that is, it induces loop closure. Yet, if this were
the case, the reversibility of the TIM reaction and the chem-
ical resemblance of its reactant and product (GAP and
DHAP) make it difficult to rationalize how product could
be released. However, NMR measurements by John
Williams and McDermott reveal that, in fact, loop motion
still occurs when TIM is binding either glycerol-3-phosphate
(a substrate analog) or 2-phosphoglycolate (a transition
state analog) and is sufficiently fast (on a time scale of 100
s) to account for the catalytic reaction rate (a turnover
C¬O
C¬O
time of 230 s).This is a clear example of how complemen-
tary information supplied by X-ray and NMR methods has
yielded important insights into an enzymatic mechanism
that neither technique alone could have provided.
d. TIM Is a Perfect Enzyme
TIM, as Jeremy Knowles demonstrated, has achieved
catalytic perfection in that the rate of bimolecular reaction
between enzyme and substrate is diffusion controlled; that
is, product formation occurs as rapidly as enzyme and sub-
strate can collide in solution, so that any increase in TIM’s
catalytic efficiency would not increase the reaction rate
(Section 14-2Bb). Because of the high interconversion effi-
ciency of GAP and DHAP, these two metabolites are
maintained in equilibrium: K ⫽ [GAP]/[DHAP] ⫽ 4.73 ⫻
10
⫺2
; that is, [DHAP] ⬎⬎ [GAP] at equilibrium. However,
as GAP is utilized in the succeeding reaction of the gly-
colytic pathway, more DHAP is converted to GAP, so that
these compounds maintain their equilibrium ratio. One
common pathway therefore accounts for the metabolism
of both products of the aldolase reaction.
Let us now take stock of where we are in our travels
down the glycolytic pathway. At this stage, the glucose,
which has been transformed into two GAPs, has com-
pleted the preparatory stage of glycolysis. This process has
required the expenditure of two ATPs. However, this in-
vestment has resulted in the conversion of one glucose to
two C
3
units, each of which has a phosphoryl group that,
with a little chemical artistry, can be converted to a “high-
energy” compound (Section 16-4Ba) whose free energy of
hydrolysis can be coupled to ATP synthesis. This energy
investment is doubly repaid in the final stage of glycolysis in
which the two phosphorylated C
3
units are transformed to
two pyruvates with the coupled synthesis of four ATPs per
glucose.
606 Chapter 17. Glycolysis
Figure 17-12 The spontaneous
decomposition of the enediol
intermediate in the TIM reaction to
form methylglyoxal through the
elimination of a phosphate group. (a)
This reaction can occur only when the
bond to the phosphate group lies
in a plane that is nearly perpendicular
to that of the enediol so as to permit
the formation of a double bond in the
intermediate enol product. (b) When
the bond to the phosphate group
lies in a plane that is nearly parallel to
that of the enediol, the p orbitals on the
resulting intermediate product would
be perpendicular to each other and
hence lack the overlap necessary to
form a bond, that is, a double bond.
The resulting unsatisfied bonding
capacity greatly increases the energy of
the reaction intermediate and hence
makes the reaction highly unfavorable.
