Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

membrane without any accompanying leakage of small
molecules or ions.
b. Eukaryotes Express a Variety of
Glucose Transporters
The erythrocyte glucose transporter, known also as
GLUT1 (for glucose transporter 1) has a highly conserved
amino acid sequence (98% sequence identity between hu-
mans and rats), which suggests that all segments of this pro-
tein are functionally significant. GLUT1 is expressed in
most tissues, although in liver and muscle, tissues that are
highly active in glucose transport, it is present in only tiny
amounts. Three other glucose transporters, GLUT2,
GLUT3, and GLUT4, have been well characterized
(GLUT5 was originally thought to be a glucose transporter
but was later shown to be a fructose transporter).They are
40 to 65% identical to GLUT1 but have different tissue
distributions. For example,GLUT2 is prominent in pancre-
atic  cells (which secrete insulin in response to increased
[glucose] in blood; Section 18-3F), liver (where its defects
result in symptoms resembling Type I glycogen storage dis-
ease; Section 18-4), and the intestine (which absorbs di-
etary glucose; Section 20-4A); GLUT3 is expressed in neu-
rons and the placenta, and GLUT4 occurs mainly in muscle
and fat cells. Note that the tissue distributions of these glu-
cose transporters correlate with the response of these tis-
sues to insulin: Liver is unresponsive to insulin (liver func-
tions, in part, to maintain the level of blood glucose; Section
18-3Fb), whereas muscle and fat cells take up glucose when
stimulated by insulin. Analysis of the human genome has
identified eight other members of the GLUT family,
GLUT6 through GLUT12 and HMIT (for H
⫹
-coupled
myo-inositol transporter), although they have yet to be
well characterized. All of them are members of the major
facilitator superfamily (MFS).
c. Cellular Glucose Uptake Is Regulated through
the Insulin-Sensitive Exocytosis/Endocytosis
of Glucose Transporters
Insulin stimulates fat and muscle cells to take up glucose.
Within 2 or 3 min after the administration of insulin to fat
cells, the J
max
for passive-mediated glucose transport into
these cells increases 20- to 30-fold, whereas the K
M
remains
constant. On withdrawal of the insulin, the rate of glucose
uptake returns to its basal level within 20 min to 2 h de-
pending on conditions. Neither the increase nor the de-
crease in the rate of glucose transport is affected by the
presence of protein synthesis inhibitors, so that these ob-
servations cannot be a consequence of the synthesis of new
glucose transporter or of a protein that inhibits it. How,
then, does insulin regulate glucose transport?
GLUT4 is the dominant glucose transporter in skeletal
muscle and adipose (fat) cells. In their basal state, these
cells store most of their GLUT4 in specialized GLUT4
storage vesicles. On insulin stimulation, these vesicles fuse
with the plasma membrane in a process known as exocyto-
sis (Fig. 20-11). The consequent increased number of
Section 20-2. Kinetics and Mechanisms of Transport 751
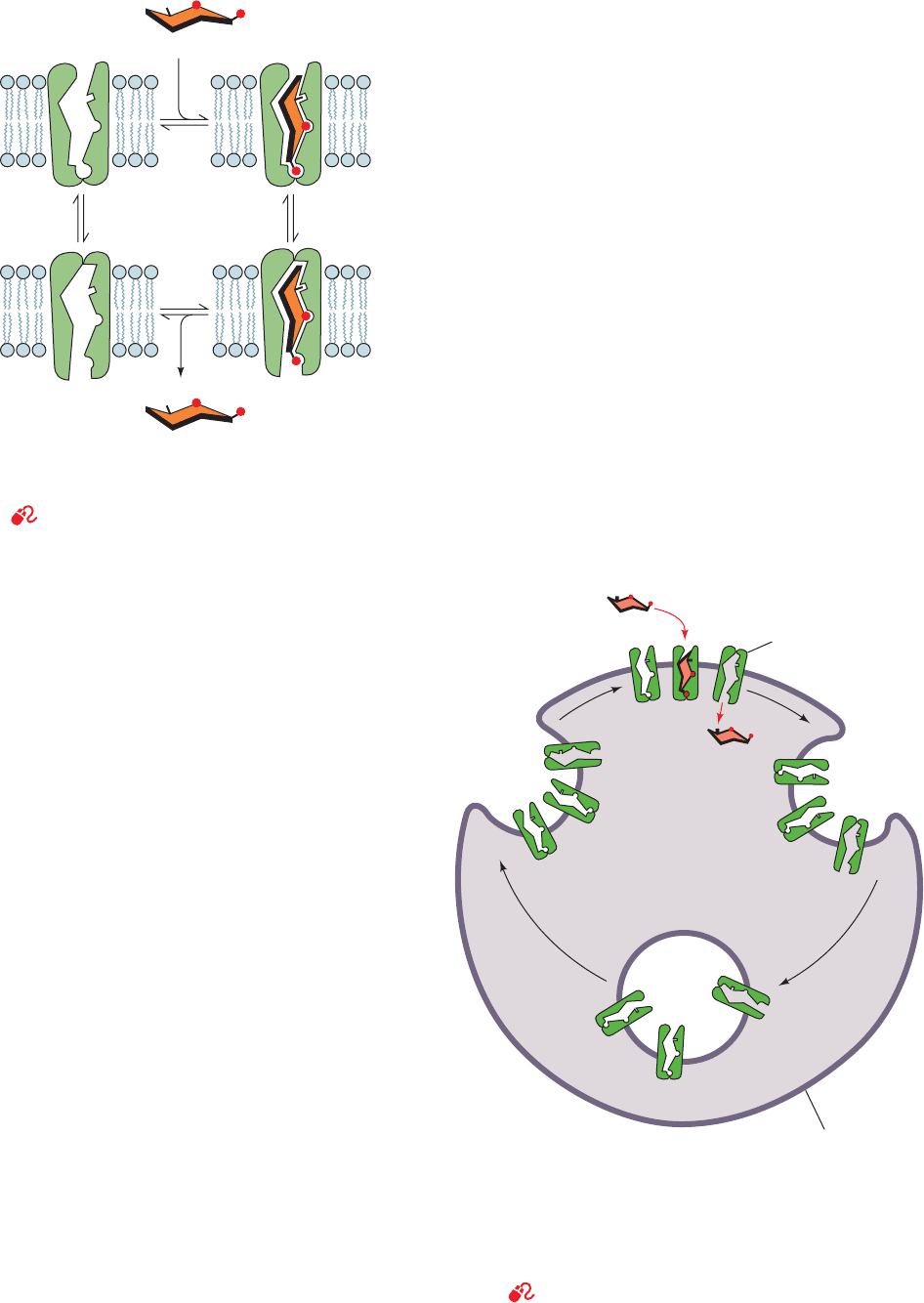
Figure 20-10 Alternating conformation model for glucose
transport. Such a system is also known as a “gated pore.” [After
Baldwin, S.A. and Lienhard, G.E., Trends Biochem. Sci. 6, 210
(1981).]
See the Animated Figures
Figure 20-11 Regulation of glucose uptake in muscle and fat
cells. Regulation is mediated by the insulin-stimulated exocytosis
(the opposite of endocytosis; Section 12-5Bc) of membranous
vesicles containing GLUT4 glucose transporters (left). On insulin
withdrawal, the process reverses itself through endocytosis
(right).
