Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.


proteases) and pharmacokinetics (they do not readily pass
through cell membranes.). Consequently, therapeutically
effective HIV-1 protease inhibitors must be peptidomimet-
ics (peptide mimics), substances that sterically and perhaps
physically, but not chemically, resemble polypeptides. The
use of peptidomimetics also permits conformational con-
straints to be imposed on a drug candidate that would not
be present in the corresponding polypeptide.
The FDA has approved ten HIV-1 protease inhibitors
(Fig. 15-41), the first of which, saquinavir, was sanctioned in
late 1995. These peptidomimetics have IC
50
’s against HIV
in culture ranging from 2 to 60 nM but have little or no ac-
tivity against human aspartic proteases (K
I
’s 10 M).
They are the first drugs to clearly prolong the lives of AIDS
victims. Their development, in each case, was a complex it-
erative process that required the design, synthesis, and
evaluation of numerous related compounds. In several
cases, these investigations capitalized on the wealth of ex-
perience gained in developing peptidomimetic inhibitors
of the aspartic protease renin and in the resulting stock-
piles of these compounds.
All the FDA-approved HIV-1 protease inhibitors ini-
tially cause a rapid and profound decline in a patient’s
plasma HIV load, which is often paralleled by immune sys-
tem recovery. However, as we saw with reverse transcrip-
tase inhibitors, mutant forms of the protease that are resist-
ant to the inhibitor being used arise, usually within 4 to 12
weeks. Moreover, such a mutant protease is likely to be re-
sistant to other HIV-1 protease inhibitors, because all of the
HIV-1 protease inhibitors are targeted to the same binding
site. This has led to the use of combination therapies in
which an HIV-1 protease inhibitor is administered together
with one, or more often, two inhibitors of other viral
processes (inhibitors of reverse transcriptase, integrase, and
viral entry into host cells). This is because any virus that
gains resistance to one drug in a regimen will be suppressed
by the other drug(s) in that regimen. In addition, the HIV-1
protease inhibitor ritonavir has been shown to be a potent
inhibitor of the cytochrome P450 isoforms (CYP3A4,5,7)
that metabolize other protease inhibitors and hence is usu-
ally prescribed in low dosage as an adjunct to another pro-
tease inhibitor to improve the latter’s pharmacokinetics.
The plasma virus levels in many patients who were placed
on combination therapy rapidly became undetectable and
have remained so for several years. This, however, does not
constitute a cure: If drug therapy is interrupted, the virus will
reappear in the plasma because certain tissues in the body
harbor latent viruses that are unaffected by and/or inaccessi-
ble to drug therapy. Thus, the presently available anti-HIV
medications must be taken for a lifetime.
Current anti-HIV therapies are by no means ideal. To
maximize their oral bioavailability, some of the different
drugs must be taken well before or after a meal but others
must be taken with a meal. To minimize the probability of
resistant forms of HIV arising, the bioavailability of each
drug must be maintained at a certain minimum level and
hence each drug must be taken on a rigid schedule. More-
over, these drugs have significant side effects, mainly
fatigue, nausea, diarrhea, tingling and numbness with riton-
avir, and kidney stones with indinavir. Consequently, nu-
merous AIDS patients fail to take their medications prop-
erly, which greatly increases the likelihood that they will
develop resistance to these drugs and infect others with
drug-resistant viruses. Finally, HIV-1 protease inhibitors,
being complex molecules, are difficult to synthesize and
therefore are relatively expensive, so that in the developing
countries in which AIDS is most prevalent, governments
and most individuals cannot afford to purchase these
drugs, even if they were to be supplied at cost. It is there-
fore important that anti-HIV therapies be developed that
are easy for patients to comply with, are inexpensive, and
ideally, will totally eliminate an HIV infection.
Chapter Summary 551
1 Catalytic Mechanisms Most enzymatic mechanisms of
catalysis have ample precedent in organic catalytic reactions.
Acid- and base-catalyzed reactions occur, respectively, through
the donation or abstraction of a proton to or from a reactant
so as to stabilize the reaction’s transition state complex.
Enzymes often employ ionizable amino acid side chains as
general acid–base catalysts. Covalent catalysis involves nucle-
ophilic attack of the catalyst on the substrate to transiently
form a covalent bond followed by the electrophilic stabiliza-
tion of a developing negative charge in the reaction’s transi-
tion state. Various protein side chains as well as certain coen-
zymes can act as covalent catalysts. Metal ions, which are
common enzymatic components, catalyze reactions by stabi-
lizing developing negative charges in a manner resembling
general acid catalysis. Metal ion–bound water molecules are
potent sources of OH
ions at neutral pH’s. Metal ions also fa-
cilitate enzymatic reactions through the charge shielding of
bound substrates. The arrangement of charged groups about
an enzymatic active site of low dielectric constant in a manner
that stabilizes the transition state complex results in the elec-
trostatic catalysis of the enzymatic reaction. Enzymes catalyze
reactions by bringing their substrates into close proximity in
reactive orientations. The enzymatic binding of the substrates
in a bimolecular reaction arrests their relative motions result-
ing in a rate enhancement.The preferential enzymatic binding
of the transition state of a catalyzed reaction over the sub-
strate is an important rate enhancement mechanism. Transi-
tion state analogs are potent competitive inhibitors because
they bind to the enzyme more tightly than does the correspon-
ding substrate.
2 Lysozyme Lysozyme catalyzes the hydrolysis of
(1 S 4)-linked poly(NAG–NAM), the bacterial cell wall
polysaccharide, as well as that of poly(NAG). Lysozyme
binds a hexasaccharide so as to distort its D-ring toward the
CHAPTER SUMMARY
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 551
half-chair conformation of the planar oxonium ion transition
state. This is followed by cleavage of the bond be-
tween the D- and E-rings as promoted by proton donation
from Glu 35. The resulting oxonium ion transition state is
electrostatically stabilized by the nearby carboxyl group of
Asp 52, which then forms a covalent bond with C1.The E-ring
leaving group is subsequently replaced by water, which in
what is essentially a reversal of the previous reaction se-
quence, attacks C1 yielding the reaction’s second product and
regenerating the enzyme. The roles of Glu 35 and Asp 52 in
lysozyme catalysis have been verified through mutagenesis
studies. Structural and binding studies indicate that strain is of
major catalytic importance in the lysozyme mechanism. Mass
spectrometry and X-ray studies have shown that the lysozyme
reaction proceeds via a covalent glycosyl–enzyme intermedi-
ate involving Asp 52 rather than by the noncovalently bound
oxonium ion intermediate postulated by the original Phillips
mechanism.
