Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

(uORFs), uORF1 to uORF4, in its 5¿ leader that precedes
the sequence encoding GCN4. Under the normal nutrient
conditions in which GCN2 is inactive, the ribosome binds
to the mRNA near its 5¿ cap and scans for the nearest AUG
initiation codon (which is in uORF1), where it forms the
48S preinitiation complex (Fig. 32-44) and commences the
translation of uORF1 (Section 32-3Cd). On terminating
translation at uORF1’s Stop codon,the presence of the sur-
rounding A U–rich sequences causes the ribosome to re-
sume scanning for the next AUG codon, where it initiates
the translation of uORF2.This process repeats until the ri-
bosome terminates at the end of uORF4, where its Stop
codon’s surrounding G C–rich sequences induce the ri-
bosome to disengage from the mRNA. Hence GCN4 is
only expressed at a low basal level. However, under the
low nutrient conditions in which GCN2 phosphorylates
eIF2 at its Ser 51, the resulting reduced level of the
eIF2 GTP ternary complex causes the 40S
subunit to scan longer distances before it can form the 48S
preinitiation complex. Consequently, ⬃50% of the ribo-
somes scan past uORF2, uORF3, and uORF4 and only ini-
tiate translation at the GCN4 AUG codon, which is there-
fore translated at a high level (uORF2 and uORF3 can be
mutationally eliminated without significantly affecting
translational control).
Mammalian homologs of GCN2 are activated under
conditions of amino acid starvation. This suggests that the
foregoing process has been conserved throughout eukary-
otic evolution.
B. Regulation of eIF4E
eIF4E (cap-binding protein) binds to the m
7
G cap of eu-
karyotic mRNAs and thereby participates in translational
initiation by helping to identify the initiating AUG codon
(Section 32-3Cd).When mammalian cells are treated with
hormones, cytokines, mitogens (substances that induce
mitosis), and/or growth factors, Ser 209 of human eIF4E is
phosphorylated via a Ras-activated MAP kinase cascade
(Sections 19-3C and 19-3D), thereby increasing eIF4E’s
affinity for capped mRNA and hence stimulating transla-
tional initiation. Ser 209 occupies a surface position on
eIF4E adjacent to the binding site for the phosphate
group of the m
7
GDP and flanking the putative binding
cleft for mRNA (Fig. 32-45b).The structure of eIF4E sug-
gests that the phosphoryl group of phosphorylated Ser
209 forms a salt bridge with Lys 159, which occupies the
other side of the putative mRNA-binding cleft, so as to
form a clamp that would help stabilize the bound mRNA.
The importance of regulating eIF4E activity is indicated
by the observations that the overexpression of eIF4E
causes the malignant transformation of rodent cell lines
and that eIF4E expression is elevated in several human
cancers.
The homologous ⬃120-residue proteins known as 4E-
BP1, 4E-BP2, and 4E-BP3 (BP for binding protein; the
first two are also known as PHAS-I and PHAS-II) inhibit
cap-dependent translation. They do so by binding on the
Met–tRNA
Met
i
opposite side of eIF4E from its mRNA-binding site, pre-
sumably to a patch of seven highly conserved surface
residues, and hence do not prevent eIF4E from binding the
m
7
G cap. Rather, they block eIF4E from binding to eIF4G
and thereby interfere with the formation of the eIF4F com-
plex that positions the 40S ribosomal subunit-bound
Met–tRNA
i
Met
on the mRNA’s initiating AUG codon (Sec-
tion 32-3Cd). In fact, the 4E-BPs and eIF4G all possess the
sequence motif YXXXXL (where is an aliphatic
residue, most often L but also M or F) through which they
bind to eIF4E.
The treatment of responsive cells with insulin or any of
several protein growth factors causes the 4E-BPs to disso-
ciate from eIF4E.This is because the presence of these hor-
mones induces the phosphorylation of the 4E-BPs at six
Ser/Thr residues via the signal transduction pathway in-
volving PI3K, PKB, and mTOR (Fig. 19-67). Evidently, the
phosphorylation of eIF4E and the 4E-BPs have similar if
not synergistic effects in the hormonal regulation of trans-
lation in eukaryotes.
C. mRNA Masking and Cytoplasmic
Polyadenylation
It has been known since the nineteenth century that early
embryonic development in animals such as sea urchins, in-
sects, and frogs is governed almost entirely by information
present in the oocyte (egg) before fertilization.Indeed, sea
urchin embryos exposed to sufficient actinomycin D (Sec-
tion 31-2Cc) to inhibit RNA synthesis without blocking
DNA replication develop normally through their early
stages without a change in their protein synthesis pro-
gram. This is in part because an unfertilized egg contains
large quantities of mRNA that is “masked” by associated
proteins to form ribonucleoprotein particles, thereby pre-
venting the mRNAs’ association with the ribosomes that
are also present. On fertilization, this mRNA is “un-
masked” in a controlled fashion, quite possibly by the de-
phosphorylation of the associated proteins, and com-
mences directing protein synthesis. Development of the
embryo can therefore start immediately on fertilization
rather than wait for the synthesis of paternally specified
mRNAs.Thus, gene expression in the early stages of devel-
opment is entirely translationally controlled; transcrip-
tional control only becomes important when transcription
is initiated.
a. Cytoplasmic Polyadenylation
Another mechanism of translational control in oocytes
and early embryos involves the polyadenylation of mRNAs
in the cytoplasm (polyadenylation usually occurs in the
nucleus, following which the mRNA is exported to the cy-
toplasm; Section 31-4Ab). A substantial number of mater-
nally supplied mRNAs in oocytes have relatively short
poly(A) tails (20–40 nt versus a usual length of ⬃250 nt).
The 3¿ untranslated region of these mRNAs contains both
the AAUAAA polyadenylation signal (which is required
for polyadenylation in the nucleus; Section 31-4Ab)
Section 32-4. Control of Eukaryotic Translation 1401
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1401
(a) (b)
Translationally dormant Translationally active
CPEB
CPEB CPSF
PAP
PABP PABP
4G
3
40S
P
CRF
CRF
4E-Cap
Maskin
Maskin
UUUUUAU AAUAAA
-AAAAAAAAAAAAAAAAAAA
UUUUUAU AAUAAA
4E
-Cap
-A
together with a so-called cytoplasmic polyadenylation
element (CPE), which has the consensus sequence
UUUUUAU. The CPE is recognized by CPE-binding pro-
tein (CPEB), which contains two RNA recognition motifs
(RRMs) as well as a zinc finger motif (Section 34-3Bl) that
contribute to its binding to the mRNA. Joel Richter discov-
ered that CPEB recruits a 931-residue protein named
maskin which, in turn, binds the eIF4E (cap-binding pro-
tein) that is bound to the mRNA’s 5¿ cap (Fig. 32-69a).
Maskin contains the same YXXXXL motif through
which the 4E-BPs and eIF4G bind to eIF4E (Section 32-4B),
thereby blocking the binding of eIF4G to eIF4E and hence
preventing the formation of the 48S preinitiation complex
(Fig. 32-44).
