Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

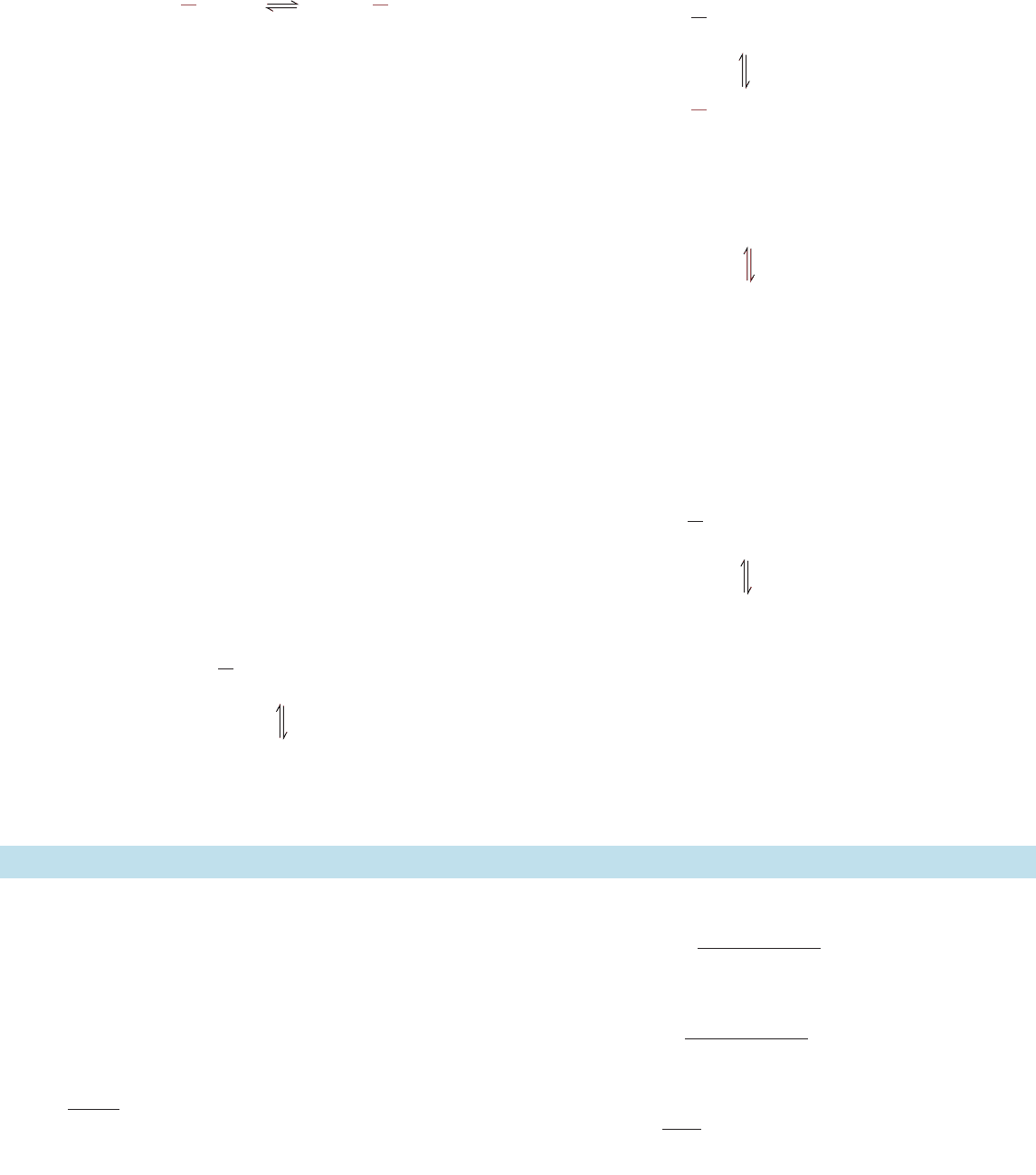
the absence of the second substrate. Consider an overall
Ping Pong reaction catalyzed by the bisubstrate enzyme E
in which, as usual, A ⫽ P¬X, Q ⫽ B¬X, and X is the
group that is transferred from one substrate to the other in
the course of the reaction. Only the first step of the reac-
tion can take place in the absence of B. If a small amount of
isotopically labeled P, denoted P*, is added to this reaction
mixture then, in the reverse reaction, P*¬X will form:
that is, isotopic exchange will occur.
In contrast, let us consider the first step of a Sequential
reaction. Here a noncovalent enzyme–substrate complex
forms:
Addition of P* cannot result in an exchange reaction
because no covalent bonds are broken in the formation of
E ⴢ P¬X; that is, there is no P released from the enzyme to
exchange with P*.The demonstration of isotopic exchange
for a bisubstrate enzyme is therefore convincing evidence
favoring a Ping Pong mechanism.
a. Isotope Exchange in Sucrose Phosphorylase and
Maltose Phosphorylase
The enzymes sucrose phosphorylase and maltose phos-
phorylase provide two clear-cut examples of how enzymat-
ically catalyzed isotopic exchange reactions are used to dif-
ferentiate kinetic mechanisms. Sucrose phosphorylase
catalyzes the overall reaction
Glucose
Glucose-1-phosphate fructose+
fructose phosphate+
Sucrose
E
E ⫹ P¬X Δ E ⴢ P¬X
Reverse reaction
E—X⫹ P*
¡
E ⫹ P*—X
Forward reaction
E ⫹ P—X
¡
E—X⫹ P
PPX
X
BB++
E
If the enzyme is incubated with sucrose and isotopically la-
beled fructose in the absence of phosphate, it is observed
that the label passes into the sucrose:
For the reverse reaction, if the enzyme is incubated with
glucose-1-phosphate and
32
P-labeled phosphate, this label
exchanges into the glucose-1-phosphate:
These observations indicate that a tight glucosyl–enzyme
complex is formed with the release of fructose, thereby es-
tablishing that the sucrose phosphorylase reaction occurs
via a Ping Pong mechanism. This finding has been conclu-
sively corroborated by the isolation and characterization of
the glucosyl–enzyme complex.
The enzyme maltose phosphorylase catalyzes a similar
overall reaction:
In contrast to sucrose phosphorylase, however, it does not
catalyze isotopic exchange between glucose-1-phosphate
and [
32
P]phosphate or between maltose and [
14
C]glucose.
Likewise, a glucosyl–enzyme complex has not been de-
tected. This evidence is consistent with maltose phos-
phorylase having a sequential mechanism.
