Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

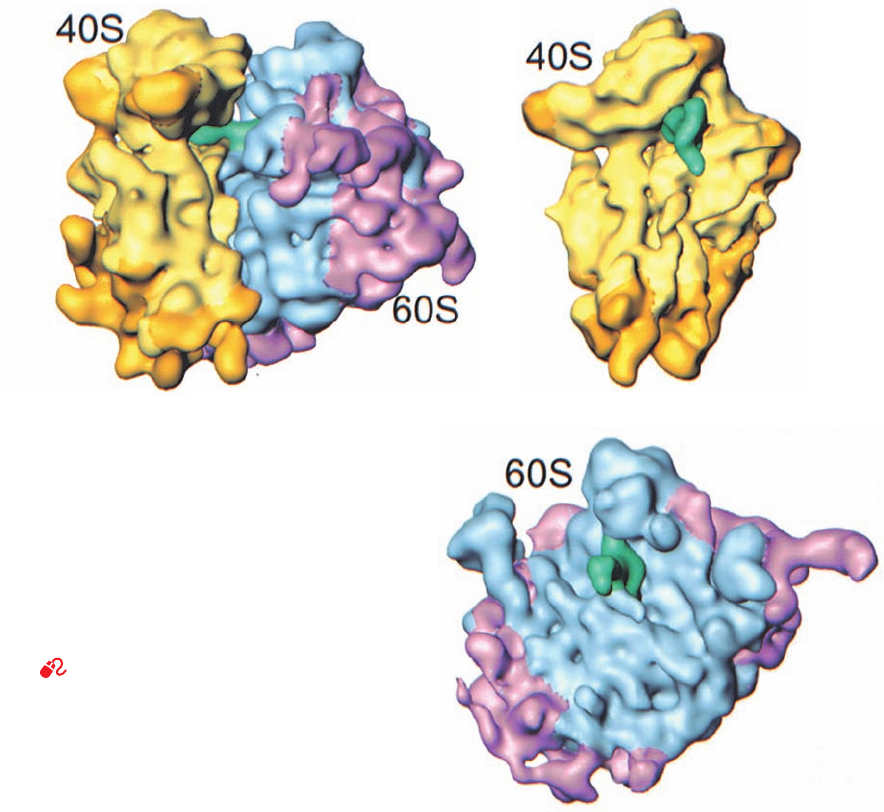
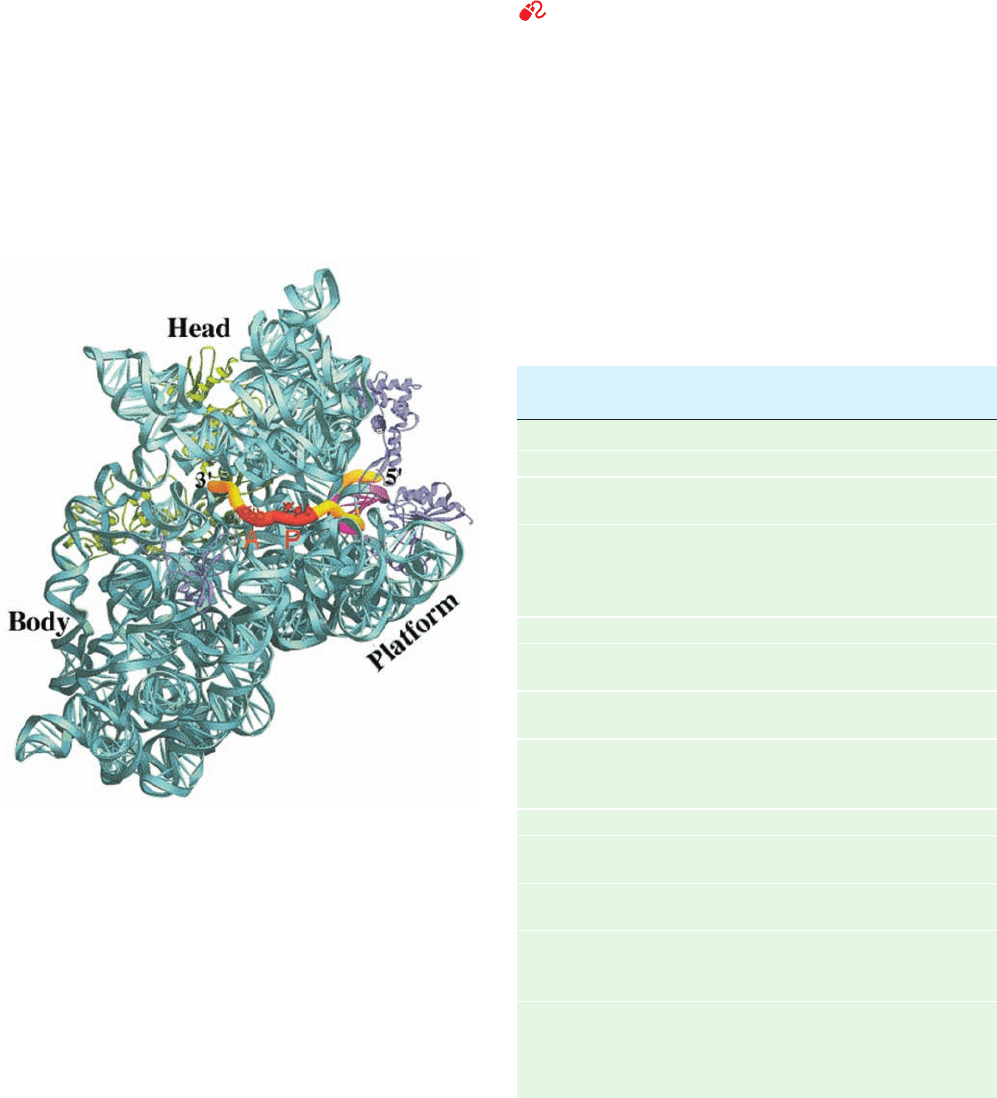
The cryo-EM–based image of the yeast 80S ribosome
(Fig. 32-36), determined at 15 Å resolution by Andrej Sali,
Günter Blobel, and Frank, reveals that there is a high de-
gree of structural conservation between eukaryotic and
prokaryotic ribosomes. Although the yeast 40S subunit
(which consists of a 1798-nt 18S rRNA and 32 proteins)
contains an additional 256 nt of RNA and 11 proteins rela-
tive to the E. coli 30S subunit (Table 32-8; 15 of the E. coli
proteins are homologous to those of yeast), both exhibit a
similar division into head, neck, body, and platform (Fig.
