Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

b. Certain Amino Acid Side Chains and Coenzymes
Can Serve as Covalent Catalysts
Enzymes commonly employ covalent catalytic mecha-
nisms as is indicated by the large variety of covalently
linked enzyme–substrate reaction intermediates that have
been isolated. For example, the enzymatic decarboxylation
of acetoacetate proceeds, much as described above,
through Schiff base formation with an enzyme Lys
residue’s ε-amino group. The covalent intermediate, in this
case, has been isolated through NaBH
4
reduction of its
imine bond to an amine, thereby irreversibly inhibiting the
enzyme. Other enzyme functional groups that participate
in covalent catalysis include the imidazole moiety of His,
the thiol group of Cys, the carboxyl function of Asp, and
the hydroxyl group of Ser. In addition, several coenzymes,
most notably thiamine pyrophosphate (Section 17-3Ba)
and pyridoxal phosphate (Section 26-1Aa), function in as-
sociation with their apoenzymes mainly as covalent cata-
lysts.
C. Metal Ion Catalysis
Nearly one-third of all known enzymes require the presence
of metal ions for catalytic activity. There are two classes of
metal ion–requiring enzymes that are distinguished by the
strengths of their ion–protein interactions:
1. Metalloenzymes contain tightly bound metal ions,
most commonly transition metal ions such as Fe
2
,Fe
3
,
Cu
2
,Zn
2
,Mn
2
, or Co
3
.
2. Metal-activated enzymes loosely bind metal ions
from solution, usually the alkali and alkaline earth metal
ions Na
,K
,Mg
2
, or Ca
2
.
Metal ions participate in the catalytic process in three ma-
jor ways:
1. By binding to substrates so as to orient them prop-
erly for reaction.
2. By mediating oxidation–reduction reactions through
reversible changes in the metal ion’s oxidation state.
3. By electrostatically stabilizing or shielding negative
charges.
In this section we shall be mainly concerned with the third
aspect of metal ion catalysis. The other forms of enzyme-
mediated metal ion catalysis are considered in later chapters
in conjunction with discussions of specific enzyme mecha-
nisms.
a. Metal Ions Promote Catalysis through
Charge Stabilization
In many metal ion–catalyzed reactions, the metal ion
acts in much the same way as a proton to neutralize nega-
tive charge, that is, it acts as a Lewis acid.Yet metal ions are
often much more effective catalysts than protons because
metal ions can be present in high concentrations at neutral
pH’s and can have charges greater than 1. Metal ions have
therefore been dubbed “superacids.”
The decarboxylation of dimethyloxaloacetate, as cat-
alyzed by metal ions such as Cu
2
and Ni
2
, is a nonenzy-
matic example of catalysis by a metal ion:
Here the metal ion (M
n
), which is chelated by the di-
methyloxaloacetate, electrostatically stabilizes the devel-
oping enolate ion of the transition state. This mechanism is
supported by the observation that acetoacetate, which
cannot form such a chelate, is not subject to metal ion–
catalyzed decarboxylation. Most enzymes that decarboxy-
late oxaloacetate require a metal ion for activity.
b. Metal Ions Promote Nucleophilic Catalysis via
Water Ionization
A metal ion’s charge makes its bound water molecules
more acidic than free H
2
O and therefore a source of OH
ions even below neutral pH’s. For example, the water
molecule of (NH
3
)
5
Co
3
(H
2
O) ionizes according to the
reaction:
with a pK of 6.6, which is ⬃9 pH units below the pK of free
H
2
O. The resulting metal ion–bound hydroxyl group is a po-
tent nucleophile.
An instructive example of this phenomenon occurs in the
catalytic mechanism of carbonic anhydrase (Section 10-1C),
a widely occurring enzyme that catalyzes the reaction:
Carbonic anhydrase contains an essential Zn
2
ion that lies
at the bottom of an ⬃15-Å-deep active site cleft (Fig. 8-41),
where it is tetrahedrally coordinated by three evolutionar-
ily invariant His side chains and an O atom of either an
CO
2
H
2
O Δ HCO
3
H
(NH
3
)
5
Co
3
(H
2
O) Δ (NH
3
)
5
Co
3
(OH
) H
M
n+
–
OO
O
CH
3
CH
3
CO
2
O
O
–
CC CC
–
O
O
O
CH
3
CH
3
CC CH +
M
n+
M
n+
H
+
–
O
–
O
O
CH
3
CH
3
CC C
Dimethyloxaloacetate
Section 15-1. Catalytic Mechanisms 511
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 511
HCO
⫺
3
ion (Fig.15-5a) or a water molecule (Fig. 15-5b).The
enzyme has the following catalytic mechanism:
1. We begin with a water molecule bound to the protein
in the Zn
2⫹
ion’s fourth liganding position (Fig. 15-5b).This
Zn
2⫹
-polarized H
2
O ionizes in a process facilitated through
general base catalysis by His 64 in its “in” conformation.
Although His 64 is too far away from the Zn
2⫹
-bound wa-
ter to directly abstract its proton, these entities are linked
by two intervening water molecules to form a hydrogen
bonded network that is thought to act as a proton shuttle.
2. The resulting Zn
2⫹
-bound OH
⫺
ion nucleophilically
attacks the nearby enzymatically bound CO
2
, thereby con-
verting it to HCO
⫺
3
.
In doing so, the Zn
2⫹
-bound OH
⫺
group donates a hydro-
gen bond to Thr 199, which in turn donates a hydrogen
bond to Glu 106 (Fig. 15-5a). These interactions orient the
OH
⫺
group with the optimal geometry (see below) for nu-
cleophilic attack on the substrate CO
2
.
3. The catalytic site is regenerated by the exchange of
the Zn
2⫹
-bound HCO
⫺
3
reaction product for H
2
O together
C
Im
Zn
2+
O
–
Im
H
Im
O
+
O
O
C
O
H
Im
Zn
2+
Im
Im
O
–
O
H
+
Im
Zn
2+
O
–
Im
H
Im
+
H
+
O
C
O
–
O
H
2
O
Im = imidazole
N
N HH
H
O
H
H
O
H
H
Zn
2
+
Im
Im
Im
O
H
H
H
O
H H
H
O
H
Zn
2
+
Im
Im
Im
O
His 64
+
–
Im = imidazole
N
N
His 64
512 Chapter 15. Enzymatic Catalysis
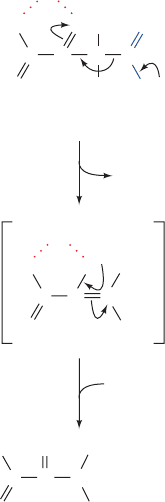
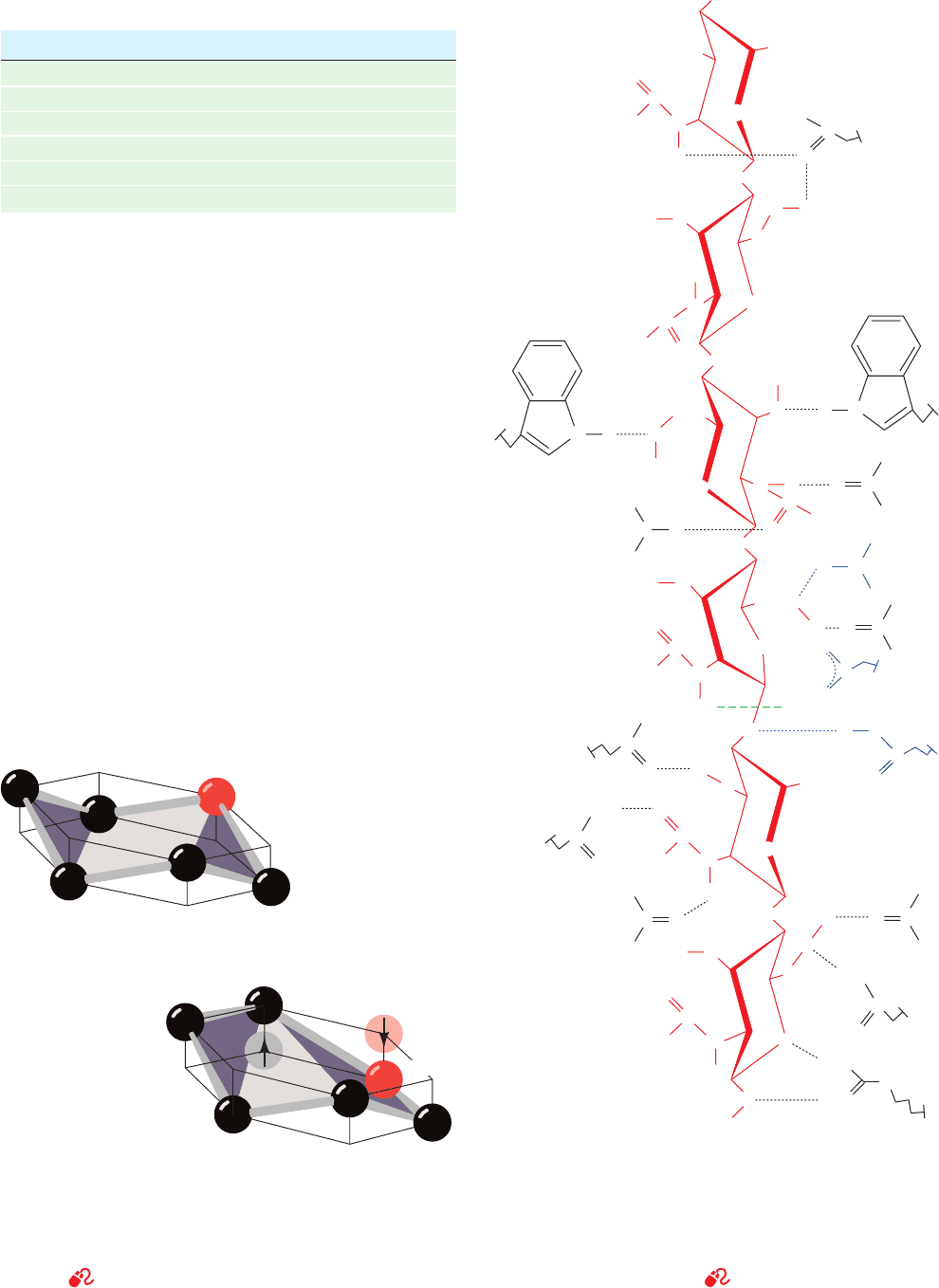
Figure 15-5 X-ray structures of human carbonic anhydrase.
