Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

phosphocreatine in a transgenic mouse liver protects the
animal against the sharp drop in [ATP] ordinarily caused
by fructose overload (Section 17-5Aa).This genetic manip-
ulation technique is being used to study mechanisms of
metabolic control in vivo.
Metabolic pathways are regulated both by controlling the
activities of regulatory enzymes (Sections 17-4 and 18-3) and
by controlling their concentrations at the level of gene ex-
pression (Sections 31-3, 32-4, and 34-3).The important ques-
tion of how hormones and diet control metabolic processes
at the level of gene expression is being addressed through
the use of transgenic animals. Reporter genes (genes whose
products are easily detected; Section 5-5Gd) are placed un-
der the influence of promoters (genetic elements that regu-
late transcriptional initiation; Section 5-4Aa) that control
the expression of specific regulatory enzymes, and the re-
sulting composite gene is expressed in animals. The trans-
genic animals can then be treated with specific hormones
and/or diets and the production of the reporter gene prod-
uct measured. For instance, in an investigation by Richard
Hanson, the promoter for the enzyme phosphoenolpyru-
vate carboxykinase (PEPCK) was attached to the structural
gene encoding growth hormone (GH). PEPCK, an impor-
tant regulatory enzyme in gluconeogenesis (the synthesis of
glucose from noncarbohydrate precursors; Section 23-1), is
normally present in liver and kidneys but not in blood. GH,
however, is secreted into the blood and its presence there
can be readily quantitated by an ELISA (Section 6-1Da).
Mice transgenic for PEPCK/GH were fed either a high-
carbohydrate/low-protein diet or a high-protein/low-carbo-
hydrate diet, which are known to decrease and increase
PEPCK activity, respectively. GH in high concentrations was
detected only in the serum of PEPCK/GH mice on a high-
protein diet, thereby indicating that the GH was synthesized
under the same dietary control as that of the PEPCK ex-
pressed by the normal gene. Thus, the activity of PEPCK in
PEPCK/GH mice can be continuously monitored, albeit in-
directly, through serum GH assays (the direct measurement
of PEPCK in mouse liver or kidney requires the sacrifice of
the animal and hence can be done only once).Such use of re-
porter genes has proved to be of great value in the study of
the genetic control of metabolism.
Section 16-3. Experimental Approaches to the Study of Metabolism 571
Figure 16-14 Pathway of arginine biosynthesis indicating the
positions of genetic blocks. All of these mutants grow in the
presence of arginine, but mutant 1 also grows in the presence of
the (nonstandard) -amino acids citrulline or ornithine and
mutant 2 grows in the presence of citrulline. This is because in
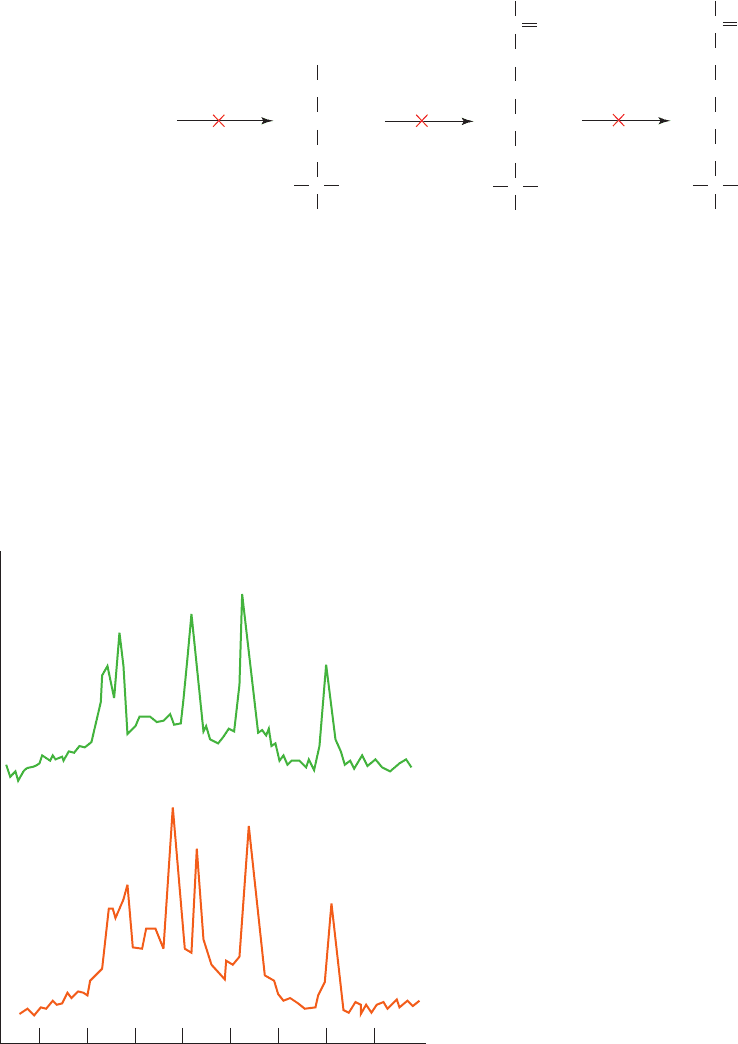
(a) Control liver
PME
PCr
15 10 5 0
PPM
–5 –10 –15 –20
ATP
P
i
γ
α
β
(b) Creatine kinase
positive liver
Figure 16-15 The expression of creatine kinase in transgenic
mouse liver as demonstrated by localized in vivo
31
P NMR.
(a) The spectrum of a normal mouse liver after the mouse had
been fed a diet supplemented with 2% creatine. The peaks
corresponding to inorganic phosphate (P
i
), the , , and
phosphoryl groups of ATP, and phosphomonoesters (PME) are
labeled. (b) The spectrum of the liver of a mouse transgenic for
creatine kinase that had been fed a diet supplemented with 2%
creatine. The phosphocreatine peak is labeled PCr. [After
Koretsky,A.P., Brosnan, M.J., Chen, L., Chen, J., and Van Dyke,
T.A., Proc. Natl. Acad. Sci. 87, 3114 (1990)].
Ornithine
Citrulline Arginine
mutant 1 mutant 2
mutant 3
NH
3
NH
3
CH
2
CH
2
CH
2
C
COO
H
NH
NH
3
CH
2
NH
2
CH
2
CH
2
C
CO
COO
H
NH
NH
NH
2
CH
2
NH
2
CH
2
CH
2
C
C
COO
H
mutant 1, an enzyme leading to the production of ornithine is
absent but enzymes farther along the pathway are normal. In
mutant 2, the enzyme catalyzing citrulline production is
defective, whereas in mutant 3 an enzyme involved in the
conversion of citrulline to arginine is lacking.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 571
Modern techniques also make it possible to insert a muta-
tion that inactivates or deletes an enzyme or control protein in
a pathway of interest in higher organisms such as mice (knock-
out mice; Section 5-5H). Knockout mice have proved useful
for studying metabolic control mechanisms. For example,
PEPCK activity is thought to be controlled exclusively by in-
creasing or decreasing its availability. Diet affects its produc-
tion, as we have seen. However, this demand-based control is
superimposed on the developmental regulation of PEPCK
production.The enzyme is not produced at all in early embryos
and only appears near birth, when gluconeogenesis is required
to supply the glucose that had been previously available in
utero. One of the proteins thought to be responsible for the de-
velopmental regulation of PEPCK production is CCAAT/en-
hancer-binding protein ␣ (C/EBP␣), a transcription factor
(Section 5-4Aa; transcriptional regulation in eukaryotes is dis-
cussed in Section 34-3B). Newborn mice homozygous for
the targeted deletion of the c/ebp gene (c/ebp knockout
mice) do not produce C/EBP and therefore do not produce
PEPCK. Consequently, their livers cannot synthesize the
glucose necessary to maintain adequate blood glucose levels
once they are disconnected from the maternal circulation.
