Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

serine proteases. The primordial trypsin gene evidently
arose before the divergence of prokaryotes and eukary-
otes.
There are three known serine proteases whose primary
and tertiary structures bear no discernible relationship to
each other or to chymotrypsin but which, nevertheless,
contain catalytic triads at their active sites whose structures
closely resemble that of chymotrypsin:
1. Subtilisin, an endopeptidase that was originally iso-
lated from Bacillus subtilis.
2. Wheat germ serine carboxypeptidase II, an exopepti-
dase whose structure is surprisingly similar to that of car-
boxypeptidase A (Fig. 8-19a) even though the latter pro-
tease has an entirely different catalytic mechanism from
that of the serine proteases (see Problem 3).
3. E. coli ClpP, which functions in the degradation of
cellular proteins (Section 32-6B).
Since the orders of the corresponding active site residues
in the amino acid sequences of the four types of serine
proteases are quite different (Fig. 15-22), it seems highly
improbable that they could have evolved from a common
ancestor serine protease. These proteins apparently consti-
tute a remarkable example of convergent evolution: Nature
seems to have independently discovered the same catalytic
mechanism at least four times. (In addition, human cy-
tomegalovirus protease, an essential protein for virus
replication that bears no resemblance to the above
proteases, has active site Ser and His residues whose rela-
tive positions are similar to those in other serine proteases
but lacks an active site Asp residue; it appears to have a
catalytic dyad.)
C. Catalytic Mechanism
See Guided Exploration 12: The catalytic mechanism of serine
proteases
The extensive active site homologies among the
various serine proteases indicate that they all have the
same catalytic mechanism. On the basis of considerable
chemical and structural data gathered in many laborato-
ries, the following catalytic mechanism has been formu-
lated for the serine proteases, here given in terms of chy-
motrypsin (Fig. 15-23):
1. After chymotrypsin has bound substrate to form
the Michaelis complex, Ser 195, in the reaction’s rate-deter-
mining step, nucleophilically attacks the scissile peptide’s
carbonyl group to form a complex known as the tetrahe-
dral intermediate (covalent catalysis). X-ray studies indi-
cate that Ser 195 is ideally positioned to carry out this nu-
cleophilic attack (proximity and orientation effects). The
imidazole ring of His 57 takes up the liberated proton,
thereby forming an imidazolium ion (general base cataly-
sis). This process is aided by the polarizing effect of the
unsolvated carboxylate ion of Asp 102, which is hydrogen
bonded to His 57 (electrostatic catalysis; see Section 15-
3Dd). Indeed, the mutagenic replacement of trypsin’s Asp
Section 15-3. Serine Proteases 531
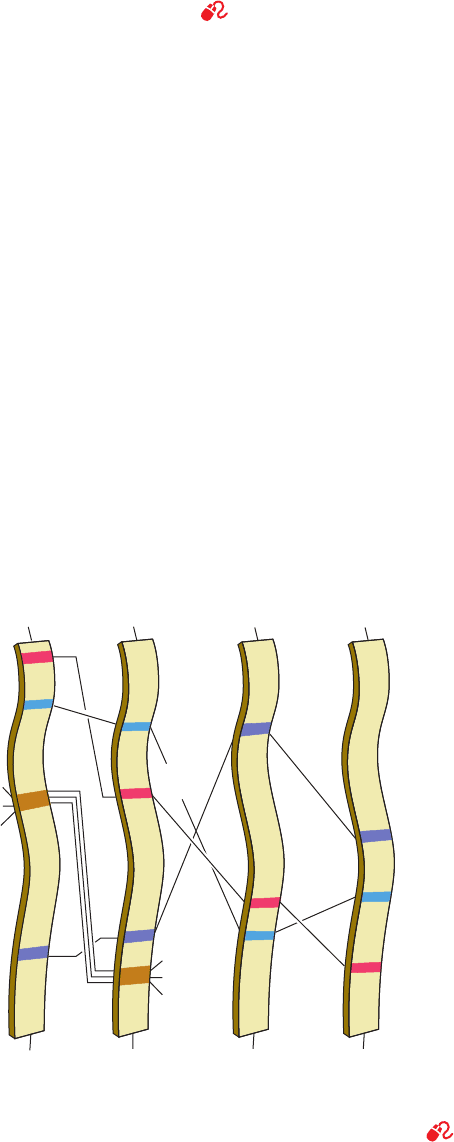
Figure 15-22 Relative positions of the active site residues in
subtilisin, chymotrypsin, serine carboxypeptidase II, and ClpP
protease. The peptide backbones of Ser 214,Trp 215, and Gly 216
in chymotrypsin, and their counterparts in subtilisin, participate
Ser 125
Leu 126
Gly 127
Ser 221
Subtilisin
His 64
Asp 32
NH
3
+
COO
–
Ser 214
Trp 215
Gly 216
Chymotrypsin
Ser 195
His 57
NH
3
+
COO
–
Asp
102
Ser
146
Serine
carboxypeptidase II
NH
3
+
COO
–
His
397
Asp
338
Ser
97
Asp
171
His
122
ClpP
protease
NH
3
+
COO
–
in substrate-binding interactions. [After Robertus, J.D.,Alden,
R.A., Birktoft, J.J., Kraut, J., Powers, J.C., and Wilcox, P.E.,
Biochemistry 11, 2449 (1972).]
See Kinemage Exercise 10-2
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 531
102 by Asn leaves the enzyme’s K
M
substantially un-
changed at neutral pH but reduces its k
cat
to ⬃0.05% of
its wild-type value. Neutron diffraction studies have
demonstrated that Asp 102 remains a carboxylate ion rather
than abstracting a proton from the imidazolium ion to form
an uncharged carboxylic acid group. The tetrahedral
532 Chapter 15. Enzymatic Catalysis
R
O
CN
H
R⬘
Enzyme–substrate
complex
Nucleophilic
attack
Asp
102
1
2
C
....
....
O
O
N
N
CH
2
His
57
CH
2
Ser
195
–
H
2
C
OH
H
H
H
H
H
H
..
3
1
4
2
Tetrahedral intermediate
R
O
–
CN
H
H
R⬘
3
1
Asp
102
C
....
....
O
O
N
N
+
CH
2
His
57
CH
2
Ser
195
–
H
2
C
O
Acyl–enzyme intermediate
New N-terminus of
cleaved polypeptide
chain
Substrate
polypeptide
R⬘NH
2
H
2
O
O
N CH
2
O
CO
H
H
Asp
102
C
....
....
O
N
CH
2
His
57
–
H
2
C
1
3
Ser
195
O
R
O
CN
H
H
R⬘
3
1
Asp
102
C
....
....
O
O
N
N
CH
2
His
57
CH
2
Ser
195
–
H
2
C
O
R
R
O
CO
H
3
+
New C-terminus
of cleaved polypeptide
chain
Active enzymeTetrahedral intermediate
Asp
102
C
....