(a) Its active site in complex with bicarbonate ion.The
polypeptide is shown in ribbon form (gold) with its side chains
shown in stick form colored according to atom type (C green,
N blue, and O red).The protein-bound Zn
2⫹
ion (cyan sphere) is
tetrahedrally liganded (gray bonds) by three invariant His side
chains and the HCO
⫺
3
ion, which is shown in ball-and-stick form.
The HCO
⫺
3
ion also interacts with the protein via van der Waals
contacts (dot surface colored according to atom type) and a
hydrogen bonded network (dashed gray lines) involving Thr 199
and Glu 106. [Based on an X-ray structure by K.K. Kannan,
Bhabha Atomic Research Center, Bombay, India. PDBid 1HCB.]
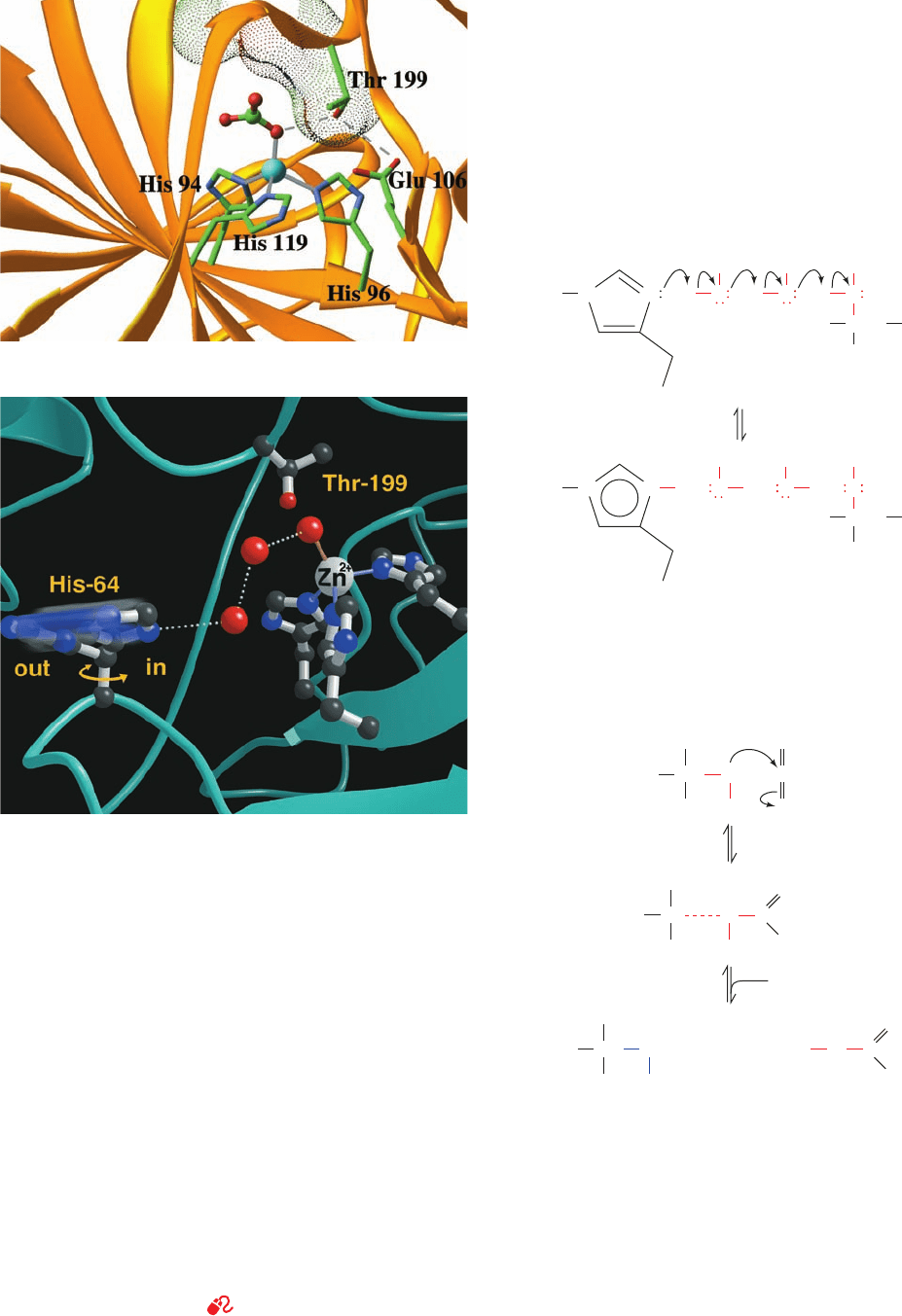
(b) The active site showing the proton shuttle through which His
64, acting as a general base, abstracts a proton from the
Zn
2⫹
-bound H
2
O to form an OH
⫺
ion.The polypeptide
backbone is shown in ribbon form (cyan), and its side chains and
several bound solvent molecules are shown in ball-and-stick
form with C black, N blue, and O red. The proton shuttle consists
of two water molecules that form a hydrogen bonded network
(dotted white lines) that bridges the Zn
2⫹
-bound OH
⫺
ion and
His 64 in its “in” conformation. On protonation, His 64 swings to
the “out” conformation. [Courtesy of David Christianson,
University of Pennsylvania.]
See Interactive Exercise 3
(a)
(b)
JWCL281_c15_506-556.qxd 10/19/10 7:19 AM Page 512
with the deprotonation of His 64. In the latter process, His
64 swings to its “out” conformation (Fig. 15-5b), which may
facilitate proton transfer to the bulk solvent.
c. Metal Ions Promote Reactions through
Charge Shielding
Another important enzymatic function of metal ions
is charge shielding. For example, the actual substrates of
kinases (phosphoryl-transfer enzymes utilizing ATP) are
Mg
2
–ATP complexes such as
rather than just ATP. Here, the Mg
2
ion’s role, in addition
to its orienting effect, is to shield electrostatically the nega-
tive charges of the phosphate groups. Otherwise, these
charges would tend to repel the electron pairs of attacking
nucleophiles, especially those with anionic character.
D. Electrostatic Catalysis
The binding of substrate generally excludes water from an
enzyme’s active site. The local dielectric constant of the ac-
tive site therefore resembles that in an organic solvent,
where electrostatic interactions are much stronger than
they are in aqueous solutions (Section 8-4A). The charge
distribution in a medium of low dielectric constant can
greatly influence chemical reactivity. Thus, as we have seen,
the pK’s of amino acid side chains in proteins may vary by
several units from their nominal values (Table 4-1) because
of the proximity of charged groups.
Although experimental evidence and theoretical analy-
ses on the subject are still sparse, there are mounting indica-
tions that the charge distributions about the active sites of
enzymes are arranged so as to stabilize the transition states
of the catalyzed reactions. Such a mode of rate enhance-
ment, which resembles the form of metal ion catalysis dis-
cussed above, is termed electrostatic catalysis. Moreover, in
several enzymes, these charge distributions apparently serve
to guide polar substrates toward their binding sites so that
the rates of these enzymatic reactions are greater than their
apparent diffusion-controlled limits (Section 14-2Bb).