In the maturation of Xenopus laevis oocytes, a process
that precedes fertilization and is stimulated by the steroid
hormone progesterone (Section 19-1Gb), a variety of
mRNAs, including those encoding several cyclins (which
participate in cell cycle control;Section 34-4Da) are transla-
tionally activated. Soon after exposure to progesterone, a
protein kinase named aurora phosphorylates the mRNA-
bound CPEB at its Ser 174. This increases CPEB’s affinity
for cleavage and polyadenylation specificity factor (CPSF;
Section 31-4Ab), which then binds to the mRNA’s
AAUAAA sequence, where it recruits poly(A) polymerase
(PAP) to lengthen the mRNA’s poly(A) tail (Fig. 32-69b).
Translational initiation and cytoplasmic polyadenyla-
tion occur simultaneously, which suggests that these
processes are linked. Indeed, Richter has shown that this
occurs through the binding to poly(A) of poly(A)-binding
protein (PABP; Section 31-4Ab), which as we saw (Section
32-3Cd), also binds to eIF4G to circularize the mRNA.The
eIF4G in this complex displaces maskin from eIF4E,
thereby permitting the formation of the 48S preinitiation
complex and hence the mRNA’s translation (Fig. 32-69b).
Mammalian cells also exhibit cell cycle–dependent cyto-
plasmic polyadenylation of mRNAs. This suggests that
translational control by polyadenylation is a general fea-
ture in animal cells.
D. Antisense Oligonucleotides
Since ribosomes cannot translate double-stranded RNA
or DNA–RNA hybrid helices, the translation of a given
mRNA can be inhibited by a segment of its complemen-
tary RNA or DNA, that is, an antisense RNA or an anti-
sense oligodeoxynucleotide, which are collectively known
as antisense oligonucleotides. Moreover, endogenous
RNase H’s (enzymes that cleave the RNA strand of
an RNA–DNA duplex; Section 31-4C) cleave an
mRNA–oligodeoxynucleotide duplex on its mRNA
strand, leaving the antisense oligodeoxynucleotide intact
for binding to another mRNA.
Since the human genome consists of ⬃3 billion bp, an
⬃15-nt oligonucleotide (which is easily synthesized; Sec-
tion 7-6Aa) should ideally be able to target any segment of
the human genome. This exquisite specificity provides the
delivery of an antisense oligonucleotide to, or its expres-
sion in, a selected tissue or organism with enormous bio-
medical and biotechnological potential. However, care
must be taken that an antisense oligonucleotide does not
also eliminate nontarget mRNAs.
Methods for the delivery of a therapeutically useful an-
tisense oligonucleotide to a target tissue are as yet in their
infancy. This is in large part because oligonucleotides are
readily degraded by the many nucleases present in an or-
ganism and because they do not readily pass through cell
membranes. Moreover, a target mRNA is likely to be asso-
ciated with cellular proteins and hence not available for
binding to other molecules. The nuclease resistance of
oligonucleotides can be increased by derivatizing them, for
example, by replacing a nonbridging oxygen at each phos-
phate group with a methyl group or an S atom so as to
yield methylphosphonate or phosphorothioate oligonu-
cleotides, although this reduces their antisense activity.
The expression of antisense oligonucleotides in the speci-
fied tissues would, of course,circumvent the delivery prob-
lem but has all the difficulties associated with gene ther-
apy (Section 5-5H).
1402 Chapter 32. Translation
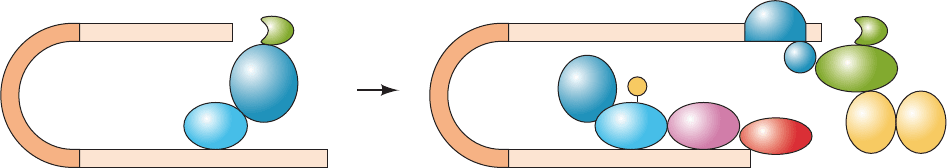
Figure 32-69 CPEB-mediated translational control. (a) In
immature Xenopus oocytes, an mRNA containing the CPE
(UUUUUAU) is bound by CPEB, which binds maskin, which
then binds eIF4E so as to prevent it from binding eIF4G, thereby
maintaining the mRNA in a translationally dormant (masked)
state. (b) In the maturation process, CPEB is phosphorylated by
an aurora protein kinase. The phosphorylated CPEB binds CPSF,
which recruits PAP to extend the mRNA’s heretofore short
poly(A) tail. PABP binds to the newly lengthened poly(A) tail
and simultaneously binds to eIF4G so as to displace maskin.This
permits the 48S preinitiation complex to assemble and hence the
translation of the mRNA to proceed. [Based on a drawing by
Mendez, R. and Richer, J.D., Nature Rev. Mol. Cell Biol. 2, 521
(2001).]
JWCL281_c32_1338-1428.qxd 9/7/10 2:23 PM Page 1402
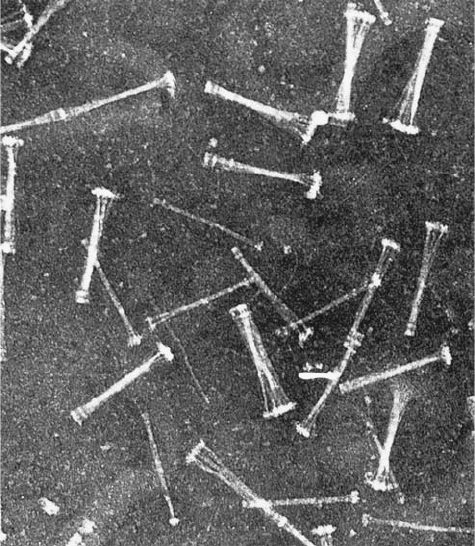
Despite the foregoing, antisense technology is begin-
ning to show success. Fomivirsen (trade name Vitravene),
a 21-nt phosphorothioate oligonucleotide that is comple-
mentary to an mRNA expressed by cytomegalovirus
(CMV), is effective in the treatment of retinitis (inflam-
mation of the retina) caused by CMV infection in individ-
uals with AIDS (CMV is an opportunistic pathogen that
rarely infects individuals with normally functioning im-
mune systems). It was approved for human use in 1998 by
the FDA, the first antisense drug so approved. A number
of antisense oligonucleotides that are mainly targeted
against genes that are overexpressed in specific cancers
and autoimmune diseases as well as other viral infections
are in clinical trials (Section 15-4Bb), although additional
antisense drugs have not yet been approved for human
use.
Antisense technology has also had some success in the
arena of biotechnology. For example, in tomatoes and
other fruits, the enzyme polygalacturonase (PG), which is
expressed during ripening, depolymerizes the pectin
(mainly polygalacturonic acid) in the cell wall. This results
in a softening of tomatoes to the point that vine-ripened
(and hence better tasting) tomatoes are unable to with-
stand the rigors of shipping and hence must be picked be-
fore they are ripe. The introduction into a tomato, via ge-
netic engineering techniques, of a gene expressing
antisense PG RNA yielded the so-called Flavr Savr tomato
that had substantially reduced PG expression and hence
remained firm after vine ripening.
