Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

Smolin, L.A. and Grosvenor, M.B, Nutrition: Science and Applica-
tions, Wiley (2008). [A good text for those interested in pursu-
ing nutritional aspects of metabolism].
Suckling, K.E. and Suckling, C.J., Biological Chemistry, Cam-
bridge University Press (1980).[Presents the organic chemistry
of biochemical reactions.]
Walsh, C., Enzymatic Reaction Mechanisms, Chapter 1, Freeman
(1979). [A discussion of the types of biochemical reactions.]
Wang, N.-D., Finegold, M.J., Bradley, A., Ou, C.N., Abdelsayed,
S.V., Wilde, M.D., Taylor, L.R., Wilson, D.R., and Darlington,
G.J., Impaired energy homeostasis in C/EBP knockout mice,
Science 269, 1108–1112 (1995).
Weckwerth, W. (Ed.), Metabolomics. Methods and Protocols,
Humana Press (2007).
Westheimer, F.H., Why nature chose phosphates, Science 235,
1173–1178 (1987).
Xia, Y., Yu, H., Jansen, R., Seringhaus, M., Baxter, S., Greenbaum,
D., Zhao,H.,and Gerstein,M.,Analyzing cellular biochemistry
in terms of molecular networks, Annu. Rev. Biochem. 73,
1051–1087 (2004).
Zhu, H., Bilgin, M., and Snyder, M., Proteomics, Annu. Rev.
Biochem. 72, 783–812 (2003).
Bioenergetics
Alberty, R.A., Standard Gibbs free energy, enthalpy and entropy
changes as a function of pH and pMg for reactions involving
adenosine phosphates, J. Biol. Chem. 244, 3290–3302 (1969).
Alberty, R.A., Calculating apparent equilibrium constants of
enzyme-catalyzed reactions at pH 7, Biochem. Ed. 28, 12–17
(2000).
Caplan, S.R., Nonequilibrium thermodynamics and its application
to bioenergetics, Curr.Top. Bioenerg. 4, 1–79 (1971).
Crabtree, B. and Taylor, D.J.,Thermodynamics and metabolism, in
Jones, M.N. (Ed.), Biochemical Thermodynamics, pp. 333–378,
Elsevier (1979).
Dickerson, R.E., Molecular Thermodynamics, Chapter 7,
Benjamin (1969). [An interesting chapter on the thermody-
namics of life.]
Henley, H.J.M., An introduction to nonequilibrium thermody-
namics, J. Chem. Ed. 41, 647–655 (1964).
Katchelsky, A. and Curran, P.F., Nonequilibrium Thermodynamics
in Biophysics, Harvard University Press (1965).
Morowitz, H.J., Foundations of Bioenergetics, Academic Press
(1978).
Problems 591
1. Glycolysis (glucose breakdown) has the overall stoichiome-
try:
whereas that of gluconeogenesis (glucose synthesis) is
What is the overall stoichiometry of the glycolytic breakdown of
1 mol of glucose followed by its gluconeogenic synthesis? Explain
why it is necessary that the pathways of these two processes be in-
dependently controlled and why they must differ by at least one
reaction.
2. It has been postulated that a trigonal bipyramidal penta-
covalent phosphorus intermediate can undergo a vibrational de-
formation process known as pseudorotation in which its apical
ligands exchange with two of its equatorial ligands via a tetragonal
pyramidal transition state:
Trigonal bipyramid
[X and Y apical]
Trigonal bipyramid
[O2 and O3 apical]
Tetragonal pyramidal
transition state
O1
O2
O3
X
Y
P
O1 O1
O2
O3
X
Y
P
O1
X
Y
O3
O2
P
O2
O3
X
Y
P
glucose 6ADP 6P
i
2NAD
2 pyruvate 6ATP 2NADH 4H
6H
2
O
¡
2 pyruvate
2ATP 2NADH 4H
2H
2
O
Glucose 2ADP 2P
i
2NAD
¡
In a nucleophilic substitution reaction, would two cycles of
pseudorotation, so as to place the leaving group (X) in an apical
position and the attacking group (Y) in an equatorial position,
lead to retention or inversion of configuration on the departure of
the leaving group?
3. One Curie (Ci) of radioactivity is defined as 3.70 10
10
dis-
integrations per second, the number that occurs in 1 g of pure
226
Ra. A sample of
14
CO
2
has a specific radioactivity of 5 Ci ⴢ
mol
1
.What percentage of its C atoms are
14
C?
4. In the hydrolysis of ATP to ADP and P
i
, the equilibrium
concentration of ATP is too small to be measured accurately. A
better way of determining and hence G°¿ of this reaction, is
to break it up into two steps whose values of G°¿ can be accu-
rately determined. This has been done using the following pair of
reactions (the first being catalyzed by glutamine synthetase):
What is the G°¿ of ATP hydrolysis according to these data?
*5. Consider the reaction catalyzed by hexokinase:
A mixture containing 40 mM ATP and 20 mM glucose was incu-
bated with hexokinase at pH 7 and 25°C.Calculate the equilibrium
concentrations of the reactants and products (see Table 16-3).
