Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

Marchese, A., Chen, C., Kim, Y.-M., and Benkovic, J.L., The in
and outs of G protein-coupled receptor trafficking, Trends
Biochem. Sci. 28, 369–376 (2003). [Reviews mechanisms of de-
sensitization.]
Okada, T., Sugihara, M., Bondar, A.-N., Elstner, M., Entel, P.,
and Buss, V., The retinal conformation and its environment in
rhodopsin in light of a new 2.2 Å crystal structure, J. Mol. Biol.
342, 571–583 (2004).
Oldham,W.M. and Hamm, H.E., Heterotrimeric G protein activa-
tion by G-protein-coupled receptors, Nature Rev. Mol. Cell
Biol. 9, 60–71 (2008); and Structural basis of function in het-
erotrimeric G proteins, Q. Rev. Biophys. 39, 117–166 (2006).
Palczewski, K., G protein–coupled receptor rhodopsin, Annu. Rev.
Biochem. 75, 743–767 (2006).
Rasmussen, S.G.F., et al., Crystal structure of the human 
2
adren-
ergic G-protein-coupled receptor, Nature, 450, 383–387 (2007);
and Cherezov, V., et al., High-resolution crystal structure of an
engineered human 
2
adrenergic G protein–coupled receptor,
Science 318, 1259–1265 (2007).
Reiter, E. and Lefkowitz, R.J., GRKs and -arrestins: roles in
receptor silencing,trafficking and signaling, Trends Endocrinol.
Metab. 17, 159–165 (2006).
Scheerer, P., Park, J.H., Hildebrand, P.W., Kim, Y.J., Krauss, N.,
Choe, H.-W., Hofmann, K.P., and Ernst, O.P., Crystal structure
of opsin in its G-protein-interacting conformation, Nature 455,
497–502 (2008). [Proposes a structural model for the activation
by opsin of its corresponding heterotrimeric G protein.]
Soundararajan, M., et al., Structural diversity in the RGS domain
and its interaction with hetertrimeric G protein ␣-subunits,
Proc. Natl. Acad. Sci. 105, 6457–6462 (2008).
Sprang, S.R. (Ed.), Mechanisms and Pathways of Heterotrimeric
G Protein Signaling, Adv. Prot. Chem. 74 (2007).
Tesmer, J.J.G. and Sprang,S.R.,The structure, catalytic mechanism
and regulation of adenylyl cyclase, Curr. Opin. Struct. Biol. 8,
713–719 (1998).
Tesmer, J.J.G., Sunahara, R.K., Gilman, A.G., and Sprang, S.R.,
Crystal structure of the catalytic domains of adenylyl cyclase in
a complex with G
s␣
ⴢ GTP␥S, Science 278, 1907–1916 (1997).
Tobin, A.B., Butcher, A.J., and Kong, K.C., Location, location, lo-
cation....Site-specific GPCR phosphorylation offers a mech-
anism for cell-type-specific signaling, Trends Pharm. Sci. 29,
5–12 (2008).
Vetter, I.R., and Wittinghofer,A.,The guanine nucleotide–binding
switch in three dimensions, Science 294, 1299–1304 (2001).
Wall, M.A., Coleman, D.E., Lee, E., Iñiguez-Lluhi, J.A., Posner,
B.A., Gilman, A.G., and Sprang, S.R., The structure of the G
protein heterotrimer G
i␣1

1
␥
2
, Cell 83, 1047–1058 (1995); and
Lambright, D.G., Sondek, J., Bohm, A., Skiba, N.P., Hamm,
H.E., and Sigler, P.B., The 2.0 Å crystal structure of a het-
erotrimeric G protein, Nature 379, 311–319 (1996).
Weis, W.I., and Kobilka, B.K., Structural insights into G-
protein-coupled receptor activation, Curr. Opin. Struct. Biol.
18, 734–740 (2008); and Deupi, X. and Kobilka, B., Activation
of G protein–coupled receptors, Adv. Protein Chem. 74,
137–165 (2007).
Willars, G.B., Mammalian RGS proteins: Multifunctional regula-
tors of cell signaling, Semin. Cell Dev. Biol. 17, 363–376 (2006).
Zhang, R.-G., Scott, D.L., Westbrook, M.L., Nance, S., Spangler,
B.D., Shipley, G.G., and Westbrook, E.M.,The three-dimensional
crystal structure of cholera toxin, J. Mol. Biol. 251, 563–573
(1995); and Merrrit, E.A., Sarfaty, S., Jobling, M.G., Chang, T.,
Holmes, R.K., Hirst,T.R., and Hol,W.G.J., Structural studies of
receptor binding by cholera toxin mutants, Protein Sci. 6,
1516–1528 (1997).
Tyrosine Kinase–Based Signaling
Alonso, A., et al., Protein tyrosine phosphatases in the human
genome, Cell 117, 699–711 (2004). [A review that enumer-
ates, classifies, and discusses the functions of all human
PTPs.]
Beene, D.L. and Scott, J.D., A-kinase anchoring proteins take
shape, Curr. Opin. Cell Biol. 19, 192–198 (2007).
Bhattacharyya, R.P., Reményi, A., Yeh, B.J., and Lim, W.A.,
Domains, motifs and scaffolds: The role of modular interac-
tions in the evolution and wiring of cell signaling circuits,
Annu. Rev. Biochem. 75, 655–680 (2006).
Boggon, T.J. and Eck, M.J., Structure and regulation of Src family
kinases, Oncogene 23, 7918–7927 (2004).
Bollen, M., Combinatorial control of protein phosphatase-1,
Trends Biochem. Sci. 26, 426–431 (2001).
Boriak-Sjodin, P.A., Margarit, S.M., Bar-Sagi, D., and Kuriyan, J.,
The structural basis of the activation of Ras by Sos, Nature 394,
337–343 (1998).
Bos, J.L., Rehmann, H., and Wittinghofer, A., GEFs and GAPs:
Critical elements in the control of small G proteins, Cell 129,
865–877 (2007).
Baselga, J.,Targeting tyrosine kinases in cancer:The second wave,
Science 312, 1175–1178 (2006).
Calderwood, S.K., Khaleque, M.A., Sawyer, D.B., and Ciocca,
D.R., Heat shock proteins in cancer: chaperones of tumorigen-
esis, Trends Biochem. Sci. 31, 164–172 (2006).
Capdeville, R., Buchdunger, E., Zimmermann, J., and Matter, A.,
Glivec (STI571, ImatinIB), a rationally developed targeted an-
ticancer drug, Nature Rev. Drug Discov. 1, 493–502 (2002).
[Glivec was the previous name of Gleevec.]
Carlisle Michel, J.J. and Scott, J.D., AKAP mediated signal
transduction, Annu. Rev. Pharmacol. Toxicol. 42, 235–257
(2002).
Cho, U.S. and Xu, W., Crystal structure of a protein phosphatase
2A heterotrimeric holoenzyme, Nature 445, 53–57 (2007); and
Xu,Y., Xing,Y., Chen,Y., Chao,Y., Lin, Z., Fan, E.,Yu, J., Stack,
S., Jeffrey, P., and Shi, Y., Structure of the protein phosphatase
2A holoenzyme, Cell 127, 1239–1251 (2006).
Chang, L. and Karin, M., Mammalian MAP kinase signaling cas-
cades, Nature 410, 37–40 (2001).
De Meyts, P., The insulin receptor: A prototype for dimeric,
allosteric membrane receptors? Trends Biochem. Sci. 33,
376–384 (2008).
Druker, B.J. and Lydon, N.B., Lessons learned from the develop-
ment of an Abl tyrosine kinase inhibitor for chronic myelogen-
ous leukemia, J. Clin. Invest. 105, 3–7 (2000).
Garcia, K.C. (Ed.), Cell Surface Receptors, Adv. Protein Chem. 68
(2004).
Griffith, J.P., Kim, J.L., Kim, E.E., Sintchak, M.D., Thomson, J.A.,
Fitzgibbon, M.J., Fleming, M.A., Caron, P.R., Hsiao, K., and
Navia, M.A., X-ray structure of calcineurin inhibited by the
immunophilin-immunosuppresant FKBP12-FK506 complex,
Cell 82, 507–522 (1995);and Huai,Q., Kim, H.-Y., Liu,Y., Zhao,
Y., Mondragon, A., Liu, J.O., and Ke, H., Crystal structure of
calcineurin–cylophilin–cyclosporin shows common but distinct
recognition of immunophilin–drug complexes, Proc. Natl.
Acad. Sci. 99, 12037–12042 (2002).
Groves, M.R., Hanlon, N., Turowski, P., Hemmings, B.A., and Bar-
ford, D., The structure of the protein phosphatase 2A PR65/A
subunit reveals the conformation of its 15 tandemly repeated
HEAT motifs, Cell 96, 99–110 (1999).