E. Catalysis through Proximity and
Orientation Effects
Although enzymes employ catalytic mechanisms that re-
semble those of organic model reactions, they are far more
catalytically efficient than these models. Such efficiency
must arise from the specific physical conditions at enzyme
catalytic sites that promote the corresponding chemical re-
actions. The most obvious effects are proximity and orien-
tation: Reactants must come together with the proper spa-
tial relationship for a reaction to occur. For example, in the
Adenine Ribose O
OOO
PPPOOO
–
O
–
O
–
O
–
Mg
2+
bimolecular reaction of imidazole with p-nitrophenyl-
acetate,
the progress of the reaction is conveniently monitored by
the appearance of the intensely yellow p-nitrophenolate ion:
[15.4]
where phenyl. Here k¿
1
, the pseudo-first-order rate con-
stant, is 0.0018 s
1
when [imidazole] 1M. However, for
the intramolecular reaction
the first-order rate constant k
2
0.043 s
1
; that is, k
2
24
k¿
1
. Thus, when the 1M imidazole catalyst is covalently at-
tached to the reactant, it is 24-fold more effective than
when it is free in solution; that is, the imidazole group in the
intramolecular reaction behaves as if its concentration is
24M. This rate enhancement has contributions from both
proximity and orientation.
a. Proximity Alone Contributes Relatively
Little to Catalysis
Let us make a rough calculation as to how the rate of a
reaction is affected purely by the proximity of its reacting
groups. Following Daniel Koshland’s treatment, we shall
make several reasonable assumptions:
1. Reactant species, that is, functional groups, are about
the size of water molecules.
2. Each reactant species in solution has 12 nearest-
neighbor molecules, as do packed spheres of identical size.
N
NH
C O
O
NO
2
NO
2N
NH
C
O
k
2
+
+
–
O
k¿
1
[p-NO
2
Ac]
d[p-NO
2
O
]
dt
k
1
[imidazole] [p-NO
2
Ac]
C
CH
3
O
O
NO
2
p-Nitrophenylacetate
Imidazole
C
CH
3
O
_
O
p-Nitrophenolate
+
N-Acetylimidazolium
NO
2
NH
N
+
NH
N
O
(p-NO
2
Ac)
(p-NO
2
O
ⴚ
)
k
1
Section 15-1. Catalytic Mechanisms 513
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 513
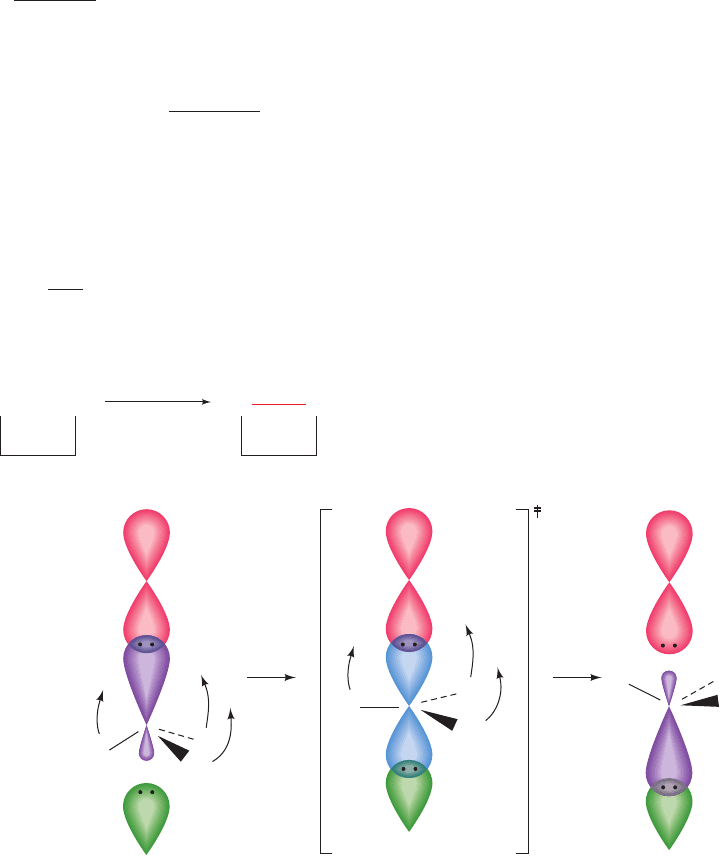
3. Chemical reactions occur only between reactants
that are in contact.
4. The reactant concentration in solution is low enough
so that the probability of any reactant species being in si-
multaneous contact with more than one other reactant
molecule is negligible.
Then the reaction:
obeys the second-order rate equation
[15.5]
where [A, B]
pairs
is the concentration of contacting mole-
cules of A and B. The value of this quantity is
[15.6]
since there are 12 ways that A can be in contact with B, and
[A]/55.5M is the fraction of sites occupied by A in water so-
lution ([H
2
O] 55.5M in dilute aqueous solutions) and
hence the probability that a molecule of B will be next to
one of A. Combining Eqs. [15.5] and [15.6] yields
[15.7]
Thus, in the absence of other effects, this model predicts
that for the intramolecular reaction,
AB AB
k
2
v k
1
a
55.5
12
b[A, B]
pairs
4.6k
1
[A, B]
pairs
[A, B]
pairs
12[A] [B]
55.5M
v
d[A¬B]
dt
k
1
[A][B] k
2
[A, B]
pairs
A B
¡
k
1
A¬B
k
2
4.6k
1
, which is a rather small rate enhancement. Fac-
tors that will increase this value other than proximity alone
clearly must be considered.
b. Properly Orienting Reactants and Arresting Their
Relative Motions Can Result in Large Catalytic
Rate Enhancements
The foregoing theory is, of course, quite simple. For ex-
ample, it does not take into account the relative orienta-
tions of the reacting molecules. Yet molecules are not
equally reactive in all directions as Koshland’s simple the-
ory assumes. Rather, they react most readily only if they
have the proper relative orientation. For example, in an S
N
2
(bimolecular nucleophilic substitution) reaction, the in-
coming nucleophile optimally attacks its target C atom
along the direction opposite to that of the bond to the leav-
ing group (Fig. 15-6). The approaches of reacting atoms
along a trajectory that deviates by as little as 10° from this
optimum direction can reduce the reaction rate by as much
as a factor of ⬃100. In a related phenomenon, a molecule
may be maximally reactive only when it assumes a confor-
mation that aligns its various orbitals in a way that minimizes
the electronic energy of its transition state, an effect termed
stereoelectronic assistance or stereoelectronic control.
Another effect that we have neglected in our treatment
of proximity is that of motions of the reacting groups with
respect to one another.Yet, in the transition state complex,
the reacting groups have little relative motion. In fact, as
Thomas Bruice demonstrated, the rates of intramolecular
reactions are greatly increased by arresting a molecule’s in-
ternal motions in a way that increases the mole fraction of
the reacting groups that are in a conformation which can
514 Chapter 15. Enzymatic Catalysis
C
X
R
R′
R′′
δ
–
C
X
R
R′
Y
–
Y
δ
–
Y
R′′
C
X
–
R
R′
R′′
sp
2
–p hybridization at carbon
Figure 15-6 The geometry of an S
N
2 reaction. The attacking
nucleophile, Y
, must approach the tetrahedrally coordinated
and hence sp
3
-hybridized C atom along the direction opposite
that of its bond to the leaving group, X, a process called backside
attack. In the transition state of the reaction, the C atom
becomes trigonal bipyramidally coordinated and hence sp
2
–p
hybridized, with the p orbital (blue) forming partial bonds to X
and Y. The three sp
2
orbitals form bonds to the C atom’s three
other substituents (R, R¿, and R–), which have shifted their
positions into the plane perpendicular to the axis
(curved arrows).Any deviation from this optimal geometry
would increase the free energy of the transition state, G
‡
, and
hence reduce the rate of the reaction (Eq. [14.15]). The transition
state then decomposes to products in which the R, R¿, and R–
have inverted their positions about the C atom, which has
rehybridized to sp
3
, and X
has been released.