See the Animated Figures
Glucose
Binding
Dissociation
Recovery Transport
Glucose
GLUT4
Exocytosis
Endocytosis
Membranous
vesicle
Stimulation
by insulin
Plasma
membrane
JWCL281_c20_744-788.qxd 6/4/10 12:14 PM Page 751
cell-surface glucose transporters results in a proportional
increase in the cell’s glucose uptake rate. On insulin with-
drawal, the process is reversed through the endocytosis
of plasma membrane-embedded glucose transporters.
The deletion or mutation of GLUT4’s N-terminal eight
residues, particularly Phe 5, causes this transporter to ac-
cumulate in the plasma membrane. A Leu-Leu sequence
and an acidic motif near GLUT4’s C-terminus are likewise
essential for its sequestration by the cell’s endocytotic ma-
chinery. The way in which insulin controls this system,
which accounts for most of insulin’s effects on muscle and
fat cells, is imperfectly understood. However, it is clear
that this mechanism involves a tyrosine phosphorylation
cascade that is triggered by the binding of insulin to the in-
sulin receptor (Section 19-3Ac and Fig. 19-67) and includes
the activation of a class IA phosphoinositide 3-kinase
(PI3K; Section 19-4Da).
F. K
⫹
Channels: Ion Discrimination
Potassium ions diffuse from the cytoplasm (where [K
⫹
] ⬎
100 mM) to the extracellular space (where [K
⫹
] ⬍ 5 mM)
through transmembrane proteins known as K
⫹
channels, a
process that underlies numerous important biological
processes including maintenance of cellular osmotic bal-
ance, neurotransmission (Section 20-5), and signal transduc-
tion (Chapter 19). Although there is a large diversity of K
⫹
channels, even within single organisms, all of them have
similar sequences, exhibit comparable permeability charac-
teristics, and most importantly, are at least 10,000-fold
more permeable to K
⫹
than Na
⫹
. Since this high selectivity
(around the same as that of valinomycin; Section 20-2Cb)
implies energetically strong interactions between K
⫹
and the
protein, how can the K
⫹
channel maintain its observed nearly
diffusion-limited throughput rate of up to 10
8
ions per second
(a 10
4
-fold greater rate than that of valinomycin)?
a. The X-Ray Structure of KcsA Reveals the Basis
of K
⫹
Channel Selectivity
KcsA, the K
⫹
channel from Streptomyces lividans, is a
tetramer of identical 158-residue subunits. The X-ray
structure of its N-terminal 125-residue segment, deter-
mined by Roderick MacKinnon, reveals that each KcsA
subunit forms two nearly parallel transmembrane helices
that are inclined ⬃25° from the normal to the membrane
plane and which are connected by an ⬃20-residue pore
region (Fig. 20-12a). As is true of all known K
⫹
channels,
four such subunits associate to form a 4-fold rotationally
symmetric assembly surrounding a central pore. The four
inner (C-terminal) helices, which largely form the pore,
pack against each other near the cytoplasmic side of the
membrane much like the poles of an inverted teepee. The
four outer helices, which face the lipid bilayer, buttress
the inner helices but do not contact the adjacent outer he-
lices. The pore regions, which each consist of a so-called
turret, pore helix, and selectivity filter, occupy the open
extracellular end of the teepee, with the pore helices
fitting in between its poles. Several K
⫹
ions and ordered
water molecules are seen to occupy the central pore (Figs.
20-12b and 20-13a).
The 45-Å-long central pore has variable width: It starts
at its cytoplasmic side (Fig. 20-12b, bottom) as an ⬃6-Å-
diameter and 18-Å-long tunnel, the so-called internal
pore, whose entrance is lined with four anionic side chains
that presumably help exclude anions (red area at the bot-
tom of Fig. 20-12b).The internal pore then widens to form
a cavity ⬃10 Å in diameter. These regions of the central
pore are both wide enough so that a K
⫹
ion could move
through them in its hydrated state. However, the upper
part of the pore, the so-called selectivity filter, narrows to
3 Å, thereby forcing a transiting K
⫹
ion to shed its waters
of hydration.The walls of the internal pore and the cavity
are lined with hydrophobic groups that interact minimally
with diffusing ions (yellow area of the pore in Fig. 20-
12b). However, the selectivity filter (red area of the pore
at the top of Fig. 20-12b) is lined with closely spaced main
chain carbonyl oxygens of residues (Fig. 20-13a, top) that
are highly conserved in all K
⫹
channels (their so-called
signature sequence, TVGYG) and whose mutations dis-
rupt the ability of the channel to discriminate between K
⫹
and Na
⫹
ions.
What is the function of the cavity? Energy calculations
indicate that an ion moving through a narrow transmem-
brane pore must surmount an energy barrier that is maximal
at the center of the membrane. The existence of the cavity
reduces this electrostatic destabilization by surrounding the
ion with polarizable water molecules (Fig. 20-12c). In addi-
tion, the C-terminal ends of the four pore helices point di-
rectly at the center of the cavity, so that their helix dipoles
impose a negative electrostatic potential on the cavity that
lowers the electrostatic barrier facing a cation crossing a
lipid bilayer.
Remarkably, the K
⫹
ion occupying the cavity is liganded
by 8 ordered water molecules located at the corners of a
square antiprism (a cube with one face twisted by 45° with
respect to the opposite face) in which the K
⫹
ion is cen-
tered (Fig. 20-13a, bottom; K
⫹
in aqueous solution was
known to have such an inner hydration shell but it had
never before been visualized). The K
⫹
ion is precisely cen-
tered in the cavity but yet its liganding water molecules are
not in van der Waals contact with the walls of the cavity. In-
deed, there is room in the cavity for ⬃40 additional water
molecules although they are unseen in the X-ray structure
because they are disordered. This disorder arises because
the cavity is lined with hydrophobic groups (mainly the
side chains of Ile 100 and Phe 103; Fig. 20-13a) that interact
but weakly with water molecules, thus allowing them to in-
teract freely with the K
⫹
ion so as to form an outer hydra-
tion shell. What, then, holds the hydrated K
⫹
ion in place?
Apparently, it is very weak indirect hydrogen bonds involv-
ing such protein groups as the hydroxyl group of Thr 107
and possibly carbonyl O atoms from the pore and inner he-
lices. The absence of such an ordered hydration complex
when Na
⫹
rather than K
⫹
occupies the cavity is indicative
of a precise geometric match between the hydrated K
⫹
and
the cavity (the ionic radii of Na
⫹
and K
⫹
are 0.95 Å and
752 Chapter 20. Transport Through Membranes
JWCL281_c20_744-788.qxd 3/17/10 1:48 PM Page 752
1.33 Å, respectively).The cavity thereby provides a high ef-
fective K
⫹
concentration (⬃2M) at the center of the mem-
brane and positions the K
⫹
ion on the pore axis ready to
enter the selectivity filter.