Hansen, G., et al.,The structure of the GM-CSF receptor complex
reveals a distinct mode of cytokine receptor activation, Cell
134, 496–507 (2008).
References 741
JWCL281_c19_671-743.qxd 6/4/10 10:57 AM Page 741
Hof, P., Pluskey, S., Dhe-Paganon, S., Eck,M.J., and Shoelson, S.E.,
Crystal structure of tyrosine phosphatase SHP-2, Cell 92,
441–450 (1998).
Hubbard, S.R., Crystal structure of the activated insulin receptor
tyrosine kinase in complex with peptide substrate and ATP
analog, EMBO J. 16, 5572–5581 (1997); and Hubbard, S.R.,
Wei, L., Ellis, L., and Hendrickson, W.A., Crystal structure of
the tyrosine kinase domain of the human insulin receptor,
Nature 372, 746–753 (1994).
Hubbard, S.R. and Miller, W.T., Receptor tyrosine kinases: mech-
anisms of activation and signaling, Curr. Opin. Cell Biol. 19,
117–123 (2007).
Karnoub, A.E. and Weinberg, R.A., Ras ongenes: split personali-
ties, Nature Rev. Mol. Cell Biol. 9, 517–531 (2008).
Kolch, W., Coordinating ERK/MAPK signaling through scaffolds
and inhibitors, Nature Rev. Mol. Cell Biol. 6, 827–838 (2005).
Li, L. and Dixon, J.E., Form, function, and regulation of protein
tyrosine phosphatases and their involvement in human dis-
ease, Semin. Immunol. 12, 75–84 (2000).
Lim, W.A., The modular logic of signaling proteins: building
allosteric switches from simple binding domains, Curr. Opin.
Struct. Biol. 12, 61–68 (2002).
Linder, J.U and Schultz, J.E., Versatility of signal transduction
encoded in dimeric adenylyl cyclases, Curr. Opin. Struct. Biol.
18, 667–672 (2008).
Maignan, S., Guilloteau, J.-P., Fromage, N., Arnoux, B., Becquart,
J., and Ducruix, A., Crystal structure of the mammalian Grb2
adaptor, Science 268, 291–293 (1995).
McKay, M.M. and Morrison, D.K., Integrating signals from RTKs
to ERK/MAPK, Oncogene 26, 3113–3121 (2007).
Musacchio, A., Sareste, M., and Wilmanns, M., High-resolution
crystal structures of tyrosine kinase SH3 domains complexed
with proline-rich peptides, Nature Struct. Biol. 1, 546–551
(1994).
Nassar, N., Horn, G., Herrmann, C., Scherer, A., McCormack, F.,
and Wittinghofer, A., The 2.2 Å crystal structure of the Ras-
binding domain of the serine/threonine kinase c-Raf1 in com-
plex with Rap1A and a GTP analogue, Nature 375, 554–560
(1995).
Neel, B.G., Gu, H., and Pao, L., The ‘Shp’ing news: SH2 domain-
containing tyrosine phosphatases in cell signaling, Trends
Biochem. Sci. 28, 284–293 (2003).
Noble, M.E.M., Endicott, J.A., and Johnson, L.N., Protein kinase
inhibitors: Insights into drug design from structure, Science
303, 1800–1805 (2004).
O’Shea, J.J., Gadino, M., and Schreiber, R.D., Cytokine signaling
in 2002: New surprises in the Jak/Stat pathway, Cell 109,
S121–S131 (2002).
Pawson,T., Dynamic control of signaling by modular adapter pro-
teins, Curr. Opin. Cell Biol. 19, 112–116 (2007).
Pawson, T. and Scott, J.D., Protein phosphorylation in signaling—
50 years and counting, Trends Biochem. Sci. 30, 286–290
(2005). [A historical review.]
Pellizzari, R., Guidi-Rontani, C., Vitale, G., Mock, M., and Monte-
cucco, C.,Anthrax lethal factor cleaves MKK3 in macrophages
and inhibits the LPS/IFN-induced release of NO and TNF,
FEBS Lett. 462, 199–204 (1999).
Scheffzek, K., Ahmadian, M.R., Kabsch, W., Wiesmüller, L.,
Lautwein, A., Schmitz, F., and Wittinghofer, A., The Ras-
RasGAP complex: Structural basis for GTPase activation and
its loss in oncogenic Ras mutants, Science 277, 333–338 (1997).
Schindler,T., Bornmann,W., Pellicenna, P., Miller,W.T., Clarkson,
B., and Kuriyan, J., Structural mechanism for STI-571 inhibi-
tion of Abelson tyrosine kinase, Science 289, 1938–1942 (2000).
[STI-571 was the original name of Gleevec.]
Schlessinger,J., Plotnikov,A.N., Ibrahimi,O.A., Eliseenkova,A.V.,
Yeh, B.K., Yayon, A., Linhardt, R.J., and Mohammadi, M.,
Crystal structure of a ternary FGF-FGFR-heparin complex re-
veals a dual role for heparin in FGF binding and dimerization,
Mol. Cell 6, 743–750 (2000).
Sebolt-Leopold, J.S. and English, J.M., Mechanisms of drug inhibi-
tion of signaling molecules, Nature 441, 457–462 (2006).
Sprang, S., GEFs: Master regulators of G-protein activation,
Trends Biochem. Sci. 26, 266–267 (2001).
Stoker, A.W., Protein tyrosine phosphatases and signaling, J. En-
docrinol. 185, 19–33 (2005).
Tiganis,T. and Bennett,A.M., Protein tyrosine kinase function: the
substrate perspective, Biochem. 402, 1–15 (2007).
Tonks, N.K., Protein tyrosine phosphatases: from genes, to func-
tion, to disease, Nature Rev. Mol. Cell Biol. 7, 833–846 (2006).
Wang, X., Lupardus, P., La Porte, S.L., and Garcia, K.C., Structural
biology of shared cytokine receptors, Annu. Rev. Immunol. 27,
29–60 (2009).
Ward, C.W., Lawrence, M.C., Streltsov, V.A., Adams, T.E., and
McKern, N.M., The insulin and EGF receptor structures: in-
sights into ligand-induced receptor activation, Trends Biochem.
Sci. 32, 129–137 (2007).
Whitesell, L. and Lindquist, S.L., Hsp90 and the chaperoning of
cancer, Nature Rev. Cancer 5, 761–772 (2005).
Whitmarsh,A.J. and Davis, R.J., Structural organization of MAP-
kinase signaling modules by scaffold proteins in yeast and
mammals, Trends Biochem. Sci. 23, 481–485 (1998).
Xu, W., Doshi, A., Lei, M., Eck, M.J., and Harrison, S.C., Crystal
structures of c-Src reveal features of its autoinhibitory mecha-
nism, Mol. Cell 3, 629–638 (1999); and Xu, W., Harrison, S.C.,
and Eck, M.J., Three dimensional structure of the tyrosine
kinase c-Src, Nature 385, 595–602 (1995).
Yaffe, M.B., Phosphotyrosine-binding domains in tyrosine trans-
duction, Nature Rev. Mol. Cell Biol. 3, 177–186 (2002).
Young, M.A., Gonfloni, F., Superti-Furga, G., Roux, B., and
Kuriyan, J., Dynamic coupling between the SH2 and SH3
domains of c-Src and Hck underlies their inactivation by
C-terminal tyrosine phosphorylation, Cell 105, 115–126 (2001).
Zhang, Z.-Y., Protein tyrosine phosphatases: structure and func-
tion, substrate specificity, and inhibitor development, Annu.
Rev. Pharmacol. Toxicol. 42, 209–234 (2002).
Zhou, M.-M., et al., Structure and ligand recognition of the phos-
photyrosine binding domain of Shc, Nature 378, 584–592 (1995).
The Phosphoinositide Cascade
Brazil, D.P., Yang, Z.-Z., and Hemmings, B.A., Advances in pro-
tein kinase B signaling: AKTion on multiple fronts, Trends
Biochem. Sci. 29, 233–242 (2004).
Brognard, J. and Newton,A.C., PHLiPPing the switch on Akt and
protein kinase C signaling, Trends Endocrinol. Metab. 19,
223–230 (2008).
Carrasco, S. and Mérida, I., Diacylglycerol, when simplicity
becomes complex, Trends Biochem. Sci. 32, 27–36 (2007).
Cho, W. and Stahelin, R.V., Membrane-protein interactions in cell
signaling and membrane trafficking, Annu. Rev. Biophys.
Biomol. Struct. 34, 119–151 (2005).
Clapham, D.E., Calcium signaling, Cell 131, 1047–1058 (2007).
Cockcroft, S. (Ed.), Biology of Phosphoinositides, Oxford (2000).
