Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

permits a cognate aminoacyl–tRNA to enter the peptidyl
transferase center. The irreversible GTPase reaction must
precede this proofreading step because otherwise the dis-
sociation of a noncognate tRNA (the release of its anti-
codon from the codon) would simply be the reverse of the
initial binding step, that is, it would be part of the initial se-
lection step rather than proofreading. GTP hydrolysis
therefore provides the second context necessary for proof-
reading; it is the entropic price the system must pay for accu-
rate tRNA selection.
F. Chain Termination
Polypeptide synthesis under the direction of synthetic
mRNAs such as poly(U) terminates with a peptidyl–tRNA
in association with the ribosome. However, the translation
of natural mRNAs, which contain the Stop codons UAA,
UGA, or UAG, results in the release of free polypeptides.
Accurate termination is essential, not only because it pre-
vents the wasteful synthesis of nonfunctional polypeptides,
but also because prematurely terminated polypeptides
may be toxic.
a. Prokaryotic Termination
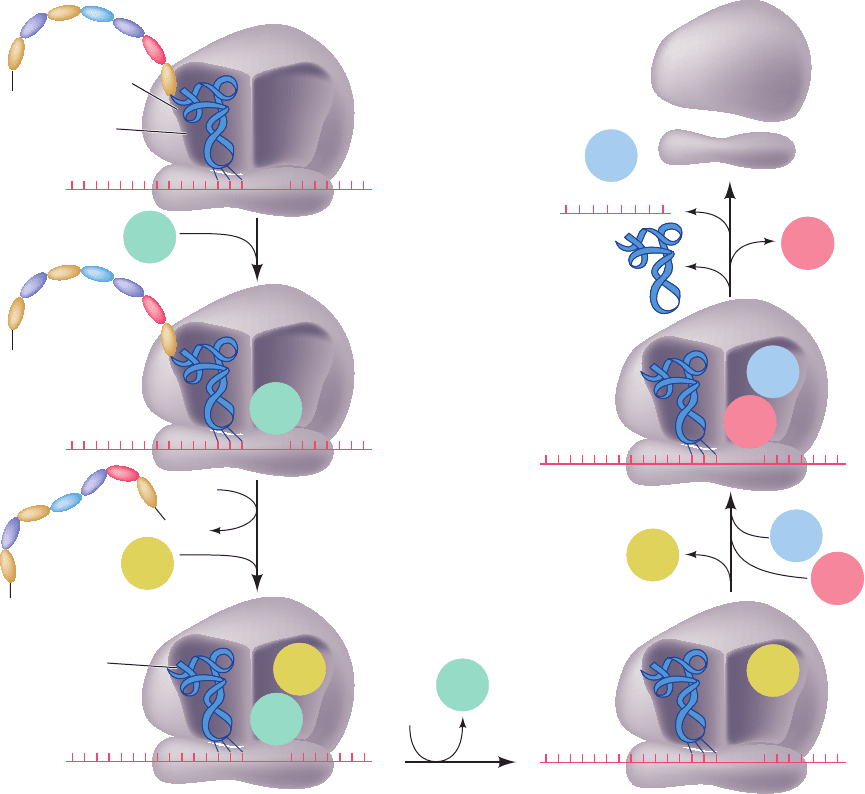
In E.coli, chain termination has several stages (Fig. 32-60):
1. The termination codons, the only codons that nor-
mally have no corresponding tRNAs, are recognized by
class I release factors (Table 32-9): RF-1 recognizes UAA
and UAG, whereas the 39% identical RF-2 recognizes
UAA and UGA. Swapping a conserved PXT tripeptide in
RF-1 with a conserved SPF tripeptide in RF-2 interchanges
their Stop codon specificities, which suggests that these
tripeptides mimic anticodons.
2. On binding to their corresponding Stop codon, RF-1
and RF-2 induce the transfer of the peptidyl group from
Section 32-3. Ribosomes and Polypeptide Synthesis 1391
P site
NH
3
+
3′
NH
3
+
NH
3
+
A site
Empty
mRNA
Nascent polypeptide
5′
UAA
3′5′
UAA
RF-1
RF-3
RF-1
3′5′
UAA
RF-1
GDP
GTP
RF-3
RF-1
GDP
Peptidyl–tRNA
COO
–
H
2
O
Uncharged
tRNA
1
2
3
Polypeptide
GDP
+
+
P
i
GTP
EF-G
4
RRF
5
3′5′
UAA
3′5′
UAA
GTP
RF-3
GDP
RF-3
+
+
GTP
EF-G
RRF
RRF
3′
5′
GDP
EF-G
50S subunit
30S subunit
Figure 32-60 Termination pathway in E. coli ribosomes. RF-1
recognizes the Stop codons UAA and UAG, whereas RF-2 (not
shown) recognizes UAA and UGA. Eukaryotic termination
follows an analogous pathway but requires only a single class I
release factor, eRF1, that recognizes all three Stop codons.
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1391
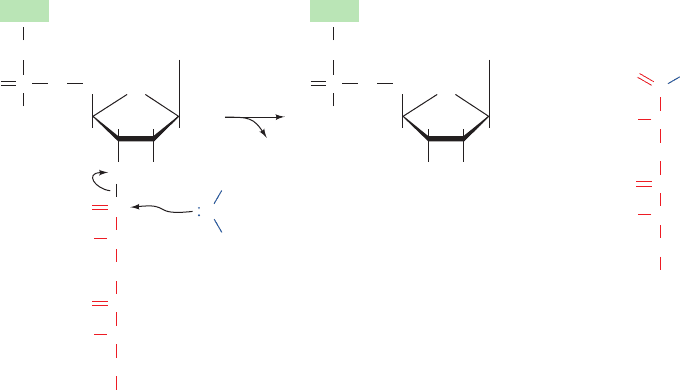
tRNA to water rather than to an aminoacyl–tRNA, thereby
releasing the completed polypeptide (Fig 32-61). This occurs
with an error rate of 10
5
without proofreading.The class I
release factors act at the ribosomal A site as is indicated by
the observations that they compete with suppressor tRNAs
for termination codons and that they cannot bind to the ri-
bosome simultaneously with EF-G.A GGQ tripeptide that
is universally conserved in all class I release factors is im-
plicated in catalyzing the hydrolysis of the peptidyl–tRNA
ester linkage (see below).
