Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

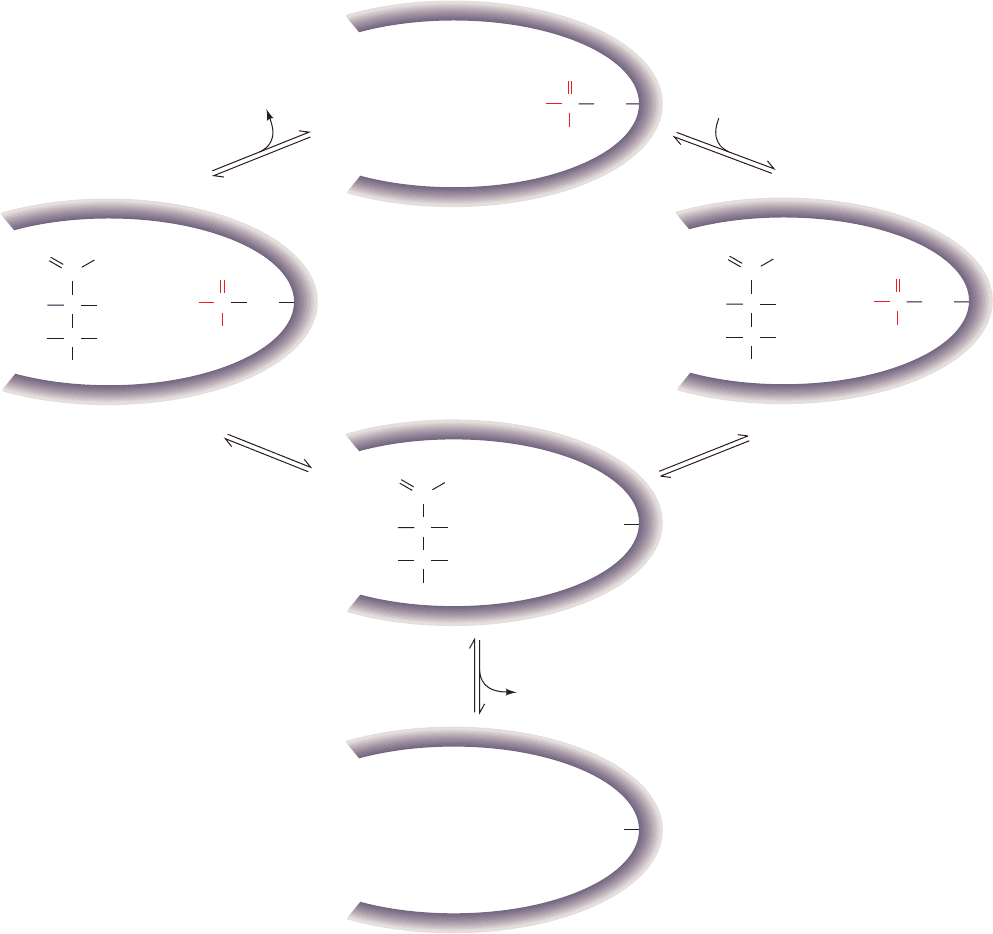
Occasionally, 2,3-BPG dissociates from the enzyme (Fig.
17-18; Step 5), leaving it in an inactive form. Trace amounts
of 2,3-BPG must therefore always be available to regener-
ate the active phosphoenzyme by the reverse reaction.
b. Glycolysis Influences Oxygen Transport
2,3-BPG specifically binds to deoxyhemoglobin and
thereby alters the oxygen affinity of hemoglobin (Section
10-1D). The concentration of 2,3-BPG in erythrocytes is
much higher (⬃5 mM) than the trace amounts required for
its use as a primer of PGM. Erythrocytes synthesize and
degrade 2,3-BPG by a detour from the glycolytic pathway,
diagrammed in Fig. 17-19. Bisphosphoglycerate mutase
catalyzes the transfer of a phosphoryl group from C1 to C2
of 1,3-BPG.The resulting 2,3-BPG is hydrolyzed to 3PG by
2,3-bisphosphoglycerate phosphatase. The rate of glycoly-
sis affects the oxygen affinity of hemoglobin through the
mediation of 2,3-BPG. Consequently, inherited defects of
Section 17-2. The Reactions of Glycolysis 611
Figure 17-18 Proposed reaction mechanism for
phosphoglycerate mutase. The active form of the enzyme
contains a phospho-His residue at the active site. (1) Formation
of an enzyme–substrate complex; (2) transfer of the enzyme-
bound phosphoryl group to the substrate; (3) rephosphorylation
Phosphoenzyme
C
OH
O
–
CH
O
CH
H
OPO
3
2–
P
–
O
O
O
–
His
C
O
–
CH
O
CH
H
OPO
3
2–
His
His
OPO
3
2–
2,3-BPG•Enzyme
complex
C
O
–
CH
O
CH
H
OH
OPO
3
2–
P
–
O
O
O
–
His
1
2
3
5
4
P
–
O
O
O
–
His
3PG
3PG•Phosphoenzyme
complex
2PG•Phosphoenzyme
complex
2PG
Dephosphoenzyme
(inactive)
2,3-BPG
of the enzyme by the other phosphoryl group of the substrate;
and (4) release of product regenerating the active
phosphoenzyme. (5) Occasionally, 2,3-BPG dissociates from the
enzyme, leaving it in an inactive, dephospho form that must be
rephosphorylated by the reverse reaction.
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 611
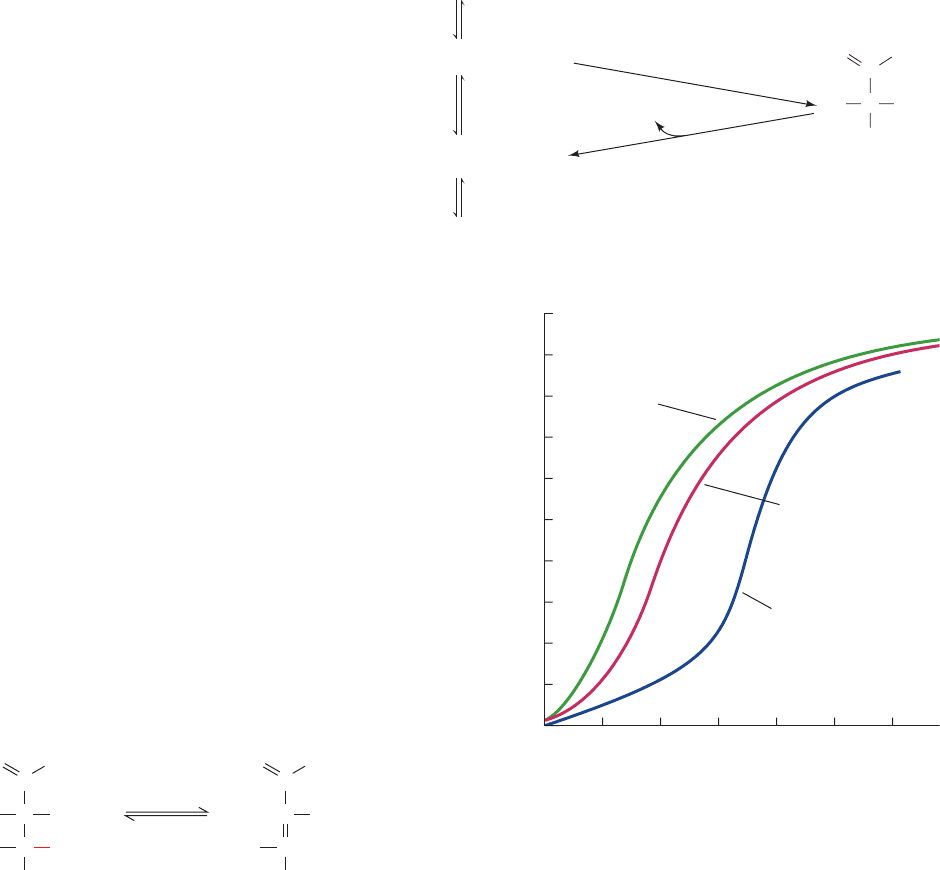
glycolysis in erythrocytes alter the capacity of the blood to
transport oxygen (Fig. 17-20). For example, the concentra-
tion of glycolytic intermediates in hexokinase-deficient
erythrocytes is less than normal because hexokinase cat-
alyzes the first reaction of glycolysis. This results in a di-
minished 2,3-BPG concentration and therefore in increased
hemoglobin oxygen affinity. Conversely, pyruvate kinase
deficiency decreases hemoglobin oxygen affinity through
the increase of 2,3-BPG resulting from the blockade of the
last reaction in glycolysis. Thus, although erythrocytes,
which lack nuclei and other organelles, have but a minimal
metabolism, this metabolism is physiologically significant.