C¬O
C¬O
H
HH
O
Parallel p orbitals
maximally
overlapped to
form a π bond
Perpendicular p orbitals
have no overlap; a π
bond cannot be formed
O bond to phosphate
in plane perpendicular
to plane of molecule
O bond to phosphate
in plane of molecule
Enol Methyl
glyoxal
Enediol
intermediate
(a)
(b)
P
i
P
i
CH
OHC
C
C
OPO
3
2⫺
O
CH
CH
H
OH
C
O
CH
O
C
H
3
C
H
H
H
O
CH
OHC
C
C
OPO
3
2⫺
O
CH
H
H
OHC
C
JWCL281_c17_593-637.qxd 6/30/10 10:53 AM Page 606
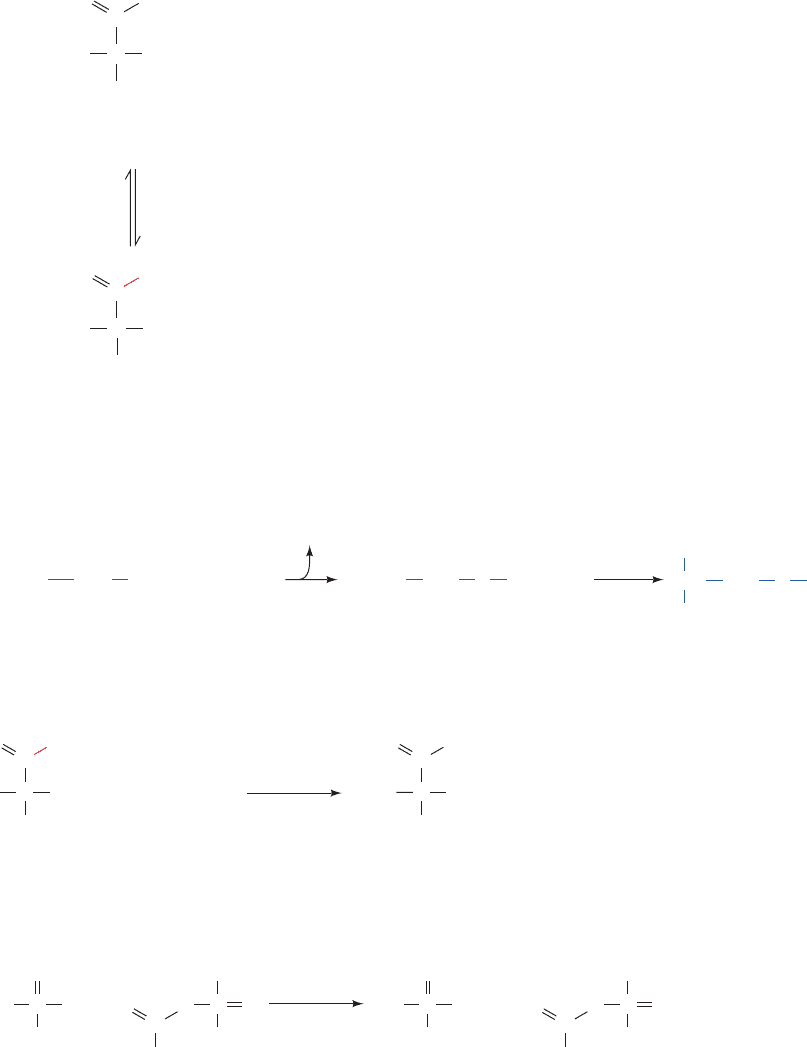
F. Glyceraldehyde-3-Phosphate Dehydrogenase:
First “High-Energy” Intermediate Formation
Reaction 6 of glycolysis involves the oxidation and phos-
phorylation of GAP by NAD
⫹
and P
i
as catalyzed by
glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
Figs. 8-45 and 8-53b):
1
C
O
H
2
C
3
CH
2
OPO
2
3
–
HOH
+ NAD
+
+ P
i
Glyceraldehyde-
3-phosphate (GAP)
1
C
O
OPO
2
3
–
2
C
3
CH
2
OPO
2
3
–
HOH
+ NADH
+
H
+
1,3-Bisphosphoglycerate
(1,3-BPG)
glyceraldehyde-3-phosphate
dehydrogenase (GAPDH)
This is the first instance of the chemical artistry alluded
to above. In this reaction, aldehyde oxidation, an exer-
gonic reaction, drives the synthesis of the acyl phosphate
1,3-bisphosphoglycerate (1,3-BPG; previously called
1,3-diphosphoglycerate). Recall that acyl phosphates are
compounds with high phosphate group-transfer potential
(Section 16-4Ba).
a. Mechanistic Studies
Several key enzymological experiments have con-
tributed to the elucidation of the GAPDH reaction mech-
anism (Fig. 17-13):
1. GAPDH is inactivated by alkylation with stoichio-
metric amounts of iodoacetate. The presence of car-
boxymethylcysteine in the hydrolysate of the resulting
alkylated enzyme (Fig. 17-13a) suggests that GAPDH has
an active site Cys sulfhydryl group.
2. GAPDH quantitatively transfers
3
H from C1 of
GAP to NAD
⫹
(Fig. 17-13b), thereby establishing that this
reaction occurs via direct hydride transfer.
3. GAPDH catalyzes exchange of
32
P between [
32
P]P
i
and the product analog acetyl phosphate (Fig. 17-13c).
Such isotope exchange reactions are indicative of an
acyl–enzyme intermediate (Section 14-5D).
