Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

The reaction pathways that comprise metabolism are
often divided into two categories:
1. Catabolism, or degradation, in which nutrients and
cell constituents are broken down exergonically to salvage
their components and/or to generate free energy.
2. Anabolism, or biosynthesis, in which biomolecules
are synthesized from simpler components.
The free energy released by catabolic processes is con-
served through the synthesis of ATP from ADP and phos-
phate or through the reduction of the coenzyme NADP
to
NADPH (Fig. 13-2). ATP and NADPH are the major free
energy sources for anabolic pathways (Fig. 16-2).
A striking characteristic of degradative metabolism is that
it converts large numbers of diverse substances (carbohy-
drates, lipids, and proteins) to common intermediates. These
intermediates are then further metabolized in a central ox-
idative pathway that terminates in a few end products. Figure
16-3 outlines the breakdown of various foodstuffs, first to
their monomeric units, and then to the common intermedi-
ate, acetyl-coenzyme A (acetyl-CoA) (Fig. 21-2).
Biosynthesis carries out the opposite process. Relatively
few metabolites, mainly pyruvate, acetyl-CoA, and the citric
acid cycle intermediates, serve as starting materials for a host
of varied biosynthetic products. In the next several chapters
we discuss many degradative and biosynthetic pathways in
detail. For now, let us consider some general characteristics
of these processes.
Five principal characteristics of metabolic pathways
stem from their function of generating products for use by
the cell:
1. Metabolic pathways are irreversible. A highly exer-
gonic reaction (having a large negative free energy change)
is irreversible; that is, it goes to completion.If such a reaction
is part of a multistep pathway, it confers directionality on the
pathway; that is, it makes the entire pathway irreversible.
2. Catabolic and anabolic pathways must differ. If two
metabolites are metabolically interconvertible, the pathway
from the first to the second must differ from the pathway
from the second back to the first:
This is because if metabolite 1 is converted to metabolite 2
by an exergonic process, the conversion of metabolite 2 to
metabolite 1 requires that free energy be supplied in order
to bring this otherwise endergonic process “back up the
hill.” Consequently, the two pathways must differ in at least
1
2
A
YX
Section 16-1. Metabolic Pathways 561
Figure 16-2 ATP and NADPH are the sources of free energy
for biosynthetic reactions. They are generated through the
degradation of complex metabolites.
Figure 16-3 Overview of catabolism. Complex metabolites
such as carbohydrates, proteins, and lipids are degraded first to
their monomeric units, chiefly glucose, amino acids, fatty acids,
and glycerol, and then to the common intermediate,
acetyl-coenzyme A (acetyl-CoA).The acetyl group is then
oxidized to CO
2
via the citric acid cycle with the concomitant
reduction of NAD
and FAD. Reoxidation of these latter
coenzymes by O
2
via the electron-transport chain and oxidative
phosphorylation yields H
2
O and ATP.
ATP
NADPH
NADP
+
ADP + HPO
2–
4
Degradation
Simple products
Complex metabolites
Biosynthesis
CO
2
H
2
O
O
2
CO
2
NH
3
Citric
Acid
Cycle
Oxidative
phosphorylation
Pyruvate
Acetyl-CoA
Glycolysis
GlucoseAmino acids Fatty acids & Glycerol
CarbohydratesProteins Lipids
FAD
NAD
+
NAD
+
ADP
NADH
ATP
FADH
2
FADH
2
NADH
FAD
NAD
+
NADH
ATP
ADP
JWCL281_c16_557-592.qxd 6/10/10 11:51 AM Page 561
one of their reaction steps. The existence of independent in-
terconversion routes, as we shall see,is an important property
of metabolic pathways because it allows independent control
of the two processes. If metabolite 2 is required by the cell, it
is necessary to “turn off” the pathway from 2 to 1 while
“turning on” the pathway from 1 to 2. Such independent
control would be impossible without different pathways.
3. Every metabolic pathway has a first committed step.
Although metabolic pathways are irreversible, most of
their component reactions function close to equilibrium.
Early in each pathway, however, there is an irreversible
(exergonic) reaction that “commits” the intermediate it
produces to continue down the pathway.
4. All metabolic pathways are regulated. Metabolic
pathways are regulated by laws of supply and demand. In
order to exert control on the flux of metabolites through a
metabolic pathway, it is necessary to regulate its rate-limiting
step. The first committed step, being irreversible, functions
too slowly to permit its substrates and products to equili-
brate (if the reaction were at equilibrium, it would not be
irreversible). Since most of the other reactions in a path-
way function close to equilibrium, the first committed step
is often one of its rate-limiting steps. Most metabolic path-
ways are therefore controlled by regulating the enzymes
that catalyze their first committed step(s). This is an effi-
cient way to exert control because it prevents the unneces-
sary synthesis of metabolites further along the pathway
when they are not required. Specific aspects of such flux
control are discussed in Section 17-4C.
5. Metabolic pathways in eukaryotic cells occur in spe-
cific cellular locations. The compartmentation of the eu-
karyotic cell allows different metabolic pathways to operate
in different locations, as is listed in Table 16-1 (these or-
ganelles are described in Section 1-2A). For example, ATP
is mainly generated in the mitochondrion but much of it is
utilized in the cytoplasm. The synthesis of metabolites in
specific membrane-bounded subcellular compartments
makes their transport between these compartments a vital
component of eukaryotic metabolism. Biological mem-
branes are selectively permeable to metabolites because of
the presence in membranes of specific transport proteins.