32-36b vs Figs. 32-32a and 32-34b). Many of the differences
between these two small ribosomal subunits are accounted
for by the 40S subunit’s additional RNA and proteins, al-
though their homologous portions exhibit several distinct
conformational differences. Similarly, the yeast 60S subunit
(Fig. 32-35c; which consists of an aggregate of 3671 nt and
45 proteins) structurally resembles the considerably
smaller (Table 32-7) prokaryotic 50S subunit (Fig. 32-32b).
The yeast ribosome exhibits 16 intersubunit bridges, 12 of
which match the 12 that were observed in the X-ray struc-
ture of the T. thermophilus ribosome, a remarkable evolu-
tionary conservation that indicates the importance of these
bridges. Moreover, the tRNA that occupies the P site of the
yeast ribosome has a conformation that more closely re-
sembles that of the P-site tRNA in the T. thermophilus ri-
bosome than that of free tRNA
Phe
.
B. Polypeptide Synthesis: An Overview
Before we commence our detailed discussion of polypep-
tide synthesis, it will be helpful to outline some of its major
features.
a. Polypeptide Synthesis Proceeds from N-Terminus
to C-Terminus
The direction of ribosomal polypeptide synthesis was es-
tablished, in 1961 by Howard Dintzis, through radioactive
labeling experiments. He exposed reticulocytes that were
actively synthesizing hemoglobin to
3
H-labeled leucine for
times less than that required to make an entire polypeptide.
The extent to which the tryptic peptides from the soluble
(completed) hemoglobin molecules were labeled increased
Section 32-3. Ribosomes and Polypeptide Synthesis 1371
Figure 32-36 Cryo-EM–based image of the yeast 80S
ribosome at 15 Å resolution. (a) The ribosome shown in side
view analogous to Fig. 32-30 of the E. coli ribosome.The small
(40S) subunit is yellow, the large (60S) subunit is cyan, and the
tRNA that is bound in the ribosomal P site is green. Portions of
this ribosome that are not homologous to the RNA or proteins
of the E. coli ribosome are shown in gold for the small subunit
and magenta for the large subunit. (b) The computationally
isolated small subunit shown in interface view analogous to the
left panel of Fig. 32-32a. (c) The computationally isolated large
subunit shown in interface view analogous to the left panel of
Fig. 32-32b. [Courtesy of Joachim Frank, State University of New
York at Albany.]
See the Interactive Exercise.