6. In aerobic metabolism, glucose is completely oxidized in
the reaction
with the coupled generation of 32 ATP molecules from 32 ADP
and 32 P
i
.Assuming the G for the hydrolysis of ATP to ADP and
Glucose 6 O
2
Δ 6CO
2
6H
2
O
ATP glucose Δ ADP glucose-6-phosphate
¢G°¿
2
14.2 kJ ⴢ mol
1
(2) Glutamate NH
4
Δ glutamine H
2
O H
¢G°¿
1
16.3 kJ ⴢ mol
1
(1) ATP glutamate NH
4
Δ ADP P
i
glutamine H
K¿
eq
,
PROBLEMS
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 591

592 Chapter 16. Introduction to Metabolism
P
i
under intracellular conditions is ⫺50 kJ ⴢ mol
⫺1
and that for the
combustion of glucose is ⫺2823.2 kJ
ⴢ mol
⫺1
, what is the efficiency
of the glucose oxidation reaction in terms of the free energy se-
questered in the form of ATP?
7. Typical intracellular concentrations of ATP, ADP, and P
i
in
muscles are 5.0, 0.5, and 1.0 mM, respectively. At 25°C and pH 7:
(a) What is the free energy of hydrolysis of ATP at these concen-
trations? (b) Calculate the equilibrium concentration ratio of
phosphocreatine to creatine in the creatine kinase reaction:
if ATP and ADP have the above concentrations. (c) What concen-
tration ratio of ATP to ADP would be required under the forego-
ing conditions to yield an equilibrium concentration ratio of phos-
phocreatine to creatine of 1? Assuming the concentration of P
i
remained 1.0 mM, what would the free energy of hydrolysis of
ATP be under these latter conditions?
*8. Assuming the intracellular concentrations of ATP, ADP,
and P
i
, are those given in Problem 7: (a) Calculate the concen-
tration of AMP at pH 7 and 25°C under the condition that the
adenylate kinase reaction:
is at equilibrium. (b) Calculate the equilibrium concentration of
AMP when the free energy of hydrolysis of ATP to ADP and P
i
is
⫺55 kJ ⴢ mol
⫺1
. Assume [P
i
] and ([ATP] ⫹ [ADP]) remain
constant.
9. Using the data in Table 16-4, list the following substances in
order of their decreasing oxidizing power: (a) fumarate
⫺
, (b) cys-
tine, (c) O
2
, (d) NADP
⫹
, (e) cytochrome c (Fe
3⫹
), and (f) lipoic
acid.
10. Calculate the equilibrium concentrations of reactants and
products for the reaction:
-hydroxybutyrate
⫺
⫹ NAD
⫹
Acetoacetate
⫺
⫹ NADH ⫹ H
⫹
Δ
2ADP Δ ATP ⫹ AMP
Creatine ⫹ ATP Δ phosphocreatine ⫹ ADP
when the initial concentrations of acetoacetate
⫺
and NADH are
0.01 and 0.005M, respectively, and -hydroxybutyrate
⫺
and NAD
⫹
are initially absent. Assume the reaction takes place at 25°C and
pH 7.
11. In anaerobic bacteria, the final metabolic electron accep-
tor is some molecule other than O
2
. A major requirement for any
redox pair utilized as a metabolic free energy source is that it pro-
vides sufficient free energy to generate ATP from ADP and P
i
.In-
dicate which of the following redox pairs are sufficiently exer-
gonic to enable a properly equipped bacterium to utilize them as
a major energy source. Assume that redox reactions forming ATP
require two electrons and that .
(a) Ethanol ⫹ NO
⫺
3
(c) H
2
⫹ S
(b) Fumarate
⫺
⫹ SO
2⫺
3
(d) Acetaldehyde ⫹ acetaldehyde
12. Calculate ⌬G°¿ for the following pairs of half-reactions at
pH 7 and 25°C.Write a balanced equation for the overall reaction
and indicate the direction in which it occurs spontaneously under
standard conditions.
(a) and
(b) (Pyruvate
⫺
⫹ 2H
⫹
/lactate
⫺
) and (NAD
⫹
⫹ H
⫹
/NADH)
*13. The chemiosmotic hypothesis (Section 22-3A) postulates
that ATP is generated in the two-electron reaction:
which is driven by a metabolically generated pH gradient in the
mitochondria.What is the magnitude of the pH gradient required
for net synthesis of ATP at 25°C and pH 7, if the steady-state
concentrations of ATP, ADP, and P
i
are 0.01, 10, and 10 mM,
respectively?
14. Gastric juice is 0.15M HCl.The blood plasma, which is the
source of this H
⫹
and Cl
⫺
, is 0.10M in Cl
⫺
and has a pH of 7.4. Cal-
culate the free energy necessary to produce the HCl in 0.1 L of
gastric juice at 37°C.
ATP ⫹ H
2
O ⫹ 2H
⫹
(high pH)
ADP ⫹ P
i
⫹ 2H
⫹
(low pH) Δ
(
1
2
O
2
⫹ 2H
⫹
>H
2
O)(H
⫹
>
1
2
H
2
)
¢e ⫽ ¢e°¿
JWCL281_c16_557-592.qxd 7/20/10 5:42 PM Page 592
593593
CHAPTER 17
Glycolysis
1 The Glycolytic Pathway
A. Historical Perspective
B. Pathway Overview
2 The Reactions of Glycolysis
A. Hexokinase: First ATP Utilization
B. Phosphoglucose Isomerase
C. Phosphofructokinase: Second ATP Utilization
D. Aldolase
E. Triose Phosphate Isomerase
F. Glyceraldehyde-3-Phosphate Dehydrogenase: First “High-
Energy” Intermediate Formation
G.Phosphoglycerate Kinase: First ATP Generation
H. Phosphoglycerate Mutase
I. Enolase: Second “High-Energy” Intermediate Formation
J. Pyruvate Kinase: Second ATP Generation
3 Fermentation: The Anaerobic Fate of Pyruvate
A. Homolactic Fermentation
B. Alcoholic Fermentation
C. Energetics of Fermentation
4 Metabolic Regulation and Control
A. Homeostasis and Metabolic Control
B. Metabolic Flux
C. Metabolic Control Analysis
D. Supply–Demand Analysis
E. Mechanisms of Flux Control
F. Regulation of Glycolysis in Muscle
5 Metabolism of Hexoses Other than Glucose
A. Fructose
B. Galactose
C. Mannose
At this point we commence our discussions of specific
metabolic pathways by considering glycolysis (Greek:
glykos, sweet; lysis, loosening), the pathway by which glu-
cose is converted via fructose-1,6-bisphosphate to pyruvate
with the generation of 2 mol of ATP per mole of glucose.