3 Serine Proteases Serine proteases constitute a wide-
spread class of proteolytic enzymes that are characterized by
the possession of a reactive Ser residue. The pancreatically
synthesized digestive enzymes trypsin, chymotrypsin, and
elastase are sequentially and structurally related but have
different side chain specificities for their substrates. All have
the same catalytic triad,Asp 102, His 57, and Ser 195, at their
active sites. The differing side chain specificities of trypsin
and chymotrypsin depend in a complex way on the structures
of the loops that connect the walls of the specificity pocket,
as well as on the charge of the side chain at the base of the
specificity pocket. Subtilisin, serine carboxypeptidase II, and
ClpP are unrelated serine proteases that have essentially the
same active site geometry as do the pancreatic enzymes.
Catalysis in serine proteases is initiated by the nucle-
ophilic attack of the active Ser on the carbonyl carbon atom
of the scissile peptide to form the tetrahedral intermediate, a
process that may be facilitated by the formation of a low-
barrier hydrogen bond between Asp 102 and His 57. The
tetrahedral intermediate, which is stabilized by its preferen-
tial binding to the enzyme’s active site, then decomposes to
the acyl–enzyme intermediate under the impetus of proton
donation from the Asp 102-polarized His 57. After the re-
placement of the leaving group by solvent H
2
O, the catalytic
process is reversed to yield the second product and the re-
generated enzyme. The active Ser is not unusually reactive
but is ideally situated to nucleophilically attack the activated
scissile peptide. The X-ray structure of the trypsin–BPTI
complex indicates the existence of the tetrahedral intermedi-
ate, whereas X-ray structures of a complex of elastase with
the heptapeptide BCM7 have visualized both the acyl–enzyme
intermediate and the tetrahedral intermediate. Enzyme–sub-
strate complexes have free energy landscapes that facilitate
the formation of the transition state.
The pancreatic serine proteases are synthesized as zymo-
gens to prevent pancreatic self-digestion. Trypsinogen is acti-
vated by a single proteolytic cleavage by enteropeptidase. The
resulting trypsin similarly activates trypsinogen as well as chy-
motrypsinogen, proelastase,and other pancreatic digestive en-
zymes. Trypsinogen’s catalytic triad is structurally intact. The
C1¬O1
zymogen’s low catalytic activity arises from a distortion of its
specificity pocket and oxyanion hole, so that it is unable to
productively bind substrate or preferentially bind the catalytic
reaction’s transition state.
4 Drug Design Drugs act by binding to and thereby mod-
ifying the functions of receptors. Many promising drug candi-
dates, which are known as lead compounds, have been found
by methods in which a large number of compounds are tested
for drug efficacy in an assay that is a suitable surrogate of the
disease/condition under consideration. Lead compounds are
then chemically manipulated in the search for compounds
with improved drug efficacy. Structure–activity relationships
(SARs) and quantitative structure–activity relationships
(QSARs) are useful tools in this endeavor. Structure-based
drug design uses the X-ray and NMR structures of drug candi-
dates in complex with their target proteins, together with a va-
riety of molecular modeling tools, to guide the search for im-
proved drug candidates. However,the advent of combinatorial
chemistry, fragment-based lead discovery, and high-throughput
screening procedures has extended the “make-many-compounds-
and-see-what-they-do” approaches to drug discovery.
In order to reach their target receptors, drugs must have
favorable pharmacokinetics, that is, they must readily traverse
numerous physical barriers in the body, avoid chemical trans-
formation by enzymes, and not be excreted too rapidly. Most
useful drugs are neither too lipophilic nor too hydrophilic so
that they can both gain access to the necessary membranes
and pass through them. Drug toxicity, dosage, efficacy, and the
nature of rare adverse reactions are determined through ex-
tensive and carefully designed clinical trials. Most drugs are
metabolically cleared through oxidative hydroxylation by one
of the numerous cytochrome P450 isozymes. This permits the
hydroxylated drugs to be enzymatically conjugated to polar
groups such as glucuronic acid and glycine, which increases
their rates of excretion by the kidneys. Drug–drug interactions
are frequently mediated by cytochromes P450.Polymorphisms
among cytochromes P450 are often responsible for the varia-
tions among individuals in their response to a given drug, in-
cluding adverse reactions.
The formulation of HIV-1 protease inhibitors to control
HIV infections is one of the major triumphs of modern drug
discovery methods. HIV are retroviruses that attack specific
immune system cells and thereby degrade the immune system
over a period of several years to the point that it is no longer
able defend against opportunistic infections. HIV-1 protease
functions to cleave the polyproteins in immature HIV-1 viri-
ons that have budded out from a host cell, thus generating the
mature, infectious form. HIV-1 protease is an aspartic pro-
tease that, as do eukaryotic aspartic proteases such as pepsin,
uses its two active site Asp residues to activate its bound lytic
water molecule as the nucleophile that attacks and thereby
cleaves specific peptide bonds in the substrate polyprotein.
All of the FDA-approved peptidomimetic inhibitors of HIV-1
protease cause a rapid and profound decrease in plasma HIV
levels, although they do not entirely eliminate the virus. They
are used in combination with inhibitors of other viral
processes to minimize the ability of the rapidly mutating HIV
to evolve drug-resistant forms.
552 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 552

References 553
General
Fersht, A., Structure and Mechanism in Protein Science, Freeman
(1999).
Frey, P.A. and Hegeman, A.D., Enzymatic Reaction Mechanisms,
Oxford (2007). [A compendium of enzymatic reactions.]
Jencks, W.P., Catalysis in Chemistry and Enzymology, Dover
(1987). [A classic and, in many ways, still current work.]
Catalytic Mechanisms
Bruice, T.C., Some pertinent aspects of mechanism as determined
with small molecules, Annu. Rev. Biochem. 45, 331–373 (1976).
