Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

b. Second Messengers Mediate Glucagon- and
Epinephrine-Stimulated Glycogen Breakdown
The response to glucagon and epinephrine involves the
release inside the cell of molecules known as second messen-
gers, that is, intracellular mediators of the externally received
hormonal message. Different receptors act to release dif-
ferent second messengers. Indeed, cAMP was identified by
Earl Sutherland as the first known instance of a second
messenger through his demonstration that glucagon and
epinephrine act at cell surfaces to stimulate adenylyl cy-
clase (AC) to increase [cAMP] [the mechanism of AC acti-
vation, as well as a discussion of other second messengers,
including Ca
2⫹
, inositol-1,4,5-trisphosphate (IP
3
), and
diacylglycerol (DAG), is elaborated in Sections 19-2D and
19-4A]. Following this discovery, it was realized that
cAMP, which is present in all forms of life, is an essential
control element in many biological processes.
When hormonal stimulation by glucagon or epineph-
rine increases the intracellular cAMP concentration, the
protein kinase A activity increases, increasing the rates of
phosphorylation of many proteins and decreasing their
dephosphorylation rates as well. A decrease in dephos-
phorylation rates, as previously noted, increases the phos-
phorylation level of phosphoprotein phosphatase in-
hibitor-1, which in turn inhibits phosphoprotein
phosphatase-1. An increase in the concentration of phos-
phorylase a also contributes to the inhibition of phospho-
protein phosphatase-1.
Because of the amplifying properties of the cyclic cas-
cades, a small change in [cAMP] results in a large change in
the fraction of enzymes in their phosphorylated forms.
When a large fraction of the glycogen metabolism enzymes
are present in their phosphorylated forms, the metabolic
flux is in the direction of glycogen breakdown, since glyco-
gen phosphorylase is active and glycogen synthase is inac-
tive. When [cAMP] decreases, phosphorylation rates de-
crease, dephosphorylation rates increase, and the fraction
of enzymes in their dephospho forms increases. The result-
ant activation of glycogen synthase and the inhibition of
glycogen phosphorylase cause a change in the flux direc-
tion toward net glycogen synthesis.
F. Maintenance of Blood Glucose Levels
An important function of the liver is to maintain the blood
concentration of glucose, the brain’s primary fuel source, at
⬃5 mM.When blood [glucose] decreases beneath this level,
usually during exercise or well after meals have been di-
gested, the liver releases glucose into the bloodstream. The
process is mediated by the hormone glucagon as follows:
1. Glucose inhibits the pancreatic ␣ cells from secreting
glucagon into the bloodstream. When the blood glucose
concentration falls, this inhibition is released causing the ␣
cells to secrete glucagon.
2. Glucagon receptors on liver cell surfaces respond to
the presence of glucagon by activating adenylate cyclase,
thereby increasing the [cAMP] inside these cells.
3. The [cAMP] increase, as described above, triggers an
increase in the rate of glycogen breakdown, leading to in-
creased intracellular [G6P].
4. G6P, in contrast to glucose, cannot pass through the
cell membrane. However, in liver, which does not employ
glucose as a major energy source, the enzyme glucose-6-
phosphatase (G6Pase) hydrolyzes G6P:
The resulting glucose enters the bloodstream, thereby in-
creasing the blood glucose concentration. Muscle and brain
cells, however, lack G6Pase so that they retain their G6P.
G6P hydrolysis requires intracellular G6P transport.
G6P is produced in the cytosol, whereas G6Pase is a TM
protein that resides in the endoplasmic reticulum (ER)
membrane with its active site in the ER lumen. G6P must
therefore be imported into the ER by a G6P translocase
before it can be hydrolyzed. The resulting glucose and P
i
are then returned to the cytosol via specific transport pro-
teins (Section 18-3G).A defect in any of the components of
this G6P hydrolysis system results in type I glycogen stor-
age disease (Section 18-4).
How does this delicately balanced system respond to
an increase in blood [glucose]? When blood sugar is high,
normally immediately after meals have been digested,
glucagon levels decrease and insulin is released from
the pancreatic  cells. The rate of glucose transport
across many cell membranes increases in response to in-
sulin (through the insulin-dependent glucose transporter
GLUT4; Section 20-2E), [cAMP] decreases, and glycogen
metabolism therefore shifts from glycogen breakdown to
glycogen synthesis. The mechanism of insulin action is
quite complex and is not fully understood (Sections 19-3
and 19-4F), but one of its target enzymes appears to be
phosphoprotein phosphatase-1.
In muscle, insulin and epinephrine have antagonistic ef-
fects on glycogen metabolism. Epinephrine promotes
glycogenolysis by activating the cAMP-dependent phos-
phorylation cascade, which stimulates glycogen breakdown
while inhibiting glycogen synthesis. Insulin, as we saw in
Section 18-3Cg, activates insulin-stimulated protein kinase
to phosphorylate site 1 on the glycogen-binding G
M
sub-
unit of phosphoprotein phosphatase-1 so as to activate this
protein to dephosphorylate the enzymes of glycogen me-
tabolism (Fig. 18-22). The storage of glucose as glycogen is
thereby stimulated through the inhibition of glycogen
breakdown and the stimulation of glycogen synthesis.
In liver,it appears that glucose and glucose-6-phosphate
themselves may be the messengers to which the glycogen
metabolism system responds. Glucose inhibits phosphory-
lase a by binding only to the active site of the enzyme’s inac-
tive T state, but in a manner different from that of substrate.
The presence of glucose therefore shifts phosphorylase a’s
T 34 R equilibrium toward the T state (Fig. 18-10, right).
This conformational shift, as we saw in Section 18-3Cg, ex-
poses the Ser 14-phosphoryl group to phosphoprotein
phosphatase-1, resulting in the demodification of phospho-
rylase a. An increase in glucose concentration therefore
G6P ⫹ H
2
O
¡
glucose ⫹ P
i
Section 18-3. Control of Glycogen Metabolism 661
JWCL281_c18_638-670.qxd 6/3/10 1:49 PM Page 661
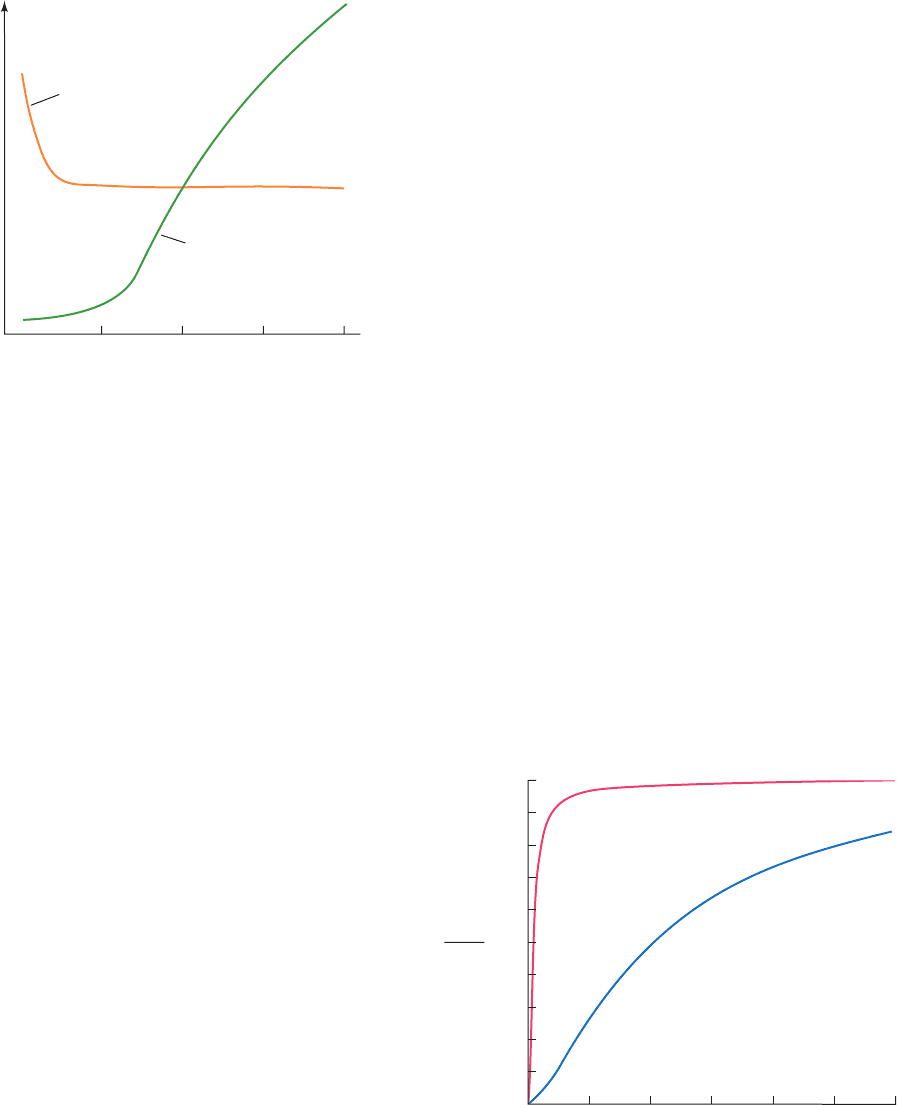
promotes inactivation of glycogen phosphorylase a
through the enzyme’s conversion to phosphorylase b
(Fig. 18-23; i.e., phosphorylase a acts as a glucose receptor).