Indeed, these mice become so hypoglycemic that they die
within 8 hours of birth. Clearly C/EBP has an important
role in the developmental regulation of PEPCK.
B. Isotopes in Biochemistry
The specific labeling of metabolites such that their inter-
conversions can be traced is an indispensable technique for
elucidating metabolic pathways. Franz Knoop formulated
this technique in 1904 to study fatty acid oxidation. He fed
dogs fatty acids chemically labeled with phenyl groups
and isolated the phenyl-substituted end products from
their urine. From the differences in these products when
the phenyl-substituted starting material contained odd and
even numbers of carbon atoms he deduced that fatty acids
are degraded in C
2
units (Section 25-2).
a. Isotopes Specifically Label Molecules without
Altering Their Chemical Properties
Chemical labeling has the disadvantage that the chemi-
cal properties of labeled metabolites differ from those of
normal metabolites. This problem is eliminated by labeling
molecules of interest with isotopes (atoms with the same
number of protons but a different number of neutrons in
their nuclei). Recall that the chemical properties of an
element are a consequence of its electron configuration
which, in turn, is determined by its atomic number, not its
atomic mass. The metabolic fate of a specific atom in a
metabolite can therefore be elucidated by isotopically
labeling that position and following its progress through the
metabolic pathway of interest.The advent of isotopic label-
ing and tracing techniques in the 1940s therefore revolu-
tionized the study of metabolism. (Isotope effects, which
are changes in reaction rates arising from the mass differ-
ences between isotopes, are in most instances negligible.
Where they are significant,most noticeably between hydro-
gen and its isotopes deuterium and tritium, they have been
used to gain insight into enzymatic reaction mechanisms.)
b. NMR Can Be Used to Study Metabolism
in Whole Animals
Nuclear magnetic resonance (NMR) detects specific
isotopes due to their characteristic nuclear spins. Among
the isotopes that NMR can detect are
1
H,
13
C,and
31
P. Since
the NMR spectrum of a particular nucleus varies with its
immediate environment, it is possible to identify the peaks
corresponding to specific atoms even in relatively complex
mixtures.
The development of magnets large enough to accom-
modate animals and humans, and to localize spectra to
specific organs, has made it possible to study metabolic
pathways noninvasively by NMR techniques. Thus,
31
P
NMR can be used to study energy metabolism in muscle by
monitoring the levels of ATP, ADP, inorganic phosphate,
and phosphocreatine (Figure 16-15). Indeed, a
31
P NMR
system has been patented to measure the muscular meta-
bolic efficiency and maximum power of race horses while
they are walking or running on a motor-driven treadmill in
order to identify promising animals and to evaluate the
efficacy of their training and nutritional programs.
Isotopically labeling specific atoms of metabolites with
13
C (which is only 1.10% naturally abundant) permits the
metabolic progress of the labeled atoms to be followed by
13
C
NMR. Figure 16-16 shows in vivo
13
C NMR spectra of a rat
liver before and after an injection of
D-[1-
13
C]glucose.The
13
C
can be seen entering the liver and then being converted to
glycogen (the storage form of glucose; Chapter 18).
1
H NMR
techniques are being used to determine the in vivo levels of a
variety of metabolites in tissues such as brain and muscle.
c. The Detection of Radioactive Isotopes
All elements have isotopes. For example, the atomic
mass of naturally occurring Cl is 35.45 D because, at least
on Earth, it is a mixture of 55%
35
Cl and 45%
36
Cl (other
isotopes of Cl are present in only trace amounts). Stable
isotopes are generally identified and quantitated by mass
spectrometry or NMR techniques. Many isotopes, how-
ever, are unstable; they undergo radioactive decay, a
process that involves the emission from the radioactive
nuclei of subatomic particles such as helium nuclei (␣ parti-
cles), electrons ( particles), and/or photons (␥ radiation).
Radioactive nuclei emit radiation with characteristic ener-
gies. For example,
3
H,
14
C, and
32
P all emit particles but
with respective energies of 0.018, 0.155, and 1.71 MeV.The
radiation from
32
P is therefore highly penetrating, whereas
that from
3
H and
14
C is not. (
3
H and
14
C, as all radioactive
isotopes, must, nevertheless, be handled with great caution
because they can cause genetic damage on ingestion.)
Radiation can be detected by a variety of techniques.
Those most commonly used in biochemical investigations
are proportional counting (known in its simplest form as
Geiger counting), liquid scintillation counting, and autora-
diography. Proportional counters electronically detect the
ionizations in a gas caused by the passage of radiation.
Moreover, they can also discriminate between particles of
different energies and thus simultaneously determine the
amounts of two or more different isotopes present.
Although proportional counters are quite simple to use,
the radiation from two of the most widely used isotopes in
572 Chapter 16. Introduction to Metabolism
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 572
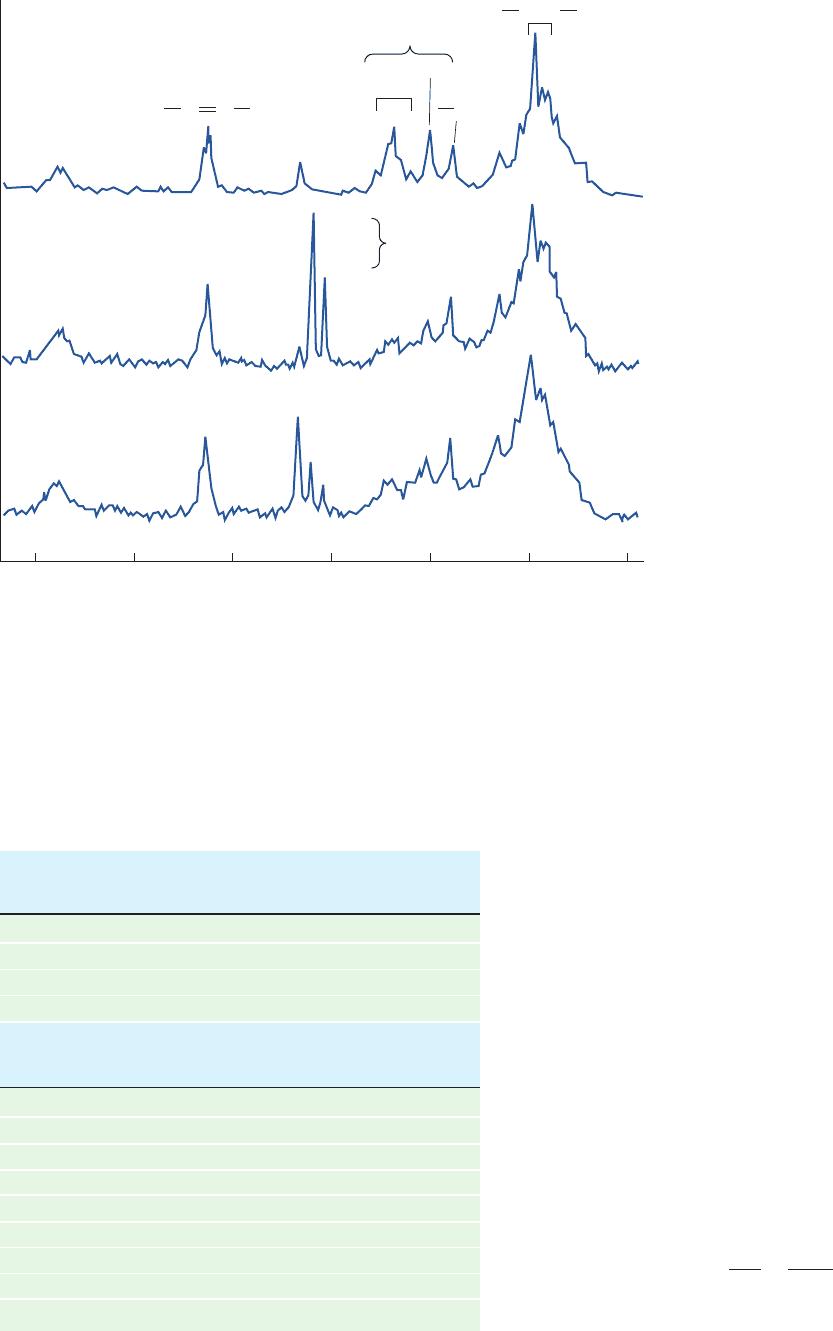
biochemical analysis,
3
H and
14
C, have insufficient pene-
trating power to enter a proportional counter’s detection
chamber with reasonable efficiency. This limitation is cir-
cumvented through liquid scintillation counting. In this
Section 16-3. Experimental Approaches to the Study of Metabolism 573
Figure 16-16 The conversion of
[1-
13
C]glucose to glycogen as observed by
localized in vivo
13
C NMR. (a) The natural
abundance
13
C NMR spectrum of the liver
of a live rat. Note the resonance
corresponding to C1 of glycogen. (b) The
13
C NMR spectrum of the liver of the same
rat ⬃5 min after it was intravenously
injected with 100 mg of [1-
13
C]glucose (90%
enriched).The resonances of the C1 atom
of both the and anomers of glucose are
clearly distinguishable from each other and
from the resonance of the C1 atom of
glycogen. (c) The
13
C NMR spectrum of the
liver of the same rat ⬃30 min after the
[1-
13
C]glucose injection.The C1 resonances
of both the and glucose anomers are
much reduced while the C1 resonance of
glycogen has increased. [After Reo, N.V.,
Siegfried, B.A., and Acherman, J.J.H., J.
