Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

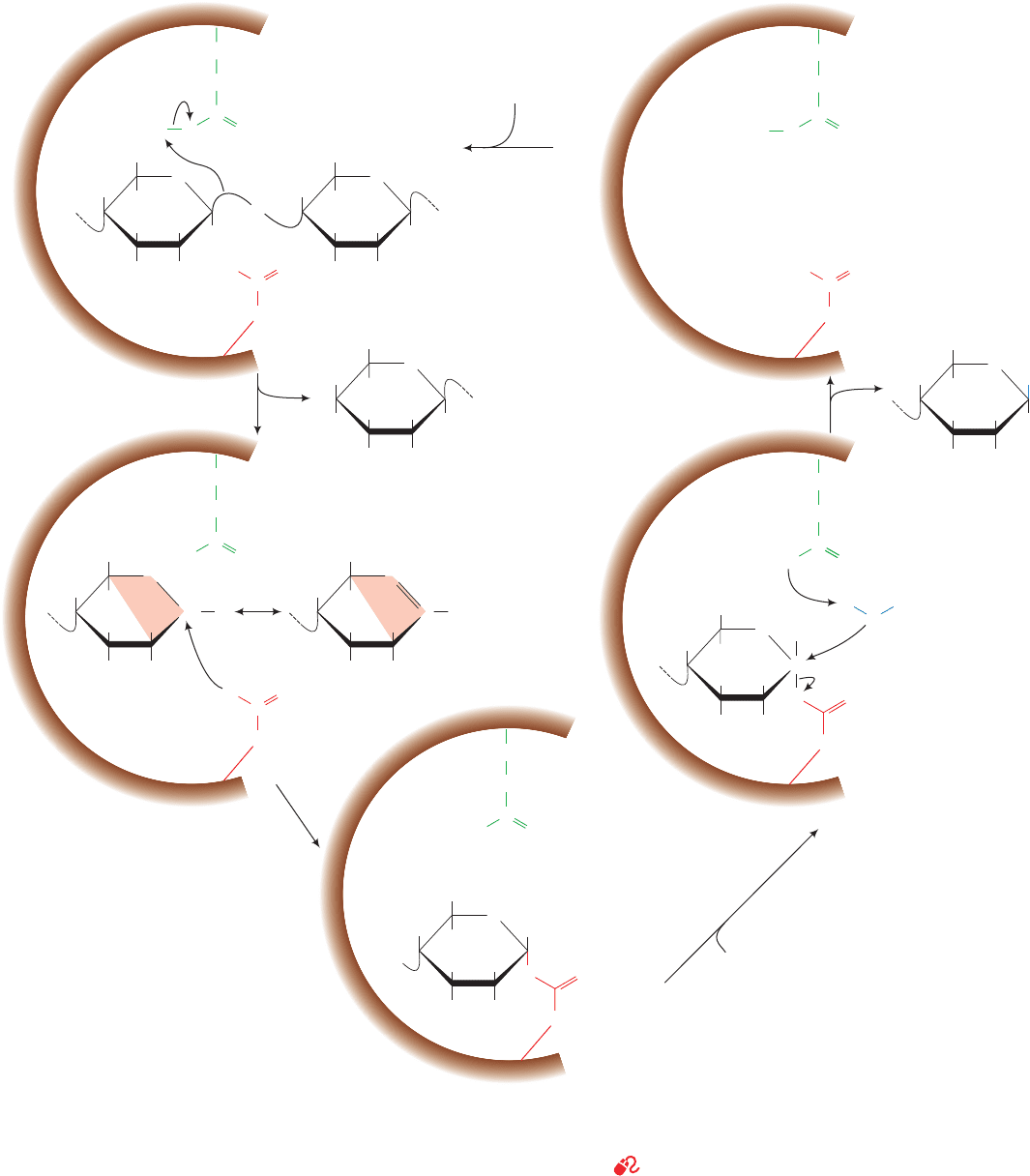
b. The Lysozyme Catalytic Mechanism Proceeds
via a Covalent Intermediate
Lysozyme’s catalytic mechanism was largely formulated
by Phillips based on the foregoing information. However,
as we shall see, further investigations have required that
important changes be made to the original version of this
mechanism. The presently accepted mechanism occurs as
follows (Fig. 15-14):
Section 15-2. Lysozyme 521
Figure 15-14 The lysozyme reaction mechanism. Glu 35 acts
as an acid catalyst, and Asp 52 acts as a covalent catalyst. Only
the substrate D- and E-rings are shown. R represents the
N-acetyl group at C2, and R¿ represents the CH
3
CHCOO
group
at C3.The resonance-stabilized oxonium ion transition state
–
O
C
O
HOCH
2
OR
⬘
H
H
H
O
H
H
R
Covalent
intermediate
CH
2
Glu35
HOCH
2
General
acid
catalysis
General
base
catalysis
Substrate
polysaccharide
Binding
Covalent
catalysis
Water binding
OR
⬘
O
–
–
O
H
H
H
H
O
H
H
C
R
OO
CH
2
CH
2
Glu35
CH
2
CH
2
Asp52
H
2
O
C
O
C
O
O
O
–
C
O
O
–
C
O
D
HOCH
2
OH
H
H
H
O
H
H
R
E
HOCH
2
OR
⬘
H
H
H
H
O
C
+
H
R
HOCH
2
OR
⬘
H
H
H
H
C
O
+
H
R
HOCH
2
OH
H
H
H
O
H
H
R
E
HO
2
1
HOCH
2
OR
⬘
OH
H
H
H
O
H
H
R
5
4
3
Oxonium ion
(transition state)
H
C
OO
–
O
C
O
–
O
C
O
O
HOCH
2
OR
⬘
H
H
H
H
O
C
H
HH
R
O
O
O
O
CH
2
Glu35
CH
2
CH
2
Glu35
CH
2
CH
2
Glu35
CH
2
CH
2
Asp52
CH
2
Asp52
CH
2
Asp52
CH
2
Asp52
requires that C1, C2, C5, and O5 be coplanar (orange shading)
creating a half-chair conformation. Step 5 includes the
participation of an oxonium ion transition state that is not
shown.
See Kinemage Exercise 9
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 521
1. Lysozyme attaches to a bacterial cell wall by binding
to a hexasaccharide unit. In the process, the D-ring is dis-
torted toward the half-chair conformation in response to
the unfavorable contacts that its ¬C6H
2
OH group would
otherwise make with the protein.
2. Glu 35 transfers its proton to the O1 atom linking the
D- and E-rings, the only polar group in its vicinity, thereby
cleaving the C1¬O1 bond (general acid catalysis).This step
converts the D-ring to a planar resonance-stabilized oxo-
nium ion transition state, whose formation is facilitated by
the strain distorting it to the half-chair conformation (catal-
ysis by the preferential binding of the transition state). The
positively charged oxonium ion is stabilized by the presence
of the nearby negatively charged Asp 52 carboxylate group
(electrostatic catalysis).The E-ring product is released.