....
O
O
N
N
CH
2
His
57
CH
2
Ser
195
–
H
2
C
OH
Asp
102
C
....
....
O
O
N
N
+
CH
2
His
57
–
O
–
CO
H
H
H
2
C
CH
2
Ser
195
O
R
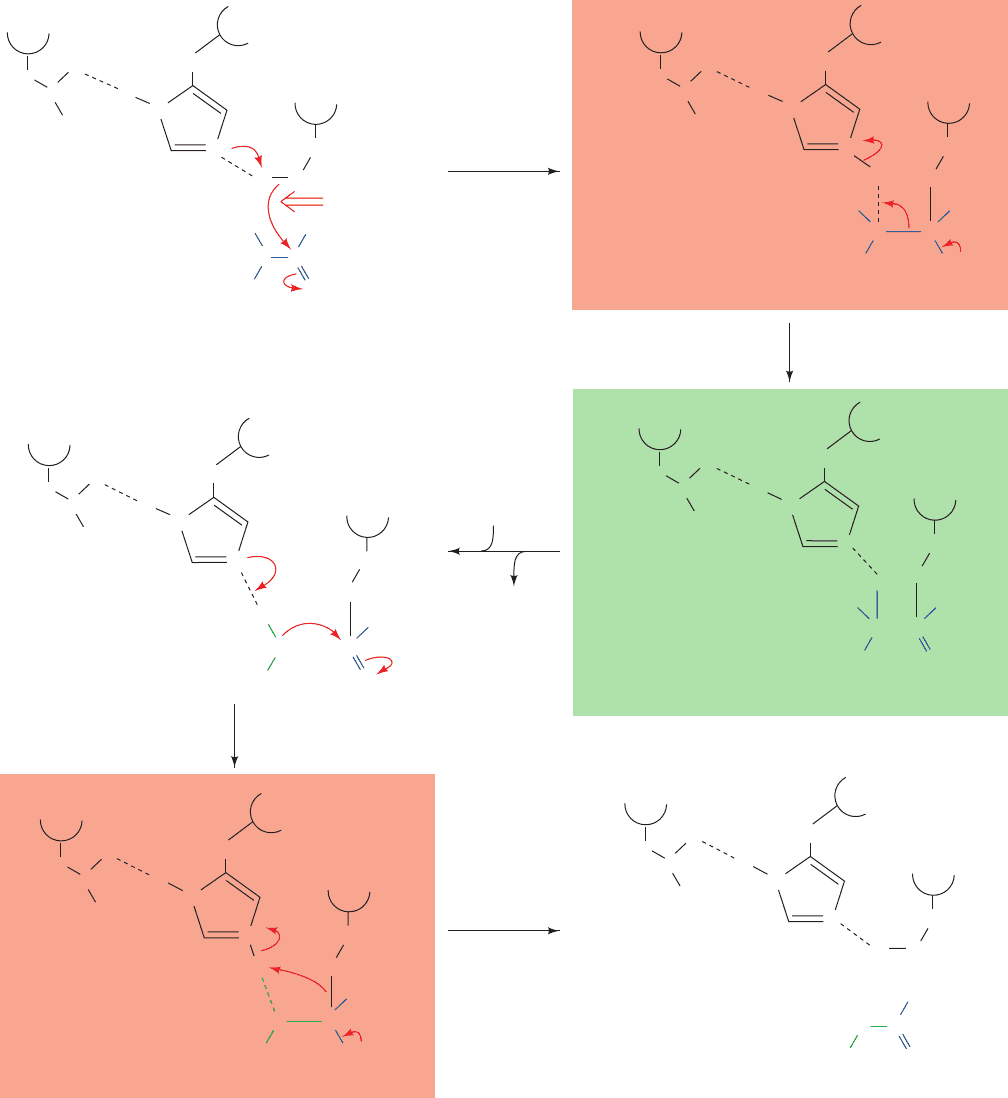
Figure 15-23 Catalytic mechanism of the serine proteases. The
reaction involves (1) the nucleophilic attack of the active site Ser
on the carbonyl carbon atom of the scissile peptide bond to form
the tetrahedral intermediate; (2) the decomposition of the
tetrahedral intermediate to the acyl–enzyme intermediate
through general acid catalysis by the active site Asp-polarized
His, followed by loss of the amine product and its replacement by
a water molecule; (3) the reversal of Step 2 to form a second
tetrahedral intermediate; and (4) the reversal of Step 1 to yield
the reaction’s carboxyl product and the active enzyme.
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 532
intermediate has a well-defined, although transient, exis-
tence. We shall see that much of chymotrypsin’s catalytic
power derives from its preferential binding of the transi-
tion state leading to this intermediate (transition state bind-
ing catalysis).
2. The tetrahedral intermediate decomposes to the
acyl–enzyme intermediate under the driving force of pro-
ton donation from N3 of His 57 (general acid catalysis).
The amine leaving group (R¿NH
2
, the new N-terminal por-
tion of the cleaved polypeptide chain) is released from the
enzyme and replaced by water from the solvent.
3 & 4. The acyl–enzyme intermediate (which, in the ab-
sence of enzyme, would be a stable compound) is rapidly
deacylated by what is essentially the reverse of the previ-
ous steps followed by the release of the resulting carboxy-
late product (the new C-terminal portion of the cleaved
polypeptide chain), thereby regenerating the active en-
zyme. In this process, water is the attacking nucleophile
and Ser 195 is the leaving group.
D. Testing the Catalytic Mechanism
The formulation of the foregoing model for catalysis by
serine proteases has prompted numerous investigations of
its validity. In this section we discuss several of the most re-
vealing of these studies.
a. The Tetrahedral Intermediate Is Mimicked in a
Complex of Trypsin with Trypsin Inhibitor
Convincing structural evidence for the existence of the
tetrahedral intermediate was provided by Robert Huber in
an X-ray study of the complex between the 58-residue pro-
tein bovine pancreatic trypsin inhibitor (BPTI) and
trypsin. BPTI binds to and inactivates trypsin, thereby pre-
venting any trypsin that is prematurely activated in the
pancreas from digesting that organ (Section 15-3E). BPTI
binds to the active site region of trypsin across a tightly
packed interface that is cross-linked by a complex network
of hydrogen bonds. This complex’s 10
13
M
1
association
constant, among the largest of any known protein–protein
interaction, emphasizes BPTI’s physiological importance.
The portion of BPTI in contact with the trypsin active
site resembles bound substrate. The side chain of BPTI Lys
15I (here “I” differentiates BPTI residues from trypsin
residues) occupies the trypsin specificity pocket (Fig.15-24a)
and the peptide bond between Lys 15I and Ala 16I is posi-
tioned as if it were the scissile peptide bond (Fig. 15-24b).