The concomitant release of phosphoprotein phosphatase-1
inhibition (recall that it specifically binds to and is thereby
inactivated by phosphorylase a), moreover, results in the
activation (dephosphorylation) of m-glycogen synthase b.
In addition,glucose is converted to G6P by glucokinase (see
below), which facilitates the dephosphorylation and activa-
tion of m-glycogen synthase b to o-glycogen synthase a.
Above a glucose concentration of 7 mM, these processes
reverse the flux of glycogen metabolism from breakdown to
synthesis. The liver can thereby store the excess glucose as
glycogen.
a. Glucokinase Forms G6P at a Rate Proportional
to the Glucose Concentration
The liver’s function in “buffering” the blood [glucose] is
made possible because this organ contains a variant of hex-
okinase (the first glycolytic enzyme; Section 17-2A) known
as glucokinase (GK; also called hexokinase D and
hexokinase IV). The hexokinase in most cells obeys
Michaelis–Menten kinetics, has a high glucose affinity
(K
M
0.1 mM; the value of [glucose] at which the enzyme
achieves half-maximal velocity; Section 14-2A), and is in-
hibited by its reaction product, G6P. GK, in contrast, has a
much lower glucose affinity (reaching half of its maximal
velocity at ⬃5 mM) and displays sigmoidal kinetics with a
Hill constant (Section 10-1Bb) of 1.5, so its activity in-
creases rapidly with the blood [glucose] over the normal
physiological range (Fig. 18-24; see Problem 7 of this chap-
ter). GK, moreover, is not inhibited by physiological concen-
trations of G6P. Consequently, the higher the blood [glu-
cose], the faster the liver converts glucose to G6P [liver
cells, unlike most cells, contain ample quantities of the
insulin-independent glucose transporter GLUT2 (Section
20-2Eb) and are therefore freely permeable to glucose;
their glucose transport rate is unresponsive to insulin].
Thus at low blood [glucose], the liver does not compete
with other tissues for the available glucose supply, whereas
at high blood [glucose],when the glucose needs of these tis-
sues are met, glucose in the liver is converted to G6P. The
excess glucose in the liver induces the inactivation of glyco-
gen phosphorylase and the release of phosphoprotein
phosphatase-1, whereas the resulting G6P allosterically
facilitates the activation of glycogen synthase via dephos-
phorylation. The net result is that the liver converts the
excess glucose to glycogen. (Note that GK is a monomeric
enzyme, so that its sigmoidal rate increase with [glucose]
is a puzzling observation in light of the various allosteric
models indicating that monomeric enzymes are inca-
pable of cooperative behavior. Since GK does not exhibit
Michaelis–Menten kinetics, the glucose concentration
when this enzyme has half its maximal activity is known as
its K
0.5
in analogy with the operational definition of K
M
.)
GK is subject to metabolic controls. Emile Van Schaftin-
gen isolated glucokinase regulatory protein (GKRP; a
625-residue monomer) from rat liver, which, in the pres-
ence of the glycolytic intermediate fructose-6-phosphate
(F6P), is a competitive inhibitor of glucokinase. Fructose-1-
phosphate (F1P), an intermediate in liver fructose metabo-
lism (Section 17-5A), overcomes this inhibition. Since
fructose is normally available only from dietary sources
(e.g., sucrose), fructose may be the signal that triggers the
uptake of dietary glucose by the liver.
662 Chapter 18. Glycogen Metabolism
Figure 18-23 The enzymatic activities of phosphorylase a and
glycogen synthase in mouse liver in response to an infusion of
glucose. Phosphorylase a is rapidly inactivated and, somewhat
later, glycogen synthase is activated. [After Stalmans, W., De
Wulf, H., Hue, L., and Hers, H.-G., Eur. J. Biochem. 41, 129
(1974).]
Figure 18-24 Comparison of the relative enzymatic activities
of hexokinase and glucokinase over the physiological blood
glucose range. The affinity of glucokinase for glucose
(K
0.5
5 mM ) is much lower than that of hexokinase
(K
M
0.1 mM) and exhibits sigmoidal rather than hyperbolic
variation with [glucose]. [The glucokinase curve was generated
using the Hill equation (Eq. [10.7]) with K 10 mM and
n 1.5 as determined by Cardenas, M.L., Rabajille, E., and
Niemeyer, H., Eur. J. Biochem. 145, 163–171 (1984).]
04826
Phosphorylase a
Glycogen
synthase
Time after glucose infusion (min)
Enzyme activity
Hexokinase
Glucokinase
0
0.0
0.2
0.4
0.6
0.8
1.0
5
[Glucose] (mM)
v
o
V
max
10 15
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 662
b. Glucokinase Regulates Glucose Homeostasis via
an Intracellular Localization Mechanism
Intracellular localization plays an important role in GK
inhibition by GKRP. GK translocates freely between the
nucleus and cytoplasm. However, GKRP is located exclu-
sively in the nucleus. When the glucose concentration is
low, GK remains bound to GKRP in the nucleus, where it is
unavailable to phosphorylate glucose. At increased con-
centrations of glucose and/or F1P, GK dissociates from
GKRP and translocates to the cytoplasm, where it phos-
phorylates glucose to G6P, starting it on its way toward
glycogen synthesis.The antagonism of GK and GKRP pro-
vides a major mechanism for controlling glucose phospho-
rylation and glycogen metabolism in the liver. Their flux
control coefficients (Section 17-4Ca) in liver cells are close
to ⫹1 for GK and ⫺1 for GKRP (a negative flux control
coefficient is indicative of inhibition). This countervailing
relationship provides a sensitive mechanism for maintain-
ing glucose homeostasis.
Phosphoglucomutase, which has a high enough activity
to equilibrate its substrate and product and therefore func-
tions in either direction, transforms G6P to G1P, which is
then converted to glycogen. Some of the G6P is also recon-
verted to glucose by the action of glucose-6-phosphatase in
what amounts to a “futile” cycle. This is apparently the en-
ergetic price of effective glucose “buffering” of the blood.
c. Fructose-2,6-Bisphosphate Activates Glycolysis
-D-Fructose-2,6-bisphosphate (F2,6P)
is also an important factor in the liver’s maintenance of
blood [glucose]. F2,6P, which is not a glycolytic metabolite,
is an extremely potent allosteric activator of animal phos-
phofructokinase (PFK) and an inhibitor of fructose bispho-
sphatase (FBPase). F2,6P, which was independently discov-
O
HHO
H
CH
2
OH
HO H
OH
2
C
–2
O
3
P
PO
3
O
2–
-D-Fructose-2,6-bisphosphate
(F2,6P)
ered in 1980 by Simon Pilkis, by Emile Van Schaftingen and
Henri-Géry Hers, and by Kosaku Uyeda, therefore stimu-
lates glycolytic flux (the F6P–FBP substrate cycle is dis-
cussed in Section 17-4Ff).