3. Once the newly synthesized polypeptide has been re-
leased from the ribosome, the class II release factor RF-3,
in its complex with GDP, binds to the ribosome at the same
site as do EF-Tu and EF-G. In fact, the X-ray structure of
RF-3 ⋅ GDP resembles that of EF-Tu ⋅ GMPPNP (Fig. 32-
47). Free RF-3 has a greater affinity for GDP than GTP but
on binding to the ribosome–RF-1/2 complex, it exchanges
its bound GDP for GTP. The resulting change in the con-
formation of RF-3, as seen in cryo-EM studies, causes it to
bind more tightly to the ribosome and expel the RF-1/2.
RF-3 is not required for cell viability although it is neces-
sary for maximum growth rate; RF-3 only accelerates the
dissociation of RF-1/2 from the ribosome by ⬃5-fold.
4. The interaction of RF-3 ⋅ GTP with the ribosome
stimulates it to hydrolyze its bound GTP, much as occurs
with EF-Tu ⋅ GTP and EF-Tu ⋅ GTP. The resulting RF-3 ⋅
GDP then dissociates from the ribosome. Subsequently, ri-
bosomal recycling factor (RRF) binds in the ribosomal A
site followed by EF-G GTP. RRF, which was discovered
by Akira Kaji, is essential for cell viability.
5. EF-G hydrolyzes its bound GTP, which causes RRF
to be translocated to the P site and the tRNAs previously
in the P and E sites (the latter not shown in Fig.32-60) to be
released. Finally, the small and large ribosomal subunits
separate, a process that is facilitated by the binding of IF-3
(Section 32-3Cc), and RRF, EF-G GDP, and mRNA are
released. The ribosomal subunits can then participate in a
new round of initiation (Fig. 32-43).
b. Eukaryotic Termination
Chain termination in eukaryotes resembles that in
prokaryotes, but it has only one class I release factor, eRF1,
that recognizes all three Stop codons. It is unrelated in se-
quence to RF-1 and RF-2. However, the eukaryotic class II
release factor, eRF3, resembles RF-3 in both sequence and
function. Nevertheless, eRF3 is essential for eukaryotic cell
viability.
c. The Ribosome Binds RF-1 and RF-2 in a
Conformation That Catalyzes Peptide Release
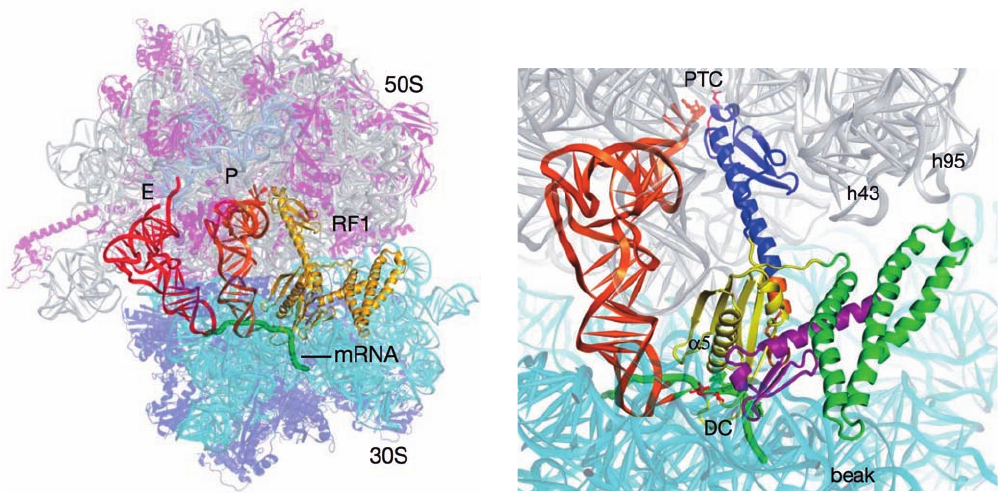
The X-ray structures of the T. thermophilus ribosome
with RF-1, an mRNA containing a UAA Stop codon, and
deacylated tRNAs in its P and E sites was determined by
Noller (Fig. 32-62a), and the closely similar structures con-
taining the tRNAs, RF-2, and an mRNA with a UAA or a
UGA Stop codon, were respectively determined by Noller
and Ramakrishnan. These are all product complexes since
they lack peptidyl groups on their P-site tRNAs. The struc-
turally similar RF-1 (Fig. 32-62b) and RF-2 each consist of
four domains with domains 2 and 4 occupying the ribo-
some’s decoding center (DC), where they contact the
mRNA’s Stop codon, and with domain 3 occupying the
peptidyl transferase center (PTC), where it interacts with
the ribose residue of the P-site tRNA’s A76 to which a pep-
tidyl group would be linked in a substrate complex. The
deletion of domain 1 does not affect peptide release activ-
ity but is required for the RF-3–facilitated dissociation of
RF-1/2 from the ribosome (see below).
1392 Chapter 32. Translation
Figure 32-61 Ribosome-catalyzed hydrolysis of peptidyl–tRNA to form a polypeptide and free tRNA.
NH
H
2
C
HH
HH
O OH
O
O
OPO
O
O
–
O
OC
C
Adenine
H
H
CHR
n
NH
CHR
n–1
NH
OC
C
O
–
O
CHR
n
NH
CHR
n–1
Peptidyl–tRNA
tRNA
H
2
C
HH
HH
HO OH
O
OPO
O
O
–
Adenine
tRNA
tRNA
+
...
...