X¬C¬Y
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 514
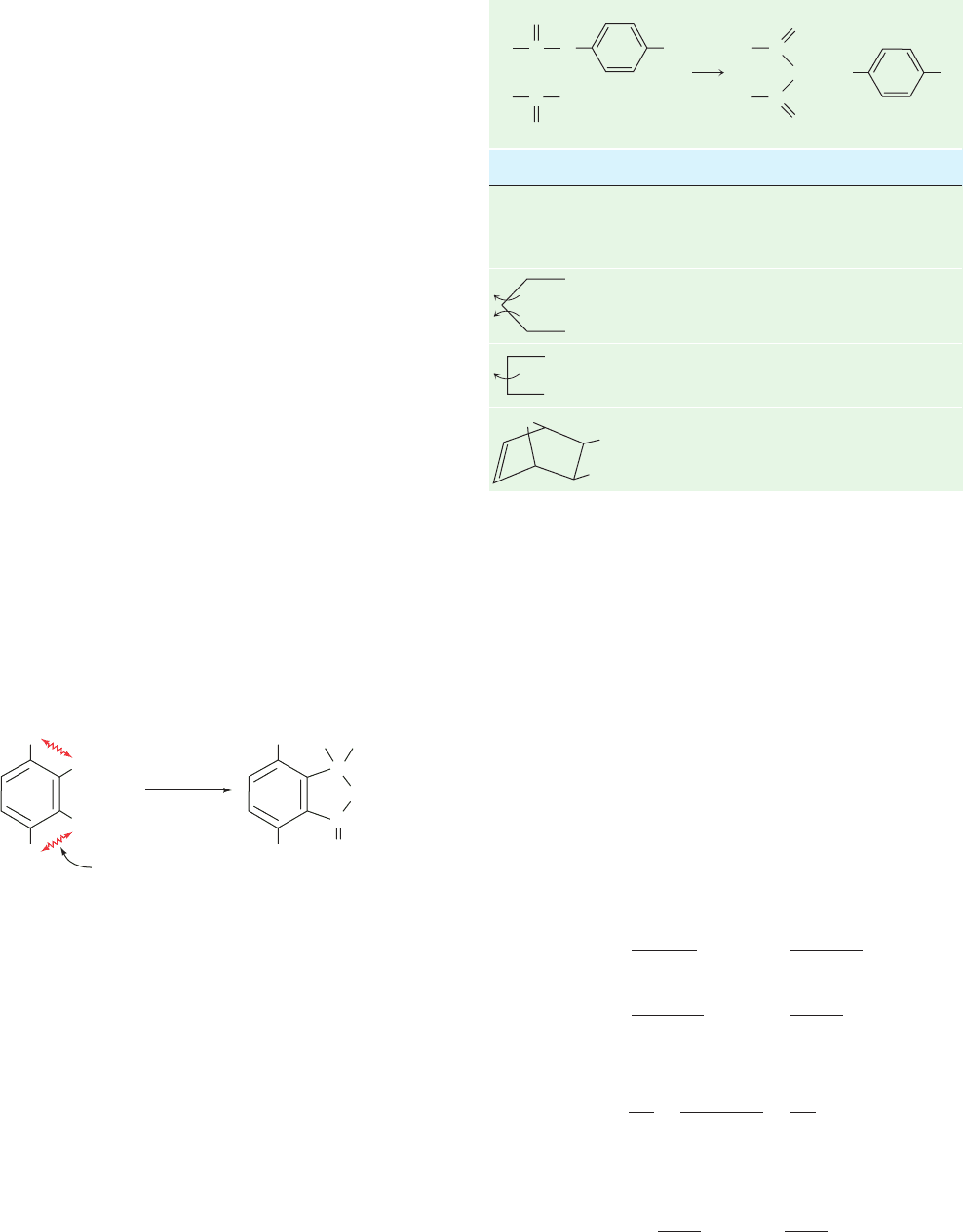
enter the transition state (Table 15-1). Similarly, when an
enzyme brings two molecules together in a bimolecular re-
action, as William Jencks pointed out, not only does it in-
crease their proximity, but it freezes out their relative
translational and rotational motions (decreases their en-
tropy), thereby enhancing their reactivity. Theoretical stud-
ies by Bruice indicate that much of this rate enhancement
can arise from the enzymatic binding of substrates in a con-
formation that readily enters the transition state.
Enzymes, as we shall see in Sections 16-2 and 16-3, bind
substrates in a manner that both aligns and immobilizes
them so as to optimize their reactivities. The free energy re-
quired to do so is derived from the specific binding free en-
ergy of substrate to enzyme.
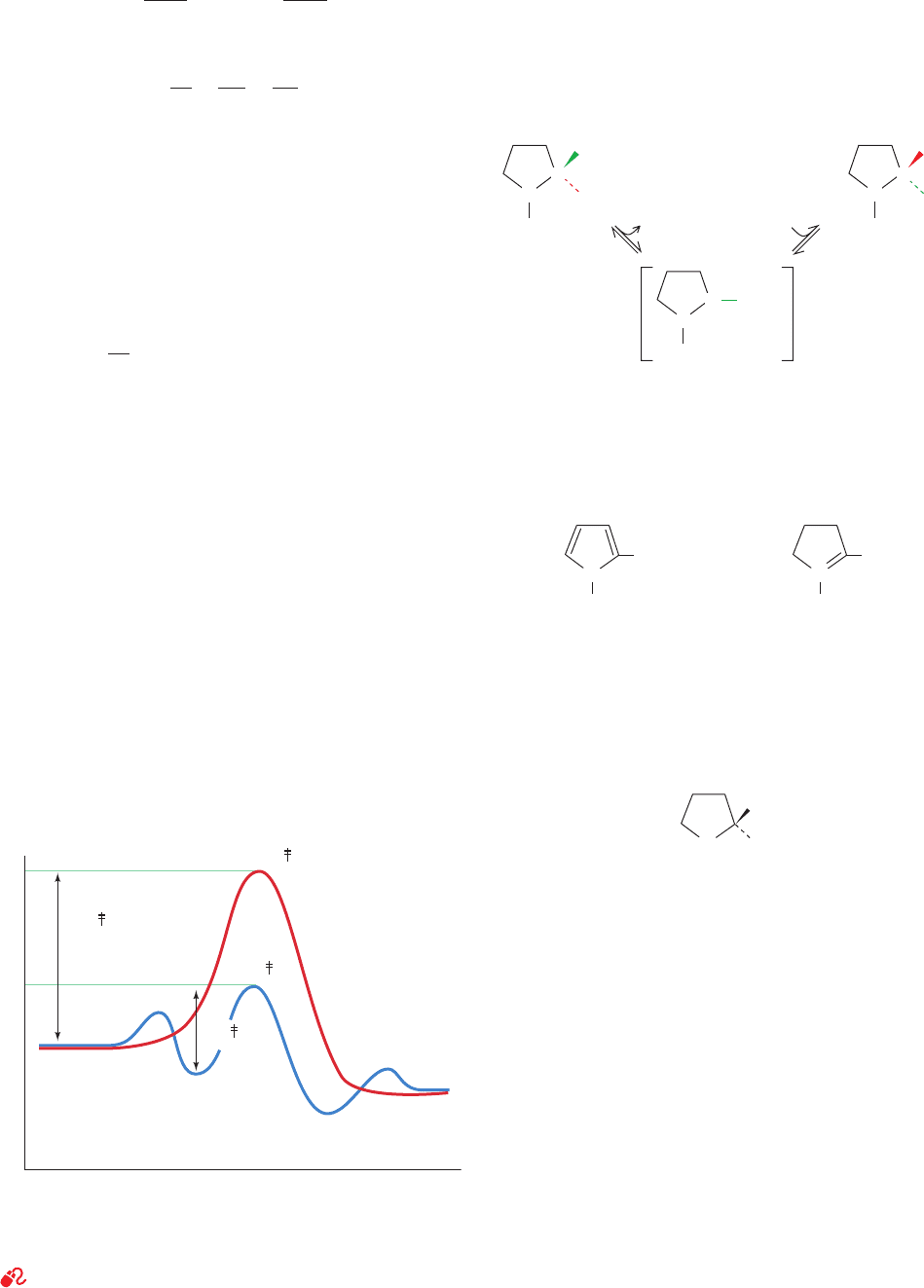
F. Catalysis by Preferential Transition State Binding
The rate enhancements effected by enzymes are often
greater than can be reasonably accounted for by the cat-
alytic mechanisms so far discussed. However, we have not
yet considered one of the most important mechanisms of
enzymatic catalysis: the binding of the transition state to an
enzyme with greater affinity than the corresponding sub-
strates or products. When taken together with the previ-
ously described catalytic mechanisms, preferential transi-
tion state binding rationalizes the observed rates of
enzymatic reactions.
The original concept of transition state binding pro-
posed that enzymes mechanically strained their substrates
toward the transition state geometry through binding sites
into which undistorted substrates did not properly fit. This
so-called rack mechanism (in analogy with the medieval
torture device) was based on the extensive evidence for the
role of strain in promoting organic reactions. For example,
the rate of the reaction,
is 315 times faster when R is CH
3
rather than when it is H
because of the greater steric repulsions between the CH
3
groups and the reacting groups. Similarly, ring opening re-
actions are considerably more facile for strained rings such
as cyclopropane than for unstrained rings such as cyclo-
hexane. In either process, the strained reactant more closely
resembles the transition state of the reaction than does the
corresponding unstrained reactant. Thus, as was first sug-
gested by Linus Pauling and further amplified by Richard
Wolfenden and Gustav Lienhard, interactions that prefer-
entially bind the transition state increase its concentration
and therefore proportionally increase the reaction rate.
