Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

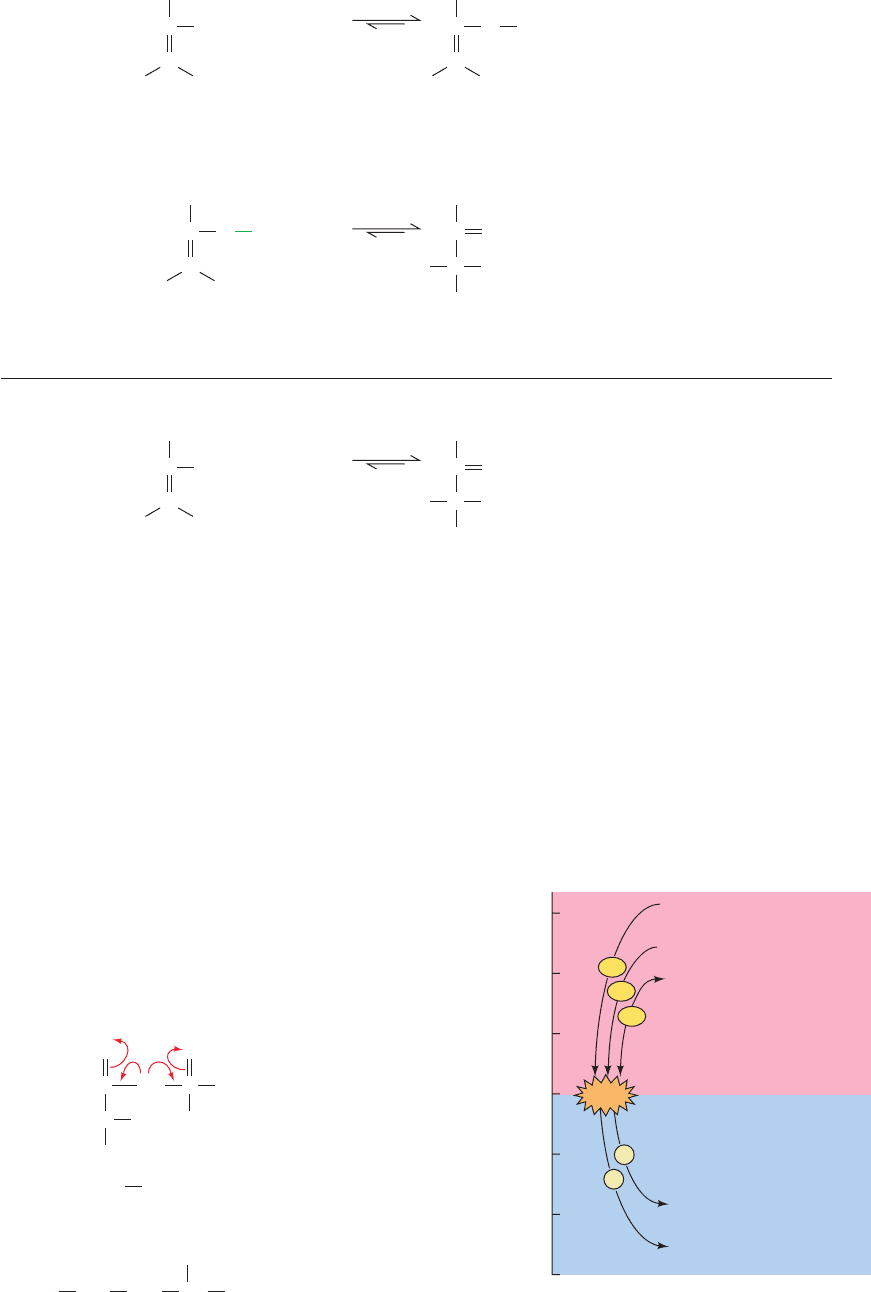
which are below ATP in Table 16-3, have no significantly
different resonance stabilization or charge separation in
comparison with their hydrolysis products. Their free ener-
gies of hydrolysis are therefore much less than those of the
preceding “high-energy” compounds.
C. The Role of ATP
As Table 16-3 indicates, in the thermodynamic hierarchy of
phosphoryl-transfer agents, ATP occupies the middle rank.
This enables ATP to serve as an energy conduit between
“high-energy” phosphate donors and “low-energy” phos-
phate acceptors (Fig. 16-27). Let us examine the general
biochemical scheme of how this occurs.
In general, the highly exergonic phosphoryl-transfer
reactions of nutrient degradation are coupled to the for-
mation of ATP from ADP and P
i
through the auspices of
various enzymes known as kinases, enzymes that catalyze
the transfer of phosphoryl groups between ATP and other
molecules. Consider the two reactions in Fig. 16-23b. If
carried out independently, these reactions would not influ-
ence each other. In the cell, however, the enzyme pyruvate
Section 16-4. Thermodynamics of Phosphate Compounds 581
Figure 16-25 Hydrolysis of phosphoenolpyruvate. The reaction is broken down into two steps,
hydrolysis and tautomerization.
Phosphoenol-
pyruvate
COO
–
C
O
+ H
2
OPO
2
3
–
C
HH
Hydrolysis
COO
–
C
O
H
H
+ HPO
2
4
–
C
HH
Pyruvate
(enol form)
Pyruvate
(keto form)
COO
–
C
O
C
HH
Tautomerization
COO
–
C
O
HC
H
H
COO
–
C
O
+
H
2
O
PO
2
3
–
C
HH
Overall reaction
+
HPO
2
4
–
COO
–
C
O
HC
H
H
⬃
⬃
ΔG° = –61.9 kJ
•
mol
–1
ΔG° = –46 kJ
•
mol
–1
ΔG° = –16 kJ
•
mol
–1
Phosphocreatine
N
R
CO
–
H
2
N
NH
NH
+
P
O
or
or
O
–
C
X
O
–
O
+
R = CH
2
X = CH
3
2
3
2
;
Phosphoarginine
CO
–
CHCH
2
CH
2
R = CH
2
X = H
;
Figure 16-26 Competing resonances in phosphoguanidines.
Figure 16-27 The flow of phosphoryl groups from
“high-energy” phosphate donors, via the ATP–ADP
system, to “low-energy” phosphate acceptors.
–60
–50
–40
–30
–20
–10
0
Phosphoenolpyruvate
1,3-Bisphosphoglycerate
Phosphocreatine
Glucose-6-phosphate
Glycerol-3-phosphate
“High-energy”
phosphate
compounds
“Low-energy”
phosphate
compounds
ΔG
°′
of hydrolysis (kJ • mol
–1
)
~P
~P
~P
P
P
ATP
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 581
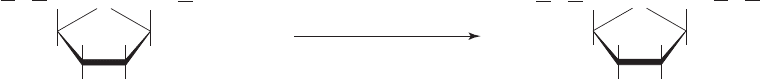
kinase couples the two reactions by catalyzing the trans-
fer of the phosphoryl group of phosphoenolpyruvate di-
rectly to ADP to result in an overall exergonic reaction
(Section 17-2J).
a. Consumption of ATP
In its role as the universal energy currency of living sys-
tems, ATP is consumed in a variety of ways:
1. Early stages of nutrient breakdown. The exergonic
hydrolysis of ATP to ADP may be enzymatically coupled
to an endergonic phosphorylation reaction to form “low-
energy” phosphate compounds.We have seen one example
of this in the hexokinase-catalyzed formation of glucose-
6-phosphate (Fig. 16-23a). Another example is the phos-
phofructokinase-catalyzed phosphorylation of fructose-6-
phosphate to form fructose-1,6-bisphosphate (Fig. 16-28).
Both of these reactions occur in the first stage of glycolysis
(Section 17-2).