3. The Asp 52 carboxylate group nucleophilically at-
tacks the now electron-poor C1 of the D ring to form a co-
valent glycosyl–enzyme intermediate (covalent catalysis).
4. Water replaces the E-ring product in the active site.
5. Hydrolysis of the covalent bond with the assistance
of Glu 35 (general base catalysis), which involves another
oxonium ion transition state, regenerates the active site
groups.The enzyme then releases the D-ring product, com-
pleting the catalytic cycle.
The double-displacement mechanism diagrammed in
Fig. 15-14 (in which the Asp 52 carboxylate group displaces
O1 from C1 of the D-ring and is, in turn,displaced by water)
allows the incoming water molecule to attach to the same
face of the D-ring as the E-ring it replaces. Consequently,
the configuration of the D-ring is retained. A single-dis-
placement reaction, in which water directly displaces O1,
would invert the configuration at C1 of the D-ring between
the substrate and product, a result that is not observed.
C. Testing the Catalytic Mechanism
The forgoing mechanism is the product of over 40 years of
enzymatic research. It differs in important ways from the
mechanism Phillips originally proposed based on his struc-
tural studies of lysozyme and a knowledge of the mecha-
nism of nonenzymatic acetal hydrolysis. In the remainder
of this section, we discuss the highlights of these enzymatic
studies to illustrate how scientific models evolve.
a. Confirming the Identities of Lysozyme’s
Catalytic Residues
The identities of lysozyme’s catalytically important
groups have been experimentally verified through site-
directed mutagenesis (Section 5-5Gc) and the use of
group-specific reagents:
Glu 35.The mutagenesis of Glu 35 to Gln yields a protein with
no detectable catalytic activity (0.1% of wild type),although
it has only a ⬃1.5-fold decrease in substrate affinity. Glu 35
must therefore be essential for lysozyme’s catalytic activity.
Asp 52. The mutagenesis of Asp 52 to Asn, which has a po-
larity comparable to that of Asp but lacks its negative
charge, yields an enzyme with no more than 5% of wild-
type lysozyme’s catalytic activity even though this mutation
causes an ⬃2-fold increase in the enzyme’s affinity for sub-
strate. Asp 52 is therefore important for enzymatic activity.
Noninvolvement of Other Amino Acid Residues. Lysozyme’s
other carboxyl groups besides Glu 35 and Asp 52 do not
participate in the catalytic process, as was demonstrated by
reacting lysozyme with carboxyl-specific reagents in the
presence of substrate. This treatment yields an almost fully
active enzyme in which all carboxyl groups but Glu 35 and
Asp 52 are derivatized. Other group-specific reagents that
modify, for instance, His, Lys, Met, or Tyr residues but in-
duce no major protein structure disruptions, cause little
change in lysozyme’s catalytic efficiency.
b. Role of Strain
Many of the mechanistic investigations of lysozyme
have had the elusive goal of establishing the catalytic role
of strain. Not all of these studies, as we shall see, supported
the Phillips mechanism, thereby stimulating a series of in-
vestigations that eventually settled this issue.
Measurements of the binding equilibria of various
oligosaccharides to lysozyme indicate that all saccharide
residues except that binding to the D subsite contribute ener-
getically toward the binding of substrate to lysozyme; bind-
ing NAM in the D subsite requires a free energy input of
12.1 kJ ⴢ mol
1
(Table 15-3). The Phillips mechanism ex-
plains this observation as being indicative of the energy
penalty of straining the D-ring from its preferred chair con-
formation toward the half-chair form.
As we discussed in Section 15-1Fa, an enzyme that cat-
alyzes a reaction by the preferential binding of its transi-
tion state has a greater binding affinity for an inhibitor that
has the transition state geometry (a transition state analog)
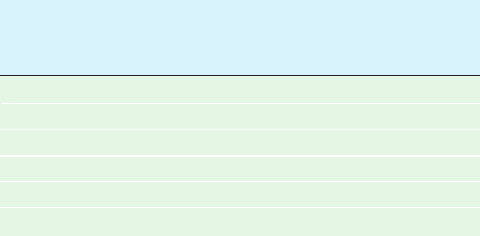
than it does for its substrate. The -lactone analog of
(NAG)
4
(Fig. 15-15) is presumably a transition state analog
of lysozyme since this compound’s lactone ring has the half-
chair conformation that geometrically resembles the pro-
posed oxonium ion transition state of the substrate’s D-ring.
X-ray studies indicate, in accordance with prediction, that
this inhibitor binds to lysozyme’s subsites
such that the lactone ring occupies the D subsite in a half-
chairlike conformation.
A¬B¬C¬D
522 Chapter 15. Enzymatic Catalysis
Table 15-3 Binding Free Energies of HEW
Lysozyme Subsites
Binding
Bound Free Energy
Site Saccharide (kJ ⴢ mol
1
)
ANAG 7.5
BNAM 12.3
CNAG 23.8
DNAM ⴙ12.1
ENAG 7.1
FNAM 7.1
Source: Chipman, D.M. and Sharon, N., Science 165, 459 (1969).
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 522

Despite the foregoing, the role of substrate distortion in
lysozyme catalysis had been questioned. Theoretical studies
by Michael Levitt and Arieh Warshel on substrate binding by
lysozyme suggested that the protein is too flexible to me-
chanically distort the D-ring of a bound substrate. Rather,
these calculations implied that transition state stabilization
occurs through the displacement by substrate of several
tightly bound water molecules from the D subsite.The result-
ing desolvation of the Asp 52 carboxylate group would signif-
icantly enhance its capacity to electrostatically stabilize the
transition state oxonium ion.This study therefore concluded
that “electrostatic strain” rather than steric strain is the more
important factor in stabilizing lysozyme’s transition state.
In an effort to obtain further experimental information
bearing on the Phillips strain mechanism, Nathan Sharon
and David Chipman determined the D subsite–binding
affinities of several saccharides by comparing the
lysozyme-binding affinities of various substrate analogs.
The NAG lactone inhibitor binds to the D subsite with 9.2
kJ ⴢ mol
1
greater affinity than does NAG. This quantity
corresponds, according to Eq. [14.15], to no more than an
⬃40-fold rate enhancement of the lysozyme reaction as a
result of strain (recall that the difference in binding energy
between a transition state analog and a substrate is indica-
tive of the enzyme’s rate enhancement arising from the
preferential binding of the transition state complex). Such
an enhancement is hardly a major portion of lysozyme’s
⬃10
8
-fold rate enhancement (accounting for only ⬃20% of
the reaction’s
; Section 14-1Cd). Moreover, an N-
acetylxylosamine (XylNAc) residue,
HOH
OH
H
H
H
H
N
-Acetylxylosamine residue
NHCOCH
3
H
O
O
¢¢G
‡
cat
which lacks the sterically hindered ¬C6H
2
OH group of
NAM and NAG, has only marginally greater binding affin-
ity for the D subsite (3.8 kJ ⴢ mol
1
) than does NAG
(2.5 kJ ⴢ mol
1
). Yet recall that the Phillips mechanism
postulates that it is the unfavorable contacts made by this
¬C6H
2
OH group that promotes D-ring distortion. Never-
theless, lysozyme does not hydrolyze saccharides with
XylNAc in the D subsite.