Polypeptide
H
+
JWCL281_c32_1338-1428.qxd 9/7/10 2:23 PM Page 1392
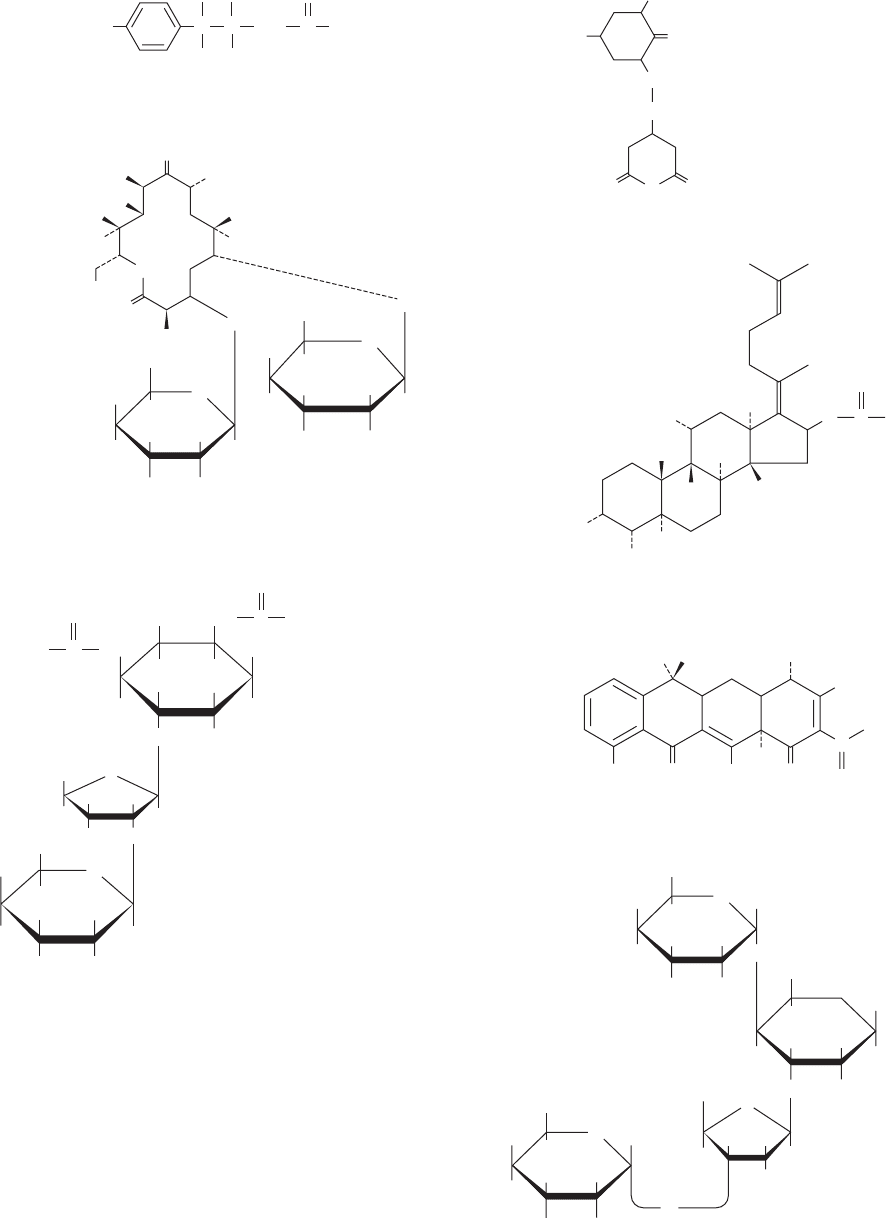
The binding of RF-1 or RF-2 in the DC causes A530 and
A1492 of the 16S RNA to flip out from their resting state
(Fig. 32-59a) as occurs with the binding of a tRNA to its
cognate codon (Fig. 32-59b). However,A1493 does not flip
out because in doing so it would clash with domain 2 of ei-
ther release factor. Instead, it stacks on A1913 of the 23S
RNA. The Stop codons are recognized by hydrogen bond-
ing and van der Waals interactions with the similarly lo-
cated PXT and SPF tripeptides on domain 2 of RF-1 and
RF-2. However, the observation that mutations in RF-2
distant from its SQF motif result in altered specificity sug-
gests that Stop codon recognition arises from a subtle bal-
ance of binding energy and conformational changes as we
have seen to be the case for codon recognition by tRNA
(Section 32-3Ea).
In the PTC, the GGQ tripeptide on domain 3 of both
RF-1 and RF-2 contacts the 3¿-terminal ribose residue
(A76) of the deacylated P-site tRNA. Both Gly residues
adopt backbone conformations that are forbidden for
other amino acid residues, which accounts for the observa-
tions that the mutation of either residue results in an up to
10
4
-fold reduction in the rate of peptide release. The main
chain NH group of the Gln residue is hydrogen bonded to
the 3¿-OH group of A76, which, it is hypothesized, positions
it to also hydrogen bond to and thereby stabilize the tran-
siton state oxyanion in the hydrolysis reaction. In agree-
ment with this hypothesis, the mutation of the Gln residue
to Pro, which lacks a main chain NH group, abolishes the
hydrolysis reaction. The side chain of the Gln residue is
pointed away from the ribose (top of Fig. 32-62b) where, it
is proposed, it helps position a water molecule for an in-
line nucleophilic attack on the scissile ester bond. In addi-
tion, the observation that peptide release is nearly abol-
ished by the removal of the 2¿-OH from the P-site tRNA’s
3¿ terminal residue suggests that peptide release utilizes a
substrate-assisted proton shuttle mechanism similar to that
of peptide bond formation (Section 32-3Di). Finally, the
binding of RF-1/2 shifts U2585 so as to expose the other-
wise protected scissile ester bond to nucleophilic attack
(Section 32-3Di). Nevertheless, the formulation of a defin-
itive mechanism for peptide release must await the X-ray
structure of a ribosome in complex with both a release fac-
tor and a peptidyl–tRNA, that is, a substrate complex.
How does the binding of a release factor to a Stop
codon in the DC induce the ⬃75-Å distant PTC to hy-
drolyze the scissile ester bond? In the X-ray structures of
RF-1 or RF-2 alone, their PXT/SPF and GGQ motifs are
only ⬃23 Å apart due to a change in conformation of their
switch–loop segments (Fig. 32-62b) relative to that in the
ribosomal complexes. The conformation of the
Section 32-3. Ribosomes and Polypeptide Synthesis 1393
Figure 32-62 X-ray structure of the T. thermophilus ribosome
in complex with RF-1, a UAA Stop codon–containing mRNA,
and deacylated tRNAs in its P and E sites. (a) The overall
structure with proteins shown in ribbon form and all RNAs
shown in ladder form but the mRNA, which is drawn in worm
form in green.The ribosome is semitransparent with its 23S RNA
gray, its 5S RNA light blue, its 16S RNA cyan, the 50S subunit’s
proteins magenta, and the 30S subunit’s proteins purple. The
tRNAs occupying the P and E sites are orange and red, and the
RF-1, which in part occupies the ribosomal A site, is yellow. (b)
Close-up of the interactions between the P-site tRNA, mRNA,
(b)
and RF-1.The ribosome, tRNA, and mRNA are drawn as in Part
a and the RF-1 is colored with its domains 1, 2, 3, and 4 green,
yellow, blue, and magenta, respectively.The so-called
switch–loop, which connects domains 3 and 4 and undergoes a
major conformational rearrangement between the free and
ribosome-bound RF-1, is orange.The PVT tripeptide implicated
in Stop codon recognition and the GGQ tripeptide implicated in
catalyzing the ester hydrolysis reaction are drawn in stick form in
red. [Courtesy of Harry Noller, University of California at Santa
Cruz. PDBids 3D5A and 3D5B.]