Glucose glucose phosphate+
E
Glucose-1-phosphate glucose+
Maltose
Glucose-1-phosphate phosphate*+
Glucose-1-phosphate* phosphate+
E
Glucose fructose fructose*+
Glucose fructose* fructose+
Sucrose
E
Appendix 501
A. The Michaelis–Menten Equation for Reversible
Reactions—Equation [14.30]
The conservation condition for the reversible reaction with
one intermediate (Section 14-2Ca) is
[14.A1]
The steady-state condition (as well as the equilibrium con-
dition) is
[14.A2]
d[ES]
dt
⫽ k
1
[E][S] ⫹ k
⫺2
[E][P] ⫺ (k
⫺1
⫹ k
2
)[ES] ⫽ 0
[E]
T
⫽ [E] ⫹ [ES]
so that
[14.A3]
Substituting this result into Eq. [14.A1] yields
[14.A4]
The velocity of the reaction is expressed
[14.A5]v ⫽⫺
d[S]
dt
⫽ k
1
[E][S] ⫺ k
⫺1
[ES]
[E]
T
⫽ a
k
⫺1
⫹ k
2
k
1
[S] ⫹ k
⫺2
[P]
⫹ 1b[ES]
[E] ⫽ a
k
⫺1
⫹ k
2
k
1
[S] ⫹ k
⫺2
[P]
b[ES]
APPENDIX Derivations of Michaelis–Menten Equation Variants
JWCL281_c14_482-505.qxd 2/22/10 8:46 AM Page 501
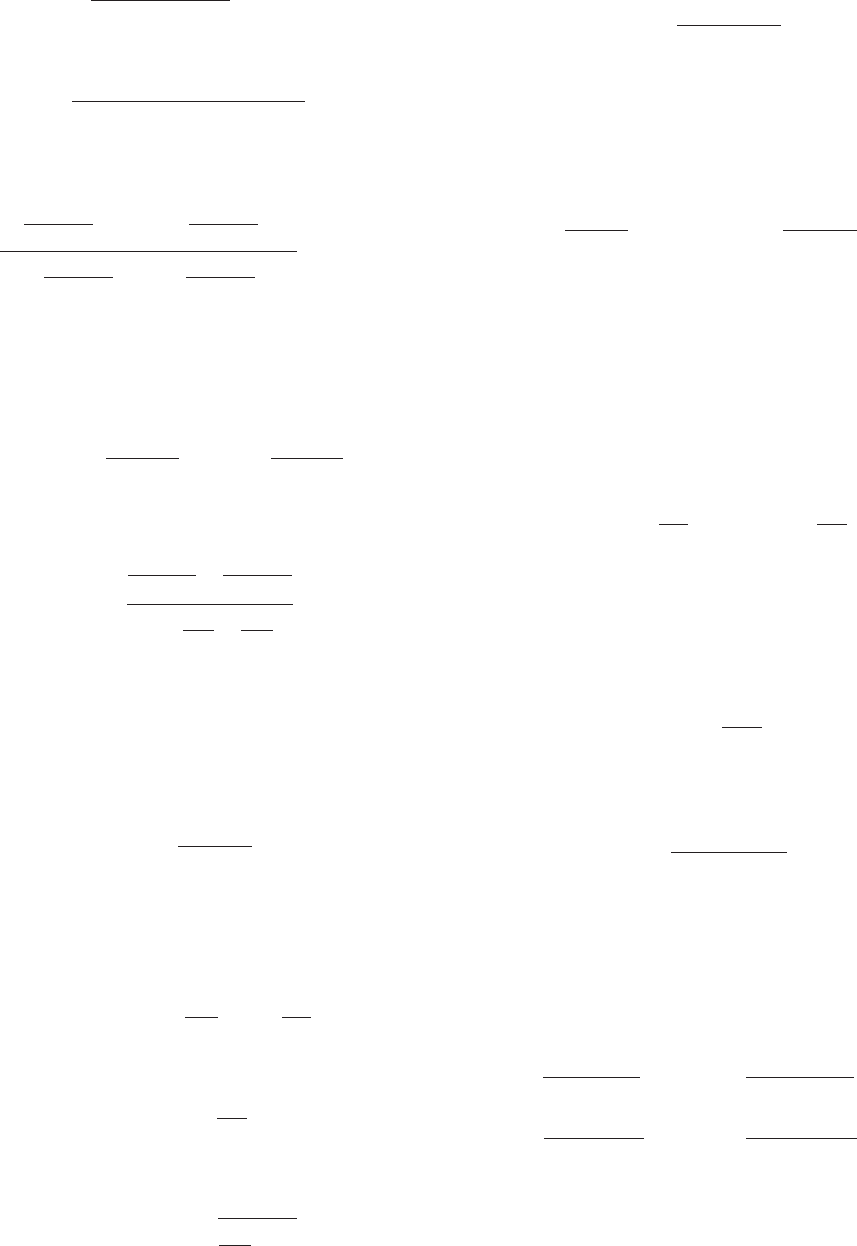
which can be combined with Eq. [14.A3] to give
[14.A6]
which, in turn, is combined with Eq. [14.A4] to yield
[14.A7]
Dividing the numerator and denominator of this equation
by (k
–1
⫹ k
2
) results in
[14.A8]
Then, if we define the following parameters analogously
with the constants of the Michaelis–Menten equation (Eqs.
[14.23] and [14.21]),
we obtain the Michaelis–Menten equation for a reversible
one-intermediate reaction:
[14.30]
B. Michaelis–Menten Equation for Uncompetitive
Inhibition—Equation [14.41]
For uncompetitive inhibition (Section 14-3B), the inhibitor
binds to the Michaelis complex with dissociation constant
[14.A9]
The conservation condition is
[14.A10]
Substituting in Eqs. [14.34] and [14.A9] yields
[14.A11]
Defining ␣¿ analogously to Eq. [14.37] as
[14.A12]
and v
o
and V
max
as in Eqs. [14.22] and [14.23], respectively,
[14.A13]v
o
⫽ k
2
[ES] ⫽
V
max
K
M
[S]
⫹ a¿
a¿ ⫽ 1 ⫹
[I]
K¿
I
[E]
T
⫽ [ES]a
K
M
[S]
⫹ 1 ⫹
[I]
K¿
I
b
[E]
T
⫽ [E] ⫹ [ES] ⫹ [ESI]
K¿
I
⫽
[ES][I]
[ESI]
v ⫽
V
f
max
[S]
K
S
M
⫺
V
r
max
[P]
K
P
M
1 ⫹
[S]
K
S
M
⫹
[P]
K
P
M
K
S
M
⫽
k
⫺1
⫹ k
2
k
1
K
P
M
⫽
k
⫺1
⫹ k
2
k
⫺2
V
f
max
⫽ k
2
[E]
T
V
r
max
⫽ k
⫺1
[E]
T
v⫽ ±
k
2
a
k
1
k
⫺1
⫹ k
2
b[S] ⫺ k
⫺1
a
k
⫺2
k
⫺1
⫹ k
2
b[P]
1⫹ a
k
1
k
⫺1
⫹ k
2
b[S] ⫹ a
k
⫺2
k
⫺1
⫹ k
2
b[P]
≤[E]
T
v ⫽ a
k
1
k
2
[S] ⫺ k
⫺1
k
⫺2
[P]
k
⫺1
⫹ k
2
⫹ k
1
[S] ⫹ k
⫺2
[P]
b[E]
T
v ⫽ a
k
1
[S](k
⫺1
⫹ k
2
)
k
1
[S] ⫹ k
⫺2
[P]
⫺ k
⫺1
b[ES]
which on rearrangement yields the Michaelis–Menten
equation for uncompetitive inhibition:
[14.41]
C. The Michaelis–Menten Equation for Mixed
Inhibition—Equation [14.45]
In mixed inhibition (Section 14-3C), the inhibitor-binding
steps have different dissociation constants:
[14.A14]
(Here, for the sake of mathematical simplicity, we are mak-
ing the thermodynamically unsupportable assumption that
EI does not react with S to form ESI. Inclusion of this reac-
tion requires a more complex derivation than that given
here but leads to results that are substantially the same.)