Biol. Chem. 259, 13665 (1984)].
Table 16-2 Some Trace Isotopes of Biochemical
Importance
Stable Isotopes
Nucleus Natural Abundance (%)
2
H 0.012
13
C 1.07
15
N 0.36
18
O 0.20
Radioactive Isotopes
Nucleus Radiation Type Half-Life
3
H 12.31 years
14
C 5715 years
22
Na
, 2.60 years
32
P 14.28 days
35
S 87.2 days
45
Ca 162.7 days
60
Co , 5.271 years
125
I 59.4 days
131
I , 8.02 days
Source: Holden, N.E., in Lide, D.R. (Ed.), Handbook of Chemistry and
Physics (90th ed.), pp. 11–57 to 266, CRC Press (2009–2010).
technique, a radioactive sample is dissolved or suspended
in a solution containing fluorescent substances that emit a
pulse of light when struck by radiation. The light is de-
tected electronically so that the number of light pulses can
be counted. The emitting nucleus can also be identified
because the intensity of a light pulse is proportional to the
radiation energy (the number of fluorescent molecules
excited by a radioactive particle is proportional to the
particle’s energy).
In autoradiography, radiation is detected by its blacken-
ing of photographic film.The radioactive sample is laid on,
or in some cases mixed with,the photographic emulsion and,
after sufficient exposure time (from minutes to months),
the film is developed. Autoradiography is widely used to
locate radioactive substances in polyacrylamide gels (e.g.,
Fig. 6-27). Position-sensitive radiation counters (electronic
film) are similarly employed.
d. Radioactive Isotopes Have Characteristic
Half-Lives
Radioactive decay is a random process whose rate for a
given isotope depends only on the number of radioactive
atoms present. It is therefore a simple first-order process
whose half-life, t
1/2
, is a function only of the rate constant, k,
for the decay process (Section 14-1Ba):
[14.5]
Because k is different for each radioactive isotope, each
has a characteristic half-life. The properties of some iso-
topes in common biochemical use are listed in Table 16-2.
t
1>2
ln 2
k
0.693
k
(a)
RCOOR′
180 120 60 0
ppm
C1 Glycogen
C1–β
Glucose
C1–α
Glucose and
glycogen
Choline
N(CH
3
)
3
CH
2
C
2
–C
5
C
6
CC
(b)
(c)
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 573
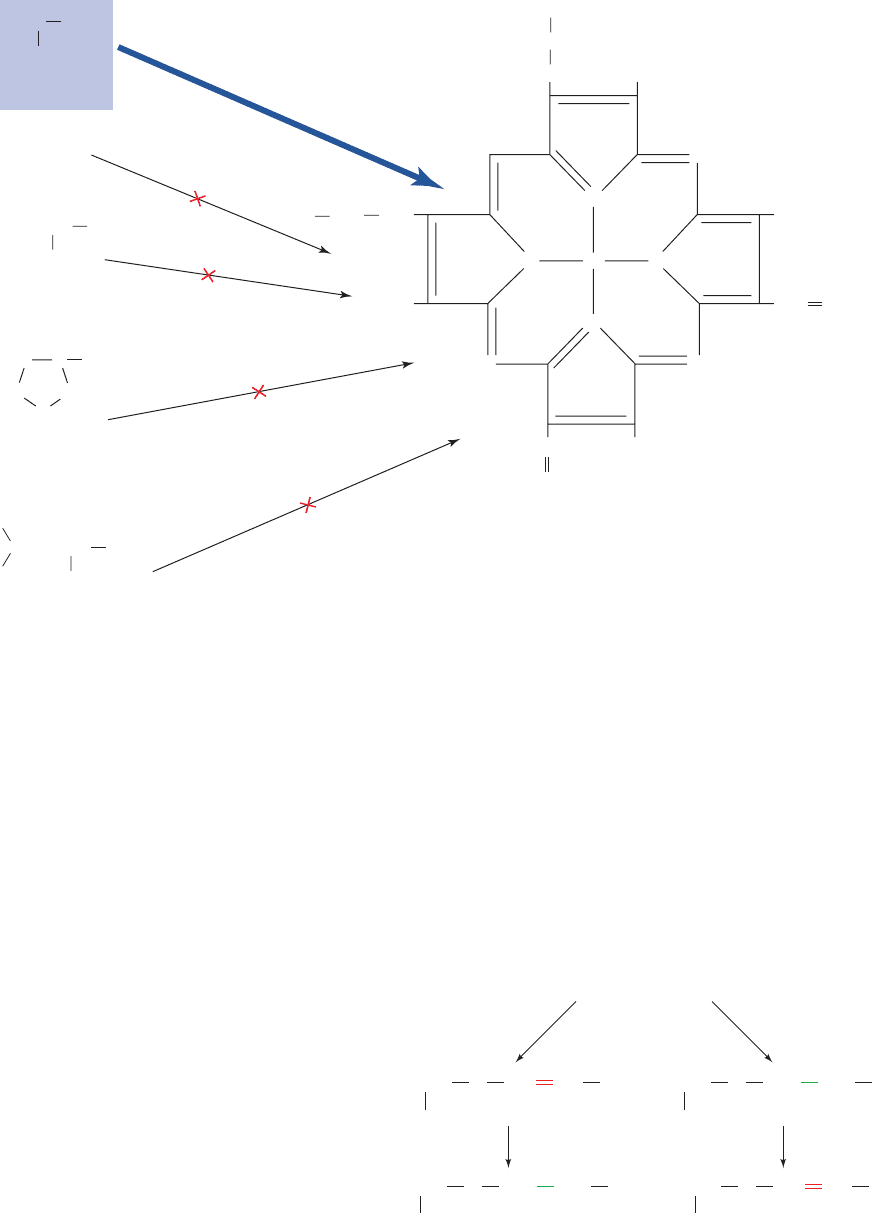
e. Isotopes Are Indispensable for Establishing the
Metabolic Origins of Complex Metabolites and
Precursor–Product Relationships
The metabolic origins of complex molecules such as
heme, cholesterol, and phospholipids may be determined
by administering isotopically labeled starting materials to
animals and isolating the resulting products. One of the
early advances in metabolic understanding resulting from
the use of isotopic tracers was the demonstration, by David
Shemin and David Rittenberg in 1945, that the nitrogen
atoms of heme are derived from glycine rather than from
ammonia,glutamic acid, proline,or leucine (Section 26-4Aa).