The apparent inconsistencies among the foregoing exper-
imental observations were largely rationalized by Michael
James’ highly accurate (1.5-Å resolution) X-ray crystal
structure determination of lysozyme in complex with
NAM–NAG–NAM. This trisaccharide binds, as expected,
to the B, C, and D subsites of lysozyme. The NAM in the
D subsite, in agreement with the Phillips mechanism, is dis-
torted to the half-chair conformation with its ¬C6H
2
OH
group in a nearly axial position due to steric clashes that
would otherwise occur with the acetamido group of the
C subsite NAG (although, contrary to the original Phillips
mechanism, Glu 35 and Trp 108 are too far away from the
¬C6H
2
OH group to contribute to this distortion). This
strained conformation is stabilized by a strong hydrogen
bond between the D-ring O6 and the backbone NH of Val
109 (Fig. 15-12; transition state stabilization). Indeed, the
mutation of Val 109 to Pro, which lacks the NH group to
make such a hydrogen bond, inactivates the enzyme.
Lysozyme’s lack of hydrolytic activity when XylNAc occ-
upies its D subsite is likewise explained by the absence of
this hydrogen bond and the consequent lesser stability of
the XylNAc ring’s half-chair transition state.
The unexpectedly small free energy differences in bind-
ing NAG, NAG lactone, and XylNAc to the D subsite are
explained by the observation that undistorted NAG and
XylNAc can be modeled into the D subsite as it occurs in
the X-ray structure of the lysozyme ⴢ NAM–NAG–NAM
complex. NAM’s bulky lactyl side chain prevents it from
binding to the D subsite in this manner.
c. Evidence for a Covalent Intermediate
The original Phillips mechanism proposed that the
lysozyme reaction proceeds via the direct attack of a water
molecule on C1 of the half-chair-distorted D-ring so as to
directly displace the E-ring (a single-displacement reac-
tion) and therefore did not involve the intermediate for-
mation of a covalent bond (much like the mechanism dia-
grammed in Fig. 15-14 but skipping Step 3). This was based
on Phillip’s observation that the distance between C1 of
the D-ring and a carboxyl O of Asp 52 (which participates
in a network of hydrogen bonds that appear to hold this
side chain in place) are too long to form a covalent bond
(minimally 2.3 Å in the NAM–NAG–NAM complex with-
out significantly disrupting the protein structure vs ⬃1.4 Å
for a C¬O single bond). The fact that the lysozyme reac-
tion proceeds with retention of configuration was attrib-
uted to the shielding of the reaction intermediate by the
enzyme, thereby preventing a water molecule from ap-
proaching the bond being cleaved from its back side as nor-
mally occurs in a single-displacement reaction. This aspect
of the original Phillips mechanism was widely accepted for
Section 15-2. Lysozyme 523
Figure 15-15 Transition state analog inhibition of lysozyme.
The -lactone analog of (NAG)
4
(left) resembles the transition
state of the lysozyme reaction (right). Note that atoms C1, C2,
C5, and O5 in each structure are coplanar (as indicated by
orange shading), consistent with the half-chair conformation
of the hexose ring.
H
OH
H
H
O
O
O
NHCOCH
3
H
CH
2
OH
(NAG)
3
␦-Lactone analog
of (NAG)
4
H
OR
H
H
H
O
C
+
NHCOCH
3
H
CH
2
OH
O
...
R =
CH
CH
3
COO
_
Lysozyme transition state
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 523
over 35 years because no enzyme–substrate covalent bond
had been detected in any of the numerous experimental
studies of hen egg white (HEW) lysozyme.
Despite the foregoing,all other -glycosidases of known
structure that cleave glycosidic linkages with net retention
of configuration at the anomeric carbon (as does HEW
lysozyme) have been shown to do so via a covalent glycosyl–
enzyme intermediate. The active sites of these so-called
retaining -glycosidases structurally resemble that of
HEW lysozyme. Moreover, there is no direct evidence in-
dicative of the existence of a long-lived oxonium ion at the
active site of any retaining -glycosidase, including HEW
lysozyme (the lifetime of a glucosyl oxonium ion in water is
⬃10
12
s, a time only slightly larger than that of a bond vi-
bration).Consequently, there had been a growing suspicion
that the HEW lysozyme reaction also proceeds via a cova-
lent intermediate, one between the D-ring’s anomeric car-
bon (C1) and the side chain carboxyl group of Asp 52 to
form an ester linkage (as generated by Step 3 in Fig. 15-14).
This intermediate presumably reacts with H
2
O in what is
essentially the reverse of the reaction leading to its forma-
tion, thereby yielding the reaction’s second product (Steps
4 and 5 of Fig. 15-14). In this double-displacement mecha-
nism, the oxonium ion is proposed to be the transition state
on the way to forming the covalent intermediate, rather
than being an intermediate itself as it was in the original
Phillips mechanism.
If, in fact, HEW lysozyme follows the mechanism in Fig.
15-14, the reason that its covalent intermediate had not
been observed is that its rate of breakdown must be much
faster than its rate of formation. Hence, if this intermedi-
ate is to be experimentally observed, its rate of formation
must be made significantly greater than its rate of break-
down.To do so, Stephen Withers capitalized on three phe-
nomena. First, if, as postulated, the reaction goes through
an oxonium ion transition state, all steps involving its for-
mation should be slowed by the electron withdrawing ef-
fects of substituting F (the most electronegative element)
at C2 of the D-ring. Second, mutating Glu 35 to Gln
(E35Q) removes the general acid–base that catalyzes the
reaction, further slowing all steps involving the oxonium
ion transition state. Third, substituting an additional F
atom at C1 of the D-ring accelerates the formation of the
intermediate because this F is a good leaving group. Mak-
ing all three of these changes should increase the rate of
formation of the proposed covalent intermediate relative
to its breakdown and hence should result in its accumula-
tion. Withers therefore incubated E35Q HEW lysozyme
with NAG-(1 S 4)-2-deoxy-2-fluoro--
D-glucopyranosyl
fluoride (NAG2FGlcF):
Electrospray ionization mass spectrometry (ESI-MS; Sec-
H F
O
OH
CH
2
OH
H
HHO
H NHCOCH
3
H
H
OH
CH
2
OH
H
H
H F
H
NAG2FGlcF
O O
tion 7-1I) of this reaction mixture revealed a sharp peak at
14,683 D, consistent with the formation of the proposed co-
valent intermediate, but no significant peak at or near the
14,314-D molecular mass of the mutant enzyme alone.