The conservation condition for this reaction scheme is
[14.A15]
so that substituting in Eqs. [14.A14]
[14.A16]
Defining ␣ and ␣¿ as in Eqs. [14.37] and [14.A12], respec-
tively, Eq. [14.A16] becomes
[14.A17]
Then substituting in Eq. [14.34]
[14.A18]
Defining v
o
and V
max
as in Eqs. [14.22] and [14.23] results in
the Michaelis–Menten equation for mixed inhibition:
[14.45]
D. The Michaelis–Menten Equation for Ionizable
Enzymes—Equation [14.47]
In the model presented in Section 14-4a to account for the
effect of pH on enzymes, the dissociation constants for
the ionizations are
[14.A19]
Protonation and deprotonation are among the fastest
known reactions, so that, with the exception of the few
enzymes with extremely high turnover numbers, it can be
reasonably assumed that all acid–base reactions are at
K
E1
⫽
[H
⫹
] [EH]
[EH
⫹
2
]
K
ES1
⫽
[H
⫹
] [ESH]
[ESH
⫹
2
]
K
E2
⫽
[H
⫹
][E
⫺
]
[EH]
K
ES2
⫽
[H
⫹
][ES
⫺
]
[ESH]
v
o
⫽
V
max
[S]
aK
M
⫹ a¿[S]
[E]
T
⫽ [ES]a
aK
M
[S]
⫹ a¿b
[E]
T
⫽ [E]a ⫹ [ES]a¿
[E]
T
⫽ [E]a1 ⫹
[I]
K
I
b⫹ [ES]a1 ⫹
[I]
K¿
I
b
[E]
T
⫽ [E] ⫹ [EI] ⫹ [ES] ⫹ [ESI]
K
I
⫽
[E][I]
[EI]
and
K¿
I
⫽
[ES][I]
[ESI]
v
o
⫽
V
max
[S]
K
M
⫹ a¿[S]
502 Chapter 14. Rates of Enzymatic Reactions
JWCL281_c14_482-505.qxd 6/3/10 12:17 PM Page 502
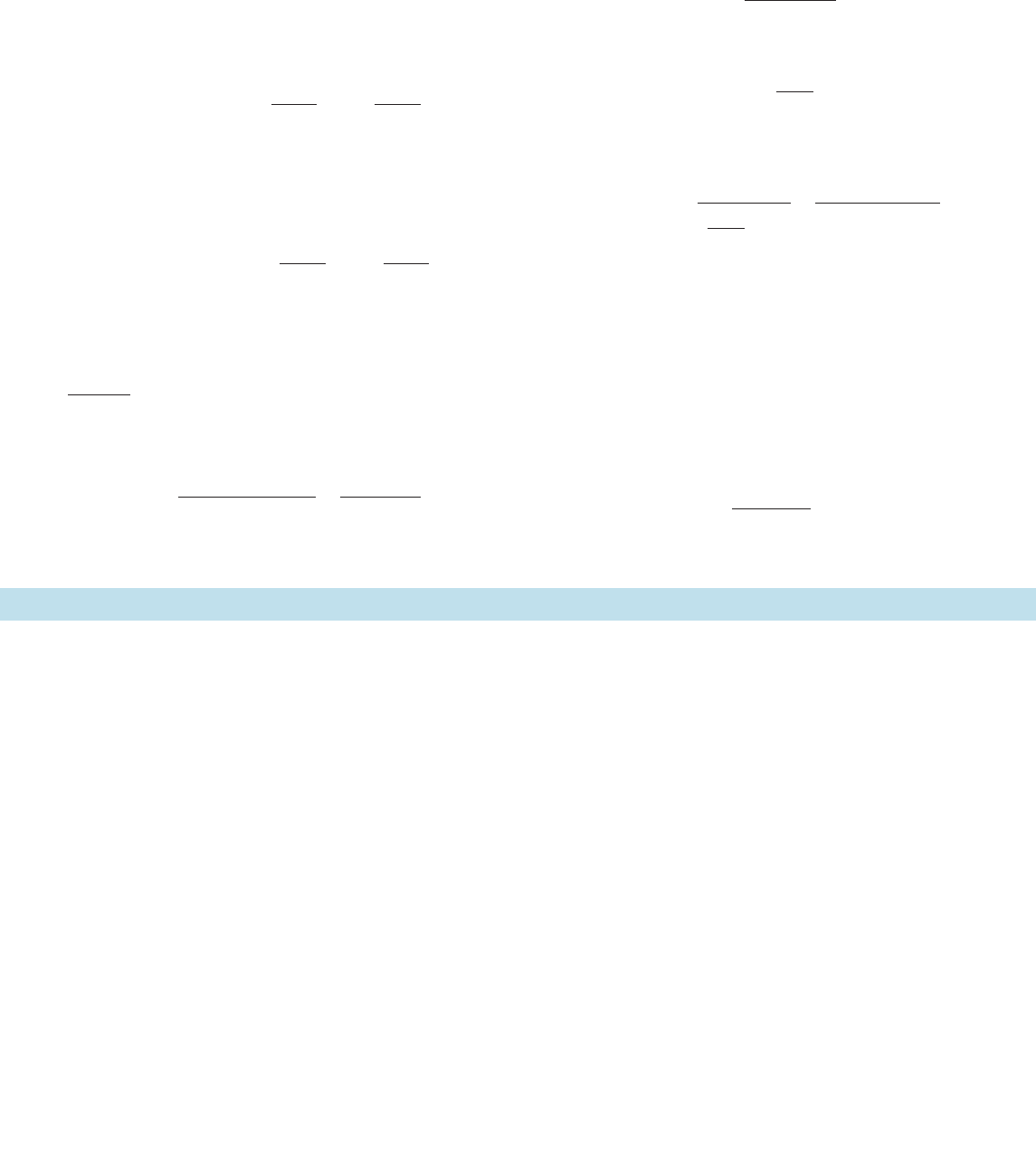
equilibrium.The conservation condition is
[14.A20]
where [E]
T
is the total enzyme present in any form,
[14.A21]
and
[14.A22]
Then making the steady-state assumption
[14.A23]
and solving for [EH]
[14.A24][EH] ⫽
(k
⫺1
⫹ k
2
)[ESH]
k
1
[S]
⫽
K
M
[ESH]
[S]
d[ESH]
dt
⫽ k
1
[EH][S] ⫺ (k
⫺1
⫹ k
2
)[ESH] ⫽ 0
⫽ [ESH]f
2
⫽ [ESH]a
[H
⫹
]
K
ES1
⫹ 1 ⫹
K
ES2
[H
⫹
]
b
[ ESH ]
T
⫽ [ESH
⫹
2
] ⫹ [ESH] ⫹ [ES
⫺
]
⫽ [EH]f
1
⫽ [EH]a
[H
⫹
]
K
E1
⫹ 1 ⫹
K
E2
[H
⫹
]
b
[EH]
T
⫽ [EH
⫹
2
] ⫹ [EH] ⫹ [E
⫺
]
[E]
T
⫽ [EH]
T
⫹ [ESH]
T
Therefore, from Eq. [14.A21],
[14.A25]
which, together with Eqs. [14.A20] and [14.A22], yields
[14.A26]
As in the simple Michaelis–Menten derivation, the initial
rate is
[14.A27]
Then defining the “apparent” values of K
M
and V
max
⫽
k
2
[E]
T
at a given pH:
[14.A28]
and
[14.A29]
the Michaelis–Menten equation modified to account for
pH effects is
[14.47]v
o
⫽
V¿
max
[S]
K¿
M
⫹ [S]
V¿
max
⫽ V
max
>f
2
K¿
M
⫽ K
M
(f
1
>f
2
)
v
o
⫽ k
2
[ESH] ⫽
k
2
[E]
T
a
K
M
f
1
[S]
b⫹ f
2
⫽
(k
2
>f
2
)[E]
T
[S]
K
M
(f
1
>f
2
) ⫹ [S]
[E]
T
⫽ [ESH] a
K
M
f
1
[S]
⫹ f
2
b
[EH]
T
⫽
K
M
[ESH]f
1
[S]
Chapter Summary 503
1 Chemical Kinetics Complicated reaction processes
occur through a series of elementary reaction steps defined as
having a molecularity equal to the number of molecules that
simultaneously collide to form products. The order of a reac-
tion can be determined from the characteristic functional form
of its progress curve.Transition state theory postulates that the
rate of a reaction depends on the free energy of formation of
its activated complex. This complex, which occurs at the free
energy maximum of the reaction coordinate, is poised be-
tween reactants and products and is therefore also known as
the transition state. Transition state theory explains that catal-
ysis results from the reduction of the free energy difference
between the reactants and the transition state.
2 Enzyme Kinetics In the simplest enzymatic mecha-
nism, the enzyme and substrate reversibly combine to form an
enzyme–substrate complex known as the Michaelis complex,
which may irreversibly decompose to form product and the re-
generated enzyme. The rate of product formation is expressed
by the Michaelis–Menten equation, which is derived under the
assumption that the concentration of the Michaelis complex is
constant, that is, at a steady state.The Michaelis–Menten equa-
tion, which has the functional form of a rectangular hyperbola,
has two parameters: V
max
, the maximal rate of the reaction,
which occurs when the substrate concentration is saturating,
and K
M
, the Michaelis constant, which has the value of the
substrate concentration at the half-maximal reaction rate.