2. Interconversion of nucleoside triphosphates. Many
biosynthetic processes, such as the synthesis of proteins
and nucleic acids, require nucleoside triphosphates other
than ATP. These include the ribonucleoside triphosphates
CTP, GTP, and UTP, which, together with ATP, are utilized,
for example, in the biosynthesis of RNA (Section 31-2) and
the deoxyribonucleoside triphosphate DNA precursors
dATP, dCTP, dGTP, and dTTP (Section 5-4C). All these
nucleoside triphosphates (NTPs) are synthesized from
ATP and the corresponding nucleoside diphosphate
(NDP) in reactions catalyzed by the nonspecific enzyme
nucleoside diphosphate kinase:
The G°¿ values for these reactions are nearly zero, as
might be expected from the structural similarities among
the NTPs. These reactions are driven by the depletion
of the NTPs through their exergonic hydrolysis in the biosyn-
thetic reactions in which they participate (Section 3-4C).
3. Physiological processes. The hydrolysis of ATP to
ADP and P
i
energizes many essential endergonic physio-
logical processes such as chaperone-assisted protein fold-
ing (Section 9-2C), muscle contraction (Section 35-3B),
and the transport of molecules and ions against concentra-
tion gradients (Section 20-3). In general, these processes
result from conformational changes in proteins (enzymes)
that occur in response to their binding of ATP. This is fol-
lowed by the exergonic hydrolysis of ATP and release of
ATP NDP Δ ADP NTP
ADP and P
i
, thereby causing these processes to be unidi-
rectional (irreversible).
4. Additional phosphoanhydride cleavage in highly
endergonic reactions. Although many reactions involving
ATP yield ADP and P
i
(orthophosphate cleavage), others
yield AMP and PP
i
(pyrophosphate cleavage). In these lat-
ter cases, the PP
i
is rapidly hydrolyzed to 2P
i
by inorganic
pyrophosphatase (G°¿ 19.2 kJ ⴢ mol
1
) so that the
pyrophosphate cleavage of ATP ultimately results in the
hydrolysis of two “high-energy” phosphoanhydride bonds.
The attachment of amino acids to tRNA molecules for pro-
tein synthesis is an example of this phenomenon (Fig. 16-29
and Section 32-2C).The two steps of the reaction involving
the amino acid are readily reversible because the free ener-
gies of hydrolysis of the bonds formed are comparable to
that of ATP hydrolysis. The overall reaction is driven to
completion by the hydrolysis of PP
i
, which is essentially ir-
reversible. Nucleic acid biosynthesis from the appropriate
NTPs also releases PP
i
(Sections 30-1A and 31-2).The free
energy changes of these vital reactions are around zero, so
the subsequent hydrolysis of PP
i
is essential to drive the
synthesis of nucleic acids.
b. Formation of ATP
To complete its intermediary metabolic function, ATP
must be replenished. This is accomplished through three
types of processes:
1. Substrate-level phosphorylation. ATP may be
formed, as is indicated in Fig. 16-23b, from phospho-
enolpyruvate by direct transfer of a phosphoryl group from
a “high-energy” compound to ADP. Such reactions, which
are referred to as substrate-level phosphorylations, most
commonly occur in the early stages of carbohydrate me-
tabolism (Section 17-2).
2. Oxidative phosphorylation and photophosphoryla-
tion. Both oxidative metabolism and photosynthesis act to
generate a proton (H
) concentration gradient across a
membrane (Sections 22-3 and 24-2D). Discharge of this
gradient is enzymatically coupled to the formation of ATP
from ADP and P
i
(the reverse of ATP hydrolysis). In oxida-
tive metabolism, this process is called oxidative phosphory-
lation, whereas in photosynthesis it is termed photophos-
phorylation. Most of the ATP produced by respiring and
photosynthesizing organisms is generated in this manner.
3. Adenylate kinase reaction. The AMP resulting from
pyrophosphate cleavage reactions of ATP is converted to
582 Chapter 16. Introduction to Metabolism
Figure 16-28 The phosphorylation of fructose-6-phosphate by ATP to form fructose-1,6-bisphosphate and ADP.
Fructose-6-phosphate
Fructose-1,6-bisphosphate
ATP ADP
2
O
3
PCH
2
CH
2
O
O
OH
H
OH
HO
H
H
HO
PO
3
2
phosphofructokinase
G = –14.2 kJ •mol
–1
2
O
3
PCH
2
CH
2
O
O
O
H
OH
HO
H
H
HO
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 582
ADP in a reaction catalyzed by the enzyme adenylate
kinase (Section 17-4Fe):
The ADP is subsequently converted to ATP through
substrate-level phosphorylation, oxidative phosphorylation,
or photophosphorylation.
c. Rate of ATP Turnover
The cellular role of ATP is that of a free energy transmit-
ter rather than a free energy reservoir. The amount of ATP
in a cell is typically only enough to supply its free energy
needs for a minute or two. Hence,ATP is continually being
hydrolyzed and regenerated. Indeed,
32
P-labeling experi-
ments indicate that the metabolic half-life of an ATP mole-
cule varies from seconds to minutes depending on the cell
type and its metabolic activity. For instance, brain cells
have only a few seconds’ supply of ATP (which, in part, ac-
counts for the rapid deterioration of brain tissue by oxygen
deprivation). An average person at rest consumes and re-
generates ATP at a rate of ⬃3 mol (1.5 kg) ⴢ h
1
and as much
as an order of magnitude faster during strenuous activity.
d. Phosphocreatine Provides a “High-Energy”
Reservoir for ATP Formation
Muscle and nerve cells, which have a high ATP turnover
(a maximally exerting muscle has only a fraction of a
second’s ATP supply), have a free energy reservoir that
functions to regenerate ATP rapidly. In vertebrates, phos-
phocreatine (Fig. 16-26) functions in this capacity. It is
synthesized by the reversible phosphorylation of creatine
by ATP as catalyzed by creatine kinase:
Note that this reaction is endergonic under standard condi-
tions. However, the intracellular concentrations of its reac-
tants and products (typically 4 mM ATP and 0.013 mM
¢G°¿ 12.6 kJ ⴢ mol
1
ATP creatine Δ phosphocreatine ADP
AMP ATP Δ 2ADP
ADP) are such that it operates close to equilibrium (G ⬇ 0).
Accordingly, when the cell is in a resting state, so that
[ATP] is relatively high, the reaction proceeds with net
synthesis of phosphocreatine, whereas at times of high
metabolic activity, when [ATP] is low, the equilibrium shifts
so as to yield net synthesis of ATP. Phosphocreatine thereby
acts as an ATP “buffer” in cells that contain creatine kinase.
A resting vertebrate skeletal muscle normally has suffi-
cient phosphocreatine to supply its free energy needs for
several minutes (but for only a few seconds at maximum
exertion). In the muscles of some invertebrates, such as
lobsters, phosphoarginine performs the same function.
These phosphoguanidines are collectively named phos-
phagens.
5 OXIDATION–REDUCTION REACTIONS
Oxidation–reduction reactions, processes involving the
transfer of electrons, are of immense biochemical signifi-
cance; living things derive most of their free energy from
them. In photosynthesis (Chapter 24), CO
2
is reduced
(gains electrons) and H
2
O is oxidized (loses electrons) to
yield carbohydrates and O
2
in an otherwise endergonic
process that is powered by light energy. In aerobic metabo-
lism, which is carried out by all eukaryotes and many
prokaryotes, the overall photosynthetic reaction is essen-
tially reversed so as to harvest the free energy of oxidation
of carbohydrates and other organic compounds in the form
of ATP (Chapter 22). Anaerobic metabolism generates
ATP, although in lower yields, through intramolecular
oxidation–reductions of various organic molecules, for
example, glycolysis (Chapter 17). In certain anaerobic
bacteria,ATP is generated through the use of non-O
2
oxidiz-
ing agents such as sulfate or nitrate. In this section we out-
line the thermodynamics of oxidation–reduction reactions
in order to understand the quantitative aspects of these
crucial biological processes.