I. Enolase: Second “High-Energy”
Intermediate Formation
In Reaction 9 of glycolysis, 2PG is dehydrated to phospho-
enolpyruvate (PEP) in a reaction catalyzed by enolase:
The enzyme forms a complex with a divalent cation such as
Mg
2
before the substrate is bound. A second divalent
metal ion then binds to the enzyme.As is mentioned in Sec-
tion 17-1A, fluoride ion inhibits glycolysis, resulting in the
accumulation of 2PG and 3PG. It does so by strongly in-
hibiting enolase in the presence of P
i
.F
and P
i
form a
tightly bound complex with the Mg
2
at the enzyme’s ac-
tive site, blocking substrate binding and thereby inactivat-
ing the enzyme. Enolase’s substrate, 2PG, therefore builds
up and, as it does so, is equilibrated with 3PG by PGM.
a. Catalytic Mechanism of Enolase
The dehydration (elimination of H
2
O) catalyzed by eno-
lase might occur in one of three ways (Fig. 16-9a): (1) The
group at C3 can leave first, generating a carbocation¬OH
2-Phosphoglycerate
(2PG)
OO
–
C
OPO
2
3
–
C
H
H
H
OH
3
2
C
1
Phosphoenolpyruvate
(PEP)
OO
–
C + H
2
O
OPO
2
3
–
C
H
H
C
enolase
at C3; (2) the C2 proton can leave first, generating a carban-
ion at C2; or (3) the reaction can be concerted. Isotope
exchange studies by Paul Boyer demonstrated that the C2
proton of 2PG exchanges with solvent 12 times faster than
the rate of PEP formation. However, the C3 oxygen ex-
changes with solvent at a rate roughly equivalent with the
overall reaction rate.This suggests the following mechanism
(Fig. 17-21):
Step 1 Rapid carbanion formation at C2 facilitated by a
general base on the enzyme. The abstracted proton can
readily exchange with the solvent, accounting for its ob-
served rapid exchange rate.
Step 2 Rate-limiting elimination of the group at
C3.This is consistent with the slow rate of exchange of this
hydroxyl group with solvent.
¬OH
612 Chapter 17. Glycolysis
Figure 17-19 The pathway for the synthesis
and degradation of 2,3-BPG in erythrocytes is a
detour from the glycolytic pathway.
Figure 17-20 The oxygen-saturation curves of hemoglobin in
normal erythrocytes (red curve) and in those from patients with
hexokinase deficiency (green) and with pyruvate kinase
deficiency (purple). [After Delivoria-Papadopoulos, M., Oski,
F.A., and Gottlieb,A.J., Science 165, 601 (1969).]
3-Phosphoglycerate
2-Phosphoglycerate
1,3-Bisphosphoglycerate
2,3-Bisphospho-
glycerate
(2,3-BPG)
Glyceraldehyde 3-phosphate
GAPDH
PGK
PGM
bisphosphoglycerate
mutase
2,3-bisphosphoglycerate
phosphatase
P
i
OO
C
CHOPO
3
2
CH
2
OPO
3
2
0 102030405060
pO
2
(torr)
Hexokinase
deficient
Pyruvate kinase
deficient
Normal
erythrocytes
100
90
80
70
60
50
40
30
20
10
0
Oxygen saturation (%)
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 612
C
C
C
C
H
H
H
PO
OO
⫺
O
OH
H
O
⫺
O
⫺
O
O
NH
3
⫹
Mg
2⫹
Mg
2⫹
PO
O
O
⫺
O
⫺
PO
O
O
⫺
O
⫺
Mg
2⫹
Mg
2⫹
Mg
2⫹
Mg
2⫹
Mg
2⫹
Mg
2⫹
N
H
2
N
H
2
Lys 345
Lys 396
C
C
C
C
C
H
H
H
⫺
O
O
⫺
OH
H
H
H
O
O
NH
3
⫹
⫹
N
H
2
1 fast
2-Phosphoglycerate (2 PG)
C
C
C
C
H
O
O
⫺
H
OH
O
O
NH
3
⫹
C
C
CH
2
H
O
O
⫺
O
⫺
H H
O
O
NH
3
⫹
⫹
N
H
2
2 slow
Delocalized carbanion intermediate
C
⫹
rapid
exchange
H
2
O HOH
Glu 211
Phosphoenolpyruvate (PEP)
PO
O
O
⫺
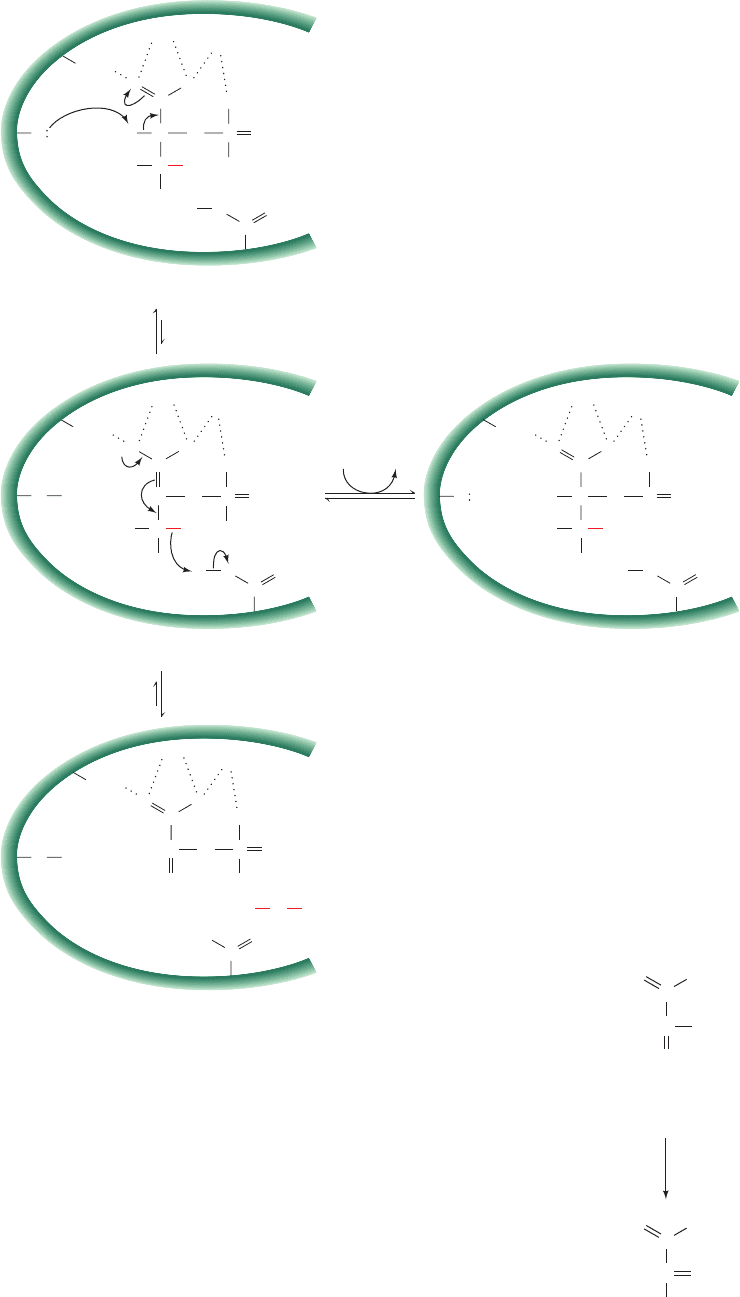
The enolase reaction (Fig. 17-21) is of mechanistic inter-
est because it involves the abstraction of the decidedly
nonacidic proton at C2 (pK ⬎ 30), followed by the elimina-
tion of an OH
⫺
ion, which is a poor leaving group. The X-
ray structure of yeast enolase in complex with two Mg
2⫹
ions and an equilibrium mixture of 2PG and PEP (eno-
lase’s substrate and product), determined by George Reed
and Ivan Rayment, reveals that enolase binds 2PG in an in-
tricate complex that involves both Mg
2⫹
ions. Mutagenic
and enzymological studies indicate that the reaction in-
volves the Lys 345 side chain functioning as a general base
and the Glu 211 side chain functioning as a general acid.