How does the K
⫹
channel discriminate so acutely be-
tween K
⫹
and Na
⫹
ions? The main chain O atoms lining the
selectivity filter form a stack of rings (Fig. 20-13a, top) that
provide a series of closely spaced sites of appropriate di-
mensions for coordinating dehydrated K
⫹
ions but not the
smaller Na
⫹
ions. If the observed diameter of the selectiv-
ity filter is rigidly maintained, it would make the energy of
a dehydrated Na
⫹
in the selectivity filter considerably
Section 20-2. Kinetics and Mechanisms of Transport 753
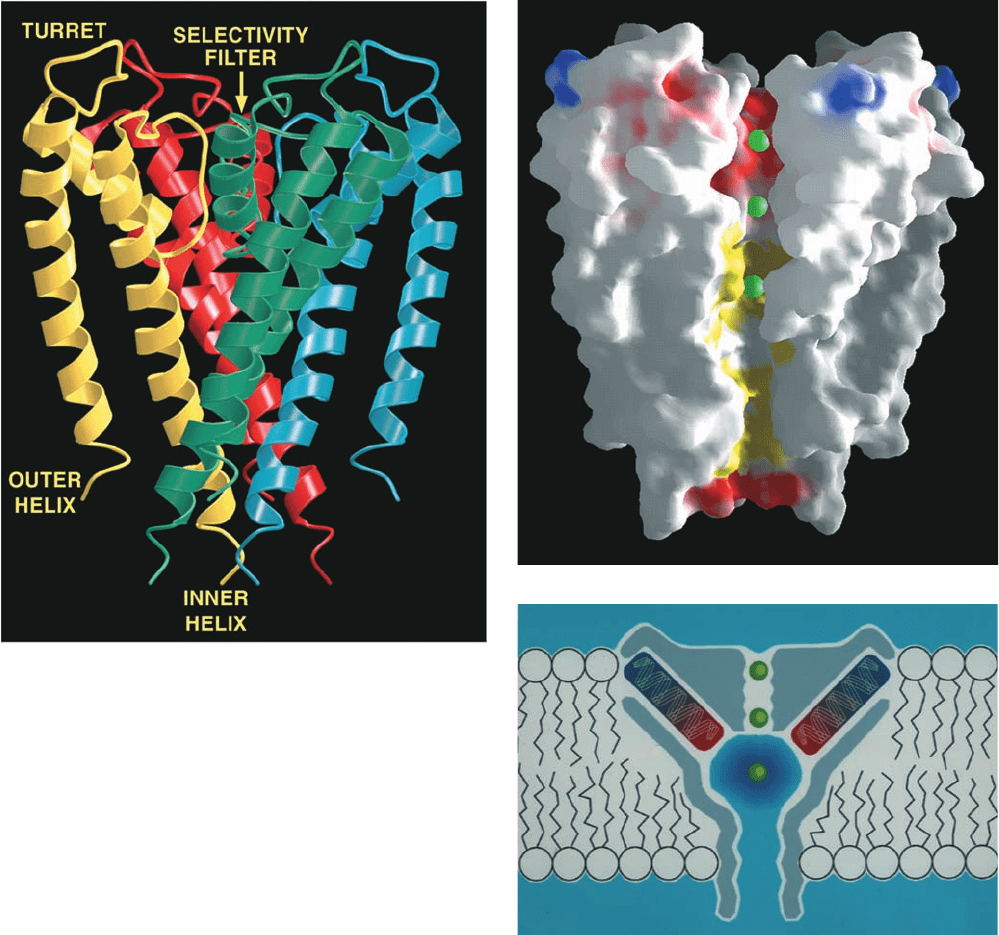
Figure 20-12 X-ray structure of the KcsA K
⫹
channel.
(a) Ribbon diagram of the tetramer as viewed from within the
plane of the membrane with the cytoplasm below and the
extracellular region above. The protein’s 4-fold axis of rotation is
vertical and each of its identical subunits is differently colored.
(b) A cutaway diagram viewed similarly to Part a in which the
K
⫹
channel is represented by its solvent-accessible surface. The
surface is colored according to its physical properties, with
negatively charged areas red, uncharged areas white, positively
charged areas blue, and hydrophobic areas of the central pore
yellow. K
⫹
ions are represented by green spheres. (c) A schematic
diagram indicating how the K
⫹
channel stabilizes a cation in the
center of the membrane. The central pore’s 10-Å-diameter aqueous
cavity (which contains ⬃50 water molecules) stabilizes a K
⫹
ion
(green spheres) in the otherwise hydrophobic membrane interior.
In addition, the C-terminal ends of the pore helices (red) all point
toward the K
⫹
ion, thereby electrostatically stabilizing it via their
dipole moments (an ␣ helix has a strong dipole moment with its
negative end pointing toward the helix’s C-terminal end because
the bond dipoles of its component carbonyl and N¬H groups are
(a)
(b)
(c)
all parallel to the helix axis with their negative ends pointing
toward its C-terminal end; Fig. 8-11).This effect is magnified by the
low dielectric constant at the center of the membrane interior.
Electrostatic calculations indicate that the cavity is tuned to
maximally stabilize monovalent cations. [Courtesy of Roderick
MacKinnon, Rockefeller University. PDBid 1BL8.]
JWCL281_c20_744-788.qxd 3/17/10 1:48 PM Page 753
higher than that of hydrated Na
⫹
and thus account for the
K
⫹
channel’s high selectivity for K
⫹
ions. However, pro-
teins are not static structures. In fact, both X-ray evidence
and molecular dynamics simulations (Section 9-4a) indi-
cate that, at physiological temperatures, the atoms forming
the KcsA selectivity filter undergo thermal excursions av-
eraging ⬃1 Å, fluctuations sufficient to snugly cradle Na
⫹
ions with little energetic cost. Instead, as free energy calcu-
lations have demonstrated, it is the electrostatic interac-
tions of the carbonyl groups with the cation and with each
754 Chapter 20. Transport Through Membranes
(a)
(b)
(c)
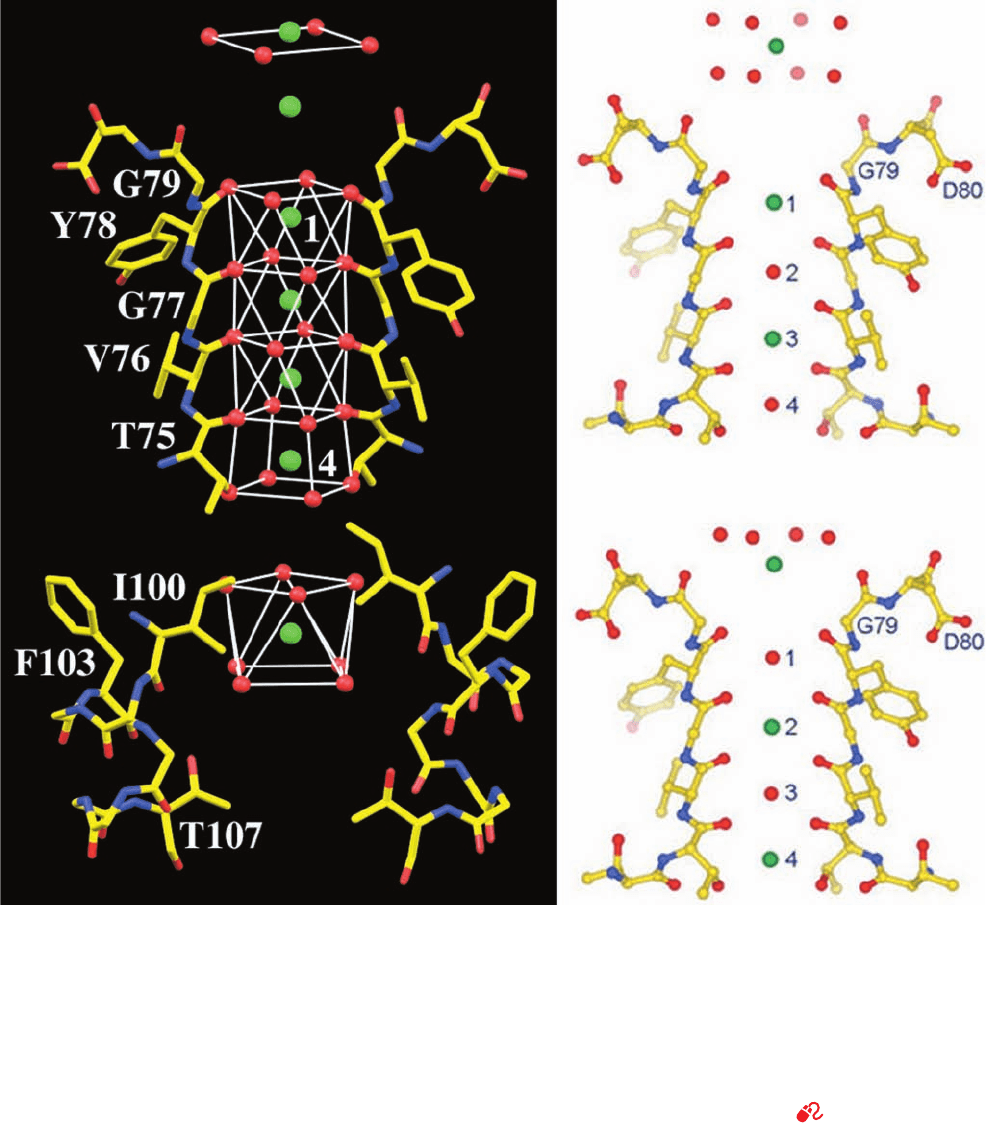
Figure 20-13 Portions of the KcsA K
⫹
channel responsible for
its ion selectivity viewed similarly to Fig. 20-12. (a) The X-ray
structure of the residues forming the cavity (bottom) and
selectivity filter (top) but with the front and back subunits
omitted for clarity. Atoms are colored according to type, with
C yellow, N blue, O red, and K
⫹
ions represented by green spheres.