The concentration of F2,6P in the cell depends on the bal-
ance between its rates of synthesis and degradation by phos-
phofructokinase-2 (PFK-2; also called 6PF-2-K) and fruc-
tose bisphosphatase-2 (FBPase-2; also called F-2,6-Pase),
respectively (Fig. 18-25). These enzyme activities are located
on different domains of a single ⬃100-kD homodimeric
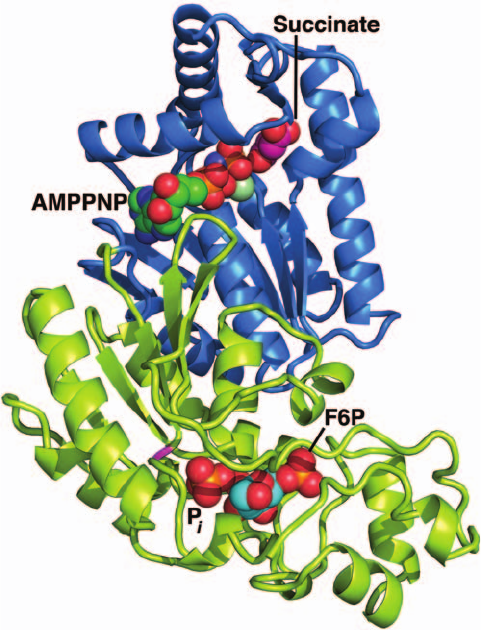
protein named PFK-2/FBPase-2. The X-ray structure of the
H256A mutant of rat testis PFK-2/FBPase-2 in complex
with F6P, P
i
, succinate, and the nonhydrolyzable ATP ana-
log adenosine-5ⴕ-(,␥-imido)triphosphate (AMPPNP)
was determined by Uyeda and Charles Hasemann (Fig. 18-
26). It indicates, in agreement with a variety of studies,
that the PFK-2 activity resides on each subunit’s 246-residue
N-terminal domain, whereas the FBPase-2 activity resides
on each subunit’s 213-residue C-terminal domain. The
succinate, which binds in the vicinity of AMPPNP’s ␥ phos-
phate group, presumably occupies the F6P binding pocket in
the PFK-2 active site, whereas the F6P and P
i
mark the F2,6P
binding site in the FBPase-2 active site. The FBPase-2 do-
main is structurally related to the glycolytic enzyme phos-
phoglycerate mutase (PGM; Section 17-2H) and shares a
common catalytic mechanism involving a covalent phospho-
His intermediate (His 256 in FBPase-2).The PFK-2 domain
is structurally related to adenylate kinase (Section 17-4Fe)
but not, as had been speculated, to PFK (also called PFK-1
to distinguish this glycolytic enzyme from PFK-2).
The enzyme activities of PFK-2/FBPase-2 are subject to
allosteric regulation by a variety of metabolic intermediates
H
H
O
–
CH
2
Adenosine-5ⴕ-(,␥-imido)triphosphate
(AMPPNP)
A
O
H
H
OH OH
O
PO
O
–
O
P
O
–
–
O
O
P NH
Section 18-3. Control of Glycogen Metabolism 663
Figure 18-25 Formation and degradation of -D-fructose-2,
6-bisphosphate as catalyzed by PFK-2 and FBPase-2. These two
enzyme activities occur on different domains of the same protein
HHO
ATP ADP
H
2
C
liver PFK-2
(dephosphoenzyme)
P
i
H
2
O
liver FBPase-2
(phosphoenzyme)
β-
D-Fructose-6-phosphate
(F6P)
O
O
–
2
O
3
P
H
CH
2
OH
HO
OH
H
HHO
H
2
C
β-D-Fructose-2,6-bisphosphate
(F2,6P)
O
O
–
2
O
3
P
H
CH
2
OH
HO
O
H
PO
2
3
–
molecule. Dephosphorylation of the liver enzyme activates
PFK-2 but deactivates FBPase-2.
JWCL281_c18_638-670.qxd 6/30/10 12:00 PM Page 663
as well as to phosphorylation/dephosphorylation by pro-
tein kinase A and a phosphoprotein phosphatase. Phos-
phorylation of the liver enzyme at its Ser 32 inhibits its
PFK-2 activity and activates its FBPase-2 activity.Thus, the
pancreatic cell’s release of glucagon in response to low
blood [glucose] results, through an increase in liver
[cAMP], in a decreased liver [F2,6P]. This situation, in turn,
decreases the PFK-1 activity, thereby inhibiting glycolysis.
Hence, the G6P resulting from the concurrent stimulation
of glycogen degradation is converted to glucose and se-
creted as described above rather than being metabolized.
Simultaneously, the deinhibition of FBPase (also called
FBPase-1 to distinguish it from FBPase-2) by the decrease
of [F2,6P] stimulates gluconeogenesis, the formation of
glucose from nonglucose precursors such as amino acids by
a pathway that effectively reverses glycolytic flux (and in
which FBPase-1 is a key regulatory enzyme; Section 23-1).
This process provides a second means of glucose produc-
tion. Conversely, when the blood [glucose] is high, cAMP
levels decrease, the liver PFK-2/FBPase-2 is dephosphory-
lated by phosphoprotein phosphatase-1 activating PFK-2,
which, in turn, causes a rise in [F2,6P]. PFK-1 is therefore
activated, FBPase-1 is inhibited, and the net glycolytic flux
changes from gluconeogenesis to glycolysis.
The F2,6P control systems in skeletal muscle and in
heart muscle function quite differently from that in liver due
to the presence in these tissues of different PFK-2/FBPase-2
isozymes. In heart and skeletal muscles, increased glyco-
gen breakdown is coordinated with increased glycolysis
rather than increased glucose secretion. This is because
phosphorylation of the heart muscle PFK-2/FBPase-2
isozyme occurs at entirely different sites (Ser 406 and Thr
475 of the 530-residue protein) from that of the liver
isozyme (Ser 32 of the 470-residue protein) and activates
rather than inhibits PFK-2. Consequently, hormones that
stimulate glycogen breakdown also increase heart muscle
[F2,6BP], thereby stimulating glycolysis as well. The skele-
tal muscle and testis isozymes lack phosphorylation
sites altogether and are therefore not subject to cAMP-
dependent phosphorylation control.