What is most remarkable about this structure is that its ac-
tive site complex assumes a conformation well along the re-
action coordinate toward the tetrahedral intermediate: The
side chain oxygen of trypsin Ser 195, the active Ser, is in
closer-than-van der Waals contact (2.6 Å) with the pyrami-
dally distorted carbonyl carbon of BPTI’s “scissile” peptide.
Despite this close contact, the proteolytic reaction cannot
proceed past this point along the reaction coordinate be-
cause of the rigidity of the active site complex and because
it is so tightly sealed that the leaving group cannot leave
and water cannot enter the reaction site.
Protease inhibitors are common in nature, where they
have protective and regulatory functions. For example, cer-
tain plants release protease inhibitors in response to insect
bites, thereby causing the offending insect to starve by in-
activating its digestive enzymes. Protease inhibitors consti-
tute ⬃10% of the nearly 200 proteins of blood serum. For
instance, ␣
1
-proteinase inhibitor, which is secreted by the
liver, inhibits leukocyte elastase (leukocytes are a type of
white blood cell; the action of leukocyte elastase is thought
to be part of the inflammatory process). Pathological vari-
ants of
1
-proteinase inhibitor with reduced activity are as-
sociated with pulmonary emphysema, a degenerative dis-
ease of the lungs resulting from the hydrolysis of its elastic
fibers. Smokers also suffer from reduced activity of their
1
-proteinase inhibitor because of the oxidation of its ac-
tive site Met residue. Full activity of this inhibitor is not re-
gained until several hours after smoking.
Section 15-3. Serine Proteases 533
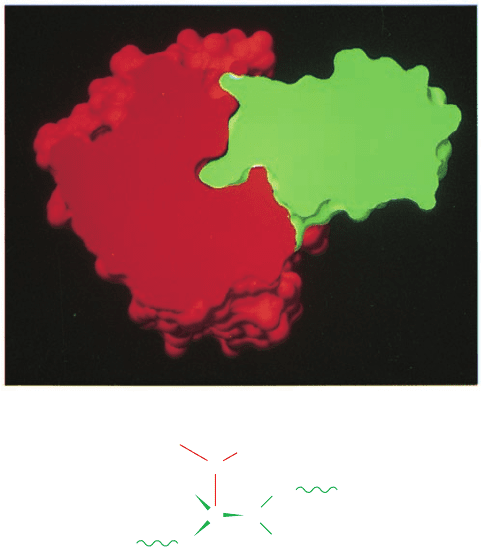
Figure 15-24 Trypsin–BPTI complex. (a) The X-ray structure
shown as a cutaway surface drawing indicating how trypsin (red)
binds BPTI (green).The green protrusion extending into the red
cavity near the center of the figure represents the Lys 15I side
chain occupying trypsin’s specificity pocket. Note the close
complementary fit of these two proteins. [Courtesy of Michael
Connolly, New York University.] (b) Trypsin Ser 195, the active
Ser, is in closer-than-van der Waals contact with the carbonyl
carbon of BPTI’s scissile peptide, which is pyramidally distorted
toward Ser 195.The normal proteolytic reaction is apparently
arrested somewhere along the reaction coordinate between the
Michaelis complex and the tetrahedral intermediate.
Ser 195
H
H
O
O
C
N
C
␣
C
␣
Ala 16I
Lys 15I
(b)
(a)
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 533
b. Serine Proteases Preferentially Bind the
Transition State
Detailed comparisons of the X-ray structures of several
serine protease–inhibitor complexes have revealed a
further structural basis for catalysis in these enzymes
(Fig. 15-25):
1. The conformational distortion that occurs with the
formation of the tetrahedral intermediate causes the car-
bonyl oxygen of the scissile peptide to move deeper into
the active site so as to occupy a previously unoccupied po-
sition, the oxyanion hole.
2. There it forms two hydrogen bonds with the enzyme
that cannot form when the carbonyl group is in its normal
trigonal conformation. These two enzymatic hydrogen
bond donors were first noted by Joseph Kraut to occupy
corresponding positions in chymotrypsin and subtilisin. He
proposed the existence of the oxyanion hole based on the
premise that convergent evolution had made the active
sites of these unrelated enzymes functionally identical.
3. The tetrahedral distortion, moreover, permits the for-
mation of an otherwise unsatisfied hydrogen bond between
the enzyme and the backbone NH group of the residue pre-
ceding the scissile peptide. Consequently, the enzyme binds
the tetrahedral intermediate in preference to either the
Michaelis complex or the acyl–enzyme intermediate.
It is this phenomenon that is responsible for much of the
catalytic efficiency of serine proteases (see below). In fact,
the reason that DIPF is such an effective inhibitor of serine
proteases is because its tetrahedral phosphate group
makes this compound a transition state analog of the
enzyme.
c. The Tetrahedral Intermediate and the Water
Molecule Attacking the Acyl–Enzyme Intermediate
Have Been Directly Observed
Most enzymatic reactions turn over far too rapidly for
their intermediate states to be studied by X-ray or NMR
techniques. Consequently, much of our structural knowl-
edge of these intermediate states derives from the study of
enzyme–inhibitor complexes or complexes of substrates
with inactivated enzymes. Yet the structural relevance of
these complexes is subject to doubt precisely because they
are catalytically unproductive.
In an effort to rectify this situation for serine pro-
teases, Janos Hajdu and Christopher Schofield searched
for peptide–protease complexes that are stable at a pH at
which the protease is inactive but that could be rendered
active by changing the pH. To do so, they screened libraries
of peptides for their ability to bind to porcine pancreatic
elastase at pH 3.5 (at which His 57 is protonated and hence
unable to act as a general base) through the use of ESI-MS
(Section 7-1I). They thereby discovered that YPFVEPI, a
heptapeptide segment of the human milk protein -casein
that is named BCM7, forms a complex with elastase, whose
mass is consistent with the formation of an ester linkage
between BCM7 and the enzyme. In the presence of
18
OH
2
at pH 7.5 (where elastase is active), the
18
O label was incor-
porated into both BCM7 and the elastase–BCM7 complex,
thereby demonstrating that the reaction of BCM7 with
elastase is reversible at this pH. Fragmentation studies by
fast atom bombardment–tandem mass spectrometry
(FAB–MS/MS; Section 7-1I) further revealed that BCM7
that had been incubated with elastase in the presence of
18
OH
2
at pH 7.5 incorporated the
18
O label into only its
C-terminal Ile residue.