Bruice, T.C. and Benkovic, S.J., Chemical basis for enzyme cataly-
sis, Biochemistry 39, 6267–6274 (2000); and Bruice, T.C. and
Lightstone, F.C., Ground state and transition state contribu-
tions to the rates of intramolecular and enzymatic reactions,
Acc. Chem. Res. 32, 127–136 (1999).
Christianson, D.W. and Cox, J.D., Catalysis by metal-activated hy-
droxide in zinc and manganese metalloenzymes, Annu. Rev.
Biochem. 68, 33–57 (1999). [Discusses the enzymatic mecha-
nism of carbonic anhydrase.]
Garcia-Viloca, M., Gao, J., Karplus, M., and Truhlar, D.G., How en-
zymes work: Analysis by modern rate theory and computer
simulations, Science 303, 186–195 (2004).
Hackney, D.D., Binding energy and catalysis, in Sigman, D.S. and
Boyer, P.D. (Eds.), The Enzymes (3rd ed.), Vol. 19, pp. 1–36,
Academic Press (1990).
Jencks, W.P., Binding energy, specificity, and enzymatic catalysis:
The Circe effect, Adv. Enzymol. 43, 219–410 (1975).
Kraut, J., Carroll, K.S., and Herschlag, D., Challenges in enzyme
mechanism and energetics, Annu. Rev. Biochem. 72, 517–571
(2003).
Lolis, E. and Petsko, G.A., Transition-state analogues in protein
crystallography: Probes of the structural source of enzyme
catalysis, Annu. Rev. Biochem. 59, 597–630 (1990).
Schramm, V.L., Enzymatic transition states and transition state
analog analogues, Curr. Opin. Struct. Biol. 15, 604–613 (2005).
Wolfenden, R., Analogue approaches to the structure of the tran-
sition state in enzyme reactions, Acc. Chem. Res. 5, 10–18
(1972).
Lysozyme
Blake, C.C.F., Johnson, L.N., Mair, G.A., North, A.C.T., Phillips,
D.C., and Sarma, V.R., Crystallographic studies of the activity
of hen egg-white lysozyme, Proc. R. Soc. London Ser. B 167,
378–388 (1967).
Chipman, D.M. and Sharon, N., Mechanism of lysozyme action,
Science 165, 454–465 (1969).
Ford, L.O., Johnson, L.N., Machin, P.A., Phillips, D.C., and Tijan,
R., Crystal structure of a lysozyme–tetrasaccharide lactone
complex, J. Mol. Biol. 88, 349–371 (1974).
Imoto, T., Johnson, L.N., North, A.C.T., Phillips, D.C., and Rupley,
J.A., Vertebrate lysozymes, in Boyer, P.D. (Ed.), The Enzymes
(3rd ed.), Vol. 7, pp. 665–868, Academic Press (1972). [An ex-
haustive review.]
Johnson, L.N., Cheetham, J., McLaughlin, P.J., Acharya, K.R.,
Barford, D., and Phillips, D.C., Protein–oligosaccharide inter-
actions: Lysozyme, phosphorylase, amylases, Curr. Top. Micro-
biol. Immunol. 139, 81–134 (1988).
Jollès, P. (Ed.), Lysozymes: Model Enzymes in Biochemistry and
Biology, Birkhaüser Verlag (1996).
Kirby, A.J., The lysozyme mechanism sorted—after 50 years, Na-
ture Struct. Biol. 8, 737–739 (2001). [Briefly summarizes the
theoretical and experimental evidence for a covalent interme-
diate in the lysozyme mechanism.]
Mooser, G., Glycosidases and glycosyltransferases, in Sigman, D.S.
(Ed.), The Enzymes (3rd ed.), Vol. 20, pp. 187–233, Academic
Press (1992). [Section II discusses lysozyme.]
Phillips, D.C.,The three-dimensional structure of an enzyme mol-
ecule, Sci.Am. 215(5), 75–80 (1966).
Schindler, M., Assaf, Y., Sharon, N., and Chipman, D.M., Mecha-
nism of lysozyme catalysis: Role of ground-state strain in sub-
site D in hen egg-white and human lysozymes, Biochemistry
16, 423–431 (1977).
Secemski, I.I., Lehrer, S.S., and Lienhard, G.E., A transition state
analogue for lysozyme, J. Biol. Chem. 247, 4740–4748 (1972).
[Binding studies on the lactone derivative of (NAG)
4
.]
Strynadka, N.C.J. and James, M.N.G., Lysozyme revisited: Crystal-
lographic evidence for distortion of an N-acetylmuramic acid
residue bound in site D, J. Mol. Biol. 220, 401–424 (1991).
Vocadlo, D.J., Davies, G.J., Laine, R., and Withers, S.G., Catalysis
by hen egg-white lysozyme proceeds via a covalent intermedi-
ate, Nature 412, 835–838 (2001).
Warshel, A. and Levitt, M., Theoretical studies of enzymatic reac-
tions; dielectric, electrostatic and steric stabilization of the car-
bonium ion in the reaction of lysozyme, J. Mol. Biol. 103,
227–249 (1976). [Theoretical indications that lysozyme cataly-
sis occurs through electrostatic rather than steric strain.]
Serine Proteases
Blow, D.M., The tortuous story of Asp…His…Ser: Structural
analysis of chymotrypsin, Trends Biochem. Sci. 22, 405–408
(1998). [A personal memoir of the structural determination of
-chymotrypsin in the years 1967 through 1969.]
Cleland, W.W., Frey, P.A., and Gerlt, J.A., The low barrier hy-
drogen bond in enzymatic catalysis, J. Biol. Chem. 273,
25529–25532 (1998).
Corey, D.R. and Craik, C.S., An investigation into the minimum
requirements for peptide hydrolysis by mutation of the cat-
alytic triad of trypsin, J.Am. Chem. Soc. 114, 1784–1790 (1992).
Ding, X., Rasmussen, B.F., Petsko, G.A., and Ringe, D., Direct
structural observation of an acyl-enzyme intermediate in the
hydrolysis of an ester substrate by elastase, Biochemistry 33,
9285–9293 (1994).