5 POST-TRANSLATIONAL
MODIFICATION
To become mature proteins, polypeptides must fold to
their native conformations, their disulfide bonds, if any,
must form, and, in the case of multisubunit proteins, the
subunits must properly combine. Moreover, as we have
seen throughout this text, many proteins are modified in
enzymatic reactions that proteolytically cleave certain
peptide bonds and/or derivatize specific residues. In this
section we shall review some of these post-translational
modifications.
A. Proteolytic Cleavage
Proteolytic cleavage is the most common type of post-
translational modification. Probably all mature proteins
have been so modified, if by nothing else than the prote-
olytic removal of their leading Met (or fMet) residue
shortly after it emerges from the ribosome. Many proteins,
which are involved in a wide variety of biological
processes, are synthesized as inactive precursors that are
activated under proper conditions by limited proteolysis.
Some examples of this phenomenon that we have encoun-
tered are the conversion of trypsinogen and chy-
motrypsinogen to their active forms by tryptic cleavages
of specific peptide bonds (Section 15-3E), and the forma-
tion of active insulin from the 84-residue proinsulin by the
excision of its internal 33-residue C chain (Section 9-1Aa).
Inactive proteins that are activated by removal of
polypeptides are called proproteins, whereas the excised
polypeptides are termed propeptides.
a. Propeptides Direct Collagen Assembly
Collagen biosynthesis is illustrative of many facets of
post-translational modification. Recall that collagen, a ma-
jor extracellular component of connective tissue, is a fi-
brous triple-helical protein whose polypeptides each con-
tain the amino acid sequence (Gly-X-Y)
n
where X is often
Pro, Y is often 4-hydroxyproline (Hyp), and 340 (Sec-
tion 8-2B). The polypeptides of procollagen (Fig. 32-70)
differ from those of the mature protein by the presence of
both N-terminal and C-terminal propeptides of ⬃100
residues whose sequences, for the most part, are unlike
those of mature collagen. The procollagen polypeptides
rapidly assemble, in vitro as well as in vivo, to form a colla-
gen triple helix. In contrast, polypeptides extracted from
mature collagen will reassemble only over a period of days,
if at all. The collagen propeptides are apparently necessary
for proper procollagen folding.
The N- and C-terminal propeptides of procollagen are
respectively removed by amino- and carboxyprocollagen
n ⬇
Section 32-5. Post-Translational Modification 1403
Figure 32-70 Electron micrograph of procollagen aggregates
that have been secreted by fibroblasts into the extracellular
medium. [Courtesy of Jerome Gross, Massachusetts General
Hospital, Harvard Medical School.]
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1403
peptidases (Fig. 32-71), which may also be specific for the
different collagen types. An inherited defect of aminopro-
collagen peptidase in cattle and sheep results in a bizarre
condition, dermatosparaxis, that is characterized by ex-
tremely fragile skin. An analogous disease in humans,
Ehlers–Danlos syndrome VII, is caused by a mutation in
one of the procollagen polypeptides that inhibits the enzy-
matic removal of its aminopropeptide. Collagen molecules
normally spontaneously aggregate to form collagen fibrils
(Figs. 8-31 and 8-32). However, electron micrographs of
dermatosparaxic skin show sparse and disorganized colla-
gen fibrils. The retention of collagen’s aminopropeptides ap-
parently interferes with proper fibril formation. (The der-
matosparaxis gene was bred into some cattle herds because
heterozygotes produce tender meat.)
b. Signal Peptides Are Removed from Nascent
Proteins by a Signal Peptidase
Many transmembrane proteins or proteins that are des-
tined to be secreted are synthesized with an N-terminal sig-
nal peptide of 13 to 36 predominantly hydrophobic residues.
As we saw in our discussion of the secretory pathway (Sec-
tion 12-4B), a signal peptide is recognized by a signal recog-
nition particle (SRP). The SRP binds a ribosome synthesiz-
ing a signal peptide to a protein pore known as the
translocon that is embedded in the membrane [the rough
endoplasmic reticulum (RER) in eukaryotes and the plasma
membrane in bacteria] and conducts the signal peptide and
its following nascent polypeptide through the translocon.
Proteins bearing a signal peptide are known as prepro-
teins or, if they also contain propeptides, as preproproteins.
Once the signal peptide has passed through the membrane,
it is specifically cleaved from the nascent polypeptide by a
membrane-bound signal peptidase. Both insulin and colla-
gen are secreted proteins and are therefore synthesized
with leading signal peptides in the form of preproinsulin
and preprocollagen. These and many other proteins are
therefore subject to three sets of sequential proteolytic
cleavages: (1) the deletion of their initiating Met residue,
(2) the removal of their signal peptides, and (3) the excision
of their propeptides.
c. Polyproteins
Some proteins are synthesized as segments of polypro-
teins, polypeptides that contain the sequences of two or
more proteins. Examples include many polypeptide hor-
mones (Section 34-3C); the proteins synthesized by many
viruses, including those causing polio (Section 33-2C) and
AIDS (Section 15-4Cb); and ubiquitin, a highly conserved
eukaryotic protein involved in protein degradation (Section
32-6Bb). Specific proteases post-translationally cleave
polyproteins to their component proteins, presumably
through the recognition of the cleavage site sequences. Some
of these proteases are conserved over remarkable evolu-
tionary distances. For instance, ubiquitin is synthesized as
several tandem repeats (polyubiquitin) that E. coli properly
cleave even though prokaryotes lack ubiquitin. Other pro-
teases have more idiosyncratic cleavage sequences. This has
allowed medicinal chemists to design inhibitors of HIV pro-
tease (which catalyzes an essential step in the viral life cycle)
that have been highly effective in attenuating if not prevent-
ing the progression of AIDS (Section 15-4Cd).
1404 Chapter 32. Translation
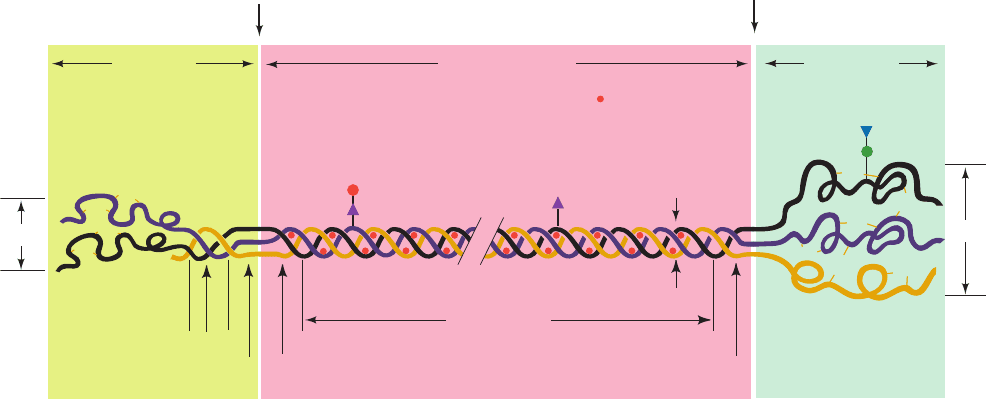
Figure 32-71 Schematic representation of the procollagen
molecule. Gal, Glc, GlcNAc, and Man, respectively, denote
galactose, glucose, N-acetylglucosamine, and mannose residues.