Let us quantitate this statement by considering the ki-
netic consequences of preferentially binding the transition
state of an enzymatically catalyzed reaction involving a sin-
gle substrate. The substrate S may react to form product P
R
R
CH
2
OH
COOH
Steric
strain
R
R
C
C
OH
2
O
O
HH
+
either spontaneously or through enzymatic catalysis:
The relationships between the various states of these two
reaction pathways are indicated in the following scheme:
K
R
K
T
where
are all association constants. Consequently,
[15.8]
According to transition state theory, Eqs. [14.7] and [14.14],
the rate of the uncatalyzed reaction can be expressed
[15.9]v
N
⫽ k
N
[S] ⫽ a
kk
B
T
h
b[S
‡
] ⫽ a
kk
B
T
h
bK
‡
N
[S]
K
T
K
R
⫽
[S][ES
‡
]
[S
‡
][ES]
⫽
K
‡
E
K
‡
N
K
‡
N
⫽
[E][S
‡
]
[E][S]
K
‡
E
⫽
[ES
‡
]
[ES]
K
R
⫽
[ES]
[E][S]
K
T
⫽
[ES
‡
]
[E][S
‡
]
ES Δ
K
‡
E
ES
‡
¡
EP
Δ
Δ
Δ
E ⫹ S Δ
K
‡
N
S
‡
⫹ E
¡
P ⫹ E
ES
¡
k
E
EP
S
¡
k
N
P
Section 15-1. Catalytic Mechanisms 515
Table 15-1 Relative Rates of Anhydride Formation for
Esters Possessing Different Degrees of
Motional Freedom in the Reaction:
O
O
O
O
O
O
R
1
CO
R
2
CO
Br
+
+
+
R
1
C
R
2
C
_
O
_
Br
Reactants
a
Relative Rate Constant
CH
3
COOBr
COOBr
COOBr
COOBr
CH
3
COO
_
COO
_
COO
_
COO
_
1.0
⬃1 ⫻ 10
3
⬃2.3 ⫻ 10
5
⬃8 ⫻ 10
7
a
Curved arrows indicate rotational degrees of freedom.
Source: Bruice, T.C. and Lightstone, F.C., Acc. Chem. Res. 32, 127 (1999).
JWCL281_c15_506-556.qxd 6/7/10 2:07 PM Page 515
Similarly, the rate of the enzymatically catalyzed reaction is
[15.10]
Therefore, combining Eqs. [15.8] to [15.10],
[15.11]
This equation indicates that the more tightly an enzyme
binds its reaction’s transition state (K
T
) relative to the sub-
strate (K
R
), the greater the rate of the catalyzed reaction (k
E
)
relative to that of the uncatalyzed reaction (k
N
); that is, catal-
ysis results from the preferential binding and therefore the
stabilization of the transition state (S
‡
) relative to that of the
substrate (S) (Fig. 15-7).
According to Eq. [14.15], the ratio of the rates of the cat-
alyzed versus the uncatalyzed reaction is expressed
[15.12]
A rate enhancement factor of 10
6
therefore requires that an
enzyme bind its transition state complex with 10
6
-fold
higher affinity than its substrate, which corresponds to a
34.2 kJ ⴢ mol
⫺1
stabilization at 25°C. This is roughly the free
energy of two hydrogen bonds. Consequently, the enzymatic
binding of a transition state (ES
‡
) by two hydrogen bonds
that cannot form in the Michaelis complex (ES) should result
in a rate enhancement of ⬃10
6
based on this effect alone.
It is commonly observed that the specificity of an en-
zyme is manifested by its turnover number (k
cat
) rather
than by its substrate-binding affinity. In other words, an en-
zyme binds poor substrates, which have a low reaction rate,
as well as or even better than good ones, which have a high
reaction rate. Such enzymes apparently use a good sub-
strate’s intrinsic binding energy to stabilize the correspon-
ding transition state; that is, a good substrate does not nec-
essarily bind to its enzyme with high affinity, but does so on
activation to the transition state.
k
E
k
N
⫽ exp[ (¢G
‡
N
⫺ ¢G
‡
E
)>RT]
k
E
k
N
⫽
K
‡
E
K
‡
N
⫽
K
T
K
R
v
E
⫽ k
E
[ES] ⫽ a
kk
B
T
h
b[ES
‡
] ⫽ a
kk
B
T
h
bK
‡
E
[ES]
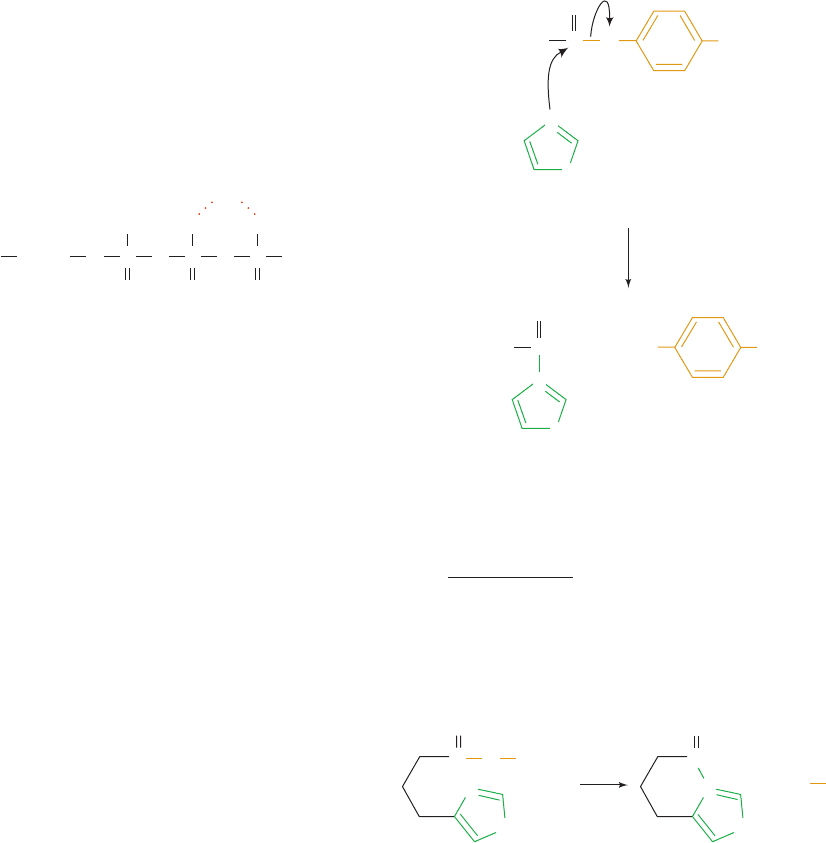
a. Transition State Analogs Are Potent
Competitive Inhibitors
If an enzyme preferentially binds its transition state, then
it can be expected that transition state analogs, stable mol-
ecules that resemble S
‡
or one of its components, are potent
competitive inhibitors of the enzyme. For example, the reac-
tion catalyzed by proline racemase from Clostridium stick-
landii is thought to occur via a planar transition state:
Proline racemase is competitively inhibited by the planar
analogs of proline, pyrrole-2-carboxylate and ⌬-1-pyrroline-
2-carboxylate,
both of which bind to the enzyme with 160-fold greater
affinity than does proline. These compounds are there-
fore thought to be analogs of the transition state in the
proline racemase reaction. In contrast, tetrahydrofuran-
2-carboxylate,
which more closely resembles the tetrahedral structure of
proline, is not nearly as good an inhibitor as these com-
pounds. A 160-fold increase in binding affinity corre-
sponds, according to Eq. [15.12], to a 12.6 kJ ⴢ mol
⫺1
in-
crease in the free energy of binding. This quantity
presumably reflects the additional binding affinity that
proline racemase has for proline’s planar transition state
over that of the undistorted molecule.
Hundreds of transition state analogs for various enzy-
matic reactions have been reported. Some are naturally oc-
curring antibiotics. Others were designed to investigate the
mechanisms of particular enzymes and/or to act as specific
enzymatic inhibitors for therapeutic or agricultural use. In-
deed, as we discuss in Section 15-4C, the theory that en-
zymes bind transition states with higher affinity than sub-
strates has led to a rational basis for drug design based on
the understanding of specific enzyme reaction mechanisms.