Lys 396 and the two Mg
2⫹
ions are thought to stabilize the
increased negative charge that develops on the carboxylate
ion in the delocalized carbanion intermediate.
J. Pyruvate Kinase: Second ATP Generation
In Reaction 10 of glycolysis, its final reaction, pyruvate ki-
nase (PK) couples the free energy of PEP hydrolysis to the
synthesis of ATP to form pyruvate:
Phosphoenolpyruvate
(PEP)
OO
–
C + ADP
OPO
2
3
–
CH
2
H
+
C
Pyruvate
OO
–
C + ATP
O
CH
3
C
+
pyruvate
kinase (PK)
Section 17-2. The Reactions of Glycolysis 613
Figure 17-21 Proposed reaction mechanism of enolase. (1)
Rapid formation of a carbanion by removal of a proton at C2 by
Lys 345 acting as a general base; this proton can rapidly exchange
with the solvent. (2) Slow elimination of H
2
O to form phospho-
enolpyruvate with general acid catalysis by Glu 211; the C3 oxy-
gen of the substrate can exchange with solvent only as rapidly as
this step occurs.
JWCL281_c17_593-637.qxd 6/30/10 10:54 AM Page 613
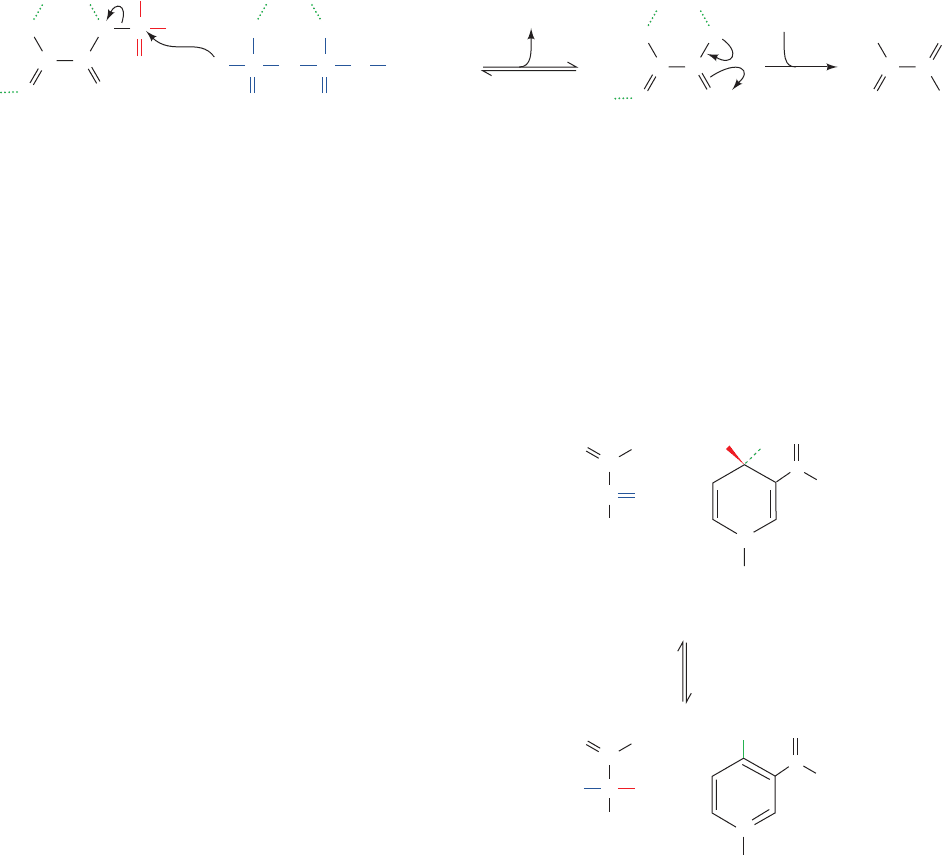
a. Catalytic Mechanism of PK
The PK reaction, which requires the participation of
both monovalent (K
⫹
) and divalent (Mg
2⫹
) cations, occurs
as follows (Fig. 17-22):
Step 1 A -phosphoryl oxygen of ADP nucleophilically
attacks the PEP phosphorus atom, thereby displacing
enolpyruvate and forming ATP. This reaction conserves
the free energy of PEP hydrolysis.
Step 2 Enolpyruvate converts to pyruvate.This enol–keto
tautomerization is sufficiently exergonic to drive the coupled
endergonic synthesis of ATP (Section 16-4Ba).
We can now see the “logic” of the enolase reaction. The
standard free energy of hydrolysis of 2PG (⌬G°¿) is only
⫺17.6 kJ ⴢ mol
⫺1
, which is insufficient to drive ATP synthe-
sis (⌬G°¿ ⫽ 30.5 kJ ⴢ mol
⫺1
for ATP synthesis from ADP
and P
i
).The dehydration of 2PG results in the formation of
a “high-energy” compound capable of such synthesis [the
standard free energy of hydrolysis of PEP is ⫺61.9
kJ ⴢ mol
⫺1
(Fig. 16-25)]. In other words, PEP is a “high-
energy” compound, 2PG is not.
3 FERMENTATION: THE ANAEROBIC
FATE OF PYRUVATE
For glycolysis to continue, NAD
⫹
, which cells have in lim-
ited quantities, must be recycled after its reduction to
NADH by GAPDH (Fig. 17-3; Reaction 6). In the presence
of oxygen, the reducing equivalents of NADH are passed
into the mitochondria for reoxidation (Chapter 22). Under
anaerobic conditions, on the other hand, the NAD
⫹
is re-
plenished by the reduction of pyruvate in an extension of
the glycolytic pathway.Two processes for the anaerobic re-
plenishment of NAD
⫹
are homolactic and alcoholic fer-
mentation, which occur in muscle and yeast, respectively.
A. Homolactic Fermentation
In muscle, particularly during vigorous activity when the
demand for ATP is high and oxygen has been depleted, lac-
tate dehydrogenase (LDH) catalyzes the oxidation of
NADH by pyruvate to yield NAD
⫹
and lactate. This reac-
tion is often classified as Reaction 11 of glycolysis:
LDH, as do other NAD
⫹
-requiring enzymes, catalyzes its
reaction with absolute stereospecificity: The pro-R (A-side)
hydrogen at C4 of NADH is stereospecifically transferred
to the re face of pyruvate at C2 to form
L- (or S-) lactate.