Note that the N-terminal propeptide has intrachain disulfide
Glc
Gal
GlcNAc
(Man)
n
N-terminal
propeptide
(150 Å)
Collagen molecule
(3000 Å)
C-terminal
propeptide
(100 Å)
Gal
15 Å
Non–triple
helical domain
Non–triple
helical domain
Triple helical
domain
Globular
domain
Triple helical domain
Non–triple
helical domain
Aminoprocollagen
peptidase
cleavage site
Carboxyprocollagen
peptidase
cleavage site
20 Å
100 Å
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
S-S
= OH
S-S
S-S
bonds while the C-terminal propeptide has both intrachain and
interchain disulfide bonds. [After Prockop, D.J., Kivirikko, K.I.,
Tuderman, L., and Guzman, N.A., New Engl. J. Med. 301, 16
(1979).]
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1404
B. Covalent Modification
Proteins are subject to specific chemical derivatizations,
both at the functional groups of their side chains and at
their terminal amino and carboxyl groups. Over 150 differ-
ent types of side chain modifications, involving all side
chains but those of Ala, Gly, Ile, Leu, Met, and Val, are
known (Section 4-3A).These include acetylations, glycosy-
lations, hydroxylations, methylations, nucleotidylations,
phosphorylations, and ADP-ribosylations as well as nu-
merous “miscellaneous” modifications.
Some protein modifications, such as the phosphoryla-
tion of glycogen phosphorylase (Section 18-1A) and the
ADP-ribosylation of eEF2 (Section 32-3Ge), modulate
protein activity. Several side chain modifications covalently
bond cofactors to enzymes, presumably to increase their
catalytic efficiency. Examples of linked cofactors that we
have encountered are N
ε
-lipoyllysine in dihydrolipoyl
transacetylase (Section 21-2Ac) and 8-histidylflavin in
succinate dehydrogenase (Section 21-3F). The attachment
of complex carbohydrates, which occur in almost infinite
variety, alter the structural properties of proteins and form
recognition markers in various types of targeting and
cell–cell interactions (Sections 11-3C, 12-3E, and 23-3B).
Modifications that cross-link proteins, such as occur in col-
lagen (Section 8-2Bc), stabilize supramolecular aggregates.
The functions of most side chain modifications, however,
remain enigmatic.
a. Collagen Assembly Requires
Chemical Modification
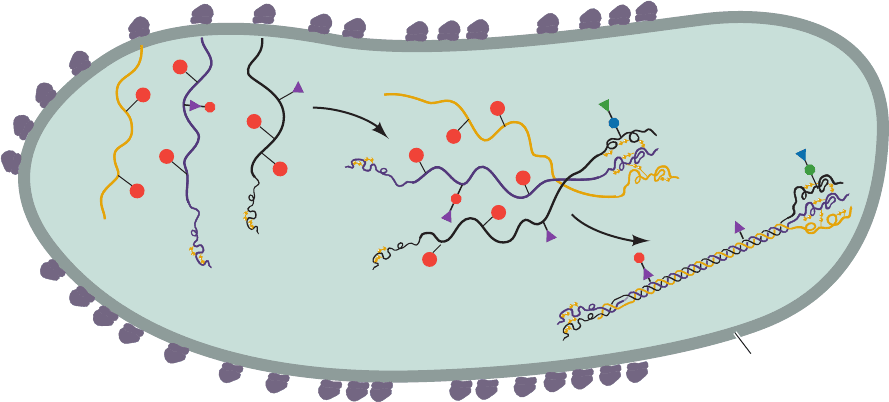
Collagen biosynthesis (Fig. 32-72) is illustrative of pro-
tein maturation through chemical modification.As the nas-
cent procollagen polypeptides pass into the RER of the fi-
broblasts that synthesized them, the Pro and Lys residues
are hydroxylated to Hyp, 3-hydroxy-Pro, and 5-hydroxy-Lys
(Hyl). The enzymes that do so are sequence specific: Prolyl
4-hydroxylase and lysyl hydroxylase act only on the Y
residues of the Gly-X-Y sequences, whereas prolyl 3-
hydroxylase acts on the X residues but only if Y is Hyp.Gly-
cosylation, which also occurs in the RER, subsequently at-
taches sugar residues to Hyl residues (Section 8-2Bb). The
folding of three polypeptides into the collagen triple helix
must follow hydroxylation and glycosylation because the
hydroxylases and glycosyl transferases do not act on helical
substrates. Moreover,the collagen triple helix denatures be-
low physiological temperatures unless stabilized by hydro-
gen bonding interactions involving Hyp residues (Section 8-
2B). Folding is also preceded by the formation of specific
interchain disulfide bonds between the carboxylpropep-
tides. This observation bolsters the previously discussed
conclusion that collagen propeptides help select and align
the three collagen polypeptides for proper folding.
The procollagen molecules pass into the Golgi appara-
tus where they are packaged into secretory vesicles (Sec-
tions 12-4C and 12-4D) and secreted into the extracellular
spaces of connective tissue. The aminopropeptides are ex-
cised just after procollagen leaves the cell and the car-
boxypropeptides are removed sometime later. The colla-
gen molecules then spontaneously assemble into fibrils,
which suggests that an important propeptide function is to
prevent intracellular fibril formation. Finally, after the ac-
tion of the extracellular enzyme lysyl oxidase, the collagen
molecules in the fibrils spontaneously cross-link (Fig. 8-33).
C. Protein Splicing: Inteins and Exteins
Protein splicing is a post-translational modification process
in which an internal protein segment (an intein) excises it-
self from a surrounding external protein, which it ligates to
form the mature extein. The portions of the unspliced
Section 32-5. Post-Translational Modification 1405
Figure 32-72 Schematic representation of procollagen
biosynthesis. Saccharides are represented as in Fig. 32-71.The
diagram does not indicate the removal of signal peptides. [After
NH
2
NH
2
Procollagen
Rough endoplasmic
reticulum
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
OH
Prockop, D.J., Kivirikko, K.I., Tuderman, L., and Guzman, N.A.,
New Engl. J. Med. 301, 18 (1979).]