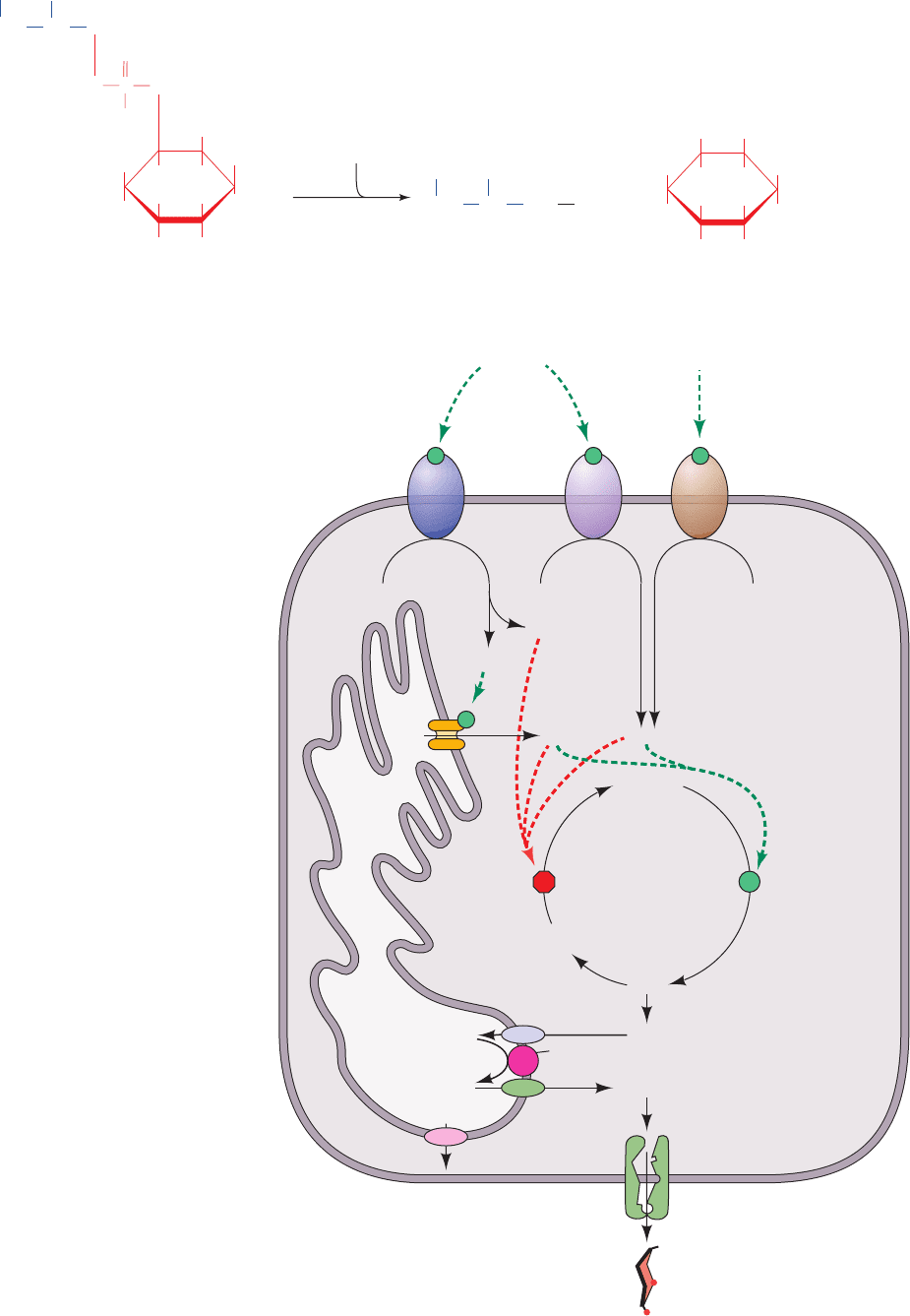
G. Response to Stress
Epinephrine and norepinephrine, which are often called
the “fight or flight” hormones, are released into the blood-
stream by the adrenal glands in response to stress. Epi-
nephrine receptors (known as -adrenergic receptors; Sec-
tion 19-1F) present on the surfaces of liver and muscle cells
respond to these hormones just as glucagon receptors re-
spond to the presence of glucagon; they activate adenylate
cyclase, thereby increasing intracellular [cAMP]. Indeed,
epinephrine also stimulates the pancreatic cells to re-
lease glucagon, which further increases liver [cAMP]. The
G6P produced by the consequent glycogen breakdown in
muscle enters the glycolytic pathway, thereby generating
ATP and helping the muscles cope with the stress that trig-
gered the epinephrine release.
The liver’s response to stress, in addition to its response
to the glucagon released by pancreatic epinephrine stimu-
lation, involves response to epinephrine stimulation via
two types of receptors, -adrenergic receptors, as discussed
above, and ␣-adrenergic receptors. -Adrenergic receptors
act by stimulating phospholipase C to release other second
messengers, namely inositol-1,4,5-trisphosphate (IP
3
), dia-
cylglycerol (DAG), and Ca
2
(Fig. 18-27a), which act to re-
inforce the cells’ response to cAMP. As we mentioned in
Section 18-3Cf, phosphorylase kinase, which both activates
phosphorylase and inactivates glycogen synthase, is fully
active only when both phosphorylated and in the presence
of increased [Ca
2
]. In addition, glycogen synthase is inac-
tivated by phosphorylation by several other Ca
2
-depend-
ent protein kinases, including protein kinase C (Section
18-3D). Protein kinase C requires both Ca
2
and DAG for
activity (Section 19-4C).This dual stimulation of receptors
in response to epinephrine causes the liver to produce
664 Chapter 18. Glycogen Metabolism
Figure 18-26 X-ray structure of the H256A mutant of rat
testis PFK-2/FBPase-2. The N-terminal PFK-2 domain is blue
and the C-terminal FBPase-2 domain is yellow-green.The bound
Mg
2
–AMPPNP, succinate, F6P, and P
i
are shown in space-filling
form colored according to atom type (AMPPNP C green,
succinate C magenta, F6P C cyan, N blue, O red, Mg
2
light
green, and P orange). The P
i
, which occupies the binding site of
the F2,6P’s 2-phosphate group, is opposite the site that would be
occupied by the side chain of the wild-type enzyme’s His 256
(magenta), to which it would be transferred in the catalytic
reaction.The succinate occupies the presumed F6P-binding
pocket of the PFK-2 domain. [Based on an X-ray structure by
Kosaku Uyeda and Charles Hasemann, University of Texas
Southwestern Medical Center. PDBid 2BIF.]
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 664
Section 18-3. Control of Glycogen Metabolism 665
Phosphatidylinositol-4,5-bisphosphate (PIP
2
)
CH
2
CH
2
CH
OR
2
OR
1
O
O
–
P
O
O
OPO
3
2–
OPO
3
2–
HO
HO
OH
H
H
H
H
H
H
CH
2
OH
CH
2
CH
OR
2
OR
1
1,2-Diacylglycerol
(DAG)
Inositol-1,4,5-triphosphate
(IP
3
)
2
–
O
3
PO
2
–
O
3
PO
OPO
3
2–
OPO
3
2–
HO
HO
OH
H
H
H
H
H
H
⫹
H
2
O
phospholipase C
(a)
β-Adrenergic
receptor
Glucagon
receptor
Epinephrine Glucagon
glycogen
synthase
phospho-
lipase C
adenylate
cyclase
adenylate
cyclase
ATP
Liver
Cell
ATP
IP
3
PIP
2
DAG
UDP–glucose
pyrophosphorylase
glycogen
phosphorylase
cAMP
Ca
2+
Glycogen
G1P
G6P
UDP–glucose
phosphoglucomutase
Glucose-6-
phosphatase
GLUT2 glucose
transporter
α-Adrenergic receptor
Glucose
Glucose
Ca
2+
G6P
T1
T3
Glucose
+
P
i
Endoplasmic
reticulum
(b)
T2
Figure 18-27 The liver’s response to
stress. (a) Stimulation of ␣-adrenergic
receptors by epinephrine activates
phospholipase C to hydrolyze
phosphatidylinositol-4,5-bisphosphate
(PIP
2
) to inositol-1,4,5-trisphosphate
(IP
3
) and diacylglycerol (DAG). (b) The
participation of two second messenger
systems: the cAMP-mediated stimulation
of glycogenolysis and inhibition of
glycogen synthesis triggered by glucagon
and -adrenergic receptor activation; and
the IP
3
, DAG, and Ca
2⫹
-mediated
stimulation of glycogenolysis and
inhibition of glycogen synthesis triggered
by ␣-adrenergic receptor activation. IP
3
stimulates the release of Ca
2⫹
from the
endoplasmic reticulum, whereas DAG,
together with Ca
2⫹
, activates protein
kinase C to phosphorylate and thereby
inactivate glycogen synthase. G6Pase
occupies the endoplasmic reticulum.
Consequently, the cytosolically produced
G6P is transported into the endoplasmic
reticulum via the T1 G6P translocase,
where it is hydrolyzed to glucose and P
i
.