The transport protein that facilitates the passage of ATP
through the mitochondrial membrane is discussed in
Section 20-4C, along with the characteristics of membrane
transport processes in general.The synthesis and utilization
of acetyl-CoA are also compartmentalized. This metabolic
intermediate is utilized in the cytosolic synthesis of fatty
acids but is synthesized in mitochondria. Yet there is no
transport protein for acetyl-CoA in the mitochondrial
membrane. How cells solve this fundamental problem is
discussed in Section 25-4D. In multicellular organisms, com-
partmentation is carried a step further to the level of tissues
and organs.The mammalian liver, for example, is largely re-
sponsible for the synthesis of glucose from noncarbohy-
drate precursors (gluconeogenesis; Section 23-1) so as to
maintain a relatively constant level of glucose in the circula-
tion, whereas adipose tissue is specialized for the storage
and mobilization of triacylglycerols. The metabolic interde-
pendence of the various organs is the subject of Chapter 27.
2 ORGANIC REACTION MECHANISMS
Almost all of the reactions that occur in metabolic path-
ways are enzymatically catalyzed organic reactions. Section
15-1 details the various mechanisms enzymes have at their
disposal for catalyzing reactions: acid–base catalysis, cova-
lent catalysis, metal ion catalysis, electrostatic catalysis,
proximity and orientation effects, and transition state bind-
ing. Few enzymes alter the chemical mechanisms of these
reactions, so much can be learned about enzymatic mecha-
nisms from the study of nonenzymatic model reactions. We
therefore begin our study of metabolic reactions by outlin-
ing the types of reactions we shall encounter and the mech-
anisms by which they have been observed to proceed in
nonenzymatic systems.
Christopher Walsh has classified biochemical reactions
into four categories: (1) group-transfer reactions; (2) oxida-
tions and reductions; (3) eliminations, isomerizations, and re-
arrangements; and (4) reactions that make or break carbon–
carbon bonds. Much is known about the mechanisms of
562 Chapter 16. Introduction to Metabolism
Table 16-1 Metabolic Functions of Eukaryotic Organelles
Organelle Function
Mitochondrion Citric acid cycle, electron transport and oxidative
phosphorylation, fatty acid oxidation, amino acid breakdown
Cytosol Glycolysis, pentose phosphate pathway, fatty acid
biosynthesis, many reactions of gluconeogenesis
Lysosomes Enzymatic digestion of cell components and ingested matter
Nucleus DNA replication and transcription, RNA processing
Golgi apparatus Post-translational processing of membrane and secretory
proteins; formation of plasma membrane and secretory vesicles
Rough endoplasmic reticulum Synthesis of membrane-bound and secretory proteins
Smooth endoplasmic reticulum Lipid and steroid biosynthesis
Peroxisomes (glyoxisomes in plants) Oxidative reactions catalyzed by amino acid oxidases and
catalase; glyoxylate cycle reactions in plants
JWCL281_c16_557-592.qxd 6/10/10 11:51 AM Page 562
these reactions and about the enzymes that catalyze them.
The discussions in the next several chapters focus on these
mechanisms as they apply to specific metabolic intercon-
versions. In this section we outline the four reaction cate-