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1405
1406 Chapter 32. Translation
C-extein
Intein
His
O
H
3
+
N
N-extein
H
3
+
N
N-extein
H
3
+
N
N-extein
O
H
O
HO
H
N
H
2
N
H
N
Ser 1
Ser
Asn
O
O
NH
2
COO
–
C-extein
HO
N
H
Ser
O
COO
–
C-extein
Intein
O
H
O
H
N
Ser 1
Ser
Asn
O
O
NH
2
COO
–
1
2
Precursor
Linear ester intermediate
4
Branched intermediate
Ligated exteins
His
H
2
N
C-extein
Intein
HO
H
N
Ser 1
Ser
Asn
O
O
O
NH
2
COO
–
His
H
2
N
Intein
HO
O
O
NH
His
O
H
3
+
N
N-extein
H
3
+
N
N-extein
C-extein
H
2
N
Ser
O
COO
–
O
Excised intein
H
2
N
H
2
O
Intein
HO
His
Excised intein
O
O
OH
NH
2
3
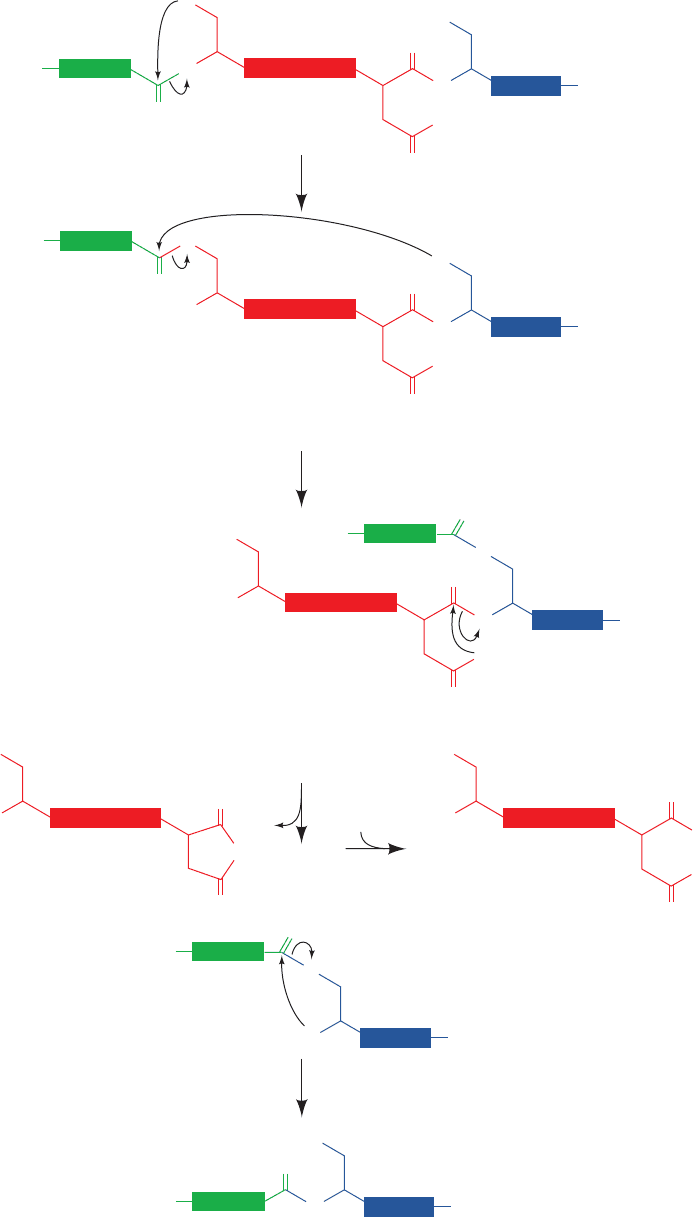
Figure 32-73 Series of reactions catalyzed by inteins to splice themselves out of a polypeptide chain.
See the text for details.
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1406
extein on the N- and C-terminal sides of the intein are
called the N-extein and the C-extein. Over 500 putative in-
teins, ranging in length from 100 to 1650 residues, have so far
been identified in archaebacteria, eubacteria, single-celled
eukaryotes, and viruses (and are registered in the Intein
Database at http://www.neb.com/neb/inteins.html). The var-
ious exteins in which these inteins are embedded exhibit no
significant sequence similarity and, in fact, can be replaced
by other polypeptides, thereby indicating that exteins do
not contain the catalytic elements that mediate protein
splicing. In contrast, the ⬃130-residue splicing elements of
inteins exhibit significant sequence similarity. All of them
have four conserved splice-junction residues: (1) a
Ser/Thr/Cys at the intein’s N-terminus; and (2 and 3) a
His–Asn/Gln dipeptide at the intein’s C-terminus; which is
immediately followed by (4) a Ser/Thr/Cys at the N-termi-
nus of the C-extein.
Protein splicing occurs via a reaction sequence that in-
volves four successive nucleophilic displacements, the first
three of which are mediated by the intein (Fig. 32-73):
1. Attack by the N-terminal intein residue (Ser, Thr, or
Cys; shown in Fig. 32-73 as Ser) on its preceding carbonyl
group, yielding a linear (thio)ester intermediate.
2. A transesterification reaction in which the ¬OH or
¬SH group on the C-extein’s N-terminal residue (shown
in Fig. 32-73 as Ser) attacks the above (thio)ester linkage,
thereby yielding a branched intermediate in which the N-
extein has been transferred to the C-extein.
3. Cleavage of the amide linkage connecting the intein
to the C-extein by cyclization of the intein’s C-terminal
Asn or Gln (shown in Fig. 32-73 as Asn). The succinimide
ring of the excised intein then spontaneously hydrolyzes to
regenerate Asn (or iso-Asn).
4. Spontaneous rearrangement of the (thio)ester link-
age between the ligated exteins to yield the more stable
peptide bond.
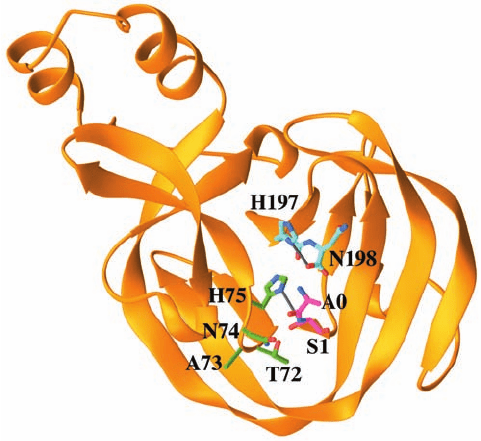
The X-ray structure of the 198-residue GyrA intein
from Mycobacterium xenopi, determined by James Sac-
chetini, indicates how this intein catalyzes the foregoing
splicing reactions. This intein’s N-terminal residue, Cys 1,
was replaced by an Ala–Ser dipeptide with the expectation
that the mutant protein would resemble the intein’s pre-
splicing state (the new N-terminal residue, Ala 0, presum-
ably represents the C-terminal residue of the N-extein).
The X-ray structure reveals that this monomeric protein
consists primarily of strands, two of which curve about
the periphery of the entire protein to give it the shape of a
flattened horseshoe (Fig. 32-74). The intein’s catalytic site
is located at the bottom of a broad and shallow cleft near
the center of this so-called -horseshoe, where the intein’s
N-terminal and C-terminal residues are in close proximity.