The water and protein O atoms that ligand the K
⫹
ions, including
those contributed by the front and back subunits, are represented
by red spheres.The coordination polyhedra formed by these
O atoms are outlined by thin white lines. (b and c) Two alternative
K
⫹
binding states of the selectivity filter, whose superposition is
presumed to be responsible for the electron density observed in
the X-ray structure of KcsA.Atoms are colored as in Part a.
Note that K
⫹
ions occupying the selectivity filter are interspersed
with water molecules and that the K
⫹
ion immediately above the
selectivity filter in Part b is farther above the protein than that in
Part c. Hence these ions maintain a constant spacing while
traversing the selectivity filter. [Part a based on an X-ray
structure by, and Parts b and c courtesy of, Roderick MacKinnon,
Rockefeller University. PDBid 1K4C.]
See Interactive
Exercise 14
JWCL281_c20_744-788.qxd 10/19/10 7:39 AM Page 754
other that confer specificity for binding K
⫹
ions. This is
consistent with the observation that no Na
⫹
-specific pro-
tein channels have evolved by refining the structure of a
KcsA-like channel.
Since the selectivity filter appears designed to specifi-
cally bind K
⫹
ions, how does it support such a high
throughput of these ions (up to 10
8
ions ⴢ s
⫺1
)? The struc-
ture in Fig. 20-13a shows what appear to be 4 K
⫹
ions in the
selectivity filter and two more just outside it on its extracel-
lular side. Such closely spaced positive ions would strongly
repel one another and hence represent a high energy situa-
tion. However, a variety of evidence suggests that this
structure is really a superposition of two sets of K
⫹
ions,
one with K
⫹
ions at the topmost position in Fig. 20-13a and
at positions 1 and 3 in the selectivity filter (Fig. 20-13b) and
the second with K
⫹
ions at the second position from the top
in Fig. 20-13a and at positions 2 and 4 in the selectivity fil-
ter (Fig. 20-13c; X-ray structures can show overlapping
atoms because they are averages of many unit cells).
Within the selectivity filter, the positions not occupied by
K
⫹
ions are instead occupied by water molecules that coor-
dinate the neighboring K
⫹
ions.
The electron density that is represented as the topmost
4 water molecules in Fig. 20-13a is highly elongated in the
vertical direction in this otherwise high-resolution (2.0 Å)
structure. Hence it is thought to actually arise from 8 water
molecules that ligand the topmost K
⫹
ion in Fig. 20-13b to
form an inner hydration shell similar to that of the K
⫹
in
the central cavity (Fig. 20-13a, bottom). Moreover, the four
water molecules liganding the topmost K
⫹
ion in Fig. 20-
13c also contribute to this electron density. This latter ring
of 4 waters provides half of the associated K
⫹
ion’s 8 lig-
anding O atoms. The others are contributed by the car-
bonyl O atoms of the 4 Gly 79 residues, which are properly
oriented to do so. It therefore appears that a dehydrated
K
⫹
ion transits the selectivity filter (moves to successive
positions in Figs. 20-13b,c) by exchanging the properly
spaced ligands extending from its walls and then exits into
the extracellular solution by exchanging protein ligands for
water molecules and hence again acquiring a hydration
shell.These ligands are spaced and oriented such that there
is little free energy change (estimated to be ⬍12 kJ ⴢ mol
⫺1
)
along the reaction coordinate via which a K
⫹
ion transits
the selectivity filter and enters the extracellular solution.
The rapid dehydration of the K
⫹
ion entering the selectiv-
ity channel from the cavity is, presumably, similarly man-
aged. The essentially level free energy landscape through-
out this process is, of course, conducive to the rapid transit
of K
⫹
ions through the ion channel and hence must be a
product of evolutionary fine-tuning. Energy calculations
indicate that mutual electrostatic repulsions between suc-
cessive K
⫹
ions, whose movements are concerted, balances
the attractive interactions holding these ions in the selec-
tivity filter and hence further facilitates their rapid transit.
G. Cl
ⴚ
Channels
Cl
⫺
channels, which occur in all cell types, permit the trans-
membrane movement of chloride ions along their concen-
tration gradient. In mammals, the extracellular Cl
⫺
concen-
tration is ⬃120 mM and the intracellular concentration is
⬃4 mM.
ClC channels form a large family of Cl
⫺
channels that
occur widely in all kingdoms of life.The X-ray structures of
ClC channels from two species of bacteria, determined by
Raimund Dutzler and MacKinnon, reveal, as biophysical
measurements had previously suggested, that ClC channels
are homodimers with each ⬃470-residue subunit forming
an anion-selective pore (Fig. 20-14). Each subunit consists
mainly of 18 mostly transmembrane ␣ helices that are re-
markably tilted with respect to the membrane plane and
have variable lengths compared to the transmembrane he-
lices in other integral proteins of known structures. The N-
and C-terminal halves of each subunit are related by a
pseudo-2-fold axis parallel to the plane of the membrane
and hence these two halves have opposite orientations in
Section 20-2. Kinetics and Mechanisms of Transport 755
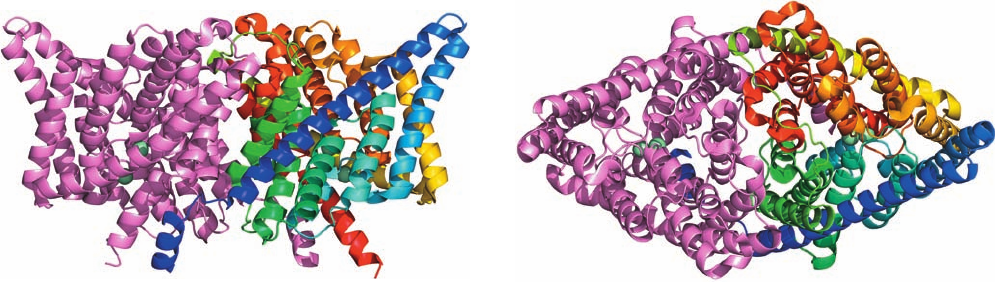
Figure 20-14 X-ray structure of the ClC Cl
⫺
channel from
E. coli. Each subunit of the homodimer contains 18 ␣ helices of
variable lengths.The subunits are drawn in ribbon form with
one colored in rainbow order from its N-terminus (blue) to its
C-terminus (red) and the other pink.The two Cl
⫺
ions bound in
the selectivity filter of each subunit are represented by pale
(a)
(b)
green spheres. (a) View from within the membrane with the
extracellular surface above and the 2-fold axis relating the two
subunits vertical. (b) View from the extracellular side of the
membrane along the molecular 2-fold axis. [Based on an X-ray
structure by Raimund Dutzler and Roderick MacKinnon,
Rockefeller University. PDBid 1OTS.]