This regenerates NAD
⫹
for participation in the GAPDH
reaction.The hydride transfer to pyruvate is from the same
face of the nicotinamide ring as that to acetaldehyde in the
alcohol dehydrogenase reaction (Section 13-2A) but from
the opposite (si) face of the nicotinamide ring as that to
GAP in the GAPDH reaction (Section 17-2F).
Mammals have two different types of LDH subunits, the
M type and the H type, which together form five tetrameric
isozymes: M
4
,M
3
H, M
2
H
2
,MH
3
, and H
4
. Although these
hybrid forms occur in most tissues, the H-type subunit pre-
dominates in aerobic tissues such as heart muscle, while the
M-type subunit predominates in tissues that are subject to
anaerobic conditions such as skeletal muscle and liver. H
4
N
R
4
53
6
1
1
2
3
2
H
R
H
S
H
+
++
C
O
–
C
O
CH
3
O
NADH
NAD
+
Pyruvate
L-Lactate
N
R
1
2
3
H
+
+
C
O
–
C
O
CH
3
HHO
lactate
dehydrogenase (LDH)
C
NH
2
O
C
NH
2
O
614 Chapter 17. Glycolysis
Figure 17-22 Mechanism of the reaction catalyzed by pyruvate
kinase. (1) Nucleophilic attack of an ADP -phosphoryl oxygen
atom on the phosphorus atom of PEP to form ATP and
PO
–
O
–
O
ATP
C
O
1
Phosphoenol-
pyruvate (PEP)
ADP
O
–
O
CH
2
Mg
2+
K
+
C
P
O
O P
O
O Adenosine
+
–
O
–
O
O
–
Mg
2+
C
O
Enolpyruvate
O
––
O
CH
2
H
+
Mg
2+
K
+
C C
O
Pyruvate
O
–
O
CH
3
C
2
ΔG°´
⫽ ⫹14.6 kJ
•
mol
⫺1
ΔG°´
⫽ ⫺46 kJ
•
mol
⫺1
Overall ΔG°´
⫽ ⫺31.4 kJ
•
mol
⫺1
enolpyruvate; and (2) tautomerization of enolpyruvate to
pyruvate.
JWCL281_c17_593-637.qxd 6/3/10 8:37 AM Page 614
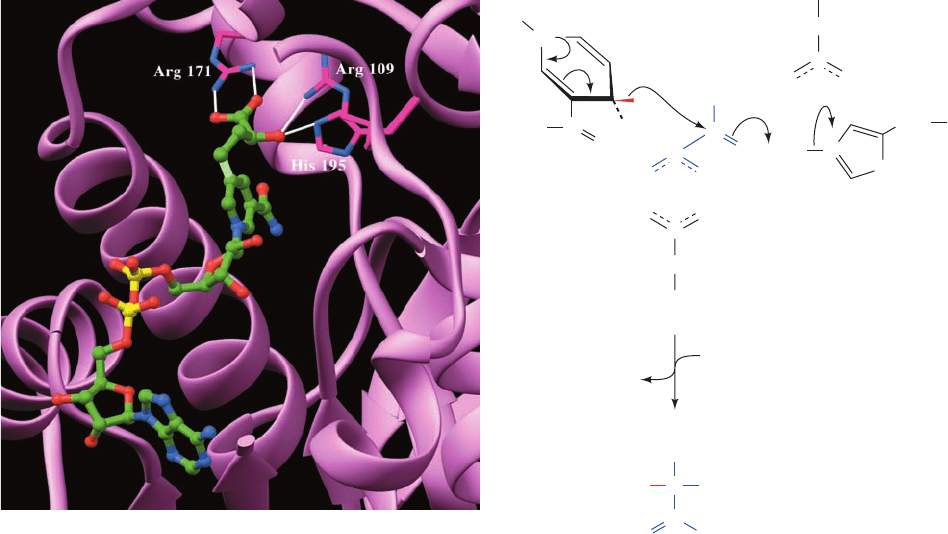
LDH has a low K
M
for pyruvate and is allosterically inhib-
ited by high levels of this metabolite, whereas the M
4
isozyme has a higher K
M
for pyruvate and is not inhibited
by it.The other isozymes have intermediate properties that
vary with the ratio of their two types of subunits. It has
therefore been proposed, although not without disagree-
ment, that H-type LDH is better adapted to function in the
oxidation of lactate to pyruvate, whereas M-type LDH is
more suited to catalyze the reverse reaction.
The X-ray structure of porcine H
4
LDH in complex with
S-lac-NAD
⫹
(a bisubstrate analog in which atom C3 of lac-
tate is covalently linked to nicotinamide atom C5 of NAD
⫹
via a CH
2
group) was determined by Michael Rossmann
(Fig. 17-23; he also determined the X-ray structure of dog-
fish M
4
LDH shown in Fig. 8-54a). Lactate atom O2, its hy-
droxyl oxygen, is hydrogen bonded to the side chains of
both Arg 109 and His 195, whereas the lactate carboxyl
group at C1 is doubly hydrogen bonded to the side chain of
Arg 171. On the basis of this structure and extensive enzy-
mological evidence, Rossmann proposed the following
mechanism for pyruvate reduction by LDH (Fig. 17-24):
The pro-R hydride is transferred from C4 of NADH’s
nicotinamide ring to C2 of pyruvate with the concomitant
transfer of a proton from the imidazolium moiety of His 195
to pyruvate O2, thereby yielding NAD
⫹
and lactate. The
proton transfer is facilitated by repulsive interactions with
the closely associated positively charged side chain of Arg
109. These interactions also serve to properly orient the
pyruvate, as does the salt bridge that the pyruvate carboxyl
group forms with the side chain of Arg 171.
The overall process of anaerobic glycolysis in muscle
can be represented:
Much of the lactate, the end product of anaerobic glycolysis,
is exported from the muscle cell via the blood to the liver,
where it is reconverted to glucose (Section 23-1C).
Contrary to the widely held belief, it is not lactate
buildup in the muscle per se that causes muscle fatigue and
soreness but the accumulation of glycolytically generated
acid (muscles can maintain their workload in the presence
of high lactate concentrations if the pH is kept constant;
but see Section 27-2B). Indeed, it is well known among
hunters that the meat of an animal that has run to exhaus-
tion before being killed has a sour taste. This is a result of
lactic acid buildup in the muscles.
2 lactate ⫹ 2ATP ⫹ 2H
2
O ⫹ 2H
⫹
Glucose ⫹ 2ADP ⫹ 2P
i
¡
Section 17-3. Fermentation: The Anaerobic Fate of Pyruvate 615
Figure 17-23 The active site region of porcine H
4
LDH in
complex with S-lac-NAD
ⴙ
, a covalent adduct of lactate and
NAD
ⴙ
. The adduct is shown in ball-and-stick form with C green,
N blue, O red, and P yellow, except for the covalent bond
between the methylene substituent to lactate atom C3 and
nicotinamide atom C5, which is light green. The three LDH side
chains that form hydrogen bonds (white lines) with the pyruvate
residue are shown in stick form with C magenta and N blue.
[Based on an X-ray structure by Michael Rossmann, Purdue
University. PDBid 5LDH.]
Figure 17-24 Reaction mechanism of lactate dehydrogenase.
The reaction involves direct hydride transfer from NADH to
pyruvate’s carbonyl carbon atom accompanied by proton
donation from the imidazolium group of His 195 to the pyruvate
carbonyl oxygen atom.The latter process is facilitated by the
positive charge on the nearby side chain of Arg 109.
N
C
O
H
2
N
.
.
R
C
O
C
OO
NH
2
NH
2
H
2
N
H
2
N
C
C
. . .
. . .
. . .
...
...