Dodson, G. and Wlodawer, A., Catalytic triads and their relatives,
Trends Biochem. Sci. 23, 347–352 (1998).
Frey, P.A., Whitt, S.A., and Tobin, J.B., A low-barrier hydrogen
bond in the catalytic triad of serine proteases, Science 264,
1927–1930 (1994).
Hammes-Schiffer, S. and Benkovic, S.J., Relating protein motion
to catalysis, Annu. Rev. Biochem. 75, 519–541 (2006); and
Benkovic, S.J., Hammes, G.G., and Hammes-Schiffer, S.,
Free-energy landscape of enzyme catalysis, Biochemistry 47,
3317–3321 (2008).
Hedstrom, L., Serine protease mechanism and specificity, Chem.
Rev. 102, 4501–4523 (2002). [A detailed review.]
Henzler-Wildman, K.A., et al., Intrinsic motions along an enzy-
matic reaction pathway, Nature 450, 838–844 (2007).
James, M.N.G., Sielecki, A.R., Brayer, G.D., Delbaere, L.T.J., and
Bauer, C.A., Structure of product and inhibitor complexes of
Streptomyces griseus protease A at 1.8 Å resolution, J. Mol.
Biol. 144, 45–88 (1980).
Kuhn, P., Knapp, M., Soltis, S.M., Ganshaw, G., Thoene, M., and
Bott, R., The 0.78 Å structure of a serine protease: Bacillus
lentus subtilisin, Biochemistry 37, 13446–13452 (1998).
REFERENCES
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 553
Liao, D.-I. and Remington, S.J., Structure of wheat serine car-
boxypeptidase II at 3.5-Å resolution, J. Biol. Chem. 265,
6528–6531 (1990).
Neurath, H., Evolution of proteolytic enzymes, Science 224,
350–357 (1984).
Perona, J.J. and Craik, C.S., Evolutionary divergence of substrate
specificity within the chymotrypsin-like serine protease fold, J.
Biol. Chem. 272, 29987–29990 (1997); and Structural basis of
substrate specificity in the serine proteases, Protein Sci. 4,
337–360 (1995).
Perrin, C.L. and Nielson, J.B., “Strong” hydrogen bonds in chem-
istry and biology, Annu. Rev. Phys. Chem. 48, 511–544 (1997).
[A detailed review which concludes that the evidence for the
importance of LBHBs in enzymatic reactions is inconclusive.]
Phillips, M.A. and Fletterick, R.J., Proteases, Curr. Opin. Struct.
Biol. 2, 713–720 (1992).
Radisky, E.S., Lee, J.M., Lu, C.-J. K., and Koshland, D.E., Jr., In-
sights into the serine protease mechanism from atomic resolu-
tion structures of trypsin reaction intermediates, Proc. Natl.
Acad. Sci. 103, 6835–6840 (2006). [Reveals the subtle motions
of the catalytic Ser and His residues, the substrate, and the hy-
drolytic water molecule that favor catalysis during the acyla-
tion reaction.]
Roberts, R.M., Mathialagan, N., Duffy, J.Y., and Smith, G.W., Reg-
ulation and regulatory role of proteinase inhibitors, Crit. Rev.
Euk. Gene Express. 5, 385–435 (1995).
Shan, S., Loh, S., and Herschlag, D., The energetics of hydrogen
bonds in model systems: Implications for enzymatic catalysis,
Science 272, 97–101 (1996).
Stroud, R.M., Kossiakoff,A.A., and Chambers, J.L., Mechanism of
zymogen activation, Annu. Rev. Biophys. Bioeng. 6, 177–193
(1977).
Wang, J., Hartling, J.A., and Flanagan, J.M., The structure of ClpP
at 2.3 Å resolution suggests a model for ATP-dependent prote-
olysis, Cell 91, 447–456 (1997).
Wilmouth, R.C., Edman, K., Neutze, R., Wright, P.A., Clifton, I.J.,
Schneider,T.R., Schofield, C.J., and Hajdu, J., X-Ray snapshots
of serine protease catalysis reveals a tetrahedral intermediate,
Nature Struct. Biol. 8, 689–694 (2001); and Wilmouth, R.C.,
Clifton, I.J., Robinson, C.V.,Roach, P.L.,Aplin, R.T.,Westwood,
N.J., Hajdu, J., and Schofield,C.J., Structure of a specific acyl-en-
zyme complex formed between -casomorphin-7 and porcine
pancreatic elastase, Nature Struct. Biol. 4, 456–461 (1997).
Drug Discovery
Ahluwalia,V.K. and Chopra, M., Medicinal Chemistry, CRC Press
(2008).
Bannwarth, W. and Hinzen, B. (Eds.), Combinatorial Chemistry.
From Theory to Applications (2nd ed.),Wiley–VCH (2006).
Brunton, L., Lazo, J., and Parker, K. (Eds.), Goodman & Gilman’s
The Pharmacologic Basis of Therapeutics (11th ed.), McGraw-
Hill (2006).
Corey, E.J., Czakó, B., and Kürti, L., Molecules and Medicine,
Wiley (2007). [Discusses the discovery, application, and mode
of action of numerous drug molecules.]
Corson,T.W. and Crews, C.M.,Molecular understanding and mod-
ern application of traditional medicines: triumphs and trials,
Cell 130, 769–774 (2007).
Debouck, C. and Metcalf, B., The impact of genomics on drug dis-
covery, Annu. Rev. Pharmacol. Toxicol. 40, 193–208 (2000).
Furge, L.L. and Guengerich, F.P., Cytochrome P450 enzymes in
drug metabolism and chemical toxicology,
Biochem. Mol. Biol.
Educ. 34, 66–74 (2006).
Ingelman-Sundberg, M., Oscarson, M., and McLellan, R.A., Poly-
morphic human cytochrome P450 enzymes: An opportunity
for individualized drug treatment, Trends Pharmacol. Sci. 20,
342–349 (1999).
Jorgenson,W.L.,The many roles of computation in drug discovery,
Science 303, 1813–1818 (2004).
Katzung, B.G. (Ed.), Basic & Clinical Pharmacology (10th ed.),
McGraw-Hill (2007).