This sequence of 10 enzymatic reactions, which is probably
the most completely understood biochemical pathway,
plays a key role in energy metabolism by providing a signif-
icant portion of the energy utilized by most organisms and
by preparing glucose, as well as other carbohydrates, for
oxidative degradation.
In our study of glycolysis, and indeed of all of metabo-
lism, we shall attempt to understand the pathway on four
levels:
1. The chemical interconversion steps, that is, the se-
quence of reactions by which glucose is converted to the
pathway’s end products.
2. The mechanism of the enzymatic conversion of each
pathway intermediate to its successor.
3. The energetics of the conversions.
4. The mechanisms controlling the flux (rate of flow) of
metabolites through the pathway.
The flux of metabolites through a pathway is remarkably
sensitive to the needs of the organism for the products of
the pathway. Through an exquisitely complex network of
control mechanisms, flux through a pathway is only as
great as required.
1 THE GLYCOLYTIC PATHWAY
An overview of glucose metabolism is diagrammed in Fig.
17-1. Under aerobic conditions, the pyruvate formed by gly-
colysis is further oxidized by the citric acid cycle (Chapter 21)
and oxidative phosphorylation (Chapter 22) to CO
2
and
water. Under anaerobic conditions, however, the pyruvate is
instead converted to a reduced end product, which is lactate
in muscle (homolactic fermentation; a fermentation is an
anaerobic biological reaction process) and ethanol CO
2
in yeast (alcoholic fermentation).
A. Historical Perspective
The fermentation of glucose to ethanol and CO
2
by yeast
(Fig. 17-2) has been a useful process since before the dawn
of recorded history. Winemaking and bread baking both
exploit this process. Yet the scientific investigation of the
mechanism of glycolysis began only in the latter half of the
nineteenth century.
In the years 1854 to 1864, Louis Pasteur established
that fermentation is caused by microorganisms. It was not
until 1897, however, that Eduard Buchner demonstrated
that cell-free yeast extracts can also carry out this process.
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 593
This discovery refuted the then widely held belief that fer-
mentation, and every other biological process, was medi-
ated by some “vital force” inherent in living matter, and
thereby brought glycolysis within the province of chem-
istry. This was a major step in the development of bio-
chemistry as a science. Although, in principle, the use of
cell-free extracts enabled a systematic “dissection” of the
reactions involved in the pathway, the complete elucida-
tion of the glycolytic pathway was still a long-range pro-
ject because analytical techniques for the isolation and
identification of intermediates and enzymes had to be de-
veloped concurrently.
In the years 1905 to 1910, Arthur Harden and William
Young made two important discoveries:
1. Inorganic phosphate is required for fermentation
and is incorporated into fructose-1,6-bisphosphate, an in-
termediate in the process.
2. A cell-free yeast extract can be separated, by dialy-
sis, into two fractions that are both required for fermenta-
tion: A nondialyzable heat-labile fraction they named zy-
mase; and a dialyzable, heat-stable fraction they called
cozymase. It was shown later by others that zymase is a
mixture of enzymes and that cozymase is a mixture of co-
factors: coenzymes such as NAD
⫹
, ATP, and ADP, as well
as metal ions.
In their efforts to identify pathway intermediates, the
early investigators of glycolysis developed a general tech-
nique of metabolic investigation that is in use today:
Reagents are found that inhibit the production of pathway
products, thereby causing the buildup of metabolites that
can then be identified as pathway intermediates. Over years
of investigation that attempted to identify glycolytic inter-
mediates, various reagents were found that inhibit the pro-
duction of ethanol from glucose in yeast extracts. The use
of different inhibitors results in the accumulation of differ-
ent intermediates. For example,the addition of iodoacetate
to fermenting yeast extracts causes the buildup of fructose-
1,6-bisphosphate, whereas addition of fluoride ion induces
the accumulation of 3-phosphoglycerate and 2-phospho-
glycerate:
C
1
O
O
–
C
2
C
3
HOH
3-Phosphoglycerate
OPO
2
3
–
H
H
C
1
O O
–
C
2
C
3
H
OH
2-Phosphoglycerate
OPO
2
3
–
H
H
594 Chapter 17. Glycolysis
Figure 17-2 Electron micrograph of yeast cells. [Biophoto
Associates/Photo Researchers, Inc.]
Figure 17-1 Glycolysis. Glycolysis converts glucose to pyruvate
while generating two ATPs. Under anaerobic conditions, alcoholic
fermentation of pyruvate occurs in yeast, whereas homolactic
fermentation occurs in muscle. Under aerobic conditions,
pyruvate is oxidized to H
2
O and CO
2
via the citric acid cycle
(Chapter 21) and oxidative phosphorylation (Chapter 22).