JWCL281_c20_744-788.qxd 3/17/10 1:48 PM Page 755
the membrane. This suggests that the ClC channel arose
through gene duplication although its two halves exhibit
only weak sequence similarity. Such antiparallel architec-
ture occurs in several types of transmembrane transport
proteins.
The ClC Cl
⫺
channel is located at the interface between
its N- and C-terminal halves. The specificity of the ClC
channel results from an electrostatic field established by
basic amino acids on the protein surface, which helps fun-
nel anions toward the pore, and by a selectivity filter
formed by the dipoles of several ␣ helices oriented with
their positively charged N-terminal ends pointing toward
the Cl
⫺
ions (opposite to their orientation in the KcsA
channel; Fig. 20-12c). This feature of the selectivity filter
helps attract Cl
⫺
ions, which are specifically coordinated by
main chain amide nitrogens and side chain hydroxyls from
Ser and Tyr residues. A positively charged residue such as
Lys or Arg, if it were present in the selectivity filter, would
probably bind a Cl
⫺
ion too tightly to facilitate its rapid
transit through the channel.
Unlike the KcsA channel, which has a central aqueous
cavity (Fig. 20-12c), the Cl
⫺
channel is hourglass-shaped,
with its narrowest part in the center of the membrane and
flanked by wider aqueous vestibules. A conserved Glu side
chain projects into the pore. This group would repel other
anions, suggesting that rapid Cl
⫺
flux requires a protein
conformational change in which the Glu side chain moves
aside. Another anion could push the Glu away, which ex-
plains why some Cl
⫺
channels appear to be activated by
Cl
⫺
ions; that is, they open in response to a certain concen-
tration of Cl
⫺
in the extracellular fluid.
H. Aquaporins
The observed rapid passage of water molecules across bio-
logical membranes had long been assumed to occur via
simple diffusion that was made possible by the small size
and high concentration of water molecules. However, cer-
tain cells, such as erythrocytes and those of the kidney, can
sustain particularly rapid rates of water transport, which
are reversibly inhibited by mercuric ion.This suggested the
existence of previously unrecognized protein pores that
conduct water through biological membranes. The first of
these proteins was discovered in 1992 by Peter Agre, who
named them aquaporins.
Aquaporins occur widely in all kingdoms of life. Plants
have up to 50 different aquaporins, which is indicative of
the importance of water transport to plant physiology. The
13 known mammalian aquaporins, AQP0 through AQP12,
are selectively expressed at high levels in tissues that rap-
idly transport water, such as kidneys, salivary glands, sweat
glands, and lacrimal glands (which produce tears). In fact,
kidneys alone employ seven different aquaporins, each
with specific locations and regulatory properties. There are
two subfamilies of aquaporins: those that permit only the
passage of water and those that also allow the passage of
small neutral molecules such as glycerol and urea and
hence are named aquaglyceroporins. Aquaporins permit
the passage of water molecules at extremely high rates (up
to ⬃3 ⫻ 10
9
per second) but, quite surprisingly, not protons
(really hydronium ions; H
3
O
⫹
), whose free passage would
discharge the cell’s membrane potential.
All known aquaporins are homotetramers, each of
whose subunits contain a water-transport channel (unlike
K
⫹
channels, whose transport channels lie along their 4-fold
axes; Section 20-2Fa). The X-ray structure of the most ex-
tensively studied aquaporin, bovine AQP1, reveals that
each of its 271-residue subunits consists mainly of six trans-
membrane ␣ helices plus two shorter helices that are com-
ponents of loops that extend only to the middle of the bi-
layer (Fig. 20-15a). Other aquaporins of known structure
have similar structures. The N- and C-terminal halves of
aquaporins are ⬃20% identical in sequence and related by
a pseudo-2-fold axis of symmetry that is parallel to the
plane of the membrane (Fig. 20-15a). Evidently, these seg-
ments arose through gene duplication. ClC channels have a
similar antiparallel architecture (Section 20-2G).
The helices in AQP1 surround an elongated hourglass-
shaped channel through the membrane (Fig. 20-16) that at
its narrowest point is ⬃2.8 Å wide, the diameter of a water
molecule. This region is formed by the side chains of the
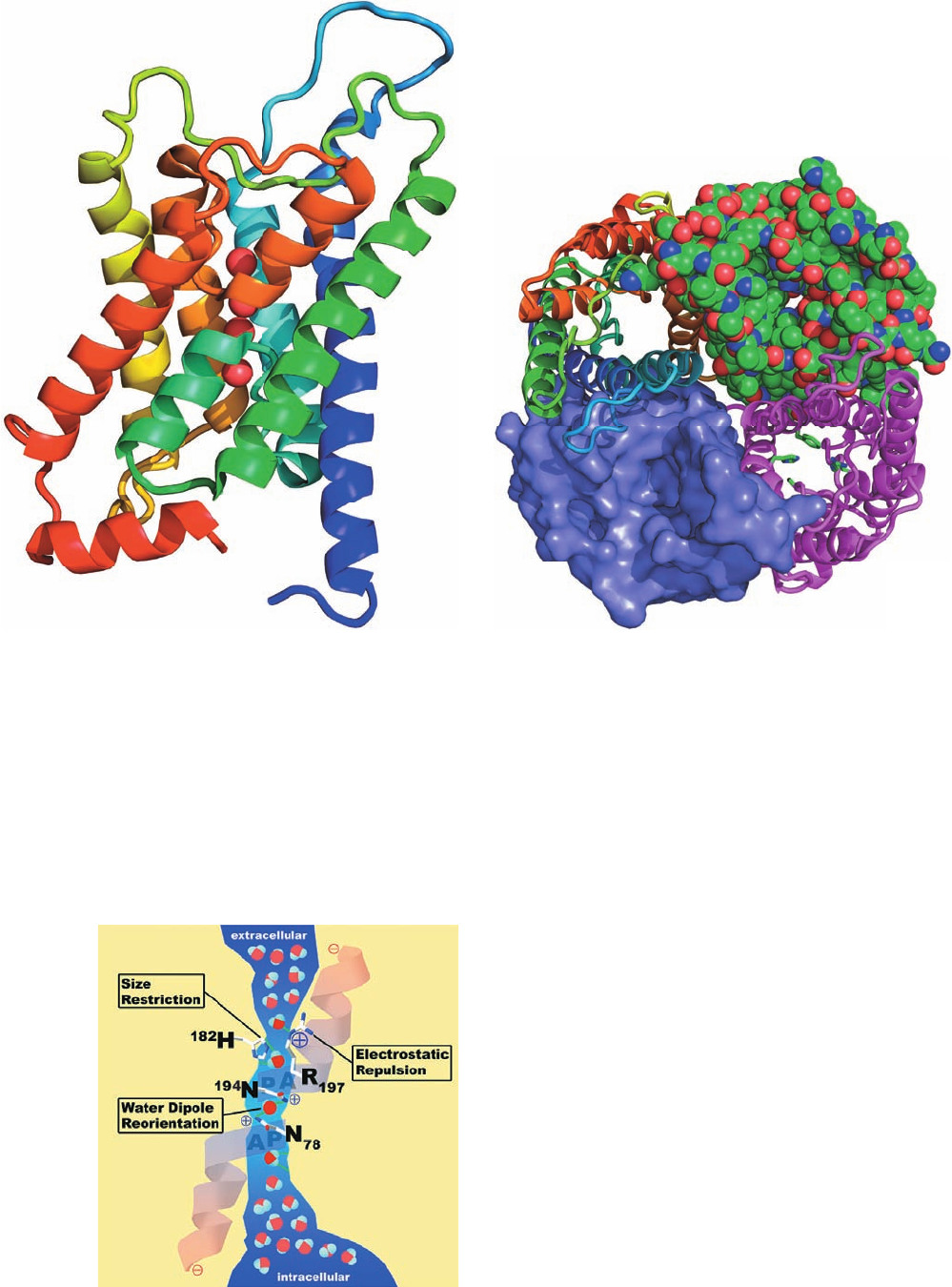
highly conserved Phe 58, His 182, and Arg 197 (Fig. 20-15b,
lower right subunit) and hence is known as the ar/R con-
striction (ar for aromatic).The side chain of Cys 191, which
also forms part of the ar/R constriction, is the site of chan-
nel blockage by the binding of mercuric ion. For a water
molecule to pass through the ar/R constriction, it must
shed its shell of associated water molecules. This is facili-
tated by the side chains of His 182 and Arg 197. The water
molecules then continue in single file through the ⬃25-Å-
long and ⬃4-Å-wide portion of the channel, which is lined
with hydrophobic groups interspersed with several hydro-
gen bonding groups. The water molecules’ lack of interac-