gories and discuss how our knowledge of their reaction
mechanisms derives from the study of model organic reac-
tions.We begin by briefly reviewing the chemical logic used
in analyzing these reactions.
A. Chemical Logic
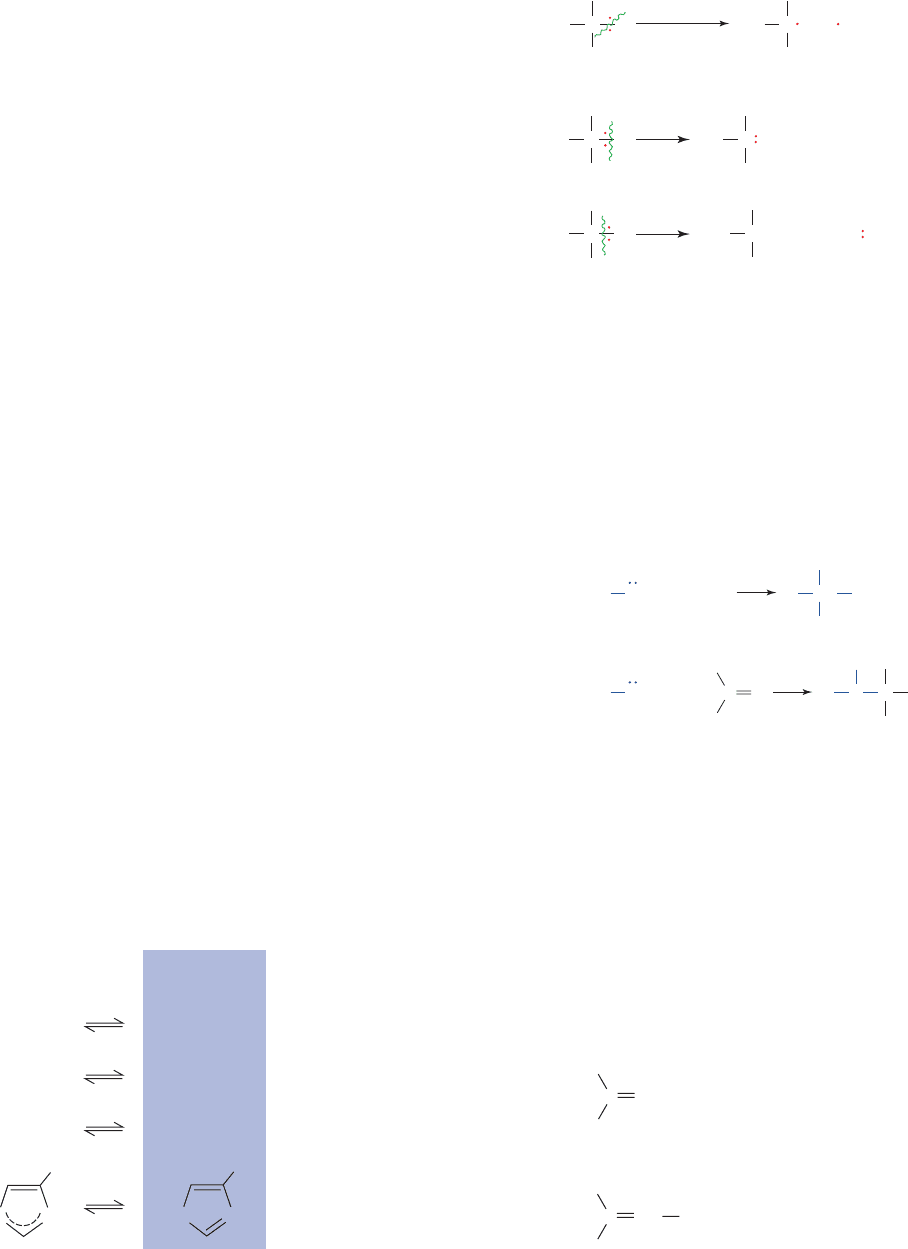
A covalent bond consists of an electron pair shared between
two atoms. In breaking such a bond, the electron pair can ei-
ther remain with one of the atoms (heterolytic bond cleav-
age) or separate such that one electron accompanies each of
the atoms (homolytic bond cleavage) (Fig. 16-4). Homolytic
bond cleavage, which usually produces unstable radicals, oc-
curs mostly in oxidation–reduction reactions. Heterolytic
C¬H bond cleavage involves either carbanion and proton
(H
) formation or carbocation (carbonium ion) and hydride
ion (H
) formation. Since hydride ions are highly reactive
species and carbon atoms are slightly more electronegative
than hydrogen atoms, bond cleavage in which the electron
pair remains with the carbon atom is the predominant mode
of bond breaking in biochemical systems. Hydride ion
abstraction occurs only if the hydride is transferred directly
to an acceptor such as NAD
or NADP
.
Compounds participating in reactions involving het-
erolytic bond cleavage and bond formation are categorized
into two broad classes: electron rich and electron deficient.
Electron-rich compounds, which are called nucleophiles
(nucleus lovers), are negatively charged or contain un-
shared electron pairs that easily form covalent bonds with
electron-deficient centers. Biologically important nucle-
ophilic groups include amino, hydroxyl, imidazole, and
sulfhydryl functions (Fig. 16-5a). The nucleophilic forms of
these groups are also their basic forms. Indeed, nucle-
ophilicity and basicity are closely related properties (Sec-
C¬H
tion 15-1Ba):A compound acts as a base when it forms a co-
valent bond with H
, whereas it acts as a nucleophile when
it forms a covalent bond with an electron-deficient center
other than H
, usually an electron-deficient carbon atom:
Electron-deficient compounds are called electrophiles
(electron lovers). They may be positively charged, contain
an unfilled valence electron shell, or contain an electroneg-
ative atom.The most common electrophiles in biochemical
Nucleophilic
reaction of an
amine
Basic reaction
of an amine RRNH
2
H
H
H
H
N
R CCO OH
H
R
R
R
R
N
RNH
2
Section 16-2. Organic Reaction Mechanisms 563
Figure 16-4 Modes of bond breaking. Homolytic cleav-
age yields radicals, whereas heterolytic cleavage yields either (i)
a carbanion and a proton or (ii) a carbocation and a hydride ion.
C¬H
Figure 16-5 Biologically important nucleophilic and
electrophilic groups. (a) Nucleophiles are the conjugate bases
of weak acids such as the hydroxyl, sulfhydryl, amino, and
Homolytic:
Heterolytic:
Radicals
Carbanion Proton
Carbocation Hydride
ion
CCCH
CC
H
C
H
CC
H
homolytic
cleavage
(i)
(ii)
H
C
H
O
Q
S
(a) Nucleophiles
ROH
O
Q
RSH
RNH
3
HN
NH
R
R
O
Q
RO
O
Q
RS
O
RNH
2
HN
N
S
S
Nucleophilic
form
H
Hydroxyl group
H
Sulfhydryl group
H
Amino group
H
Imidazole group
(b) Electrophiles
H
Protons
M
n
Metal ions
R
C
R
O
Carbonyl carbon atom
R
C
R
NH
Cationic imine (Schiff base)
imidazole groups. (b) Electrophiles contain an electron-deficient
atom (red).