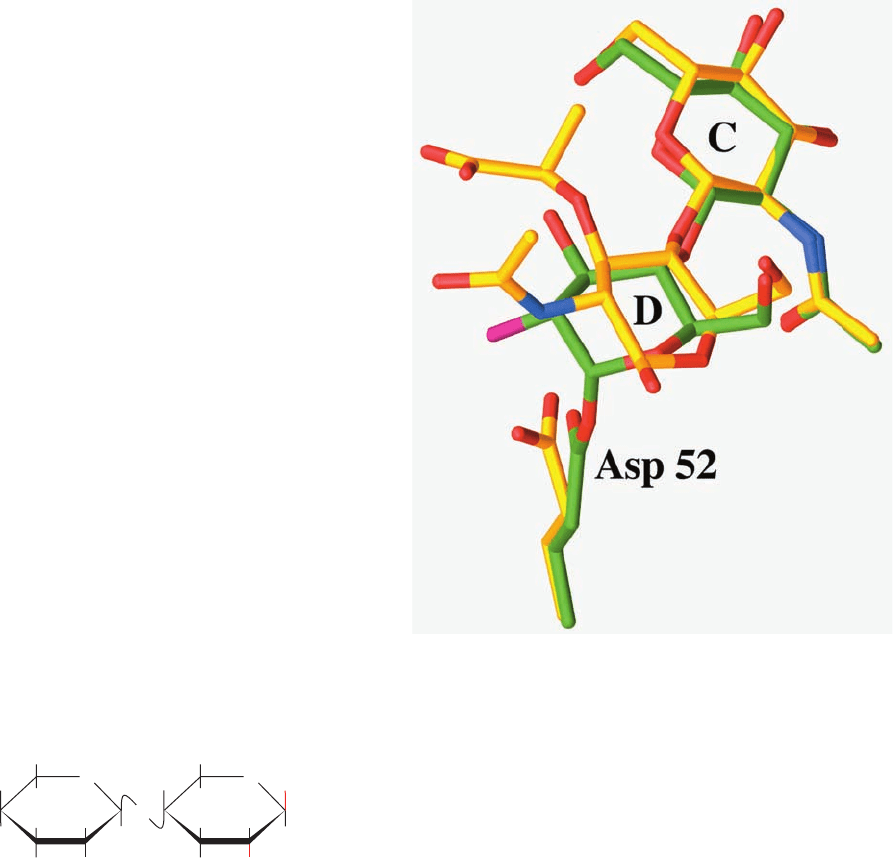
The X-ray structure of this covalent complex unam-
biguously reveals the expected ⬃1.4-Å-long covalent
bond between C1 of the D-ring NAG and a side chain
carboxyl O of Asp 52 (Fig. 15-16). This D-ring NAG
adopts an undistorted chair conformation, thus indicat-
ing that it is a reaction intermediate rather than an ap-
proximation of the transition state. The superposition of
this covalent complex with that of the above described
complex of NAM–NAG–NAM with wild-type HEW
lysozyme reveals how this covalent bond forms (Fig. 15-16).
The shortening of the 3.2-Å distance between the D-ring
NAG C1 and the Asp 52 O in the NAM–NAG–NAM
524 Chapter 15. Enzymatic Catalysis
Figure 15-16 The HEW lysozyme covalent intermediate. The
substrate C- and D-rings and Asp 52 are shown in the superposition
of the X-ray structures of the covalent complex formed by
reacting E35Q lysozyme with NAG2FGlcF (C green, N blue, O
red, and F magenta) and the noncovalent complex of wild-type
lysozyme with NAM–NAG–NAM (C yellow, N blue, and O red).
Note that the covalent bond between Asp 52 and C1 of the
D-ring forms when the D-ring in the noncovalent complex relaxes
from its distorted half-chair conformation to an undistorted chair
conformation and that the side chain of Asp 52 undergoes an
⬃45° rotation about its bond. [Based on X-ray structures
by David Vocadlo and Stephen Withers, University of British
Columbia,Vancouver, Canada; and Michael James, University of
Alberta, Edmonton, Canada. PDBids 1H6M and 9LYZ.]
C
¬C
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 524
complex to ⬃1.4 Å in the covalent complex is almost en-
tirely a consequence of the relaxation of the D-ring from
the half-chair to the chair conformation combined with an
⬃45°rotation of the Asp 52 side chain about its C
¬C
bond; the positions of the D-ring O4 and O6 atoms are es-
sentially unchanged. Hence, over 35 years after its formu-
lation, it was shown that the Phillips mechanism must be al-
tered to take into account the transient formation of this
covalent glycosyl–enzyme ester intermediate (covalent
catalysis). Keep in mind, however, that in order to form
this covalent linkage, the D-ring must pass through an ox-
onium-like transition state, which requires it to transiently
assume the half-chair conformation.
3 SERINE PROTEASES
Our next example of enzymatic mechanisms is a diverse
group of proteolytic enzymes known as the serine pro-
teases (Table 15-4). These enzymes are so named because
they have a common catalytic mechanism characterized by
the possession of a peculiarly reactive Ser residue that is
essential for their enzymatic activity. The serine proteases
are the most thoroughly understood family of enzymes as a
result of their extensive examination over a nearly 60-year
period by kinetic, chemical, physical, and genetic tech-
niques. In this section, we mainly study the best character-
ized serine proteases, chymotrypsin, trypsin, and elastase.
We also consider how these three enzymes, which are syn-
thesized in inactive forms, are physiologically activated.
A. Kinetics and Catalytic Groups
Chymotrypsin, trypsin, and elastase are digestive enzymes
that are synthesized by the pancreatic acinar cells (Fig. 1-10c)
and secreted, via the pancreatic duct, into the duodenum
(the small intestine’s upper loop). All of these enzymes
catalyze the hydrolysis of peptide (amide) bonds but with
different specificities for the side chains flanking the scissile
(to be cleaved) peptide bond (recall that chymotrypsin is
specific for a bulky hydrophobic residue preceding the scis-
sile peptide bond, trypsin is specific for a positively charged
residue, and elastase is specific for a small neutral residue;
Table 7-2).Together, they form a potent digestive team.
a. Ester Hydrolysis as a Kinetic Model
That chymotrypsin can act as an esterase as well as a
protease is not particularly surprising because the chemical
mechanisms of ester and amide hydrolysis are almost iden-
tical. The study of chymotrypsin’s esterase activity has led
to important insights concerning this enzyme’s catalytic
mechanism. Kinetic measurements by Brian Hartley of the
chymotrypsin-catalyzed hydrolysis of p-nitrophenylacetate
indicated that the reaction occurs in two phases (Fig. 15-17):
1. The “burst phase,” in which the highly colored p-
nitrophenolate ion is rapidly formed in amounts stoichio-
metric with the quantity of active enzyme present.
2. The “steady-state phase,” in which p-nitrophenolate
is generated at a reduced but constant rate that is inde-
pendent of substrate concentration.