Section 17-2. The Reactions of Glycolysis 607
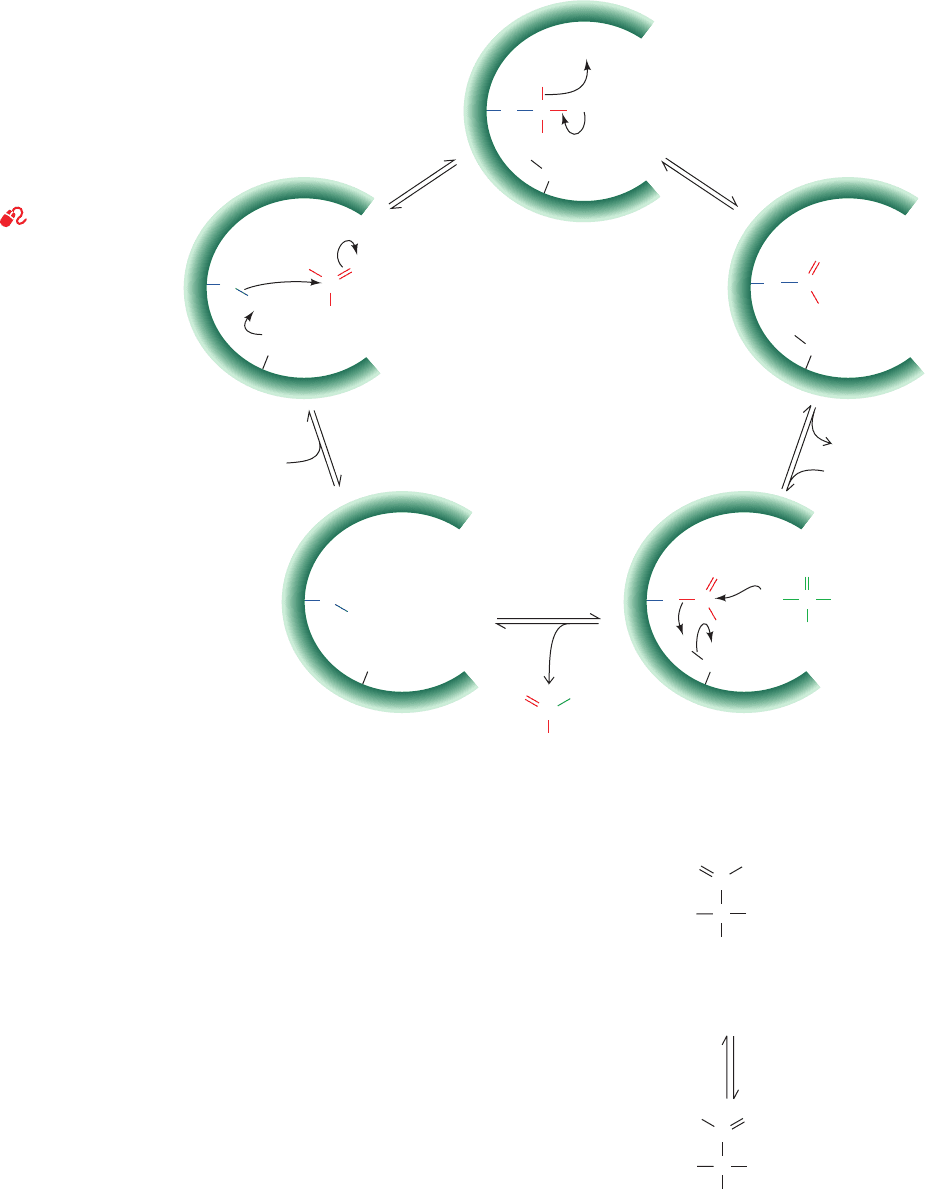
Figure 17-13 Some reactions employed in elucidating the
enzymatic mechanism of GAPDH. (a) The reaction of
iodoacetate with an active site Cys residue. (b) Quantitative
1,3-Bisphosphoglycerate
(1,3-BPG)
C
OH
3
H
CH
O
CH
2
SH
(a)
Enzyme
+ ICH
2
COO
–
CH
2
S
Enzyme
CH
2
COO
–
CH
2
COO
–
HI
GAPDH
Active site
Cys
Iodoacetate
protein
hydrolysis
CH
COO
–
NH
3
+
CH
2
S +
Other
amino
acids
Carboxy-
methylcysteine
(b)
+ NAD
+
[1-
3
H]GAP
C
OHCH
O
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
GAPDH
+ NAD
3
H
(c)
PHO
O
O
–
O
–
+
C
OO
P
O
–
O
–
O
CH
3
GAPDH
PHO
O
O
–
O
–
+
C
OO
P
O
–
O
–
O
CH
3
32 32
Acetyl phosphate
+ P
i
OPO
3
2
–
tritium transfer from substrate to NAD
⫹
.(c) The enzyme-
catalyzed exchange of
32
P from phosphate to acetyl phosphate.