These parameters may be graphically determined using the
Lineweaver–Burk plot. Physically more realistic models of
enzyme mechanisms than the Michaelis–Menten model as-
sume the enzymatic reaction to be reversible and to have one
or more intermediates. The functional form of the equations
describing the reaction rates for these models is independent
of their number of intermediates, so that the models cannot be
differentiated using only steady-state kinetic measurements.
3 Inhibition Enzymes may be inhibited by competitive
inhibitors, which compete with the substrate for the enzymatic
binding site. The effect of a competitive inhibitor may be re-
versed by increasing the substrate concentration. An uncom-
petitive inhibitor inactivates a Michaelis complex on binding
to it. The maximal rate of an uncompetitively inhibited en-
zyme is a function of inhibitor concentration, and therefore
the effect of an uncompetitive inhibitor cannot be reversed by
increasing substrate concentration. In mixed inhibition, the in-
hibitor binds to both the enzyme and the enzyme–substrate
complex to form a complex that is catalytically inactive. The
rate equation describing this situation has characteristics of
both competitive and uncompetitive reactions.
4 Effects of pH The rate of an enzymatic reaction is a
function of hydrogen ion concentration. At any pH, the rate
of a simple enzymatic reaction can be described by the
Michaelis–Menten equation. However, its parameters V
max
and K
M
vary with pH. By the evaluation of kinetic rate curves
CHAPTER SUMMARY
JWCL281_c14_482-505.qxd 2/19/10 2:22 PM Page 503

as a function of pH, the pK’s of an enzyme’s ionizable binding
and catalytic groups can be determined, which may help iden-
tify these groups.
5 Bisubstrate Reactions The majority of enzymatic reac-
tions are bisubstrate reactions in which two substrates react to
form two products. Bisubstrate reactions may have Ordered or
Random Sequential mechanisms or Ping Pong Bi Bi mecha-
nisms, among others. The initial rate equations for any of these
mechanisms involve five parameters, which are analogous to
either Michaelis–Menten equation parameters or equilibrium
constants. The various bisubstrate mechanisms may be experi-
mentally differentiated according to the forms of their double-
reciprocal plots and from the nature of their product inhibition
patterns. Isotope exchange reactions provide an additional,
nonkinetic method of differentiating bisubstrate mechanisms.
Chemical Kinetics
Atkins, P.W. and de Paula, J., Physical Chemistry for the Life Sci-
ences, Chapters 6–8, Freeman (2006). [Most physical chemistry
textbooks have similar coverage.]
Hammes, G.G., Principles of Chemical Kinetics, Academic Press
(1978).
Laidler, K.J., Chemical Kinetics (3rd ed.), Harper & Row (1987).
Enzyme Kinetics
Biswanger,H., Enzyme Kinetics: Principles and Methods (2nd ed.),
Wiley–VCH (2008).
Cleland, W.W., Steady state kinetics, in Boyer, P.D. (Ed.), The En-
zymes (3rd ed.), Vol. 2, pp. 1–65, Academic Press (1970); and
Steady-state kinetics, in Sigman, D.S. and Boyer, P.D. (Eds.),The
Enzymes (3rd ed.),Vol. 19, pp. 99–158, Academic Press (1990).
Cleland, W.W., Determining the mechanism of enzyme-catalyzed
reactions by kinetic studies, Adv. Enzymol. 45, 273 (1977).
Cornish-Bowden, A., Fundamentals of Enzyme Kinetics (Revised
ed.), Portland Press (1995). [A lucid and detailed account of
enzyme kinetics.]
Copeland, R.A., Enzymes, VCH (1996).
Dixon, M. and Webb, E.C., Enzymes (3rd ed.), Chapter IV,
Academic Press (1979). [An almost exhaustive treatment of
enzyme kinetics.]
Fersht, A., Structure and Mechanism in Protein Science, Chapters
3–7, Freeman (1999).
Gutfreund, H., Kinetics for the Life Sciences: Receptors,Transmit-
ters, and Catalysts, Cambridge University Press (1995).
Knowles, J.R.,The intrinsic pK
a
-values of functional groups in en-
zymes: Improper deductions from the pH-dependence of
steady state parameters, CRC Crit. Rev. Biochem. 4, 165
(1976).
Marangoni, A.G., Enzyme Kinetics. A Modern Approach, Wiley
(2002).
Piszkiewicz, D., Kinetics of Chemical and Enzyme Catalyzed Reac-
tions, Oxford University Press (1977). [A highly readable dis-
cussion of enzyme kinetics.]
Purich, D.L. (Ed.), Contemporary Enzyme Kinetics and Mecha-
nism (2nd ed.),Academic Press (1996) [A collection of articles
on advanced topics.]
Schulz,A.R., Enzyme Kinetics, Cambridge (1994).
Segel, I.H., Enzyme Kinetics, Wiley–Interscience (1993). [A
detailed and understandable treatise providing full explana-
tions of many aspects of enzyme kinetics.]
Tinoco, I., Jr., Sauer, K., Wang, J.C., and Puglisi, J.D., Physical
Chemistry. Principles and Applications for Biological Sciences
(4th ed.), Chapters 7 and 8, Prentice-Hall (2002).
REFERENCES
504 Chapter 14. Rates of Enzymatic Reactions
1. The hydrolysis of sucrose:
takes the following time course.
Sucrose ⫹ H
2
O
¡
glucose ⫹ frutose
PROBLEMS
Time (min) [Sucrose] (M)
0 0.5011
30 0.4511
60 0.4038
90 0.3626
130 0.3148
180 0.2674
Determine the first-order rate constant and the half-life of the re-
action. Why does this bimolecular reaction follow a first-order
rate law? How long will it take to hydrolyze 99% of the sucrose
initially present? How long will it take if the amount of sucrose
initially present is twice that given in the table?
2. By what factor will a reaction at 25°C be accelerated if a cat-
alyst reduces the free energy of its activated complex by 1 kJ ⴢ
mol
⫺1
; by 10 kJ ⴢ mol
⫺1
?
[S] (1) v
o
(2) v
o
(3) v
o
(mM)(M ⴢ s
⫺1
)(M ⴢ s
⫺1
)(M ⴢ s
⫺1
)
1 2.5 1.17 0.77
2 4.0 2.10 1.25
5 6.3 4.00 2.00
10 7.6 5.7 2.50
20 9.0 7.2 2.86
(a)Determine K
M
and V
max
for the enzyme. For each inhibitor de-
termine the type of inhibition and K
I
and/or K¿
I
. What additional
information would be required to calculate the turnover number
3. For a Michaelis–Menten reaction, k
1
⫽ 5 ⫻ 10
7
M
⫺1
ⴢ s
⫺1
,
k
–1
⫽ 2 ⫻ 10
4
s
⫺1
, and k
2
⫽ 4 ⫻ 10
2
s
⫺1
. Calculate K
S
and K
M
for
this reaction. Does substrate binding achieve equilibrium or the
steady state?
*4. The following table indicates the rates at which a substrate
reacts as catalyzed by an enzyme that follows the Michaelis–Menten
mechanism: (1) in the absence of inhibitor; (2) and (3) in the
presence of 10 mM concentration, respectively, of either of two
inhibitors.Assume [E]
T
is the same for all reactions.
JWCL281_c14_482-505.qxd 2/22/10 8:46 AM Page 504
of the enzyme? (b) For [S] ⫽ 5 mM, what fraction of the enzyme
molecules have a bound substrate in the absence of inhibitor, in
the presence of 10 mM inhibitor of type (2), and in the presence of
10 mM inhibitor of type (3)?