(a)
JWCL281_c32_1338-1428.qxd 9/7/10 2:23 PM Page 1393

switch–loop observed in the ribosomal complexes is only
possible when a Stop codon is recognized. This is because
the flipping out of A1493, which only occurs when a tRNA
binds its cognate codon in the DC (Fig. 32-59), or the fail-
ure of A1913 to stack on A1493, alters the binding pocket
for the ribosomally bound switch–loop. Noller has there-
fore proposed that the binding of a Stop codon by its cor-
responding release factor and the consequent rearrange-
ment of both its switch–loop and the DC cooperatively
permit the GGQ motif to bind to the PTC in a way that
catalyzes peptide release.
d. RRF Binds in the Ribosomal A Site
The X-ray structure of T. thermophilus ribosomal recy-
cling factor (RRF), determined by Yoshikazu Nakamura,
reveals it to be a two-domain structure that resembles
tRNA in its overall shape (Fig. 32-63). The comparison of
this structure with those of several other bacterial RRFs in-
dicates that the two linkers connecting the RRF domains
are flexible such that domain II can rotate about the axis of
the three-helix bundle forming domain I.
The X-ray structure of the T. thermophilus ribosome in
complex with RRF in its A site, the anticodon stem–loop
(ASL) of tRNA
Phe
in its P site, tRNA
f
Met
in its E site, and an
mRNA with a UAG Stop codon in the A site was deter-
mined by Ramakrishnan (Fig. 32-64). Domain I of the RRF
spans the A and P sites of the 50S ribosome, a position in
which the tip of its domain I would clash with a tRNA in
the P site (and which rationalizes why a ribosomal complex
of RRF and a tRNA in the P site has not been crystallized).
This suggests that RRF binding forces a tRNA bound in
the P site into the P/E hybrid binding state (Section 32-
3Dl). Previous structural studies suggested that RRF bind-
ing induced changes in the bridges connecting the small
and large subunits (Section 32-3Ae). However, no such
changes are observed in the above structure. Perhaps they
occur in the P/E hybrid state.
e. GTP Hydrolysis Speeds Up Ribosomal Processes
What is the role of the GTP hydrolysis reactions medi-
ated by the various ribosomally associated G proteins (IF-
2, EF-Tu, EF-G, and RF-3 in bacteria)? Translation occurs
in the absence of GTP, albeit slowly, so that the free energy
of the peptidyl transferase reaction is sufficient to drive the
entire translational process. Moreover, none of the GTP
1394 Chapter 32. Translation
Figure 32-63 X-ray structure of T. thermophilus RRF. This
monomeric protein is drawn in ribbon form colored in rainbow
order from its N-terminus (blue) to its C-terminus (red). [Based
on an X-ray structure by Yoshikazu Nakamura,The University of
Tokyo, Japan. PDBid 1EH1.]
Figure 32-64 X-ray structure of the T. thermophilus ribosome
in complex with RRF in its A site, the anticodon stem–loop
(ASL) of tRNA
Phe
in its P site, tRNA
f
Met
in its E site, and an
mRNA with a UAG Stop codon in the A site. The ribosomal
RNAs are shown as semitransparent surface diagrams with 23S
RNA light blue, 5S RNA blue-green, and 16S RNA yellow.The
ribosomal proteins are drawn in ribbon form with 50S subunit
proteins blue and 30S subunit proteins tan.The RRF, tRNAs, and
mRNA are represented by their surface diagrams with domains I
and II of RRF red and blue, the mRNA magenta, the P-site ASL
purple, and the E-site tRNA
f
Met
gray. [Courtesy of Venki
Ramakrishnan, MRC Laboratory of Molecular Biology, Cam-
bridge, U.K. PDBids 2V46 and 2V47.]
JWCL281_c32_1338-1428.qxd 8/19/10 10:06 PM Page 1394
hydrolysis reactions yields a “high-energy” covalent inter-
mediate as does, say, ATP hydrolysis in numerous biosyn-
thetic reactions. Instead, the ribosomal binding of a
G-protein induces it to hydrolyze its bound GTP to GDP
resulting a conformational change that causes the ribo-
some to carry out a particular process (
binding for IF-2, accommodation for EF-Tu, translocation
for EF-G, and RF-1/2 release for RF-3) and release the re-
sulting G-protein ⋅ GDP complex. The high rate and irre-
versibility of the GTP hydrolysis reaction ensures that the
various complex ribosomal processes to which it is coupled,
initiation, elongation, and termination, will themselves be
fast and irreversible. In essence, G-protein GTP com-
plexes act as Maxwell’s demons to trap the ribosome in
functionally productive conformations. Hence, as we saw to
be the case for ribosomal proofreading (Section 32-3Eb),
the ribosome utilizes the free energy of GTP hydrolysis to
gain a more ordered (lower entropy) state rather than a
higher energy state as often occurs in ATP-dependent
processes.
G. Protein Synthesis Inhibitors: Antibiotics
Antibiotics are bacterially, fungally, or synthetically pro-
duced substances that inhibit the growth of microorganisms.
Antibiotics are known to inhibit a variety of essential
biological processes, including DNA replication (e.g.,
ciprofloxacin; Section 29-3Cd), transcription (e.g., rif-
amycin B; Section 31-2Bb), and bacterial cell wall synthesis
(e.g., penicillin; Section 11-3Bb). However, the majority of
known antibiotics, including a great variety of medically
useful substances, block translation. This situation is pre-
sumably a consequence of the translational machinery’s
enormous complexity, which makes it vulnerable to disrup-
tion in many ways. Antibiotics have also been useful in an-
fMet–tRNA
Met
f
alyzing ribosomal mechanisms because, as we have seen for
puromycin (Section 32-3Df), the blockade of a specific
function often permits its biochemical dissection into its
component steps.Table 32-10 and Fig.32-65 present several
medically significant and/or biochemically useful transla-
tional inhibitors. We study the mechanisms of a few of the
best characterized of them below.
a. Streptomycin
Streptomycin, which was discovered in 1944 by Selman
Waksman, is a medically important member of a family of
antibiotics known as aminoglycosides that inhibit prokary-
otic ribosomes in a variety of ways. At low concentrations,
streptomycin induces the ribosome to characteristically
misread mRNA: One pyrimidine may be mistaken for the
other in the first and second codon positions and either
pyrimidine may be mistaken for adenine in the first posi-
tion. This inhibits the growth of susceptible cells but does
not kill them. At higher concentrations, however, strepto-
mycin prevents proper chain initiation and thereby causes
cell death.
Certain streptomycin-resistant mutants (str
R
) have ri-
bosomes with an altered protein S12 compared with
streptomycin-sensitive bacteria (str
S
). Intriguingly, a
change in base C912 of 16S rRNA (which lies in its central
domain; Fig. 32-27a) also confers streptomycin resistance.