(a) (b)
(c)
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1371
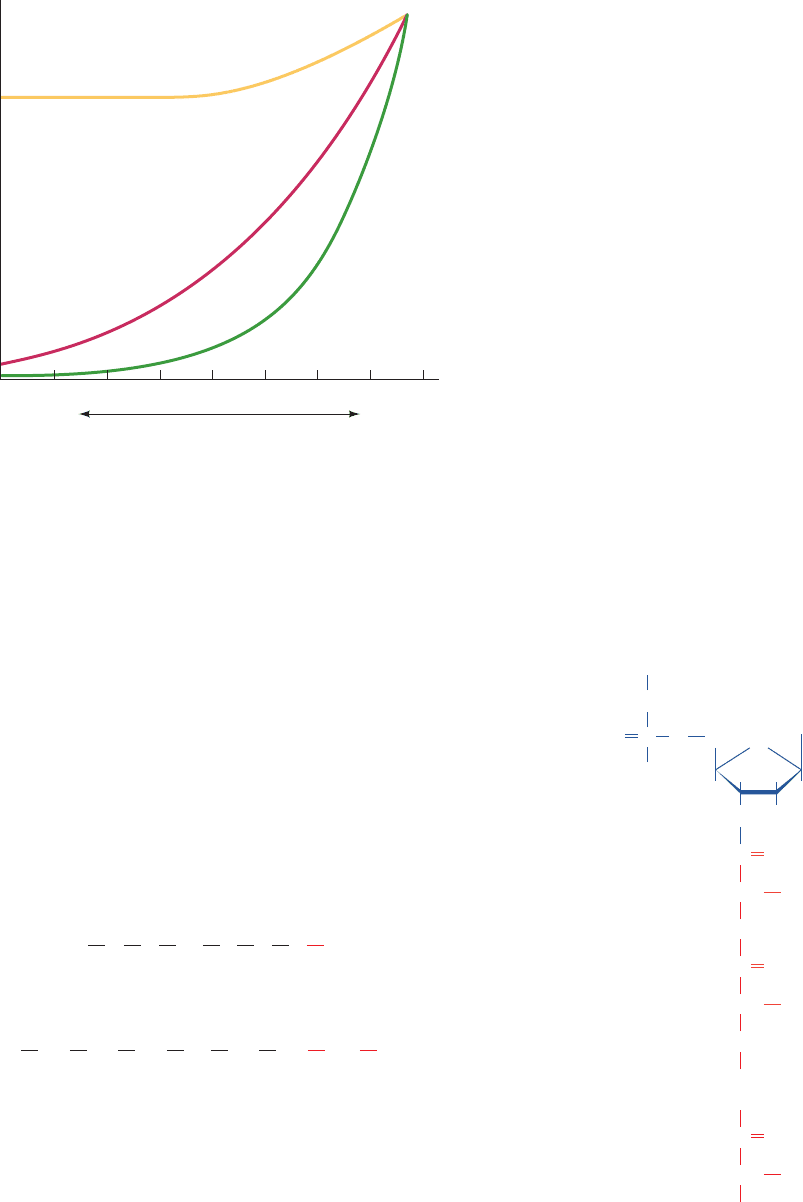
with their proximity to the C-terminus (Fig. 32-37). Incom-
ing amino acids must therefore be appended to a growing
polypeptide’s C-terminus; that is, polypeptide synthesis pro-
ceeds from N-terminus to C-terminus.
b. Ribosomes Read mRNA in the 5¿S3¿ Direction
The direction in which the ribosome reads mRNAs was
determined through the use of a cell-free protein synthe-
sizing system in which the mRNA was poly(A) with a 3¿-
terminal C.
Such a system synthesizes a poly(Lys) that has a C-terminal
Asn.
This, together with the knowledge that AAA and AAC
code for Lys and Asn and the polarity of polypeptide syn-
thesis, indicates that the ribosome reads mRNA in the 5¿S
3¿ direction. Since mRNA is synthesized in the 5¿S3¿ di-
rection, this accounts for the observation that, in prokary-
otes, ribosomes initiate translation on nascent mRNAs
(Section 31-3).
c. Active Translation Occurs on Polyribosomes
Electron micrographs, as Rich discovered, reveal that ri-
bosomes engaged in protein synthesis are tandemly
arranged on mRNAs like beads on a string (Fig. 31-27) to
...
H
3
N
+
Lys Lys Lys Lys Lys Asn COO
_
5
3
A
A
A
AAAC
...
form assemblies known as polyribosomes (polysomes).
Polysomes arise because once an active ribosome has
cleared its initiation site on an mRNA, a second ribosome
can initiate at that site.
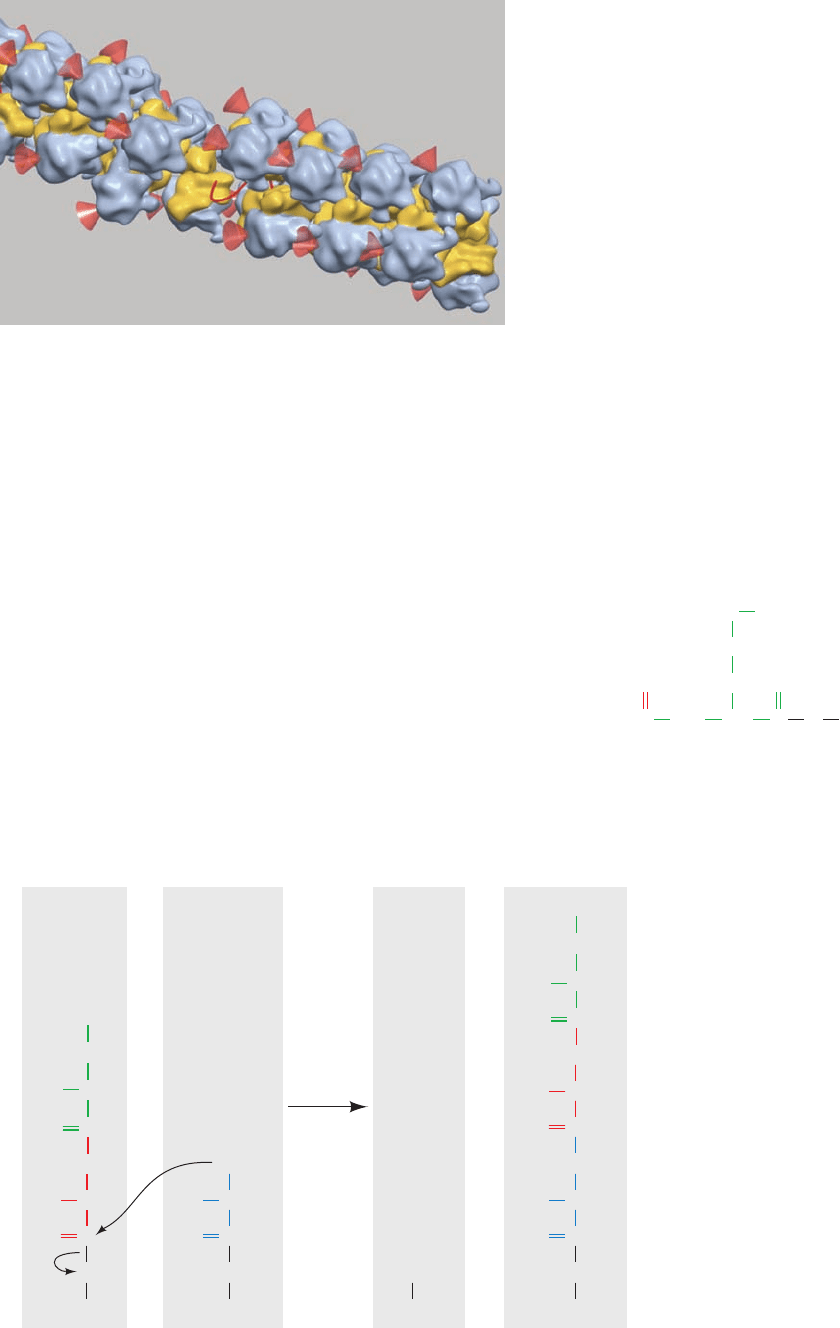
Cryo-EM–based image reconstruction of E. coli
polysomes, by Ulrich Hartl and Wolfgang Baumeister, has
revealed that adjacent ribosomes are densely packed along
the mRNA and have staggered or pseudohelical arrange-
ments (Fig. 32-38). The mRNA is located along the inside
of these assemblies, the tRNA entrance sites are accessible,
and the polypeptide exit tunnel on each ribosome faces
the cytosol. Modeling suggests that such an arrangement
maximizes the distance between the nascent polypeptide
chains exiting neighboring ribosomes. This reduces the
ability of these incompletely folded polypeptides to non-
specifically aggregate and hence increases the yield of na-
tively folded protein.