Di Paolo, G. and De Camilli, P., Phosphoinositides in cell regula-
tion and membrane dynamics, Nature 443, 651–657 (2006).
Dekker, L.V. (Ed.), Protein Kinase C (2nd ed.), Kluwer Acade-
mic/Plenum Publishers (2004).
Ferguson, K.M., Lemmon, M.A., Schlessinger, M.A., and Sigler,
P.B., Structure of the high affinity complex of inositol trisphos-
phate with a phospholipase C pleckstrin homology domain,
Cell 83, 1037–1046 (1995).
742 Chapter 19. Signal Transduction
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 742

Gallegos, L.L. and Newton, A.C., Spaciotemporal dynamics of
lipid signaling: Protein kinase C as a paradigm, IUBMB Life
60, 782–786 (2008).
Harden, T.K. and Sondek, J., Regulation of phospholipase C
isozymes by Ras superfamily GTPases, Annu. Rev. Pharmacol.
Toxicol. 46, 355–379 (2006).
Huang,C.-H., Mandelker,D., Schmidt-Kittler, O., Samuels,Y.,Vel-
culescu, V.E., Kinzler, K.W., Vogelstein, B., Gabelli, S.B., and
Amzel, L.M., The structure of a human p110/p85 complex
elucidates the effects of oncogenic PI3K mutations, Science
318, 1744–1748 (2007).[p110 is the catalytic subunit of PI3K.]
Hurley, J.H. and Misra, S., Signaling and subcellular targeting by
membrane-binding domains, Annu. Rev. Biophys. Biomol. Struct.
29, 49–79 (2000).
Jezyk, M.R., Snyder, J.T., Gershberg, S., Worthylake, D.K., Harden,
T.K., and Sondek, J., Crystal structure of Rac1 bound to its
effector phospholipase C-2, Nature Struct. Mol. Biol. 13,
1135–1140 (2006); and Hicks, S.N., Jezyk, M.R., Gershberg, S.,
Seifer, J.P., Harden, T.K., and Sondek, J., General and versatile
autoinhibition of PLC isoenzymes, Mol. Cell 31, 383–394 (2008).
Katso, R., Okkenhaug, K.,Ahmadi, K.,White, S.,Timms, J., and Wa-
terfield, M.D., Cellular function of phosphoinositide
3-kinases: Implications for development, immunity, homeo-
stasis, and cancer, Annu. Rev. Cell Dev. Biol. 17, 615–675 (2001).
Kok, K., Geering, B., and Vanhaesebroeck, B., Regulation of phos-
phoinositide 3-kinase expression in health and disease, Trends
Biochem. Sci. 34, 115–127 (2009).
Kutateladze,T. and Overduin, M., Structural mechanism of endo-
some docking by the FYVE domain, Science 291, 1793–1796
(2001).
Lee, J.-O., Yang, H., Georgescu, M.-M., Di Cristofano, A., Mae-
hama, T., Shi, Y., Dixon, J.E., Pandolfi, P., and Pavletich, N.P.,
Crystal structure of the PTEN tumor suppressor: Implications
for its phosphoinositide phosphatase activity and membrane
association, Cell 99, 323–344 (1999).
Maehama, T., Taylor, G.S., and Dixon, J.E., PTEN and myotubu-
larin: Novel phosphoinositide phosphatases, Annu. Rev.
Biochem. 70, 247–279 (2001).
Manning, B.D. and Cantley, L.C.,AKT/PKB signaling: Navigating
downstream, Cell 129, 1261–1274 (2007).
Michell, R.H., Inositol derivatives: evolution and function, Nature
Rev. Mol. Cell Biol. 9, 151–161 (2008).
Patterson,R.L.,van Rossum,D.B., Nikolaidis, N.,Gill,D.L., and Sny-
der,S.H., Phospholipase C-: diverse roles in receptor-mediated
calcium signaling, Trends Biochem. Sci. 32, 688–697 (2005).
Rhee, S.G., Regulation of phosphoinositide-specific phospholi-
pase C, Annu. Rev. Biochem. 70, 281–312 (2001).
Salmena, L., Carracedo, A., and Pandolfi, P.P., Tenets of PTEN
tumor suppression, Cell 133, 403–414 (2008).
Saltiel, A.R. and Pessin, J.E., Insulin signaling pathways in time
and space, Trends Cell Biol. 12, 65–71 (2002).
Steinberg, S.F., Structural basis of protein kinase C isoform func-
tion, Physiol. Rev. 88, 1341–1378 (2008). [A detailed review.]
Vanhaesebroek, B., Leevers, S.J., Ahmadi, K., Timms, J., Katso, R.,
Driscoll, P.C.,Woscholski, R., Parker, P.J., and Waterfield, M.D.,
Synthesis and function of 3-phosphorylated inositol lipids,
Annu. Rev. Biochem. 70, 535–632 (2001).
Walker, E.H., Persic, O., Ried, C., Stephens, L., and Williams, R.L.,
Structural insights into phosphoinositide 3-kinase catalysis and
signaling, Nature 402, 313–320 (1999); and Pacold, M.E., et al.,
Crystal structure and functional analysis of Ras binding to its
effector phosphoinositide 3-kinase , Cell 103, 931–943 (2000).
Weng, G., Bhalla, U.S., and Iyengar, R., Complexity in biological
signaling systems, Science 284, 92–96 (1999).
Zick, Y., Insulin resistance: a phosphorylation-based uncoupling
of insulin signaling, Trends Cell Biol. 11, 437–441 (2001).
Problems 743
1. Explain the following observations: (a) Thyroidectomized
rats, when deprived of food, survive for 20 days while normal rats
starve to death within 7 days. (b) Cushing’s syndrome, which re-
sults from excessive secretion of adrenocortical steroids, can be
caused by a pituitary tumor. (c) Diabetes insipidus, which is char-
acterized by unceasing urination and unquenchable thirst, results
from an injury to the pituitary. (d) The growth of malignant tu-
mors derived from sex organs may be slowed or even reversed by
the surgical removal of the gonads and the adrenal glands.
2. How does the presence of the nonhydrolyzable GTP analog
GMPPNP affect cAMP-dependent receptor systems?
3. Explain why individuals who regularly handle dynamite
(which is nitroglycerin soaked into an absorbant such as wood
pulp) as part of their jobs have an unusually high incidence of
heart attacks on weekends.
4. A dose-dependent side effect of sildenafil (Viagra) is the
transient impairment of blue/green color discrimination. What is
the biochemical basis for this phenomenon?
5. Retroviruses bearing oncogenes will infect cells from their
corresponding host animal but will usually not transform them.
Yet these retroviruses will readily transform immortalized cells
derived from the same organism. Explain.
6. Explain why mutations of the Arg residue in G
s
that is
ADP-ribosylated by cholera toxin are oncogenic mutations. Why
doesn’t cholera toxin cause cancer?
7. Would the following alterations to Src be oncogenic? Ex-
plain. (a) The deletion or inactivation of the SH3 domain. (b) The
mutation of Tyr 416 to Phe. (c) The mutation of Tyr 527 to
Phe. (d) The replacement of Src residues 249 to 253 with the
sequence APTMP.
8. JIP-1 was originally so named because,when it was first char-
acterized by overexpression in mammalian cells, it appeared to act
as a “JNK inhibitor protein.”What is the basis of this observation?
9. Why does pertussis toxin appear to inhibit certain isozymes
of PLC? Identify these isozymes.
10. Phosphatidylethanolamine and PIP
2
containing identical
fatty acyl residues can be hydrolyzed with the same efficiency by a
certain phospholipase C. Will the hydrolysis products of the two
lipids have the same effect on protein kinase C? Explain.
11. PKC’s autoinhibitory pseudosubstrate occurs at its N-
terminus, whereas that of MLCK occurs at its C-terminus (Fig.
18-20). To further investigate this phenomenon, a colleague pro-
poses to construct a PKC with its pseudosubstrate attached to the
protein’s C-terminus with a sufficiently long linker so that the
pseudosubstrate could bind in the enzyme’s active site. Would you
expect this variant PKC to be activatable? Explain.