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 563
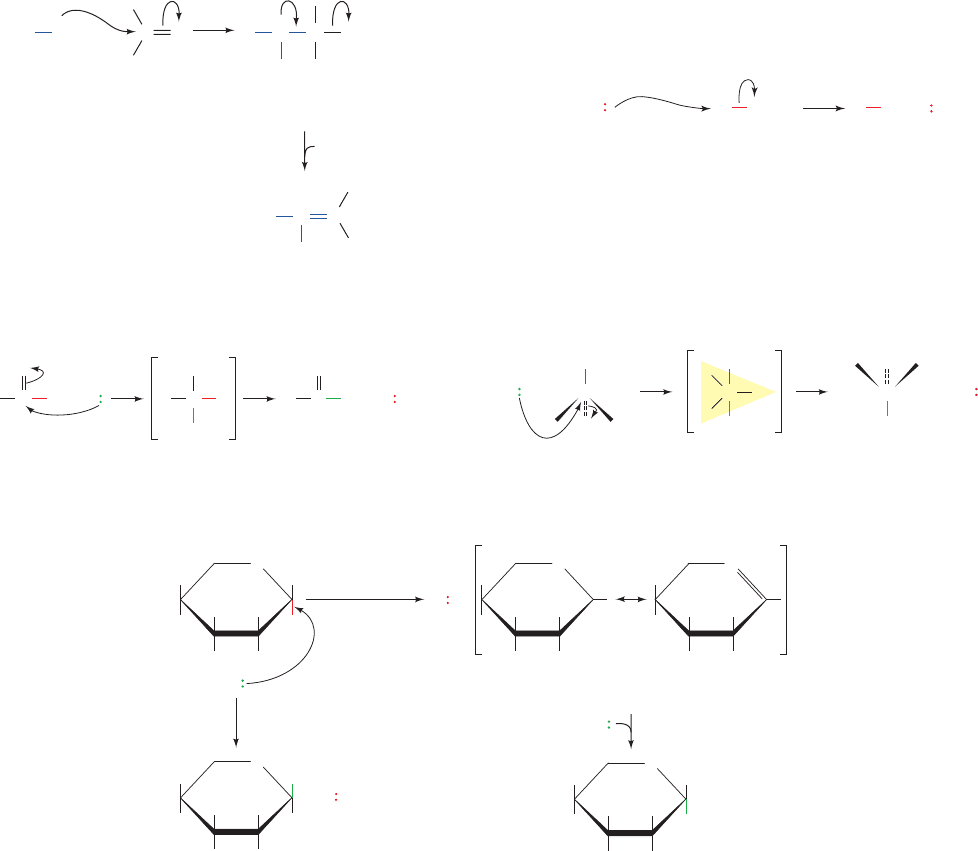
systems are H
, metal ions, the carbon atoms of carbonyl
groups, and cationic imines (Fig. 16-5b).
Reactions are best understood if the electron pair re-
arrangements involved in going from reactants to products
can be traced. In illustrating these rearrangements we shall
use the curved arrow convention in which the movement of
an electron pair is symbolized by a curved arrow emanating
from the electron pair and pointing to the electron-
deficient center attracting the electron pair. For example,
imine formation, a biochemically important reaction
between an amine and an aldehyde or ketone, is represented:
NH
2
OC
H
+
R
N
H
R
R
R
R
COH
R+
Amine Aldehyde
or
ketone
Carbinolamine
intermediate
R
R
N
H
C
R +
+
H
2
O
Imine
O
O
O
O
In the first reaction step, the amine’s unshared electron
pair adds to the electron-deficient carbonyl carbon atom
while one electron pair from its double bond trans-
fers to the oxygen atom. In the second step, the unshared
electron pair on the nitrogen atom adds to the electron-
deficient carbon atom with the elimination of water. At all
times, the rules of chemical reason prevail: For example,
there are never five bonds to a carbon atom or two bonds
to a hydrogen atom.
B. Group-Transfer Reactions
The group transfers that occur in biochemical systems in-
volve the transfer of an electrophilic group from one nucle-
ophile to another:
They could equally well be called nucleophilic substitution
reactions. The most commonly transferred groups in bio-
chemical reactions are acyl groups, phosphoryl groups, and
glycosyl groups (Fig. 16-6):
Nucleophile Electrophile–
nucleophile
YYAAXX
C“O
564 Chapter 16. Introduction to Metabolism
Figure 16-6 Types of metabolic group-transfer reactions.
(a) Acyl group transfer involves addition of a nucleophile (Y) to
the electrophilic carbon atom of an acyl compound to form a
tetrahedral intermediate. The original acyl carrier (X) is then
expelled to form a new acyl compound. (b) Phosphoryl group
transfer involves the in-line (with the leaving group) addition of
a nucleophile (Y) to the electrophilic phosphorus atom of a
tetrahedral phosphoryl group. This yields a trigonal bipyramidal
intermediate whose apical positions are occupied by the leaving
group (X) and the attacking group (Y). Elimination of the leaving
O
C
XR
O
C
Y X
R
O
C
Y
XRY
Tetrahedral
intermediate
(a)
X
(c)
O
Y
O
Y
O
O O
double
displacement
(S
N
1)
Resonance-stabilized
carbocation (oxonium ion)
single
displacement (S
N
2)
Y
Y
X
X
P
Y
Y
X
P
O
O
O
O
O
O
X
(b)
O
P
O
O
Trigonal
bipyramid
intermediate
Y
X
group to complete the transfer reaction results in the phosphoryl
group’s inversion of configuration. (c) Glycosyl group transfer
involves the substitution of one nucleophilic group for another at
C1 of a sugar ring.This reaction usually occurs via a double
displacement mechanism in which the elimination of the original
glycosyl carrier (X) is accompanied by the intermediate formation
of a resonance-stabilized carbocation (oxonuim ion) followed by
the addition of the adding nucleophile (Y).The reaction also
may occur via a single displacement mechanism in which Y directly
displaces X with inversion of configuration.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 564
1. Acyl group transfer from one nucleophile to another
almost invariably involves the addition of a nucleophile to
the acyl carbonyl carbon atom so as to form a tetrahedral
intermediate (Fig. 16-6a). Peptide bond hydrolysis, as cat-
alyzed, for example, by chymotrypsin (Section 15-3C), is a
familiar example of such a reaction.
2. Phosphoryl group transfer proceeds via the in-line
addition of a nucleophile to a phosphoryl phosphorus atom
to yield a trigonal bipyramidal intermediate whose apexes
are occupied by the adding and leaving groups (Fig. 16-6b).
The overall reaction results in the tetrahedral phosphoryl
group’s inversion of configuration. Indeed, chiral phospho-
ryl compounds have been shown to undergo just such an
inversion. For example, Jeremy Knowles has synthesized
ATP made chiral at its -phosphoryl group by isotopic sub-
stitution and demonstrated that this group is inverted on its
transfer to glucose in the reaction catalyzed by hexokinase
(Fig. 16-7).