d. Chain Elongation Occurs by the Linkage of the
Growing Polypeptide to the Incoming tRNA’s Amino
Acid Residue
During polypeptide synthesis, amino acid residues are
sequentially added to the C-terminus of the nascent, ribo-
somally bound polypeptide chain. If the growing polypep-
tide is released from the ribosome by treatment with high
salt concentrations, its C-terminal residue is invariably es-
terified to a tRNA molecule as a peptidyl–tRNA:
The nascent polypeptide must therefore grow by being
transferred from the peptidyl–tRNA to the incoming
aminoacyl–tRNA to form a peptidyl–tRNA with one more
H
2
C
HH
HH
O OH
O
PO
O
–
O
O
OC
CH R
n
NH
OC
CH R
n–1
NH
.
.
.
OC
CH R
1
NH
3
+
Peptidyl–tRNA
Adenine
tRNA
1372 Chapter 32. Translation
Figure 32-37 Demonstration that polypeptide synthesis
proceeds from the N-terminus to the C-terminus. Rabbit
reticulocytes were incubated with [
3
H]leucine. The curves show
the distribution of [
3
H]Leu among the tryptic peptides from the
subunit of soluble rabbit hemoglobin after the indicated
incubation times.The numbers on the horizontal axis are peptide
identifiers arranged from N-terminus to C-terminus. [After
Dintzis, H.M., Proc. Natl. Acad. Sci. 47, 255 (1961).]
13 24 1 17 3 9 18 12 27
Incubation time
60 min
7 min
4 min
Relative amount of
3
H
Peptide number, β chain
C-terminusN-terminus
JWCL281_c32_1338-1428.qxd 9/7/10 2:22 PM Page 1372
residue (Fig. 32-39). Apparently, the ribosome has at least
two tRNA-binding sites: the so-called peptidyl or P site,
which binds the peptidyl–tRNA, and the aminoacyl or A
site, which binds the incoming aminoacyl–tRNA (Fig. 32-
39). Consequently, after the formation of a peptide bond,
the newly deacylated P-site tRNA must be released and re-
placed by the newly formed peptidyl–tRNA from the A
site, thereby permitting a new round of peptide bond for-
mation. The finding by Knud Nierhaus that each ribosome
can bind up to three deacylated tRNAs but only two
aminoacyl–tRNAs indicates, however, that the ribosome
has a third tRNA-binding site, the exit or E site, which
transiently binds the outgoing deacylated tRNA. All three
sites, as we have seen (Fig. 32-34), extend over both riboso-
mal subunits.