The Ala 0¬Ser 1 peptide bond, the bond cleaved in Reac-
tion 1 of the protein splicing process (Fig. 32-73) assumes
the cis conformation (Fig. 8-2), a rare high-energy confor-
mation (except when the peptide bond is followed by Pro)
that destabilizes this bond. Its amide nitrogen atom is hy-
drogen bonded to the side chain of the highly conserved
His 75. Hence His 75 is well positioned to donate a proton
that would promote the breakdown of the tetrahedral in-
termediate in Reaction 1.The side chains of Thr 72 and Asn
74 appear well positioned to stabilize this tetrahedral inter-
mediate in a manner resembling that of the oxyanion hole
in serine proteases (Section 15-3Db). The position of Ser 1
and a modeled Thr at the intein’s C-terminus is consistent
with Reaction 2 of the splicing process. The side chain of
the invariant His 197 is hydrogen bonded to the carboxy-
late of the C-terminal Asn 198 and hence is positioned to
protonate the peptide bond cleaved in Reaction 3.
a. Most Inteins Encode a Homing Endonuclease
What is the biological function of inteins? Nearly all in-
teins contain polypeptide inserts forming so-called homing
endonucleases. These are site-specific endonucleases that
make a double-strand break in genes that are homologous to
their corresponding extein but which lack inteins. The break
initiates the double-strand break repair of the DNA via re-
combination (Section 30-6Ag). Since the intein-containing
gene is likely to be the only other gene in the cell containing
extein-like sequences, the intein gene is copied into the
break.Thus, most inteins mediate a highly specific transposi-
tion or “homing” of the genes that insert them in similar
sites. The intein’s protease and endonuclease activities ap-
pear to have a symbiotic relationship: The protease activity
excises the intein from the host protein, thereby preventing
deleterious effects on the host, whereas the endonuclease
Section 32-5. Post-Translational Modification 1407
Figure 32-74 X-ray structure of the M. xenopi Gyr A intein in
which Cys 1 was replaced by an Ala 0–Ser 1 dipeptide. The
protein is drawn in ribbon form with its N-terminal Ala 0–Ser 1
dipeptide and its C-terminal His 197–Asn 198 dipeptide as well
as the side chains of residues 72 through 75 drawn in stick form
colored according to atom type (C of residues 0–1 magenta, C of
residues 72–75 green, C of residues 197–198 cyan, N blue, and O
red). Hydrogen bonds are represented by thin gray bonds.
[Based on an X-ray structure by James Sacchetini, Texas A&M
University. PDBid 1AM2.]
JWCL281_c32_1338-1428.qxd 8/19/10 10:06 PM Page 1407

activity assures the mobility of the intein gene. Thus intein
genes appear to be molecular parasites (junk DNA) that
only function to propagate themselves. Indeed, homing en-
donucleases are also encoded by certain types of introns.
6 PROTEIN DEGRADATION
The pioneering work of Henry Borsook and Rudolf
Schoenheimer around 1940 demonstrated that the compo-
nents of living cells are constantly turning over. For exam-
ple, adult humans normally turn over ⬃2% of their pro-
teins per day. Proteins have lifetimes that range from as
short as a few minutes to weeks or more. In any case, cells
continuously synthesize proteins from and degrade them to
their component amino acids. The function of this seem-
ingly wasteful process is 2-fold: (1) to eliminate abnormal
proteins whose accumulation would be harmful to the cell,
and (2) to permit the regulation of cellular metabolism by
eliminating superfluous enzymes and regulatory proteins.
Indeed, since the level of an enzyme depends on its rate of
degradation as well as its rate of synthesis, controlling a
protein’s rate of degradation is as important to the cellular
economy as is controlling its rate of synthesis. In this section
we consider the processes of intracellular protein degrada-
tion and their consequences.
A. Degradation Specificity
Cells selectively degrade abnormal proteins. For example,
hemoglobin that has been synthesized with the valine ana-
log -amino--chlorobutyrate
has a half-life in reticulocytes of ⬃10 min, whereas normal
hemoglobin lasts the 120-day lifetime of the red cell (which
makes it perhaps the longest lived cytoplasmic protein).
Likewise, unstable mutant hemoglobins are degraded soon
after their synthesis, which, for reasons explained in Sec-
tion 10-3A, results in the hemolytic anemia characteristic
of these molecular disease agents. Bacteria also selectively
degrade abnormal proteins. For instance, amber and ochre
mutants of -galactosidase have half-lives in E. coli of only
a few minutes, whereas the wild-type enzyme is almost in-
definitely stable. Most abnormal proteins, however, proba-
bly arise from the chemical modification and/or sponta-
neous denaturation of these fragile molecules in the cell’s
reactive environment rather than by mutations or the rare
errors in transcription or translation. The ability to elimi-
nate damaged proteins selectively is therefore an essential re-
cycling mechanism that prevents the buildup of substances
that would otherwise interfere with cellular processes.
Normal intracellular proteins are eliminated at rates
that depend on their identities. A given protein is elimi-
Cl
CH
3
CH
CH
3
CH
H
3
N
+
H
3
N
+
CH
CH
COO
_
COO
_
H
3
C
-Amino--chlorobutyrate Valine
nated with first-order kinetics, indicating that the mole-
cules being degraded are chosen at random rather than ac-
cording to their age. The half-lives of different enzymes in
a given tissue vary substantially as is indicated for rat liver
in Table 32-11. Remarkably, the most rapidly degraded en-
zymes all occupy important metabolic control points,
whereas the relatively stable enzymes have nearly constant
catalytic activities under all physiological conditions. The
susceptibilities of enzymes to degradation have evidently
evolved together with their catalytic and allosteric properties
so that cells can efficiently respond to environmental
changes and metabolic requirements. The criteria through
which native proteins are selected for degradation are con-
sidered in Section 32-6B.
The rate of protein degradation in a cell also varies with
its nutritional and hormonal state. Under conditions of nu-
tritional deprivation, cells increase their rate of protein
degradation so as to provide the necessary nutrients for in-
dispensable metabolic processes. The mechanism that in-
creases degradative rates in E. coli is the stringent response
(Section 31-3I). A similar mechanism may be operative in
eukaryotes since, as happens in E. coli, increased rates of
degradation are prevented by antibiotics that block protein
synthesis.
B. Degradation Mechanisms
Eukaryotic cells have dual systems for protein degradation:
lysosomal mechanisms and ATP-dependent cytosolically
based mechanisms. We consider both mechanisms below.
a. Lysosomes Mostly Degrade Proteins
Nonselectively
Lysosomes are membrane-encapsulated organelles
(Section 1-2Ad) that contain ⬃50 hydrolytic enzymes, in-
cluding a variety of proteases known as cathepsins. The
lysosome maintains an internal pH of ⬃5 and its enzymes
have acidic pH optima. This situation presumably protects
1408 Chapter 32. Translation
Table 32-11 Half-Lives of Some Rat Liver Enzymes
Enzyme Half-Life (h)
Short-Lived Enzymes
Ornithine decarboxylase 0.2
RNA polymerase I 1.3
Tyrosine aminotransferase 2.0
Serine dehydratase 4.0
PEP carboxylase 5.0
Long-Lived Enzymes
Aldolase 118
GAPDH 130
Cytochrome b 130
LDH 130
Cytochrome c 150
Source: Dice, J.F. and Goldberg,A.L., Arch. Biochem. Biophys. 170,
214 (1975).
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1408
the cell against accidental lysosomal leakage since lysoso-
mal enzymes are largely inactive at cytosolic pH’s.