*5. Ethanol in the body is oxidized to acetaldehyde
(CH
3
CHO) by liver alcohol dehydrogenase (LADH). Other alco-
hols are also oxidized by LADH. For example, methanol, which is
mildly intoxicating, is oxidized by LADH to the quite toxic prod-
uct formaldehyde (CH
2
O).The toxic effects of ingesting methanol
(a component of many commercial solvents) can be reduced by
administering ethanol. The ethanol acts as a competitive inhibitor
of the methanol by displacing it from LADH. This provides suffi-
cient time for the methanol to be harmlessly excreted by the kid-
neys. If an individual has ingested 100 mL of methanol (a lethal
dose), how much 100 proof whiskey (50% ethanol by volume)
must he imbibe to reduce the activity of his LADH toward
methanol to 5% of its original value? The adult human body con-
tains ⬃40 L of aqueous fluids throughout which ingested alcohols
are rapidly and uniformly mixed. The densities of ethanol and
methanol are both 0.79 g ⴢ cm
⫺3
.Assume the K
M
values of LADH
for ethanol and methanol to be 1.0 ⫻ 10
⫺3
M and 1.0 ⫻ 10
⫺2
M,re-
spectively, and that K
I
⫽ K
M
for ethanol.
6. The K
M
of a Michaelis–Menten enzyme for a substrate is
1.0 ⫻ 10
⫺4
M. At a substrate concentration of 0.2M, v
O
⫽ 43 M ⴢ
min
⫺1
for a certain enzyme concentration. However, with a
substrate concentration of 0.02M, v
o
has the same value. (a) Using
numerical calculations, show that this observation is accurate.
(b) What is the best range of [S] for measuring K
M
?
7. Why are uncompetitive and mixed inhibitors generally con-
sidered to be more effective in vivo than competitive inhibitors?
8. Explain why an exact fit to a kinetic model of the experi-
mental parameters describing a reaction does not prove that the
reaction follows the model.
9. An enzyme that follows the model for pH effects presented
in Section 14-4a has pK
ES1
⫽ 4 and pK
ES2
⫽ 8. What is the pH at
which V¿
max
is a maximum for this enzyme? What fraction of V
max
does V¿
max
achieve at this pH?
10. Derive the initial rate equation for a Rapid Equilibrium
Random Bi Bi reaction.Assume the equilibrium constants K
A
S
and
K
B
S
for binding A and B to the enzyme are independent of whether
the other substrate is bound (an assumption that constrains K
B
M
⫽ K
B
S
in Eq. [14.49]).
*11. Consider the following variation of a Ping Pong Bi Bi
mechanism.
Assume that the substrate-binding reactions are in rapid equilibrium,
that both [A] ⬎⬎ [E]
T
and [B] ⬎⬎ [E]
T
, that neither product re-
lease reaction is reversible, and that the steady-state approxima-
tion is valid. (a) Derive an expression for v
o
in terms of K
A
S
, K
B
S
, k
2
,
and k
4
. (b) Indicate the form of the double-reciprocal plots for
1/v
o
versus 1/[A] for various values of [B]. (c) Indicate the form of
the double-reciprocal plots for 1/v
o
versus 1/[B] for various values
of [A].
12. Creatine kinase catalyzes the reaction
which functions to regenerate ATP in muscle. Rabbit muscle
creatine kinase exhibits the following kinetic behavior. In the
absence of both products, plots of 1/v
o
versus 1/[MgADP
⫺
] at
different fixed concentrations of phosphocreatine yield lines
that intersect to the left of the 1/v
o
axis. Similarly, plots of 1/v
o
versus 1/[phosphocreatine] in the absence of product at differ-
ent fixed concentrations of MgADP
⫺
yield lines that intersect
to the left of the 1/v
o
axis. In the absence of one of the reaction
products, MgATP
2⫺
or creatine, plots of 1/v
o
versus 1/[MgADP
⫺
]
at different concentrations of the other product intersect on the
1/v
o
axis.The same is true of the plots of 1/v
o
versus 1/[phospho-
creatine]. Indicate a kinetic mechanism that is consistent with
this information.
MgADP
⫺
⫹ phosphocreatine Δ MgATP
2⫺
⫹ creatine
K
A
S
⫽
[E][A]
[EA]
and
K
B
S
⫽
[F][B]
[FB]
E
AP
EEA F
K
S
A
k
2
FB
B
K
S
B
Q
k
4
Problems 505
JWCL281_c14_482-505.qxd 2/22/10 8:47 AM Page 505

506
CHAPTER 15
Enzymatic
Catalysis
1 Catalytic Mechanisms
A. Acid–Base Catalysis
B. Covalent Catalysis
C. Metal Ion Catalysis
D. Electrostatic Catalysis
E. Catalysis through Proximity and Orientation Effects
F. Catalysis by Preferential Transition State Binding
2 Lysozyme
A. Enzyme Structure
B. Catalytic Mechanism
C. Testing the Catalytic Mechanism
3 Serine Proteases
A. Kinetics and Catalytic Groups
B. X-Ray Structures
C. Catalytic Mechanism
D. Testing the Catalytic Mechanism
E. Zymogens
4 Drug Design
A. Techniques of Drug Discovery
B. Introduction to Pharmacology
C. HIV Protease and Its Inhibitors
Enzymes, as we have seen, cause rate enhancements that
are orders of magnitude greater than those of the best
chemical catalysts. Yet they operate under mild conditions
and are highly specific as to the identities of both their sub-
strates and their products. These catalytic properties are so
remarkable that many nineteenth century scientists con-
cluded that enzymes have characteristics that are not
shared by substances of nonliving origin. To this day, there
are few enzymes for which we understand in more than
cursory detail how they achieve their enormous rate accel-
erations. Nevertheless, it is now abundantly clear that the
catalytic mechanisms employed by enzymes are identical
to those used by chemical catalysts. Enzymes are simply
better designed.
In this chapter we consider the nature of enzymatic
catalysis. We begin by discussing the underlying principles
of chemical catalysis as elucidated through the study of or-
ganic reaction mechanisms. We then embark on a detailed
examination of the catalytic mechanisms of several of the
best characterized enzymes: lysozyme and the serine pro-
teases. Their study should lead to an appreciation of the in-
tricacies of these remarkably efficient catalysts as well as of
the experimental methods used to elucidate their proper-
ties. We end with a discussion of how drugs are discovered
and tested, a process that depends heavily on the principles
of enzymology since many drug targets are enzymes. In do-
ing so, we consider how therapeutically effective inhibitors
of HIV-1 protease were discovered.
1 CATALYTIC MECHANISMS
Catalysis is a process that increases the rate at which a reac-
tion approaches equilibrium. Since, as we discussed in Sec-
tion 14-1Cb, the rate of a reaction is a function of its free
energy of activation (G
‡
), a catalyst acts by lowering the
height of this kinetic barrier; that is, a catalyst stabilizes the
transition state with respect to the uncatalyzed reaction.
There is, in most cases, nothing unique about enzymatic
mechanisms of catalysis in comparison to nonenzymatic
mechanisms. What apparently make enzymes such powerful
catalysts are two related properties: their specificity of sub-
strate binding combined with their optimal arrangement of
catalytic groups. An enzyme’s arrangement of binding and
catalytic groups is, of course, the product of eons of evolu-
tion: Nature has had ample opportunity to fine-tune the
performances of most enzymes.
The types of catalytic mechanisms that enzymes employ
have been classified as:
1. Acid–base catalysis.
2. Covalent catalysis.
3. Metal ion catalysis.
4. Electrostatic catalysis.
5. Proximity and orientation effects.
6. Preferential binding of the transition state complex.
In this section, we examine these various phenomena. In
doing so we shall frequently refer to the organic model
compounds that have been used to characterize these cat-
alytic mechanisms.