JWCL281_c17_593-637.qxd 6/3/10 8:16 AM Page 607
David Trentham has proposed a mechanism for GAPDH
based on this information and the results of kinetic studies
(Fig. 17-14):
Step 1 GAP binds to the enzyme.
Step 2 The essential sulfhydryl group, acting as a nucle-
ophile, attacks the aldehyde to form a thiohemiacetal.
Step 3 The thiohemiacetal undergoes oxidation to an
acyl thioester by direct transfer of a hydride to NAD
.This
intermediate, which has been isolated, has a high group-
transfer potential. The energy of aldehyde oxidation has not
been dissipated but has been conserved through the synthe-
sis of the thioester and the reduction of NAD
to NADH.
Step 4 Another molecule of NAD
replaces NADH.
Step 5 The thioester intermediate undergoes nucle-
ophilic attack by P
i
to regenerate free enzyme and form
1,3-BPG. This “high-energy” mixed anhydride generates
ATP from ADP in the next reaction of glycolysis.
G. Phosphoglycerate Kinase: First ATP Generation
Reaction 7 of the glycolytic pathway results in the first for-
mation of ATP together with 3-phosphoglycerate (3PG) in
a reaction catalyzed by phosphoglycerate kinase (PGK):
(Note: The name “kinase” is given to any enzyme that trans-
fers a phosphoryl group between ATP and a metabolite.
Nothing is implied as to the exergonic direction of transfer.)
C
OHCH
O
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
3
2
1
1,3-Bisphosphoglycerate
(1,3-BPG)
+ ADP
OPO
3
2
–
C
OHCH
O
3
2
1
3-Phosphoglycerate
(3PG)
+ ATP
–
O
phosphoglycerate
kinase (PGK)
Mg
2+
608 Chapter 17. Glycolysis
Figure 17-14 Enzymatic mechanism of glyceraldehyde-
3-phosphate dehydrogenase. (1) GAP binds to the enzyme;
(2) the active site sulfhydryl group forms a thiohemiacetal
with the substrate; (3) NAD
oxidizes the thiohemiacetal
to a thioester; (4) the newly formed NADH is replaced
on the enzyme by NAD
; and (5) P
i
attacks the
thioester, forming the acyl phosphate
product, 1,3-BPG, and
regenerating the
active enzyme.
See the
Animated Figures
H
S
NAD
+
...
Thiohemiacetal
intermediate
R
+
C
H
O
–
1
Acyl thioester
intermediate
R
+
C
O
2
NADH
NAD
+
P
i
3
4
C
O
R
OPO
3
2
–
B
C
H
R
O
H
S
NAD
+
...
B
..
Enzyme–substrate
complex
S
NADH
...
B
H
R
C
O
P
–
O
O
O
–
OH
S
NAD
+
...
+
B
H
H
S
NAD
+
...
B
..