tion with the hydrophobic walls of the channel facilitates
their rapid passage through the channel, whereas the hy-
drogen bonding groups reduce the energy barrier to water
transport. It is the balancing of these opposing factors that
is presumably responsible for aquaporin’s selective perme-
ability to water and its rapid transport rate.
If water were to pass through aquaporin as an uninter-
rupted chain of hydrogen-bonded molecules, then protons
would pass even more rapidly through the channel via pro-
ton jumping (Fig. 2-10; in order for more than one such se-
ries of proton jumps to occur, each water molecule in the
chain must reorient such that one of its protons forms a hy-
drogen bond to the next water molecule in the chain).
However, aquaporin interrupts this process by forming hy-
drogen bonds from the side chain NH
2
groups of the highly
conserved Asn 78 and Asn 194, to a water molecule that is
centrally located in the channel (Fig. 20-16). Consequently,
although this central water molecule can readily donate
hydrogen bonds to its neighboring water molecules in the
hydrogen bonded chain, it cannot accept one from them
nor reorient, thereby severing the “proton-conducting
wire.” Both of these Asn residues occur in the sequence
Asn-Pro-Ala (NPA), the signature sequence of aquaporins,
in which the Ala is located at the N-terminal end of each of
the half-spanning helices.
756 Chapter 20. Transport Through Membranes
JWCL281_c20_744-788.qxd 6/4/10 12:14 PM Page 756
Section 20-2. Kinetics and Mechanisms of Transport 757
Figure 20-15 X-ray structure of the aquaporin AQP1 from
bovine erythrocytes. (a) Ribbon diagram of an aquaporin
subunit colored in rainbow order from its N-terminus (blue) to
its C-terminus (red).The view is from within the membrane with
its extracellular surface above and along the subunit’s pseudo-2-
fold axis of symmetry. Note that the two helices closest to the
viewer (orange and blue-green) are both portions of loops that
extend only to the center of the bilayer.The four water
molecules that occupy the central portion of AQP1’s water-
transport channel are represented by red spheres. (b) View of the
aquaporin homotetramer from the extracellular surface along its
Figure 20-16 Schematic drawing of the water-conducting pore
of bovine aquaporin AQP1. The pore is viewed from within the
membrane with the extracellular surface above. The positions of
residues critical for preventing the passage of protons, other ions,
and small molecule solutes are indicated. [Courtesy of Peter
Agre, Johns Hopkins School of Medicine.]
(a)
(b)
4-fold axis.The subunit in the upper right is drawn in space-
filling form with C green, N blue, and O red; that in the upper left
is drawn in ribbon form colored in rainbow order from its
N-terminus (blue) to its C-terminus (red), that in the lower left is
represented by its solvent-accessible surface; and that in the
lower right displays the side chains forming the ar/R constriction
(those of Phe 58, His 182, Cys 191, and Arg 197) in stick form.
Each subunit forms a water-transport channel, which is most
clearly visible in the subunit drawn in space-filling form. [Based
on an X-ray structure by Bing Jap, University of California at
Berkeley. PDBid 1J4N.]
JWCL281_c20_744-788.qxd 6/4/10 12:15 PM Page 757
3 ATP-DRIVEN ACTIVE TRANSPORT
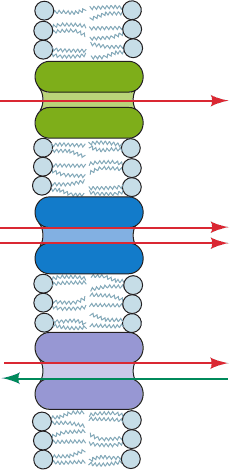
Mediated transport is categorized according to the stoi-
chiometry of the transport process (Fig. 20-17):
1. A uniport involves the movement of a single mole-
cule at a time. Maltoporin and GLUT1 are uniports.
2. A symport simultaneously transports two different
molecules in the same direction.
3. An antiport simultaneously transports two different
molecules in opposite directions.
The electrical character of ion transport is further spec-
ified as:
1. Electroneutral (electrically silent) if there is simulta-
neous charge neutralization, either by symport of oppo-
sitely charged ions or antiport of similarly charged ions.
Aquaporin is electroneutral.
2. Electrogenic if the transport process results in a
charge separation across the membrane. KcsA and ClC are
electrogenic.
Since the glucose concentration in blood plasma is gen-
erally higher than that in cells, GLUT1 normally transports
glucose into the erythrocyte, where it is metabolized via
glycolysis. Many substances, however, are available on one
side of a membrane in lower concentrations than are re-
quired on the other side of the membrane. Such substances
must be actively and selectively transported across the
membrane against their concentration gradients.
Active transport is an endergonic process that is often
coupled to the hydrolysis of ATP. How is this coupling ac-
complished? In endergonic biosynthetic reactions, it often
occurs through the direct phosphorylation of a substrate by
ATP; for example, the formation of UTP in the synthesis of
glycogen (Section 18-2B). Membrane transport, however,
is usually a physical rather than a chemical process; the
transported molecule is not chemically altered. Determin-
ing the mechanism by which the free energy of ATP hy-
drolysis is coupled to endergonic physical processes has
therefore been a challenging problem.