Section 16-5. Oxidation–Reduction Reactions 583
Figure 16-29 Pyrophosphate cleavage in the synthesis of an
aminoacyl–tRNA. Here the squiggle (⬃) represents a “high-
energy” bond. In the first reaction step, the amino acid is
adenylylated by ATP. In the second step, a tRNA molecule
AMP P
H
C + ⬃⬃P
ATP
P ⬃P
2P
i
inorganic
pyrophosphatase
H
2
O
⬃
O
C
Aminoacyl–adenylate
tRNA
AMP
C
NH
3
+
NH
3
+
NH
3
+
Amino acid
O
–
O
PP
i
H
C
R
AMP
O
C
H
C
R
tRN
A
Aminoacyl–tRNA
R
displaces the AMP moiety to form an aminoacyl–tRNA. The
exergonic hydrolysis of pyrophosphate (G°¿ 19.2
kJ ⴢ mol
1
) drives the reaction forward.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 583
A. The Nernst Equation
Oxidation–reduction reactions (also known as redox or ox-
idoreduction reactions) resemble other types of chemical
reactions in that they involve group transfer. For instance,
hydrolysis transfers a functional group to water.In oxidation–
reduction reactions, the “groups” transferred are electrons,
which are passed from an electron donor (reductant or re-
ducing agent) to an electron acceptor (oxidant or oxidizing
agent). For example, in the reaction
Cu
, the reductant, is oxidized to Cu
2
while Fe
3
, the oxi-
dant, is reduced to Fe
2
.
Redox reactions may be divided into two half-reactions
or redox couples, such as
whose sum is the above whole reaction.These half-reactions
occur during oxidative metabolism in the vital mitochon-
drial electron transfer mediated by cytochrome c oxidase
(Section 22-2C5). Note that for electrons to be transferred,
both half-reactions must occur simultaneously. In fact,
the electrons are the two half-reactions’ common interme-
diate.
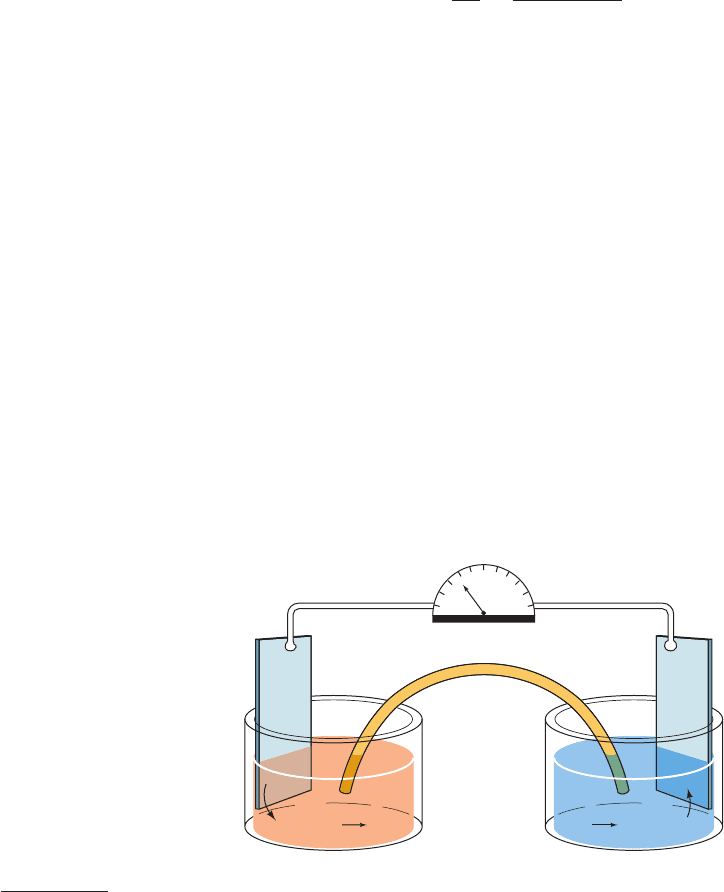
a. Electrochemical Cells
A half-reaction consists of an electron donor and its
conjugate electron acceptor; in the oxidation half-reaction
shown above, Cu
is the electron donor and Cu
2
is its con-
jugate electron acceptor.Together these constitute a conju-
gate redox pair analogous to the conjugate acid–base pair
(HA and A
) of a Brønsted acid (Section 2-2A).An impor-
tant difference between redox pairs and acid–base pairs,
however, is that the two half-reactions of a redox reaction,
each consisting of a conjugate redox pair, may be physically
separated so as to form an electrochemical cell (Fig. 16-30).
In such a device, each half-reaction takes place in its sepa-
rate half-cell, and electrons are passed between half-cells
as an electric current in the wire connecting their two elec-
trodes. A salt bridge is necessary to complete the electrical
circuit by providing a conduit for ions to migrate in the
maintenance of electrical neutrality.
The free energy of an oxidation–reduction reaction is
particularly easy to determine through a simple measure-
ment of the voltage difference between its two half-cells.
Consider the general redox reaction:
in which n electrons per mole of reactants are transferred
from reductant (B
red
) to oxidant ( ). The free energy of
this reaction is expressed, according to Eq. [3.15], as
[16.2]
Equation [3.12] indicates that, under reversible conditions,
[16.3]¢G w¿ w
el
¢G ¢G° RT ln a
[A
red
][B
ox
n
]
[A
ox
n
][B
red
]
b
A
n
ox
A
n
ox
B
red
Δ A
red
B
n
ox
Cu
Δ Cu
2
e
(oxidation)
Fe
3
e
Δ Fe
2
(reduction)
Fe
3
Cu
Δ Fe
2
Cu
2
where w¿, the non-pressure–volume work, is, in this case,
w
el
, the electrical work required to transfer the n moles of
electrons through the electric potential difference .This,
according to the laws of electrostatics, is
[16.4]
where f, the faraday, is the electrical charge of 1 mol of
electrons (1 f 96,485 C ⴢ mol
1
96,485 J ⴢ V
1
ⴢ mol
1
,
where C and V are the symbols for coulomb and volt).
Thus, substituting Eq. [16.4] into Eq. [16.3],
[16.5]
Combining Eqs. [16.2] and [16.5], and making the analo-
gous substitution for G°, yields the Nernst equation:
[16.6]
which was originally formulated in 1881 by Walther Nernst.
Here e, the electromotive force (emf) or redox potential,
may be described as the “electron pressure” that the elec-
trochemical cell exerts. The quantity e°, the redox poten-
tial when all components are in their standard states, is
called the standard redox potential. If these standard states
refer to biochemical standard states (Section 3-4Ba), then
e° is replaced by e°¿. Note that a positive e in
Eq. [16.5] results in a negative G; in other words, a posi-
tive e is indicative of a spontaneous reaction, one that can
do work.
B. Measurements of Redox Potentials
The free energy change of a redox reaction may be deter-
mined, as Eq. [16.5] indicates, by simply measuring its redox
potential with a voltmeter (Fig. 16-30). Consequently,
voltage measurements are commonly employed to charac-
terize the sequence of reactions comprising a metabolic
¢
¢¢¢
¢
¢
¢e ¢e°
RT
nf
ln a
[A
red
][B
ox
n
]
[A
ox
n
][B
red
]
b
¢G nf ¢e
w
el
nf ¢e
¢e
584 Chapter 16. Introduction to Metabolism
Figure 16-30 Example of an electrochemical cell. The half-cell
undergoing oxidation (here Cu
S Cu
2
e
) passes the
liberated electrons through the wire to the half-cell undergoing
reduction (here e
Fe
3
S Fe
2
). Electroneutrality in the two
half-cells is maintained by the transfer of ions through the
electrolyte-containing salt bridge.