GAP
5
JWCL281_c17_593-637.qxd 6/3/10 8:16 AM Page 608
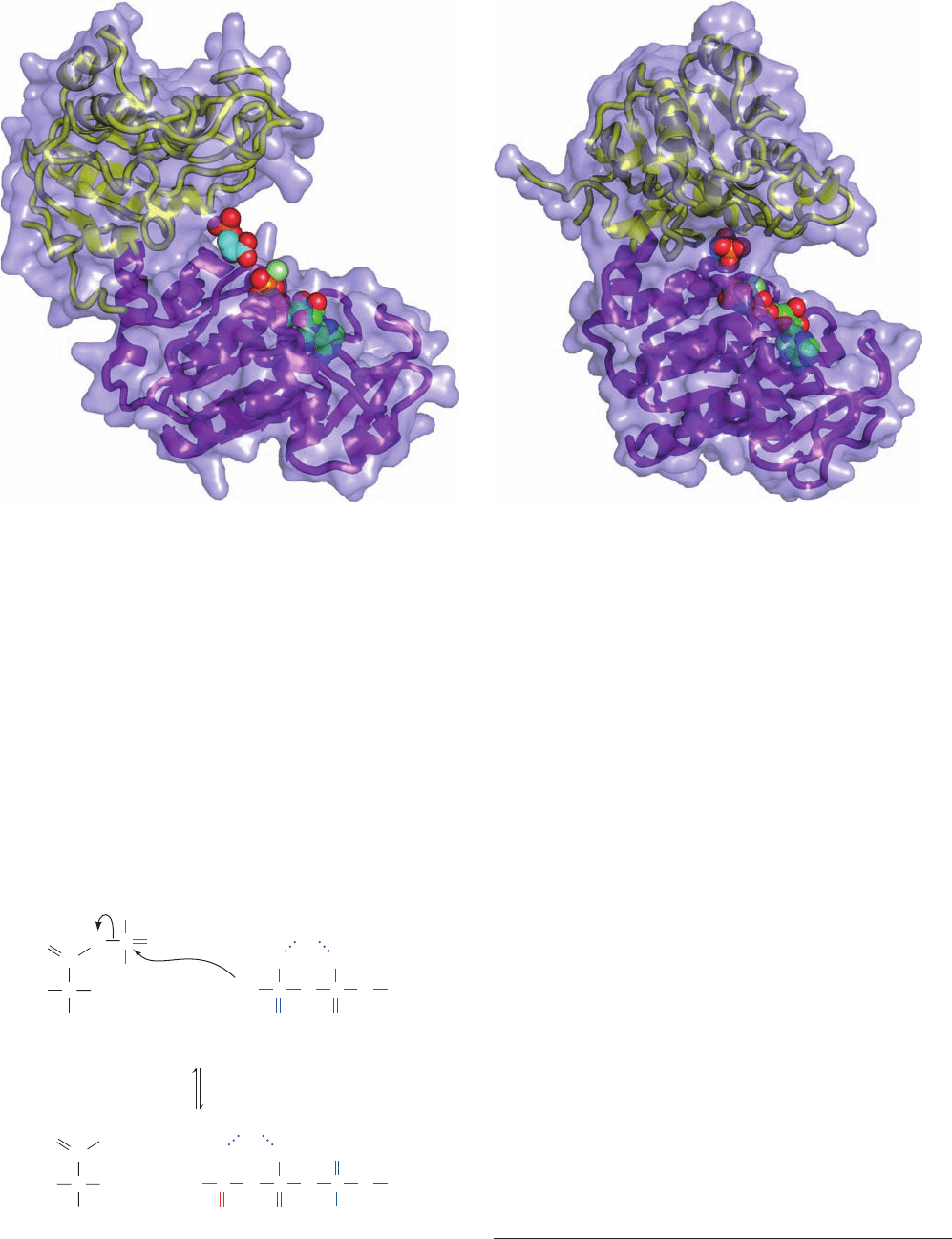
The X-ray structure of yeast PGK in complex with
Mg
2⫹
–ATP and 3PG, its enzyme–product complex, was de-
termined by Herman Watson (Fig. 17-15a). Note its con-
spicuously bilobal appearance and the close approach be-
tween the ATP’s ␥-phosphate group and one of the 3PG’s
carboxylate oxygen atoms. The 3PG and the Mg–ATP are
respectively bound to PGK’s N- and C-terminal domains.
The X-ray structure of PGK from the thermophile Ther-
motoga maritima in complex with 3PG and the nonhy-
drolyzable ATP analog AMPPNP (ATP with the O atom
bridging its  and ␥ phosphate groups replaced by an NH
group) reveals that its two domains have swung together
relative to those in the 50% identical yeast PGK (Fig. 17-
15b). This forms the catalytic site and permits the sub-
strates to react in a water-free environment, much as oc-
curs with hexokinase (Section 17-2Aa).