H
–
H
H
NH
NH
Arg 171
Arg 109
N
+
NH
CH
2
His 195
PyruvateNADH
H
+
NAD
+
C
OH
O
–
CH
O
CH
3
CH
3
+
+
L-Lactate
JWCL281_c17_593-637.qxd 6/10/10 1:06 PM Page 615
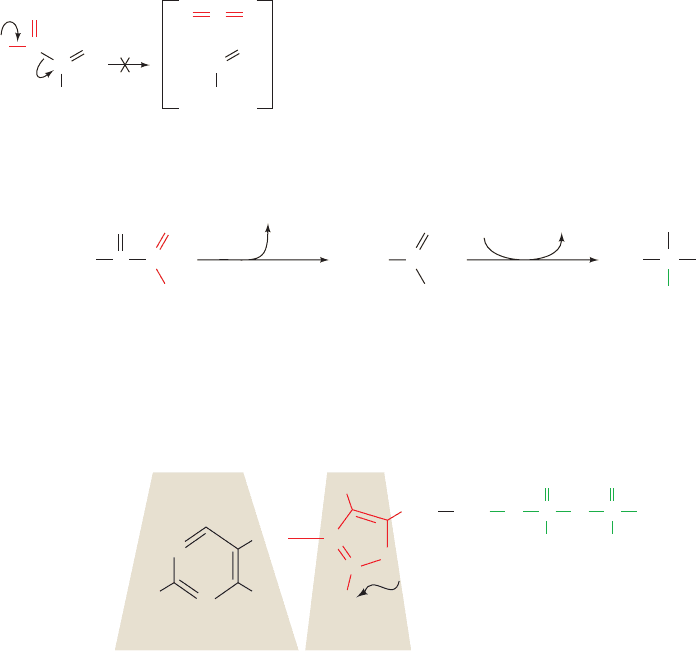
B. Alcoholic Fermentation
Under anaerobic conditions in yeast, NAD
is regenerated
in a manner that has been of importance to mankind for
thousands of years: the conversion of pyruvate to ethanol
and CO
2
. Ethanol is, of course, the active ingredient of wine
and spirits; CO
2
so produced leavens bread. From the point
of view of the yeast, however, alcoholic fermentation has a
practical benefit that homolactic fermentation cannot sup-
ply: Yeast employ ethanol as a kind of antibiotic to elimi-
nate competing organisms. This is because yeast can grow
in ethanol concentrations 12% (2.5M), whereas few
other organisms can survive in 5% ethanol (recall that
ethanol is a widely used antiseptic).
a.
TPP Is an Essential Cofactor of Pyruvate
Decarboxylase
Yeast produces ethanol and CO
2
via two consecutive re-
actions (Fig. 17-25). The first reaction is the decarboxyla-
tion of pyruvate to form acetaldehyde and CO
2
as cat-
alyzed by pyruvate decarboxylase (PDC; an enzyme not
present in animals). PDC contains the coenzyme thiamine
pyrophosphate [TPP; Fig. 17-26; also called thiamin
diphosphate (ThDP)], which it binds tightly but noncova-
lently.The coenzyme is employed because decarboxylation
of an -keto acid such as pyruvate requires the buildup of
negative charge on the carbonyl carbon atom in the transi-
tion state, an unstable situation:
C
O
O
O
–
R
–
C
O
R
C
C
OO
+
This transition state may be stabilized by delocalization of
the developing negative charge into a suitable “electron
sink.” The amino acid residues of proteins function poorly
in this capacity but TPP does so easily.
The “business” end of TPP is the thiazolium ring (Fig.
17-26). Its group is relatively acidic because of the
adjacent positively charged quaternary nitrogen atom,
which electrostatically stabilizes the carbanion formed on
dissociation of the proton. This dipolar carbanion (or ylid)
is the active form of the coenzyme.The mechanism of PDC
catalysis is as follows (Fig. 17-27):
Step 1 Nucleophilic attack by the ylid form of TPP on
the carbonyl carbon of pyruvate to form a covalent adduct.
Step 2 Departure of CO
2
to generate a resonance-
stabilized carbanion adduct in which the thiazolium ring of
the coenzyme acts as an electron sink.
Step 3 Protonation of the carbanion.
Step 4 Elimination of the TPP ylid to form acetalde-
hyde and regenerate the active enzyme.
This mechanism has been corroborated by the isolation of
the hydroxyethylthiamine pyrophosphate intermediate
(Fig. 17-27).
The X-ray structure of PDC in complex with TPP (Fig.
17-28),which was determined by William Furey and Martin
Sax, has suggested a role for TPP’s aminopyrimidine ring in
the formation of the active ylid. Ylid formation requires a
base to remove the C2 proton. Yet PDC has no basic side
chain that is properly positioned to do so.The amino group
of the enzyme-bound TPP’s aminopyrimidine ring is suit-
ably positioned to accept this proton; however,its pK is too
low to do so efficiently and one of its protons sterically
C2¬H
616 Chapter 17. Glycolysis
Figure 17-25 The two reactions of alcoholic fermentation. (1) Decarboxylation of pyruvate to
form acetaldehyde is followed by (2) reduction of the acetaldehyde to ethanol by NADH.
Figure 17-26 Thiamine pyrophosphate. The thiazolium ring constitutes its catalytically active
functional group.
Pyruvate
pyruvate
decarboxylase
O
O
CCCH
3
O
–
Acetaldehyde
O
CCH
3
Ethanol
C
H
H
OH
CH
3
H
1
CO
2
alcohol
dehydrogenase
2
NAD
+
NADH
N
N
PPO
O
O
–
O
O
O
–
O
–
CH
2
CH
2
CH
3
C
N
+
S
H
H
3
C
CH
2
NH
2
1
1
2
2
3
3
4
4
5
5
6
acidic
proton
Thiazolium
ring
Aminopyrimidine
ring
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 616
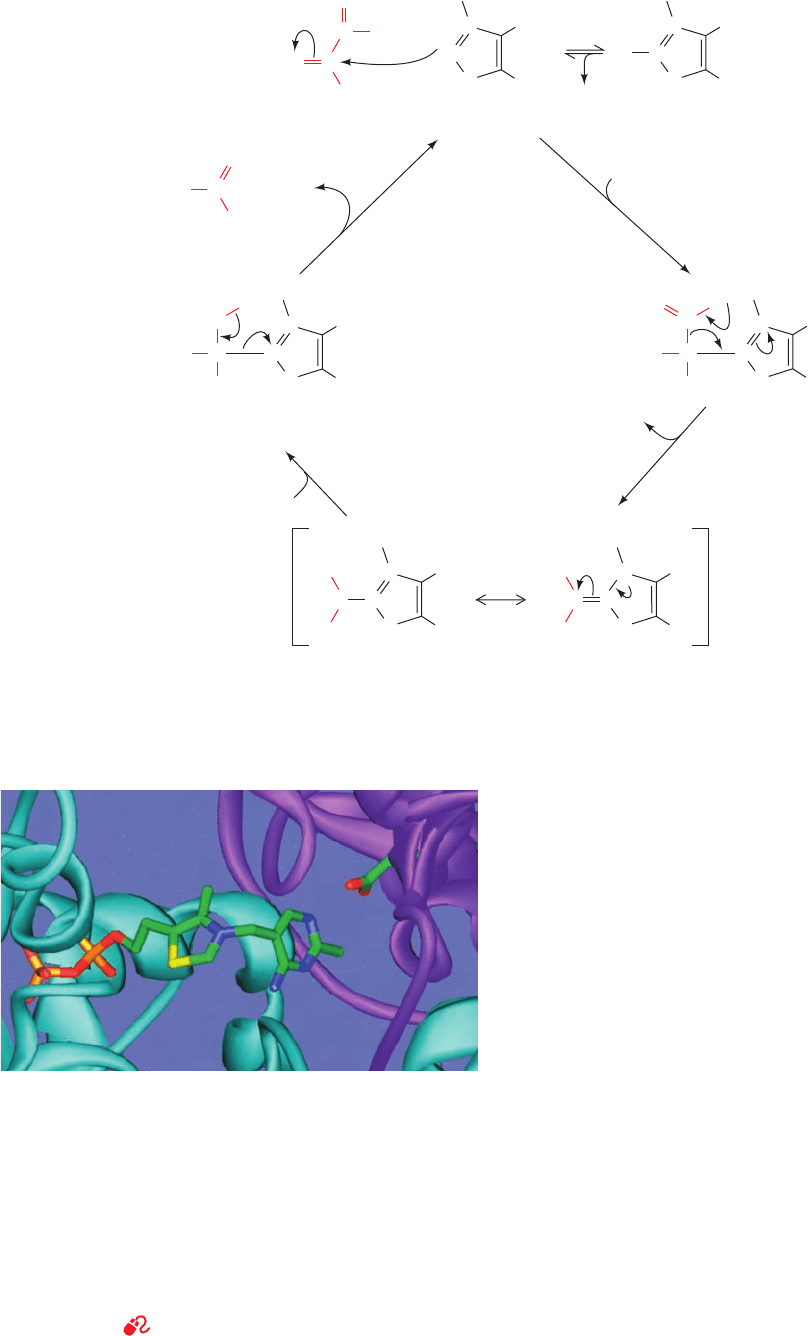
clashes with the C2 proton. It is therefore proposed that
the aminopyrimidine is converted to its imino tautomeric
form on the enzyme’s surface in a reaction involving pro-
ton donation by Glu 51 (Fig. 17-29). The imine, in turn, ac-
cepts a proton from C2, thereby forming the ylid, with tau-
tomerization back to the amino form. The participation of
N1¿ and the 4¿-amino group of the aminopyrimidine is sup-
ported by experiments showing that TPP analogs missing
either of these functionalities are catalytically inactive.