Salt
bridge
e
–
+ Fe
3+
Cu
+
Fe
2+
Pt Pt
Cu
2+
+ e
–
Voltmeter
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 584
electron-transport pathway (such as mediates, e.g., oxida-
tive metabolism; Chapter 22).
Any redox reaction can be divided into its component
half-reactions:
where, by convention, both half-reactions are written as re-
ductions. These half-reactions can be assigned reduction
potentials, e
A
and e
B
,in accordance with the Nernst equation:
[16.7a]
[16.7b]
For the redox reaction of any two half-reactions:
[16.8]
Thus, when the reaction proceeds with A as the electron
acceptor and B as the electron donor, and
similarly for .¢e
¢e° ⫽ e°
A
⫺ e°
B
¢e° ⫽ e°
(e
⫺
acceptor)
⫺ e°
(e
⫺
donor)
e
B
⫽ e
B
° ⫺
RT
nf
ln a
[B
red
]
[B
ox
n⫹
]
b
e
A
⫽ e
A
° ⫺
RT
nf
ln a
[A
red
]
[A
ox
n⫹
]
b
B
n⫹
ox
⫹ ne
⫺
Δ B
red
A
n⫹
ox
⫹ ne
⫺
Δ A
red
Reduction potentials, like free energies, must be defined
with respect to some arbitrary standard. By convention,
standard reduction potentials are defined with respect to
the standard hydrogen half-reaction
in which H
⫹
at pH 0, 25°C,and 1 atm is in equilibrium with
H
2
(g) that is in contact with a Pt electrode. This half-cell is
arbitrarily assigned a standard reduction potential of ⫽ 0 V
(1 V ⫽ 1 J ⴢ C
⫺1
). For the biochemical convention, we like-
wise define the standard (pH ⫽ 0) hydrogen half-reaction
as having so that the hydrogen half-cell at the bio-
chemical standard state (pH ⫽ 7) has
(Table 16-4). When is positive, ⌬G is negative (Eq.
[16.5]), indicating a spontaneous process. In combining
two half-reactions under standard conditions, the direc-
tion of spontaneity therefore involves the reduction of the
redox couple with the more positive standard reduction
potential. In other words, the more positive the standard re-
duction potential, the greater the tendency for the redox
couple’s oxidized form to accept electrons and thus become
reduced.
¢e
e°¿ ⫽⫺0.421 V
e¿ ⫽ 0
e°
2H
⫹
⫹ 2e
⫺
Δ H
2
(g)
Section 16-5. Oxidation–Reduction Reactions 585
Half-Reaction (V)
0.815
0.42
0.385
0.295
0.29
0.235
0.22
0.077
0.045
0.031
⫺0.040
⫺0.166
⫺0.185
⫺0.197
⫺0.219
⫺0.23
⫺0.29
⫺0.315
⫺0.320
⫺0.340
⫺0.346
⫺0.421
⫺0.454
⫺0.581Acetate
⫺
⫹ 3H
⫹
⫹ 2e
⫺
Δ acetaldehyde ⫹ H
2
O
SO
2⫺
4
⫹ 2H
⫹
⫹ 2e
⫺
Δ SO
2⫺
3
⫹ H
2
O
H
⫹
⫹ e
⫺
Δ
1
2
H
2
Acetoacetate
⫺
⫹ 2H
⫹
⫹ 2e
⫺
Δ -hydroxybutyrate
⫺
Cystine ⫹ 2H
⫹
⫹ 2e
⫺
Δ 2 cysteine
NADP
⫹
⫹ H
⫹
⫹ 2e
⫺
Δ NADPH
NAD
⫹
⫹ H
⫹
⫹ 2e
⫺
Δ NADH
Lipoic acid ⫹ 2H
⫹
⫹ 2e
⫺
Δ dihydrolipoic acid
S ⫹ 2H
⫹
⫹ 2e
⫺
Δ H
2
S
FAD ⫹ 2H
⫹
⫹ 2e
⫺
Δ FADH
2
(free coenzyme)
Acetaldehyde ⫹ 2H
⫹
⫹ 2e
⫺
Δ ethanol
Pyruvate
⫺
⫹ 2H
⫹
⫹ 2e
⫺
Δ lactate
⫺
Oxaloacetate
⫺
⫹ 2H
⫹
⫹ 2e
⫺
Δ malate
⫺
FAD ⫹ 2H
⫹
⫹ 2e
⫺
Δ FADH
2
(in flavoproteins)
Fumarate
⫺
⫹ 2H
⫹
⫹ 2e
⫺
Δ succinate
⫺
Ubiquinone ⫹ 2H
⫹
⫹ 2e
⫺
Δ ubiquinol
Cytochrome b(Fe
3⫹
) ⫹ e
⫺
Δ cytochrome b(Fe
2⫹
) (mitochondrial)
Cytochrome c
1
(Fe
3⫹
) ⫹ e
⫺
Δ cytochrome c
1
(Fe
2⫹
)
Cytochrome c(Fe
3⫹
) ⫹ e
⫺
Δ cytochrome c(Fe
2⫹
)
Cytochrome a(Fe
3⫹
) ⫹ e
⫺
Δ cytochrome a(Fe
2⫹
)
O
2
(g) ⫹ 2H
⫹
⫹ 2e
⫺
Δ H
2
O
2
Cytochrome a
3
(Fe
3⫹
) ⫹ e
⫺
Δ cytochrome a
3
(Fe
2⫹
)
NO
⫺
3
⫹ 2H
⫹
⫹ 2e
⫺
Δ NO
⫺
2
⫹ H
2
O
1
2
O
2
⫹ 2H
⫹
⫹ 2e
⫺
Δ H
2
O
e°¿
Table 16-4 Standard Reduction Potentials of Some Biochemically Important
Half-Reactions
Source: Mostly from Loach, P.A., in Fasman, G.D. (Ed.), Handbook of Biochemistry and Molecular Biology
(3rd ed.), Physical and Chemical Data, Vol. I, pp. 123–130, CRC Press (1976).
JWCL281_c16_557-592.qxd 6/30/10 10:34 AM Page 585

a. Biochemical Half-Reactions Are
Physiologically Significant
The biochemical standard reduction potentials of
some biochemically important half-reactions are listed in
Table 16-4. The oxidized form of a redox couple with a
large positive standard reduction potential has a high affin-
ity for electrons and is a strong electron acceptor (oxidizing
agent), whereas its conjugate reductant is a weak electron
donor (reducing agent). For example, O
2
is the strongest
oxidizing agent in Table 16-4, whereas H
2
O, which tightly
holds its electrons, is the table’s weakest reducing agent.
The converse is true of half-reactions with large negative
standard reduction potentials. Since electrons sponta-
neously flow from low to high reduction potentials, they
are transferred, under standard conditions, from the re-
duced products in any half-reaction in Table 16-4 to the ox-
idized reactants of any half-reaction above it (although this
may not occur at a measurable rate in the absence of a suit-
able enzyme). Thus, in biological systems, the approximate
lower limit for a standard reduction potential is 0.421 V
because reductants with a lesser value of would reduce
protons to H
2
. However, reducing centers in proteins that
are protected from water may have lower potentials. Note
that the Fe
3
ions of the various cytochromes tabulated in
Table 16-4 have significantly different redox potentials.
This indicates that the protein components of redox
enzymes play active roles in electron-transfer reactions by
modulating the redox potentials of their bound redox-active
centers.