They showed this by feeding rats these
15
N-labeled nutri-
ents, isolating the heme in their blood, and analyzing it for
15
N content. Only when the rats were fed [
15
N]glycine did
the heme contain
15
N (Fig. 16-17). This technique was also
used to demonstrate that all of cholesterol’s carbon atoms
are derived from acetyl-CoA (Section 25-6A).
Isotopic tracers are also useful in establishing the order
of appearance of metabolic intermediates, their so-called
precursor–product relationships. An example of such an
analysis concerns the biosynthesis of the complex phos-
pholipids called plasmalogens and alkylacylglycerophos-
pholipids (Section 25-8Ab). Alkylacylglycerophospholipids
are ethers, whereas the closely related plasmalogens are
vinyl ethers.Their similar structures brings up the interesting
question of their biosynthetic relationship: Which is the
precursor and which is the product? Two possible modes of
synthesis can be envisioned (Fig. 16-18):
I. The starting material is converted to the vinyl ether
(plasmalogen), which is then reduced to yield the ether
(alkylacylglycerophospholipid). Accordingly, the vinyl
ether would be the precursor and the ether the product.
574 Chapter 16. Introduction to Metabolism
Figure 16-17 The metabolic origin of the nitrogen atoms in heme. Only [
15
N]glycine, of many
15
N-labeled metabolites, is an
15
N-labeled heme precursor.
Figure 16-18 Two possible pathways for the biosynthesis of
ether– and vinyl ether–containing phospholipids. (I) The vinyl
ether is the precursor and the ether is the product. (II) The ether
is the precursor and the vinyl ether is the product.
Glutamate
Proline
Leucine
Glycine
H
2
C
NH
3
COO
15
NH
4
15
OOCCH
2
CH
2
CH
CH
2
CH
2
CH
3
CH
2
CH
2
CH
3
CH
3
CH
2
CH
2
COO
OOC
CHCH
2
CH
NH
3
COO
15
NH
3
15
NH
15
COO
C
H
2
CH
2
C
H
3
C
H
3
C
H
3
C
H
2
C
COO
H
HC
CH
CH
NN Fe
N
N
CH
Heme
CH
2
Starting materials
Scheme I
reduction
Scheme II
Vinyl ether
Vinyl ether
Ether
Ether
CHCHO
R
CH
2
R
CHCHO
R
CH
2
R
CH
2
CH
2
O
R
CH
2
R
CH
2
CH
2
O
R
CH
2
R
oxidation
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 574
II. The ether is formed first and then oxidized to yield
the vinyl ether.The ether would then be the precursor and
the vinyl ether the product.
Precursor–product relationships can be most easily
sorted out through the use of radioactive tracers. A pulse of
the labeled starting material is administered to an organ-
ism and the specific radioactivities of the resulting meta-
bolic products are followed with time (Fig. 16-19):
(here the * represents the radioactive label). Metabolic
pathways, as we shall see in Section 16-6Ba, normally oper-
ate in a steady state; that is, the throughput of metabolites
in each of its reaction steps is equal. Moreover, the rates of
most metabolic reactions are first order for a given sub-
strate. Making these assumptions, we note that the rate of
change of B’s radioactivity, [B*], is equal to the rate of pas-
sage of label from A* to B* less the rate of passage of label
from B* to the pathway’s next product:
[16.1]
where k is the pseudo-first-order rate constant for both the
conversion of A to B and the conversion of B to its prod-
uct, and t is time. Inspection of this equation indicates the
criteria that must be met to establish that A is the precur-
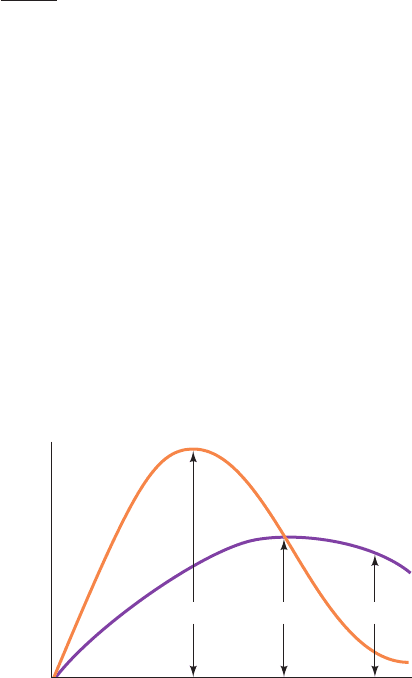
sor of B (Fig. 16-19):
1. Before the radioactivity of the product [B*] is maxi-
mal, d[B*]>dt 0, so [A*] [B*]; that is, while the radioac-
tivity of a product is rising, it should be less than that of its
precursor.
2. When [B*] is maximal, d[B*]>dt 0, so [A*] [B*];
that is, when the radioactivity of a product is at its peak, it
should be equal to that of its precursor. This result also im-
plies that the radioactivity of a product peaks after that of its
precursor.
d[B*]
dt
k[A*] k[B*] k([A*] [B*])
Starting material*
¡
A*
¡
B*
¡
later products*
3. After [B*] begins to decrease, d[B*]>dt 0, so [A*]
[B*]; that is, after the radioactivity of a product has peaked,
it should remain greater than that of its precursor.
Such a determination of the precursor–product rela-
tionship between alkylacylglycerophospholipid and plas-
malogen, using
14
C-labeled starting materials, indicated
that the ether is the precursor and the vinyl ether is the
product (Fig. 16-18, Scheme II).
C. Isolated Organs, Cells, and Subcellular Organelles
In addition to understanding the chemistry and catalytic
events that occur at each step of a metabolic pathway, it is
important to learn where a given pathway occurs within an
organism. Early workers studied metabolism in whole ani-
mals. For example, the role of the pancreas in diabetes was
established by Frederick Banting and Charles Best in 1921
by surgically removing that organ from dogs and observing
that these animals then developed the disease.
The metabolic products produced by a particular organ
can be studied by organ perfusion or in tissue slices. In or-
gan perfusion, a specific organ is surgically removed from
an animal and the organ’s arteries and veins are connected
to an artificial circulatory system. The composition of the
material entering the organ can thereby be controlled and
its metabolic products monitored. Metabolic processes can
be similarly studied in slices of tissue thin enough to be
nourished by free diffusion in an appropriate nutrient solu-
tion. Otto Warburg pioneered the tissue slice technique in
the early twentieth century through his studies of respiration,
in which he used a manometer to measure the changes in
gas volume above tissue slices as a consequence of their O
2
consumption.
A given organ or tissue generally contains several cell
types. Cell sorters are devices that can separate cells ac-
cording to type once they have been treated with the en-
zymes trypsin and collagenase to destroy the intercellular
matrix that binds them into a tissue. This technique allows
further localization of metabolic function. A single cell
type may also be grown in tissue culture for study. Al-
though culturing cells often results in their loss of differen-
tiated function, techniques have been developed for main-
taining several cell types that still express their original
characteristics.
As discussed in Section 16-1, metabolic pathways in eu-
karyotes are compartmentalized in various subcellular or-
ganelles (Table 16-1). For example, oxidative phosphoryla-
tion occurs in the mitochondrion, whereas glycolysis and
fatty acid biosynthesis occur in the cytosol. Such observa-
tions are made by breaking cells open and fractionating
their components by differential centrifugation (Section
6-1B),possibly followed by zonal ultracentrifugation through
a sucrose density gradient or by equilibrium density gradi-
ent ultracentrifugation in a CsCl density gradient, which, re-
spectively, separate particles according to their size and
density (Section 6-5B). The cell fractions are then analyzed
for biochemical function.