Lysosomes recycle intracellular constituents by fusing
with membrane-enclosed bits of cytoplasm known as au-
tophagic vacuoles and subsequently breaking down their
contents. They similarly degrade extracellular substances
that the cell takes up via endocytosis (Section 12-5Bc).The
existence of these processes has been demonstrated
through the use of lysosomal inhibitors. For example, the
antimalarial drug chloroquine
is a weak base that, in uncharged form, freely penetrates
the lysosome where it accumulates in charged form,
thereby increasing the intralysosomal pH and inhibiting
lysosomal function. The treatment of cells with chloro-
quine reduces their rate of protein degradation. Similar ef-
fects arise from treatment of cells with cathepsin inhibitors
such as the polypeptide antibiotic antipain.
Lysosomal protein degradation in well-nourished cells
appears to be nonselective. Lysosomal inhibitors do not af-
fect the rapid degradation of abnormal proteins or short-
lived enzymes. Rather, they prevent the acceleration of
nonselective protein breakdown on starvation. However,
the continued nonselective degradation of proteins in
starving cells would rapidly lead to an intolerable deple-
tion of essential enzymes and regulatory proteins. Lyso-
somes therefore also have a selective pathway, which is
activated only after a prolonged fast, that takes up and de-
grades proteins containing the pentapeptide Lys-Phe-Glu-
Arg-Gln (KFERQ) or a closely related sequence. Such
KFERQ proteins are selectively lost in fasting animals
from tissues that atrophy in response to fasting (e.g., liver
and kidney) but not from tissues that do not do so (e.g.,
brain and testes). KFERQ proteins are specifically bound
in the cytosol and delivered to the lysosome by a 73-kD
peptide recognition protein (prp73), a member of the 70-
kD heat shock protein (Hsp70) family (Section 9-2C).
Both normal and pathological processes are associated
with increased lysosomal activity. Diabetes mellitus (Sec-
tion 27-4B) stimulates the lysosomal breakdown of pro-
teins. Similarly, muscle wastage caused by disuse, denerva-
tion, or traumatic injury arises from increased lysosomal
activity. The regression of the uterus after childbirth, in
which this muscular organ reduces its mass from 2 kg to
_
OOCCHNH
Phe
O
OO
O
C
NHCHC
NHCHC NHCHC H
Arg
ArgVal
Antipain
Cl
NH
CH CH
2
CH
2
CH
2
CH
3
N
N(C
2
H
5
)
2
Chloroquine
50 g in 9 days, is a striking example of this process. Many
chronic inflammatory diseases, such as rheumatoid arthri-
tis, involve the extracellular release of lysosomal enzymes
that break down the surrounding tissues.
b. Ubiquitin Marks Proteins Selected
for Degradation
It was initially assumed that protein degradation in eu-
karyotic cells is primarily a lysosomal process.Yet, reticulo-
cytes, which lack lysosomes, selectively degrade abnormal
proteins. The observation that protein breakdown is inhib-
ited under anaerobic conditions led to the discovery of a
cytosolically based ATP-dependent proteolytic system that
is independent of the lysosomal system. This phenomenon
was thermodynamically unexpected since peptide hydroly-
sis is an exergonic process.
The analysis of a cell-free rabbit reticulocyte system
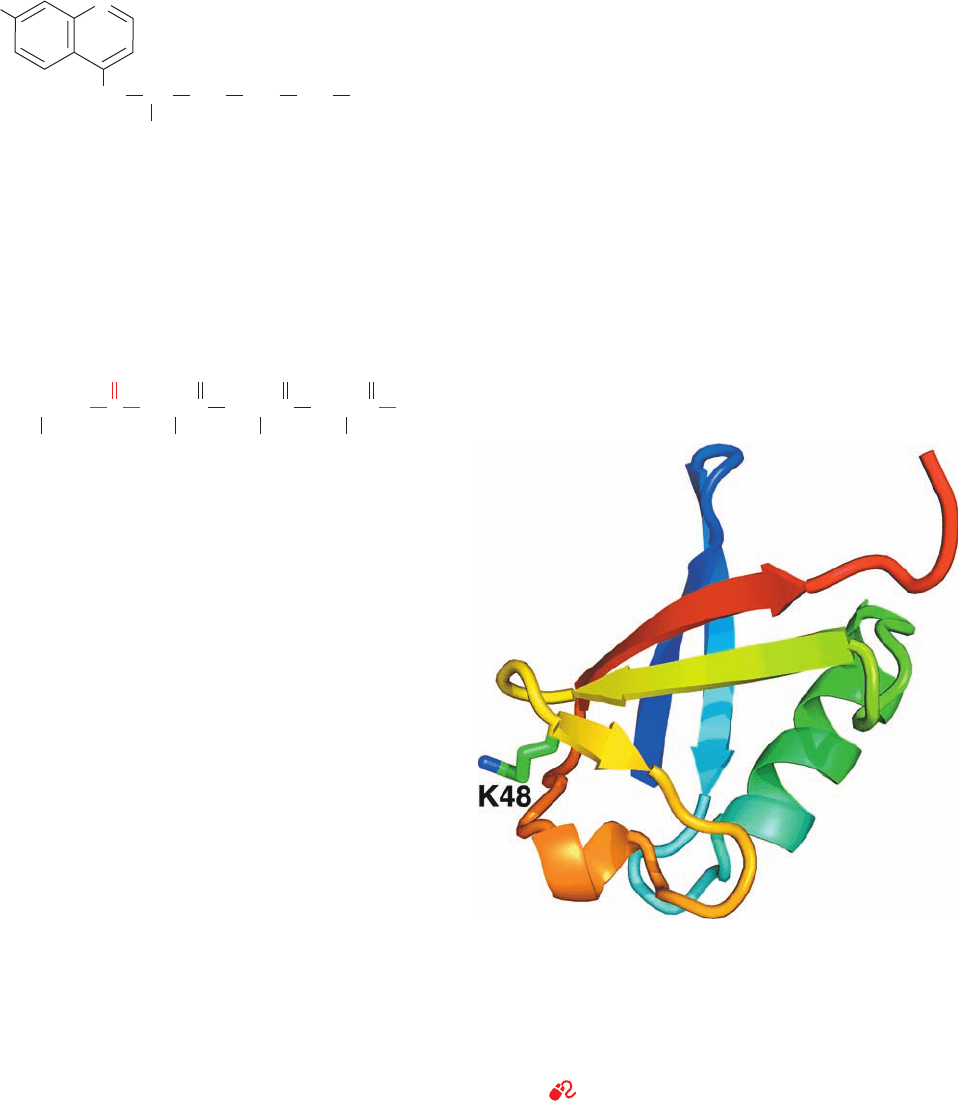
demonstrated that ubiquitin (Fig. 32-75) is required for
ATP-dependent protein degradation. This 76-residue
monomeric protein, so named because it is ubiquitous as well
as abundant in eukaryotes, is the most highly conserved pro-
tein known: It is identical in such diverse organisms as hu-
mans, toad, trout, and Drosophila and differs in only three
residues between humans and yeast. Evidently, ubiquitin is
all but uniquely suited to an essential cellular function.