CH
3
H
2
O
2H
+
NO
2
C
chymotrypsin
O
O
CH
3
CO
–
O
–
O
NO
2
p-Nitrophenylacetate
p-NitrophenolateAcetate
+
Section 15-3. Serine Proteases 525
Table 15-4 A Selection of Serine Proteases
Enzyme Source Function
Trypsin Pancreas Digestion of proteins
Chymotrypsin Pancreas Digestion of proteins
Elastase Pancreas Digestion of proteins
Thrombin Vertebrate serum Blood clotting
Plasmin Vertebrate serum Dissolution of blood clots
Kallikrein Blood and tissues Control of blood flow
Complement C1 Serum Cell lysis in the immune response
Acrosomal protease Sperm acrosome Penetration of ovum
Lysosomal protease Animal cells Cell protein turnover
Cocoonase Moth larvae Dissolution of cocoon after metamorphosis
-Lytic protease Lysobacter enzymogenes Possibly digestion
Proteases A and B Streptomyces griseus Possibly digestion
Subtilisin Bacillus subtilis Possibly digestion
Source: Stroud, R.M., Sci.Am. 231(1), 86 (1974).
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 525
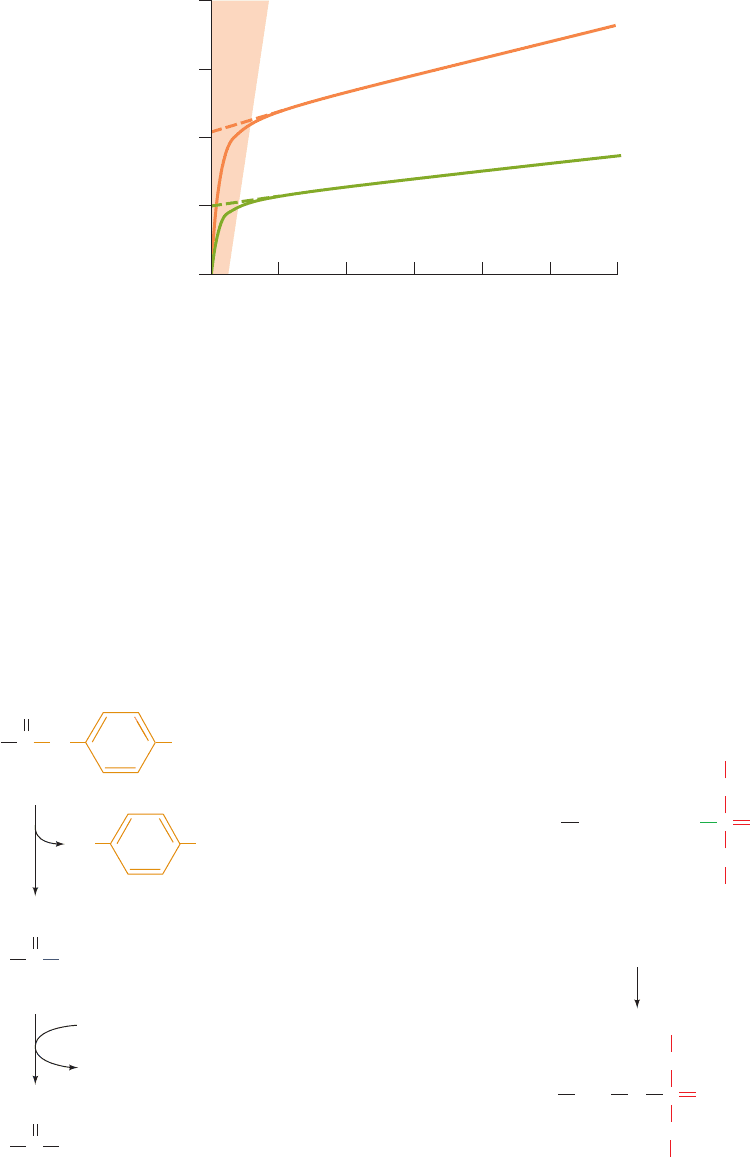
These observations have been interpreted in terms of a two-
stage reaction sequence in which the enzyme (1) rapidly re-
acts with the p-nitrophenylacetate to release p-nitrophenolate
ion forming a covalent acyl–enzyme intermediate that (2) is
slowly hydrolyzed to release acetate:
Chymotrypsin evidently follows a Ping Pong Bi Bi mecha-
nism (Section 14-5A). Chymotrypsin-catalyzed amide hy-
drolysis has been shown to follow a reaction pathway similar
to that of ester hydrolysis but with the first step of the reac-
tion, enzyme acylation, being rate determining rather than
the deacylation step.
CH
3
NO
2
C O
O
CH
3
C Enzyme
Enzyme
O
–
O
CH
3
CO
–
O
NO
2
p-Nitrophenylacetate
p-Nitrophenolate
Acetate
H
2
O
H
+
+
+
Enzyme
Chymotrypsin
Acyl–enzyme intermediate
fast
slow
b. Identification of the Catalytic Residues
Chymotrypsin’s catalytically important groups were
identified by chemical labeling studies.These are described
below.
Ser 195. A diagnostic test for the presence of the active Ser
of serine proteases is its reaction with diisopropylphospho-
fluoridate (DIPF):
which irreversibly inactivates the enzyme. Other Ser
residues, including those on the same protein, do not react
with DIPF. DIPF reacts only with Ser 195 of chymotrypsin,
thereby demonstrating that this residue is the enzyme’s ac-
tive Ser.
Diisopropylphospho-
fluoridate (DIPF)
DIP–Enzyme
CH
2
OH
+
P
O
O
F O
CH(CH
3
)
2
CH(CH
3
)
2
CH
2
(Active Ser)
+
P
O
O
O
CH(CH
3
)
2
CH(CH
3
)
2
O
HF
(Active Ser)
526 Chapter 15. Enzymatic Catalysis
Figure 15-17 Time course of p-nitrophenylacetate hydrolysis
as catalyzed by two different concentrations of chymotrypsin.
The enzyme rapidly binds substrate and releases the first
product, p-nitrophenolate ion, but the second product, acetate
ion, is released more slowly. Consequently, the rate of
p-nitrophenolate generation begins rapidly (burst phase) but
0
1
2
3
4
2 4 6 8 10 12
Time (min)
0.8 mg ⋅ mL
–1
0.4 mg ⋅ mL
–1
[p-Nitrophenolate] (mM)
Steady state
phase
Burst
phase
slows as acyl–enzyme complex accumulates until the rate of
p-nitrophenolate generation approaches that of acetate release
(steady state).The extrapolation of the steady state curve to zero
time (dashed lines) indicates the initial concentration of active
enzyme. [After Hartley, B.S. and Kilby, B.A., Biochem. J. 56, 294
(1954).]
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 526
The use of DIPF as an enzyme inactivating agent came
about through the discovery that organophosphorus com-
pounds such as DIPF are potent nerve poisons. The neu-
rotoxicity of DIPF arises from its ability to inactivate
acetylcholinesterase, a serine esterase that catalyzes the
hydrolysis of acetylcholine:
Acetylcholine is a neurotransmitter: It transmits nerve im-
pulses across the synapses (junctions) between certain
types of nerve cells (Sections 12-4Da and 20-5Cb). The in-
activation of acetylcholinesterase prevents the otherwise
rapid hydrolysis of the acetylcholine released by a nerve
impulse and thereby interferes with the regular sequence
of nerve impulses. DIPF is of such great toxicity to humans
that it has been used militarily as a nerve gas. Related com-
pounds, such as parathion and malathion,
are useful insecticides because they are far more toxic to
insects than to mammals.