3. Glycosyl group transfer involves the substitution of
one nucleophilic group for another at C1 of a sugar ring
(Fig. 16-6c). This is the central carbon atom of an acetal.
Chemical models of acetal reactions generally proceed
via acid-catalyzed cleavage of the first bond to form a
resonance-stabilized carbocation at C1 (an oxonium ion).
The lysozyme-catalyzed hydrolysis of bacterial cell wall
polysaccharides (Section 15-2Bb) is such a reaction.
C. Oxidations and Reductions
Oxidation–reduction (redox) reactions involve the loss or
gain of electrons. The thermodynamics of these reactions
is discussed in Section 16-5. Many of the redox reactions
that occur in metabolic pathways involve bond
cleavage with the ultimate loss of two bonding electrons
by the carbon atom. These electrons are transferred to an
electron acceptor such as NAD
(Fig. 13-2). Whether
these reactions involve homolytic or heterolytic bond
cleavage has not always been rigorously established. In
most instances heterolytic cleavage is assumed when radi-
cal species are not observed. It is useful, however, to visu-
alize redox bond cleavage reactions as hydride
transfers as diagrammed below for the oxidation of an
alcohol by NAD
:
For aerobic organisms, the terminal acceptor for the
electron pairs removed from metabolites by their oxida-
tion is molecular oxygen (O
2
). Recall that this molecule is a
ground state diradical species whose unpaired electrons
have parallel spins. The rules of electron pairing (the Pauli
exclusion principle) therefore dictate that O
2
can only ac-
cept unpaired electrons; that is, electrons must be trans-
ferred to O
2
one at a time (in contrast to redox processes
in which electrons are transferred in pairs). Electrons that
are removed from metabolites as pairs must therefore be
passed to O
2
via the electron-transport chain one at a
time. This is accomplished through the use of conjugated
coenzymes that have stable radical oxidation states and
can therefore undergo both 1e
and 2e
redox reactions.
One such coenzyme is flavin adenine dinucleotide (FAD;
General
base
Alcohol NAD
ⴙ
General
acid
Ketone NADH
B
B H H
H
H
H
R
N
O
O
C
C
OC
C
H
R
R
NH
2
NH
2
H
H
H
N
R
O
R
R
C¬H
C¬H
Section 16-2. Organic Reaction Mechanisms 565
Figure 16-7 The phosphoryl-transfer reaction catalyzed by
hexokinase. During its transfer to the 6-OH group of glucose, the
-phosphoryl group of ATP made chiral by isotopic substitution
undergoes inversion of configuration via a trigonal bipyramidal
intermediate.
P
Glucose
O ADP
ADP
ADP
P
O
O
O
O
O
O
O
O
P
H
H
H
H
OH
OH
OH
H
HO
O
H
H
H
H
H
OH
OH
OH
HO
CH
2
OH
O
16
16
16
18
18
18
17
17
O
17
γ
Glucose
ATP
Trigonal
bipyramid
intermediate
H
2
CO
Glucose-6-phosphate
O
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 565
Fig. 16-8). Flavins (substances that contain the isoallox-
azine ring) can undergo two sequential one-electron trans-
fers or a simultaneous two-electron transfer that bypasses
the semiquinone state.
D. Eliminations, Isomerizations, and
Rearrangements
a. Elimination Reactions Form Carbon–Carbon
Double Bonds
Elimination reactions result in the formation of a dou-
ble bond between two previously single-bonded saturated
centers. The substances eliminated may be H
2
O, NH
3
,an
alcohol (ROH), or a primary amine (RNH
2
).The dehydra-
tion of an alcohol, for example, is an elimination reaction:
Bond breaking and bond making in this reaction may pro-
ceed via one of three mechanisms (Fig. 16-9a): (1) con-
certed; (2) stepwise with the bond breaking first to
form a carbocation; or (3) stepwise with the bond
breaking first to form a carbanion.
Enzymes catalyze dehydration reactions by either of
two simple mechanisms: (1) protonation of the OH group
by an acidic group (acid catalysis) or (2) abstraction of the
proton by a basic group (base catalysis). Moreover, in a step-
wise reaction, the charged intermediate may be stabilized
C¬H
C¬O
H
H
H
OH
H
R
R
H
R
R
CC CC H
2
O
566 Chapter 16. Introduction to Metabolism
Figure 16-8 The molecular formula and reactions of the
coenzyme flavin adenine dinucleotide (FAD). The term “flavin”
is synonymous with the isoalloxazine system.The
D-ribitol
residue is derived from the alcohol of the sugar
D-ribose. The
FAD may be half-reduced to the stable radical FADH or fully
reduced to FADH
2
(boxes). Consequently, different
FAD-containing enzymes cycle between different oxidation
states of FAD. FAD is usually tightly bound to its enzymes, so
that this coenzyme is normally a prosthetic group rather than a
cosubstrate as is, for example, NAD
. Consequently, although
humans and other higher animals are unable to synthesize the
isoalloxazine component of flavins and hence must obtain it in
their diets [for example, in the form of riboflavin (vitamin B
2
)],
riboflavin deficiency is quite rare in humans. The symptoms of
riboflavin deficiency, which are associated with general
malnutrition or bizarre diets, include an inflamed tongue, lesions
in the corners of the mouth, and dermatitis.