The details of the chain elongation process are dis-
cussed in Section 32-3D. Chain initiation and chain termi-
nation, which are special processes, are examined in Sec-
tions 32-3C and 32-3F, respectively. In all of these sections
we shall first consider the process of interest in E. coli and
then compare it with the analogous eukaryotic activity.
C. Chain Initiation
a. fMet Is the N-Terminal Residue of Prokaryotic
Polypeptides
The first indication that the initiation of translation re-
quires a special codon, since identified as AUG (and, in
prokaryotes, occasionally GUG), was the observation that
almost half of the E. coli proteins begin with the otherwise
uncommon amino acid Met. This was followed by the dis-
covery of a peculiar form of Met–tRNA
Met
in which the
Met residue is N-formylated:
CH
2
HC CHNH
O
CH
2
S CH
3
C
O
O tRNA
f
Met
N-Formylmethionine–tRNA
f
Met
(fMet–tRNA
f
Met
)
Section 32-3. Ribosomes and Polypeptide Synthesis 1373
Figure 32-38 Cryo–EM–based image of an E. coli polysome.
The mRNA (which is mostly occluded) is represented by a red
line, the small ribosomal subunits are yellow, the large subunits
are blue-gray, and the red cones point to the polypeptide exit
tunnel on each large subunit.The assembly shown has a pseudo-
helical arrangement of ribosomes in which the center-to-center
distance between adjacent ribosomes averages ⬃230 Å.
Polysomes with somewhat different although equally densely
packed arrangements of ribosomes have also been observed.
[Courtesy of Ulrich Hartl and Wolfgang Baumeister, Max Planck
Institute of Biochemistry, Martinsreid, Germany.]
Figure 32-39 Ribosomal peptidyl transferase
reaction forming a peptide bond. The ribosome
catalyzes the nucleophilic attack of the amino
group of the aminoacyl–tRNA in the A site on
the peptidyl–tRNA ester in the P site, thereby
forming a new peptide bond and transferring
the nascent polypeptide to the A-site tRNA,
while displacing the P-site tRNA.
CH
NH
NH
R
n
R
n–1
C
...
O
CH
CO
O
tRNA
(n)
P site A site P site A site
NH
2
R
n+1
CH
CO
O
tRNA
(n+1)
:
OH
tRNA
(n)
CH
NH
NH
R
n
R
–1
C
...