His 57. A second catalytically important residue was dis-
covered through affinity labeling. In this technique, a sub-
strate analog bearing a reactive group specifically binds at
the enzyme’s active site, where it reacts to form a stable co-
valent bond with a nearby susceptible group (these reac-
tive substrate analogs have therefore been described as the
“Trojan horses” of biochemistry). The affinity labeled
groups can subsequently be identified by peptide mapping
(Section 7-1J). Chymotrypsin specifically binds tosyl-
L-
phenylalanine chloromethyl ketone (TPCK),
CH
3
CH
2
CH CCH
2
ClS
O O
O
NH
Parathion
O
2
N
P
O
O
O
S
CH
2
CH
3
CH
2
CH
3
CH
2
P
O
O
S
CH
3
CH
3
S
CH
O
C
CH
2
CH
3
O
C
CH
2
CH
3
O
O
Malathion
(CH
3
)
3
NCH
2
CH
2
CH
3
H
2
OO
O
–
O
acetylcholinesterase
O
C +
+
(CH
3
)
3
NCH
2
CH
2
CH
3
COH +
+
Acetylcholine
Choline
because of its resemblance to a Phe residue (one of chy-
motrypsin’s preferred residues;Table 7-2).Active site–bound
TPCK’s chloromethyl ketone group is a strong alkylating
agent; it reacts with His 57 (Fig. 15-18), thereby inactivat-
ing the enzyme. The TPCK reaction is inhibited by -
phenylpropionate,
a competitive inhibitor of chymotrypsin that presumably
competes with TPCK for its enzymatic binding site. More-
over,the TPCK reaction does not occur in 8M urea, a dena-
turing reagent, or with DIP–chymotrypsin, in which the ac-
tive site is blocked.These observations establish that His 57
is an essential active site residue of chymotrypsin.
B. X-Ray Structures
Bovine chymotrypsin, bovine trypsin, and porcine elastase
are strikingly homologous: The primary structures of these
⬃240-residue monomeric enzymes are ⬃40% identical and
their internal sequences are even more alike (in compari-
son, the and chains of human hemoglobin have a 44%
sequence identity). Furthermore, all of these enzymes have
an active Ser and a catalytically essential His as well as simi-
lar kinetic mechanisms. It therefore came as no surprise
when their X-ray structures all proved to be closely related.
To most conveniently compare the structures of these
three digestive enzymes, they have been assigned the same
amino acid residue numbering scheme. Bovine chy-
motrypsin is synthesized as an inactive 245-residue precur-
sor named chymotrypsinogen that is proteolytically con-
verted to chymotrypsin (Section 15-3Ea). In what follows,
the numbering of the amino acid residues in chymotrypsin,
trypsin, and elastase will be that of the corresponding
residues in bovine chymotrypsinogen.
The X-ray structure of bovine chymotrypsin was eluci-
dated in 1967 by David Blow. This was followed by the
CH
2
CH
2
COO
–
-Phenylpropionate
Section 15-3. Serine Proteases 527
N
CH
2
N
CH
2
H
Chymotrypsin
CH
2
Chymotrypsin
C
CH
2
R
O
Cl
C
R
O
HCl
His 57
TPCK
N
N
O
+
Figure 15-18 Reaction of TPCK with chymotrypsin to
alkylate His 57.
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 527
528 Chapter 15. Enzymatic Catalysis
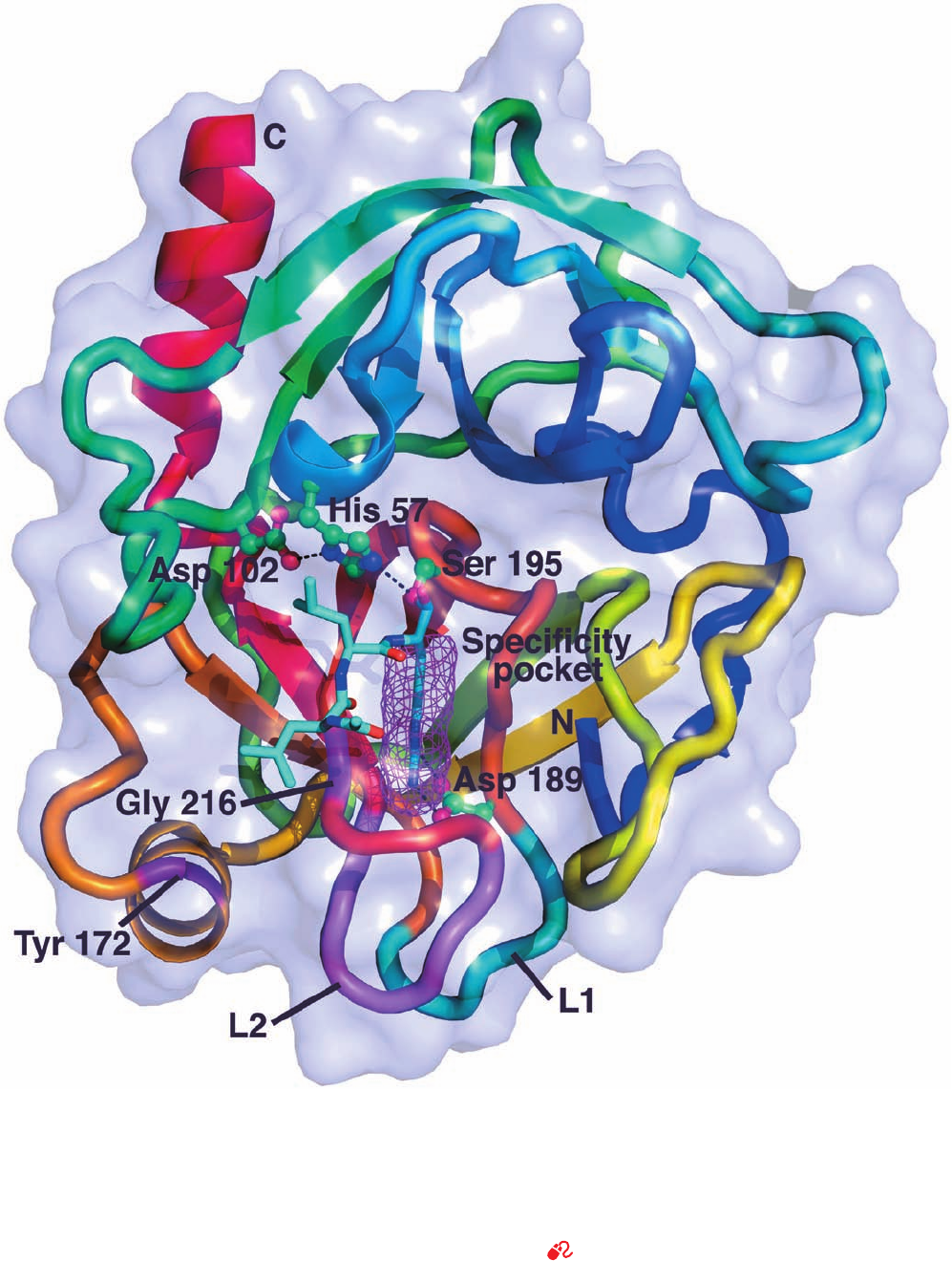
Figure 15-19 X-ray structure of bovine trypsin in covalent
complex with its inhibitor leupeptin. The protein, viewed looking
into its upper barrel, is represented by its transparent
molecular surface with its polypeptide chain in ribbon form
colored in rainbow order from its N-terminus (blue) to its
C-terminus (red), but with loop L1 (residues 185–188)
blue-green, loop L2 (residues 221–225) violet, and Tyr 172 and
Gly 216 purple. The side chains of the catalytic triad (Ser 195, His
57, and Asp 102) and Asp 189 are drawn in ball-and-stick form
colored according to atom type (C green, N blue, O red) with
hydrogen bonds represented by dashed black lines. Leupeptin
(acetyl-Leu-Leu-Arg in which the terminal carboxyl group is
replaced by ) is drawn in stick form (C cyan, N blue, O
red) with its Arg side chain occupying the enzyme’s specificity
pocket (magenta mesh). [Based on an X-ray structure by Daniel
Koshland, Jr., University of California at Berkeley. PDBid
2AGI.]