PROBLEMS
JWCL281_c19_671-743.qxd 3/16/10 7:17 PM Page 743

744
CHAPTER 20
Transport through
Membranes
1 Thermodynamics of Transport
2 Kinetics and Mechanisms of Transport
A. Nonmediated Transport
B. Kinetics of Mediated Transport: Glucose Transport
Into Erythrocytes
C. Ionophores
D. Maltoporin: The Structural Basis of Sugar Discrimination
E. Passive-Mediated Glucose Transport
F. K
⫹
Channels: Ion Discrimination
G. Cl
⫺
Channels
H. Aquaporins
3 ATP-Driven Active Transport
A. (Na
⫹
–K
⫹
)–ATPase of Plasma Membranes
B. Ca
2⫹
–ATPase
C. (H
⫹
–K
⫹
)–ATPase of Gastric Mucosa
D. Group Translocation
E. ABC Transporters
4 Ion Gradient–Driven Active Transport
A. Na
⫹
–Glucose Symport
B. Lactose Permease
C. ATP–ADP Translocator
5 Neurotransmission
A. Voltage-Gated Ion Channels
B. Action Potentials
C. Neurotransmitters and Their Receptors
Metabolism occurs within cells that are separated from
their environments by plasma membranes. Eukaryotic
cells, in addition, are compartmentalized by intracellular
membranes that form the boundaries and internal struc-
tures of their various organelles. The nonpolar cores of bi-
ological membranes make them highly impermeable to
most ionic and polar substances, so that these substances
can traverse membranes only through the action of specific
transport proteins. Such proteins are therefore required to
mediate all transmembrane movements of ions, such as
Na
⫹
,K
⫹
,Ca
2⫹
, and Cl
⫺
, as well as metabolites such as pyru-
vate, amino acids, sugars, and nucleotides, and even water
(despite its relatively high permeability in bilayers; Section
12-2B). Transport proteins are also responsible for all bio-
logical electrochemical phenomena such as neurotransmis-
sion. In this chapter, we discuss the thermodynamics, kinet-
ics, and chemical mechanisms of these membrane transport
systems and end with a discussion of the mechanism of
neurotransmission.
1 THERMODYNAMICS OF TRANSPORT
As we saw in Section 3-4A, the free energy of a solute, A,
varies with its concentration:
[20.1]
where is the chemical potential (partial molar free en-
ergy) of A (the bar indicates quantity per mole) and is
the chemical potential of its standard state. Strictly speak-
ing, this equation applies only to ideal solutions; for non-
ideal (real) solutions, molar concentrations must be re-
placed by activities (Appendix to Chapter 3). In the dilute
(millimolar) solutions that are characteristic of laboratory
conditions, the activity of a substance closely approaches
its molar concentration in value. However, this is not the
case in the highly concentrated cellular milieu (Appendix
to Chapter 3). Yet it is difficult to determine the activity of
a substance in a cellular compartment. Hence, in the fol-
lowing derivations, we shall make the simplifying assump-
tion that activities are equal to molar concentrations.
The diffusion of a substance between two sides of a
membrane
thermodynamically resembles a chemical equilibration.
A difference in the concentrations of the substance on
two sides of a membrane generates a chemical potential
difference:
[20.2]
Consequently, if the concentration of A outside the mem-
brane is greater than that inside, for the transfer of A
from outside to inside will be negative and the spontaneous
net flow of A will be inward. If, however, [A] is greater inside
than outside, is positive and an inward net flow of A can
only occur if an exergonic process, such as ATP hydrolysis, is
coupled to it to make the overall free energy change negative.
¢G
A
¢G
A
¢G
A
⫽ G
A
(in) ⫺ G
A
(out) ⫽ RT lna
[A]
in
[A]
out
b
A(out) Δ A(in)
G
A
°¿
G
A
G
A
⫺ G
A
°¿ ⫽ RT ln[A]
JWCL281_c20_744-788.qxd 3/17/10 1:47 PM Page 744
a. Membrane Potentials Arise from Transmembrane
Concentration Differences of Ionic Substances
The permeabilities of biological membranes to ions
such as H
⫹
,Na
⫹
,K
⫹
,Cl
⫺
, and Ca
2⫹
are controlled by spe-
cific membrane-embedded transport systems that we shall
discuss in later sections. The resulting charge differences
across a biological membrane generate an electric potential
difference, ⌬⌿ ⫽ ⌿(in) ⫺⌿(out), where ⌬⌿ is termed the
membrane potential. Consequently, if A is ionic, Eq. [20.2]
must be amended to include the electrical work required to
transfer a mole of A across the membrane from outside to
inside:
[20.3]
where Z
A
is the ionic charge of A; f, the Faraday constant,
is the charge of one mole of electrons (96,485 C ⴢ mol
⫺1
);
and is now termed the electrochemical potential of A.
Membrane potentials in living cells can be measured di-
rectly with microelectrodes. ⌬⌿ values of ⫺100 mV (inside
negative) are not uncommon (note that 1 V ⫽ 1 J ⴢ C
⫺1
).
Thus the last term of Eq. [20.3] is often significant for ionic
substances.
2 KINETICS AND MECHANISMS
OF TRANSPORT
Thermodynamics indicates whether a given transport
process will be spontaneous but, as we saw for chemical and
enzymatic reactions, provides no indication of the rates of
these processes. Kinetic analyses of transport processes to-
gether with mechanistic studies have nevertheless permit-
ted these processes to be characterized. There are two
types of transport processes: nonmediated transport and
mediated transport. Nonmediated transport occurs through
simple diffusion. In contrast, mediated transport occurs
through the action of specific carrier proteins that are vari-
ously called carriers, permeases, porters, translocases,
translocators, and transporters. Mediated transport is fur-
ther classified into two categories depending on the ther-
modynamics of the system:
1. Passive-mediated transport or facilitated diffusion in
which specific molecules flow from high concentration to
low concentration so as to equilibrate their concentration
gradients.
2. Active transport in which specific molecules are
transported from low concentration to high concentration,
that is, against their concentration gradients. Such an en-
dergonic process must be coupled to a sufficiently exer-
gonic process to make it favorable.
In this section, we consider the nature of nonmediated
transport and then compare it to passive-mediated trans-
port as exemplified by ionophores, porins, glucose trans-
porters, K
⫹
channels, Cl
⫺
channels, and aquaporins. Active
transport is examined in succeeding sections.
G
A
¢G
A
⫽ RT lna
[A]
in
[A]
out
b⫹ Z
A
f ¢
A. Nonmediated Transport
The driving force for the nonmediated flow of a substance A
through a medium is A’s electrochemical potential gradient.
This relationship is expressed by the Nernst–Planck
equation:
[20.4]
where J
A
is the flux (rate of passage per unit area) of A, x is
distance, is the electrochemical potential gradient
of A, and U
A
is its mobility (velocity per unit force) in the
medium. If we assume, for simplicity, that A is an un-
charged molecule so that is given by Eq. [20.1], the
Nernst–Planck equation reduces to
[20.5]
where D
A
K RTU
A
is the diffusion coefficient of A in the
medium of interest. This is Fick’s first law of diffusion,
which states that a substance diffuses in the direction that
eliminates its concentration gradient, d[A]/dx, at a rate pro-
portional to the magnitude of this gradient.
For a membrane of thickness x, Eq. [20.5] is approxi-
mated by
[20.6]
where D
A
is the diffusion coefficient of A inside the mem-
brane and P
A
⫽ D
A
/x is termed the membrane’s perme-
ability coefficient for A. The permeability coefficient is in-
dicative of the solute’s tendency to transfer from the
aqueous solvent to the membrane’s nonpolar core. It
should therefore vary with the ratio of the solute’s solubil-
ity in a nonpolar solvent resembling the membrane’s core
(e.g., olive oil) to that in water, a quantity known as the
solute’s partition coefficient between the two solvents. In-
deed, the fluxes of many nonelectrolytes across erythrocyte
membranes vary linearly with their concentration differences
across the membrane as predicted by Eq. [20.6] (Fig. 20-1).