(Some mutant bacteria are not only resistant to strepto-
mycin but dependent on it; they require it for growth.) In
partial diploid bacteria that are heterozygous for strepto-
mycin resistance (str
R
/str
S
), streptomycin sensitivity is
dominant. This puzzling observation is explained by the
finding that, in the presence of streptomycin, str
S
ribo-
somes remain bound to initiation sites, thereby excluding
str
R
ribosomes from these sites. Moreover, the mRNAs
in these blocked complexes are degraded after a few
Section 32-3. Ribosomes and Polypeptide Synthesis 1395
Table 32-10 Some Ribosomal Inhibitors
Inhibitor Action
Chloramphenicol Inhibits peptidyl transferase on the prokaryotic large subunit
Cycloheximide Inhibits peptidyl transferase on the eukaryotic large subunit
Erythromycin Inhibits translocation by the prokaryotic large subunit
Fusidic acid Inhibits elongation in prokaryotes by binding to EF-G GDP in a way that prevents its dissociation
from the large subunit
Paromomycin Increases the ribosomal error rate
Puromycin An aminoacyl–tRNA analog that causes premature chain termination in prokaryotes and eukaryotes
Streptomycin Causes mRNA misreading and inhibits chain initiation in prokaryotes
Tetracycline Inhibits the binding of aminoacyl–tRNAs to the prokaryotic small subunit
Diphtheria toxin Catalytically inactivates eEF2 by ADP-ribosylation
Ricin/abrin/-sarcin Ricin and abrin are poisonous plant glycosidases that catalytically inactivate the eukaryotic large subunit
by hydrolytically depurinating a specific highly conserved A residue of the 28S RNA, which is located on
the so-called sarcin–ricin loop that forms a critical part of the ribosomal factor–binding center; -sarcin
is a fungal protein that cleaves a specific phosphodiester bond in the sarcin–ricin loop
JWCL281_c32_1338-1428.qxd 8/19/10 10:06 PM Page 1395
1396 Chapter 32. Translation
O
2
N
OH
C
C C
O
H
H
CH
2
OH
NH CHCl
2
Chloramphenicol
H
3
C
CH
3
CH
2
O
O
O
CHOH
N
H
Cycloheximide
O
O
O
O
O
H
3
C
H
3
C
HO
HO
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
OH
N(CH
3
)
2
H
H
H
H
HOH
H
O
OCH
3
H
HO
H
H
H
H
O
CH
3
Erythromycin
H
3
C
CH
3
CH
3
CH
3
COOH
CH
3
CH
3
OCCH
3
H
H
H
HO
HO
Fusidic acid
OH OH
OH
OH
OO
O
C
OH
NH
2
H
3
C
N(CH
3
)
2
Tetracycline
O
O
H
HH
H
HOH
2
C
OH
OH H
HO
H
H
HNH
2
NH
2
NH
2
H
O
O
H
HO
H
H
O
H
H
CH
2
OH
OH H
HO
H
CH
2
NH
2
HNH
2
H
O
O
H
Paromomycin
H
H
CH
2
OH
H
3
CNH
H
HO
O
H
H
OH
H
H
H
OH
HHO
HN HH
2
N
OH
H
O
Streptomycin
H
O
CHO H
H
3
C
H
OH O
NH C
C
NH
+
2
NH
+
2
NH
2
Figure 32-65 Selection of antibiotics that act as translational inhibitors.
JWCL281_c32_1338-1428.qxd 8/19/10 10:06 PM Page 1396
minutes, which allows the str
S
ribosomes to bind to newly
synthesized mRNAs as well.
b. Chloramphenicol
Chloramphenicol, the first of the “broad-spectrum” an-
tibiotics, inhibits the peptidyl transferase activity on the
large subunit of prokaryotic ribosomes. However, its clini-
cal uses are limited to only severe infections because of its
toxic side effects, which are caused, at least in part, by the
chloramphenicol sensitivity of mitochondrial ribosomes.
The 23S RNA is implicated in chloramphenicol binding by
the observation that some of its mutants are chloram-
phenicol resistant. Indeed, X-ray studies indicate that
chloramphenicol binds in the large subunit’s polypeptide
exit tunnel in the vicinity of the A site. This explains why
chloramphenicol competes for binding with the 3¿ end of
aminoacyl–tRNAs as well as with puromycin (whose ribo-
somal binding site overlaps that of chloramphenicol) but
not with peptidyl–tRNAs. These observations suggest that
chloramphenicol inhibits peptidyl transfer by interfering
with the interactions of ribosomes with A site–bound
aminoacyl–tRNAs.
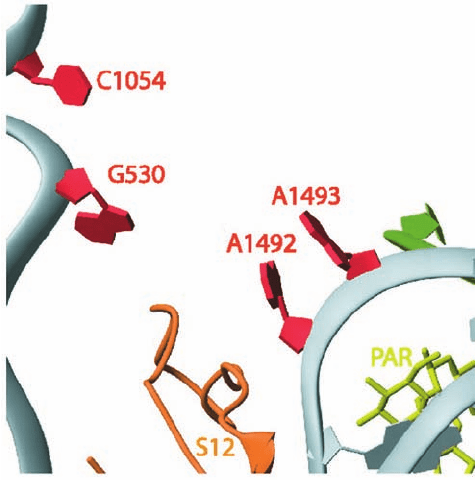
c. Paromomycin
Paromomycin, a clinically useful aminoglycoside antibi-
otic, increases the ribosomal error rate. The X-ray structure
of the 30S subunit in complex with paromomycin (Fig. 32-66)
reveals that it binds to the interior of the RNA loop in
which the bases of A1492 and A1493 are normally stacked
(Fig. 32-59a).This causes these bases to flip out of the loop
and assume a conformation resembling that in the
codon–anticodon–30S subunit complex (Fig. 32-59b). In-
deed, this codon–anticodon–30S subunit complex is not
significantly disturbed by the binding of paromomycin. As
we have seen in Section 32-3Ea, the 30S subunit employs
A1492 and A1493 to ascertain whether the first two
codon–anticodon base pairs are Watson–Crick base pairs,
that is, whether the incoming tRNA is cognate to the codon
in the A site. Noncognate tRNAs normally have insuffi-
cient codon–anticodon binding energy to flip A1492 and
A1493 out of the loop and consequently are rejected by the
ribosome. However, the binding of paromomycin to the
30S subunit pays the energetic price of these base flips.This
facilitates the ribosomal acceptance (stabilizes the binding)
of near-cognate aminoacyl–tRNAs and hence the erro-
neous incorporation of their amino acid residues into the
polypeptide being synthesized.
d. Tetracycline
Tetracycline and its derivatives are broad-spectrum
antibiotics that bind to the small subunit of prokaryotic ri-
bosomes, where they inhibit aminoacyl–tRNA binding. An
X-ray structure of tetracycline in complex with the 30S
subunit reveals that tetracycline mainly binds in a crevice
comprised of only the 3¿ major domain of 16S RNA (Fig.