H/D exchange experiments followed by
1
H NMR analysis
of the exchange products indicate that when TPP is bound
to PDC in complex with the substrate analog pyruvamide
(CH
3
¬CO¬CO¬NH
2
), its rate of exchange to form the
active species (ylid) is much greater (⬎6 ⫻ 10
2
s
⫺1
) than
the enzyme’s catalytic rate (k
cat
⫽ 10 s
⫺1
). Moreover, the
mutation of PDC’s Glu 51 to Gln reduces the rate of H/D
exchange to 1.7 s
⫺1
, thereby supporting Glu 51’s postulated
function of donating a proton to N1¿ of TPP’s aminopyri-
dine ring.
b. Beriberi Is a Thiamine Deficiency Disease
The ability of TPP’s thiazolium ring to add to carbonyl
groups and act as an “electron sink” makes it the coenzyme
most utilized in ␣-keto acid decarboxylations. TPP is also
Section 17-3. Fermentation: The Anaerobic Fate of Pyruvate 617
Figure 17-27 Reaction mechanism of pyruvate decarboxylase.
(1) Nucleophilic attack by the ylid form of TPP on the carbonyl
carbon of pyruvate; (2) departure of CO
2
to generate a
Figure 17-28 A portion of the X-ray structure of pyruvate
decarboxylase from Saccharomyces uvarum (brewer’s yeast) in
complex with its TPP cofactor. The enzyme’s identical
563-residue subunits form a tightly associated dimer, two of
which associate loosely to form a tetramer.The TPP and the side
chain of Glu 51 are shown in stick form with C green, N blue, O
red, S yellow, and P gold. The TPP binds in a cavity situated
between the dimer’s two subunits (cyan and magenta), where it
hydrogen bonds to Glu 51. [Based on an X-ray structure by
William Furey and Martin Sax, Veterans Administration Medical
Center and University of Pittsburgh, Pittsburgh, Pennsylvania.
PDBid 1PYD.]
See Interactive Exercise 9
CH
3
C
N
+
S
CO
CH
3
C O
–
O
R⬘
–
R
CH
3
C
N
+
S
R⬘
R
H
Pyruvate TPP (ylid form) TPP
C
O
–
CHO
O
CH
3
C
N
+
S
R⬘
R
CH
3
1
CO
2
2
3
O
H
C
H
CH
3
C
N
+
S
R⬘
R
CH
3
Hydroxyethylthiamine
pyrophosphate
4
H C
O
CH
3
A
cetaldehyde
CH
3
C
N
..
S
R⬘
R
C
HO
CH
3
C
N
S
R⬘
R
–
C
HO
H
3
CH
3
C
+
Resonance-stabilized carbanion
H
+
+
H
+
H
+
H
+
resonance-stabilized carbanion; (3) protonation of the carbanion;
and (4) elimination of the TPP ylid and release of product.
JWCL281_c17_593-637.qxd 10/19/10 7:26 AM Page 617
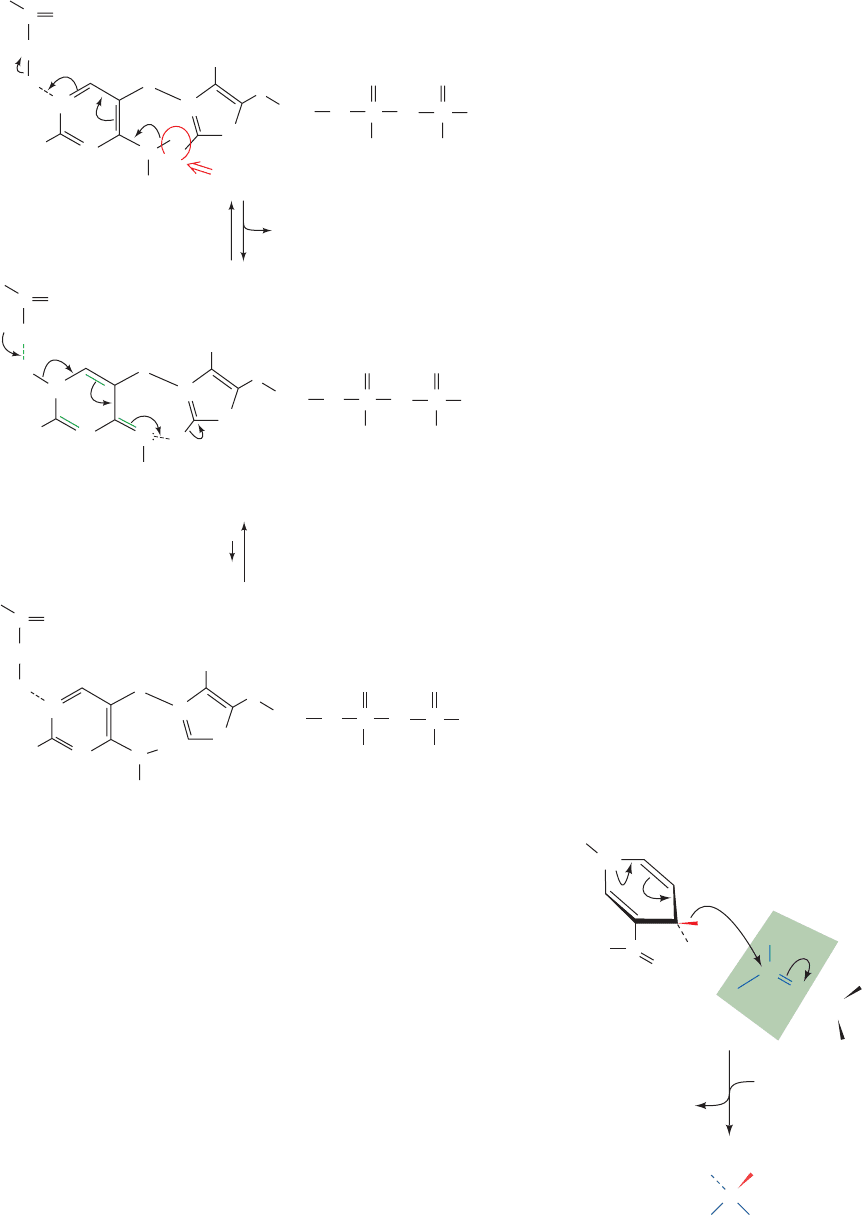
involved in decarboxylation reactions that we shall encounter
in other metabolic pathways. Consequently, thiamine (vita-
min B
1
), which is neither synthesized nor stored in signifi-
cant amounts by the tissues of most vertebrates, is required
in their diets. Its deficiency in humans results in an ultimately
fatal condition known as beriberi (Sinhalese for weakness)
that is characterized by neurological disturbances causing
pain, paralysis and atrophy (wasting) of the limbs, and/or
cardiac failure resulting in edema (the accumulation of fluid
in tissues and body cavities).Beriberi was particularly preva-
lent in the late nineteenth and early twentieth centuries in
the rice-consuming areas of Asia after the introduction of
steam-powered milling machines that polished the rice
grains to remove their coarse but thiamine-containing outer
layers (the previously used milling procedures were less effi-
cient and hence left sufficient thiamine on the grains). Par-
boiling rice before milling, a process common in India,
causes the rice kernels to absorb nutrients from their outer
layers, thereby decreasing the incidence of beriberi. Once
thiamine deficiency was recognized as the cause of beriberi,
enrichment procedures were instituted so that today it has
ceased to be a problem except in areas undergoing famine.