Lipinski, C. and Hopkins, A., Navigating chemical space for biol-
ogy and medicine, Nature 432, 855–861 (2004). [Discusses
strategies of discovering new drugs.]
MacCoss, M. and Baillie, T.A., Organic chemistry in drug discov-
ery, Science 303, 1810–1813 (2004).
Ohlstein, E.H., Ruffolo, R.R., Jr., and Elliott, J.D., Drug discovery
in the next millennium, Annu. Rev. Pharmacol. Toxicol. 40,
177–191 (2000).
Patrick, G.L, An Introduction to Medicinal Chemistry (3rd ed.),
Oxford University Press (2005).
Smith, D.A. and van der Waterbeemd, H., Pharmacokinetics and
metabolism in early drug design, Curr. Opin. Chem. Biol. 3,
373–378 (1999).
Terrett, N.O., Combinatorial Chemistry, Oxford University Press
(1998).
White, R.E., High-throughput screening in drug metabolism and
pharmacokinetic support of drug discovery, Annu. Rev. Phar-
macol.Toxicol. 40, 133–157 (2000).
Williams, P.A., Cosme, J., Ward, A., Angove, H.C., Matak, D., and
Jhoti, H., Crystal structure of human cytochrome P450 2C9
with bound warfarin, Nature 424, 464–468 (2003).
Wong, L.-L., Cytochrome P450 monooxygenases, Curr. Opin.
Chem. Biol. 2, 263–268 (1998).
Zartler, E.R. and Shapiro, M.J., Fragment-Based Drug Discovery.
A Practical Approach, Wiley (2008).
HIV-1 Protease and Other Aspartic Proteases
Davies, D.R., The structure and function of the aspartic proteases,
Annu. Rev. Biophys. Biophys. Chem. 19, 189–215 (1990).
Erickson, J.W. and Burt, S.K., Structural mechanisms of HIV
drug resistance, Annu. Rev. Pharmacol. Toxicol. 36, 545–571
(1996).
Flexner, C., Dual protease inhibitor therapy in HIV-infected pa-
tients: Pharmacological rationale and clinical benefits, Annu.
Rev. Pharmacol. Toxicol. 40, 649–674 (2000).
Kling, J., Blocking HIV’s “scissors,” Modern Drug Discovery 3(2),
37–45 (2000).
Meek, T.D., Catalytic mechanisms of the aspartic proteases, in
Sinnott, M. (Ed.), Comprehensive Biological Catalysis, Vol. 1,
pp. 327–344,Academic Press (1998).
Richman, D.D., HIV chemotherapy, Nature 410, 995–1001 (2001).
Tomesselli,A.G.,Thaisrivongs, S., and Heinrikson, R.L., Discovery
and design of HIV protease inhibitors as drugs for treatment
of AIDS, Adv. Antiviral Drug Design 2, 173–228 (1996).
Turner, B.G. and Summers, M.F.,Structural biology of HIV, J. Mol.
Biol. 285, 1–32 (1999). [A review.]
Wilk, T. and Fuller, S.D., Towards the structure of human im-
munodeficiency virus: Divide and conquer? Curr. Opin. Struct.
Biol. 9, 231–243 (1999).
Wlodawer, A., Rational approach to AIDS drug design through
structural biology, Annu. Rev. Med. 53, 595–614 (2001); and
Wlodawer,A. and Vondrasek, J., Inhibitors of HIV-1 protease:
A major success of structure-assisted drug design, Annu. Rev.
Biophys. Biomol. Struct. 27, 249–284 (1998).
554 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 2/19/10 9:28 PM Page 554
Problems 555
1. Explain why ␥-pyridone is not nearly as effective a catalyst
for glucose mutarotation as is ␣-pyridone.What about -pyridone?
2. RNA is rapidly hydrolyzed in alkaline solution to yield a
mixture of nucleotides whose phosphate groups are bonded to ei-
ther the 2¿ or the 3¿ positions of the ribose residues. DNA, which
lacks RNA’s 2¿ OH groups, is resistant to alkaline degradation.
Explain.
3. Carboxypeptidase A, a Zn
2⫹
-containing enzyme, hydrolyzes
the C-terminal peptide bonds of polypeptides (Fig 8-19a). In the
enzyme–substrate complex, the Zn
2⫹
ion is coordinated to three
enzyme side chains, the carbonyl oxygen of the scissile peptide
bond, and a water molecule. A plausible model for the enzyme’s
reaction mechanism that is consistent with X-ray and enzymolog-
ical data is diagrammed in Fig. 15-42. What are the roles of the
Zn
2⫹
ion and Glu 270 in this mechanism?
4. In the following lactonization reaction,
the relative reaction rate when R ⫽ CH
3
is 3.4 ⫻ 10
11
times that
when R ⫽ H. Explain.
*5. Derive the analog of Eq. [15.11] for an enzyme that cat-
alyzes the reaction:
Assume the enzyme must bind A before it can bind B:
6. Explain, in thermodynamic terms, why an “enzyme” that
stabilizes its Michaelis complex as much as its transition state does
not catalyze a reaction.
7. Suggest a transition state analog for proline racemase that
differs from those discussed in the text. Justify your suggestion.
8. Wolfenden has stated that it is meaningless to distinguish
between the “binding sites” and the “catalytic sites” of enzymes.
Explain.
9. Explain why oxalate (
⫺
OOCCOO
⫺
) is an inhibitor of ox-
aloacetate decarboxylase.