Three types of ATP hydrolyzing, transmembrane pro-
teins have been identified that actively transport cations:
1. P-type ATPases are located mostly in plasma mem-
branes and are so named because they are phosphorylated
by ATP during the transport process. P-type ATPases are
known that transport H
⫹
,Na
⫹
,K
⫹
,Ca
2⫹
,Cu
2⫹
,Cd
2⫹
, and
Mg
2⫹
against their concentration gradients. They are distin-
guished from the other types of cation-translocating ATPases
by their inhibition by vanadate ( , a phosphate analog;
see Problem 8 in this chapter).
2. F-type ATPases (F
1
F
0
) function to translocate pro-
tons into mitochondria and bacterial cells, which in turn
powers ATP synthesis.They are discussed in Section 22-3C.
3. V-type ATPases are located in plant vacuolar mem-
branes and acidic vesicles, such as animal lysosomes, and
are homologous to the F-type ATPases.
Anions are transported by a fourth type of ATPase, the so-
called A-type ATPases. In this section, we discuss P-type
ATPases. We also examine a bacterial active transport
process, in which the molecules transported are concomi-
tantly phosphorylated, and the ABC transporters, which
transport a wide variety of substances across membranes.
In the next section, we study secondary active transport
systems, so called because they utilize the free energy of
electrochemical gradients generated by ion-pumping
ATPases to transport ions and neutral molecules against
their concentration gradients.
A. (Na
⫹
–K
⫹
)–ATPase of Plasma Membranes
One of the most thoroughly studied active transport sys-
tems is the (Na
⫹
–K
⫹
)–ATPase of plasma membranes. This
transmembrane protein, which was first isolated in 1957 by
Jens Skou, is often called the (Na
⫹
–K
⫹
) pump because it
pumps Na
⫹
out of and K
⫹
into the cell with the concomitant
hydrolysis of intracellular ATP. Unlike most P-type ATPases,
which are monomeric,(Na
⫹
–K
⫹
)–ATPases consist of ␣ and
 subunits. The ⬃1000-residue, nonglycosylated ␣ subunit
contains the enzyme’s ATP and ion binding sites. It is
highly conserved (98% identical among mammals) and
homologous to single-subunit P-type ATPases such as
the Ca
2⫹
-ATPase (Section 20-3B). The ⬃300-residue, gly-
cosylated  subunit facilitates the correct insertion of the
␣ subunit into the plasma membrane and has been impli-
cated in K
⫹
transport.
The overall stoichiometry of the (Na
⫹
–K
⫹
)–ATPase re-
action is
3Na
⫹
(out) ⫹ 2K
⫹
(in) ⫹ ADP ⫹ P
i
3Na
⫹
(in) ⫹ 2K
⫹
(out) ⫹ ATP ⫹ H
2
O Δ
VO
3⫺
4
758 Chapter 20. Transport Through Membranes
Figure 20-17 Uniport, symport, and antiport translocation
systems.
A (in)
B (in)
Symport
A (out)
B (out)
A (out)
B (out)
A (in)
B (in)
Antiport
A (in)
Uniport
A (out)
JWCL281_c20_744-788.qxd 3/17/10 1:48 PM Page 758
The (Na
⫹
–K
⫹
)–ATPase is therefore an electrogenic an-
tiport: Three positive charges exit the cell for every two
that enter. This extrusion of Na
⫹
enables animal cells to
control their water content osmotically; without function-
ing (Na
⫹
–K
⫹
) pumps, animal cells, which lack cell walls,
would swell and burst (recall that lipid bilayers are perme-
able to H
2
O; Section 12-2Ba). Moreover, the electrochemi-
cal potential gradient generated by the (Na
⫹
–K
⫹
) pump is
responsible for the electrical excitability of nerve cells
(Section 20-5Ba) and provides the free energy for the ac-
tive transport of glucose and amino acids into some cells
(Section 20-4A). In fact, all cells expend a large fraction of
the ATP they produce (typically 30% and up to 70% in
nerve cells) to maintain their required cytosolic Na
⫹
and K
⫹
concentrations.
a. ATP Phosphorylates an Essential Asp during the
Transport Process
The free energy of ATP hydrolysis powers the ender-
gonic transport of Na
⫹
and K
⫹
against an electrochemical
gradient. In coupling these two processes, a kinetic barrier
must somehow be erected against the “downhill” transport
of Na
⫹
and K
⫹
along their ion concentration gradients,
while simultaneously facilitating their “uphill” transport. In
addition, futile ATP hydrolysis must be prevented in the
absence of uphill transport. How the enzyme does so is by
no means well understood, although many of its mechanis-
tic aspects have been elucidated.
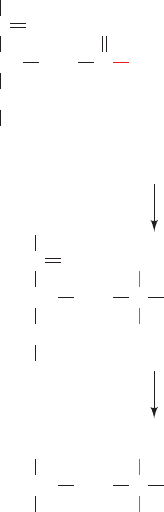
A key discovery was that the protein is phosphorylated
by ATP in the presence of Na
⫹
during the transport
process. The use of chemical trapping techniques demon-
strated that this phosphorylation occurs on an Asp residue
to form a highly reactive aspartyl phosphate intermediate.
For instance, sodium borohydride reduces acyl phosphates
to their corresponding alcohols. In the case of an aspartyl
phosphate residue, the alcohol is homoserine. By use of
[
3
H]NaBH
4
to reduce the phosphorylated enzyme, radioac-
tive homoserine was, in fact, isolated from the acid hy-
drolysate (Fig. 20-18). The phosphorylated residue, Asp
374, begins the highly conserved sequence DKTG that oc-
curs in the central region of the polypeptide chain.
b. The (Na
⫹
–K
⫹
)–ATPase Has Two Major
Conformational States
The observations that ATP phosphorylates the
(Na
⫹
–K
⫹
)–ATPase only in the presence of Na
⫹
, while the
aspartyl phosphate residue is only subject to hydrolysis in
the presence of K
⫹
, led to the realization that the enzyme
has two major conformational states, E1 and E2. These
states have different tertiary structures, different catalytic
activities, and different ligand specificities:
1. E1 has an inward-facing high-affinity Na
⫹
binding
site (K
M
⫽ 0.2 mM, well below the intracellular [Na
⫹
]) and
reacts with ATP to form the activated product E1
⬃
P only
when Na
⫹
is bound.
2. E2¬P has an outward-facing high-affinity K
⫹
bind-
ing site (K
M
⫽ 0.05M, well below the extracellular [K
⫹
])
and hydrolyzes to form P
i
⫹ E2 only when K
⫹
is bound.