Figure 17-16 indicates a reaction mechanism for PGK
that is consistent with its observed sequential kinetics. The
terminal phosphoryl oxygen of ADP nucleophilically at-
tacks the C1 phosphorus atom of 1,3-BPG to form the re-
action product.
The energetics of the overall GAPDH–PGK reaction
pair are
¢G°¿ ⫽⫺12.1 kJ ⴢ mol
⫺1
GAP ⫹ P
i
⫹ NAD
⫹
⫹ ADP
¡
3PG ⫹ NADH ⫹ ATP
¢G°¿ ⫽⫺18.8 kJ ⴢ mol
⫺1
1,3-BPG ⫹ ADP
¡
3PG ⫹ ATP
¢G°¿ ⫽ ⫹6.7 kJ ⴢ mol
⫺1
GAP ⫹ P
i
⫹ NAD
⫹
¡
1,3-BPG ⫹ NADH
Section 17-2. The Reactions of Glycolysis 609
Figure 17-15 X-ray structures of phosphoglycerate kinase
(PGK). (a) The yeast enzyme in complex with 3PG and
Mg
2⫹
–ATP. (b) The T. maritima enzyme in complex with 3PG
and Mg
2⫹
–AMPPNP. In both structures, the enzyme is
represented by its transparent molecular surface with its
embedded ribbon diagram colored with its N-terminal domain
yellow and its C-terminal domain purple. The Mg
2⫹
–ATP,
Mg
2⫹
–AMPPNP, and 3PG are drawn in space-filling form
Figure 17-16 Mechanism of the PGK reaction.
1,3-Bisphosphoglycerate
3-Phosphoglycerate
Mg
2ⴙ
–ADP
Mg
2⫹
Mg
2ⴙ
–ATP
⫹
⫹
OOOP
O
⫺
O
⫺
C
CHOH
CH
2
OPO
3
2⫺
⫺
OOP
O
⫺
OO
O
⫺
OP Adenosine
O
C
CHOH
CH
2
OPO
3
2⫺
Mg
2⫹
⫺
O OP
O
⫺
O O
O
⫺
OP
O
⫺
Adenosine
O
O
⫺
OP
(a)
(b)
colored according to atom type (ATP and AMPPNP C green,
3PG C cyan, N blue, O red, P orange, and Mg
2⫹
pale green). Note
the similar overall appearance of PGK and hexokinase (Fig.
17-5), although these proteins unrelated. [Based on X-ray
structures by Herman Watson, University of Bristol, U.K.; and
Günter Auerbach and Robert Huber, Max-Planck-Institut für
Biochemie, Martinsreid, Germany. PDBids 3PGK and 1VPE.]
JWCL281_c17_593-637.qxd 6/3/10 8:36 AM Page 609
Although the GAPDH reaction is endergonic, the
strongly exergonic nature of the transfer of a phosphoryl
group from 1,3-BPG to ADP makes the overall synthesis
of NADH and ATP from GAP, P
i
,NAD
⫹
, and ADP
favorable.
H. Phosphoglycerate Mutase
In Reaction 8 of glycolysis, 3PG is converted to 2-phospho-
glycerate (2PG) by phosphoglycerate mutase (PGM):
A mutase catalyzes the transfer of a functional group from
one position to another on a molecule. This reaction is a
necessary preparation for the next reaction in glycolysis,
which generates a “high-energy” phosphoryl compound for
use in ATP synthesis.