CH
2
CH
2
NH
2
N
N
N
N
OOO
O
C
O
O
C
C
CH
2
PP
O
H
HH
HO OH
O
D-Ribitol
Riboflavin
Flavin adenine dinucleotide (FAD)
(oxidized or quinone form)
HO H
H
H
HO
HO
H
N
H
7, 8-Dimethylisoalloxazine
Adenosine
FADH
2
(reduced or hydroquinone form)
R
N
N
O
O
H
3
C
H
3
C
N
H
N
O
10
10a
4a
9a
8a
7a
5a
8
7
6
9
5
4
1
3
2
NN
O
H
3
C
H
3
C
N
N
H
H
R
N
N
O
O
H
3
C
H
3
C
N
H
N
H
H
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 566
by an oppositely charged active site group (electrostatic
catalysis). The glycolytic enzyme enolase (Section 17-2I) and
the citric acid cycle enzyme fumarase (Section 21-3G)
catalyze such dehydration reactions.
Elimination reactions may take one of two possible
stereochemical courses (Fig. 16-9b): (1) trans (anti) elimi-
nations, the most prevalent biochemical mechanism, and
(2) cis (syn) eliminations, which are biochemically less
common.
b. Biochemical Isomerizations Involve Intramolecular
Hydrogen Atom Shifts
Biochemical isomerization reactions involve the in-
tramolecular shift of a hydrogen atom so as to change the
location of a double bond. In such a process, a proton is re-
moved from one carbon atom and added to another. The
metabolically most prevalent isomerization reaction is the
aldose–ketose interconversion, a base-catalyzed reaction
that occurs via enediolate anion intermediates (Fig. 16-10).
The glycolytic enzyme phosphoglucose isomerase cat-
alyzes such a reaction (Section 17-2B).
Racemization is an isomerization reaction in which a hy-
drogen atom shifts its stereochemical position at a molecule’s
only chiral center so as to invert that chiral center (e.g., the
racemization of proline by proline racemase;Section 15-1Fa).
Such an isomerization is called an epimerization in a mole-
cule with more than one chiral center.
c. Rearrangements Produce Altered
Carbon Skeletons
Rearrangement reactions break and reform bonds
so as to rearrange a molecule’s carbon skeleton.There are
few such metabolic reactions. One is the conversion of
L-methylmalonyl-CoA to succinyl-CoA by methylmalonyl-
CoA mutase, an enzyme whose prosthetic group is a
vitamin B
12
derivative:
This reaction is involved in the oxidation of fatty acids with
an odd number of carbon atoms (Section 25-2Ec) and sev-
eral amino acids (Section 26-3Ec).
E. Reactions That Make and Break
Carbon–Carbon Bonds
Reactions that make and break carbon–carbon bonds form
the basis of both degradative and biosynthetic metabolism.
The breakdown of glucose to CO
2
involves five such cleav-
ages, whereas its synthesis involves the reverse process.
Such reactions, considered from the synthetic direction, in-
volve addition of a nucleophilic carbanion to an elec-
trophilic carbon atom. The most common electrophilic
H
H
HHCC
COO
C S CoA
O
H
H
HHCC
COO
CCC
C
CCC
C
SCoA
methylmalonyl-
CoA mutase
C
O
L
-Methylmalonyl-CoA Succinyl-CoA
Carbon skeleton rearrangement
C¬C
Section 16-2. Organic Reaction Mechanisms 567
Figure 16-9 Possible elimination reaction mechanisms using
dehydration as an example. Reactions may be (a) either
concerted, stepwise via a carbocation intermediate, or stepwise
via a carbanion intermediate; and may occur with (b) either trans
(anti) or cis (syn) stereochemistry.
Figure 16-10 Mechanism of aldose–ketose isomerization. The
reaction occurs with acid–base catalysis and proceeds via
cis-enediolate intermediates.
H
R
H
R
CC
H
R
H
R
CC
H
R
H
R
CC
R
H
H
R
CC
H
R
H
R
CC
H
H
CC
R
H
OH
R
H
OH
OH
H
OH
H
H
CC
R
H
OH
R
Stepwise via a carbocation
Concerted
H
H
CC
R R
H
OH
R
H
H
CC
H
R
Stepwise via a carbanion
H
H
H
H
CC
R
H
OH
R
OH
C
H
C
R
H
OH
R
(a)
(b)
trans (anti)
H
OH
H
H
CC
R
H
OH
R
cis (syn)
BH
Ketose
Aldose
cis-Enediolate intermediates
B H
H C
CB
R
H
O
O
H
H
H
C
R
C
O
O
H
H
C
R
C
O
O
HO
H
C
R
C
O
BH
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 567
carbon atoms in such reactions are the sp
2
-hybridized car-
bonyl carbon atoms of aldehydes, ketones, esters, and CO
2
:
Stabilized carbanions must be generated to add to these
electrophilic centers. Three examples are the aldol con-
densation (catalyzed, e.g., by aldolase; Section 17-2D),
Claisen ester condensation (citrate synthase; Section 21-3A),
and the decarboxylation of -keto acids (isocitrate dehy-
C
CCOHO
C
drogenase, Section 21-3C; and fatty acid synthase, Section
25-4C). In nonenzymatic systems, both the aldol condensa-
tion and Claisen ester condensation involve the base-
catalyzed generation of a carbanion to a carbonyl group
(Fig. 16-11a,b). The carbonyl group is electron withdraw-
ing and thereby provides resonance stabilization by form-
ing an enolate (Fig. 16-12a). The enolate may be further
stabilized by neutralizing its negative charge. Enzymes do
so through hydrogen bonding or protonation (Fig. 16-12b),
conversion of the carbonyl group to a protonated Schiff
base (covalent catalysis; Fig. 16-12c), or by its coordination
568 Chapter 16. Introduction to Metabolism
Figure 16-11 Examples of C
—
C bond formation and cleavage
reactions. (a) Aldol condensation, (b) Claisen ester
condensation, and (c) decarboxylation of a -keto acid. All three
(a) Aldol condensation
(b) Claisen ester condensation
(c) Decarboxylation of a -keto acid
BB RC
C
R
H
O
H H
R
RRCC
C
C
R
O
H
H
H
O
R
C
C
R
O
H
B RR
R
CC
H HRC
C
H H
O
O
BB
H C
H
H
O
C SCoA H
C
H
H
O
C SCoA
Addition to
electrophilic
center [as
in (a)]
Addition to
electrophilic
center [as
in (a)]
C
H
H
O
C SCoA
Ketone
Acetyl-CoA
-Keto acid
Resonance-stabilized
enolate
Resonance-
stabilized
carbanion
(enolate)
Resonance-
stabilized
enolate
Second ketone
(electrophilic center)
RC CO
O
O
CH
2
RC
O
CH
2
RC
O
CH
2
CO
2
types of reactions involve generation of a resonance-stabilized
carbanion followed by addition of this carbanion to an
electrophilic center.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 568
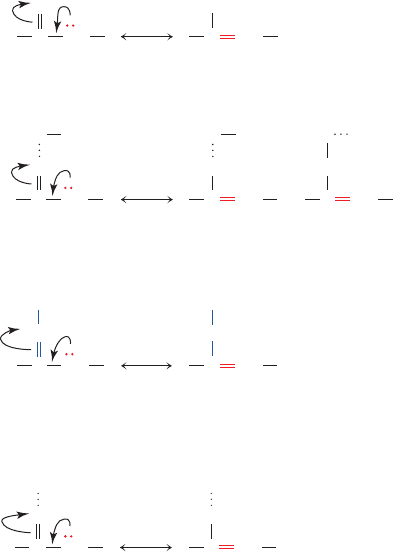
complex network of regulatory processes renders meta-
bolic pathways remarkably sensitive to the needs of the or-
ganism; the output of a pathway is generally only as great
as required.