534 Chapter 15. Enzymatic Catalysis
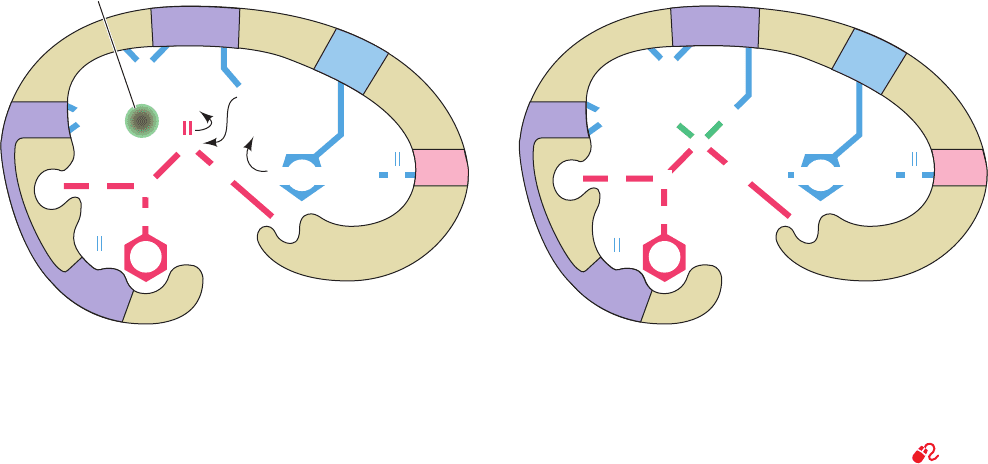
Figure 15-25 Transition state stabilization in the serine
proteases. (a) In the Michaelis complex, the trigonal carbonyl
carbon of the scissile peptide is conformationally constrained
from binding in the oxyanion hole (upper left). (b) In the
tetrahedral intermediate, the now charged carbonyl oxygen of
the scissile peptide (the oxyanion) has entered the oxyanion
hole, thereby hydrogen bonding to the backbone NH groups of
C
O
N
H
NHNH
Oxyanion hole
N
H
Ser 195
His 57
Asp
102
Gly 193
N
H
R C
α
C
β
O
H
CNHN
O
C
R′
O
C
HN
(a)
Ser 195
His 57
Asp
102
N
H
R C
α
C
β
R′
(b)
+NHHN
O
C
O
–
Gly 193
O
C
O
Gly 193
...
Gly 193
.....
...
...
...
...
NH
.. ..
–
O
–
O
Gly 193 and Ser 195.The consequent conformational distortion
permits the NH group of the residue preceding the scissile
peptide bond to form an otherwise unsatisfied hydrogen bond to
Gly 193. Serine proteases therefore preferentially bind the
tetrahedral intermediate. [After Robertus, J.D., Kraut, J., Alden,
R.A., and Birktoft, J.J., Biochemistry 11, 4302 (1972).]
See
Kinemage Exercise 10-3
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 534
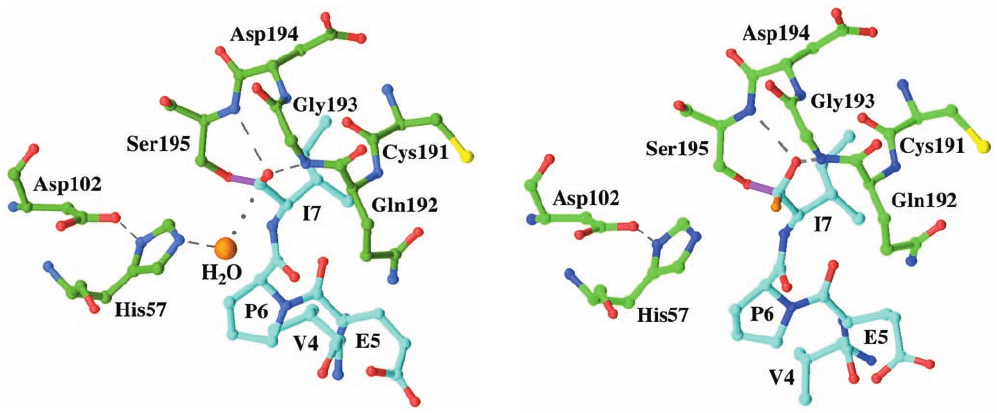
The X-ray structure of the BCM7–elastase complex at
pH 5 (Fig. 15-26a) revealed that BCM7’s C-terminal car-
boxyl group, in fact, forms an ester linkage with elastase’s
Ser 195 side chain hydroxyl group to form the expected
acyl–enzyme intermediate. Moreover, this X-ray structure
reveals the presence of a bound water molecule that appears
poised to nucleophilically attack the ester linkage (the
distance from this water molecule to BCM7’s C-terminal
C atom is 3.1 Å and the line between them is nearly per-
pendicular to the plane of the acyl group). His 57, which is
hydrogen bonded to this water molecule, is properly posi-
tioned to abstract one of its protons, thereby activating it
for the nucleophilic attack (general base catalysis).The car-
bonyl O atom of the acyl group occupies the enzyme’s
oxyanion hole such that it is hydrogen bonded to the main
chain N atoms of both Ser 195 and Gly 193. This is in agree-
ment with spectroscopic measurements indicating that the
acyl–enzyme intermediate’s carbonyl group is, in fact,
hydrogen bonded to the oxyanion hole. It was initially
assumed that the oxyanion hole acts only to stabilize the
tetrahedral oxyanion transition state that resides near
the tetrahedral intermediate on the catalytic reaction coor-
dinate. However, it now appears that the oxyanion hole
also functions to polarize the carbonyl group of the
acyl–enzyme intermediate toward an oxyanion (electro-
static catalysis).
The catalytic reaction was initiated in crystals of the
BCM7–elastase complex by transferring them to a buffer at
pH 9.After soaking in this buffer for 1 min,the crystals were
rapidly frozen in liquid N
2
(196°C), thereby arresting the
enzymatic reaction (recall that the catalytically essential
collective motions of proteins cease at such low tempera-
tures; Section 9-4a). The X-ray structure of such a frozen
crystal (Fig. 15-26b) revealed that the above acyl–enzyme
intermediate had converted to the tetrahedral intermedi-
ate, whose oxyanion, as expected, remained hydrogen
bonded to the N atoms of Ser 195 and Gly 193. Comparison
of this crystal structure with that of the acyl–enzyme inter-
mediate reveals that the enzyme’s active site residues do
not significantly change their positions in the conversion
from the acyl–enzyme intermediate to the tetrahedral inter-
mediate. However, the peptide substrate must do so out of
steric necessity when the trigonal planar acyl group con-
verts to the tetrahedral oxyanion (compare Figs. 15-26a and
15-26b). In response, several enzyme residues that contact
the peptide but which are distant from the active site also
shift their positions (not shown in Fig. 15-26).
d. The Role of the Catalytic Triad: Low-Barrier
Hydrogen Bonds
The earlier literature postulated that the Asp 102-
polarized His 57 side chain directly abstracts a proton
Section 15-3. Serine Proteases 535
Figure 15-26 X-ray structures of porcine pancreatic elastase in
complex with the heptapeptide BCM7 (YPFVEPI). The residues
of elastase are specified by the three-letter code and those of
BCM7 are specified by the one-letter code. (a) The complex at
pH 5.The enzyme’s active site residues and the heptapeptide
(whose N-terminal three residues are disordered) are shown in
ball-and-stick form with elastase C green, BCM7 C cyan, N blue,
O red, S yellow, and the bond between the Ser 195 O atom and
the C-terminal C atom of BCM7 lavender.The enzyme-bound
water molecule, which appears poised to nucleophilically attack
the acyl–enzyme’s carbonyl C atom, is represented by an orange
sphere. The dashed gray lines represent the catalytically
important hydrogen bonds and the dotted gray line indicates the
trajectory that the bound water molecule presumably follows in
nucleophilically attacking the acyl group’s carbonyl C atom.