10. In light of the information given in this chapter, why are
enzymes such large molecules? Why are active sites almost always
E ⫹ A ⫹ B Δ EA ⫹ B Δ EAB S EP
A ⫹ B S P
R
OH
R
CCH
2
COO
⫺
R
R
O
C
C
CH
2
O
R R
PROBLEMS
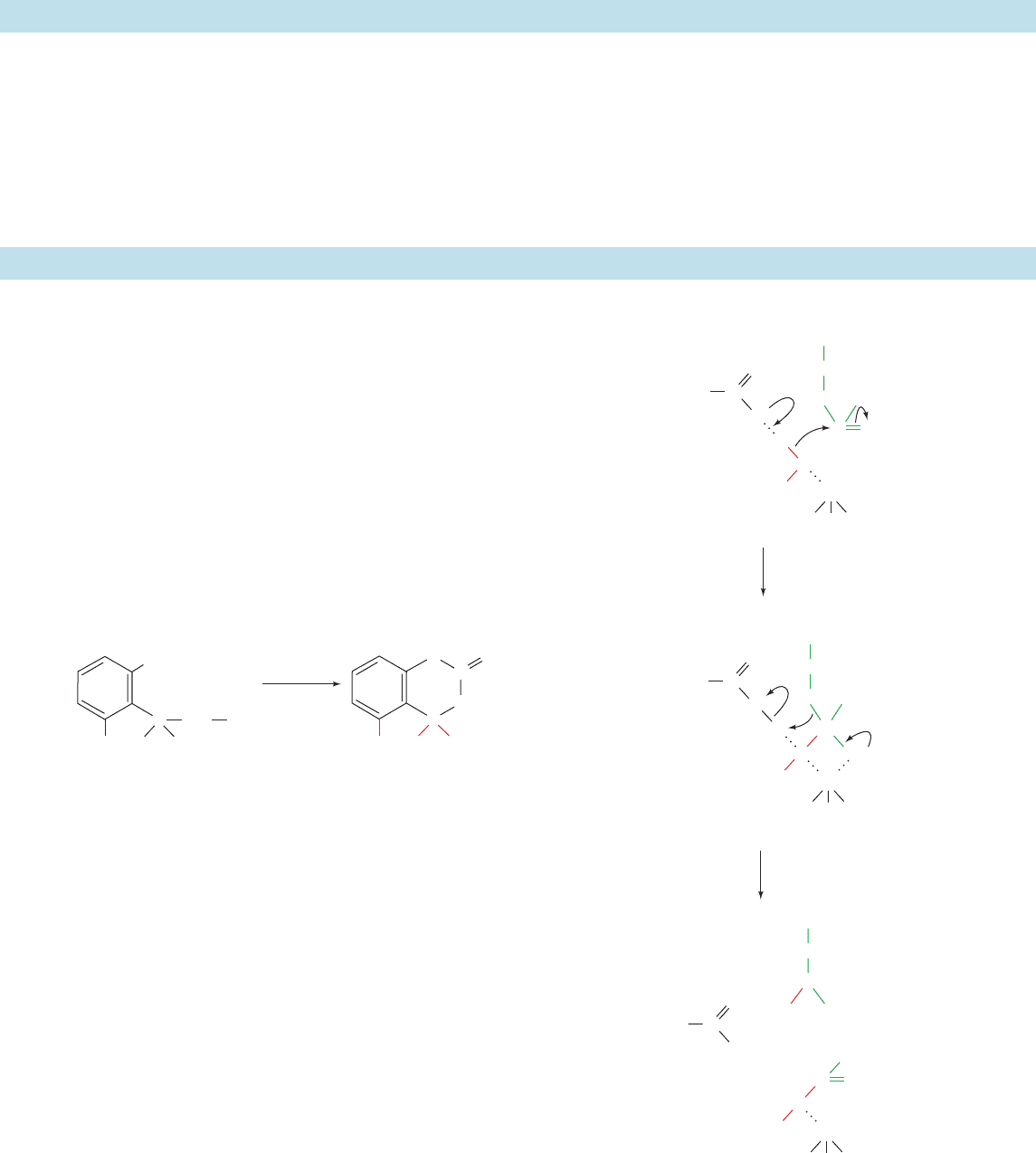
Figure 15-42 Mechanism of carboxypeptidase A.
Glu 270 C
CO
O
O
–
H
H
attack of
water
Zn
2+
CO
2
–
CHR
NH R⬘
Michaelis complex
Glu 270 C
C
O
O
O
–
O
–
H
H H
H
scissle bond
scission
Zn
2+
CO
2
–
CHR
NH R⬘
CO
2
–
CHR
N
O
Glu 270 C
O
O
–
CO
O
H
Zn
2+
R⬘
Tetrahedral intermediate
Enzyme-product complex
+
Bioinformatics Exercises are available at www.wiley.com/college/voet.
Chapter 12
Enzyme Inhibitors and Rational Drug Design.
1. Dihydrofolate Reductase. Examine the structure of an enzyme with an inhibitor bound to it.
2. HIV Protease. Compare the structures of complexes containing HIV protease and an inhibitor.
3. Pharmacogenomics and Single Nucleotide Polymorphisms. Use online databases to find information on cytochrome P450 poly-
morphisms.
BIOINFORMATICS EXERCISES
JWCL281_c15_506-556.qxd 6/24/10 7:47 AM Page 555
located in clefts or depressions in enzymes rather than on protru-
sions?
11. Predict the effects on lysozyme catalysis of changing Phe
34, Ser 36, and Trp 108 to Arg, assuming that this change does not
significantly alter the structure of the protein.
*12. The incubation of (NAG)
4
with lysozyme results in the
slow formation of (NAG)
6
and (NAG)
2
. Propose a mechanism for
this reaction. What aspect of the lysozyme mechanism is sup-
ported by this reaction?
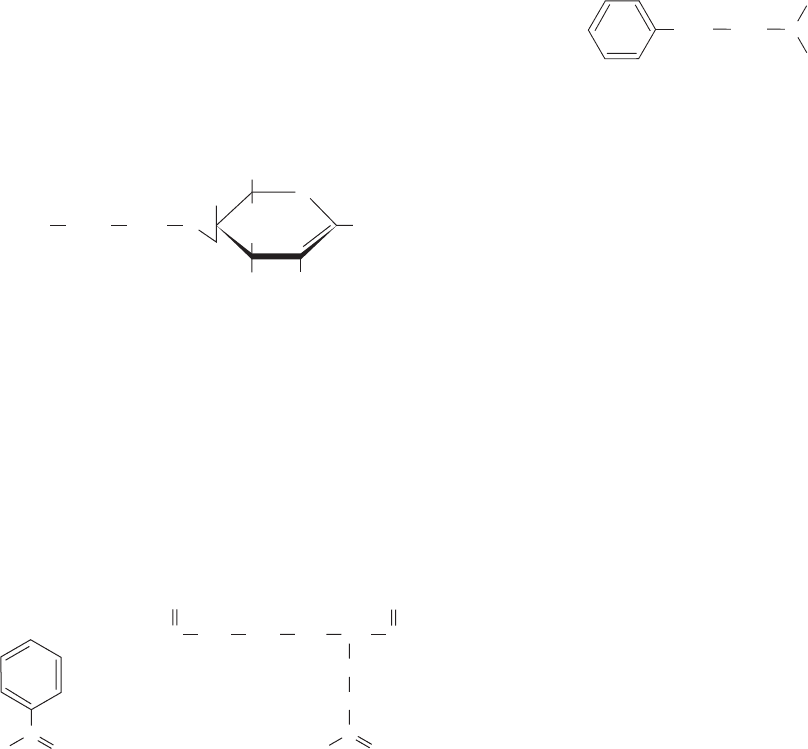
13. How would the lysozyme binding affinity of the following
(1 S 4)-linked tetrasaccharide compare with that of NAG–
NAM–NAG–NAM? Explain.