Section 16-3. Experimental Approaches to the Study of Metabolism 575
Figure 16-19 The flow of a pulse of radioactivity from precursor
to product. At point 1, product radioactivity (B*, purple) is
increasing and is less than that of its precursor (A*, orange); at
point 2, product radioactivity is maximal and is equal to that of
its precursor; and at point 3, product radioactivity is decreasing
and is greater than that of its precursor.
Time after addition of labeled starting material
[A*]
1
[B*]
Specific radioactivity
2 3
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 575
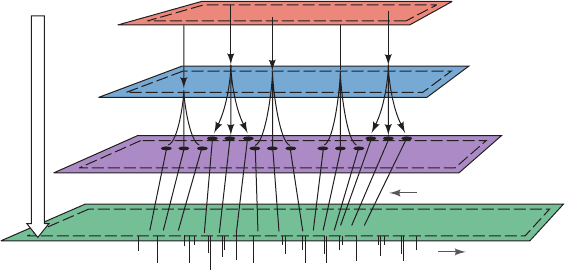
D. Systems Biology
Metabolism has traditionally been studied by hypothesis-
based research: isolating individual enzymes and metabo-
lites and assembling them into metabolic pathways as
guided by experimentally testable hypotheses. This is a re-
ductionist approach: the explanation of the workings of a
system in terms of its component parts. A different, so-
called integrative approach, systems biology, has recently
emerged with the advent of complete genome sequences,
the development of rapid and sensitive techniques for ana-
lyzing large numbers of gene transcripts, proteins, and
metabolites all at once, and the development of new com-
putational and mathematical tools. Systems biology is
discovery-based: collecting and integrating enormous
amounts of data in searchable databases so that the prop-
erties and dynamics of entire biological networks can be
analyzed. As a result, our understanding of the path from
genotype to phenotype has expanded. In addition to the
central dogma of molecular biology (Section 5-4), that a
single gene composed of DNA is transcribed to mRNA,
which is translated to a single protein that influences
metabolism, we are increasingly taking into account the
genome, transcriptome, proteome, and metabolome (the
complete set of a cell’s metabolites) and their interrelation-
ships (Fig. 16-20). The term bibliome (Greek: biblion,
book) has even been coined to denote the systematic
incorporation of pre-existing information about reaction
mechanisms and metabolic pathways. In the following
paragraphs we discuss some of these emerging technolo-
gies and new fields of study.
a. Transcriptomics
The overall metabolic capabilities of an organism are
encoded by its genome (its entire complement of genes). In
principle, it should be possible to reconstruct a cell’s meta-
bolic activities from its genomic sequence. However, at
present, this can be done only in a general sense. For exam-
ple, the 4.0-Mb genome of Vibrio cholerae, the bacterium
that causes cholera, contains a large repertoire of genes en-
coding transport proteins and enzymes for catabolizing a
576 Chapter 16. Introduction to Metabolism
Figure 16-20 The relationship between genotype and
phenotype. The path from genetic information (genotype) to
metabolic function (phenotype) has several steps. Portions of the
genome are transcribed to produce the transcriptome, which
wide range of nutrients. This is consistent with the compli-
cated lifestyle of V. cholerae, which can live on its own, in
association with zooplankton, or in the human gastroin-
testinal tract (where it causes cholera; Section 19-2Cd).
However, a simple catalog of an organism’s genes does not
reveal how these genes function. Thus, some genes are
expressed continuously at high levels, whereas others are
expressed rarely, for example, only when the organism
encounters a particular metabolite.
Creating an accurate picture of gene expression is the
goal of transcriptomics, the study of a cell’s transcriptome
(its entire complement of mRNAs). Identifying and quan-
tifying all the transcripts from a single cell type reveals
which genes are active. Cells transcribe thousands of genes
at once so this study requires the use of DNA microarray
technology (Section 7-6B). For example, Fig. 7-39 shows a
DNA microarray that indicates the differences in gene ex-
pression between yeast grown in the presence and absence
of glucose.
Differences in the expression of particular genes have
been correlated with many developmental processes or
growth patterns. For example, DNA microarrays have been
used to profile the patterns of gene expression in tumor
cells because different types of tumors express different
types and amounts of proteins (Section 34-3B). This infor-
mation is useful in choosing how best to treat a cancer.
b. Proteomics
The correlation between the amount of a particular
mRNA and the amount of its protein product is imperfect.
This is because the various mRNAs and their correspon-
ding proteins are synthesized and degraded at different
rates. Furthermore, many proteins are post-translationally
modified, sometimes in several different ways (e.g., by
phosphorylation or glycosylation). Consequently, the num-
ber of unique proteins in a cell exceeds the number of
unique mRNAs.
A more reliable way than transcriptomics to assess gene
expression is to examine a cell’s proteome, the complete
set of proteins that the cell synthesizes. This proteomics
Metabolome
Genome
DNA
Genotype
Transcriptome
Proteome
Phenotype
Metabolites
Substrates
mRNA
Enzyme
directs the synthesis of the proteome, whose various activities are
responsible for synthesizing and degrading the components of
the metabolome.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 576
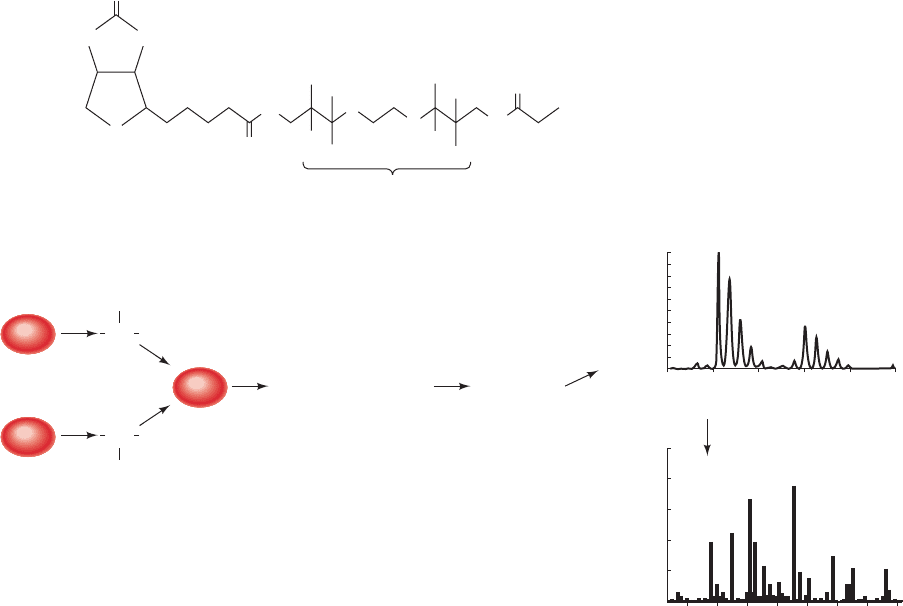
approach requires that the proteins first be separated,usually
by two-dimensional (2D) gel electrophoresis (Section 6-4D).
Individual proteins are then identified by using tandem
mass spectrometry to obtain amino acid sequence infor-
mation (Section 7-1Ia) and correlating it with protein
sequence databases. All the proteins that are contained in
a cell or tissue under a given set of conditions can thereby
be catalogued.
One can compare all the proteins synthesized by a cell
under two different sets of conditions by using different
isotopically labeled reagents that are either contained in
the growth medium (e.g., deuterated amino acids) or that
are reacted with the cell extract. One technique for label-
ing cellular proteins uses isotope-coded affinity tags
(ICATs), which are analogous to the differently fluoresc-
ing dyes that are used to label cDNAs.