Electron-transfer reactions are of great biological
importance. For example, in the mitochondrial electron-
transport chain (Section 22-2), the primary source of ATP
in eukaryotes, electrons are passed from NADH (Fig. 13-2)
along a series of electron acceptors of increasing reduction
potential (many of which are listed in Table 16-4) to O
2
.
ATP is generated from ADP and P
i
by coupling its synthe-
sis to this free energy cascade. NADH thereby functions as
an energy-rich electron-transfer coenzyme. In fact, the oxi-
dation of one NADH to NAD
supplies sufficient free en-
ergy to generate 2.5 ATPs (Section 22-2Bb). The NAD
/
NADH redox couple functions as the electron acceptor in
many exergonic metabolite oxidations. In serving as the
electron donor in ATP synthesis, it fulfills its cyclic role as a
free energy conduit in a manner analogous to ATP. The
metabolic roles of redox coenzymes are further discussed
in succeeding chapters.
C. Concentration Cells
A concentration gradient has a lower entropy (greater or-
der) than the corresponding uniformly mixed solution and
therefore requires the input of free energy for its formation.
Consequently, discharge of a concentration gradient is an
exergonic process that may be harnessed to drive an ender-
gonic reaction. For example, discharge of a proton concen-
tration gradient (generated by the reactions of the electron-
transport chain) across the inner mitochondrial
membrane drives the enzymatic synthesis of ATP from
ADP and P
i
(Section 22-3). Likewise, nerve impulses,
e°¿
(e°¿)
which require electrical energy, are transmitted through
the discharge of [Na
] and [K
] gradients that nerve cells
generate across their cell membranes (Section 20-5B).
Quantitation of the free energy contained in a concentra-
tion gradient is accomplished by use of the concepts of
electrochemical cells.
The reduction potential and free energy of a half-cell
vary with the concentrations of its reactants. An electro-
chemical cell may therefore be constructed from two
half-cells that contain the same chemical species but at
different concentrations. The overall reaction for such an
electrochemical cell may be represented
[16.9]
and, according to the Nernst equation, since when
the same reaction occurs in both cells,
Such concentration cells are capable of generating electri-
cal work until they reach equilibrium.This occurs when the
concentration ratios in the half-cells become equal (K
eq
1).
The reaction constitutes a sort of mixing of the two half-
cells; the free energy generated is a reflection of the en-
tropy of this mixing.The thermodynamics of concentration
gradients as they apply to membrane transport is discussed
in Section 20-1.
6 THERMODYNAMICS OF LIFE
One of the last refuges of vitalism, the doctrine that biolog-
ical processes are not bound by the physical laws that gov-
ern inanimate objects, was the belief that living things can
somehow evade the laws of thermodynamics.This view was
partially refuted by elaborate calorimetric measurements
on living animals that are entirely consistent with the
energy conservation predictions of the first law of thermo-
dynamics. However, the experimental verification of the
second law of thermodynamics in living systems is more
difficult. It has not been possible to measure the entropy of
living matter because the heat, q
p
, of a reaction at a con-
stant T and P is only equal to T S if the reaction is carried
out reversibly (Eq. [3.8]). Obviously, the dismantling of a
living organism to its component molecules for such a
measurement would invariably result in its irreversible
death. Consequently, the present experimentally verified
state of knowledge is that the entropy of living matter is
less than that of the products to which it decays.
In this section we consider the special aspects of the
thermodynamics of living systems. Knowledge of these
matters, which is by no means complete, has enhanced our
understanding of how metabolic pathways are regulated,
how cells respond to stimuli, and how organisms grow and
change with time.
¢e
RT
nf
ln a
[A
n
ox
(half-cell 2) ][A
red
(half-cell 1) ]
[A
n
ox
(half-cell 1) ] [A
red
(half-cell 2) ]
b
¢e° 0
A
n
ox
(half-cell 2) A
red
(half-cell 1)
A
n
ox
(half-cell 1) A
red
(half-cell 2) Δ
586 Chapter 16. Introduction to Metabolism
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 586
A. Living Systems Cannot Be at Equilibrium
Classical or equilibrium thermodynamics (Chapter 3)
applies largely to reversible processes in closed systems.
The fate of any isolated system, as we discussed in Section
3-4A, is that it must inevitably reach equilibrium. For ex-
ample, if its reactants are in excess, the forward reaction
will proceed faster than the reverse reaction until equilib-
rium is attained (G 0). In contrast, open systems may
remain in a nonequilibrium state as long as they are able to
acquire free energy from their surroundings in the form of
reactants, heat, or work. While classical thermodynamics
provides invaluable information concerning open systems
by indicating whether a given process can occur sponta-
neously, further thermodynamic analysis of open systems
requires the application of the more recently elucidated
principles of nonequilibrium or irreversible thermodynamics.
In contrast to classical thermodynamics, this theory explic-
itly takes time into account.
Living organisms are open systems and therefore can
never be at equilibrium. As indicated above, they continu-
ously ingest high-enthalpy, low-entropy nutrients, which
they convert to low-enthalpy, high-entropy waste products.
The free energy resulting from this process is used to do
work and to produce the high degree of organization char-
acteristic of life. If this process is interrupted, the organism
ultimately reaches equilibrium, which for living things is
synonymous with death. For example, one theory of aging
holds that senescence results from the random but in-
evitable accumulation in cells of genetic defects that inter-
fere with and ultimately disrupt the proper functioning of
living processes. [The theory does not, however, explain
how single-celled organisms or the germ cells of multicellu-
lar organisms (sperm and ova), which are in effect immor-
tal, are able to escape this so-called error catastrophe.]
Living systems must maintain a nonequilibrium state
for several reasons:
1. Only a nonequilibrium process can perform useful
work.
2. The intricate regulatory functions characteristic of
life require a nonequilibrium state because a process at
equilibrium cannot be controlled (similarly, a ship that is
dead in the water will not respond to its rudder).
3. The complex cellular and molecular systems that
conduct biological processes can be maintained only in the
nonequilibrium state. Living systems are inherently unsta-
ble because they are degraded by the very biochemical re-
actions to which they give rise. Their regeneration, which
must occur almost simultaneously with their degradation,
requires the continuous influx of free energy. For example,
the ATP-generating consumption of glucose (Section 17-2),
as has been previously mentioned, occurs with the initial
consumption of ATP through its reactions with glucose to
form glucose-6-phosphate and with fructose-6-phosphate
to form fructose-1,6-bisphosphate. Consequently, if metab-
olism is suspended long enough to exhaust the available
ATP supply, glucose metabolism cannot be resumed. Life
therefore differs in a fundamental way from a complex
machine such as a computer. Both require a throughput of
free energy to be active. However, the function of the ma-
chine is based on a static structure, so that the machine can
be repeatedly switched on and off. Life, in contrast, is based
on a self-destructing but self-renewing process, which once
interrupted, cannot be reinitiated.
B. Nonequilibrium Thermodynamics and
the Steady State
In a nonequilibrium process, something (such as matter,
electrical charge, or heat) must flow, that is, change its spa-
tial distribution. In classical mechanics, the acceleration of
mass occurs in response to force. Similarly, flow in a ther-
modynamic system occurs in response to a thermodynamic
force (driving force), which results from the system’s
nonequilibrium state. For example, the flow of matter in
diffusion is motivated by the thermodynamic force of a con-
centration gradient; the migration of electrical charge (elec-
tric current) occurs in response to a gradient in an electric
field (a voltage difference); the transport of heat results
from a temperature gradient; and a chemical reaction
results from a difference in chemical potential. Such flows
are said to be conjugate to their thermodynamic force.