Glucose
Fructose-1,6-
bisphosphate
2Pyruvate
2ADP
+ 2P
i
2NAD
+
2NADH
2ATP
2
Anaerobic
homolactic
fermentation
Anaerobic
alcoholic
fermentation
2Lactate
CO
2
+ 2Ethanol6CO
2
+
6H
2
O
2NADH
2NAD
+
2NADH
2NAD
+
2NADH
2NAD
+
6O
2
Oxidative
phosphorylation
Citric Acid
Cycle
Aerobic oxidation
Glycolysis
JWCL281_c17_593-637.qxd 7/20/10 5:44 PM Page 594

The mechanisms by which these inhibitors act are dis-
cussed in Sections 17-2Da and 17-2I, respectively.
One remarkable finding of these studies was that the
same intermediates and enzyme activities could be isolated
not only from yeast, but from a great variety of other or-
ganisms. With few exceptions (see Problem 11 in this chap-
ter), living things all metabolize glucose by identical path-
ways. In spite of their enormous diversity, they share a
common biochemistry.
By 1940, the efforts of many investigators had come to
fruition with the elucidation of the complete pathway of
glycolysis. The work of three of these individuals, Gustav
Embden, Otto Meyerhof, and Jacob Parnas, has been com-
memorated in that glycolysis is alternatively known as the
Embden–Meyerhof–Parnas pathway. Other major contrib-
utors to the elucidation of this pathway were Carl and
Gerty Cori, Carl Neuberg, Robert Robison, and Otto
Warburg.
B. Pathway Overview
Before beginning our detailed discussion of the enzymes
of glycolysis, let us first take a moment to survey the
overall pathway as it fits in with animal metabolism as a
whole. Glucose usually arises in the blood as a result of
the breakdown of higher polysaccharides (Sections 11-
2B, 11-2Db, and 18-1) or from its synthesis from noncar-
bohydrate sources (gluconeogenesis; Section 23-1). The
fate of nonglucose hexoses is discussed in Section 17-5.
Glucose enters most cells by specific carriers that trans-
port it from the exterior of the cell into the cytosol (Sec-
tion 20-2E). The enzymes of glycolysis are located in the
cytosol, where they are only loosely associated, if at all,
with cell structures such as membranes. However, there is
considerable circumstantial evidence that successive en-
zymes in the glycolytic pathway loosely associate, pre-
sumably to facilitate the efficient transfer of intermedi-
ates between enzymes. Such associations of functionally
related enzymes have been referred to as metabolons.
Nevertheless, no actual complexes of glycolytic enzymes
have yet been isolated.
Glycolysis converts glucose to two C
3
units (pyruvate) of
lower free energy in a process that harnesses the released
free energy to synthesize ATP from ADP and P
i
. This
process requires a pathway of chemically coupled phosphoryl-
transfer reactions (Sections 16-4 and 16-6).Thus the chem-
ical strategy of glycolysis is