The P
i
and glucose are then returned to
the cytosol by the T2 and T3 transporters,
respectively, and the glucose is exported
from the cell via the GLUT2 glucose
transporter.
JWCL281_c18_638-670.qxd 6/10/10 1:09 PM Page 665

G6P, which is hydrolyzed by G6Pase, resulting in the re-
lease of glucose into the bloodstream, thereby further fuel-
ing the muscles (Fig. 18-27b).
4 GLYCOGEN STORAGE DISEASES
With glycogen metabolism being such a finely controlled
system, it is not surprising that genetically determined en-
zyme deficiencies result in disease states. The study of
these disease states and the enzyme deficiencies that cause
them has provided insights into the system’s balance. In
this sense, genetic diseases are valuable research tools.
Conversely, the biochemical characterization of the path-
ways affected by a genetic disease often leads, as we shall
see, to useful strategies for its treatment. Many diseases
have been characterized that result from inherited defi-
ciencies of one or another of the enzymes of glycogen me-
tabolism. These defects are listed in Table 18-1 and dis-
cussed in this section.
Type I: Glucose-6-Phosphatase Deficiency
(von Gierke’s Disease)
G6Pase catalyzes the final step leading to the release of
glucose into the bloodstream by the liver. Deficiency of
this enzyme results in an increase of intracellular [G6P],
which leads to a large accumulation of glycogen of normal
structure in the liver and kidney (recall that G6P inhibits
glycogen phosphorylase and activates glycogen synthase)
and an inability to increase blood glucose concentration in
response to glucagon or epinephrine. Similar difficulties
occur when there are defects in the protein that transports
glucose across the liver cell plasma membrane (Section
20-2Eb) or in any of the proteins that transport glucose,
G6P, or P
i
across the endoplasmic reticulum membrane
(Section 18-3F; Fig. 18-27b). The symptoms of Type I
glycogen storage disease include massive liver enlarge-
ment, severe hypoglycemia (low blood sugar) after a few
hours’ fast, and a general failure to thrive. Treatment of
the disease has included drug-induced inhibition of glu-
cose uptake by the liver to increase blood [glucose], con-
tinuous intragastric feeding overnight, again to increase
blood [glucose], oral administration of uncooked corn
starch (which is only slowly broken down to glucose), and
surgical transposition of the portal vein, which ordinarily
feeds the liver directly from the intestines, so as to allow
this glucose-rich blood to reach peripheral tissues before it
reaches the liver.This latter treatment has the added ben-
efit of allowing the tissues to receive more glucose while
decreasing the storage of this glucose as liver glycogen.
Liver transplantation has also been successful in the few
patients in which this treatment has been tried.
A gene therapy protocol (Section 5-5H) is being devel-
oped to correct type I glycogen storage disease. G6Pase-
deficient knockout mice (Section 5-5H) have been treated
with a viral vector containing the mouse G6Pase gene.This
treatment, which delivers G6Pase to the livers of these
mice, greatly increases their survival rate and corrects the
metabolic abnormalities associated with this glycogen stor-
age disease.
Type II: ␣-1,4-Glucosidase Deficiency
(Pompe’s Disease)
This is the most devastating glycogen storage disease. It
results in a large accumulation of glycogen of normal
structure in the lysosomes of all cells and causes death by
cardiorespiratory failure, usually before the age of 1 year.
We have not discussed ␣-1,4-glucosidase in the sections on
the pathways of glycogen synthesis and breakdown since it
is not among those enzymes. It occurs in lysosomes, where
it functions to hydrolyze the disaccharide maltose (Section
11-2B) and linear oligosaccharides, as well as the outer
branches of glycogen, thereby yielding free glucose. How-
ever, this second pathway of glycogen metabolism is not
quantitatively important. The reason that lysosomes nor-
mally take up and degrade glycogen granules is unknown.
666 Chapter 18. Glycogen Metabolism
Table 18-1 Hereditary Glycogen Storage Diseases
Type Enzyme Deficiency Tissue Common Name Glycogen Structure
I Glucose-6-phosphatase Liver von Gierke’s disease Normal
II ␣-1,4-Glucosidase All lysosomes Pompe’s disease Normal
III Amylo-1,6-glucosidase All organs Cori’s disease Outer chains missing
(glycogen debranching enzyme) or very short
IV Amylo-(1,4 S 1,6)- Liver, probably Andersen’s disease Very long
transglycosylase all organs unbranched chains
(glycogen branching enzyme)
V Glycogen phosphorylase Muscle McArdle’s disease Normal
VI Glycogen phosphorylase Liver Hers’ disease Normal
VII Phosphofructokinase Muscle Tarui’s disease Normal
VIII Phosphorylase kinase Liver X-Linked phosphorylase Normal
kinase deficiency
IX Phosphorylase kinase All tissues Normal
0 Glycogen synthase Liver Normal, deficient
in quantity
JWCL281_c18_638-670.qxd 6/30/10 12:00 PM Page 666
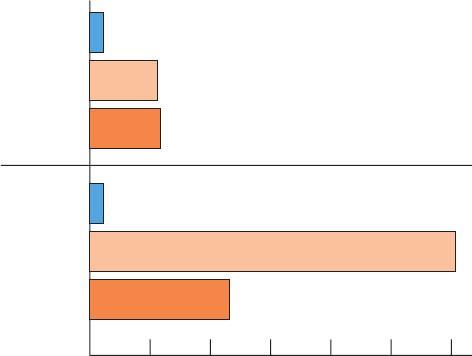
Type III: Amylo-1,6-Glucosidase (Glycogen
Debranching Enzyme) Deficiency (Cori’s Disease)
In this disease, glycogen of abnormal structure contain-
ing very short outer chains accumulates in both liver and
muscle since, in the absence of debranching enzyme, the
glycogen cannot be further degraded. Its hypoglycemic
symptoms are similar to, but not as severe as, those of von
Gierke’s disease (Type I). The low blood sugar, which in
this case is a result of the decreased efficiency of glycogen
breakdown, is treated with frequent feeding and a high-
protein diet [in response to low blood sugar, the liver,
through gluconeogenesis (Section 23-1), synthesizes glucose
from amino acids]. For unknown reasons, the symptoms of
Cori’s disease often disappear at puberty.
Type IV: Amylo-(1,4 S 1,6)-Transglycosylase
(Glycogen Branching Enzyme) Deficiency
(Andersen’s Disease)
This is one of the most severe glycogen storage diseases;
victims rarely survive past the age of 5 years because of
liver dysfunction. Glycogen concentration in liver is not in-
creased but its structure is abnormal, with very long un-
branched chains resulting from the lack of glycogen
branching enzyme. This decreased branching greatly
reduces the solubility of glycogen. It has been suggested
that the liver dysfunction may be caused by a “foreign
body” immune reaction to the abnormal glycogen.
Type V: Muscle Glycogen Phosphorylase Deficiency
(McArdle’s Disease)
We have mentioned this condition in connection with
the realization that glycogen synthesis and breakdown
must occur by different pathways (Section 18-2). Its major
symptom, which is most severely manifested in early adult-
hood, is painful muscle cramps on exertion.This situation is
a result of the inability of the glycogen breakdown system
to provide sufficient fuel for glycolysis to keep up with the
metabolic demand for ATP. Studies by
31
P NMR on human
forearm muscle have noninvasively corroborated this con-
clusion by demonstrating that exercise in individuals with
McArdle’s disease leads to elevated muscle ADP levels
compared to those of normal individuals (Fig. 18-28). Curi-
ously, if McArdle’s victims continue their exertions after a
short rest, their cramps subside. This “second wind” effect
has been attributed to the muscles’ switch from anaerobic
to aerobic metabolism as well as vasodilation, which gives
the muscles increased access to the glucose and fatty acids
in the blood for use as alternative fuels to glycogen. Liver
glycogen phosphorylase is normal in these individuals, im-
plying the presence of different glycogen phosphorylase
isozymes in muscle and liver.