An ICAT contains three functional elements: an
iodoacetyl group to react with Cys residues, a linker that
contains either 8 hydrogen (light) or 8 deuterium (heavy)
atoms, and biotin, a coenzyme (Section 23-1Ab) that is
used as a biotechnology tool because of its extremely tight
binding to the protein avidin (K 10
15
M; Fig. 16-21a).
Avidin is immobilized on a chromatographic resin so that
the ICAT-labeled peptides can be isolated by biotin/avidin
affinity chromatography (Section 6-3C).
The ICAT procedure is illustrated in Fig. 16-21b.Two
protein mixtures representing two different growth condi-
tions are treated with light (d0) or heavy (d8) versions of
the ICAT reagent. The labeled protein mixtures are com-
bined and digested with trypsin to form Cys-containing la-
beled peptides, which are then isolated by biotin/avidin
affinity chromatography. Individual peptides are separated
by liquid chromatography and detected by mass spectrom-
etry (LC/MS). The ratio of the intensities of the light and
heavy peptide signals indicates the relative peptide abun-
dance in the two samples. Tandem mass spectrometry
Section 16-3. Experimental Approaches to the Study of Metabolism 577
Figure 16-21 The isotope-coded affinity tag (ICAT) method
for quantitative proteome analysis. (a) An example of an ICAT
reagent that contains an iodoacetyl reactive group, a linker, and a
biotin residue. X denotes the position of hydrogen (d0) or
deuterium (d8). (b) The ICAT strategy for differential labeling of
proteins expressed by cells under two different sets of conditions.
(1) Proteins from states A and B are respectively treated with
the light (d0) and heavy (d8) forms of the ICAT reagent. (2) The
labeled protein mixtures are combined. (3) The labeled proteins
are digested with trypsin to form Cys-containing labeled
peptides.These peptides are then purified by biotin/avidin
Two cell states:
Reduce and label cysteines
with ICAT reagent
Digest and affinity
purify labeled peptides
Analyze by
LC/MS and
MS/MS
200
1405.0 1426.0
1417
1409
Percentage intensityPercentage intensity
0
100
100
400 600
Mass (m/z)
Mass (m/z)
800
Biotin Reactive group
Linker
O
X
X
X
X
X
X
X
X
R(d0)-biotin
(b)
(a)
R(d8)-biotin
1
Quantify by MS
Identify by MS/MS
Cys
A
B
Cys
NHHN
S
O
NH
NH
O
I
O
O
3
2
1
4
5
affinity chromatography. The purified peptides are analyzed by
mass spectrometry in two ways: (4) Liquid chromatography
followed by mass spectrometry (LC/MS) is used to quantitate
the peptides.The ratio of the signal intensities from the
corresponding light and heavy peptides indicates the relative
peptide abundance in the two mixtures. (5) Tandem mass
spectrometry (MS/MS) is used to determine the amino acid
sequence of each peptide and to thereby identify the protein
from which it is derived by comparing the peptide’s sequence to
those in a database of all known proteins.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 577
(MS/MS) is then used to sequence each peptide and deter-
mine its identity.This method was used to identify many of
the yeast proteins whose mRNA concentrations increased
or decreased when glucose was absent from the growth
medium (Fig. 7-39). A hope for the future is that samples
from diseased and normal subjects can be compared in this
manner to find previously undetected disease markers that
would allow early diagnosis of various diseases.
c. Metabolomics
In order to describe a cell’s functional state (its pheno-
type) we need, in addition to the cell’s genome, transcrip-
tome, and proteome, a quantitative description of all of the
metabolites it contains under a given set of conditions, its
metabolome. However, a cell or tissue contains thousands
of metabolites with greatly varying properties, so that iden-
tifying and quantifying all these substances is a daunting
task, requiring many different analytical tools. Conse-
quently, this huge undertaking is often subdivided. For ex-
ample, lipidomics is the subdiscipline of metabolomics
aimed at identifying and characterizing all lipids in a cell un-
der a particular set of conditions, including how these lipids
influence membrane structure, cell signaling, gene expres-
sion, cell–cell interactions, etc., whereas glycomics similarly
identifies and characterizes all the carbohydrates in a cell.
A recently constructed model of the human
metabolome—based on 1496 protein-encoding genes, 2004
proteins, 2766 metabolites, and 3311 metabolic and trans-
port reactions—has been used to simulate 288 known meta-
bolic functions in a variety of cell and tissue types. This in
silico (computerized) model is expected to provide a frame-
work for future advances in human systems biology.
4 THERMODYNAMICS OF
PHOSPHATE COMPOUNDS
The endergonic processes that maintain the living state are
driven by the exergonic reactions of nutrient oxidation. This
coupling is most often mediated through the syntheses of a
few types of “high-energy” intermediates whose exergonic
consumption drives endergonic processes. These intermedi-
ates therefore form a sort of universal free energy “currency”
through which free energy–producing reactions “pay for” the
free energy–consuming processes in biological systems.
Adenosine triphosphate (ATP; Fig. 16-22), which occurs
in all known life-forms, is the “high-energy” intermediate
that constitutes the most common cellular energy currency.
Its central role in energy metabolism was first recognized in
1941 by Fritz Lipmann and Herman Kalckar. ATP consists
of an adenosine moiety to which three phosphoryl groups
( ) are sequentially linked via a phosphoester bond
followed by two phosphoanhydride bonds. Adenosine
diphosphate (ADP) and 5ⴕ-adenosine monophosphate
(AMP) are similarly constituted but with only two and
one phosphoryl units, respectively.
In this section we consider the nature of phosphoryl-trans-
fer reactions, discuss why some of them are so exergonic, and
outline how the cell consumes and regenerates ATP.
¬PO
2
3
A. Phosphoryl-Transfer Reactions
Phosphoryl-transfer reactions,
are of enormous metabolic significance. Some of the most
important reactions of this type involve the synthesis and
hydrolysis of ATP:
where P
i
and PP
i
, respectively, represent orthophosphate
and pyrophosphate in any of their ioniza-
tion states. These highly exergonic reactions are coupled to
numerous endergonic biochemical processes so as to drive
them to completion. Conversely, ATP is regenerated by cou-
pling its formation to a more highly exergonic metabolic
process (the thermodynamics of coupled reactions is dis-
cussed in Section 3-4C).
To illustrate these concepts, let us consider two exam-
ples of phosphoryl-transfer reactions.The initial step in the
metabolism of glucose is its conversion to glucose-6-phos-
phate (Section 17-2A). Yet the direct reaction of glucose
and P
i
is thermodynamically unfavorable (Fig. 16-23a). In
biological systems, however, this reaction is coupled to the
exergonic hydrolysis of ATP, so the overall reaction is ther-
modynamically favorable. ATP can be similarly rege-
nerated by coupling its synthesis from ADP and P
i
to the
even more exergonic hydrolysis of phosphoenolpyruvate
(Fig. 16-23b; Section 17-2J).
The bioenergetic utility of phosphoryl-transfer reactions
stems from their kinetic stability to hydrolysis combined
with their capacity to transmit relatively large amounts of
free energy. The G°¿ values of hydrolysis of several phos-
phorylated compounds of biochemical importance are tab-
ulated in Table 16-3. The negatives of these values are often
referred to as phosphate group-transfer potentials; they
(P
2
O
4
7
)(PO
4
3
)
ATP H
2
O Δ AMP PP
i
ATP H
2
O Δ ADP P
i
R
1
¬O¬PO
3
2
R
2
¬OH Δ R
1
¬OH R
2
¬O¬PO
3
2
578 Chapter 16. Introduction to Metabolism
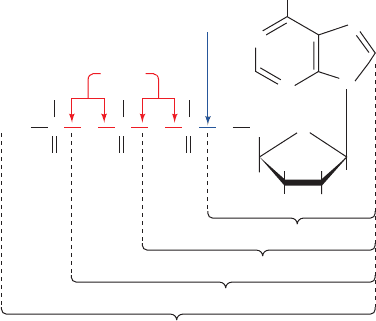
Figure 16-22 The structure of ATP indicating its relationship
to ADP, AMP, and adenosine. The phosphoryl groups, starting with
that on AMP, are referred to as the , , and phosphates. Note the
difference between phosphoester and phosphoanhydride bonds.