Moreover,their permeability coefficients, as obtained from
the slopes of plots such as Fig. 20-1, correlate rather well
J
A
⫽
D
A
x
([A]
out
⫺ [A]
in
) ⫽ P
A
([A]
out
⫺ [A]
in
)
J
A
⫽⫺D
A
(d[A])>dx
G
A
dG
A
>dx
J
A
⫽⫺[A]U
A
(dG
A
>dx)
Section 20-2. Kinetics and Mechanisms of Transport 745
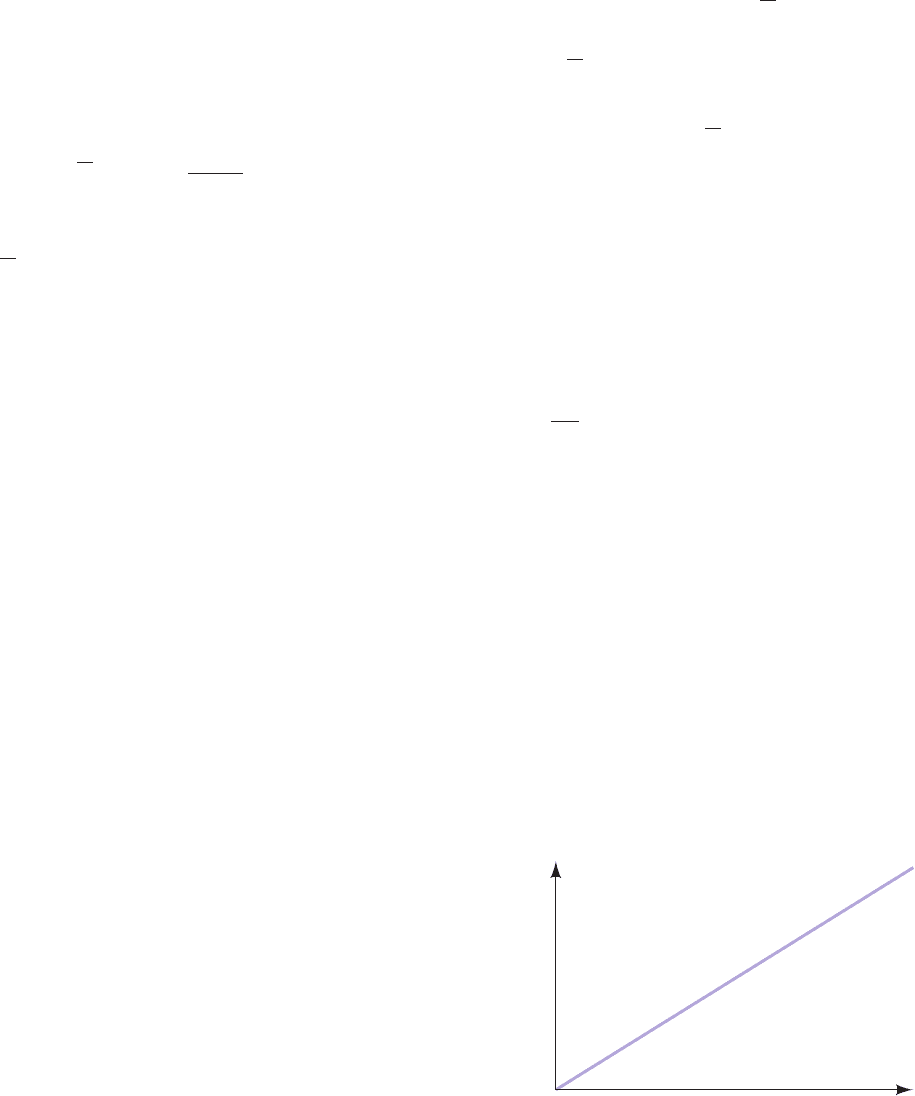
[ A ]
out
– [ A ]
in
J
A
Slope = P
A
Figure 20-1 Linear relationship between diffusional flux (J
A
)
and ([A]
out
⫺ [A]
in
) across a semipermeable membrane. See
Eq. [20.6].
JWCL281_c20_744-788.qxd 6/4/10 12:13 PM Page 745
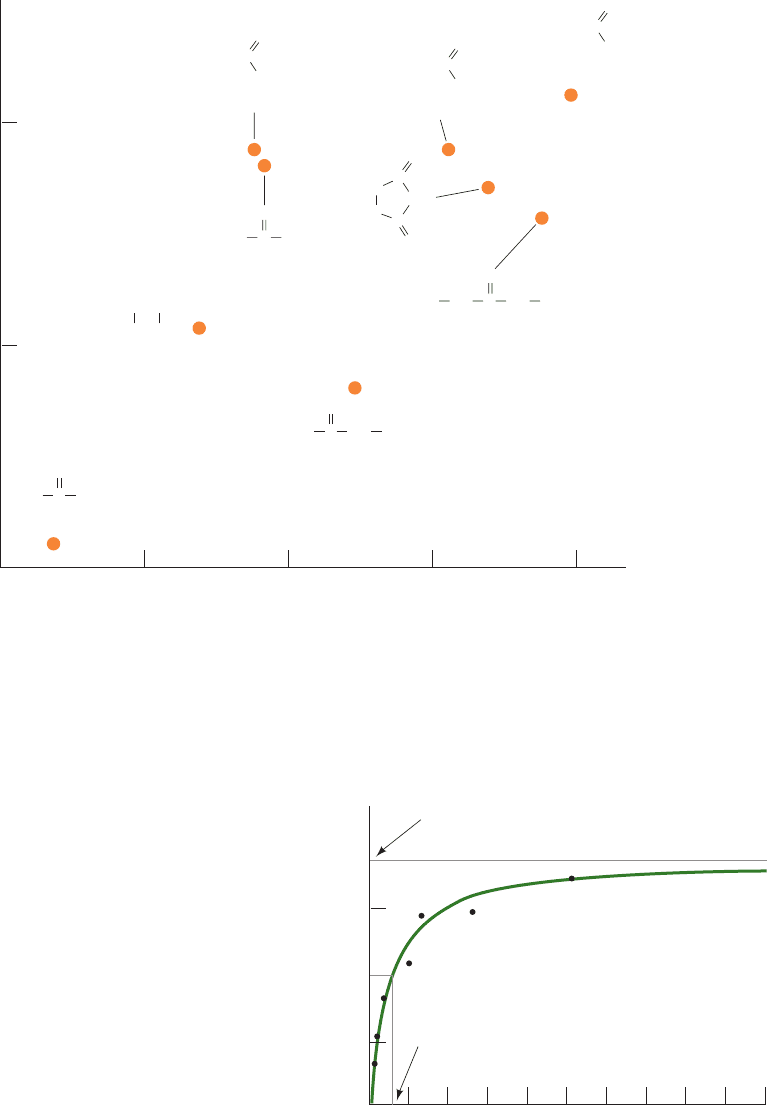
with their measured partition coefficients between nonpo-
lar solvents and water (Fig. 20-2).
B. Kinetics of Mediated Transport: Glucose
Transport Into Erythrocytes
Despite the success of the foregoing model in predicting
the rates at which many molecules pass through mem-
branes, there are numerous combinations of solutes and
membranes that do not obey Eq. [20.6]. The flux in such a
system is not linear with the solute concentration differ-
ence across the corresponding membrane (Fig. 20-3) and,
furthermore, the solute’s permeability coefficient is much
larger than is expected on the basis of its partition coeffi-
cient. Such behavior indicates that these solutes are con-
veyed across membranes in complex with carrier molecules;
that is, they undergo mediated transport.
The system that transports glucose across the erythro-
cyte membrane provides a well-characterized example of
passive-mediated transport: It invariably transports glu-
cose down its concentration gradient but not at the rate
predicted by Eq. [20.6]. Indeed, the erythrocyte glucose
transporter exhibits four characteristics that differentiate
mediated from nonmediated transport: (1) speed and
746 Chapter 20. Transport Through Membranes
Figure 20-2 Permeability correlates with membrane solubility.
The permeability coefficients of various organic molecules in
plasma membranes from the alga Nitella mucronata versus their
partition coefficients between olive oil and water (a measure of a
molecule’s polarity).This more or less linear log–log plot
Figure 20-3 Variation of glucose flux into human erythrocytes
with the external glucose concentration at 5°C. The black dots
are experimentally determined data points, and the solid green
line is computed from Eq. [20.7] with J
max
⫽ 1.0 ⫻ 10
⫺6
mM ⴢ
cm ⴢ s
⫺1
and K
M
⫽ 0.5 mM.The nonmediated glucose flux
increases linearly with [glucose] (Fig. 20-1) but would not visibly
depart from the baseline on the scale of this drawing. [Based on
data from Stein,W.D., Movement of Molecules across
Membranes, p. 134, Academic Press (1967).]
Permeability coefficient (cm
.
s
–1
)
0.001 0.010.0001
Oil–water partition coefficient
Ethylurea
Urea
Ethylene glycol
Acetamide
Succinimide
N,N'-Diethylurea
n-Butyramide
Propionamide
Formamide
10
–7
10
–6
10
–5
NH
2
CH
2
CH
2
H
2
NC
O
HO OH
NHH
2
NCH
2
CH
3
C
O
NHHN
NH
CH
2
CH
3
CH
3
CH
2
CH
3
CH
2
C
C
C
H
2
C
H
2
C
C
O
O
O
NH
2
CH
3
C
O
NH
2
HC
O
NH
2
O
CH
3
CH
2
CH
2
C
NH
2
O
indicates that the rate-limiting step for the nonmediated entry of
a molecule into a cell is its passage through the membrane’s
hydrophobic core. [Based on data from Collander, R., Physiol.
Plant. 7, 433–434 (1954).]