Type VI: Liver Glycogen Phosphorylase Deficiency
(Hers’ Disease)
Patients with a deficiency of liver phosphorylase have
symptoms similar to those with mild forms of Type I glyco-
gen storage disease. The hypoglycemia in this case results
from the inability of glycogen phosphorylase to respond to
the need for glucose production by the liver.
Type VII: Muscle Phosphofructokinase Deficiency
(Tarui’s Disease)
The result of a deficiency of the glycolytic enzyme PFK-
1 in muscle is an abnormal buildup of the glycolytic
metabolites G6P and F6P. High concentrations of G6P in-
crease the activities of glycogen synthase (G6P activates
glycogen synthase and inactivates glycogen phosphory-
lase) and UDP–glucose pyrophosphorylase (G6P is in
equilibrium with G1P, a substrate for the enzyme) so that
glycogen accumulates in muscle. Other symptoms are
similar to those of Type V glycogen storage disease, mus-
cle phosphorylase deficiency, since PFK deficiency pre-
vents glycolysis from keeping up with the ATP demand of
muscle contraction.
Type VIII: Liver Phosphorylase Kinase Deficiency
(X-Linked Phosphorylase Kinase Deficiency)
Some individuals with symptoms of Type VI glycogen
storage disease have liver phosphorylase of normal struc-
ture. However, they have a defective phosphorylase kinase,
which results in their inability to convert phosphorylase b
to phosphorylase a. The gene encoding the subunit of
phosphorylase kinase resides on the X chromosome and
hence Type VIII disease is X-linked rather than autosomal,
as are the other glycogen storage diseases.
Type IX: Phosphorylase Kinase Deficiency
Phosphorylase kinase deficiency, when it is autosomal
recessive, is caused by a mutation in one of the genes
encoding a , , or subunit of phosphorylase kinase. Since
different organs contain different isozymes of phosphory-
lase kinase, the symptoms and severity of Type IX disease
vary with the affected organs.
Section 18-4. Glycogen Storage Diseases 667
Figure 18-28 The ADP concentration in human forearm
muscles during rest and following exertion in normal individuals
and those with McArdle’s disease. The ADP concentration was
determined from
31
P NMR measurements on intact forearms.
[After Radda, G.K., Biochem. Soc.Trans. 14, 522 (1986).]
McArdle's
patients
Normal
individuals
Rest
Light exercise
Rest
Upon cramps after light exercise
After “second wind”
Heavy exercise
leading to exhaustion
080
[ADP] (μM)
16040 120 200180
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 667

1 Glycogen Breakdown In animals, when glucose is not
needed as a source of metabolic energy, it is stored, predomi-
nantly in liver and muscle cells, as glycogen, an ␣(1 S 4)-linked
glucan with ␣(1 S 6) branches every 8 to 14 residues. Glycogen
breakdown to glucose-6-phosphate (G6P) is a two-step process.
Glycogen phosphorylase catalyzes the phosphorolysis of the gly-
cosidic linkage of a terminal glucosyl residue to form glucose-1-
phosphate (G1P). Phosphoglucomutase interconverts G1P and
G6P. Glycogen debranching enzyme allows complete degrada-
tion of glycogen by catalyzing the transfer of three-residue chains
onto the nonreducing ends of other chains and catalyzing the hy-
drolysis of the remaining ␣(1 S 6)-linked glucosyl unit to glucose.
2 Glycogen Synthesis Glycogen is synthesized from
G6P by a pathway different from that of glycogen breakdown.
G6P is converted to G1P under the influence of phosphoglu-
comutase. UDP–glucose pyrophosphorylase utilizes UTP to
convert G1P to UDP–glucose, the activated intermediate in
glycogen synthesis. The hydrolysis of the PP
i
product by inor-
ganic pyrophosphatase drives the reaction to completion. Glu-
cosyl units are transferred from UDP–glucose to the C4 OH
group of a terminal residue on a growing glycogen chain by
glycogen synthase. The chain is initiated on glycogenin.
Branching occurs through the action of glycogen branching
enzyme, which transfers
⬃7-residue segments of ␣(1 S 4)-
linked chains to the C6 OH group of a glucosyl residue on
the same or another glycogen chain.
3 Control of Glycogen Metabolism The rates at which
glycogen is synthesized by glycogen synthase and degraded by
glycogen phosphorylase are controlled by the levels of their al-
losteric effectors such as ATP,AMP,G6P, and glucose. Superim-
posed on this allosteric control is control by the phosphoryla-
tion/dephosphorylation of these enzymes. The kinases and
phosphatases that catalyze these modifications are part of am-
plifying cascades that are ultimately controlled by the hor-
mones glucagon, insulin, and epinephrine, and by Ca
2⫹
.
Glucagon and epinephrine stimulate glycogen breakdown by
stimulating adenylate cyclase to increase the intracellular
¬
¬
[cAMP]. cAMP is a “second messenger” that activates protein
kinase A (PKA), which, through its activation of phosphory-
lase kinase, results in the phosphorylation of both glycogen
phosphorylase and glycogen synthase. Phosphorylation activates
glycogen phosphorylase but inactivates glycogen synthase. In ad-
dition, epinephrine causes an increase in the concentrations of
other second messengers, namely, inositol-1,4,5-trisphosphate
(IP
3
), diacylglycerol (DAG), and Ca
2⫹
, that reinforce the cAMP-
dependent responses. Ca
2⫹
, which is also released into muscle cy-
tosol by nerve impulses,binds to calmodulin so as to induce this
protein to activate protein kinases via an intrasteric mecha-
nism by which Ca
2⫹
–CaM extracts autoinhibitory sequences
from the kinases’ active sites.A decrease in [cAMP] and/or the
presence of insulin leads to the activation of phosphoprotein
phosphatase-1 to dephosphorylate glycogen phosphorylase
and glycogen synthase.
The phosphoproteins that participate in glycogen metabolism
are dephosphorylated through the action of phosphoprotein
phosphatase-1, which is only active when it is associated with a
glycogen particle through the intermediacy of glycogen-binding
G
M
or G
L
subunits. When [glucose] is high, the liver synthesizes
glucose-6-phosphate (G6P) and ultimately glycogen from glu-
cose via the action of glucokinase (GK), which has kinetic prop-
erties distinct from those of other hexokinases.When [glucose] is
low, GK is inhibited by glucokinase regulatory protein (GKRP)
and G6Pase hydrolyzes the G6P product of glycogen breakdown
(which is favored at low [glucose]) for export to other tissues.The
concentration in liver of F2,6P, an activator of PFK and an in-
hibitor of FBPase, is also dependent on the rates of cAMP-
dependent phosphorylation and dephosphorylation. It is both
synthesized and degraded through the action of PFK-2/FBPase-2,
whose enzymatic activities are oppositely controlled by both
allosteric regulation and phosphorylation/ dephosphorylation.
4 Glycogen Storage Diseases Glycogen storage dis-
eases are caused by a genetic deficiency of one or another of
the enzymes of glycogen metabolism. Ten different deficien-
cies of varying severity have been reported in humans.
668 Chapter 18. Glycogen Metabolism
CHAPTER SUMMARY
Type 0: Liver Glycogen Synthase Deficiency
The activity of liver glycogen synthase is extremely low
in individuals with this disease and hence they exhibit hy-
perglycemia after meals and hypoglycemia at other times.
However, the primary lesion may not always be in the syn-
thase itself because other metabolic defects may lead to an
imbalance of the glycogen synthase cyclic cascade.The root
cause of Type 0 glycogen storage disease is still under in-
vestigation. Muscle glycogen synthase deficiency disease
has recently been described. Its victims are easily fatigued
and develop heart abnormalities that may cause sudden
cardiac arrest. Note these are the only known glycogen
storage diseases in which there is a deficiency rather than
an overabundance of glycogen.This suggests that the com-
plete absence of glycogen is lethal.