32-27a) and which is located in the neck of the 30S subunit
just above its A site.This permits the initial screening of the
aminoacyl–tRNA to proceed but physically blocks its ac-
commodation into the peptidyl transferase (A/A) site after
EF-Tu–catalyzed GTP hydrolysis has occurred, resulting in
the release of the tRNA. Hence, in addition to preventing
protein synthesis, tetracycline binding causes the unpro-
ductive hydrolysis of GTP which, since this occurs every
time a cognate aminoacyl–tRNA binds to the ribosome,
poses an enormous energetic drain on the cell. The nu-
cleotides forming the tetracycline binding site are poorly
conserved in eukaryotic ribosomes, thereby accounting for
tetracycline’s bacterial specificity.
Tetracycline also blocks the stringent response (Section
31-3I) by inhibiting (p)ppGpp synthesis. This indicates that
deacylated tRNA must bind to the A site in order to acti-
vate stringent factor.
Tetracycline-resistant bacterial strains have become
quite common, thereby precipitating a serious clinical
problem. Resistance is often conferred by a decrease in
bacterial cell membrane permeability to tetracycline rather
than any alteration of ribosomal components.
e. Diphtheria Toxin
Diphtheria is a disease resulting from bacterial infection
by Corynebacterium diphtheriae that harbor the bacterio-
phage corynephage . Diphtheria was a leading cause of
childhood death until the late 1920s when immunization be-
came prevalent. Although the bacterial infection is usually
confined to the upper respiratory tract, the bacteria secrete
a phage-encoded protein, known as diphtheria toxin (DT),
which is responsible for the disease’s lethal effects. Diphthe-
ria toxin specifically inactivates the eukaryotic elongation
factor eEF2, thereby inhibiting eukaryotic protein synthesis.
Section 32-3. Ribosomes and Polypeptide Synthesis 1397
Figure 32-66 X-ray structure of the 30S ribosome in complex
with the antibiotic paromomycin. The view and coloring are the
same as those in Fig. 32-59 with the paromomycin (PAR) drawn
in stick form in yellow-green. [Courtesy of Venki Ramakrishnan,
MRC Laboratory of Molecular Biology, Cambridge, U.K. PDBid
1IBK.]
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1397
The pathogenic effects of diphtheria are prevented, as
was discovered in the 1880s, by immunization with toxoid
(formaldehyde-inactivated toxin). Individuals who have
contracted diphtheria are treated with antitoxin from
horse serum, which binds to and thereby inactivates DT, as
well as with antibiotics to combat the bacterial infection.
DT is a member of the family of bacterial toxins that in-
cludes cholera toxin (CT) and pertussis toxin (PT; Section
19-2Ce). It is a monomeric 535-residue protein that is readily
cleaved past its Arg residues 190, 192, and 193 by trypsin
and trypsinlike enzymes. This hydrolysis occurs around the
time diphtheria toxin encounters its target cell, yielding
two fragments, A and B, which, nevertheless, remain linked
by a disulfide bond. The B fragment’s C-terminal domain
binds to a specific receptor on the plasma membrane of
susceptible cells, thereby inducing DT’s uptake into the en-
dosome (Fig. 12-91) via receptor-mediated endocytosis
(Section 12-5Bc; free fragment A is devoid of toxic activ-
ity).The endosome’s low pH of 5 triggers a conformational
change in the B fragment’s N-terminal domain, which then
inserts into the endosomal membrane so as to facilitate the
entry of the A fragment into the cytoplasm. The disulfide
bond linking the A and B subunits is then cleaved by the
cytoplasm’s reducing environment.
Within the cytosol, the A fragment catalyzes the ADP-
ribosylation of eEF2 by NAD
,
thereby inactivating this elongation factor. Since the A
fragment acts catalytically, one molecule is sufficient to
ADP-ribosylate all of a cell’s eEF2s, which halts protein
synthesis and kills the cell. Only a few micrograms of diph-
theria toxin are therefore sufficient to kill an unimmu-
nized individual.
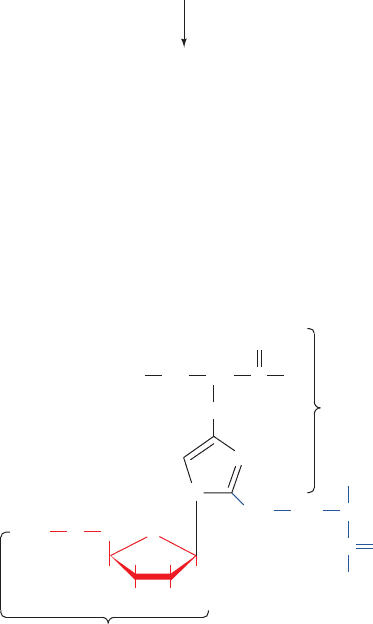
Diphtheria toxin specifically ADP-ribosylates a modi-
fied His residue on eEF2 known as diphthamide:
CH
2
CH
2
CH
2
NH CH
O
HH
H
H
N
N
OH OH
ADP-Ribosylated diphthamide
OADP
ADP-ribosyl group
His residue
CH
2
NH
2
CH
CO
C
O
N(CH
3
)
3
+
diphtheria toxin
(inactive)
(active)
+ NAD
+
eEF2
A
DP-ribosyl-eEF2 + Nicotinamide + H
+
Diphthamide occurs only in eEF2 (not even in its bacter-
ial counterpart, EF-G), which accounts for the specificity
of diphtheria toxin in exclusively modifying eEF2 (recall
that CT ADP-ribosylates a specific Arg residue on G
s
and PT ADP-ribosylates a specific Cys residue on G
ia
;
Section 19-2C). Since diphthamide occurs in all eukary-
otic eEF2s, it probably is essential to eEF2 activity. Yet,
certain mutant cultured animal cells, which have unim-
paired capacity to synthesize proteins, lack the enzymes
that post-translationally modify His to diphthamide
(although mutating the diphthamide His to Asp, Lys, or
Arg inactivates translation). Perhaps the diphthamide
residue has a control function.