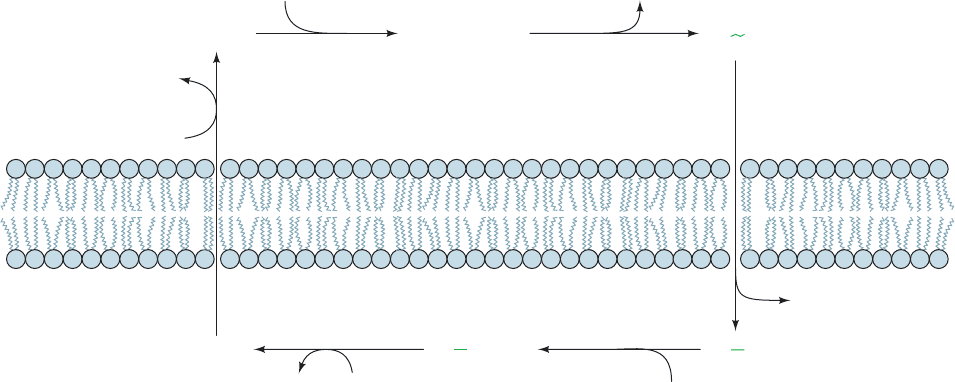
c. An Ordered Sequential Kinetic Reaction
Mechanism Accounts for the Coupling of Active
Transport with ATP Hydrolysis
The (Na
⫹
–K
⫹
)–ATPase is thought to operate in accor-
dance with the following ordered sequential reaction
scheme (Fig. 20-19):
1. E1 ⴢ ATP, which acquired its ATP inside the cell,
binds 3Na
⫹
to yield the ternary complex E1 ⴢ ATP ⴢ 3Na
⫹
.
2. The ternary complex reacts to form the “high-energy”
aspartyl phosphate intermediate E1
⬃
P ⴢ 3Na
⫹
.
3. This “high-energy” intermediate relaxes to its “low-
energy” conformation, E2¬P ⴢ 3Na
⫹
, and releases its
bound Na
⫹
outside the cell; that is, Na
⫹
is transported
through the membrane.
4. E2¬P binds 2K
⫹
from outside the cell to form
E2¬P ⴢ 2K
⫹
.
5. The phosphate group is hydrolyzed,yielding E2 ⴢ 2K
⫹
.
6. E2 ⴢ 2K
⫹
changes conformation to E1, binds ATP,
and releases its 2K
⫹
inside the cell, thereby completing the
transport cycle.
The enzyme appears to have only one set of cation binding
sites, which apparently changes both its orientation and its
specificity during the course of the transport cycle.
Section 20-3. ATP-Driven Active Transport 759
Figure 20-18 Reaction of [
3
H]NaBH
4
with phosphorylated
(Na
⫹
–K
⫹
)–ATPase. The isolation of [
3
H]homoserine following
acid hydrolysis of the protein indicates that the original phospho-
rylated amino acid residue is Asp.
A
spartyl phosphate
residue
Homoserine
CH CH
2
OPO
3
C
O
NaB
NH
CO
acid hydrolysis
–
+
3
H
4
CH CH
2
OHC P
i
NH
CO
+
3
H
3
H
CH CH
2
OHC
NH
3
COO
–
3
H
3
H
+
JWCL281_c20_744-788.qxd 6/4/10 1:20 PM Page 759
The obligatory order of the reaction requires that ATP
can be hydrolyzed only as Na
⫹
is transported “uphill.”
Conversely, Na
⫹
can be transported “downhill” only if ATP
is concomitantly synthesized. Consequently, although each
of the above reaction steps is, in fact, individually re-
versible, the cycle, as is diagrammed in Fig. 20-19, circulates
only in the clockwise direction under normal physiological
conditions; that is, ATP hydrolysis and ion transport are
coupled processes. Note that the vectorial (unidirectional)
nature of the reaction cycle results from the alternation of
the steps of the exergonic ATP hydrolysis reaction (Step 2,
Step 5, and ATP binding in Step 6) with the steps of the en-
dergonic ion transport process (Step 1, Steps 3 ⫹ 4, and K
⫹
release in Step 6).Thus, neither reaction can go to comple-
tion unless the other one also does.
d. Mutual Destabilization Accounts for the
Rate of Na
⫹
and K
⫹
Transport
The above ordered kinetic mechanism accounts only for
the coupling of active transport with ATP hydrolysis. In or-
der to maintain a reasonable rate of transport, the free ener-
gies of all its intermediates must be roughly equal. If some
intermediates were much more stable than others, the stable
intermediates would accumulate, thereby severely reducing
the overall transport rate. For example, in order for Na
⫹
to
be transported out of the cell, uphill, its binding must be
strong to E1 on the inside and weak to E2 on the outside.
Strong binding means greater stability and a potential bot-
tleneck.This difficulty is counteracted by the phosphoryla-
tion of E1 ⴢ 3Na
⫹
and its subsequent conformational
change to yield the low Na
⫹
affinity E2¬P (Steps 2 and 3,
Fig. 20-19). Likewise, the strong binding of K
⫹
to E2¬P on
the outside is attenuated by its dephosphorylation and con-
formational change to yield the low K
⫹
affinity E1 (Steps 5
and 6, Fig. 20-19). It is these mutual destabilizations that
permit Na
⫹
and K
⫹
to be transported at a rapid rate.
e. The X-Ray Structure of the (Na
⫹
–K
⫹
)–ATPase
Chikashi Toyoshima determined the X-ray structure of
shark (Na
⫹
–K
⫹
)–ATPase in complex with K
⫹
ions, an
MgF
4
2⫺
ion (a P
i
mimic), and a 74-residue subunit named
FXYD that functions as a tissue-specific regulator.This X-
ray structure (Fig.20-20) is that of the E2¬P ⴢ 2K
⫹
complex
(Fig. 20-19). The ␣ subunit of this ⬃160-Å-long protein
consists of a transmembrane domain (M) composed of 10
helices (␣M1–␣M10) of varied lengths and, from top to
bottom in Fig. 20-20, three well-separated cytoplasmic do-
mains: the nucleotide-binding domain (N), which binds
ATP; the actuator domain (A), so named because it partic-
ipates in the transmission of major conformational changes
(see below); and the phosphorylation domain (P), which
contains the protein’s phosphorylatable Asp residue. The
 subunit’s single transmembrane helix is tilted ⬃32° from
the normal to the plane of the membrane. The FXYD
subunit also has a single transmembrane helix but it is
nearly perpendicular to the plane of the membrane. The
MgF
4
2⫺
ion marks the ATPase’s catalytic site and is coordi-
nated by conserved residues from both its A and P do-
mains. Two K
⫹
ions are located ⬃4.1 Å apart in a common
binding cavity near the center of the ␣ subunit’s transmem-
brane domain that is formed, in large part, by the partial
unwinding of helices ␣M5 and ␣M7, and where they are
each liganded by several main chain carbonyl and side
chain oxygen atoms. The same cavity is implicated in bind-
ing the three Na
⫹
ions bound to the enzyme’s E1 form,with
two of these binding sites probably formed by the same
side chains that coordinate the K
⫹
ions and the third such
site formed, in part, by the side chains of the ␣ subunit’s
760 Chapter 20. Transport Through Membranes
Figure 20-19 Kinetic scheme for the active transport of Na
⫹
and K
⫹
by (Na
⫹
–K
⫹
)–ATPase.
Here (in) refers to the cytosol and (out) refers to the exterior of the cell.
Mg
2+
Mg
2+
Na
+
binding
H
2
O
1 Formation of
“high-energy” aspartyl
phosphate intermediate
2
Na
+
transport3
K
+
binding4
Phosphate
hydrolysis
5
K
+
transport and
ATP
binding
6
E1 • ATP E1 • ATP• 3Na
+
E1
P • 3Na
+
ATP
Inside
3Na
+
(in)
2K
+
(in)
Outside
3Na
+
(out)
ADP
P
i
2K
+
(out)
E
2
P • 2K
+
E2 • 2K
+
E2
P
JWCL281_c20_744-788.qxd 6/4/10 12:15 PM Page 760