Since many of the glycogen storage diseases have simi-
lar clinical symptoms, these diseases are best diagnosed via
genetic testing.
REFERENCES
General
Boyer,P.D. and Krebs, E.G. (Eds.),The Enzymes (3rd ed.),Vol. 17,
Academic Press (1986). [Contains detailed articles on the en-
zymes of glycogen metabolism and their control.]
Roach, P.J., Glycogen and its metabolism, Curr. Mol. Med. 2,
101–120 (2002).
Glycogen Metabolism
Browner, M.F. and Fletterick, R.J., Phosphorylase: A biological
transducer, Trends Biochem. Sci. 17, 66–71 (1992).
Buchbinder, J.L., Rath, V.L., and Fletterick, R.J., Structural rela-
tionships among regulated and unregulated phosphorylases,
Annu. Rev. Biophys. Biomol. Struct. 30, 191–209 (2001).
JWCL281_c18_638-670.qxd 6/3/10 1:50 PM Page 668
References 669
Dai, J.-B., Liu, Y., Ray, W.J., Jr., and Konno, M., The crystal struc-
ture of muscle phosphoglucomutase refined at 2.7-angstrom
resolution, J. Biol. Chem. 267, 6322–6337 (1992).
Gibbons, B.J., Roach,P.J., and Hurley,T.D., Crystal structure of the
autocatalytic initiator of glycogen biosynthesis, glycogenin,
J. Mol. Biol. 319, 463–477 (2002); and Hurley, T.D., Stout,
S., Miner, E., Zhou,J., and Roach, P.J., Requirements for catalysis
in mammalian glycogenin, J. Biol. Chem. 280, 23892–23899
(2005).
Johnson, L.N., Glycogen phosphorylase: Control by phosphoryla-
tion and allosteric effectors, FASEB J. 6, 2274–2282 (1992); and
Rabbit muscle glycogen phosphorylase b. The structural basis
of activation and catalysis, in Harding, J.J. and Crabbe, M.J.C.
(Eds.), Post-Translational Modifications of Proteins, pp. 81–151,
CRC Press (1993).
Johnson, L.N. and Barford, D., Glycogen phosphorylase, J. Biol.
Chem. 265, 2409–2412 (1990).
Madsen, N.B., Glycogen phosphorylase and glycogen synthetase,
in Kuby, S.A. (Ed.), A Study of Enzymes, Vol. II, pp. 139–158,
CRC Press (1991).
Meléndez-Hevia, E., Waddell, T.G., and Shelton, E.D., Optimiza-
tion of molecular design in the evolution of metabolism: The
glycogen molecule, Biochem. J. 295, 477–483 (1993).
Palm, D., Klein, H.W., Schinzel, R.S., Bucher, M., and Helmreich,
E.J.M., The role of pyridoxal 5¿-phosphate in glycogen phos-
phorylase catalysis, Biochemistry 29, 1099–1107 (1990).
Smythe, C. and Cohen, P., The discovery of glycogenin and the
priming mechanism for glycogen biosynthesis, Eur. J. Biochem.
200, 625–631 (1991).
Sprang, S.R., Acharya, K.R., Goldsmith, E.J., Stuart, D.I., Varvill,
K., Fletterick, R.J., Madsen, N.B., and Johnson, L.N., Structural
changes in glycogen phosphorylase induced by phosphoryla-
tion, Nature 336, 215–221 (1988).
Sprang, S.R., Withers, S.G., Goldsmith, E.J., Fletterick, R.J., and
Madsen, N.B., Structural basis for the activation of glycogen
phosphorylase b by adenosine monophosphate. Science 254,
1367–1371 (1991).
Calmodulin and Its Control of Glycogen Metabolism
Babu, Y.S., Sack, J.S., Greenough, T.J., Bugg, C.E., Means, A.R.,
and Cook, W.J., Three-dimensional structure of calmodulin,
Nature 315, 37–40 (1985).
Crivici, A. and Ikura, M., Molecular and structural basis of target
recognition by calmodulin, Annu. Rev. Biophys. Biomol. Struct.
25, 85–116 (1995).
Ikura, M., Clore, G.M., Gronenborn, A.M., Zhu, G., and Bax, A.,
Solution structure of a calmodulin-target peptide complex by
multidimensional NMR, Science 256, 632–638 (1992); and
Meador,W.E., Means, A.R., and Quiocho, F.A.,Target enzyme
recognition by calmodulin: 2.4 Å structure of a calmodulin-
peptide complex, Science 257, 1251–1255 (1992).
James, P., Vorherr, T., and Carafoli, E., Calmodulin-binding do-
mains: Just two faced or multifaceted? Trends Biochem. Sci. 20,
38–42 (1995).
Nakayama, S. and Kretsinger, R.H., Evolution of the EF-hand
family of proteins, Annu. Rev. Biophys. Biomol. Struct. 23,
473–507 (1994).
Protein Kinases and Protein Phosphatases
Bollen, M., Keppens, S., and Stalmans, W., Specific features of
glycogen metabolism in the liver,Biochem.J. 336, 19–31 (1998).
Bossemeyer, D., Engh, R.A., Kinzel, V., Ponstingl, H., and Huber,
R., Phosphotransferase and substrate binding mechanism of
the cAMP-dependent protein kinase catalytic subunit from
porcine heart as deduced from the 2.0 Å structure of the com-
plex with Mn
2
adenyl imidodiphosphate and inhibitor pep-
tide PKI (5–24), EMBO J. 12, 849–859 (1993).
Egloff, M.P., Johnson, D.F., Moorhead, G., Cohen, P.T.W., Cohen,
P., and Barford, D., Structural basis for the recognition of regu-
latory subunits by the catalytic subunit of protein phosphatase
1, EMBO J. 16, 1876–1887 (1997).
Goldberg, J., Huang, H., Kwon,Y., Greengard, P., Nairn, A.C., and
Kuriyan, J., Three-dimensional structure of the catalytic sub-
unit of protein serine/threonine phosphatase-1, Nature 376,
745–753 (1995).
Johnson, L.N., Lowe, E.D., Noble, M.E.M., and Owen, D.J., The
structural basis for substrate recognition and control by pro-
tein kinases, FEBS Lett. 430, 1–11 (1998).
Kim, C., Cheng, C.Y., Saldanha, S.A., and Taylor, S.S., PKA-I
holoenzyme structure reveals a mechanism for cAMP-
dependent activation, Cell 130, 1032–1043 (2007).
Kobe, B. and Kemp, B.E., Active site-directed protein regulation,
Nature 402, 373–376 (1999). [Discusses intrasteric regulation.]
Lowe, E.D., Noble, M.E.M., Skamnaki, V.T., Oikonomakos, N.G.,
Owen, D.J., and Johnson, L.N., The crystal structure of a phos-
phorylase kinase peptide substrate complex: Kinase substrate
recognition, EMBO J. 16, 6646–6658 (1997).
Manning, G., Whyte, D.B., Martinez, R., Hunter, T., and
Sundarsanum, S., The protein kinase complement of the
human genome, Science 298, 1912–1934 (2002).
Nordlie, R.C., Foster, J.D., and Lange, A.J., Regulation of glucose
production by the liver, Annu. Rev. Nutr. 19, 379–406 (1999).
Smith, C.M., Radzio-Andzelm, E., Akamine, M.P., Madhusudan,
and Taylor, S.S.,The catalytic subunit of cAMP-dependent pro-
tein kinase: Prototype for an extended network of communica-
tion, Prog. Biophys. Mol. Biol. 71, 313–341 (1999).
Su, Y., Dostmann, W.R.G., Herberg, F.W., Durick, K., Xuong, N.,
Ten Eyck, L., Taylor, S.S., and Varughese, K.I., Regulatory sub-
unit of protein kinase A: Structure of deletion mutant with
cAMP binding domains, Science 269, 807–813 (1995).