4 CONTROL OF EUKARYOTIC
TRANSLATION
The rates of ribosomal initiation on prokaryotic mRNAs
differ by factors of up to 100, a variation that is largely a
consequence of their different Shine–Dalgarno se-
quences. Moreover, the genes forming an operon are of-
ten expressed in decreasing molar amounts from the
operon’s 5¿ end to its 3¿ end. For example, the proteins
specified by the E. coli lac operon (Section 31-1Ab), -
galactosidase, galactose permease, and thiogalactoside
transacetylase, are produced in molar ratios of 10:5:2.
Such polarity may arise when the initiation codon of a
gene that lacks a Shine–Dalgarno sequence is very near
the Stop codon of its upstream gene, a situation that oc-
curs most often when the Stop codon overlaps the initia-
tion codon as in the sequence AUGA. The translation of
the upstream gene will then be required for the transla-
tion of the downstream gene, a phenomenon termed
translational coupling. The polarity arises because a ribo-
some often dissociates from the mRNA on encountering
the upstream gene’s Stop codon.Alternatively, an mRNA
may fold in a way that masks an internal Shine–Dalgarno
sequence, for example, by the base pairing of a segment
adjacent to the Shine–Dalgarno sequence to a down-
stream element of the preceding gene. Such Shine–Dal-
garno sequences only become available when a ribosome
that is translating the preceding gene disrupts the folded
structure.
Genetic expression in prokaryotes is largely transcrip-
tionally controlled (Section 31-3). This is apparently be-
cause prokaryotic mRNAs have lifetimes of only a few
minutes and, hence, it is a more efficient use of resources to
control their transcription. Nevertheless, the expression of
certain prokaryotic genes is translationally controlled,
most notably those encoding the ribosomal proteins
(which comprise 10% of cellular proteins), which must be
produced in equimolar amounts. The production of riboso-
mal proteins is controlled, in part, through a process in
which a ribosomal protein binds to the mRNA of the
operon encoding it in the vicinity of a translational start
site located near the mRNA’s 5¿ end so as to inhibit its
translational initiation. However, each such protein binds
1398 Chapter 32. Translation
JWCL281_c32_1338-1428.qxd 9/7/10 2:23 PM Page 1398
more tightly to an rRNA in forming the ribosome. Conse-
quently, only when there is an excess of that protein will it
inhibit its own translation as well as those of other proteins
encoded by its operon.
Eukaryotic cells, whose mRNAs have lifetimes of hours
or days, respond to many of their needs through transla-
tional control. In this section, we examine how eukaryotic
translation is regulated via the phosphorylation/dephos-
phorylation of eIF2 and eIF4E. We then consider transla-
tional control by mRNA masking and cytoplasmic
polyadenylation and end by discussing the uses of anti-
sense oligonucleotides.
A. Regulation of eIF2
Four important pathways for the regulation of translation
in eukaryotes involve the phosphorylation of the con-
served Ser 51 on the subunit of eIF2 (eIF2; recall that
eIF2 is an trimer that conducts to the 40S
ribosomal subunit, and the resulting complex scans the
bound mRNA for the initiating AUG codon to form the
48S preinitiation complex; Section 32-3Cd). The so-called
eIF2 kinases that do so share a conserved kinase domain
but have unique regulatory domains.
a. Heme Availability Controls Globin Translation
Reticulocytes synthesize protein, almost exclusively he-
moglobin, at an exceedingly high rate and are therefore a
favorite subject for the study of eukaryotic translation. He-
moglobin synthesis in fresh reticulocyte lysates proceeds
normally for several minutes but then abruptly stops be-
cause of the inhibition of translational initiation and the
consequent polysome disaggregation. This process is pre-
vented by the addition of heme [a mitochondrial product
(Section 26-4A) that this in vitro system cannot synthe-
size], thereby indicating that globin synthesis is regulated by
heme availability. The inhibition of globin translational ini-
tiation is also reversed by the addition of the eukaryotic
initiation factor eIF2 and by high levels of GTP.
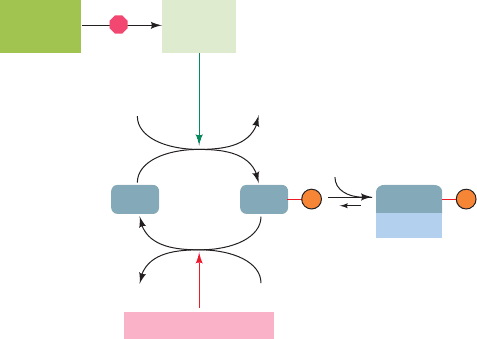
In the absence of heme, reticulocyte lysates accumulate
an eIF2 kinase named heme-regulated inhibitor [HRI;
also called heme-controlled repressor (HCR)]. HRI is a
homodimer whose 629-residue subunits each contain two
heme-binding sites. When heme is plentiful, both of these
sites are occupied and the protein, which is autophospho-
rylated at several Ser and Thr residues, is inactive. How-
ever, when heme is scarce, one of these sites loses its
bound heme, thereby activating HRI to autophosphory-
late itself at several additional sites and to phosphorylate
Ser 51 of eIF2.
Phosphorylated eIF2 can participate in the ribosomal
initiation process in much the same way as unphosphory-
lated eIF2. This puzzling observation was clarified by the
discovery that GDP does not dissociate from phosphory-
lated eIF2 at the completion of the initiation process as it
normally does through a process facilitated by eIF2B act-
ing as a GEF (Fig. 32-44). This is because phosphorylated
eIF2 forms a much tighter complex with eIF2B than does
unphosphorylated eIF2. This sequesters eIF2B (Fig. 32-
Met–tRNA
Met
i
67), which is present in lesser amounts than eIF2, thereby
preventing the regeneration of the eIF2 GTP required
for translational initiation.The presence of heme reverses
this process by inhibiting HRI, whereon the phosphory-
lated eIF2 molecules are reactivated through the action
of eIF2 phosphatase, which is unaffected by heme. The
reticulocyte thereby coordinates its synthesis of globin
and heme.
b. Interferons Protect against Viral Infection
Interferons are cytokines that are secreted by virus-infected
vertebrate cells. On binding to surface receptors of other cells,
interferons convert them to an antiviral state,which inhibits the
replication of a wide variety of RNA and DNA viruses. In-
deed, the discovery of interferons in the 1950s arose from the
observation that virus-infected individuals are resistant to in-
fection by a second type of virus.