J
glucose
(mM
.
cm
.
s
–1
× 10
6
)
1.0
0.5
0
2468
100
[Glucose] mM
J
max
= 1.0 × 10
–6
mM
.
cm
.
s
–1
K
M
1
/
2
J
max
JWCL281_c20_744-788.qxd 3/17/10 1:47 PM Page 746
specificity, (2) saturation kinetics, (3) susceptibility to com-
petitive inhibition, and (4) susceptibility to chemical inacti-
vation. In the following paragraphs we shall see how the
erythrocyte glucose transporter exhibits these qualities.
a. Speed and Specificity
Table 20-1 indicates that the permeability coefficients of
D-glucose and D-mannitol in synthetic bilayers, and that of
D-mannitol in the erythrocyte membrane, are in reasonable
agreement with the values calculated from the diffusion
and partition coefficients of these sugars between water
and olive oil. However, the experimentally determined
permeability coefficient for
D-glucose in the erythrocyte
membrane is four orders of magnitude greater than its pre-
dicted value. The erythrocyte membrane must therefore
contain a system that rapidly transports glucose and that can
distinguish
D-glucose from D-mannitol.
b. Saturation Kinetics
The concentration dependence of glucose transport in-
dicates that its flux obeys the relationship
[20.7]
This saturation function has a familiar hyperbolic form
(Fig. 20-3). We have seen it in the equation describing the
binding of O
2
to myoglobin (Eq. [10.4]) and in the
Michaelis–Menten equation describing the rates of enzy-
matic reactions (Eq. [14.24]). Here, as before, K
M
may be
defined operationally as the concentration of glucose
when the transport flux is half of its maximal rate, J
max
/2.
This observation of saturation kinetics for glucose trans-
port was the first evidence that a specific, saturatable
J
A
⫽
J
max
[A]
K
M
⫹ [A]
number of sites on the membrane were involved in the
transport of any substance.
The transport process can be described by a simple four-
step kinetic scheme involving binding, transport, dissocia-
tion, and recovery (Fig. 20-4). Its binding and dissociation
steps are analogous to the recognition of a substrate and
the release of product by an enzyme. The mechanisms of
transport and recovery are discussed in Section 20-2D.
c. Susceptibility to Competitive Inhibition
Many compounds structurally similar to D-glucose in-
hibit glucose transport. A double-reciprocal plot (Section
14-2B) for the flux of glucose into erythrocytes in the pres-
ence or absence of 6-O-benzyl-
D-galactose (Fig. 20-5)
shows behavior typical of competitive inhibition of glucose
transport (competitive inhibition of enzymes is discussed
in Section 14-3A). Susceptibility to competitive inhibition
indicates that there is a limited number of sites available for
mediated transport.
Section 20-2. Kinetics and Mechanisms of Transport 747
Table 20-1 Permeability Coefficients of Natural and Synthetic
Membranes to
D-Glucose and D-Mannitol at 25°C
Permeability Coefficient
(cm ⴢ s
⫺1
)
Membrane Preparation
D-Glucose D-Mannitol
Synthetic lipid bilayer 2.4 ⫻ 10
⫺10
4.4 ⫻ 10
⫺11
Calculated nonmediated
diffusion 4 ⫻ 10
⫺9
3 ⫻ 10
⫺9
Intact human erythrocyte 2.0 ⫻ 10
⫺4
5 ⫻ 10
⫺9
Source: Jung, C.Y., in Surgenor, D. (Ed.), The Red Blood Cell, Vol. 2,
p. 709,Academic Press (1975).
Figure 20-4 General kinetic scheme for membrane transport.
The scheme involves four steps: binding, transport, dissociation,
and recovery. T is the transport protein whose binding site for
solute A is located on either the inner or the outer side of the
membrane at any one time.
T
out
T
out
• A (out)
T
in
T
in
•
A (in)
1. Binding
3. Dissociation
TransportRecovery4. 2.
A (out)
A (in)
1/J
max
– 1/K
M
1/[Glucose]
1/
J
glucose
Glucose + 10 mM
6-O-benzyl-
D-galactose
Glucose alone
Figure 20-5 Double-reciprocal plots for the net flux of glucose
into erythrocytes in the presence and absence of 6-O-benzyl-
D-
galactose. The pattern is that of competitive inhibition. [After
Barnett, J.E.G., Holman, G.D., Chalkley, R.A., and Munday,
K.A., Biochem. J. 145, 422 (1975).]
JWCL281_c20_744-788.qxd 3/17/10 1:47 PM Page 747
d. Susceptibility to Chemical Inactivation
Treatment of erythrocytes with HgCl
2
, which reacts with
protein sulfhydryl groups
and thus inactivates many enzymes, causes the rapid, saturat-
able flux of glucose to disappear so that its permeability con-
stant approaches that of mannitol. The erythrocyte glucose
transport system’s susceptibility to such protein-modifying
agents indicates that it, in fact, is a protein.
All of the above observations indicate that glucose
transport across the erythrocyte membrane is mediated by a
limited number of protein carriers. Before we discuss the
mechanism of this transport system, however, we shall ex-
amine some simpler models of facilitated diffusion.
C. Ionophores
Our understanding of mediated transport has been en-
hanced by the study of ionophores (Greek: phoros, bearer),
substances that vastly increase the permeability of mem-
branes to particular ions.
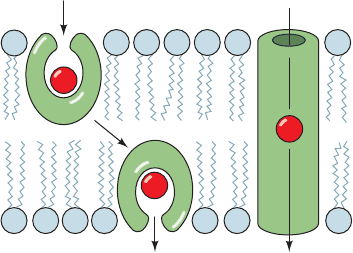
a. Ionophores May Be Carriers or Channel Formers
Ionophores are organic molecules of diverse types,
many of which are antibiotics of bacterial origin. Cells and
organelles actively maintain concentration gradients of
various ions across their membranes (Section 20-3A). The
antibiotic properties of ionophores arise from their ten-
dency to discharge these vital concentration gradients.
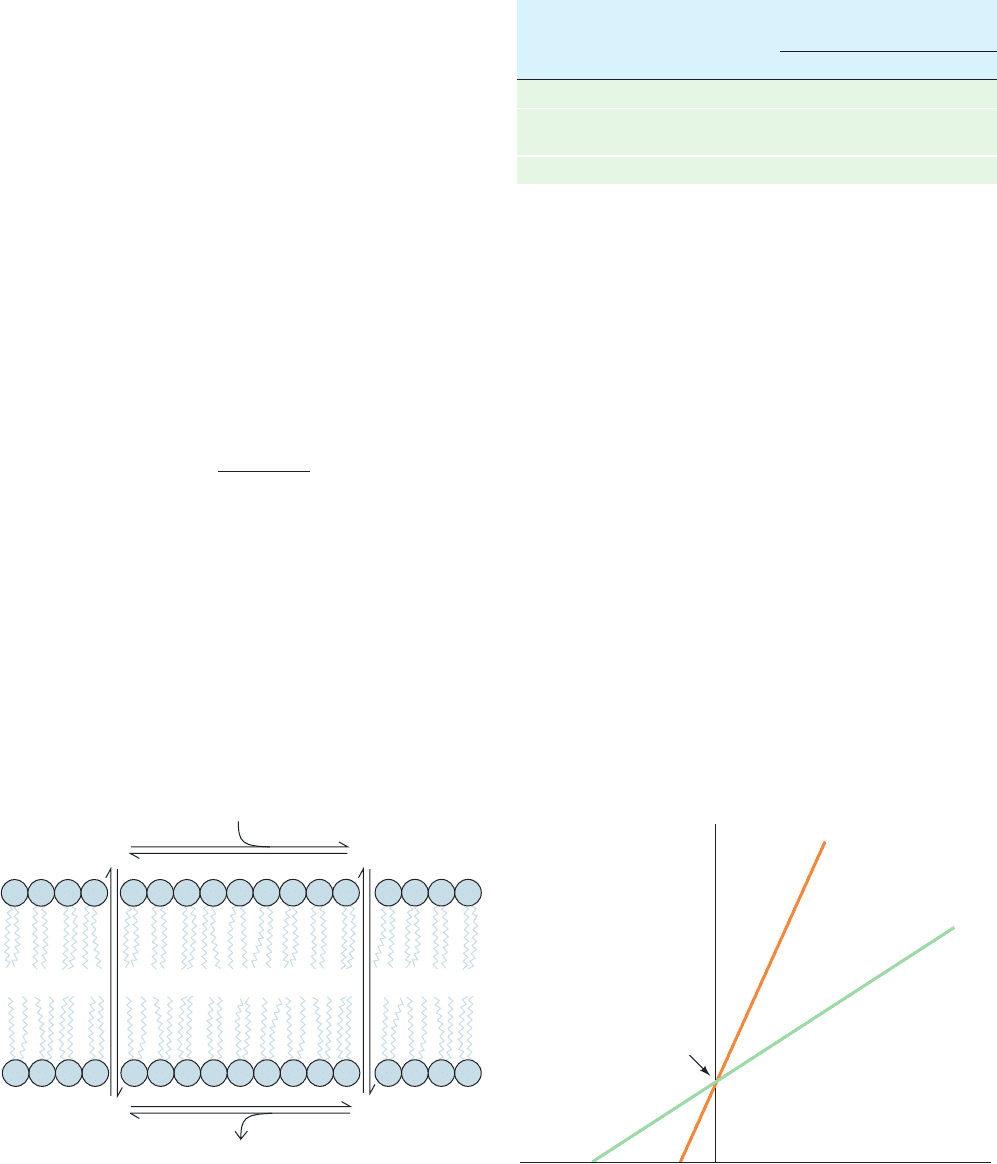
There are two types of ionophores:
1. Carriers, which increase the permeabilities of mem-
branes to their selected ion by binding it, diffusing through the
membrane,and releasing the ion on the other side (Fig. 20-6a).