O
COO
–
H
Tetrahydrofuran-2-carboxylate
Pyrrole-2-carboxylate ⌬-1-Pyrroline-2-carboxylate
N
H
COO
–
N
H
COO
–
+
N
C
H
H
COO
_
L-Proline
H
+
N
C
H
H
COO
_
D-Proline
H
+
N
C
_
H
COO
_
Planar transition
state
516 Chapter 15. Enzymatic Catalysis
ES
ΔG
E
ΔG
N
Reaction coordinate
G
E + S
E + S
E + P
ES
EP
Figure 15-7 Reaction coordinate diagrams for a hypothetical
enzymatically catalyzed reaction involving a single substrate
(blue) and the corresponding uncatalyzed reaction (red).
See the Animated Figures
JWCL281_c15_506-556.qxd 6/24/10 7:47 AM Page 516
2 LYSOZYME
In the following two sections, we shall investigate the cat-
alytic mechanisms of several well-characterized enzymes.
In doing so, we shall see how enzymes apply the catalytic
principles described in Section 15-1. You should note that
the great catalytic efficiency of enzymes arises from their si-
multaneous use of several of these catalytic mechanisms.
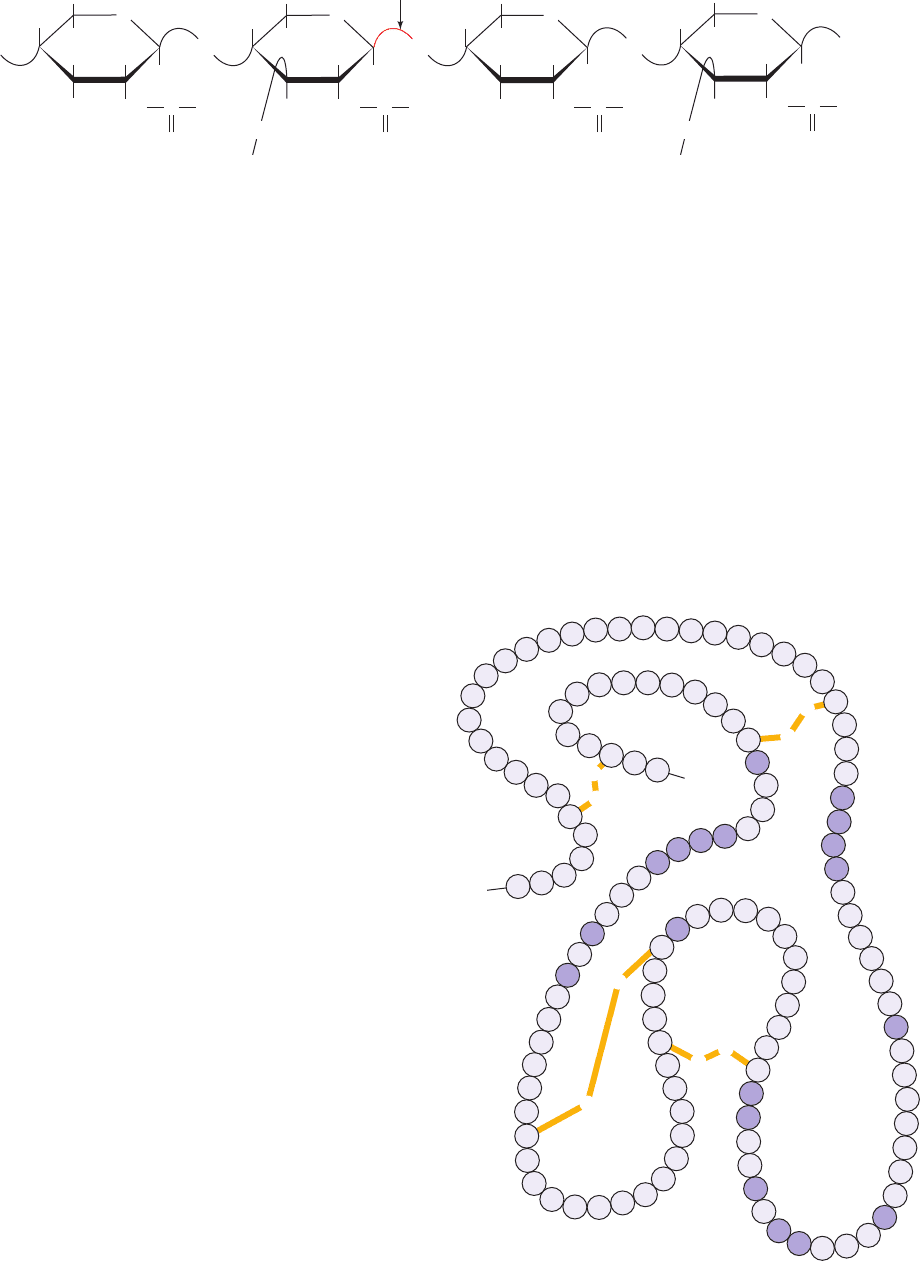
Lysozyme is an enzyme that destroys bacterial cell walls.
It does so, as we saw in Section 11-3Ba, by hydrolyzing the
(1 S 4) glycosidic linkages from N-acetylmuramic acid
(NAM) to N-acetylglucosamine (NAG) in the alternating
NAM–NAG polysaccharide component of cell wall pepti-
doglycans (Fig. 15-8). It likewise hydrolyzes (1 S 4)-linked
poly(NAG) (chitin), a cell wall component of most fungi.
Lysozyme occurs widely in the cells and secretions of ver-
tebrates, where it may function as a bactericidal agent.
However, the observation that few pathogenic bacteria are
susceptible to lysozyme alone has prompted the suggestion
that this enzyme mainly helps dispose of bacteria after they
have been killed by other means.
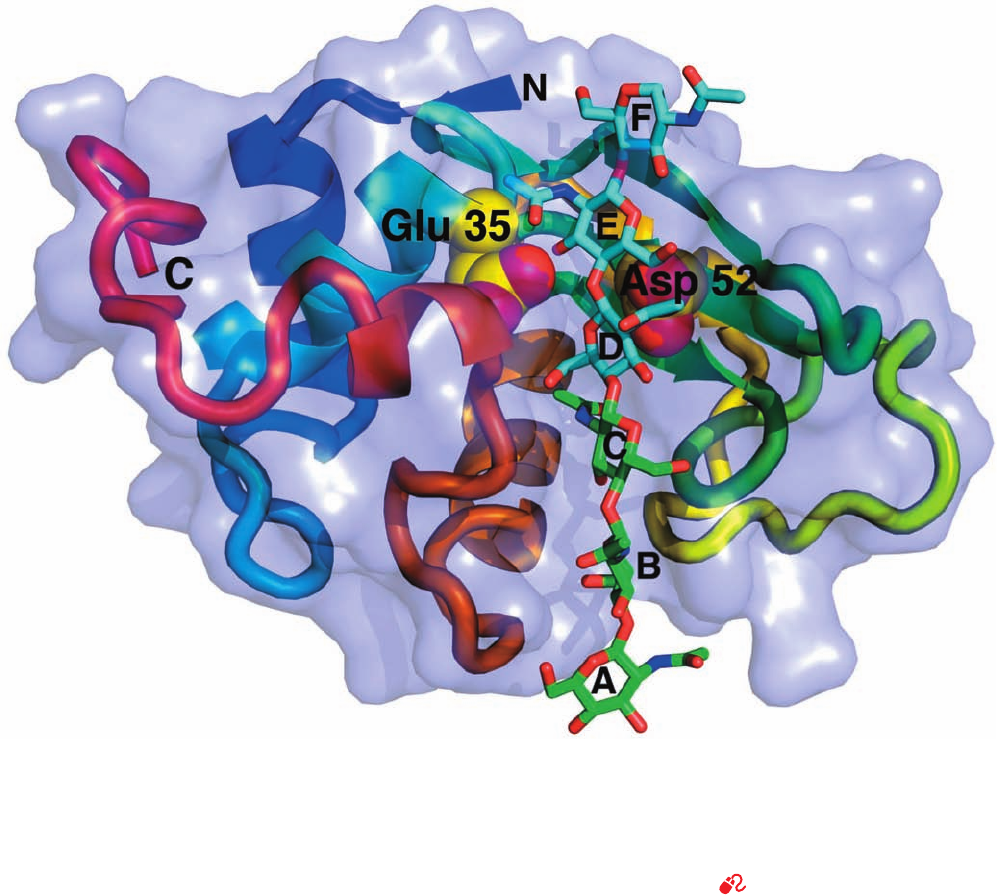
Hen egg white (HEW) lysozyme is the most widely
studied species of lysozyme and is one of the mechanisti-
cally best understood enzymes. It is a rather small (14.7 kD)
and readily available protein (an egg contains ⬃5 g of it),
whose single polypeptide chain consists of 129 amino acid
residues and is internally cross-linked by four disulfide
bonds (Fig. 15-9). HEW lysozyme catalyzes the hydrolysis
of its substrate at a rate that is ⬃10
8
-fold greater than that
of the uncatalyzed reaction.