1. Add phosphoryl groups to the glucose.
2. Chemically convert phosphorylated intermediates
into compounds with high phosphate group-transfer
potentials.
3. Chemically couple the subsequent hydrolysis of reac-
tive substances to ATP synthesis.
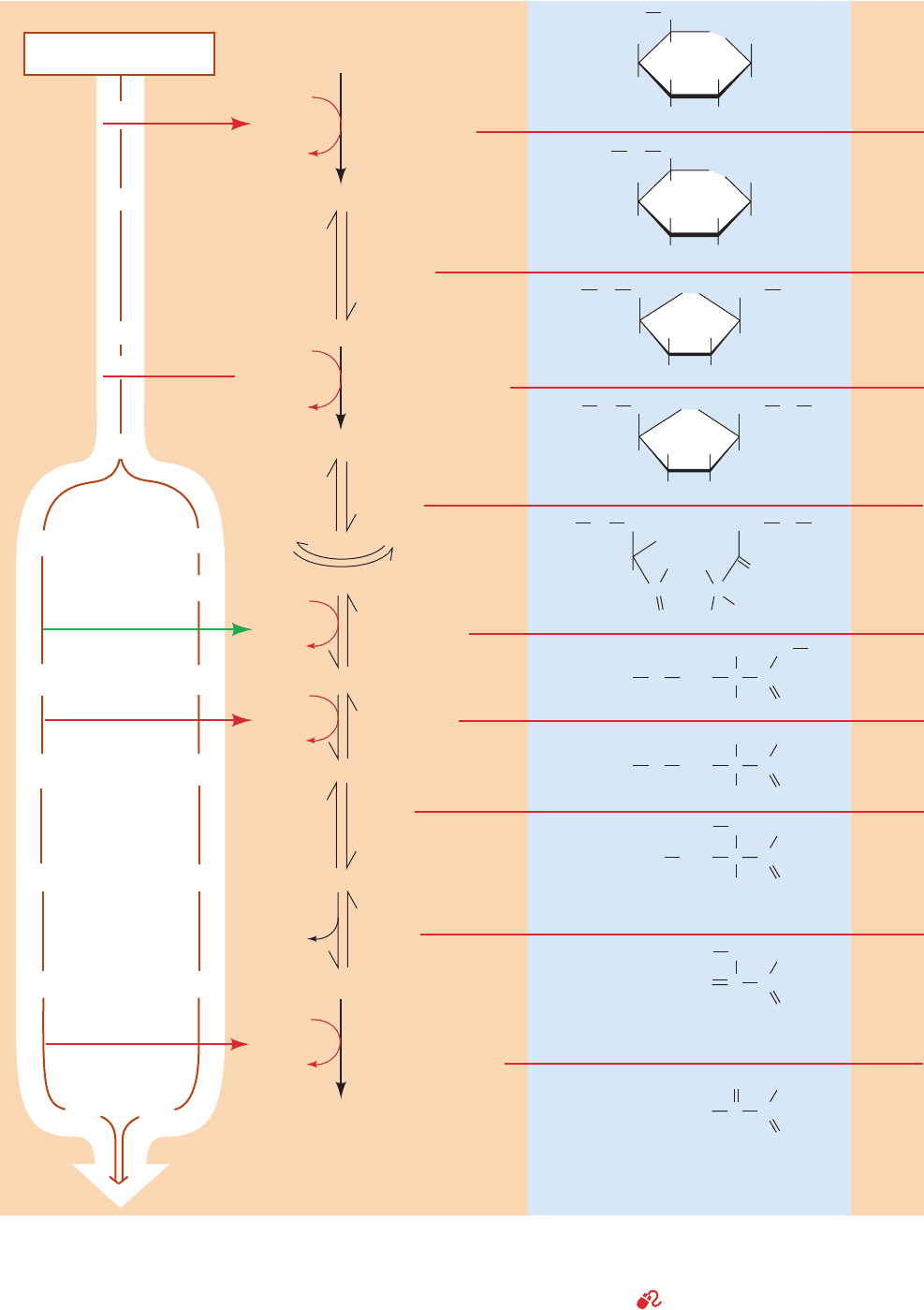
The 10 enzyme-catalyzed reactions of glycolysis are dia-
grammed in Fig. 17-3. Note that ATP is used early in the
pathway to synthesize phosphoryl compounds (Reactions
1 and 3) but is later resynthesized (Reactions 7 and 10).
Glycolysis may therefore be considered to occur in two
stages:
Stage I (Reactions 1–5): A preparatory stage in which
the hexose glucose is phosphorylated and cleaved to yield
two molecules of the triose glyceraldehyde-3-phosphate.
This process utilizes two ATPs in a kind of energy invest-
ment.
Stage II (Reactions 6–10): The two molecules of glycer-
aldehyde-3-phosphate are converted to pyruvate, with con-
comitant generation of four ATPs. Glycolysis therefore has
a net profit of two ATPs per glucose: Stage I consumes two
ATPs; Stage II produces four ATPs.
The overall reaction is
a. The Oxidizing Power of NAD
ⴙ
Must Be Recycled
NAD
⫹
is the primary oxidizing agent of glycolysis. The
NADH produced by this process (Fig. 17-3, Reaction 6)
must be continually reoxidized to keep the pathway sup-
plied with NAD
⫹
. There are three common ways that this
occurs (Fig. 17-1, bottom):
1. Under anaerobic conditions in muscle, NAD
⫹
is re-
generated when NADH reduces pyruvate to lactate (ho-
molactic fermentation; Section 17-3A).
2. Under anaerobic conditions in yeast, pyruvate is de-
carboxylated to yield CO
2
and acetaldehyde and the latter
is reduced by NADH to yield NAD
⫹
and ethanol (alco-
holic fermentation; Section 17-3B).
3. Under aerobic conditions, the mitochondrial oxida-
tion of each NADH to NAD
⫹
yields 2.5 ATPs (Section
22-2A).
Thus, in aerobic glycolysis, NADH may be thought of as a
“high-energy” compound, whereas in anaerobic glycolysis
its free energy of oxidation is dissipated as heat.
2 THE REACTIONS OF GLYCOLYSIS
See Guided Exploration 14: Glycolysis overview In this section
we examine the reactions of glycolysis more closely, de-
scribing the properties of the individual enzymes and their
mechanisms. In Section 17-3 we consider the anaerobic fate
of pyruvate. Finally, in Section 17-4 we consider the ther-
modynamics of the entire process and address the problem
of how the flux of metabolites through the pathway is con-
trolled. As we study the individual glycolytic enzymes we
shall encounter many organic reaction mechanisms (Sec-
tion 16-2). Indeed, the study of organic reaction mecha-
nisms has been invaluable in understanding the mecha-
nisms by which enzymes catalyze reactions.