For net transport to occur, the uncomplexed ionophore
must then return to the original side of the membrane
ready to repeat the process. Carriers therefore share the
common property that their ionic complexes are soluble in
nonpolar solvents.
2. Channel formers, which form transmembrane chan-
nels or pores through which their selected ions can diffuse
(Fig. 20-6b).
Both types of ionophores transport ions at a remarkable
rate. For example,a single molecule of the carrier antibiotic
valinomycin transports up to 10
4
K
⫹
ions per second across
a membrane. Channel formers have an even greater ion
throughput; for example, each membrane channel com-
posed of the antibiotic gramicidin A permits the passage of
over 10
7
K
⫹
ions ⴢ s
⫺1
. Clearly, the presence of either type
of ionophore, even in small amounts, greatly increases the
permeability of a membrane toward the specific ions trans-
ported. However, since ionophores passively permit ions to
diffuse across a membrane in either direction, their effect
can only be to equilibrate the concentrations of their selected
ions across the membrane.
Carriers and channel formers are easily distinguished
experimentally through differences in the temperature
RSH ⫹ HgCl
2
¡
RS¬Hg¬Cl ⫹ HCl
dependence of their action. Carriers depend on their abil-
ity to diffuse freely across the membrane. Consequently,
cooling a membrane below its transition temperature (the
temperature below which it becomes a gel-like solid; Sec-
tion 12-2Cb) essentially eliminates its ionic permeability in
the presence of carriers. In contrast, membrane permeabil-
ity in the presence of channel formers is rather insensitive
to temperature because, once in place, channel formers
need not move to mediate ion transport.
b. The K
⫹
–Valinomycin Complex Has a Polar Interior
and a Hydrophobic Exterior
Valinomycin, a product of several strains of Strepto-
myces bacteria that specifically binds K
⫹
(and the biologi-
cally unimportant Rb
⫹
), is perhaps the best characterized
carrier ionophore. It is a cyclic depsipeptide that contains
both
D- and L-amino acid residues (Fig. 20-7a; a depsipep-
tide contains ester linkages as well as peptide bonds). The
X-ray structure of valinomycin’s K
⫹
complex (Fig. 20-7b)
indicates that the K
⫹
is octahedrally coordinated by the car-
bonyl groups of its six Val residues, which also form its ester
linkages. The cyclic, intramolecularly hydrogen bonded
valinomycin backbone follows a zigzag path that surrounds
the K
⫹
coordination shell with a sinuous molecular bracelet.
Its methyl and isopropyl side chains project outward from
the bracelet to provide the spheroidal complex with a
hydrophobic exterior that makes it soluble in nonpolar
solvents and in the hydrophobic cores of lipid bilayers.
Uncomplexed valinomycin has a more open conformation
than its K
⫹
complex, which presumably facilitates the rapid
binding of K
⫹
.
K
⫹
(ionic radius, r ⫽ 1.33 Å) and Rb
⫹
(r ⫽ 1.49 Å) fit
snugly into valinomycin’s coordination site. However, the
rigidity of the valinomycin complex makes this site too
large to accommodate Na
⫹
(r ⫽ 0.95 Å) or Li
⫹
(r ⫽ 0.60 Å)
properly; that is, valinomycin’s six carbonyl oxygen atoms
cannot simultaneously coordinate these ions. Complexes of
these ions with water are therefore energetically more
favorable than their complexes with valinomycin. This
748 Chapter 20. Transport Through Membranes
Figure 20-6 Ion transport modes of ionophores. (a) Carrier
ionophores transport ions by diffusing through the lipid bilayer.
(b) Channel-forming ionophores span the membrane with a
channel through which ions can diffuse.
(a) Carrier ionophore (b) Channel-forming
ionophore
JWCL281_c20_744-788.qxd 3/17/10 1:47 PM Page 748
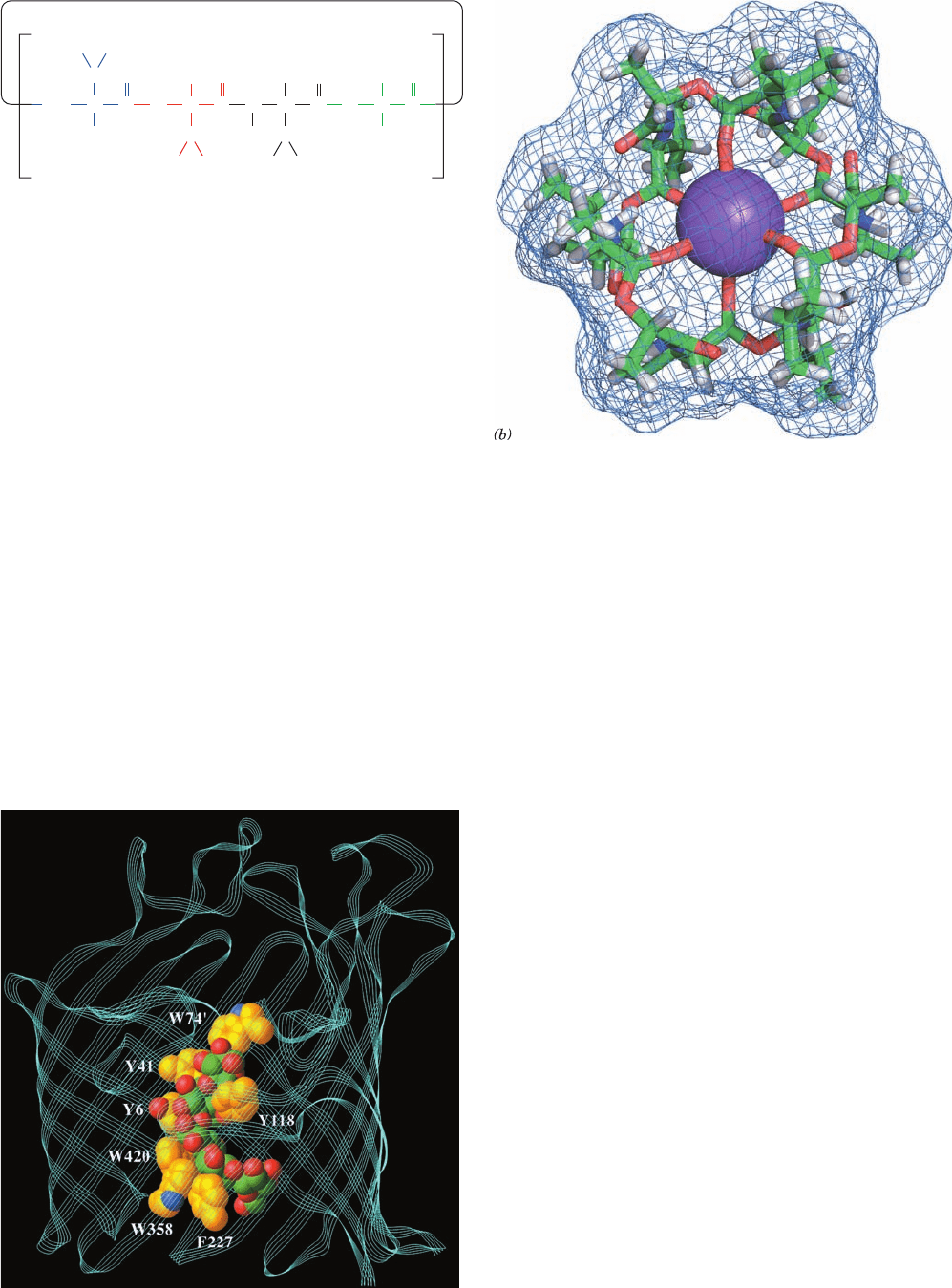
accounts for valinomycin’s 10,000-fold greater binding
affinity for K
⫹
over Na
⫹
. No other substance discriminates
more acutely between Na
⫹
and K
⫹
. A variety of carrier
ionophores with similar characteristics but with different
chemical structures and metal ion specificities are known.
D. Maltoporin: The Structural Basis
of Sugar Discrimination
The porins are homotrimeric transmembrane proteins
that facilitate the transport of small molecules and ions
across the outer membranes of gram-negative bacteria
and mitochondria. Each subunit consists mainly of a 16- to
22-stranded antiparallel  barrel that forms a solvent-
accessible channel along the barrel axis (Section 12-3Ad). In
the E.coli OmpF porin (Fig.12-27), this ⬃50-Å-long channel
is constricted near its center to an elliptical pore that has a
minimum cross section of 7 ⫻ 11 Å. Consequently, solutes
of more than ⬃600 D are too large to pass through this
channel.