There are three families of interferons: type or leuko-
cyte interferon (165 residues; leukocytes are white blood
cells), the related type or fibroblast interferon (166
residues; fibroblasts are connective tissue cells), and type
or lymphocyte interferon (146 residues; lymphocytes are
immune system cells). Interferon synthesis is induced by the
double-stranded RNA (dsRNA) that is generated during in-
fection by both DNA and RNA viruses, as well as by the syn-
thetic dsRNA poly(I) poly(C). Interferons are effective an-
tiviral agents in concentrations as low as 3 10
14
M, which
makes them among the most potent biological substances
known. Moreover, they have far wider specificities than an-
tibodies raised against a particular virus. They have there-
fore elicited great medical interest, particularly since some
cancers are virally induced (Section 19-3B). Indeed, they
are in clinical use against certain tumors and viral infec-
tions.These treatments are made possible by the production
of large quantities of these otherwise quite scarce proteins
through recombinant DNA techniques (Section 5-5G).
Interferons prevent viral proliferation largely by inhibit-
ing protein synthesis in infected cells (lymphocyte interferon
Section 32-4. Control of Eukaryotic Translation 1399
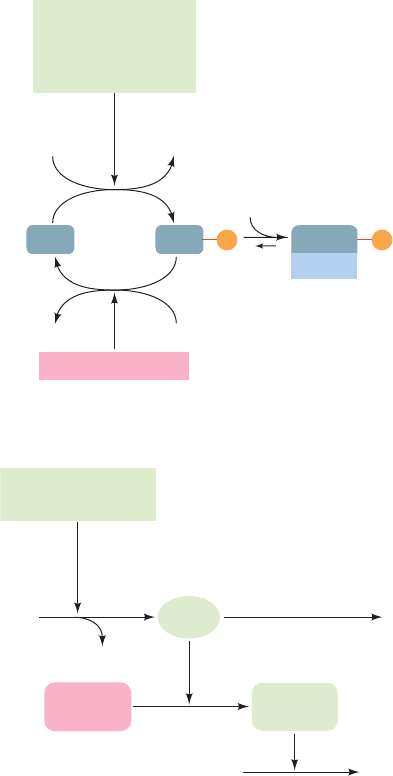
Figure 32-67 Model for heme-controlled protein synthesis in
reticulocytes.
H
2
OP
i
ATP ADP
HRI
(active)
Inhibited
by heme
pro-HRI
(inactive)
eIF2 phosphatase
eIF2 eIF2
eIF-2B
eIF2
eIF2B
P P
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1399
H
2
OP
i
ATP ADP
Double-stranded
RNA-activated
protein kinase (PKR)
eIF2 Phosphatase
eIF2 eIF2
eIF2B
eIF2
eIF2B
dsRNA
Interferon-induced
2,5A synthetase
dsRNA
RNase L
(inactive)
ATP
PP
i
2,5 A
(2',5')-phospho-
diesterase
ATP + AMP
RNase L
(active)
mRNA Nucleotides
(a)
(b)
Inhibition of Translation
mRNA Degradation
P P
also modulates the immune response). They do so in two
independent ways (Fig. 32-68):
1. Interferons induce the production of an eIF2 ki-
nase, double-stranded RNA–activated protein kinase
[PKR; also known as double-stranded RNA–activated in-
hibitor (DAI); 551 residues], which on binding dsRNA,
dimerizes and autophosphorylates itself. This activates
PKR to phosphorylate eIF2 at its Ser 51, thereby inhibit-
ing ribosomal initiation and hence the proliferation of
viruses in virus-infected cells. The importance of PKR to
cellular antiviral defense is indicated by the observation
that many viruses express inhibitors of PKR.
2. Interferons also induce the synthesis of (2,5)-
oligoadenylate synthetase (2,5A synthetase). In the pres-
ence of dsRNA, this enzyme catalyzes the synthesis from
ATP of the unusual oligonucleotide pppA(2p5A)
n
where
n 1 to 10. This compound, 2,5-A, activates a preexisting
endonuclease, RNase L, to degrade mRNA, thereby inhibit-
ing protein synthesis. 2,5-A is itself rapidly degraded by an
enzyme named (2,5)-phosphodiesterase so that it must be
continually synthesized to maintain its effect.
The independence of the 2,5-A and PKR systems is
demonstrated by the observation that the effect of 2,5-A
on protein synthesis is reversed by added mRNA but not
by added eIF2. [Recall that RNA interference (RNAi; Sec-
tion 31-4At) constitutes an alternative dsRNA-based an-
tiviral defense.]
c. PERK Prevents the Buildup of Unfolded Proteins
in the ER
PKR-like endoplasmic reticulum kinase (PERK), a
1087-residue transmembrane protein, resides in the endo-
plasmic reticulum (ER) membrane of all multicellular eu-
karyotes. It is repressed by its binding to the ER-resident
chaperone BiP (Section 12-4Bf).When the ER contains an
excessive amount of unfolded proteins (caused by various
forms of stress such as high temperatures), BiP dissociates
from PERK, thereby activating PERK to phosphorylate
eIF2 at its Ser 51 and hence inhibit translation. Thus
PERK functions to protect the cell from the irreversible
damage caused by the accumulation of unfolded proteins
in the ER.
Wolcott–Rallison syndrome is a genetic disease charac-
terized mainly by insulin-dependent (type I) diabetes that
develops in early infancy (type I diabetes usually first ap-
pears in childhood; Section 27-4B). It is caused by muta-
tions in the catalytic domain of PERK. This results in the
death of pancreatic cells, in which PERK is particularly
abundant. Multiple systemic disorders subsequently occur
including osteoporosis (reduction in the quantity of bone)
and growth retardation.
d. GCN2 Regulates Amino Acid Biosynthesis
GCN2 (1590 residues),the sole eIF2 kinase in yeast, is a
transcriptional activator of the gene encoding GCN4, a tran-
scriptional activator of numerous yeast genes, many of which
encode enzymes that participate in amino acid biosynthetic
pathways. The C-terminal domain of GCN2, which resem-
bles histidyl–tRNA synthetase (HisRS), preferentially binds
uncharged tRNAs (whose presence is indicative of an insuf-
ficient supply of amino acids). The binding of an uncharged
tRNA to this HisRS-like domain activates the adjacent
eIF2 kinase domain and thereby inhibits translational initi-
ation, although at only a modest level.
Despite this inhibition of yeast protein synthesis, acti-
vated GCN2 induces the expression of GCN4. This seem-
ingly paradoxical property of GCN2, as Alan Hinnebusch
explained, arises from the fact that GCN4 mRNA contains
four short so-called upstream open reading frames
1400 Chapter 32. Translation
Figure 32-68 The action of interferon. In interferon-treated
cells, the presence of dsRNA, which normally results from a viral
infection, causes (a) the inhibition of translational initiation and
(b) the degradation of mRNA, thereby blocking translation and
preventing virus replication.
JWCL281_c32_1338-1428.qxd 8/19/10 10:06 PM Page 1400