A thermodynamic force may also promote a nonconju-
gate flow under the proper conditions. For example, a gra-
dient in the concentration of matter can give rise to an elec-
tric current (a concentration cell), heat (such as occurs on
mixing H
2
O and HCl), or a chemical reaction (the mito-
chondrial production of ATP through the dissipation of a
proton gradient). Similarly, a gradient in electrical poten-
tial can motivate a flow of matter (electrophoresis), heat
(resistive heating), or a chemical reaction (the charging of
a battery). When a thermodynamic force stimulates a non-
conjugate flow, the process is called energy transduction.
a. Living Things Maintain the Steady State
Living systems are, for the most part, characterized by
being in a steady state. By this it is meant that all flows in the
system are constant, so that the system does not change with
time. Some environmental steady-state processes are
schematically illustrated in Fig. 16-31. Ilya Prigogine, a
pioneer in the development of irreversible thermodynamics,
has shown that a steady-state system produces the maximum
amount of useful work for a given energy expenditure under
the prevailing conditions. The steady state of an open system
is therefore its state of maximum thermodynamic efficiency.
Furthermore, in analogy with Le Châtelier’s principle (Sec-
tion 3-4A), slight perturbations from the steady state give
rise to changes in flows that counteract these perturbations
so as to return the system to the steady state. The steady state
of an open system is therefore analogous to the equilibrium
state of an isolated system; both are stable states.
In the following chapters we shall see that many biological
regulatory mechanisms function to maintain a steady state.
For example, the flow of reaction intermediates through a
metabolic pathway is often inhibited by an excess of final prod-
uct and stimulated by an excess of starting material through
the allosteric regulation of its key enzymes (Section 13-4).
Section 16-6. Thermodynamics of Life 587
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 587
Living things have apparently evolved so as to take maximum
thermodynamic advantage of their environments.
C. Thermodynamics of Metabolic Control
a. Enzymes Selectively Catalyze Required Reactions
Biological reactions are highly specific; only reactions that
lie on metabolic pathways take place at significant rates de-
spite the many other thermodynamically favorable reactions
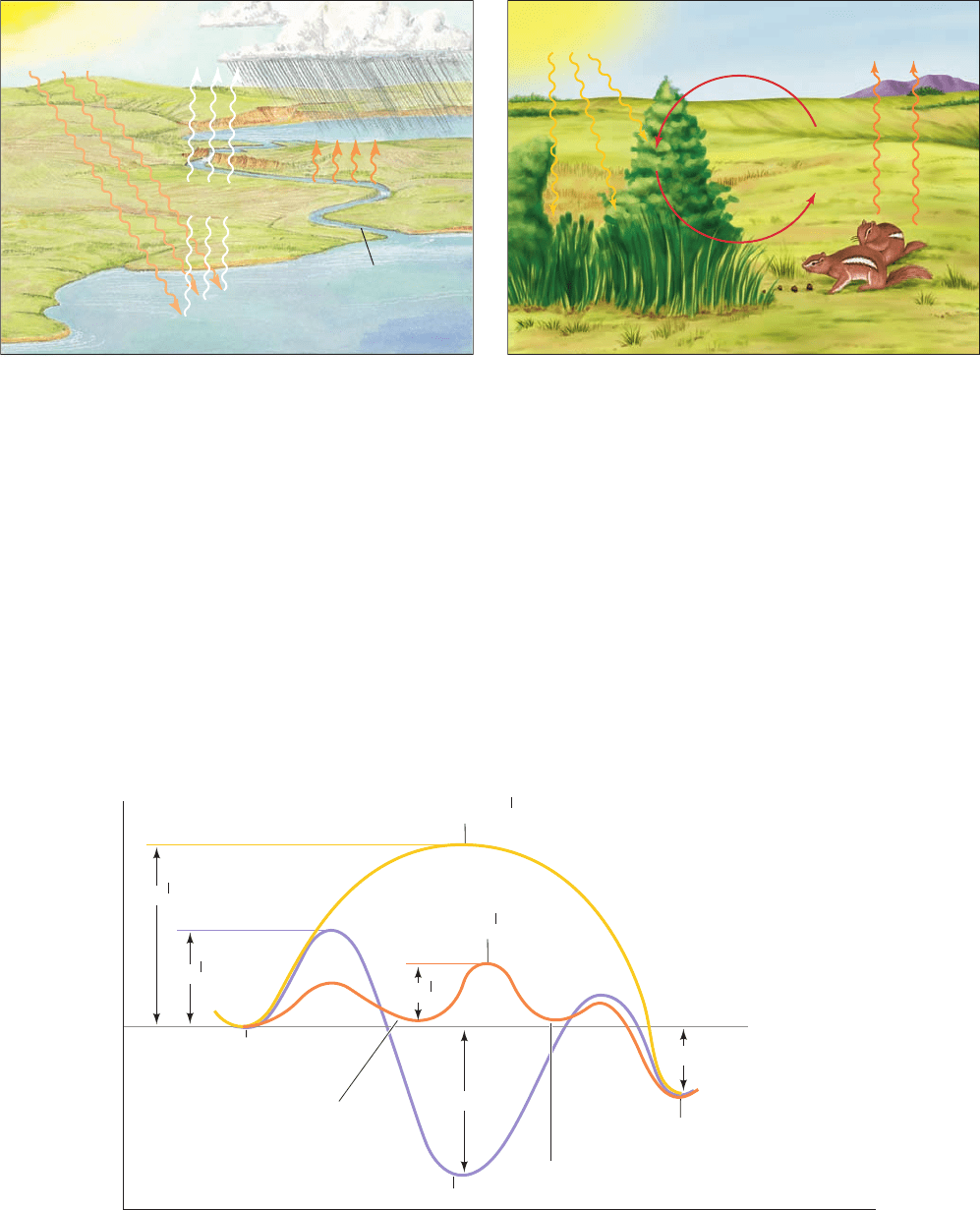
that are also possible. As an example, let us consider the re-
actions of ATP, glucose, and water. Two thermodynamically
favorable reactions that ATP can undergo are phosphoryl
transfer to form ADP and glucose-6-phosphate,and hydroly-
sis to form ADP and P
i
(Fig.16-23a).The free energy profiles
of these reactions are diagrammed in Fig.16-32.ATP hydrol-
ysis is thermodynamically favored over the phosphoryl
transfer to glucose. However, their relative rates are deter-
mined by their free energies of activation to their transition
588 Chapter 16. Introduction to Metabolism
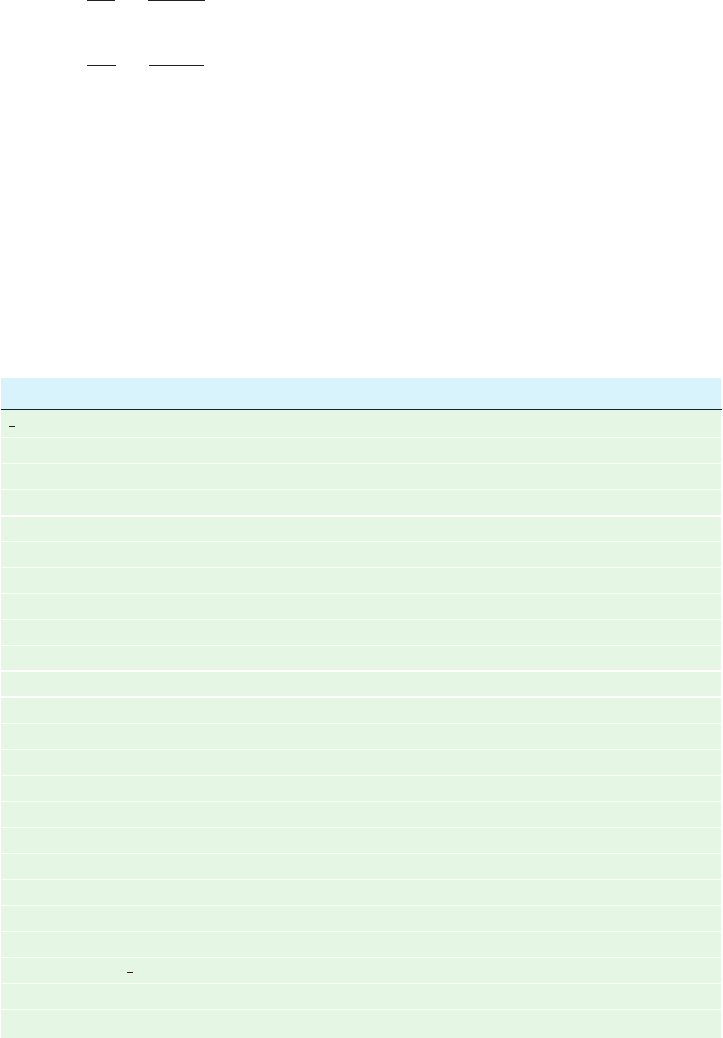
Figure 16-31 Two examples of open systems in a steady state.