As you might well imagine, the elucidation of a meta-
bolic pathway on all of these levels is a complex process, in-
volving contributions from a variety of disciplines. Most of
the techniques used to do so involve somehow perturbing
the system and observing the perturbation’s effect on
growth or on the production of metabolic intermediates.
One such technique is the use of metabolic inhibitors that
block metabolic pathways at specific enzymatic steps.
Another is the study of genetic abnormalities that interrupt
specific metabolic pathways. Techniques have also been de-
veloped for the dissection of organisms into their compo-
nent organs, tissues, cells, and subcellular organelles, and for
the purification and identification of metabolites as well as
the enzymes that catalyze their interconversions. The use of
isotopic tracers to follow the paths of specific atoms and
molecules through the metabolic maze has become routine.
Techniques utilizing NMR technology are able to trace
metabolites noninvasively as they react in vivo. This section
outlines the use of these various techniques.
A. Metabolic Inhibitors, Growth Studies, and
Biochemical Genetics
a. Pathway Intermediates Accumulate in the
Presence of Metabolic Inhibitors
The first metabolic pathway to be completely traced was
the conversion of glucose to ethanol in yeast by a process
known as glycolysis (Section 17-1A). In the course of these
studies, certain substances, called metabolic inhibitors, were
found to block the pathway at specific points, thereby caus-
ing preceding intermediates to build up. For instance,
iodoacetate causes yeast extracts to accumulate fructose-
1,6-bisphosphate, whereas fluoride causes the buildup of
two phosphate esters, 3-phosphoglycerate and 2-phospho-
glycerate. The isolation and characterization of these inter-
mediates was vital to the elucidation of the glycolytic path-
way: Chemical intuition combined with this information led
to the prediction of the pathway’s intervening steps. Each
of the proposed reactions was eventually shown to occur
in vitro as catalyzed by a purified enzyme.
b. Genetic Defects Also Cause Metabolic
Intermediates to Accumulate
Archibald Garrod’s realization, in the early 1900s, that
human genetic diseases are the consequence of deficien-
cies in specific enzymes (Section 1-4Cd) also contributed to
the elucidation of metabolic pathways. For example, on the
ingestion of either phenylalanine or tyrosine, individuals with
the largely harmless inherited condition known as alcap-
tonuria, but not normal subjects, excrete homogentisic acid
in their urine (Section 26-3Hd). This is because the liver of
alcaptonurics lacks an enzyme that catalyzes the breakdown
of homogentisic acid. Another genetic disease, phenylke-
tonuria (Section 26-3Hd), results in the accumulation of
Section 16-3. Experimental Approaches to the Study of Metabolism 569
Figure 16-12 Stabilization of carbanions. (a) Carbanions
adjacent to carbonyl groups are stabilized by the formation of
enolates. (b) Carbanions adjacent to carbonyl groups hydrogen
bonded to general acids are stabilized electrostatically or by
charge neutralization. (c) Carbanions adjacent to protonated
imines (Schiff bases) are stabilized by the formation of enamines.
(d) Metal ions stabilize carbanions adjacent to carbonyl groups
by the electrostatic stabilization of the enolate.
to a metal ion (metal ion catalysis; Fig. 16-12d).The decar-
boxylation of a -keto acid does not require base catalysis
for the generation of the resonance-stabilized carbanion;
the highly exergonic formation of CO
2
provides its driving
force (Fig. 16-11c).
3 EXPERIMENTAL APPROACHES
TO THE STUDY OF METABOLISM
A metabolic pathway can be understood at several levels:
1. In terms of the sequence of reactions by which a spe-
cific nutrient is converted to end products, and the energet-
ics of these conversions.
2. In terms of the mechanisms by which each intermedi-
ate is converted to its successor. Such an analysis requires
the isolation and characterization of the specific enzymes
that catalyze each reaction.