(b) The complex after being brought to pH 9 for 1 min and then
rapidly frozen in liquid nitrogen.The various groups in the
structure are represented and colored as in Part a. Note that the
water molecule in Part a has become a hydroxyl substituent
(orange) to the carbonyl C atom, thereby yielding the tetrahedral
intermediate. [Based on X-ray structures by Christopher
Schofield and Janos Hajdu, University of Oxford, U.K. PDBids
(a) 1HAX and (b) 1HAZ.]
(a)
(b)
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 535
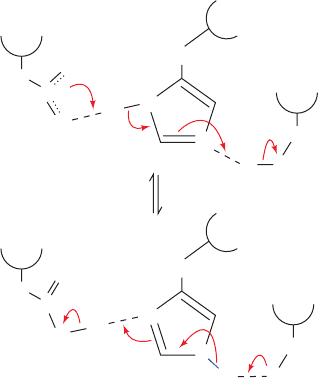
from Ser 195, thereby converting its weakly nucleophilic
¬CH
2
OH group to a highly nucleophilic alkoxide ion,
¬CH
2
O
:
In the process, the anionic charge of Asp 102 was thought
to be transferred, via a tautomeric shift of His 57, to Ser
195. The catalytic triad was therefore originally named the
charge relay system. It is now realized,however,that such a
mechanism is implausible because an alkoxide ion (pK
15) has far greater proton affinity than does His 57 (pK ⬇
7, as measured by NMR techniques). How, then, can Asp
102 nucleophilically activate Ser 195?
A possible solution to this conundrum has been
pointed out by W.W. Cleland and Maurice Kreevoy and,
independently, by John Gerlt and Paul Gassman. Proton
transfers between hydrogen bonded groups
only occur at physiologically reasonable rates when the
pK of the proton donor is no more than 2 or 3 pH units
greater than that of the protonated form of the proton
acceptor (the height of the kinetic barrier, G
‡
, for the
protonation of an acceptor by a more basic donor in-
creases with the difference between the pK’s of the
donor and acceptor). However, when the pK’s of the hy-
drogen bonding donor (D) and acceptor (A) groups are
nearly equal, the distinction between them breaks down:
The hydrogen atom becomes more or less equally shared
between them . Such low-barrier hydrogen
bonds (LBHBs) are unusually short and strong (they are
also known as short, strong hydrogen bonds): They have,
as studies of model compounds in the gas phase indicate,
association free energies as high as 40 to 80
(D
p
H
p
A)
(D¬H
p
A)
Asp
102
His
57
H
2
C
O
O
O
C
–
H
H
N
N
CH
2
CH
2
Ser
195
Asp
102
His
57
H
2
C
O
O
O
C
H
H
N
N
CH
2
CH
2
Ser
195
–
"Charge relay system"
kJ ⴢ mol
1
versus the 12 to 30 kJ ⴢ mol
1
for normal
hydrogen bonds (the energy of the normally covalent
bond is subsumed into the low-barrier hydrogen
bonding system) and a length of 2.55 Å for
and 2.65 Å for versus 2.8 to 3.1 Å
for normal hydrogen bonds.
LBHBs are unlikely to exist in dilute aqueous solution
because water molecules, which are excellent hydrogen
bonding donors and acceptors, effectively compete with
and A for hydrogen bonding sites. However, LB-
HBs may exist in nonaqueous solution and in the active
sites of enzymes that exclude bulk solvent water. If so, an
effective enzymatic “strategy” would be to convert a
weak hydrogen bond in the Michaelis complex to a
strong hydrogen bond in the transition state, thereby fa-
cilitating proton transfer while applying the difference in
the free energy between the normal and low-barrier hy-
drogen bonds to preferentially binding the transition
state. In fact, as Perry Frey has shown, the NMR spec-
trum of the proton linking His 57 to Asp 102 in chy-
motrypsin (which exhibits a particularly large downfield
chemical shift indicative of deshielding) is consistent
with the formation of an LBHB in the transition state
(Fig. 15-25b; the pK’s of protonated His 57 and Asp 102
are nearly equal in the anhydrous environment of the ac-
tive site complex). This presumably promotes proton
transfer from Ser 195 to His 57 as in the charge relay
mechanism. Moreover, an ultrahigh (0.78 Å) resolution
X-ray structure of Bacillus lentus subtilisin by Richard
Bott reveals that the hydrogen bond between His 64 and
Asp 32 of its catalytic triad has an unusually short
distance of 2.62 0.01 Å and that its H atom is nearly
centered between the N and O atoms (note that this
highly accurate protein X-ray structure is one of the very
few in which H atoms are observed and in which short
distances are confidently measured).
Although several studies, such as the foregoing, have re-
vealed the existence of unusually short hydrogen bonds in
enzyme active sites, it is far more difficult to demonstrate
experimentally that they are unusually strong, as LBHBs
are predicted to be. In fact, several studies of the strengths
of unusually short hydrogen bonds in organic model
compounds in nonaqueous solutions suggest that these
hydrogen bonds are not unusually strong. Consequently, a
lively debate has ensued as to the catalytic significance of
LBHBs. Yet if enzymes do not form LBHBs, it remains to
be explained how, in numerous widely accepted enzymatic
mechanisms that we shall encounter, the conjugate base of
an acidic group can abstract a proton from a far more basic
group.
e. Much of a Serine Protease’s Catalytic Activity
Arises from Preferential Transition State Binding
Despite the foregoing, blocking the action of the
catalytic triad through the specific methylation of His 57
by treating chymotrypsin with methyl-p-nitrobenzene
sulfonate
D
p
A
N
p
O
D¬H
N¬H
p
OO¬H
p
O
D
p
A
D¬H
536 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 536
yields an enzyme that is a reasonably good catalyst: It en-
hances the rate of proteolysis by as much as a factor of 2
10
6
over the uncatalyzed reaction, whereas the native en-
zyme has a rate enhancement factor of ⬃10
10
. Similarly, the
mutation of Ser 195, His 57, or even all three residues of the
catalytic triad yields enzymes that enhance proteolysis
rates by ⬃5 10
4
-fold over that of the uncatalyzed reac-
tion. Evidently, the catalytic triad provides a nucleophile
and is an alternate source and sink of protons (general
acid–base catalysis). However, a large portion of chy-
motrypsin’s rate enhancement must be attributed to its pref-
erential binding of the catalyzed reaction’s transition state.
f. Enzymes Have Free Energy Landscapes That
Facilitate Catalysis
How does an enzyme-catalyzed reaction reach its tran-
sition state? Recall that, under physiological conditions,
proteins are highly dynamic entities with structural fluc-
tuations having periods ranging from ⬃10
15
s for bond
vibrations to 1 s or more for triggered conformational
changes (Section 9-4). The turnover times for enzymatic
reactions, 1/k
cat
, are mostly in the range 1 s to 1 s (Table
14-1), but yet the lifetime of a transition state is only
around that of a bond vibration (Section 14-1Cb). Thus,
even for a reaction with a turnover time as little as 1 s,
every atom in the enzyme–substrate complex undergoes
approximately 10
6
/10
15
10
9
vibrational excursions be-
tween turnovers. Apparently, the transition state is an
arrangement of substrate and catalytic groups that occurs
extremely rarely through the fluctuations of its component
atoms.