Maltoporin is a bacterial porin that facilitates the diffu-
sion of maltodextrins [the ␣(1 S 4)-linked glucose oligosac-
charide degradation products of starch; e.g., maltose (Fig.
11-13)].The X-ray structure of E. coli maltoporin (Fig. 20-8),
determined by Tilman Schirmer, reveals that maltoporin is
structurally similar to OmpF porin (Fig. 12-27), but with an
18-stranded rather than a 16-stranded antiparallel  barrel
Section 20-2. Kinetics and Mechanisms of Transport 749
Figure 20-7 Valinomycin. (a) This cyclic depsipeptide (has
both ester and amide bonds) contains both
D- and L-amino acids.
(b) The X-ray structure of valinomycin in complex with a K
⫹
ion
shown in stick form colored according to atom type (C green, H
white, N blue, O red, and K
⫹
purple) and embedded in its
molecular surface. Note that the K
⫹
ion is octahedrally
coordinated by the carbonyl atoms of valinomycin’s six Val
residues and that the surface of the complex is largely covered
with methyl groups. [Based on an X-ray structure by Max
Dobler, ETH, Zürich, Switzerland.]
C
C
HN
Valinomycin
CH
3
H
3
C
C
O
N
C
C
C
C
O
C
O
O
O
O
H
H
H
3
C
CH
CH
HH
CH
H
3
C
CH
3
H
3
C
CH
3
3
H
L-Val D-Val L-Lactic
acid
D-Hydroxy-
isovaleric
acid
(a)
()
Figure 20-8 X-ray structure of a subunit of E. coli maltoporin
in complex with a maltodextrin of six glucosyl units (Glc
6
). The
structure is viewed from within the bacterial outer membrane
with its extracellular surface above. The polypeptide backbone is
represented by a multithreaded ribbon (cyan).The Glc
6
(only
five of whose glucosyl units are observed) and the aromatic side
chains lining the constricted region of the protein’s centrally
located transport channel are shown in space-filling form colored
according to atom type (protein side chain C gold, glucosyl
C green, N blue, and O red). Note the pronounced left-handed
helical twist of the Glc
6
unit.The so-called greasy slide, which
consists of the aromatic side chains of six residues (W74¿ is
contributed by an overhanging loop from an adjacent subunit),
conforms closely to this shape. The side chain of Y118 protrudes
into the channel opposite the greasy slide so as to allow only the
transit of near planar groups such as glucosyl residues.The
maltodextrin’s hydroxyl groups are arranged in two strips flank-
ing the greasy slide (only one of which is seen here) that form an
extensive hydrogen bonded network with mainly charged side
chains (not shown). [Based on an X-ray structure by Tilman
Schirmer, University of Basel, Switzerland. PDBid 1MPO.]
JWCL281_c20_744-788.qxd 6/4/10 12:13 PM Page 749
enclosing each subunit’s transport channel.Three long loops
from the extracellular face of each maltoporin subunit fold
inward into the barrel, thereby constricting the channel near
the center of the membrane to a diameter of ⬃5 Å (which is
considerably smaller than OmpF’s aperture) and giving the
channel an hourglasslike cross section. The channel is lined
on one side with a series of six contiguous aromatic side
chains arranged in a left-handed helical path that matches
the left-handed helical curvature of ␣-amylose (Fig. 11-18).
This “greasy slide” extends from the channel’s vestibule
floor, through its constriction, to its periplasmic outlet.
The way in which oligosaccharides interact with malto-
porin was investigated by determining the X-ray structures
of maltoporin in its complexes with the maltodextrins Glc
2
(maltose), Glc
3
, Glc
6
, and sucrose (a glucose–fructose
disaccharide; Fig. 11-13).Two Glc
2
molecules, one Glc
3
mole-
cule, and a Glc
5
segment of Glc
6
occupied the maltoporin
channel in contact and conformity with the greasy slide.
Thus the hydrophobic faces of the maltodextrin’s glycosyl
residues stack on aromatic side chains, as is often observed
in complexes of sugars with proteins. The glucose hydroxyl
groups, which are arranged in two strips along opposite
edges of the maltodextrins, form numerous hydrogen
bonds with polar side chains that line these strips. Six of
these seven polar side chains are charged, which probably
strengthens their hydrogen bonds, as has also been ob-
served in complexes of sugars with proteins. Tyr 118, which
protrudes into the channel opposite the greasy slide, appar-
ently functions as a steric barrier that permits only the pas-
sage of near-planar groups such as glucosyl residues. Thus
the hook-shaped sucrose, which maltoporin transports
quite slowly, binds to maltoporin with only its glucose
residue inserted into the constricted part of the channel
and its bulky fructose residue extending into the extracel-
lular vestibule.
The above structures suggest a model for the selective
transport of maltodextrins by maltoporin. At the start of
the translocation process, the entering glucosyl residue in-
teracts with the readily accessible end of the greasy slide in
the extracellular vestibule of the channel. Further translo-
cation along the helical channel requires the maltodextrin
to follow a screwlike path that maintains the helical struc-
ture of the oligosaccharide, much like the movement of a
bolt through a nut, thereby excluding molecules of compa-
rable size that have different shapes. The translocation
process is unlikely to encounter any large energy barrier
due to the smooth surface of the greasy slide and the mul-
tiple polar groups at the channel constriction that would
permit the essentially continuous exchange of hydrogen
bonds as a maltodextrin moves through the constriction.
Thus, maltoporin can be regarded as an enzyme that cat-
alyzes the translocation of its substrate from one compart-
ment to another.
E. Passive-Mediated Glucose Transport
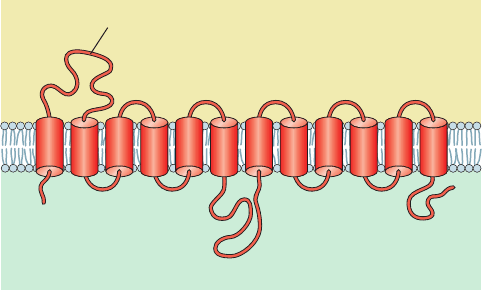
The human erythrocyte glucose transporter is a 492-residue
glycoprotein which, according to sequence hydropathy
analysis (Sections 8-4C and 12-3Aa), has 12 membrane-
spanning ␣ helices (Fig. 20-9) that are thought to form a
hydrophobic cylinder. Five of these helices (3, 5, 7, 8, and 11)
are amphipathic and hence most likely form a hydrophilic
channel through which glucose is transported. A highly
charged 66-residue domain located between helices 6 and
7, together with the 43-residue C-terminal domain, occupy
the cytoplasm, whereas a 34-residue carbohydrate-bearing
domain located between helices 1 and 2 is externally lo-
cated. The glucose transporter accounts for 2% of erythro-
cyte membrane proteins and runs as band 4.5 in SDS–PAGE
gels of erythrocyte membranes (Section 12-3Da; it is not
visible on the gel depicted in Fig. 12-37 because the het-
erogeneity of its oligosaccharides makes the protein
band diffuse).
a. Glucose Transport Occurs via a Gated
Pore Mechanism
The erythrocyte glucose transporter has glucose binding
sites on each side of the erythrocyte membrane but these
have different steric requirements. Thus, John Barnett
showed that 1-propylglucose will not bind to the extracellular
surface of the glucose transporter but will bind to its cytoplas-
mic surface, whereas the converse is true of 6-propylglucose.
He therefore proposed that the glucose transporter has
two alternate conformations: one with the glucose binding
site facing the external cell surface, requiring O1 contact
and leaving O6 free, and the other with the glucose binding
site facing the cytoplasm, requiring O6 contact and leaving
O1 free (Fig. 20-10). Transport apparently takes place by
binding glucose to the protein on one face of the membrane,
followed by a conformational change that closes the first site
while exposing the other. Glucose can then dissociate from
the protein, having been translocated across the membrane.
The transport cycle of this so-called gated pore is completed
by the reversion of the glucose transporter to its initial con-
formation in the absence of bound glucose. Since this cycle
can occur in either direction, the direction of net glucose
transport is from high to low glucose concentrations. The
glucose transporter thereby provides a means of equili-
brating the glucose concentration across the erythrocyte
750 Chapter 20. Transport Through Membranes
Figure 20-9 Predicted secondary structure and membrane
orientation of the glucose transporter.
C
Inside
–
–
–
–
+
+
+
+
Outside
Glycosylation site
N
121110987654321
JWCL281_c20_744-788.qxd 3/18/10 12:20 PM Page 750