14. A major difficulty in investigating the properties of the
pancreatic serine proteases is that these enzymes, being proteins
themselves, are self-digesting. This problem is less severe, how-
ever, for solutions of chymotrypsin than it is for solutions of
trypsin or elastase. Explain.
15. The comparison of the active site geometries of chy-
motrypsin and subtilisin under the assumption that their similari-
ties have catalytic significance has led to greater mechanistic un-
derstanding of both these enzymes. Discuss the validity of this
strategy.
16. Benzamidine (K
I
1.8 10
5
M) and leupeptin (K
I
1.8 10
7
M; Fig. 15-19)
CH
3
C
C
NH
2
+
H
2
N
NH
2
+
H
2
N
Benzamidine
O
Leu
Leu NH CH CH
O
(CH
2
)
3
NH
C
Leupeptin
H
H
CH
2
OH
H
H NHCOCH
3
H
O
NAGNAG NAM
O
are both specific competitive inhibitors of trypsin. Explain their
mechanisms of inhibition. Design leupeptin analogs that inhibit
chymotrypsin and elastase.
17. Trigonal boronic acid derivatives have a high tendency to
form tetrahedral adducts. 2-Phenylethyl boronic acid
is an inhibitor of subtilisin and chymotrypsin. Indicate the struc-
ture of these enzyme–inhibitor complexes.
18. Tofu (bean curd), a high-protein soybean product that is
widely consumed in China and Japan, is prepared in such a way as
to remove the trypsin inhibitor present in soybeans. Explain the
reason(s) for this treatment.
19. Explain why mutating all three residues of trypsin’s cat-
alytic triad has essentially no greater effect on the enzyme’s cat-
alytic rate enhancement than mutating only Ser 195.
20. Explain why chymotrypsin is not self-activating as is trypsin.
21. Does Lipinski’s “rule of five” predict that a hexapeptide
would be a therapeutically effective drug? Explain.
22. The preferred antidote for acetaminophen overdose is N-
acetylcysteine. Explain why the administration of this substance,
which must occur within 8 to 16 hours of the overdose, is an effec-
tive treatment.
23. Why would the activation of HIV-1 protease before the
virus buds from its host cell be disadvantageous to the virus?
Explain.
CH
2
CH
2
B
OH
OH
2–Phenylethyl boronic acid
556 Chapter 15. Enzymatic Catalysis
JWCL281_c15_506-556.qxd 6/5/10 9:10 AM Page 556
PART IV
METABOLISM
Schematic diagram of the major
pathways of energy metabolism.
JWCL281_c16_557-592.qxd 7/28/10 6:22 PM Page 557
This page intentionally left blank
559
CHAPTER 16
Introduction to
Metabolism
1 Metabolic Pathways
2 Organic Reaction Mechanisms
A. Chemical Logic
B. Group-Transfer Reactions
C. Oxidations and Reductions
D. Eliminations, Isomerizations, and Rearrangements
E. Reactions That Make and Break Carbon–Carbon Bonds
3 Experimental Approaches to the Study of Metabolism
A. Metabolic Inhibitors, Growth Studies,
and Biochemical Genetics
B. Isotopes in Biochemistry
C. Isolated Organs, Cells, and Subcellular Organelles
D. Systems Biology
4 Thermodynamics of Phosphate Compounds
A. Phosphoryl-Transfer Reactions
B. Rationalizing the “Energy” in “High-Energy” Compounds
C. The Role of ATP
5 Oxidation–Reduction Reactions
A. The Nernst Equation
B. Measurements of Redox Potentials
C. Concentration Cells
6 Thermodynamics of Life
A. Living Systems Cannot Be at Equilibrium
B. Nonequilibrium Thermodynamics and the Steady State
C. Thermodynamics of Metabolic Control
Living organisms are not at equilibrium. Rather,they require
a continuous influx of free energy to maintain order in a uni-
verse bent on maximizing disorder. Metabolism is the over-
all process through which living systems acquire and utilize
the free energy they need to carry out their various func-
tions. They do so by coupling the exergonic reactions of nutri-
ent oxidation to the endergonic processes required to main-
tain the living state such as the performance of mechanical
work, the active transport of molecules against concentra-
tion gradients, and the biosynthesis of complex molecules.
How do living things acquire this necessary free energy?
And what is the nature of the energy coupling process?
Phototrophs (plants and certain bacteria; Section 1-1A)
acquire free energy from the sun through photosynthesis, a
process in which light energy powers the endergonic reac-
tion of CO
2
and H
2
O to form carbohydrates and O
2
(Chapter 24). Chemotrophs obtain their free energy by ox-
idizing organic compounds (carbohydrates, lipids, proteins)
obtained from other organisms, ultimately phototrophs.
This free energy is most often coupled to endergonic reac-
tions through the intermediate synthesis of “high-energy”
phosphate compounds such as adenosine triphosphate
(ATP; Section 16-4). In addition to being completely oxi-
dized, nutrients are broken down in a series of metabolic
reactions to common intermediates that are used as precur-
sors in the synthesis of other biological molecules.