A. Enzyme Structure
The elucidation of an enzyme’s mechanism of action re-
quires a knowledge of the structure of its enzyme–substrate
complex. This is because, even if the active site residues
have been identified through chemical, physical, and ge-
netic means, their three-dimensional arrangements relative
to the substrate as well as to each other must be known for
an understanding of how the enzyme works. However, an
enzyme binds its good substrates only transiently before it
catalyzes a reaction and releases the products. Conse-
quently, most of our knowledge of enzyme–substrate com-
plexes derives from X-ray studies of enzymes in complex
with inhibitors or poor substrates that remain stably bound
to the enzyme for the several hours that are usually re-
quired to measure a protein crystal’s X-ray diffraction in-
tensities (although techniques for measuring X-ray intensi-
ties in less than 1 s have been developed). The large
solvent-filled channels that occupy much of the volume of
most protein crystals (Section 8-3Aa) often permit the for-
mation of enzyme–inhibitor complexes by the diffusion of
inhibitor molecules into crystals of the native protein.
The X-ray structure of HEW lysozyme,which was eluci-
dated by David Phillips in 1965, was the second structure of
a protein and the first of an enzyme to be determined at
Section 15-2. Lysozyme 517
Figure 15-8 The alternating NAG–NAM polysaccharide component of bacterial cell walls.
The position of the lysozyme cleavage site is shown.
Figure 15-9 Primary structure of HEW lysozyme. The amino
acid residues that line the substrate-binding pocket are shown in
dark purple.
H
H
H
H
H
HH
O
H
O
O
O
O
...
...
Lysozyme
cleavage
O
NAG NAM NAG NAM
14
OH
CH
2
OH
H
H
H NH
O
C CH
3
O
CH
3
CHCOO
–
2
3
5
6
1
CH
2
OH
H
H
H NH
O
C CH
3
O
OH
CH
2
OH
H
H
H NH
O
C CH
3
O
CH
2
OH
H
H
H NH
O
C CH
3
O
CH
3
CHCOO
–
O
H
3
N
+
COO
–
S
S
S
S
S
S
S
S
1
10
20
120
30
129
110
100
70
50
90
40
80
60
Lys
Val
Phe
Gly
Arg
Cys
Gly
Arg
Arg
Cys
Glu
Leu
Ala
Ala
Ala
Met
Lys
Arg
His
Gly
Leu
Asp
Gln
Ala
Trp
Ile
Asp
Val
Asn
Asn
Trp
Val
Cys
Cys
Leu
Ala
Ala
Lys
Lys
Phe
Asn
Thr
Gln
Ala
Arg
Asn
Thr
Asp
Gly
Ser
Thr
Tyr
Gly
Ile
Ile
Ser
Ser
Ser
Ser
Ser
Asp
Ile
Ile
Thr
Ala
Ala
Lys
Lys
Val
Ser
Val
Asn
Ala
Leu
Leu
Arg
Cys
Cys
Cys
Cys
Asp
Asn
Asn
Gly
Met
Gly
Gly
Arg
Ser
Arg
Arg
Trp
Asn
Asn
Asn
Thr
Pro
Pro
Ile
Thr
Phe
Glu
Ser
Asn
Asn
Asp
Leu
Gln
Asn
Trp
Trp
Leu
Asp
Gly
Asp
Val
Trp
Ala
Ala
Arg
Tyr
Tyr
Thr
Ser
Leu
Arg
Gly
Gly
Gly
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 517
high resolution.The protein molecule is roughly ellipsoidal
in shape with dimensions 30 ⫻ 30 ⫻ 45 Å (Fig. 15-10). Its
most striking feature is a prominent cleft, the substrate-
binding site, that traverses one face of the molecule. The
polypeptide chain contains five helical segments as well as
a three-stranded antiparallel  sheet that comprises much
of one wall of the binding cleft. As expected, most of the
nonpolar side chains are in the interior of the molecule, out
of contact with the aqueous solvent.
a. The Nature of the Binding Site
NAG oligosaccharides of less than five residues are but
very slowly hydrolyzed by HEW lysozyme (Table 15-2), al-
though these substrate analogs bind to the enzyme’s active
site and are thus its competitive inhibitors. The X-ray struc-
ture of the (NAG)
3
–lysozyme complex reveals that (NAG)
3
is bound at substrate subsites A, B, and C in Fig. 15-10.This
inhibitor associates with the enzyme through strong hydrogen
bonding interactions, some of which involve the acetamido
groups of residues A and C, as well as through close-fitting
hydrophobic contacts. In an example of induced-fit ligand
binding (Section 10-4C), there is a slight (⬃1 Å) closure of
lysozyme’s binding cleft on binding (NAG)
3
.
b. Lysozyme’s Catalytic Site Was Identified through
Model Building
(NAG)
3
takes several weeks to hydrolyze under the in-
fluence of lysozyme. It was therefore assumed that the
complex revealed by X-ray analysis is unproductive; that is,
the enzyme’s catalytic site occurs at neither the nor
the glycosidic bonds. [Presumably, the rare occasions
when (NAG)
3
is hydrolyzed occur when it binds produc-
tively at the catalytic site.]
In order to locate lysozyme’s catalytic site, Phillips used
model building to investigate how a larger substrate could bind
to the enzyme. Lysozyme’s active site cleft is long enough to
B¬C
A¬B
518 Chapter 15. Enzymatic Catalysis
Figure 15-10 X-ray structure of HEW lysozyme in complex
with (NAG)
6
. The protein is represented by its transparent
molecular surface with its polypeptide chain in ribbon form
colored in rainbow order from its N-terminus (blue) to its
C-terminus (red).The (NAG)
6
, which is drawn in stick form with
its sugar residues designated A, at its nonreducing end, through
F, at its reducing end, binds in a deep cleft in the enzyme surface.
Rings A, B, and C (colored according to atom type with C green,
N blue, and O red) are observed in the X-ray structure of the
complex of (NAG)
3
with lysozyme; the positions of rings D, E,
and F (C cyan, N blue, O red) were inferred by model building.
The side chains of lysozyme’s active site residues, Glu 35 and Asp
52, which are drawn in space-filling form (C yellow, O red),
catalyze the hydrolysis of the glycosidic bond between rings D
and E. [Based on an X-ray structure by David Phillips, Oxford
University. PDBid 1HEW.]
See Interactive Exercise 6 and
Kinemage Exercise 9.
JWCL281_c15_506-556.qxd 10/19/10 7:20 AM Page 518
accommodate (NAG)
6
, which the enzyme rapidly hydrolyzes
(Table 15-2). However, the fourth NAG residue (the D-ring in
Fig. 15-10) appeared unable to bind to the enzyme because its
C6 and O6 atoms too closely contact Glu 35, Trp 108, and the
acetamido group of the C-ring. This steric interference could
be relieved by distorting the glucose ring from its normal chair
conformation to that of a half-chair (Fig. 15-11). This distortion,
which renders atoms C1, C2, C5, and O5 of residue D coplanar,
moves the ¬C6H
2
OH group from its normal equatorial posi-
tion to an axial position where it makes no close contacts and can
hydrogen bond to the backbone carbonyl group of Gln 57 and
the amido group of Val 109 (Fig. 15-12). Continuing the model
building,Phillips found that the E- and F-rings apparently bind
to the enzyme without distortion and with a number of favor-
able hydrogen bonding and van der Waals contacts.
We are almost in a position to identify lysozyme’s cat-
alytic site. In the enzyme’s natural substrate, every second
Section 15-2. Lysozyme 519
Table 15-2 Rates of HEW Lysozyme-Catalyzed Hydrolysis
of Selected Oligosaccharide Substrate Analogs
Compound k
cat
(s
⫺1
)
(NAG)
2
2.5 ⫻ 10
⫺8
(NAG)
3
8.3 ⫻ 10
⫺6
(NAG)
4
6.6 ⫻ 10
⫺5
(NAG)
5
0.033
(NAG)
6
0.25
(NAG–NAM)
3
0.5
Source: Imoto, T., Johnson, L.N., North,A.C.T., Phillips, D.C., and Rupley,
J.A., in Boyer, P.D. (Ed.), The Enzymes (3rd ed.),Vol. 7, p. 842, Academic
Press (1972).
Figure 15-11 Chair and half-chair conformations. Hexose
rings normally assume the chair conformation. It is postulated,
however, that binding by lysozyme distorts the D-ring into the
half-chair conformation such that atoms C1, C2, C5, and O5 are
coplanar.
See the Animated Figures
Figure 15-12 Interactions of lysozyme with its substrate. The
view is into the binding cleft with the heavier edges of the rings
facing the outside of the enzyme and the lighter ones against the
bottom of the cleft. [Illustration, Irving Geis. Image from the Irving
Geis Collection, Howard Hughes Medical Institute. Reprinted with
permission.] Based on an X-ray structure by David Phillips, Oxford
University, U.K. PDBid 4LYZ.]