(a) A constant flow of water in the river occurs under the
influence of the force of gravity. The water level in the reservoir
is maintained by rain, the major source of which is the
evaporation of seawater. Hence the entire cycle is ultimately
powered by the sun. (b) The steady state of the biosphere is
Figure 16-32 Reaction coordinate diagrams. These are (1) the
reaction of ATP and water (purple curve), and the reaction of
ATP and glucose (2) in the presence (orange curve) and (3) in
the absence (yellow curve) of an appropriate enzyme.Although
(a)
Radiant energy
from the sun
Rain
Heat loss
Water
vapor
Sea
River flowing
under steady
state conditions
(gravity)
Heat loss
Breakdown of
carbohydrates
Photosynthesis
CO
2
+
H
2
O
(b)
Radiant energy
from the sun
ATP + H
2
O
+ glucose
Reaction coordinate
G
ADP + H
2
O + glucose-6-P
ADP + P
i
+ glucose
(ATP•Glucose)
enzymatic
+ H
2
O
(ATP•Glucose)
enzymatic
(ADP•Glucose-6-P)
enzymatic
=
(ATP•Glucose)
uncatalyzed
+ H
2
O
=
ΔG
3
=
ΔG
1
ΔG
1
=
ΔG
2
ΔG
2
, ΔG
3
=
similarly maintained by the sun. Plants harness the sun’s radiant
energy to synthesize carbohydrates from CO
2
and H
2
O. The
eventual metabolism of the carbohydrates by the plants or by the
animals that eat them results in the release of their stored free
energy and the return of the CO
2
and H
2
O to the environment to
complete the cycle.
the hydrolysis of ATP is a more exergonic reaction than the
phosphorylation of glucose (G
1
is more negative than G
2
), the
latter reaction is predominant in the presence of a suitable
enzyme because it is kinetically favored .(¢G
‡
2
¢G
‡
1
)
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 588

states (G
‡
values; Section 14-1Cb) and the relative concen-
trations of glucose and water.The larger G
‡
, the slower the
reaction. In the absence of enzymes, G
‡
for the phosphoryl-
transfer reaction is greater than that for hydrolysis, so the
hydrolysis reaction predominates (although neither reaction
occurs at a biologically significant rate).
The free energy barriers of both of the nonenzymatic re-
actions are far higher than that of the enzyme-catalyzed
phosphoryl transfer to glucose. Hence enzymatic forma-
tion of glucose-6-phosphate is kinetically favored over the
nonenzymatic hydrolysis of ATP. It is the role of an enzyme,
in this case hexokinase, to selectively reduce the free energy
of activation of a chemically coupled reaction so that it ap-
proaches equilibrium faster than the more thermodynami-
cally favored uncoupled reaction.
b. Many Enzymatic Reactions Are Near Equilibrium
Although metabolism as a whole is a nonequilibrium
process, many of its component reactions function close to
equilibrium. The reaction of ATP and creatine to form
phosphocreatine (Section 16-4Cd) is an example of such a
reaction. The ratio [creatine]/[phosphocreatine] depends
on [ATP] because creatine kinase, the enzyme catalyzing
this reaction, has sufficient activity to equilibrate the reac-
tion rapidly. The net rate of such an equilibrium reaction is
effectively controlled by varying the concentrations of its
reactants and/or products.
c. Pathway Throughput Is Regulated by Controlling
Enzymes Operating Far from Equilibrium
Other biological reactions function far from equilibrium.
For example, the phosphofructokinase reaction (Fig. 16-28)
has an equilibrium constant of but under physio-
logical conditions in rat heart muscle has the mass action ra-
tio [fructose-1,6-bisphosphate][ADP]/[fructose-6-phos-
phate][ATP] 0.03, which corresponds to G 25.7
kJ ⴢ mol
1
(Eq. [3.15]). This situation arises from a buildup
of reactants because there is insufficient phosphofructoki-
nase activity to equilibrate the reaction. Changes in sub-
strate concentrations therefore have relatively little effect
on the rate of the phosphofructokinase reaction; the en-
zyme is close to saturation. Only changes in the activity of
the enzyme, through allosteric interactions, for example, can
significantly alter this rate.An enzyme such as phosphofruc-
tokinase is therefore analogous to a dam on a river. Sub-
strate flux (rate of flow) is controlled by varying its activity
(allosterically or by other means), much as a dam controls
the flow of a river below the dam by varying the opening of
its floodgates (when the water levels on the two sides of the
dam are different, that is, when they are not at equilibrium).
Understanding of how reactant flux in a metabolic path-
way is controlled requires knowledge of which reactions
are functioning near equilibrium and which are far from it.
Most enzymes in a metabolic pathway operate near equi-
librium and therefore have net rates that are sensitive only
to their substrate concentrations. However, as we shall see
in the following chapters (particularly in Section 17-4), cer-
tain enzymes, which are strategically located in a metabolic
pathway, operate far from equilibrium. These enzymes,
which are targets for metabolic regulation by allosteric inter-
actions and other mechanisms, are responsible for the main-
tenance of a stable steady-state flux of metabolites through
the pathway. This situation, as we have seen, maximizes the
pathway’s thermodynamic efficiency.
K¿
eq
300
Chapter Summary 589
1 Metabolic Pathways Metabolic pathways are series of
consecutive enzymatically catalyzed reactions that produce
specific products for use by an organism. The free energy re-
leased by degradation (catabolism) is, through the intermedi-
acy of ATP and NADPH, used to drive the endergonic
processes of biosynthesis (anabolism). Carbohydrates, lipids,
and proteins are all converted to the common intermediate
acetyl-CoA, whose acetyl group is then converted to CO
2
and
H
2
O through the action of the citric acid cycle and oxidative
phosphorylation.A relatively few metabolites serve as starting
materials for a host of biosynthetic products. Metabolic path-
ways have five principal characteristics: (1) Metabolic path-
ways are irreversible; (2) if two metabolites are interconvertible,
the synthetic route from the first to the second must differ
from the route from the second to the first; (3) every meta-
bolic pathway has an exergonic first committed step; (4) all
metabolic pathways are regulated, usually at the first commit-
ted step; and (5) metabolic pathways in eukaryotes occur in
specific subcellular compartments.