O
CH
CO
NH
R
+1
CH
CO
O
tRNA
Peptidyl–tRNA Aminoacyl–tRNA Uncharged tRNA Peptidyl–tRNA
n
n
+1)(n
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1373
U
A
C
U
U
A
U
A
C
C
G
U
G
A
U
A
A
C
A
U
A
U
U
C
A
C
A
U
A
A
A
A
A
G
C
U
A
C
C
A
A
C
A
A
G
U
U
C
U
U
C
C
C
A
A
A
A
C
A
A
U
C
C
A
U
A
U
A
A
C
A
G
G
A
G
U
A
G
U
G
A
G
G
G
G
G
A
G
G
G
G
G
G
A
G
G
A
G
G
A
G
G
G
A
G
G
A
G
A
G
G
A
U
A
U
G
U
G
C
G
U
U
G
G
A
G
C
U
C
A
A
A
G
C
G
A
A
U
U
U
A
A
U
A
G
U
C
A
U
G
A
A
U
C
A
A
G
A
A
U
A
U
G
A
G
A
A
A
G
U
U
A
U
C
A
A
C
U
U
C
G
U
G
U
U
C
C
G
U
U
U
C
U
C
A
A
A
A
A
A
G
A
A
A
A
A
A
A
A
U
U
U
U
U
U
U
U
U
U
U
G
G
G
G
G
G
G
G
G
G
G
G
A
A
A
U
G
G
G
G
C
A
C
G
A
C
C
U
C
C
C
A
A
G
A
A
C
U
U
U
A
U
A
A
A
G
C
A
A
C
U
A
U
A
G
U
U
C
U
A
G
C
C
U
C
C
U
U
A
G
G
U
U
A
A
A
A
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
–
araB
g
alE
lacI
lacZ
Q phage replicase
X174 phage A protein
R17 phage coat protein
Ribosomal S12
Ribosomal L10
trpE
trp leader
Initiation
codon
3 end of 16S rRNA
AUUCCUCCACUAG
–
HO
3
5
The N-formylmethionine residue (fMet) already has an
amide bond and can therefore only be the N-terminal
residue of a polypeptide. In fact, polypeptides synthesized
in an E. coli–derived cell-free protein synthesizing system
always have a leading fMet residue. fMet must therefore be
E. coli’s initiating residue.
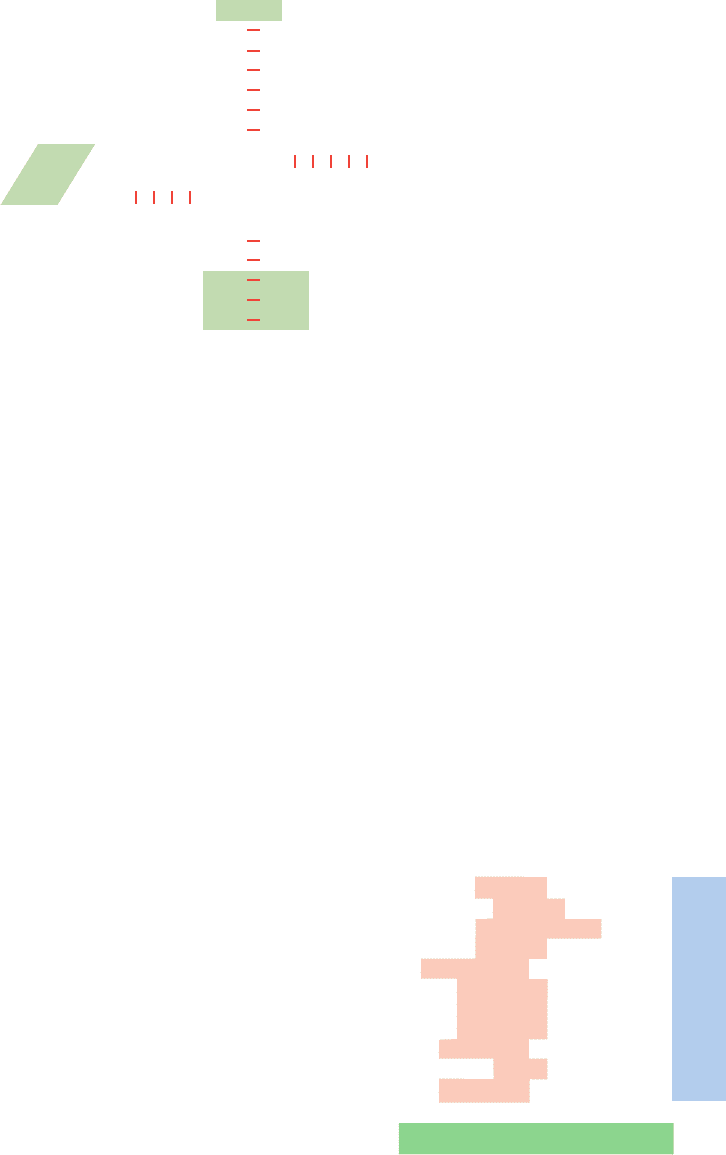
The tRNA that recognizes the initiation codon,
tRNA
f
Met
(Fig. 32-40), differs from the tRNA that carries
internal Met residues, tRNA
m
Met
, although they both recog-
nize the same codon. In E. coli, uncharged (deacylated)
tRNA
f
Met
is first aminoacylated with methionine by the same
MetRS that charges tRNA
m
Met
.The resulting Met–tRNA
f
Met
is specifically N-formylated to yield fMet–tRNA
f
Met
in an
enzymatic reaction that employs N
10
-formyltetrahydrofo-
late (Section 26-4D) as its formyl donor. The formylation
enzyme does not recognize Met–tRNA
m
Met
. The X-ray
structures of E. coli tRNA
f
Met
and yeast tRNA
Phe
(Fig.32-11b)
are largely similar but differ conformationally in their ac-
ceptor stems and anticodon loops. Perhaps these structural
differences permit tRNA
f
Met
to be distinguished from
tRNA
m
Met
in the reactions of chain initiation and elongation
(see Section 32-3D).
E. coli proteins are post-translationally modified by a de-
formylase, which hydrolytically deformylates their fMet
residue,and, in many proteins, by the subsequent removal of
the resulting N-terminal Met.This processing usually occurs
on the nascent polypeptide, which accounts for the obser-
vation that mature E. coli proteins all lack fMet.
b. Base Pairing between mRNA and the 16S rRNA
Helps Select the Translational Initiation Site
AUG codes for internal Met residues as well as the ini-
tiating Met residue of a polypeptide. Moreover, mRNAs
usually contain many AUGs (and GUGs) in different read-
ing frames. Clearly, a translational initiation site must be
specified by more than just an initiation codon. This occurs
in two ways: (1) the masking of AUGs that are not initia-
tion codons by mRNA secondary structure; and (2) inter-
actions between the mRNA and the 16S rRNA that select
the initiating AUG as we now discuss.
The 16S rRNA contains a pyrimidine-rich sequence at
its 3¿ end. This sequence, as John Shine and Lynn Dalgarno
pointed out in 1974, is partially complementary to a purine-
rich tract of 3 to 10 nucleotides, the Shine–Dalgarno
sequence, that is centered ⬃10 nucleotides upstream from
the start codon of nearly all known prokaryotic mRNAs
(Fig. 32-41). Base pairing interactions between an mRNA’s
1374 Chapter 32. Translation
Figure 32-40 Nucleotide sequence of E. coli shown
in cloverleaf form. The shaded boxes indicate the significant
differences between this initiator tRNA and noninitiator tRNAs
such as yeast tRNA
Ala
(Fig. 32-8). [After Woo, N.M., Roe, B.A.,
and Rich,A., Nature 286, 346 (1980).]