See Kinemage Exercise 10-1
¬CHO
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 528
determination of the structures of bovine trypsin (Fig.15-19)
by Robert Stroud and Richard Dickerson, and porcine
elastase by David Shotton and Herman Watson. Each of
these proteins is folded into two structurally similar do-
mains, each of which have extensive regions of antiparallel
 sheets arranged in a 6-stranded  barrel, but contain lit-
tle helix. The catalytically essential His 57 and Ser 195 are
located in a cleft between the  barrels, as is the invariant (in
all serine proteases) Asp 102, which is buried in a solvent-
inaccessible pocket. These three residues form a hydrogen
bonded constellation referred to as the catalytic triad (Figs.
15-19 and 15-20).
a. The Structural Basis of Substrate Specificity Can
Be Quite Complex
The X-ray structures of the above three enzymes
suggest the basis for their differing substrate specificities
(Fig. 15-21):
1. In chymotrypsin, the bulky aromatic side chain of the
preferred Phe, Trp, or Tyr residue (Table 7-2) that con-
tributes the carbonyl group of the scissile peptide fits
snugly into a slitlike hydrophobic pocket, the specificity
pocket, that is located near the catalytic groups (Fig. 15-19).
Section 15-3. Serine Proteases 529
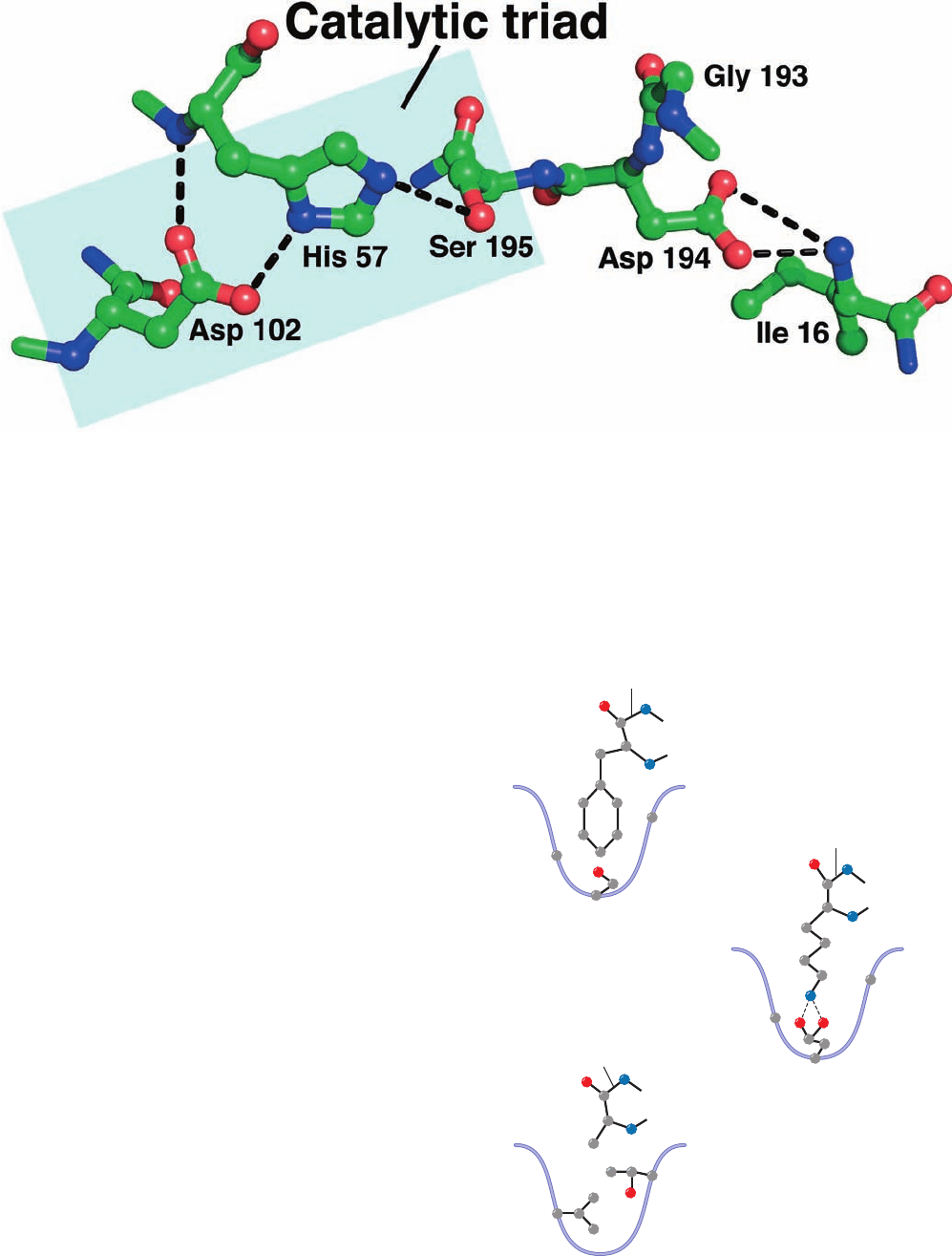
Figure 15-20 The active site residues of trypsin. Residues are
drawn in ball-and-stick form with C green, N blue, and O red and
viewed similarly to Fig. 15-19. The catalytic triad consists of Ser
Figure 15-21 Specificity pockets of three serine proteases. The
side chains of key residues that determine the size and nature of
the specificity pocket are shown along with a representative
substrate for each enzyme. Chymotrypsin prefers to cleave
peptide bonds following large hydrophobic side chains; trypsin
prefers Lys or Arg; and elastase prefers Ala, Gly, or Val. [After a
drawing in Branden, C. and Tooze, J., Introduction to Protein
Structure (2nd ed.), Garland Publishing, p. 213 (1999).]