A. Acid–Base Catalysis
General acid catalysis is a process in which partial proton
transfer from a Brønsted acid (a species that can donate
protons; Section 2-2A) lowers the free energy of a reaction’s
transition state. For example, an uncatalyzed keto–enol
tautomerization reaction occurs quite slowly as a result of
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 506
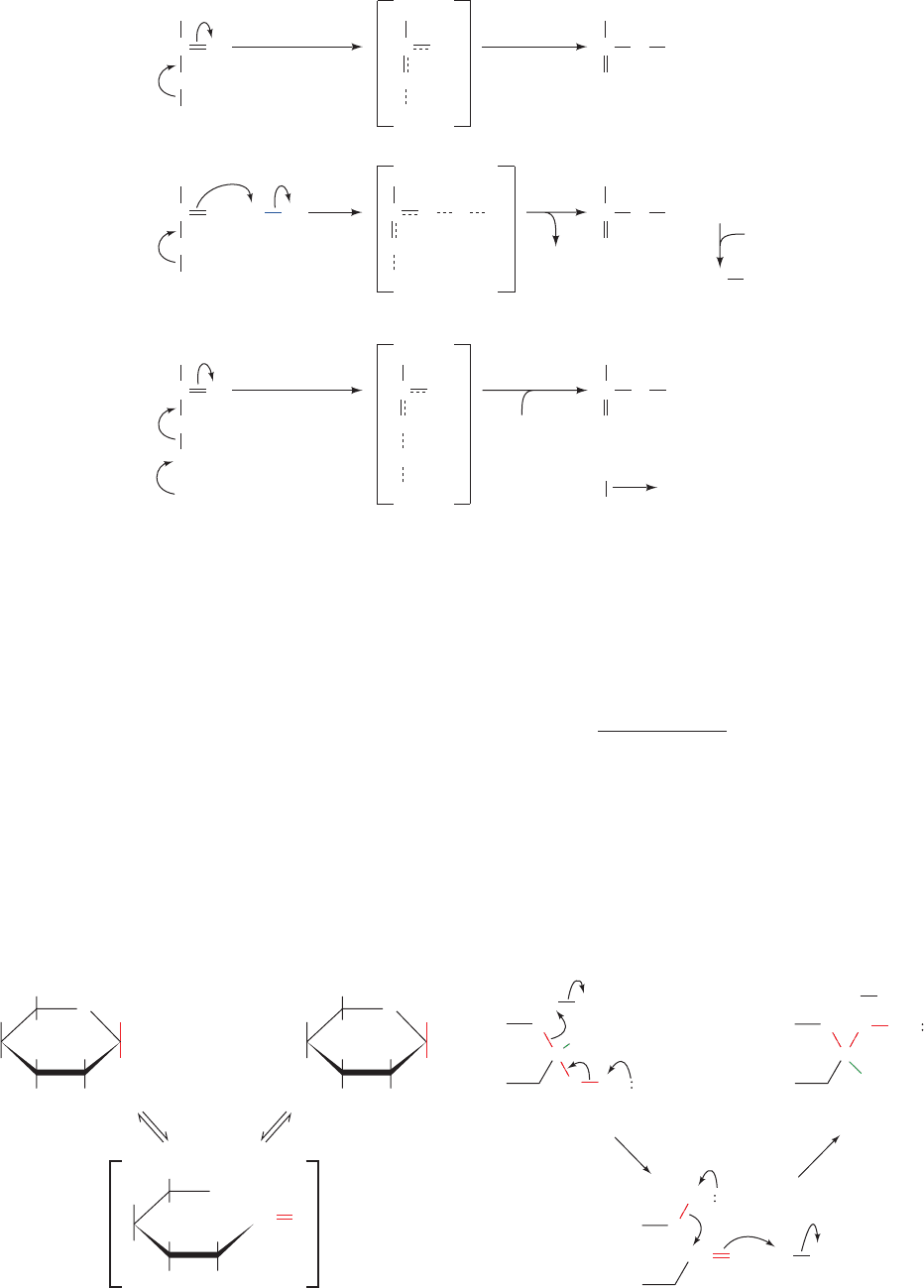
the high energy of its carbanionlike transition state (Fig.
15-1a). Proton donation to the oxygen atom (Fig. 15-1b),
however, reduces the carbanion character of the transition
state, thereby catalyzing the reaction. A reaction may also
be stimulated by general base catalysis if its rate is in-
creased by partial proton abstraction by a Brønsted base (a
species that can combine with a proton; Fig.15-1c). Some re-
actions may be simultaneously subject to both processes: a
concerted general acid–base catalyzed reaction.
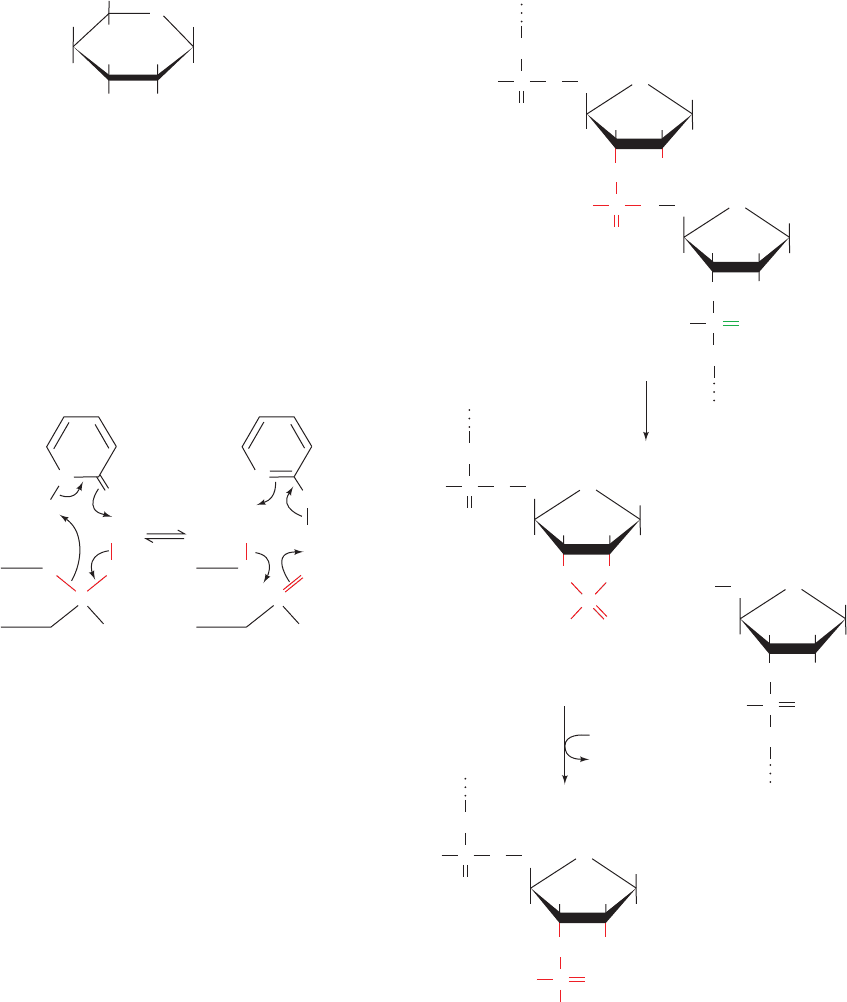
a. Mutarotation Is Catalyzed by Acids and by Bases
The mutarotation of glucose provides an instructive ex-
ample of acid–base catalysis. Recall that a glucose molecule
can assume either of two anomeric cyclic forms through the
intermediacy of its linear form (Section 11-1B):
H H
OH
CH
2
OH
H
HO
OH
H OH
H
O
␣-D-Glucose
[␣]
D
20
= 112.2⬚
H
OH
CH
2
OH
H
HO
CHO
H OH
H
OH
Linear form
H OH
OH
CH
2
OH
H
HO
H
H OH
H
O
-D-Glucose
[␣]
D
20
= 18.7⬚
In aqueous solvents, the initial rate of mutarotation of ␣-D-
glucose, as monitored by polarimetry (Section 4-2A), is ob-
served to follow the relationship:
[15.1]
where k
obs
is the reaction’s apparent first-order rate con-
stant. The mutarotation rate increases with the concentra-
tions of general acids and general bases; they are thought
to catalyze mutarotation according to the mechanism:
H
H
A
O
OHB
–
C
␣-D-Glucose
HA
O
CH OHB
Linear form
H
H
O
B
–
A
O
H
C
-D-Glucose
–
v ⫽⫺
d[␣-
D-glucose]
dt
⫽ k
obs
[␣-D-glucose]
Section 15-1. Catalytic Mechanisms 507
Figure 15-1 Mechanisms of keto–enol tautomerization. (a) Uncatalyzed, (b) general acid
catalyzed, and (c) general base catalyzed.