tRNA
Met
f
Figure 32-41 Some translational initiation sequences
recognized by E. coli ribosomes. The mRNAs are aligned
according to their initiation codons (blue shading).Their
Shine–Dalgarno sequences (red shading) are complementary,
counting G U pairs, to a portion of the 16S rRNA’s 3¿ end
E. coli tRNA
f
Met
A
OH
C
C
A
A
C
G
C
C
C
C
G
C
G
G
G
G
CGGCC
GUCGG
U
A
A
T
ψ
C
A
U
G
CGAG
GCUC
G
A
G
C
C
C
U
C
G
G
G
CA
A
U
A
C
U
A
G
C
C
U
G
G
A
D
33
G
A
30
m G
C
45
50
pC
15
17a
18
10
4
70
19
m
40
60
57
s
7
U
Unpaired bases
3 consecutive
G
•C base pairs
Extra base,
no D
(green shading; below). [After Steitz, J.A., in Chambliss, G.,
Craven, G.R., Davies, J., Davis, K., Kahan, L., and Nomura, M.
(Eds.), Ribosomes. Structure, Function and Genetics, pp. 481–482,
University Park Press (1979).]
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1374
Shine–Dalgarno sequence and the 16S rRNA apparently per-
mit the ribosome to select the proper initiation codon. Thus
ribosomes with mutationally altered anti-Shine–Dalgarno
sequences often have greatly reduced ability to recognize
natural mRNAs, although they efficiently translate mRNAs
whose Shine–Dalgarno sequences have been made comple-
mentary to the altered anti-Shine–Dalgarno sequences.
Moreover,treatment of ribosomes with the bactericidal pro-
tein colicin E3 (produced by E. coli strains carrying the E3
plasmid), which specifically cleaves a 49-nucleotide frag-
ment from the 3¿ terminus of 16S rRNA, yields ribosomes
that cannot initiate new polypeptide synthesis but can com-
plete the synthesis of a previously initiated chain.
The X-ray structure of the 70S ribosome reveals, in
agreement with Fig. 32-30, that a 27-nt segment of the
mRNA is wrapped in a groove that encircles the neck of
the 30S subunit (Fig. 32-42). The mRNA codons in the A
and P sites are exposed on the interface side of the 30S sub-
unit (as in Fig. 32-34b), whereas its 5¿ and 3¿ ends are bound
in tunnels composed of RNA and protein. The mRNA’s
Shine–Dalgarno sequence, which is located near its 5¿ end,
is base paired, as expected, with the 16S RNA’s anti-
Shine–Dalgarno sequence, which is situated close to the E
site.The resulting double helical segment is accommodated
in a cleft formed by both RNA and protein elements of the
16S subunit’s head, neck, and platform (Fig. 32-26).
c. Prokaryotic Initiation Is a Three-Stage Process
That Requires the Participation of Soluble Protein
Initiation Factors
See Guided Exploration 28: Translational initiation Intact ribo-
somes do not directly bind mRNA so as to initiate polypep-
tide synthesis. Rather, initiation is a complex process in
which the two ribosomal subunits and fMet–tRNA
f
Met
as-
semble on a properly aligned mRNA to form a complex that
is competent to commence chain elongation. This assembly
process also requires the participation of protein initiation
factors that are not permanently associated with the ribo-
some. Initiation in E. coli involves three initiation factors
designated IF-1, IF-2, and IF-3 (Table 32-9). Their exis-
tence was discovered when it was found that washing small
ribosomal subunits with 1M ammonium chloride solution,
Section 32-3. Ribosomes and Polypeptide Synthesis 1375
Figure 32-42 Path of mRNA through the ribosomal 30S
subunit as viewed from its interface side. The 16S RNA is cyan,
and the 27-nt mRNA is represented in worm form with its
A- and P-site codons orange and red, the Shine–Dalgarno helix
(which includes a segment of 16S RNA) magenta, and its
remaining segments yellow. The S3, S4, and S5 proteins are
green, the S7, S11, and S12 proteins are purple, and the
remaining ribosomal proteins have been omitted for clarity. The
S3, S4, and S5 proteins, which in part form the tunnel through
which the mRNA enters the ribosome, may function as a helicase
to remove secondary structure from the mRNA that would
otherwise interfere with tRNA binding. [Courtesy of Gloria
Culver, Iowa State University. Based on an X-ray structure by
Harry Noller, University of California at Santa Cruz. PDBid
1JGO.]
Table 32-9 The Soluble Protein Factors of E. coli
Protein Synthesis
Number of
Factor Residues
a
Function
Initiation Factors
IF-1 71 Assists IF-3 binding
IF-2 890 Binds initiator
tRNA and GTP
IF-3 180 Releases mRNA and
tRNA from recycled
30S subunit and aids
new mRNA binding
Elongation Factors
EF-Tu 393 Binds aminoacyl–tRNA
and GTP
EF-Ts 282 Displaces GDP from
EF-Tu
EF-G 703 Promotes translocation
through GTP binding
and hydrolysis
Release Factors
RF-1 360 Recognizes UAA and
UAG Stop codons
RF-2 365 Recognizes UAA and
UGA Stop codons
RF-3 528 Stimulates RF-1/RF-2
release via GTP
hydrolysis
RRF 185 Together with EF-G,
induces ribosomal
dissociation to small
and large subunits
a
All E. coli translational factors are monomeric proteins.