2NADH ⫹ 2 pyruvate ⫹ 2ATP ⫹ 2H
2
O ⫹ 4H
⫹
Glucose ⫹ 2NAD
⫹
⫹ 2ADP ⫹ 2P
i
¡
Section 17-1. The Glycolytic Pathway 595
JWCL281_c17_593-637.qxd 6/3/10 8:16 AM Page 595
ATP
Glucose
O
C C
O
O
CH
2
H
OH H
HOH
HO
HH
OH
O
6
5
4
32
1
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
2
CH
3
CH
2
CH
2
O
H
OH H
HOH
HO
HH
OH
O
6
5
4
32
1
G6P
HO H
HOH
O OH
1
2
34
5
6
F6P
H HO
HO
H
H
OH
O
1
2
5
6
FBP
O PO
3
2
–
PO
3
2
–
PO
3
2
–
O
OH
H
H
O
3(1)
2
1(3)
1(4)
2(5)
3(6)
GAP
DHAP
H
O
OH
H
DHAPGAP
O
OH
3
2
1
1,3-BPG
C
O
O
–
O
–
O
–
O
–
3PG
2PG
C C
O
PEP
C C
O
O
Pyruvate
hexokinase (HK)
phosphoglucose
isomerase
(PGI)
phosphofructokinase
(PFK)
aldolase
glyceraldehyde-3-
phosphate
dehydrogenase
(GAPDH)
phosphoglycerate
kinase (PGK)
phosphoglycerate
mutase
(PGM)
enolase
pyruvate kinase (PK)
Glucose-6-phosphate (G6P)
Fructose-6-phosphate (F6P)
Fructose-1,6-bisphosphate (FBP)
triose phosphate isomerase (TIM)
1,3-Bisphosphoglycerate (1,3-BPG)
3-Phosphoglycerate (3PG)
2-Phosphoglycerate (2PG)
Phosphoenolpyruvate (PEP)
O
ADP
Mg
2+
Mg
2+
Mg
2+
Mg
2+
Mg
2+
, K
+
Glucose
ATP
ADP
ATP
ADP
ATP
ADP
Glucose
Pyr
PEP
Pyr
PEP
2PG2PG
3PG3PG
1,3-BPG
DHAP
GAP
GAP
2ADP
2ADP
2NAD
+
+ 2P
i
G6P
F6P
FBP
H
2
O
Pyruvate
HO
1
2
3
4
–2
O
3
P
–
2
O
3
P
–
2
O
3
P
–
2
O
3
P
–
2
O
3
P
–2
O
3
P
–
2
O
3
P
–
2
O
3
P
34
O
NAD
+
NADH
+ H
+
+
6
7
8
9
10
+
GAP DHAP
ATP
ATP
H
O
C
OH
H
C
O
HO
C
H
O
O
+
P
i
5
C
C
1,3-BPG
H HO
+
(Reactions 1 and 3). Stage II (Reactions 6–10): The two
molecules of glyceraldehyde-3-phosphate are converted to
pyruvate with the concomitant generation of four ATPs
(Reactions 7 and 10).
See the Animated Figures
596
Figure 17-3 Degradation of glucose via the glycolytic pathway.
Glycolysis may be considered to occur in two stages. Stage I
(Reactions 1–5): Glucose is phosphorylated and cleaved to form
two molecules of the triose glyceraldehyde-3-phosphate. This
requires the expenditure of two ATPs in an “energy investment”
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 596
Note that the X-ray structure of each of the 10 glycolytic
enzymes has been reported.All of these enzymes are either
homodimers or homotetramers with D
2
symmetry (Section
8-5B), whose subunits consist mainly of / domains (Sec-
tion 8-3Bi).
A. Hexokinase: First ATP Utilization
Reaction 1 of glycolysis is the transfer of a phosphoryl
group from ATP to glucose to form glucose-6-phosphate
(G6P) in a reaction catalyzed by hexokinase (HK):
A kinase is an enzyme that transfers phosphoryl groups be-
tween ATP and a metabolite (Section 16-4C).The metabo-
lite that serves as the phosphoryl group acceptor for a spe-
cific kinase is identified in the prefix of the kinase name.
HK is a relatively nonspecific enzyme contained in all cells
that catalyzes the phosphorylation of hexoses such as
D-
glucose,
D-mannose, and D-fructose.Liver cells also contain
glucokinase, which catalyzes the same reaction but which is
primarily involved in the maintenance of blood glucose
levels (Section 18-3Fa). The second substrate for HK, as
with other kinases, is an Mg
2
–ATP complex. In fact, un-
complexed ATP is a potent competitive inhibitor of HK. In
what follows, we shall rarely refer to this Mg
2
require-
ment, but keep in mind that it is essential for kinase enzy-
matic activity (other divalent metal ions such as Mn
2
often satisfy the metal ion requirements of kinases in vitro,
but Mg
2
is the normal physiological species).
a. Kinetics and Mechanism of the
Hexokinase Reaction
Hexokinase has a Random Bi Bi mechanism in which
the enzyme forms a ternary complex with glucose and
Mg
2
–ATP before the reaction occurs. The Mg
2
, by com-
plexing with the phosphate oxygen atoms, is thought to
shield their negative charges, making the phosphorus atom
CH
2
OH
H
OH H
HOH
HO
HH
OH
O
+ ATP
hexokinase
Mg
2+
Glucose
CH
2
H
OH H
HOH
HO
HH
OH
O
+ ADP
Glucose-6-phosphate
(G6P)
+ H
+
OPO
2
–
3
more accessible for the nucleophilic attack of the
group of glucose (Fig. 17-4).
An important mechanistic question is why does HK cat-
alyze the transfer of a phosphoryl group from ATP to glu-
cose to yield G6P, but not to water to yield ADP P
i
(ATP
hydrolysis)? Water is certainly small enough to fit into the
phosphoryl acceptor group’s enzymatic binding site. Fur-
thermore, phosphoryl transfer from ATP to water is more
exergonic than it is to glucose (Table 16-3), particularly
since [H
2
O] 55.5M and [glucose] 5 to 10 mM in vivo.
Yet HK catalyzes phosphoryl transfer to glucose 40,000
times faster than it does to water.
The answer was provided by Thomas Steitz via X-ray
structural studies of yeast HK. Comparison of the X-ray
structures of HK and the glucose–HK complex indicates
that glucose induces a large conformational change in HK
(Fig. 17-5). The two lobes that form its active site cleft
swing together by up to 11.5 Å so as to engulf the glucose
in a manner that suggests the closing of jaws. This move-
ment places the ATP in close proximity to the
group of glucose and excludes water from the active site
(catalysis by proximity effects; Section 15-1E). If the cat-
alytic and reacting groups were in the proper position for
reaction while the enzyme was in the open position (Fig.