N
CH
2
N
NH
2
N
N
OO
O
O
–
H
H
HH
HO
Adenosine
OH
POPO
Phosphoanhydride
bonds
Phosphoester
bond
P
OO
O
–
O
–
–
O
γ
β
α
AMP
ADP
ATP
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 578
are a measure of the tendency of phosphorylated com-
pounds to transfer their phosphoryl groups to water. Note
that ATP has an intermediate phosphate group-transfer
potential. Under standard conditions, the compounds
above ATP in Table 16-3 can spontaneously transfer a
phosphoryl group to ADP to form ATP, which can, in turn,
spontaneously transfer a phosphoryl group to the hydroly-
sis products (ROH form) of the compounds below it.
a. ⌬G of ATP Hydrolysis Varies with pH, Divalent
Metal Ion Concentration, and Ionic Strength
The G of a reaction varies with the total concentra-
tions of its reactants and products and thus with their ionic
states (Eq. [3.15]). The G’s of hydrolysis of phosphory-
lated compounds are therefore highly dependent on pH,
divalent metal ion concentration (divalent metal ions such
as Mg
2
have high phosphate-binding affinities), and ionic
strength. Reasonable estimates of the intracellular values
of these quantities as well as of [ATP], [ADP], and [P
i
]
(which are generally on the order of millimolar) indicate
that ATP hydrolysis under physiological conditions has
G ⬇ 50 kJ ⴢ mol
1
rather than the 30.5 kJ ⴢ mol
1
of its
G°¿. Nevertheless, for the sake of consistency in compar-
ing reactions, we shall usually refer to the latter value.
The above situation for ATP is not unique. It is impor-
tant to keep in mind that within a given cell, the concentra-
tions of most substances vary both with location and time.
Indeed, the concentrations of many ions, coenzymes, and
metabolites commonly vary by several orders of magnitude
across membranous organelle boundaries. Unfortunately, it
is usually quite difficult to obtain an accurate measurement
of the concentration of any particular chemical species in a
specific cellular compartment. The G’s for most in vivo
reactions are therefore little more than estimates.
Section 16-4. Thermodynamics of Phosphate Compounds 579
Figure 16-23 Some overall coupled reactions involving ATP.
(a) The phosphorylation of glucose to form glucose-6-
phosphate and ADP. (b) The phosphorylation of ADP by
phosphoenolpyruvate to form ATP and pyruvate. Each reaction
Phosphoenolpyruvate
Pyruvate
Endergonic
half-reaction 1
(a)
(b)
Exergonic
half-reaction 2
Exergonic
half-reaction 1
Overall
coupled reaction
P
i
P
i
glucose
glucose
glucose-6-P
glucose-6-P
ATP
H
2
O
CH
2
CH
3
C
O
C
COO
–
COO
–
OP
O
2
–
+13.8
–30.5
+30.5
–31.4
–16.7
– 61.9
H
2
O
H
2
O
++
+
ATP
ATP
H
2
O+
ATP +
+
+
ADP
+
CH
3
C
O
COO
–
+
P
i
+
P
i
+
ADP +
ADP
ADP
Overall
coupled reaction
3
CH
2
C
COO
–
OP
O
2
–
3
Endergonic
half-reaction 2
ΔG° (kJ
•
mol
–1
)
ΔG° (kJ
•
mol
–1
)
Table 16-3 Standard Free Energies of Phosphate Hydrolysis
of Some Compounds of Biological Interest
Compound G°¿ (kJ ⴢ mol
1
)
Phosphoenolpyruvate 61.9
1,3-Bisphosphoglycerate 49.4
ATP (S AMP ⴙ PP
i
) ⴚ45.6
Acetyl phosphate 43.1
Phosphocreatine 43.1
ATP (S ADP ⴙ P
i
) ⴚ30.5
Glucose-1-phosphate 20.9
PP
i
19.2
Fructose-6-phosphate 13.8
Glucose-6-phosphate 13.8
Glycerol-3-phosphate 9.2
Source: Mostly from Jencks, W.P., in Fasman, G.D. (Ed.), Handbook of
Biochemistry and Molecular Biology (3rd ed.), Physical and Chemical
Data,Vol. I, pp. 296–304, CRC Press (1976).
has been conceptually decomposed into a direct phosphorylation
step (half-reaction 1) and a step in which ATP is hydrolyzed
(half-reaction 2). Both half-reactions proceed in the direction in
which the overall reaction is exergonic (G 0).
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 579
B. Rationalizing the “Energy”
in “High-Energy” Compounds
Bonds whose hydrolysis proceeds with large negative values
of G°¿ (customarily more negative than 25 kJ ⴢ mol
1
)
are often referred to as “high-energy” bonds or “energy-
rich” bonds and are frequently symbolized by the squiggle
(⬃).Thus ATP may be represented as AR¬P⬃P⬃P, where
A, R, and P symbolize adenyl, ribosyl, and phosphoryl
groups, respectively.Yet, the phosphoester bond joining the
adenosyl group of ATP to its -phosphoryl group appears
to be not greatly different in electronic character from the
so-called “high-energy” bonds bridging its and phos-
phoryl groups. In fact, none of these bonds have any un-
usual properties, so the term “high-energy” bond is some-
what of a misnomer. (In any case, it should not be confused
with the term “bond energy,” which is defined as the energy
required to break, not hydrolyze, a covalent bond.) Why
then, should the phosphoryl-transfer reactions of ATP be so
exergonic? The answer comes from the comparison of the
stabilities of the reactants and products of these reactions.