3. In terms of the control mechanisms that regulate the
flow of metabolites through the pathway. An exquisitely
Carbanion
Carbanion Zn
2ⴙ
–stabilized
enolate
Enolate
Hydrogen-bonded
carbonyl
Hydrogen-bonded
enolate or enol
Schiff base
carbanion (imine)
Schiff base
(enamine)
(a)
(b)
(c)
(d)
CCH
O
CCH
O
C
O
H
B
CCH or
O
H
B
CCH
O
HB
CCH
NH
NH
C
CH
C
O
Zn
2
Zn
2
CCH
O
CH
CH
CH
JWCL281_c16_557-592.qxd 6/10/10 11:51 AM Page 569
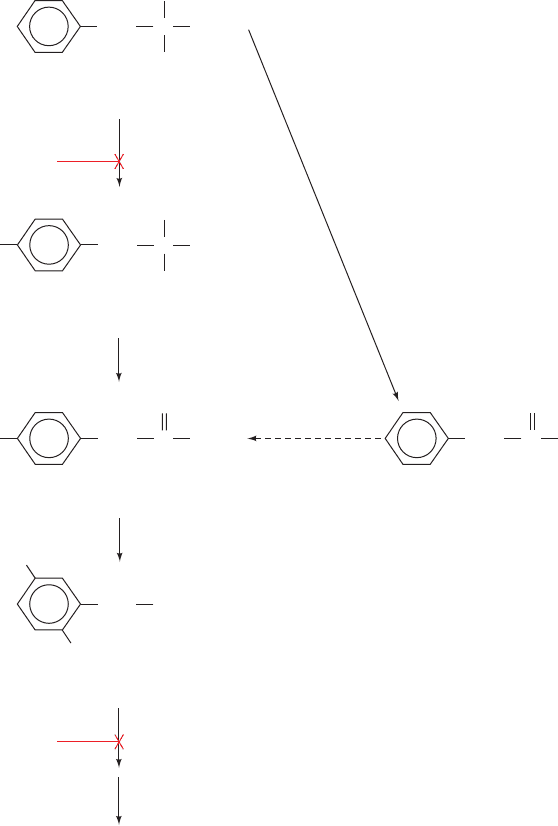
phenylpyruvate in the urine (and which,if untreated, causes
severe mental retardation in infants). Ingested phenylala-
nine and phenylpyruvate appear as phenylpyruvate in the
urine of affected subjects, whereas tyrosine is metabolized
normally. The effects of these two abnormalities suggested
the pathway for phenylalanine metabolism diagrammed in
Fig. 16-13. However, the supposition that phenylpyruvate
but not tyrosine occurs on the normal pathway of pheny-
lalanine metabolism because phenylpyruvate accumulates
in the urine of phenylketonurics has proved incorrect.This
indicates the pitfalls of relying solely on metabolic blocks
and the consequent buildup of intermediates as indicators
of a metabolic pathway. In this case, phenylpyruvate for-
mation was later shown to arise from a normally minor
pathway that becomes significant only when the pheny-
lalanine concentration is abnormally high, as it is in
phenylketonurics.
c. Metabolic Blocks Can Be Generated by
Genetic Manipulation
Early metabolic studies led to the astounding discovery
that the basic metabolic pathways in most organisms are es-
sentially identical. This metabolic uniformity has greatly fa-
cilitated the study of metabolic reactions. A mutation that
inactivates or deletes an enzyme in a pathway of interest can
be readily generated in rapidly reproducing microorganisms
through the use of mutagens (chemical agents that induce
genetic changes;Section 32-1A), X-rays, or genetic engineer-
ing techniques (Section 5-5). Desired mutants are identified
by their requirement of the pathway’s end product for
growth. For example, George Beadle and Edward Tatum
proposed a pathway of arginine biosynthesis in the mold
Neurospora crassa based on their analysis of three arginine-
requiring auxotrophic mutants (mutants requiring a specific
nutrient for growth), which were isolated after X-irradiation
(Fig. 16-14). This landmark study also conclusively demon-
strated that enzymes are specified by genes (Section 1-4Cd).
d. Genetic Manipulations of Higher Organisms
Provide Metabolic Insights
Transgenic organisms (Section 5-5H) constitute valu-
able resources for the study of metabolism. They can be
used to both create metabolic blocks and to express genes in
tissues where they are not normally present. For example,
creatine kinase catalyzes the formation of phosphocreatine
(Section 16-4Cd), a substance that functions to generate
ATP rapidly when it is in short supply. This enzyme is nor-
mally present in many tissues, including brain and muscle,
but not in liver.The introduction of the gene encoding cre-
atine kinase into the liver of a mouse causes the liver to
synthesize phosphocreatine when the mouse is fed crea-
tine, as demonstrated by localized in vivo NMR techniques
(Fig. 16-15; NMR is discussed below). The presence of
570 Chapter 16. Introduction to Metabolism
Figure 16-13 Pathway for phenylalanine degradation.
It was originally hypothesized that phenylpyruvate was a
pathway intermediate based on the observation that
phenylketonurics excrete ingested phenylalanine
and phenylpyruvate as phenylpyruvate. Further studies, however,
demonstrated that phenylpyruvate is not a homogentisate
precursor; rather, phenylpyruvate production is significant only
when the phenylalanine concentration is abnormally high.
Instead, tyrosine is the normal product of phenylalanine
degradation.
H
C
NH
+
3
CH
2
Phenylalanine
COO
–
H
C
NH
+
3
CH
2
Tyrosine
COO
–
HO
O
C
CH
2
COO
–
HO
p-Hydroxyphenylpyruvate Phenylpyruvate
CH
2
COO
–
HO
OH
Homogentisate
H
2
O + CO
2
Defective in
alcaptonuria
Originally unknown;
defective in
phenylketonuria
nonexistent:
originally
thought to
exist and be
defective in
phenylketonurics
secondary
pathway
O
C
CH
2
COO
–
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 570