C
1
O
O
–
C
2
C
3
HOH
3-Phosphoglycerate
(3PG)
OPO
2
3
–
H
H
C
1
O O
–
C
2
C
3
H
OH
2-Phosphoglycerate
(2PG)
OPO
2
3
–
H
H
phosphoglycerate
mutase (PGM)
a. Reaction Mechanism of PGM
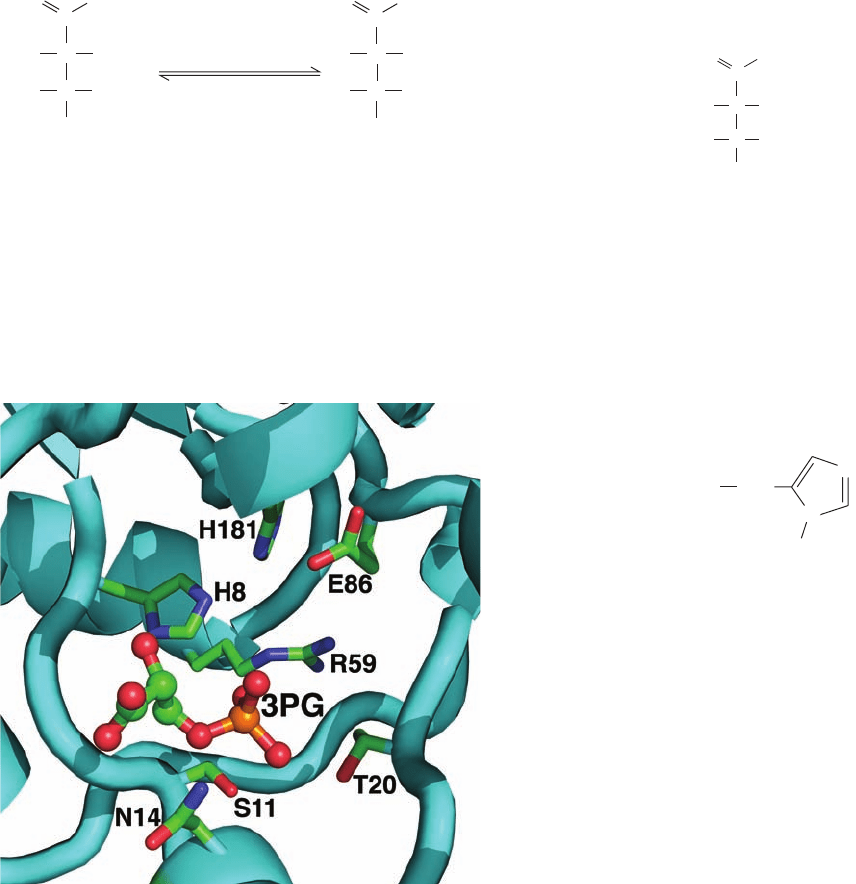
At first sight, the reaction catalyzed by PGM appears to
be a simple intramolecular phosphoryl transfer.This is not
the case, however. The active enzyme has a phosphoryl
group at its active site, which it transfers to the substrate to
form a bisphospho intermediate. This intermediate then
rephosphorylates the enzyme to form the product and re-
generate the active phosphoenzyme. The following experi-
mental data permitted the elucidation of PGM’s enzymatic
mechanism:
1. Catalytic amounts of 2,3-bisphosphoglycerate (2,3-
BPG; previously known as 2,3-diphosphoglycerate)
are required for enzymatic activity; that is, 2,3-BPG acts as
a reaction primer.
2. Incubation of the enzyme with catalytic amounts of
32
P-labeled 2,3-BPG yields a
32
P-labeled enzyme. Zelda
Rose demonstrated that this was a result of the phosphoryl-
ation of a His residue:
3. The enzyme’s X-ray structure shows His at the active
site (Fig. 17-17). In the active enzyme, His 8 is phosphoryl-
ated.
These data are consistent with a mechanism in which
the active enzyme contains a phospho-His residue at the
active site (Fig. 17-18):
Step 1 3PG binds to the phosphoenzyme in which His 8
is phosphorylated.
Step 2 This phosphoryl group is transferred to the sub-
strate, resulting in an intermediate 2,3-BPG ⴢ enzyme
complex.
Steps 3 and 4 The complex decomposes to form the
product 2PG with regeneration of the phosphoenzyme.
The phosphoryl group on 3PG therefore ends up on the C2
of the next 3PG to undergo reaction.
CH
2
Enzyme
N
N
PO
2
3
–
Phospho-His residue
C
2
C
3
H
HOPO
3
2⫺
C
1
OPO
3
2⫺
H
O
O
⫺
2,3-Bisphosphoglycerate
(2,3-BPG)
610 Chapter 17. Glycolysis
Figure 17-17 The active site region of yeast phosphoglycerate
mutase (dephospho form). The substrate, 3PG, which is drawn in
ball-and-stick form with C green, O red, and P orange, binds to
an ionic pocket whose side chains are drawn in stick form with C
green, N blue, and O red. His 8 is phosphorylated in the active
enzyme. [Based on an X-ray structure by Jennifer Littlechild,
University of Exeter, U.K. PDBid 1QHF.]
JWCL281_c17_593-637.qxd 6/10/10 1:06 PM Page 610