Taylor, S.S., Knighton, D.R., Zheng, J., Sowadski, J.M., Gibbs, C.S.,
and Zoller, M.J., A template for the protein kinase family,
Trends Biochem. Sci. 18, 84–89 (1993); and Taylor, S.S.,
Knighton, D.R., Zheng, J., Ten Eyck, L.F., and Sowadski, J.M.,
Structural framework for the protein kinase family, Annu.Rev.
Cell Biol. 8, 429–462 (1992).
Villafranca, J.E., Kissinger, C.R., and Parge, H.E., Protein serine/
threonine phosphatases, Curr. Opin. Biotech. 7, 397–402 (1996).
Glucose-6-Phosphatase, Glucokinase, and
PFK-2/FBPase-2
Cornish-Bowden, A. and Cárdenas, M.L., Hexokinase and “glu-
cokinase” in liver metabolism, Trends Biochem. Sci. 16,
281–282 (1991).
de la Iglesia, N., Mukhtar, M., Seoane,J., Guinovart, J.J., and Agius,
L.,The role of the regulatory protein of glucokinase in the glu-
cose sensory mechanism of the hepatocyte, J. Biol. Chem. 275,
10597–10603 (2000).
Iynedjian, P.B., Mammalian glucokinase and its gene, Biochem. J.
293, 1–13 (1993). [Reviews the function and control of gluco-
kinase.]
Okar, D.A., Manzano, À., Navarro-Sabatè, A., Riera, L., Bartrons,
R., and Lange, A.J., PFK-2/FBPase-2: maker and breaker of
the essential biofactor fructose-2,6-bisphosphate, Trends
Biochem. Sci. 26, 30–35 (2001).
Pilkis, S.J., 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase:
a metabolic signaling enzyme, Annu. Rev. Biochem. 64,
799–835 (1995).
Rousseau, G.G. and Hue, L., Mammalian 6-phosphofructo-2-
kinase/fructose-2,6-bisphosphatase: A bifunctional enzyme
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 669

that controls glycolysis, Prog. Nucleic Acid Res. Mol. Biol. 45,
99–127 (1993).
Van Schaftingen, E. and Gerin, I.,The glucose-6-phosphatase sys-
tem, Biochem. J. 362, 513–532 (2002).
Van Schaftingen, E.,Vandercammen,A., Detheux, M., and Davies,
D.R.,The regulatory protein of liver glucokinase, Adv. Enzyme
Reg. 32, 133–148 (1992).
Yuan, M.H., Mizuguchi, H., Lee, Y.-H., Cook, P.F., Uyeda, K., and
Hasemann, C.A., Crystal structure of the H256A mutant of
rat testis fructose-6-phosphate-2-kinase/fructose-2,6-bisphos-
phatase, J. Biol. Chem. 274, 2176–2184 (1999); and Haseman,
C.A., Istvan, E.S., Uyeda, K., and Deisenhofer, J., The crystal
structure of the bifunctional enzyme 6-phosphofructo-2-
kinase/fructose-2,6-bisphosphatase reveals distinct homolo-
gies, Structure 4, 1017–1029 (1996).
Glycogen Storage Diseases
Bartram, C., Edwards, R.H.T., and Beynon, R.J., McArdle’s
disease–muscle glycogen phosphorylase deficiency, Biochim.
Biophys. Acta 1272, 1–13 (1995). [A review.]
Chen, Y.-T., Glycogen storage diseases, in Valle, D. (Ed.), The
Online Metabolic and Molecular Bases of Inherited Disease,
Chapter 71, http://www.ommbid.com/. [Begins with a review of
glycogen metabolism.]
Kollberg, G.,Tulinius, M., Gilljam,T., Östman-Smith, I., Forsander,
G., Jotorp, P., Oldfors,A., and Holme, E., Cardiomyopathy and
exercise intolerance in muscle glycogen storage disease 0, New
Engl. J. Med. 357, 1507–1514 (2007).
Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.
nlm.nih.gov/sites/entrez?db=OMIM [A comprehensive com-
pendium of human genes and genetic phenotypes containing
information on all known Mendelian disorders, including the
glycogen storage diseases, and involving 12,000 genes.]
Radda, G.K., Control of bioenergetics: From cells to man by phos-
phorus nuclear-magnetic-resonance spectroscopy, Biochem.
Soc.Trans.14, 517–525 (1986). [Discusses the noninvasive diag-
nosis of McArdle’s disease by
31
P NMR.]
Zingone,A., Hiraiwa, H., Pan, C.-J., Lin, B., Chen, H.,Ward, J.M., and
Chou, J.Y., Correction of glycogen storage disease type 1a in a
mouse model by gene therapy, J.Biol.Chem. 275, 828–832 (2000).
670 Chapter 18. Glycogen Metabolism
PROBLEMS
1. A glycogen molecule consisting of 100,000 glucose residues
is branched, on average, every 10 residues with one branch per
tier. (a) How many reducing ends does it have? (b) How many
tiers of branches does it have, on average?
2. A mature glycogen particle typically has 12 tiers of
branches with 2 branches per tier and 13 residues per branch.How
many glucose residues are in such a particle?
3. Calculations based on the volume of a glucose residue and
the branching pattern of cellular glycogen indicate that a glycogen
molecule could have up to 28 branching tiers before becoming im-
possibly dense. What are the advantages of such a molecule and
why is it not found in vivo?
4. The free energy of hydrolysis of an (1 S 4) glycosidic
bond is 15.5 kJ ⴢ mol
1
, whereas that of an (1 S 6) glycosidic
bond is 7.1 kJ ⴢ mol
1
. Use these data to explain why glycogen
debranching includes three reactions [breaking and reforming
(1 S 4) bonds and hydrolyzing (1 S 6) bonds], while glycogen
branching requires only two reactions [breaking (1 S 4) bonds
and forming (1 S 6) bonds].
5. The complete metabolic oxidation of glucose to CO
2
and
O
2
yields 32 ATPs (Section 22-2Bc). What is the fractional ener-
getic cost of storing glucose as glycogen and later metabolizing the
glycogen rather than directly metabolizing the glucose? (Recall
that glycogen’s branched structure results in its degradation to
92% G1P and 8% glucose.)
6. What are the effects of the following on the rates of glyco-
gen synthesis and glycogen degradation: (a) increasing the Ca
2
concentration, (b) increasing the ATP concentration, (c) inhibit-
ing adenylate cyclase, (d) increasing the epinephrine concentra-
tion, and (e) increasing the AMP concentration?
7. Show that hexokinase activity but not glucokinase activity is
insensitive to blood [glucose] over the physiological range. Calcu-
late the ratio of glucokinase to hexokinase activities when [glu-
cose] is 2 mM (hypoglycemic), 5 mM (normal), and 25 mM (dia-
betic). Assume that K
M
0.1 mM for hexokinase and that both
enzymes have the same V
max
.
8. Compare the properties of a bicyclic cascade with those of
a monocyclic cascade.
9. The V
max
of muscle glycogen phosphorylase is much larger
than that of liver. Discuss the functional significance of this
phenomenon.
10. How does epinephine act on muscles to prepare them for
“fight or flight”?
*11. A complication of glycogen metabolism that we have not
discussed is that many protein kinases, including phosphorylase
kinase, are autophosphorylating; that is,they can specifically phos-
phorylate and thereby activate themselves. Discuss how this phe-
nomenon affects glycogen metabolism, taking into consideration
the possibilities that phosphorylase kinase autophosphorylation
may be an intramolecular or an intermolecular process.
12. Explain the symptoms of von Gierke’s disease.
13. A sample of glycogen from a patient with liver disease is
incubated with P
i
, normal glycogen phosphorylase, and normal
debranching enzyme. The ratio of glucose-1-phosphate to glucose
formed in this reaction mixture is 100. What is the patient’s most
likely enzymatic deficiency? What is the probable structure of the
patient’s glycogen?
JWCL281_c18_638-670.qxd 2/26/10 2:24 PM Page 670