2 Organic Reaction Mechanisms Almost all metabolic
reactions fall into four categories: (1) group-transfer reactions;
(2) oxidation–reduction reactions; (3) eliminations, isomeriza-
tions, and rearrangements; and (4) reactions that make or break
carbon–carbon bonds. Most of these reactions involve het-
erolytic bond cleavage or formation occurring through the addi-
tion of nucleophiles to electrophilic carbon atoms. Group-trans-
fer reactions therefore involve transfer of an electrophilic group
from one nucleophile to another.The main electrophilic groups
transferred are acyl groups, phosphoryl groups, and glycosyl
groups.The most common nucleophiles are amino, hydroxyl, im-
idazole, and sulfhydryl groups. Electrophiles participating in
metabolic reactions are protons, metal ions, carbonyl carbon
atoms, and cationic imines. Oxidation–reduction reactions
involve loss or gain of electrons. Oxidation at carbon usually
involves bond cleavage, with the ultimate loss by C of
the two bonding electrons through their transfer to an elec-
tron acceptor such as NAD
. The terminal electron acceptor
in aerobes is O
2
. Elimination reactions are those in which a
double bond is created from two saturated carbon cen-
ters with the loss of H
2
O, NH
3
, ROH, or RNH
2
. Dehydration
reactions are the most common eliminations. Isomerizations
involve shifts of double bonds within molecules. Rearrange-
ments are biochemically uncommon reactions in which in-
tramolecular bonds are broken and reformed to produce
new carbon skeletons. Reactions that make and break
bonds form the basis of both degradative and biosynthetic
C¬C
C¬C
C“C
C¬H
CHAPTER SUMMARY
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 589

590 Chapter 16. Introduction to Metabolism
metabolism. In the synthetic direction, these reactions involve
addition of a nucleophilic carbanion to an electrophilic carbon
atom. The most common electrophilic carbon atom is the car-
bonyl carbon, whereas carbanions are usually generated by re-
moval of a proton from a carbon atom adjacent to a carbonyl
group or by decarboxylation of a -keto acid.
3 Experimental Approaches to the Study of Metabolism
Experimental approaches employed in elucidating metabolic
pathways include the use of metabolic inhibitors, growth stud-
ies, and biochemical genetics. Metabolic inhibitors block path-
ways at specific enzymatic steps. Identification of the resulting
intermediates indicates the course of the pathway. Mutations,
which occur naturally in genetic diseases or can be induced by
mutagens, X-rays, or genetic engineering, may also result in the
absence or inactivity of an enzyme. Modern genetic techniques
make it possible to express foreign genes in higher organisms
(transgenic animals) or inactivate (knock out) a gene and study
the effects of these changes on metabolism. When isotopic la-
bels are incorporated into metabolites and allowed to enter a
metabolic system, their paths may be traced from the distribu-
tion of label in the intermediates. NMR is a noninvasive tech-
nique that may be used to detect and study metabolites in vivo.
Studies on isolated organs,tissue slices,cells, and subcellular or-
ganelles have contributed enormously to our knowledge of the
localization of metabolic pathways. Systems biology endeavors
to quantitatively describe the properties and dynamics of bio-
logical networks as a whole through the integration of genomic,
transcriptomic, proteomic, and metabolomic information.
4 Thermodynamics of Phosphate Compounds Free en-
ergy is supplied to endergonic metabolic processes by the ATP
produced via exergonic metabolic processes.ATP’s 30.5 kJ ⴢ
mol
1
G°¿ of hydrolysis is intermediate between those of
“high-energy” metabolites such as phosphoenolpyruvate and
“low-energy” metabolites such as glucose-6-phosphate. The
“high-energy” phosphoryl groups are enzymatically trans-
ferred to ADP, and the resulting ATP, in a separate reaction,
phosphorylates “low-energy” compounds. ATP may also un-
dergo pyrophosphate cleavage to yield PP
i
, whose subsequent
hydrolysis adds further thermodynamic impetus to the reaction.
ATP is present in too short a supply to act as an energy reser-
voir. This function, in vertebrate nerve and muscle cells, is car-
ried out by phosphocreatine, which under low-ATP conditions
readily transfers its phosphoryl group to ADP to form ATP.
5 Oxidation–Reduction Reactions The half-reactions of
redox reactions may be physically separated to form two elec-
trochemical half-cells.The redox potential for the reduction of
A by B,
in which n electrons are transferred, is given by the Nernst
equation
The redox potential of such a reaction is related to the reduc-
tion potentials of its component half-reactions, and , by
If , then has a greater electron affinity than does
.The reduction potential scale is defined by arbitrarily set-
ting the reduction potential of the standard hydrogen half-cell
to zero. Redox reactions are of great metabolic importance.
For example, the oxidation of NADH yields 2.5 ATPs through
the mediation of the electron-transport chain.
6 Thermodynamics of Life Living organisms are open sys-
tems and therefore cannot be at equilibrium.They must contin-
uously dissipate free energy in order to carry out their various
functions and preserve their highly ordered structures. The
study of nonequilibrium thermodynamics has indicated that the
steady state, which living processes maintain, is the state of max-
imum efficiency under the constraints governing open systems.
Control mechanisms that regulate biological processes preserve
the steady state by regulating the activities of enzymes that are
strategically located in metabolic pathways.
B
n
ox
A
n
ox
e
A
e
B
¢e e
A
e
B
e
B
e
A
¢e ¢e°
RT
nf
lna
[A
red
][B
n
ox
]
[A
n
ox
][B
red
]
b
A
n
ox
B
red
Δ A
red
B
n
ox
Metabolic Studies
Aebersold, R., Quantitative proteome analysis: Methods and ap-
plications, J. Infect. Dis. 182 (supplement 2), S315–S320 (2003).
Beadle, G.W., Biochemical genetics, Chem. Rev. 37, 15–96 (1945).
[A classic review summarizing the “one gene–one enzyme”
hypothesis.]
Campbell A.M. and Heyer L.J., Discovering Genomics, Pro-
teomics and Bioinformatics (2nd ed.), Pearson Benjamin Cum-
mings, New York (2007). [An interactive introduction to these
subjects.]
Cerdan, S. and Seelig, J., NMR studies of metabolism, Annu. Rev.
Biophys. Biophys. Chem. 19, 43–67 (1990).
Choi, S. (Ed.), Introduction to Systems Biology, Humana Press
(2007).
Cooper, T.G., The Tools of Biochemistry, Chapter 3, Wiley-
Interscience (1977). [A presentation of radiochemical tech-
niques.]
Duarte, N.C., Becker, S.A., Jamshidi, N., Thiele, I., Mo, M.L., Vo,
T.D., Srivas, R., and Palsson, B. Ø., Global reconstruction of the
human metabolic network based on genomic and bibliomic
data, Proc. Natl.Acad. Sci. 104, 1777–1782 (2007).
Freifelder, D., Biophysical Chemistry (2nd ed.), Chapters 5 and 6,
Freeman (1982). [A discussion of the principles of radioactive
counting and autoradiography.]
Go,V.L.W.,Nguyen,C.T.H., Harris, D.M.,and Lee, W.-N.P.,Nutrient-
gene interaction: Metabolic genotype-phenotype relationship,
J. Nutr. 135, 2016s–3020s (2005).
Hevesy, G., Historical sketch of the biological application of tracer
elements, Cold Spring Harbor Symp. Quant. Biol. 13, 129–150
(1948).
Jeffrey, F.M.H., Rajagopal, A., Malloy, C.R., and Sherry, A.D.,
13
C-NMR:A simple yet comprehensive method for analysis of in-
termediary metabolism, Trends Biochem. Sci. 16, 5–10 (1991).
Michal, G. (Ed.), Biochemical Pathways. An Atlas of Biochemistry
and Molecular Biology, Wiley (1999). [An encyclopedic com-
pendium of metabolic pathways.]
Shemin, D. and Rittenberg,D.,The biological utilization of glycine
for the synthesis of the protoporphyrin of hemoglobin, J. Biol
Chem. 166, 621–625 (1946).
Shulman, R.G. and Rothman, D.L.,
13
C NMR of intermediary me-
tabolism: Implications for systematic physiology, Annu. Rev.
Physiol. 63, 15–48 (2001).
REFERENCES
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 590