Proteins, as we have seen, are designed by evolution to
fold to their native states via a series of conformational
adjustments that follow funnel-shaped free energy land-
scapes (Section 9-1Ch). A variety of structural, muta-
tional, and theoretical studies indicate that enzyme–sub-
His
57
NH
N
CH
2
3
1
His
57
NH
N
+
CH
2
CH
3
3
1
+
O
2
N
S
+
O
2
N
SO
3
–
O
O
O
CH
3
Methyl-p-nitrobenzene
sulfonate
strate complexes are similarly evolutionarily designed to
structurally rearrange themselves through a progression of
conformational changes that lead to the formation of the
transition state. This explains, for example, why the muta-
tion of a residue far from the active site of an enzyme that
does not appear to have a structurally important role, may
nevertheless significantly reduce the rate of the reaction
that the enzyme catalyzes. Such a mutation perturbs ex-
tended hydrogen bonding networks and long range elec-
trostatic interactions in a way that alters the entire en-
zyme’s spectrum of thermal motions. This changes the
enzyme’s free energy landscape so as to reduce the prob-
ability that it will achieve the transition state in a given
time period.
E. Zymogens
Most proteolytic enzymes are biosynthesized as somewhat
larger inactive precursors known as zymogens (enzyme
precursors, in general, are known as proenzymes). In the
case of digestive enzymes, the reason for this is clear: If
these enzymes were synthesized in their active forms, they
would digest the tissues that synthesized them. Indeed,
acute pancreatitis, a painful and sometimes fatal condition
that can be precipitated by pancreatic trauma, is character-
ized by the premature activation of the digestive enzymes
synthesized by this gland.
a. Serine Proteases Are Autocatalytically Activated
Trypsin, chymotrypsin, and elastase are activated ac-
cording to the following pathways:
Trypsin. The activation of trypsinogen, the zymogen of
trypsin, occurs as a two-stage process when trypsinogen en-
ters the duodenum from the pancreas. Enteropeptidase, a
single-pass transmembrane serine protease that is located
in the duodenal mucosa, specifically hydrolyzes trypsino-
gen’s Lys 15¬Ile 16 peptide bond, thereby excising its N-
terminal hexapeptide (Fig. 15-27). This yields the active en-
zyme, which has Ile 16 at its N-terminus. Since this
Section 15-3. Serine Proteases 537
Figure 15-27 Activation of trypsinogen to form trypsin.
Proteolytic excision of the N-terminal hexapeptide is catalyzed
by either enteropeptidase or trypsin.The chymotrypsinogen
residue numbering is used here; that is, Val 10 is actually
trypsinogen’s N-terminus and Ile 16 is trypsin’s N-terminus.
(Asp)
4
+
Lys
Trypsinogen
Val
H
3
N
10 15
Ile
16
. . .
enteropeptidase or
trypsin
Val
(Asp)
4
+
Lys
Val
H
3
N
+
Ile
. . .
Val
Trypsin
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 537
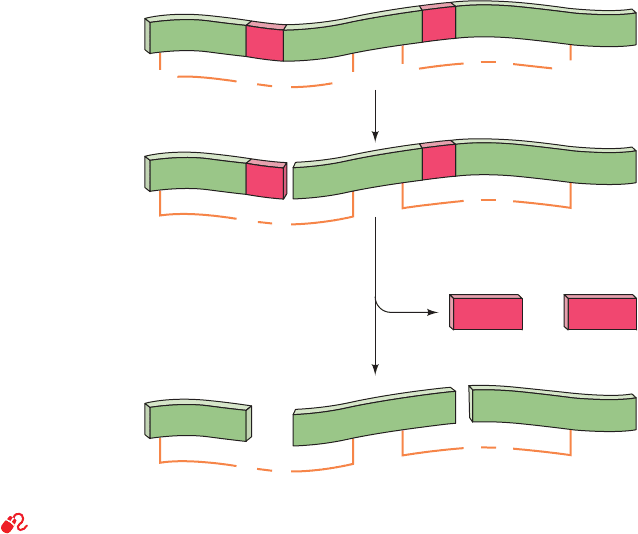
activating cleavage occurs at a trypsin-sensitive site (recall
that trypsin cleaves after Arg and Lys residues), the small
amount of trypsin produced by enteropeptidase also cat-
alyzes trypsinogen activation, generating more trypsin, etc.;
that is, trypsinogen activation is autocatalytic.
Chymotrypsin. Chymotrypsinogen is activated by the spe-
cific tryptic cleavage of its Arg 15¬Ile 16 peptide bond to
form -chymotrypsin (Fig. 15-28). -Chymotrypsin subse-
quently undergoes autolysis (self-digestion) to specifically
excise two dipeptides, Ser 14–Arg 15 and Thr 147–Asn 148,
thereby yielding the equally active enzyme ␣-chymotrypsin
(heretofore and hereafter referred to as chymotrypsin).
The biochemical significance of this latter process, if any, is
unknown.