See Kinemage Exercise 9
Chair conformation
C4
C5
C2
C3
C1
O5
C4
Half-chair conformation
C5
C1
C2
C3
O5
O
O
O
O
OH
O
C
D
CH
2
O
CH
2
OH
C
C
C
Ala
107
Asn
59
Trp
63
Trp
62
NAG
NAG
D ring in
half-chair
conformation
NAM
O
CH
2
H
3
C
H
3
C
H
HN
N
CH
2
O
N
H
Lysozyme cuts
H
B
A
NH
H
HO
O
O
C
O
HN
Asp 101
O
O
C
NH
HN
O
C
H
3
C
O
C
C
H
3
C
N
H
OR
OR
O
O
O
C
Gln
57
Gln
57
O
C
Phe
34
O
O
Glu
35
C
O
CH
2
OH
NAG
NAM
NAM
CH
2
CH
3
NH
2
F
E
O
H
O
O
O
O
C
C
H
3
C
N
H
N
H
H
OR
O
O
H
Asn
44
NH
2
O
C
Asn
37
Arg
114
H
2
N
H
2
N
H
2
N
–
+
NH
H
Val
109
H
O
O
O
Asp 52
C
–
O
O
H
Glu 35
C
O
O
JWCL281_c15_506-556.qxd 8/10/10 10:27 AM Page 519
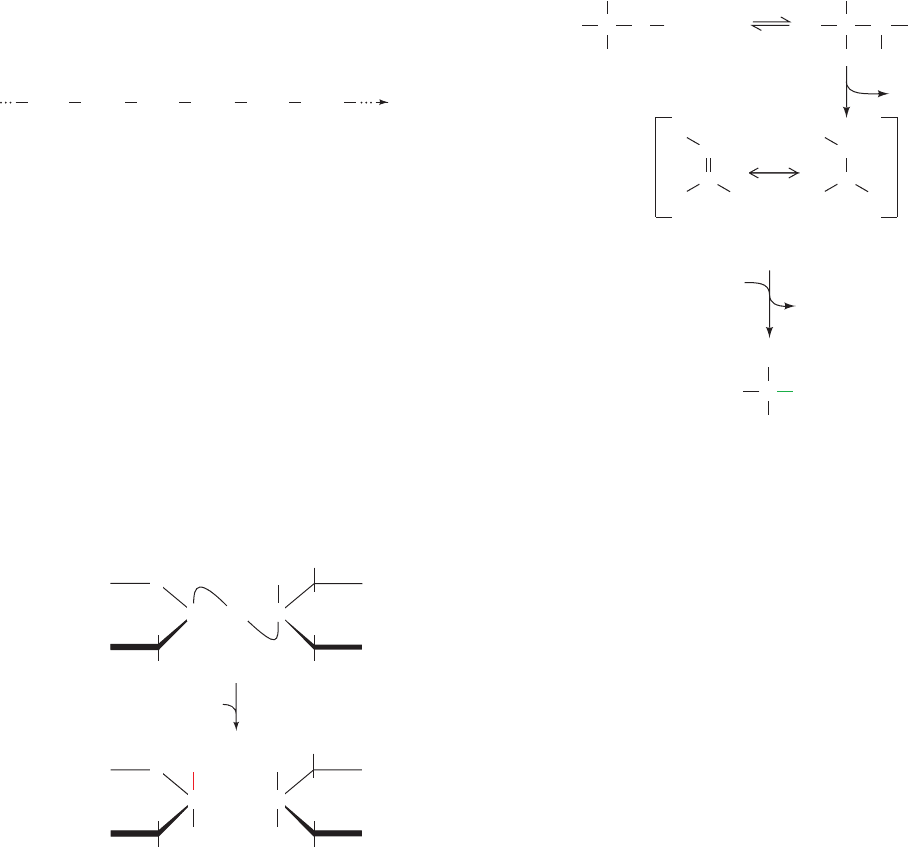
residue is an NAM. Model building, however, indicated
that its lactyl side chain cannot be accommodated in the
binding subsites of either residues C or E. Hence,the NAM
residues must bind to the enzyme in subsites B, D, and F.
The observation that lysozyme hydrolyzes (1 S 4) linkages
from NAM to NAG indicates that bond cleavage occurs
either between rings B and C or rings D and E. Since (NAG)
3
is stably bound to but not cleaved by the enzyme while span-
ning subsites B and C, the probable cleavage site is between
rings D and E.This conclusion is supported by John Rupley’s
observation that lysozyme nearly quantitatively hydrolyzes
(NAG)
6
between the second and third residues from its re-
ducing terminus (the end with a free C1¬OH), just as is ex-
pected if the enzyme has six saccharide-binding subsites and
cleaves its bound substrate between rings D and E.
The bond that lysozyme cleaves was identified by carry-
ing out the lysozyme-catalyzed hydrolysis of (NAG)
3
in
H
2
18
O. The resulting product had
18
O bonded to the C1
atom of its newly liberated reducing terminus, thereby
demonstrating that bond cleavage occurs between C1 and
the bridge oxygen O1:
Thus, lysozyme catalyzes the hydrolysis of the C1¬O1
bond of a bound substrate’s D residue. Moreover,this reac-
tion occurs with retention of configuration, so that the D-
ring product remains the anomer.
B. Catalytic Mechanism
It remains to identify lysozyme’s catalytic groups.The reac-
tion catalyzed by lysozyme, the hydrolysis of a glycoside, is
the conversion of an acetal to a hemiacetal. Nonenzymatic
acetal hydrolysis is an acid-catalyzed reaction that involves
the protonation of a reactant oxygen atom followed by
cleavage of its C¬O bond (Fig. 15-13). This results in the
formation of a resonance-stabilized carbocation that is
called an oxonium ion. To attain maximum orbital overlap,
and thus resonance stabilization, the oxonium ion’s R and
R¿ groups must be coplanar with its C, O, and H atoms
(stereoelectronic assistance). The oxonium ion then adds
water to yield the hemiacetal and regenerate the acid cata-
lyst. In searching for catalytic groups on an enzyme that
mediates acetal hydrolysis, we should therefore seek a
H
NAc
O
C
1
H
NAc
O
C
H
18
OH
+
OH
H
C
HO
CH
2
OH
H
OH
H
C
4
H
CH
2
OH
H
O
1
lysozyme
18
OH
2
H
DE
DE
NAG NAM
A
NAG
C
NAG
EB
NAM
D
NAM
F
reducing
end
()
potential acid catalyst and possibly a group that could fur-
ther stabilize an oxonium ion intermediate.
a. Glu 35 and Asp 52 Are Lysozyme’s
Catalytic Residues
The only functional groups in the immediate vicinity of
lysozyme’s reaction center that have the required catalytic
properties are the side chains of Glu 35 and Asp 52,
residues that are invariant in the family of lysozymes of
which HEW lysozyme is the prototype. These side chains,
which are disposed to either side of the (1 S 4) glycosidic
linkage to be cleaved (Fig. 15-10), have markedly different
environments. Asp 52 is surrounded by several conserved
polar residues with which it forms a complex hydrogen
bonded network. Asp 52 is therefore predicted to have a
normal pK; that is, it should be unprotonated and hence
negatively charged throughout the 3 to 8 pH range in which
lysozyme is catalytically active. In contrast, the carboxyl
group of Glu 35 is nestled in a predominantly nonpolar
pocket, where, as we discussed in Section 15-1D, it is likely to
remain protonated at unusually high pH’s for carboxyl
groups. Indeed, neutron diffraction studies, which provide
similar information to X-ray diffraction studies but also
reveal the positions of hydrogen atoms, indicate that Glu
35 is protonated at physiological pH. The closest ap-
proaches in the X-ray structures between the carboxyl O
atoms of both Asp 52 and Glu 35 and the C1¬O1 bond of
NAG D-ring are ⬃3 Å, which makes them the prime candi-
dates for electrostatic and acid catalysts, respectively.
520 Chapter 15. Enzymatic Catalysis
C
OR
H O R
O
+
R
R
+ H
+
C
OR
HO
H
+
R
C
HR
O
R
C
+
HR
ROH
Acetal
Resonance-stabilized
carbocation (oxonium ion)
H
2
O
C
OR
H OH
R
Hemiacetal
H
+
R
Figure 15-13 Mechanism of the nonenzymatic acid-catalyzed
hydrolysis of an acetal to a hemiacetal. The reaction involves the
protonation of one of the acetal’s oxygen atoms followed by
cleavage of its bond to form an alcohol (R–OH) and a
resonance-stabilized carbocation (oxonium ion).The addition of
water to the oxonium ion forms the hemiacetal and regenerates
the H
catalyst. Note that the oxonium ion’s C, O, H, R, and R¿
atoms all lie in the same plane.
C¬O
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 520