A remarkable property of living systems is that, despite
the complexity of their internal processes, they maintain a
steady state. This is strikingly demonstrated by the observa-
tion that, over a 40-year time span, a normal human adult
consumes literally tons of nutrients and imbibes over 20,000 L
of water, but does so without significant weight change. This
steady state is maintained by a sophisticated set of metabolic
regulatory systems. In this introductory chapter to metabo-
lism, we outline the general characteristics of metabolic
pathways, study the main types of chemical reactions that
comprise these pathways, and consider the experimental
techniques that have been most useful in their elucidation.
We then discuss the free energy changes associated with re-
actions of phosphate compounds and oxidation–reduction
reactions. Finally we consider the thermodynamic nature
of biological processes, that is, what properties of life are
responsible for its self-sustaining character.
1 METABOLIC PATHWAYS
Metabolic pathways are series of consecutive enzymatic re-
actions that produce specific products. Their reactants, inter-
mediates, and products are referred to as metabolites. Since
an organism utilizes many metabolites, it has many meta-
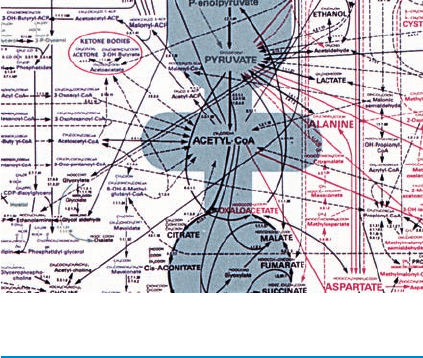
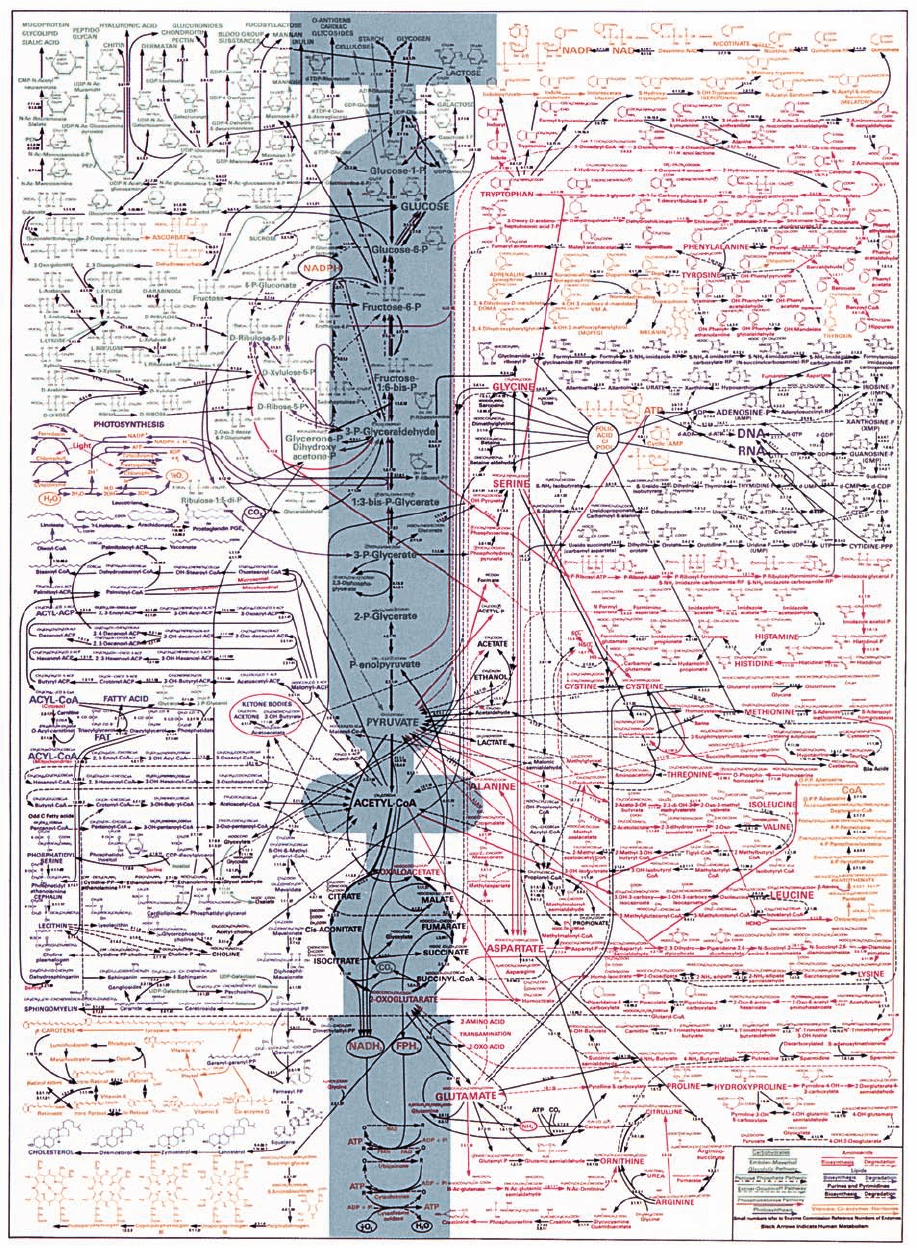
bolic pathways. Figure 16-1 shows a metabolic map for a
typical cell with many of its interconnected pathways. Each
reaction on the map is catalyzed by a distinct enzyme, of
which there are ⬃4000 known.At first glance, this network
seems hopelessly complex. Yet, by focusing on its major
areas in the following chapters, for example, the main
pathways of glucose oxidation (the shaded areas of Fig.
16-1), we shall become familiar with its most important av-
enues and their interrelationships. Maps of metabolic path-
ways in a more readable form can be found on the Web
at http://www.expasy.org/cgi-bin/search-biochem-index,
http://www.iubmb-nicholson.org/, and http://www.genome.
ad.jp/kegg/metabolism.html.
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 559
560 Chapter 16. Introduction to Metabolism
Figure 16-1 Map of the major metabolic pathways in a typical cell. The main pathways of
glucose metabolism are shaded. [Designed by Donald Nicholson. Published by BDH Ltd., Poole
2, Dorset, England.]
JWCL281_c16_557-592.qxd 2/26/10 11:10 AM Page 560