17-5a),ATP hydrolysis would almost certainly be the dom-
inant reaction. This conclusion is confirmed by the obser-
vation that xylose, which differs from glucose only by the
lack of the group,
greatly enhances the rate of ATP hydrolysis by HK (pre-
sumably xylose induces the activating conformational
change while water occupies the binding site of the missing
H
H
H
H
H
OH
OH
-
D-Xylose
OH
H
HO
O
5
4
32
1
¬C6H
2
OH
¬C6H
2
OH
C6¬OH
Section 17-2. The Reactions of Glycolysis 597
Figure 17-4 The nucleophilic attack of the group of
glucose on the ␥ phosphate of an Mg
2
–ATP complex. The
position of the Mg
2
ion is shown as an example; its actual
position(s) has not been conclusively established. In any case, the
Mg
2
functions to shield the negatively charged groups of ATP
and thereby facilitates the nucleophilic attack.
C6¬OH
PO
O
–
Adenosine
O
O
O
–
O
O
P
P
O
–
O
O
–
Mg
2+
...
...
CH
2
H
OH H
HOH
HO
HH
OH
O
Glucose
O
H
ATP
O
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 597
hydroxymethyl group). Clearly, this substrate-induced con-
formational change in HK is responsible for the enzyme’s
specificity. In addition, the active site polarity is reduced by
exclusion of water, thereby expediting the nucleophilic re-
action process. Other kinases have the same deeply clefted
structure as HK (e.g., Section 17-2G) and undergo confor-
mational changes on binding their substrates. This suggests
that all kinases have similar mechanisms for maintaining
specificity.
B. Phosphoglucose Isomerase
Reaction 2 of glycolysis is the conversion of G6P to
fructose-6-phosphate (F6P) by phosphoglucose isomerase
(PGI; also called glucose-6-phosphate isomerase). This is
the isomerization of an aldose to a ketose:
Since G6P and F6P both exist predominantly in their cyclic
forms (Fig. 11-4 shows these structures for the unphos-
phorylated sugars), the reaction requires ring opening, fol-
lowed by isomerization, and subsequent ring closure. The
determination of the enzyme’s pH dependence led to a hy-
pothesis for amino acid side chain participation in the cat-
alytic mechanism. The catalytic rate exhibits a bell-shaped
pH dependence curve with characteristic pK’s of 6.7 and
9.3, which suggests the catalytic participation of both a His
and a Lys (Section 14-4). Indeed, comparison of the amino
acid sequences of PGI from several different organisms re-
veals that both a His and Lys are conserved. However, a
Glu residue is also conserved, and as we have seen for
lysozyme (Section 15-2Ba), Glu can have an unusually high
pK under certain conditions. In fact, the X-ray structure of
PGI reveals that Glu 216 and His 388 form a hydrogen
bonded catalytic dyad (resembling the interaction of the
Asp and His residues in the catalytic triad of serine pro-
teases; Fig. 15-20), which facilitates the action of His 388 as
an acid–base catalyst.
A proposed reaction mechanism for the PGI reaction in-
volves general acid–base catalysis by the enzyme (Fig.17-6):
Step 1 Substrate binding.
Step 2 An acid, presumably the Lys ε-amino group, cat-
alyzes ring opening.
Step 3 A base, presumably the imidazole portion of the
His–Glu dyad, abstracts the acidic proton from C2 to form
H
OH H
HOH
HO
H H
OH
O
Glucose-6-phosphate (G6P)
phosphoglucose
isomerase (PGI)
H OH
H HO
HO
H
O
CH
2
OH
Fructose-6-phosphate (F6P)
52
1
34
1
23
4
5
6
–2
O
3
POCH
2
–2
O
3
POCH
2
6
598 Chapter 17. Glycolysis
(a)
(b)
Figure 17-5 Conformational changes in yeast hexokinase on
binding glucose. The enzyme is represented by its transparent
molecular surface with its embedded ribbon diagram colored
with its large domain purple and its small domain yellow. (a)
Free hexokinase. (b) Hexokinase in complex with glucose drawn
in space-filling form with C green and O red. Note the prominent
bilobal appearance of the free enzyme. In the enzyme–substrate
complex these lobes have swung together by a 17° rotation to
engulf the substrate. [Based on an X-ray structure by Igor
Polikarpov, Instituto de Física em São Carlos, Brazil. PDBids
1IG8 and 3B8A.]
See Interactive Exercise 8
JWCL281_c17_593-637.qxd 10/19/10 7:25 AM Page 598
Section 17-2. The Reactions of Glycolysis 599
a cis-enediolate intermediate (this proton is acidic because
it is to a carbonyl group).
Step 4 The proton is replaced on C1 in an overall pro-
ton transfer. Protons abstracted by bases are labile and ex-
change rapidly with solvent protons. Nevertheless, Irwin
Rose confirmed this step by demonstrating that [2-
3
H]G6P
is occasionally converted to [1-
3
H]F6P by intramolecular
proton transfer before the
3
H has had a chance to exchange
with the medium.
Step 5 Ring closure to form the product, which is subse-
quently released to yield free enzyme, thereby completing
the catalytic cycle.
PGI, like most enzymes, catalyzes reactions with nearly
absolute stereospecificity. To appreciate this, let us compare
the proposed enzymatic reaction mechanism with that in
the nonenzymatic base-catalyzed isomerization of glucose,
fructose, and mannose (Fig. 17-7). Glucose and mannose
are epimers of one another because they differ only with
Figure 17-6 Reaction mechanism of
phosphoglucose isomerase. The active
site catalytic residues (BH
and B¿) are
thought to be Lys and a His–Glu dyad,
respectively.