However, beriberi occasionally develops in chronic alco-
holics due to their penchant for drinking but not eating.
c. Reduction of Acetaldehyde
and Regeneration of NAD
ⴙ
The acetaldehyde formed by the decarboxylation of
pyruvate is reduced to ethanol by NADH in a reaction cat-
alyzed by alcohol dehydrogenase (ADH). Each subunit of
the tetrameric yeast ADH (YADH) binds one NADH and
one Zn
2⫹
ion. The Zn
2⫹
ion functions to polarize the car-
bonyl group of acetaldehyde (Fig. 17-30), so as to stabilize
the developing negative charge in the transition state of the
reaction (the role of metal ions in enzymes is discussed in
Section 15-1C). This facilitates the transfer of NADH’s
pro-R hydrogen (the same atom that LDH transfers) to
acetaldehyde’s re face, forming ethanol with the transferred
hydrogen in the pro-R position (Section 13-2A).
Both homolactic and alcoholic fermentation have the
same function: the anaerobic regeneration of NAD
⫹
for
continued glycolysis. Their main difference is in their meta-
bolic products.
Mammalian liver ADH (LADH) functions to metabo-
lize the alcohols anaerobically produced by intestinal flora
as well as those from external sources (the direction of the
ADH reaction varies with the relative concentrations of
618 Chapter 17. Glycolysis
H
C
2
N
⫹
N
S
N
N
H
H
O
C
Glu 51
O
2
3
4
5
1
⫺
O
C
Glu 51
O
3⬘
6⬘
4⬘
5⬘
1⬘
2⬘
N
CH
3
H
3
C
CH
2
CH
2
O
P
PO
O
O
O
⫺
O
⫺
O
⫺
H
H
N
⫹
H
⫺
Ylid
H
Imine Predominant form
fast
Steric clash
H
⫹
O
P
PO
O
O
O
⫺
O
⫺
O
⫺
H
C
2
N
S
N
N
H
2
3
1
3
⬘
6⬘
4⬘
5⬘
1⬘
2⬘
CH
3
H
3
C
CH
2
CH
2
H
O
C
Glu 51
O
N
N
⫹
O
P
PO
O
O
O
⫺
O
⫺
O
⫺
H
C
2
N
S
N
N
H
2
3
1
3
⬘
6⬘
4⬘
5⬘
1⬘
2⬘
CH
3
H
3
C
CH
2
CH
2
H
4
5
4
5
Figure 17-29 The formation of the active ylid form of TPP in
the pyruvate decarboxylase reaction. This reaction requires the
participation of TPP’s aminopyrimidine ring together with
general acid catalysis by Glu 51.The predominant form of the
cofactor on the enzyme is the imine, but the rate of formation of
the active ylid is fast relative to the enzyme’s catalytic rate.
C
O
H
Zn
2+
Ethanol
Acetaldehyde
NADH
H
+
NAD
+
...
...
S-Cys
S-Cys
N-His
CH
3
C
H
H
H
3
C OH
N
C
O
R
H
H
H
2
N
.
.
Figure 17-30 The reaction mechanism of alcohol
dehydrogenase involves direct hydride transfer of the pro-R
hydrogen of NADH to the re face of acetaldehyde.
JWCL281_c17_593-637.qxd 6/3/10 8:37 AM Page 618
ethanol and acetaldehyde). Each subunit of this dimeric
enzyme binds one NAD
⫹
and two Zn
2⫹
ions, although only
one of these ions participates directly in catalysis. There is
significant amino acid sequence similarity between YADH
and LADH, so it is quite likely that both enzymes have the
same general mechanism.
C. Energetics of Fermentation
Thermodynamics permits us to dissect the process of fer-
mentation into its component parts and to account for the
free energy changes that occur.This enables us to calculate
the efficiency with which the free energy of degradation of
glucose is utilized in the synthesis of ATP. The overall reac-
tion of homolactic fermentation is
(⌬G°¿ is calculated from the data in Table 3-4 using Eqs.
[3.19] and [3.21] adapted for 2H
⫹
ions.) For alcoholic fer-
mentation, the overall reaction is
Each of these reactions is coupled to the net formation of
two ATPs, which requires ⌬G°¿ ⫽⫹61 kJ ⴢ mol
⫺1
of glucose
consumed (Table 16-3). Dividing the ⌬G°¿ of ATP forma-
tion by that of lactate formation indicates that homolactic
fermentation is 31% “efficient”; that is, 31% of the free en-
ergy released by this process under standard biochemical
conditions is sequestered in the form of ATP. The rest is
dissipated as heat, thereby making the process irreversible.
Likewise, alcoholic fermentation is 26% efficient under
biochemical standard state conditions. Actually, under
physiological conditions, where the concentrations of reac-
tants and products differ from those of the standard state,
these reactions have free energy efficiencies of ⬎50%.
a. Glycolysis Is Used for Rapid ATP Production
Anaerobic fermentation utilizes glucose in a profligate
manner compared to oxidative phosphorylation: Fermenta-
tion results in the production of 2 ATPs per glucose, whereas
oxidative phosphorylation yields 32 ATPs per glucose
(Chapter 22). This accounts for Pasteur’s observation that
yeast consumes far more sugar when growing anaerobically
than when growing aerobically (the Pasteur effect; Section
22-4C). However, the rate of ATP production by anaerobic
glycolysis can be up to 100 times faster than that of oxidative
phosphorylation. Consequently, when tissues such as muscle
are rapidly consuming ATP, they regenerate it almost entirely
by anaerobic glycolysis. (Homolactic fermentation does not
really “waste” glucose since the lactate so produced is aero-
bically reconverted to glucose by the liver; Section 23-1C).
Skeletal muscles consist of both slow-twitch (Type I) and
fast-twitch (Type II) fibers. Fast-twitch fibers, so called be-
cause they predominate in muscles capable of short bursts
of rapid activity, are nearly devoid of mitochondria, so that
they must obtain nearly all of their ATP through anaerobic
¢G°¿ ⫽⫺235 kJ ⴢ mol
⫺1
of glucose
Glucose
¡
2CO
2
⫹ 2 ethanol
¢G°¿ ⫽⫺196 kJ ⴢ mol
⫺1
of glucose
Glucose
¡
2 lactate ⫹ 2H
⫹
glycolysis, for which they have a particularly large capacity.