Proteins that are selected for degradation are so marked
by covalently linking them to ubiquitin. This process, which
is reminiscent of amino acid activation (Section 32-2C),
Section 32-6. Protein Degradation 1409
Figure 32-75 X-ray structure of human ubiquitin. The
polypeptide is drawn in ribbon form colored in rainbow order
from its N-terminus (blue) to its C-terminus (red) with the side
chain of Lys 48 shown in stick form with C green and N blue. This
␣⫹architecture is known as a -Grasp fold because its 
sheets appear to grasp its ␣ helix. [Based on an X-ray structure
by Charles Bugg, University of Alabama at Birmingham. PDBid
1UBQ.]
See Interactive Exercise 46.
JWCL281_c32_1338-1428.qxd 10/19/10 8:24 AM Page 1409
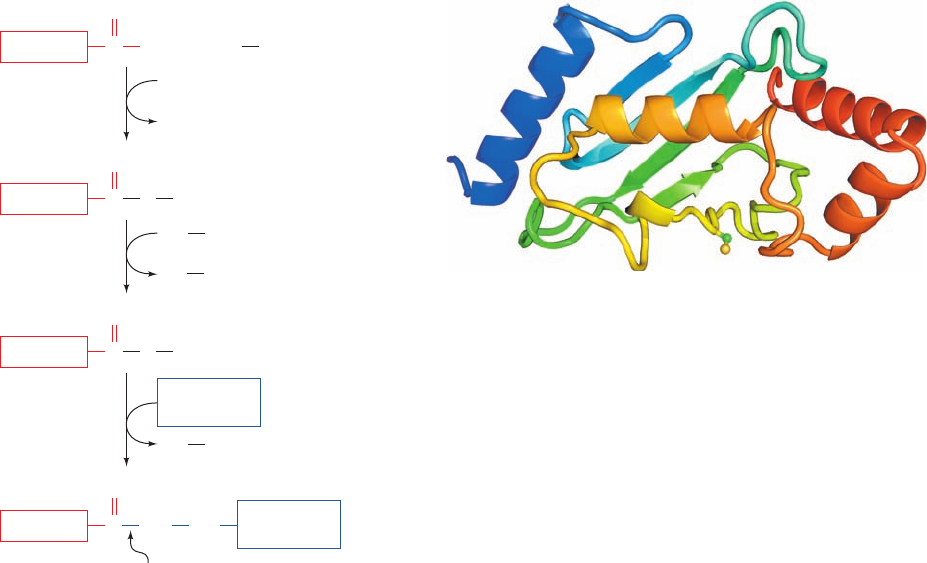
occurs in a three-step pathway that was elucidated notably
by Avram Hershko, Aaron Ciechanover, and Irwin Rose
(Fig. 32-76):
1. In an ATP-requiring reaction, ubiquitin’s terminal
carboxyl group is conjugated, via a thioester bond, to
ubiquitin-activating enzyme (E1), a homodimer of
⬃1050-residue subunits. In this process, the substrate
protein’s terminal carboxyl group is initially adenyly-
lated and then transferred to the E1 Cys¬SH group with
the elimination of AMP. Most organisms, including yeast
and humans, have only one type of E1.
2. The ubiquitin is then transferred to a specific Cys
sulfhydryl group on one of numerous proteins named
ubiquitin-conjugating enzymes (E2s; 11 in yeast and over
20 in mammals). The various E2’s are characterized by
⬃150-residue catalytic cores containing the active site Cys
that exhibit at least 25% sequence identities and which
mainly vary by the presence or absence of N- and/or C-
terminal extensions that exhibit little sequence identity to
each other. The X-ray and NMR structures of several
species of E2 reveal that their catalytic cores all assume
closely similar / structures (e.g., Fig. 32-77) in which
most of the identical residues are clustered on one surface
near the ubiquitin-accepting Cys residue, where they pre-
sumably interact with ubiquitin and E1.
3. Ubiquitin–protein ligase (E3) transfers the activated
ubiquitin from E2 to a Lys ε-amino group of its target pro-
tein, thereby forming an isopeptide bond. Each of the many
E3s present in eukaryotic cells mediates the ubiquitination
(alternatively, ubiquitylation) of a specific set of proteins
and thereby marks them for degradation. Each E3 is
served by one or a few specific E2s. Most E3s are members
of two unrelated families, those containing a HECT do-
main (HECT for homologous to E6AP C-terminus) and
those containing a so-called RING domain (also called a
RING-finger domain; RING for really interesting new
gene), although some E2s react well with members of both
families. The human genome contains 28 HECT genes and
616 RING genes, more than its number of protein kinase
genes (518), which is indicative of the specialized and
varied functions of E3s (although not all RING domain–
containing proteins are E3s). HECT domain E3s are
modularly constructed with a unique N-terminal domain
that interacts with its target proteins via their so-called
ubiquitination signals (usually short polypeptide segments;
see below) and an ⬃350-residue HECT domain that medi-
ates E2 binding and catalyzes the ubiquitination reaction.
RING domains, which are implicated in recognizing a sub-
strate protein’s ubiquitination signal, are 40- to 60-residue
motifs that each bind two structurally but not catalytically
implicated Zn
2
ions via a total of 8 Cys and His residues in
a characteristic consensus sequence (much like the zinc fin-
ger motifs in certain DNA-binding proteins, Section 34-
3Bl). RING domain–containing E3s may consist of a sin-
gle subunit or may be multisubunit proteins in which the
RING domain is contained in one subunit. HECT
E3–mediated ubiquitination occurs via the transfer of
ubiquitin from E2 to a conserved Cys residue on the
HECT domain followed by its transfer to the substrate
protein Lys side chain. In contrast, RING domain E3s act
1410 Chapter 32. Translation
Figure 32-76 Reactions involved in the attachment of
ubiquitin to a protein. In the first part of the process, ubiquitin’s
terminal carboxyl group is joined, via a thioester linkage, to E1 in
a reaction driven by ATP hydrolysis. The activated ubiquitin is
subsequently transferred to a sulfhydryl group of an E2 and
then, in a reaction catalyzed by an E3, to a Lys ε-amino group on
a condemned protein, thereby flagging the protein for
proteolytic degradation by the 26S proteasome.
Figure 32-77 X-ray structure of an E2 protein from
Arabidopsis thaliana. The protein is drawn in ribbon form
colored in rainbow order from its N-terminus (blue) to its
C-terminus (red).The side chain of Cys 88, to which ubiquitin is
covalently linked, is shown in ball-and-stick form with C green
and S yellow. [Based on an X-ray structure by William Cook,
University of Alabama at Birmingham. PDBid 2AAK.]
Ubiquitin C O
–
+ E1 SH
O
ATP
AMP
+ PP
i
1
Ubiquitin C NH Lys
O
Ubiquitin C SE1
SHE2
SHE2
SHE1
O
2
Ubiquitin C SE2
E3
Isopeptide
bond
O
3
Condemned
protein
Condemned
protein
JWCL281_c32_1338-1428.qxd 9/7/10 2:23 PM Page 1410