CH
2
CO
R
H
O
δ
–
CH
2
δ
–
C
R
H
δ+
Keto Transition state Enol
CH
2
CO
R
H
CH
2
CO
R
H
O
δ
–
CH
2
δ
–
C
R
H
δ+
CH
2
CO
R
H+ H A H
δ+
A
δ
–
H
+
+ A
–
H A
H
2
O
+ OH
–
CH
2
CO
R
H
O
δ
–
CH
2
δ
–
C
R
H
δ+
CH
2
CO
R
H
+
B
H
+
+
+
B
+
..
B
..
H
+
H
B
δ+
(a)
(b)
(c)
JWCL281_c15_506-556.qxd 6/7/10 2:04 PM Page 507
This model is consistent with the observation that in apro-
tic solvents such as benzene, 2,3,4,6-O-tetramethyl-␣-
D-
glucose (a less polar benzene-soluble analog)
does not undergo mutarotation. Yet, the reaction is cat-
alyzed by the addition of phenol, a weak benzene-soluble
acid, together with pyridine, a weak benzene-soluble base,
according to the rate equation:
[15.2]
Moreover, in the presence of ␣-pyridone, whose acid and
base groups can rapidly interconvert between two tau-
tomeric forms and are situated so that they can simultane-
ously catalyze mutarotation,
the reaction follows the rate law
[15.3]
where k¿ 7000M k. This increased rate constant indi-
cates that -pyridone does, in fact, catalyze mutarotation in
a concerted fashion since 1M -pyridone has the same cat-
alytic effect as impossibly high concentrations of phenol
and pyridine (e.g., 70M phenol and 100M pyridine).
Many types of biochemically significant reactions are
susceptible to acid and/or base catalysis. These include the
hydrolysis of peptides and esters, the reactions of phosphate
groups, tautomerizations, and additions to carbonyl groups.
The side chains of the amino acid residues Asp, Glu, His,
Cys, Tyr, and Lys have pK’s in or near the physiological pH
range (Table 4-1), which we shall see permits them to act in
the enzymatic capacity of general acid and/or base catalysts
in analogy with known organic mechanisms. Indeed, the
ability of enzymes to arrange several catalytic groups about
their substrates makes concerted acid–base catalysis a com-
mon enzymatic mechanism.
b. The RNase A Reaction Incorporates General
Acid–Base Catalysis
Bovine pancreatic ribonuclease A (RNase A) provides
an illuminating example of enzymatically mediated general
acid–base catalysis. This digestive enzyme functions to
v k¿ [-pyridone] [tetramethyl--
D-glucose]
␣-Pyridone
Glucose
N
H
O
O
O
C
H
H
N
O
O
O
C
H
H
H
v k[phenol] [pyridine] [tetramethyl--D-glucose]
HH
OCH
3
CH
2
OCH
3
H
H
3
CO
OH
H OCH
3
H
O
2,3,4,6-O-Tetramethyl-␣-D-glucose
hydrolyze RNA to its component nucleotides.The isolation
of 2ⴕ,3ⴕ-cyclic nucleotides from RNase A digests of RNA
indicates that the enzyme mediates the following reaction
sequence:
The RNase A reaction exhibits a pH rate profile that
peaks near pH 6 (Fig. 15-2).Analysis of this curve (Section
14-4b), together with chemical derivatization and X-ray
studies, indicates that RNase A has two essential His
residues, His 12 and His 119, which act in a concerted man-
ner as general acid and base catalysts (the structure of
RNase A is sketched in Fig. 9-2). Evidently, the RNase A
reaction is a two-step process (Fig. 15-3):
1. His 12, acting as a general base, abstracts a proton from
an RNA 2¿-OH group, thereby promoting its nucleophilic
–
OPO
O
O
OH
HH
HH
O
OCH
2
–
OPO
O
CH
2
–
OPO
O
Base
5
4 1
3
2
O
OH
HH
HH
O
Base
RNA
–
OPO
O
OO
HH
HH
O
OCH
2
–
O
P
HO
O
CH
2
–
OPO
O
Base
–
OPO
O
HH
HH
O
OCH
2
Base
O
–
OPO
O
–
OOH
OH
HH
HH
O
Base
2ⴕ,3ⴕ-Cyclic nucleotide
+
H
2
O
H
+
508 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 508
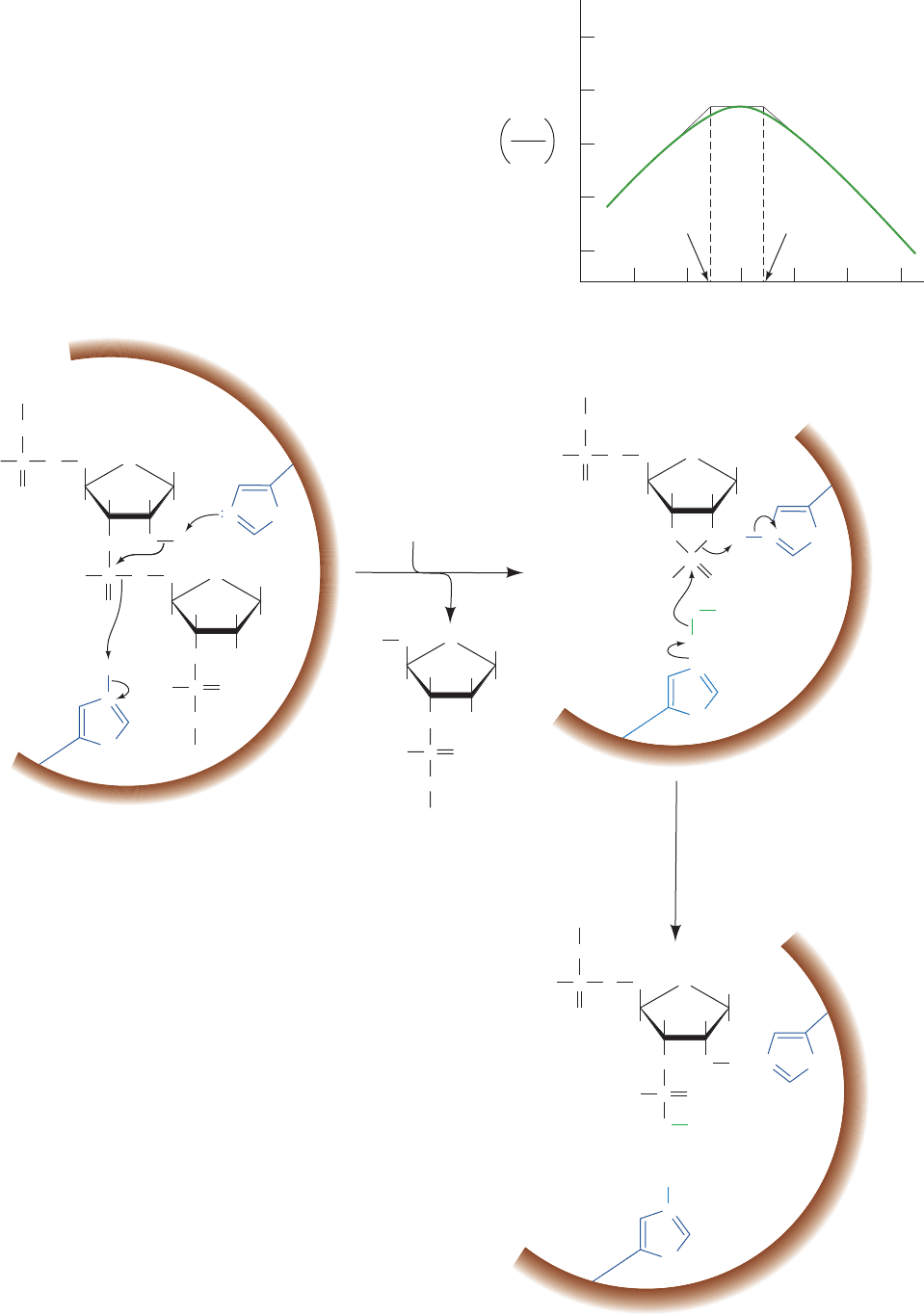
Section 15-1. Catalytic Mechanisms 509
Figure 15-2 The pH dependence of Vⴕ
max
/Kⴕ
M
in the RNase
A–catalyzed hydrolysis of cytidine-2ⴕ,3ⴕ-cyclic phosphate.