Elastase. Proelastase, the zymogen of elastase, is activated
similarly to trypsinogen by a single tryptic cleavage that ex-
cises a short N-terminal polypeptide.
b. Biochemical “Strategies” That Prevent Premature
Zymogen Activation
Trypsin activates pancreatic procarboxypeptidases A
and B and prophospholipase A
2
(the action of phospholi-
pase A
2
is outlined in Section 25-1) as well as the pancreatic
serine proteases. Premature trypsin activation can conse-
quently trigger a series of events that lead to pancreatic
self-digestion. Nature has therefore evolved an elaborate
defense against such inappropriate trypsin activation. We
have already seen (Section 15-3Da) that pancreatic trypsin
inhibitor binds essentially irreversibly to any trypsin
formed in the pancreas so as to inactivate it. Furthermore,
the trypsin-catalyzed activation of trypsinogen (Fig. 15-27)
occurs quite slowly, presumably because the unusually
large negative charge of its highly evolutionarily conserved
N-terminal hexapeptide repels the Asp at the back of
trypsin’s specificity pocket (Fig. 15-21). Finally, pancreatic
zymogens are stored in intracellular vesicles called zymo-
gen granules whose membranous walls are thought to be
resistant to enzymatic degradation.
c. Zymogens Have Distorted Active Sites
Since the zymogens of trypsin, chymotrypsin, and elastase
have all their catalytic residues, why aren’t they enzymati-
cally active? Comparisons of the X-ray structures of
trypsinogen with that of trypsin and of chymotrypsinogen
with that of chymotrypsin show that on activation, the newly
liberated N-terminal Ile 16 residue moves from the surface of
the protein to an internal position, where its free cationic
amino group forms an ion pair with the invariant anionic Asp
194 (Fig. 15-20). Aside from this change, however, the struc-
tures of these zymogens closely resemble those of their cor-
responding active enzymes. Surprisingly, this resemblance in-
cludes their catalytic triads, an observation which led to the
discovery that these zymogens are actually catalytically ac-
tive, albeit at a very low level. Careful comparisons of the cor-
responding enzyme and zymogen structures revealed the
reason for this low activity: The zymogens’ specificity pockets
and oxyanion holes are improperly formed such that, for ex-
ample,the amide NH of chymotrypsinogen’s Gly 193 points in
the wrong direction to form a hydrogen bond with the tetrahe-
dral intermediate (see Fig. 15-25). Hence, the zymogens’ very
low enzymatic activity arises from their reduced ability to
bind substrate productively and to stabilize the tetrahedral
intermediate. These observations provide further structural
evidence favoring the role of preferred transition state bind-
ing in the catalytic mechanism of serine proteases.
538 Chapter 15. Enzymatic Catalysis
Cys
Cys
Cys
Chymotrypsinogen
(inactive)
π-Chymotrypsin
(active)
1
122
136
201
245
Cys
S
S
S
S
SS
1
15 16
122
136
201
245
S
S
α-Chymotrypsin
(active)
S
S
1
16
122
136
146 149
201
245
S
S
trypsin
chymotrypsin
14 15 + 147 148
Ser Arg Thr Asn
Arg Ile
Leu
Ile
13
Tyr Ala
Figure 15-28 Activation of chymotrypsinogen by proteolytic cleavage. Both - and -chymotrypsin
are enzymatically active.
See Kinemage Exercise 10-4
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 538

4 DRUG DESIGN
The improvements in medical care over the past several
decades are, in large measure, attributable to the develop-
ment of a huge variety of drugs, which have eliminated or
greatly relieved numerous human ailments. Such medica-
tions include antibiotics (which have enormously reduced
the impact of infectious diseases), anti-inflammatory
agents (which reduce the effects of inflammatory diseases
such as arthritis), analgesics and anesthetics (which make
modern surgical techniques possible), agents that reduce
the incidence and severity of cardiovascular disease and
stroke, antidepressants, antipsychotics, agents that inhibit
stomach acid secretion (which prevent stomach ulcers and
heartburn), agents to combat allergies and asthma, im-
munosuppressants (which make organ transplants possi-
ble), agents used for cancer chemotherapy, and a great va-
riety of other substances.
Early human cultures almost certainly recognized both
the beneficial and toxic effects of indigenous plant and an-
imal products and used many of them as “medications.”
Unfortunately, most of these substances were useless or
even harmful.Although there were sporadic attempts over
the 2500 years preceding the modern era to formulate ra-
tional systems of drug discovery, they had little success be-
cause they were based mainly on unfounded theories and
superstition (e.g., the doctrine of signatures stated that if a
plant resembles a particular body part, it must be designed
by nature to influence that body part) rather than observa-
tion and experiment. Consequently, at the beginning of the
20th century, only three known drugs, apart from folk med-
icines, were effective in treating specific diseases: (1) Digi-
talis, a heart stimulant extracted from the foxglove plant
(Section 20-3Af), was used to treat various heart condi-
tions; (2) quinine (Section 26-4Ak), obtained from the bark
and roots of the Cinchona tree, was used to treat malaria;
and (3) mercury was used to treat syphilis (a cure that was
often worse than the disease). It was not until several
decades later that the rise of the scientific method coupled
to the rapidly increasing knowledge of physiology, bio-
chemistry, and chemistry led to effective methods of drug
discovery. In fact, the vast majority of drugs in use today
were discovered and developed in the past four decades.
In this section we discuss the elements of drug discovery
and pharmacology (Greek: pharmacon, drug; the science of
drugs, including their composition, uses, and effects).The sec-
tion ends with a consideration of one of the major successes
of modern drug discovery methods, HIV protease inhibitors.
A. Techniques of Drug Discovery
Most drugs act by modifying the function of a particular re-
ceptor in the body or in an invading pathogen. In most
cases, the receptor is a protein to which the drug specifically
binds. It may be an enzyme, a transmembrane channel that
transports a specific substance into or out of a cell (Chapter
20), and/or a protein that participates in an inter- or intra-
cellular signaling pathway (Chapter 19). In all of these
cases, a substance that in binding to a receptor modulates its
function is known as an agonist, whereas a substance that
binds to a receptor without affecting its function but blocks
the binding of agonists is called an antagonist. The bio-
chemical and physiological effects of a drug and its mecha-
nism of action are referred to as its pharmacodynamics.
a. Drug Discovery Is a Complex Procedure
How are new drugs discovered? Nearly all drugs that
have been in use for over 15 years were discovered by
screening large numbers of synthetic compounds and natu-
ral products for the desired effect. Drug candidates that are
natural products are usually discovered by the fractionation
of the organisms in which they occur,which are often plants
used in folk remedies of the conditions of interest. Humans
having the condition whose treatment is being sought can-
not be used as “guinea pigs” in this initial screening process,
and even guinea pigs or other laboratory animals such as
mice or dogs (if they can be made to be suitable models of
the condition under consideration) are too expensive to use
on the many thousands of compounds that are usually
tested. Thus, in vitro screens are initially used, such as the
degree of binding of a drug candidate to an enzyme that is
implicated in a disease of interest, toxicity toward the target
bacteria in the search for a new antibiotic, or effects on a
line of cultured mammalian cells. However, as the number
of drug candidates is winnowed down, more sensitive
screens such as testing in animals are employed.
A drug candidate that exhibits a desired effect is called a
lead compound (or,colloquially, a lead; pronounced leed).A
good lead compound binds to its target receptor with a dis-
sociation constant, K
D
1 M. Such a high affinity is neces-
sary to minimize a drug’s less specific binding to other
macromolecules in the body and to ensure that only low
doses of the drug need be taken. For enzyme inhibitors, the
dissociation constant is the inhibitor’s K
I
or K¿
I
(Section 14-3).