Muscles designed to contract slowly and steadily, in contrast,
are enriched in slow-twitch fibers that are rich in mitochon-
dria and obtain most of their ATP through oxidative phos-
phorylation. (Fast- and slow-twitch fibers were originally
known as white and red fibers, respectively, because other-
wise pale colored muscle tissue, when enriched with mito-
chondria, takes on the red color characteristic of their heme-
containing cytochromes. However, fiber color has been
shown to be an imperfect indicator of muscle physiology.)
In a familiar example, the flight muscles of migratory
birds such as ducks and geese, which need a continuous en-
ergy supply, are rich in slow-twitch fibers and therefore
such birds have dark breast meat. In contrast, the flight
muscles of less ambitious fliers, such as chickens and
turkeys, which are used only for short bursts (often to es-
cape danger), consist mainly of fast-twitch fibers that form
white meat. In humans, the muscles of sprinters are rela-
tively rich in fast-twitch fibers, whereas distance runners
have a greater proportion of slow-twitch fibers (although
their muscles have the same color). World class distance
runners have a remarkably high capacity to generate ATP
aerobically. This was demonstrated by the noninvasive
31
P
NMR monitoring of the ATP, P
i
, phosphocreatine, and pH
levels in their exercising but untrained forearm muscles.
These observations suggest that the muscles of these ath-
letes are better endowed genetically for endurance exer-
cise than those of “normal” individuals.
4 METABOLIC REGULATION
AND CONTROL
Living organisms, as we saw in Section 16-6, are thermody-
namically open systems that tend to maintain a steady state
rather than reaching equilibrium (death for living things).
Thus the flux (rate of flow) of intermediates through a meta-
bolic pathway is constant; that is, the rates of synthesis and
breakdown of each pathway intermediate maintain it at a
constant concentration. Such a state, it will be recalled, is
one of maximum thermodynamic efficiency (Section 16-6Ba).
Regulation of the steady state (homeostasis) must be main-
tained in the face of changes in flux through the pathway in
response to changes in demand.
The terms metabolic control and metabolic regulation
are often used interchangeably. However, for our purposes
we shall give them different definitions: Metabolic regula-
tion is the process by which the steady-state flow of
metabolites through a pathway is maintained, whereas
metabolic control is the influence exerted on the enzymes
of a pathway in response to an external signal in order to
alter the flux of metabolites.
A. Homeostasis and Metabolic Control
There are two reasons why metabolic flow must be con-
trolled:
I. To provide products at the rate they are needed, that
is, to balance supply with demand.
Section 17-4. Metabolic Regulation and Control 619
JWCL281_c17_593-637.qxd 6/3/10 8:37 AM Page 619

II. To maintain the steady-state concentrations of the
intermediates in a pathway within a narrow range (home-
ostasis).
Organisms maintain homeostasis for several reasons:
1. In an open system, such as metabolism, the steady
state is the state of maximum thermodynamic efficiency
(Section 16-6Ba).
2. Many intermediates participate in more than one
pathway, so that changing their concentrations may disturb
a delicate balance.
3. The rate at which a pathway can respond to a control
signal slows if large changes in intermediate concentrations
are involved.
4. Large changes in intermediate concentrations may
have deleterious effects on cellular osmotic properties.
The concentrations of intermediates and the level of
metabolic flux at which a pathway is maintained vary with
the needs of the organism through a highly responsive
system of precise controls. Such pathways are analogous
to rivers that have been dammed to provide a means of
generating electricity. Although water is continually flow-
ing in and out of the lake formed by the dam, a relatively
constant water level is maintained. The rate of water out-
flow from the lake is precisely controlled at the dam and
is varied in response to the need for electrical power. In
this section, we examine the mechanisms by which meta-
bolic pathways in general, and the glycolytic pathway in
particular, are controlled in response to biological energy
needs.
B. Metabolic Flux
Since a metabolic pathway is a series of enzyme-catalyzed
reactions, it is easiest to describe the flux of metabolites
through the pathway by considering its reaction steps indi-
vidually. The flux of metabolites, J, through each reaction
step is the rate of the forward reaction, v
f
, less that of the
reverse reaction, v
r
:
[17.1]
At equilibrium, by definition, there is no flux (J 0), al-
though v
f
and v
r
may be quite large. At the other extreme,
in reactions that are far from equilibrium, v
f
v
r
, so that
the flux is essentially equal to the rate of the forward reac-
tion, J ⬇ v
f
. The flux throughout a steady-state pathway is
constant and is set (generated) by the pathway’s rate-
determining step (or steps). Consequently, control of flux
through a metabolic pathway requires: (1) that the flux
through this flux-generating step vary in response to the or-
ganism’s metabolic requirements and (2) that this change in
flux be communicated throughout the pathway to maintain
a steady state.
The classic description of metabolic control and regula-
tion is that every metabolic pathway has a rate-limiting
step and is regulated by controlling the rate of this pivotal
J v
f
v
r
enzyme. These so-called regulatory enzymes are almost in-
variably allosteric enzymes subject to feedback inhibition
(Section 13-4) and are often also controlled by covalent
modification (which we discuss in Section 18-3).
Several questions arise. Are these regulatory enzymes
really rate limiting for the pathway? Is there really only
one step in the pathway that is rate limiting, or might there
be a number of enzymes contributing to the regulation of
the pathway? Does controlling these enzymes really con-
trol the flux of metabolites through the pathway or is the
function of feedback inhibition really to maintain a steady
state? These are complicated questions with complicated
answers.
C. Metabolic Control Analysis
While it has been common practice to assume that every
metabolic pathway has a rate-limiting step, experiments
suggest that the situation becomes more complex when
these pathways are combined in a living organism. Hence,
it is important to develop methods to quantitatively ana-
lyze metabolic systems in order to establish mechanisms of
control and regulation. Metabolic control analysis, devel-
oped by Henrik Kacser and Jim Burns and independently
by Reinhart Heinrich and Tom Rapoport, provides a
framework for considering these problems. It is a way of
quantitatively describing the behavior of metabolic sys-
tems in response to various perturbations.
a. The Flux Control Coefficient Measures the
Sensitivity of the Flux to the Change
in Enzyme Concentration
Metabolic control analysis makes no a priori assump-
tion that only one step is rate limiting. Instead, it defines a
flux control coefficient, C
J
(where J is an index, not an ex-
ponent), to measure the sensitivity of flux to a change in
enzyme concentration. The flux control coefficient is de-
fined as the fractional change in flux, J, with respect to the
fractional change in enzyme concentration, [E]:
[17.2]
(recall that 0x/x 0 ln x).
The flux control coefficient is the analog of the kinetic or-
der of a reaction. If a reaction is first order in substrate con-
centration, [S], then doubling [S] doubles the rate of the re-
action, whereas if the reaction is zero order in [S] (e.g., in a
saturated enzymatic reaction), then the reaction rate is in-
sensitive to the value of [S]. Similarly, if the flux control coef-
ficient of an enzyme is 1, then doubling the concentration of
the enzyme, [E], doubles the flux through the pathway and if
it is zero, the flux is insensitive to the value of [E]. Of course,
the flux control coefficient may have some intermediate
value between 0 and 1. For example, if a 10% increase in the
enzyme concentration increases the flux by only 7.5%, the
flux control coefficient would be 0.075/0.10 0.75.
The flux through a metabolic system is generally con-
trolled by more than one enzyme. Consequently, the flux
C
J
0J>J
0[E]>[E]
0 ln J
0 ln[E]
⬇
¢J>J
¢[E]>[E]
620 Chapter 17. Glycolysis
JWCL281_c17_593-637.qxd 2/26/10 1:38 PM Page 620