V¿
max
/K¿
M
is given in units of M
1
ⴢ s
1
.Analysis of this curve
(Section 14-4b) suggests the catalytic participation of groups with
pK’s of 5.4 and 6.4. [After del Rosario, E.J. and Hammes, G.G.,
Biochemistry 8, 1887 (1969).]
Figure 15-3 The bovine pancreatic RNase A–catalyzed
hydrolysis of RNA is a two-step process with the intermediate
formation of a 2ⴕ,3ⴕ-cyclic nucleotide.
O
Base
H
H
O
H
H
CH
2
O
P
O
–
O
O
3
2
5
4 1
O
P
O
H
O
Base
H
H
OH
H
H
CH
2
O
P
O
–
O
O
RNA
P
O
–
O
N
NH
His 12
H
N
N
H
O
P
O
–
O
HO
H
2
O
1
O
P
O
–
O
O
H
N
+
NH
P
–
O
O
H
O
H
N
N
H
2′,3′-Cyclic nucleotide
2
His 119
+
O
Base
O
HH
HH
CH
2
O
O
Base
OH
HH
HH
CH
2
O
S
...
...
...
...
O
P
O
–
O
O
H
H
O
H
N
H
N
+
O
P
–
O
N
NH
O
Base
O
HH
HH
CH
2
O
S
...
987654
log
1
3
5
pH
pK
E1
pK
E2
K
M
V
max
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 509
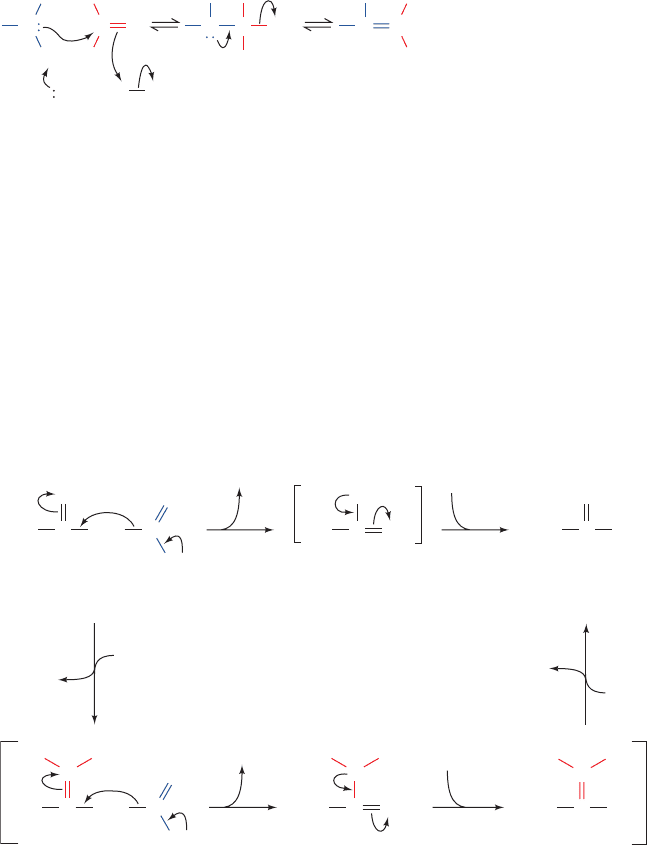
attack on the adjacent phosphorus atom while His 119, act-
ing as a general acid, promotes bond scission by protonat-
ing the leaving group.
2. The 2¿,3¿-cyclic intermediate is hydrolyzed through
what is essentially the reverse of the first step in which water
replaces the leaving group. Thus His 12 acts as a general
acid and His 119 as a general base to yield the hydrolyzed
RNA and the enzyme in its original state.
B. Covalent Catalysis
Covalent catalysis involves rate acceleration through the
transient formation of a catalyst–substrate covalent bond.
The decarboxylation of acetoacetate, as chemically cat-
alyzed by primary amines, is an example of such a process
(Fig. 15-4). In the first stage of this reaction, the amine nu-
cleophilically attacks the carbonyl group of acetoacetate to
form a Schiff base (imine bond).
The protonated nitrogen atom of the covalent intermediate
then acts as an electron sink (Fig. 15-4, bottom) so as to re-
duce the otherwise high-energy enolate character of the tran-
sition state. The formation and decomposition of the Schiff
base occur quite rapidly, so that these steps are not rate de-
termining in this reaction sequence.
a. Covalent Catalysis Has Both Nucelophilic and
Electrophilic Stages
As the preceding example indicates, covalent catalysis
may be conceptually decomposed into three stages:
NNCC COOH OH++
HH
H
HA
B
N
H
+
–
Schiff
base
1. The nucleophilic reaction between the catalyst and
the substrate to form a covalent bond.
2. The withdrawal of electrons from the reaction center
by the now electrophilic catalyst.
3. The elimination of the catalyst, a reaction that is es-
sentially the reverse of stage 1.
Reaction mechanisms are somewhat arbitrarily classified
as occurring with either nucleophilic catalysis or elec-
trophilic catalysis depending on which of these effects pro-
vides the greater driving force for the reaction, that is,
which catalyzes its rate-determining step. The primary
amine–catalyzed decarboxylation of acetoacetate is clearly
an electrophilically catalyzed reaction since its nucleophilic
phase, Schiff base formation, is not its rate-determining
step. In other covalently catalyzed reactions, however, the
nucleophilic phase may be rate determining.
The nucleophilicity of a substance is closely related to
its basicity. Indeed, the mechanism of nucleophilic catalysis
resembles that of general base catalysis except that, instead
of abstracting a proton from the substrate, the catalyst nu-
cleophilically attacks it so as to form a covalent bond. Conse-
quently, if covalent bond formation is the rate-determining
step of a covalently catalyzed reaction, the reaction rate
tends to increase with the covalent catalyst’s basicity (pK).
An important aspect of covalent catalysis is that the
more stable the covalent bond formed, the less facilely it
will decompose in the final steps of a reaction. A good co-
valent catalyst must therefore combine the seemingly con-
tradictory properties of high nucleophilicity and the ability
to form a good leaving group, that is, to easily reverse the
bond formation step. Groups with high polarizabilities
(highly mobile electrons), such as imidazole and thiol func-
tions, have these properties and hence make good covalent
catalysts.
510 Chapter 15. Enzymatic Catalysis
Figure 15-4 The decarboxylation of
acetoacetate. The uncatalyzed reaction
mechanism is shown at the top and the
reaction mechanism as catalyzed by
primary amines is shown at the bottom.
CH
3
CH
2
CO
2
C C
O
N
O
–
N
CH
3
CH
2
C CH
3
CH
3
C
H
+
RH
RH
N
RH
Schiff base
(imine)
OH
RNH
2
RNH
2
OH
Acetoacetate Enolate
Acetone
CH
3
CH
2
CO
2
C C
O
O
O
–
CH
3
CH
2
C CH
3
CH
3
C
H
+
O
O
–
..
JWCL281_c15_506-556.qxd 2/19/10 9:27 PM Page 510