Other common measures of the effect of a drug are the
IC
50
, the inhibitor concentration at which an enzyme ex-
hibits 50% of its maximal activity; the ED
50
, the effective
dose of a drug required to produce a therapeutic effect in
50% of a test sample;the TD
50
, the mean toxic dose required
to produce a particular toxic effect in animals; and the LD
50
,
the mean lethal dose required to kill 50% of a test sample.
For an inhibitor of an enzyme that follows
Michaelis–Menten kinetics, the IC
50
is determined by meas-
uring the ratio v
I
/v
o
for several values of [I] at constant [S],
where v
I
is the initial velocity of the enzyme when the in-
hibitor concentration is [I]. By dividing Eq. [14.24] by Eq.
[14.38] with ␣ defined according to Eq. [14.37], we see that
[15.13]
When v
I
/v
o
0.5 (50% inhibition),
[15.14]
Consequently, if the measurements of v
I
/v
o
are made with
[S] K
M
, then [IC
50
] K
I
.
[I] [IC
50
] K
I
a1
[S]
K
M
b
v
I
v
o
K
M
[S]
K
M
a [S]
K
M
[S]
K
M
a1
[I]
K
I
b [S]
Section 15-4. Drug Design 539
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 539

The ratio TD
50
/ED
50
is defined as a drug’s therapeutic
index, the ratio of the dose of the drug that produces toxi-
city to that which produces the desired effect. It is, of
course, preferable that a drug have a high therapeutic in-
dex, but this is not always possible.
b. Cathepsin K Is a Drug Target for Osteoporosis
The development of genomic sequencing techniques
(Section 7-2B) and hence the characterization of tens of
thousands of previously unknown genes is providing an
enormous number of potential drug targets. For example,
osteoporosis (Greek: osteon, bone poros, porous), a con-
dition that afflicts mostly postmenopausal women, is char-
acterized by the progressive loss of bone mass leading to a
greatly increased frequency of bone fracture, most often of
the hip, spine, and wrist. Bones consist of a protein matrix
that is 90% type I collagen (Section 8-2B), in which
spindle- or plate-shaped crystals of hydroxyapatite,
Ca
5
(PO
4
)
3
OH, are embedded. Bones are by no means
static structures. They undergo continuous remodeling
through the countervailing action of two types of bone
cells: osteoblasts (Greek:blast, germ cell),which synthesize
bone’s protein matrix in which its mineral component is
laid down; and osteoclasts (Greek: clast, broken), which
solubilize mineralized bone matrix through the secretion
of proteolytic enzymes into an extracellular bone resorp-
tion pit, which is maintained at pH 4.5. The acidic solution
dissolves the bone’s mineral component, thereby exposing
its protein matrix to proteolytic degradation. Osteoporosis
arises when bone resorption outstrips bone formation.
In the search for a drug target for osteoporosis, a cDNA
library (Sections 5-5E and 5-5Fa) was prepared from an
osteoclastoma (a cancer derived from osteoclasts; normally
osteoclasts are very rare cells). Around 4% of these cDNAs
encode a heretofore unknown protease, which was named
cathepsin K (cathepsins are proteases that occur in the lyso-
some). Further studies, both at the cDNA and protein levels,
indicated that cathepsin K is only expressed at high levels in
osteoclasts. Microscopic examination of osteoclasts that had
been stained with antibodies directed against cathepsin K
revealed that this enzyme is localized at the contact site be-
tween osteoclasts and the bone resorption pit. Subsequently,
it was shown that mutations in the gene encoding cathepsin
K are the cause of pycnodysostosis, a rare hereditary disease
which is characterized by hardened and fragile bones, short
stature, skull deformities, and osteoclasts that demineralize
bone normally but do not degrade its protein matrix. Evi-
dently, cathepsin K functions to degrade the protein matrix
of bone and hence is an attractive drug target for the treat-
ment of osteoporosis. Indeed, several cathepsin K inhibitors
are in clinical trials (Section 15-4Bb).
c. SARs and QSARs Are Useful Tools for
Drug Discovery
A lead compound is used as a point of departure to design
more efficacious compounds. Experience has shown that
even minor modifications to a drug candidate can result in
major changes in its pharmacological properties. Thus, one
might place methyl, chloro, hydroxyl, or benzyl groups at
various places on a lead compound in an effort to improve
its pharmacodynamics. For most drugs in use today, 5 to 10
thousand related compounds were typically synthesized in
generating the medicinally useful drug.These were not ran-
dom procedures but were guided by experience as medici-
nal chemists tested various derivatives of a lead compound:
For those compounds that had improved efficacy, deriva-
tives were made and tested; etc. This process has been sys-
tematized through the use of structure–activity relation-
ships (SARs): the determination, via synthesis and
screening, of which groups on a lead compound are impor-
tant for its drug function and which are not. For example, if
a phenyl group on a lead compound interacts hydrophobi-
cally with a flat region of its receptor, then hydrogenating
the phenyl ring to form a nonplanar cyclohexane ring will
yield a compound with reduced affinity for the receptor.
A logical extension of the SAR concept is to quantify it,
that is, to determine a quantitative structure–activity rela-
tionship (QSAR). This idea is based on the premise that
there is a relatively simple mathematical relationship be-
tween the biological activity of a drug and its physicochem-
ical properties. For instance, if the hydrophobicity of a drug
is important for its biological activity, then changing the
substituents on the drug so as to alter its hydrophobicity
will affect its activity. A measure of the substance’s hy-
drophobicity is its partition coefficient, P, between the two
immiscible solvents, octanol and water, at equilibrium:
[15.15]
Biological activity may be expressed as 1/C, where C is the
drug concentration required to achieve a specified level of
biological function (e.g., IC
50
).Then a plot of log 1/C versus
log P (the use of logarithms keeps the plot on a manage-
able scale) for a series of derivatives of the lead compound
having a relatively small range of log P values often indi-
cates a linear relationship (Fig. 15-29a), which can there-
fore be expressed:
[15.16]
Here k
1
and k
2
are constants, whose optimum values in this
QSAR can be determined by computerized curve-fitting
methods. For compounds with a larger range of log P val-
ues, it is likely that a plot of log 1/C versus log P will have a
maximum value (Fig. 15-29b) and hence be better de-
scribed by a quadratic equation:
[15.17]
Of course, the biological activities of few substances de-
pend only on their hydrophobicities. A QSAR can there-
fore simultaneously take into account several physico-
chemical properties of substituents such as their pK values,
van der Waals radii, hydrogen bonding energy, and confor-
mation.The values of the constants for each of the terms in
a QSAR is indicative of the contribution of that term to the
drug’s activity. The use of QSARs to optimize the biologi-
cal activity of a lead compound has proven to be a valuable
tool in drug discovery.
log
a
1
C
b k
1
(log P)
2
k
2
log P k
3
log a
1
C
b k
1
log P k
2
P
concentration of drug in octanol
concentration of drug in water
540 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 540