–
Phe
Lys
Ala
Gly
216
Gly
216
Thr 216
Gly
226
Gly
226
Val 226
Ser 189
Asp 189
Chymotrypsin
Trypsin
Elastase
Scissile bond
Scissile bond
Scissile bond
+
195, His 57, and Asp 102. [Based on an X-ray structure by Daniel
Koshland, Jr., University of California at Berkeley. PDBid
2AGI.]
JWCL281_c15_506-556.qxd 6/7/10 2:08 PM Page 529
2. In trypsin, the residue corresponding to chy-
motrypsin Ser 189, which lies at the back of the specificity
pocket, is the anionic residue Asp (Fig. 15-19).The cationic
side chains of trypsin’s preferred residues, Arg or Lys, can
therefore form ion pairs with this Asp residue. The rest of
chymotrypsin’s specificity pocket is preserved in trypsin so
that it can accommodate the bulky side chains of Arg and
Lys.
3. Elastase is so named because it rapidly hydrolyzes
the otherwise nearly indigestible Ala, Gly, and Val-rich
protein elastin (a connective tissue protein with rubber-
like elastic properties). Elastase’s specificity pocket is
largely occluded by the side chains of a Val and a Thr
residue that replace two Gly’s lining this pocket in both
chymotrypsin and trypsin. Consequently elastase, whose
specificity pocket is better described as a depression,
specifically cleaves peptide bonds after small neutral
residues, particularly Ala. In contrast, chymotrypsin and
trypsin hydrolyze such peptide bonds extremely slowly be-
cause these small substrates cannot be sufficiently immo-
bilized on the enzyme surface for efficient catalysis to
occur (Section 15-1E).
Thus, for example, trypsin catalyzes the hydrolysis of pep-
tidyl amide substrates with an Arg or Lys residue preceding
the scissile bond with an efficiency, as measured by k
cat
/K
M
(Section 14-2Ba), that is 10
6
-fold greater than that for the
corresponding Phe-containing substrates. Conversely, chy-
motrypsin catalyzes the hydrolysis of substrates after Phe,
Trp, and Tyr residues 10
4
-fold more efficiently than after
the corresponding Lys-containing substrates.
Despite the foregoing, the mutagenic change in trypsin
of Asp 189 S Ser (D189S) by William Rutter did not
switch its specificity to that of chymotrypsin but instead
yielded a poor, nonspecific protease. Moreover, even re-
placing the other three residues in trypsin’s specificity
pocket that differ from those in chymotrypsin, with those
of chymotrypsin, fails to yield a significantly improved en-
zyme. However, trypsin is converted to a reasonably active
chymotrypsin-like enzyme when, in addition to the forego-
ing changes (collectively designated S1), both of its two
surface loops that connect the walls of the specificity
pocket (Fig. 15-19), L1 (residues 185–188) and L2 (residues
221–225), are replaced by those of chymotrypsin (termed
Tr S Ch[S1 L1 L2]). Although this mutant enzyme
still has a low substrate-binding affinity, K
S
, the additional
mutation Y172W in a third surface loop yields an enzyme
(Tr S Ch[S1 L1 L2 Y172W]) that has 15% of chy-
motrypsin’s catalytic efficiency. Curiously, these loops,
whose sequences are largely conserved in each enzyme,
are not structural components of either the specificity
pocket or the extended substrate binding site in chy-
motrypsin or in trypsin (Fig. 15-19).
Careful comparisons, by Charles Craik and Robert Flet-
terick, of the X-ray structures of chymotrypsin and trypsin
with those of the closely similar Tr S Ch[S1 L1 L2]
and Tr S Ch[S1 L1 L2 Y172W] in complex with a
Phe-containing chloromethyl ketone inhibitor reveal the
structural basis of substrate specificity in trypsin and chy-
motrypsin. Efficient catalysis in the serine proteases re-
quires that the enzyme’s active site be structurally intact
and that the substrate’s scissile bond be properly posi-
tioned relative to the catalytic triad and other components
of the active site (see below). The above mutagenic
changes do not affect the structure of the catalytic triad or
those portions of the active site that bind the substrate’s
leaving group (that segment on the C-terminal side of the
scissile bond). However, the main chain conformation of
the conserved Gly 216 (which forms two hydrogen bonds
to the backbone of the third residue before the substrate’s
scissile bond in an antiparallel pleated sheet–like
arrangement) differs in trypsin and chymotrypsin and
adopts a chymotrypsin-like structure in both hybrid pro-
teins. Evidently, if Gly 216 adopts a trypsin-like conforma-
tion, the scissile bond in Phe-containing substrates is mis-
oriented for efficient catalysis. Thus, despite the fact that
Gly 216 is conserved in trypsin and chymotrypsin, the dif-
fering structures of loop L2 in the two enzymes maintain it
in distinct conformations.
Loop L1, which interacts with L2 in both trypsin and
chymotrypsin, is largely disordered in the X-ray structure
of Tr S Ch[S1 L1 L2]. Modeling a trypsin-like L1 into
Tr S Ch[S1 L1 L2] results in severe steric clashes
with the chymotrypsin-like L2. Thus, the requirement of a
chymotrypsin-like L1 for the efficient catalysis by
Tr S Ch[S1 L1 L2] appears to arise from the need to
permit L2 to adopt a chymotrypsin-like conformation.
The side chain of Tyr 172 extends toward the base of
the specificity pocket. The improvement in substrate
binding affinity of Tr S Ch[S1 L1 L2 Y172W]
over Tr S Ch[S1 L1 L2] arises from structural re-
arrangements in this region of the enzyme caused by the
increased bulk and different hydrogen bonding require-
ments of Trp versus Tyr. These changes appear to improve
both the structural stability of residues forming the speci-
ficity pocket and their specificity for chymotrypsin-like
substrates. These results therefore highlight an important
caveat for genetic engineers: Enzymes are so exquisitely tai-
lored to their functions that they often respond to mutagenic
tinkering in unexpected ways.
b. Evolutionary Relationships Among
Serine Proteases
We have seen that sequence and structural homologies
among proteins reveal their evolutionary relationships
(Sections 7-3 and 9-6). The great similarities among chy-
motrypsin, trypsin, and elastase indicate that these proteins
evolved through gene duplications of an ancestral serine
protease followed by the divergent evolution of the resulting
enzymes (Section 7-3C).
Several serine proteases from various sources provide
further insights into the evolutionary relationships among
the serine proteases. Streptomyces griseus protease A
(SGPA) is a bacterial serine protease of chymotryptic
specificity that exhibits extensive structural similarity, al-
though only ⬃20% sequence identity, with the pancreatic
530 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 530