Figure 17-7 Base-catalyzed isomerization of glucose, mannose, and fructose. In the
absence of enzyme, this reaction is nonstereospecific.
H
O
H
H
H
OH
HO
OH
H*
POCH
2
–2
O
3
H
B
B
+
1
23
4
5
6
Glucose-6-phosphate (G6P)
H
H
+
ring
opening
2
H
H
H
H
O
HO
OH
H*
POCH
2
–2
O
3
B
H
H
O
H
H
H
H
O
HO
OH
H*
POCH
2
–2
O
3
B
+
B
H
H
O
–
C
3
O
H
OH
H
OH
H
H
H*
POCH
2
–2
O
3
B
Fructose-6-phosphate (F6P)
B
+
H
H
+
ring
closure
5
1
H
H
H
H
O
HO
OH
H*
POCH
2
–2
O
3
B
B
H
OH
HO
OH
C
cis-Enediolate
intermediate
4
exchange of
H
+
with
medium
F6P
G6P
O O
O
O
>
B
>
>
>
>
> >
C
OH
H
H C
C
OH
HO
H
OH
OH
H
C
CH
2
OH
C
C
OH
H
HO C
C
H
HO
H
OH
OH
H
C
CH
2
OH
C
C
O
H
H
C
C
OH
HO
H
OH
OH
H
C
CH
2
OH
C
C
OH
OH
1
2
3
4
5
6
O
HC
H
OH
HO
OH
H
H
OHH
C
C
C
C
CH
2
OH
1
2
3
4
5
6
1
2
3
4
5
6
1
2
3
4
5
6
Glucose cis-Enediolate
intermediate
Mannose
Fructose
OH
JWCL281_c17_593-637.qxd 2/26/10 1:37 PM Page 599
600 Chapter 17. Glycolysis
respect to their configuration at one chiral center, C2 (Sec-
tion 11-1A). In the enediolate intermediate, as well as in
the linear form of fructose, C2 lacks chirality. Therefore, in
nonenzymatic systems, base-catalyzed isomerization of
glucose also results in racemization of C2 with the produc-
tion of mannose. In the presence of PGI, however,
1
H
NMR measurements indicate that the rate of the isomer-
ization reaction is several orders of magnitude greater than
that of the epimerization reaction. Evidently, PGI shields
the face of the enediolate to which H
⫹
must be added to
form mannose-6-phosphate.
C. Phosphofructokinase: Second ATP Utilization
In Reaction 3 of glycolysis, phosphofructokinase (PFK)
phosphorylates F6P to yield fructose-1,6-bisphosphate
[FBP or F1,6P; previously known as fructose-1,6-
diphosphate (FDP)]:
This reaction is similar to the hexokinase reaction (Reac-
tion 1 in Fig. 17-3; Section 17-2A). PFK catalyzes the nucle-
ophilic attack by the group of F6P on the elec-
trophilic ␥-phosphorus atom of the Mg
2⫹
–ATP complex.
C1¬OH
H OH
H HO
HO
H
O
CH
2
Fructose-1,6-bisphosphate
(FBP)
H OH
H HO
HO
H
O
CH
2
OH
Fructose-6-phosphate
(F6P)
52
1
34
phosphofructokinase (PFK)
Mg
2+
+ ATP
ADP
+ H
+
2
34
5
1
6
–2
O
3
POCH
2
–2
O
3
POCH
2
6
+
OPO
2
–
3
PFK plays a central role in the control of glycolysis be-
cause it catalyzes one of the pathway’s rate-determining re-
actions. In many organisms the activity of PFK is enhanced
allosterically by several substances, including AMP, and in-
hibited allosterically by several other substances, including
ATP and citrate. The control of PFK is exquisitely com-
plex; the mechanism by which it regulates the glycolytic
pathway is examined in Section 17-4F.
D. Aldolase
Aldolase catalyzes Reaction 4 of glycolysis, the cleavage of
FBP to form the two trioses glyceraldehyde-3-phosphate
(GAP) and dihydroxyacetone phosphate (DHAP):
This reaction is an aldol cleavage (retro aldol condensa-
tion) whose nonenzymatic base-catalyzed mechanism is
shown in Fig. 17-8. Note that aldol cleavage between C3
and C4 of FBP requires a carbonyl at C2 and a hydroxyl at
C4. Hence, the “logic” of Reaction 2 in the glycolytic path-
way, the isomerization of G6P to F6P, is clear.Aldol cleav-
age of G6P would have resulted in products of unequal car-
bon chain length,while aldol cleavage of FBP results in two
C
OH
O
CH
H
Glyceraldehyde-
3-phosphate
(GAP)
3
2
1
Dihydroxyacetone
phosphate (DHAP)
+
Fructose-
1,6-bisphosphate
(FBP)
OHCH
6
5
OHCH
HCHO
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
OPO
3
2
–
CH
2
C O
2
1
3
4
aldolase
HO
1
C O
2
3
Figure 17-8 Mechanism for base-catalyzed aldol cleavage. Aldol condensation occurs by the
reverse mechanism.
R
C O
CH
2
C
R⬘
OHH
OH
–
OH
–
HOH
1
R
C O
CH
2
C
R⬘
O
–
H
2
R
C O
CH
2
–
R
C O
–
CH
2
CH
3
+
C
R⬘
OH
Product 1
Enolate
3
H
2
O
R
C O
Product 2
JWCL281_c17_593-637.qxd 6/3/10 8:16 AM Page 600