Several different factors appear to be responsible for
the “high-energy” character of phosphoanhydride bonds
such as those in ATP (Fig. 16-24):
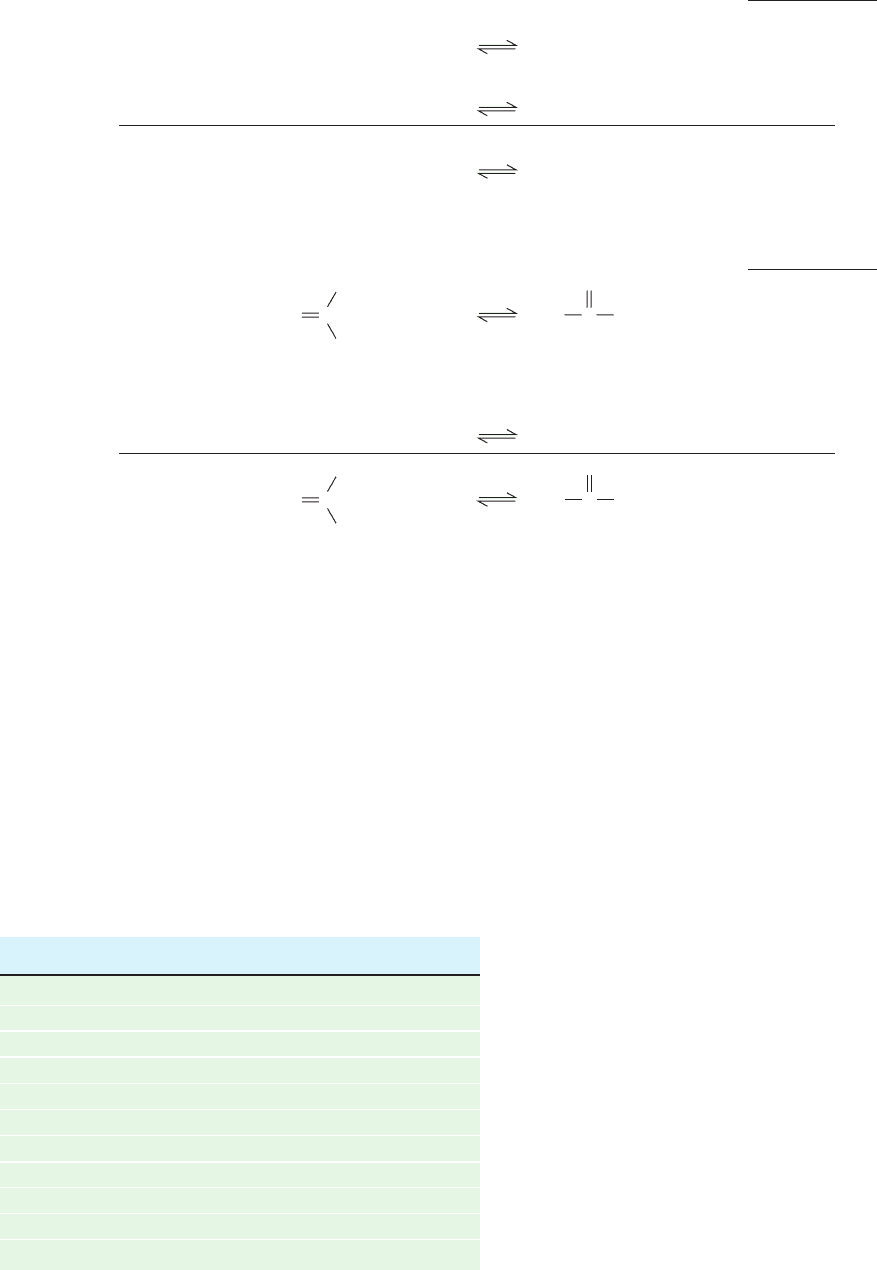
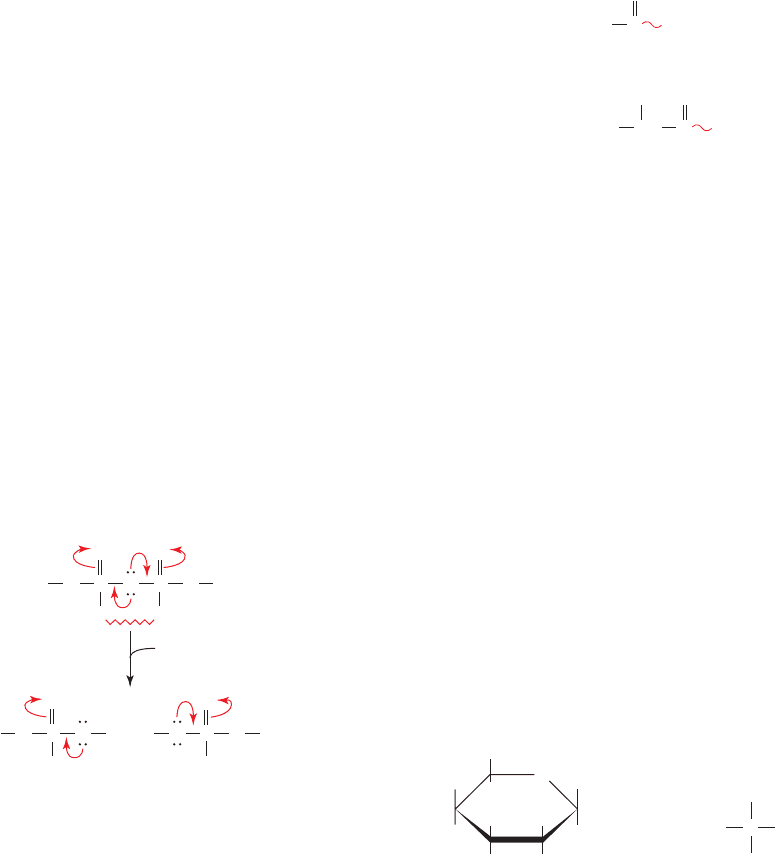
1. The resonance stabilization of a phosphoanhydride
bond is less than that of its hydrolysis products. This is
because a phosphoanhydride’s two strongly electron-
withdrawing phosphoryl groups must compete for the lone
pair of electrons of its bridging oxygen atom, whereas this
competition is absent in the hydrolysis products. In other
words, the electronic requirements of the phosphoryl
groups are less satisfied in a phosphoanhydride than in its
hydrolysis products.
2. Of perhaps greater importance is the destabilizing
effect of the electrostatic repulsions between the charged
groups of a phosphoanhydride in comparison to that of its
hydrolysis products. In the physiological pH range, ATP
has three to four negative charges whose mutual electro-
static repulsions are partially relieved by ATP hydrolysis.
3. Another destabilizing influence, which is difficult to as-
sess, is the smaller solvation energy of a phosphoanhydride in
comparison to that of its hydrolysis products. Some estimates
suggest that this factor provides the dominant thermody-
namic driving force for the hydrolysis of phosphoanhydrides.
A further property of ATP that suits it to its role as an
energy intermediate stems from the relative kinetic stabil-
ity of phosphoanhydride bonds to hydrolysis. Most types of
anhydrides are rapidly hydrolyzed in aqueous solution.
Phosphoanhydride bonds, however, have unusually large
free energies of activation. Consequently, ATP is reason-
ably stable under physiological conditions but is readily
hydrolyzed in enzymatically mediated reactions.
a. Other “High-Energy” Compounds
The compounds in Table 16-3 with phosphate group-
transfer potentials significantly greater than that of ATP
have additional destabilizing influences:
1. Acyl phosphates. The hydrolysis of acyl phosphates
(mixed phosphoric–carboxylic anhydrides), such as acetyl
phosphate and 1,3-bisphosphoglycerate,
is driven by the same competing resonance and differential
solvation influences that function in the hydrolysis of phos-
phoanhydrides. Apparently these effects are more pro-
nounced for acyl phosphates than for phosphoanhydrides.
2. Enol phosphates. The high phosphate group-transfer
potential of an enol phosphate, such as phosphoenolpyru-
vate (Fig. 16-23b), derives from its enol hydrolysis product
being less stable than its keto tautomer. Consider the hy-
drolysis reaction of an enol phosphate as occurring in two
steps (Fig. 16-25). The hydrolysis step is subject to the
driving forces discussed above. It is therefore the highly
exergonic enol–keto conversion that provides phospho-
enolpyruvate with the added thermodynamic impetus to
phosphorylate ADP to form ATP.
3. Phosphoguanidines. The high phosphate group-trans-
fer potentials of phosphoguanidines, such as phosphocrea-
tine and phosphoarginine, largely result from the compet-
ing resonances in their guanidino group, which are even
more pronounced than they are in the phosphate group of
phosphoanhydrides (Fig. 16-26). Consequently, phospho-
creatine can phosphorylate ADP (see Section 16-4Cd).
Compounds such as glucose-6-phosphate or glycerol-3-
phosphate,
H
H
OH
OH
α-
D-Glucose-6-phosphate L-Glycerol-3-phosphate
OH
CH
2
OPO
2
3
–
CH
2
OH
O
H
H
HC
HO
HO
H
CH
2
OPO
2
3
–
1,3-Bisphosphoglycerate
Acetyl phosphate
CH
3
OPO
3
2
C
O
CH OPO
3
22
O
3
POCH
2
C
OOH
580 Chapter 16. Introduction to Metabolism
OO
O
O
O
O
OPP
H
2
O
or
or
O
O
O
O
O
PPHH OO O
Figure 16-24 Resonance and electrostatic stabilization in a
phosphoanhydride and its hydrolysis products. The competing
resonances (curved arrows from the central O) and
charge–charge repulsions (zigzag line) between the phosphoryl
groups of a phosphoanhydride decrease its stability relative to its
hydrolysis products.
JWCL281_c16_557-592.qxd 6/10/10 11:52 AM Page 580