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1375
1
+
IF-1
30S subunit
2
IF-2
GTP
IF-2
GTP
+
3′5′
IF-2
•
GTP
•
fMet – tRNA
f
Met
IF-2
GDP
AUG
5′ 3′
AUG
Shine–Dalgarno
sequence
30S Initiation complex
50S subunit
AUG
A site
P site
5′ 3′
70S Initiation complex
IF-1
3
+ P
i
IF-3
IF-3
IF-1
IF-3
IF-3
+
fMet
fMet
fMet
mRNA
which removes the initiation factors but not the “perma-
nent” ribosomal proteins, prevents initiation.
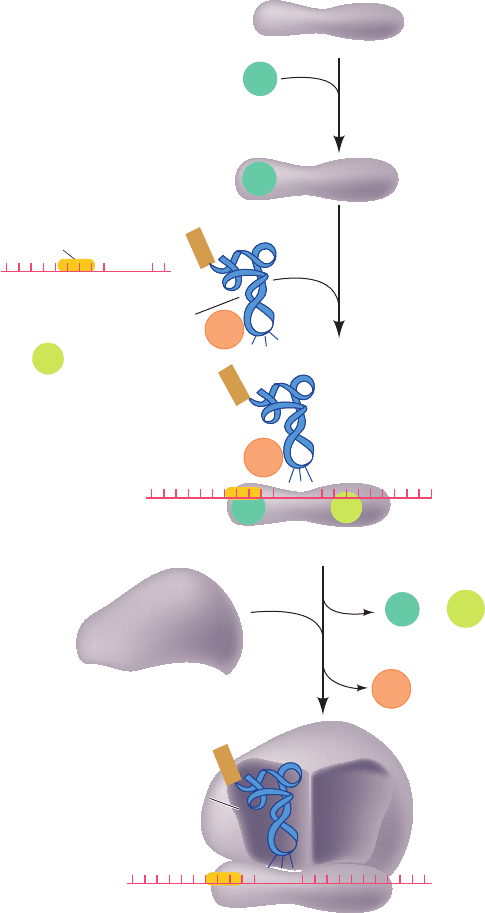
The initiation sequence in E. coli ribosomes has three
stages (Fig. 32-43):
1. On completing a cycle of polypeptide synthesis, the
30S and 50S subunits are separated (Section 32-3Fa). IF-3
then binds to the 30S subunit so as to prevent the reassocia-
tion of the 50S subunit. The X-ray structure of the 30S sub-
unit in complex with the C-terminal domain of IF-3 (which
by itself prevents the association of the 30S and 50S sub-
units), determined by Yonath and François Franceschi, indi-
cates that IF-3 binds to the upper end of the platform (Fig.
32-26) on its solvent (back) side. Hence IF-3 does not func-
tion by physically blocking the binding of the 50S subunit.
2. mRNA and IF-2 in a ternary complex with GTP and
that is accompanied by IF-1 subsequently
bind to the 30S subunit in either order. Hence,
recognition must not be mediated by a
codon–anticodon interaction. This interaction, neverthe-
less, helps bind to the ribosome. IF-1 binds
in the A site where it may function to prevent the inappro-
priate or premature binding of a tRNA. IF-3 also functions
in this stage of the initiation process: it destabilizes the
binding of tRNAs that lack the three G C pairs in the an-
ticodon stem of (Fig. 32-40) and helps discrimi-
nate between matched and mismatched codon–anticodon
interactions.
3. Last, in a process that is preceded by IF-1 and IF-3
release, the 50S subunit joins the 30S initiation complex in
a manner that stimulates IF-2 to hydrolyze its bound GTP
to GDP P
i
. This irreversible reaction conformationally
rearranges the 30S subunit and releases IF-2 for participa-
tion in further initiation reactions.
IF-2 is a member of the superfamily of regulatory GTPases
such as Ras and hence is a G protein (Section 19-2A). The
30S initiation complex therefore functions as its GAP
(GTPase-activating protein; Section 19-2Ca).
Initiation results in the formation of an
mRNA ribosome complex in which the occu-
pies the ribosome’s P site while its A site is poised to accept an
incoming aminoacyl–tRNA (an arrangement similar to that
at the conclusion of a round of elongation; Section 32-3D).
In fact, is the only tRNA that directly enters the P
site. All other tRNAs must do so via the A site during chain
elongation (Section 32-3D). This arrangement was estab-
lished through the use of the antibiotic puromycin as is dis-
cussed in Section 32-3Df.
d. Eukaryotic Initiation Is Far More Complicated
than That of Prokaryotes
Although translational initiation in eukaryotes superfi-
cially resembles that in prokaryotes, it is, in fact, a far more
complicated process. Whereas prokaryotic initiation only
requires the assistance of three monomeric initiation fac-
tors, that in eukaryotes involves the participation of at least
12 initiation factors (designated eIFn; “e” for eukaryotic)
that consist of at least 26 polypeptide chains. Eukaryotic
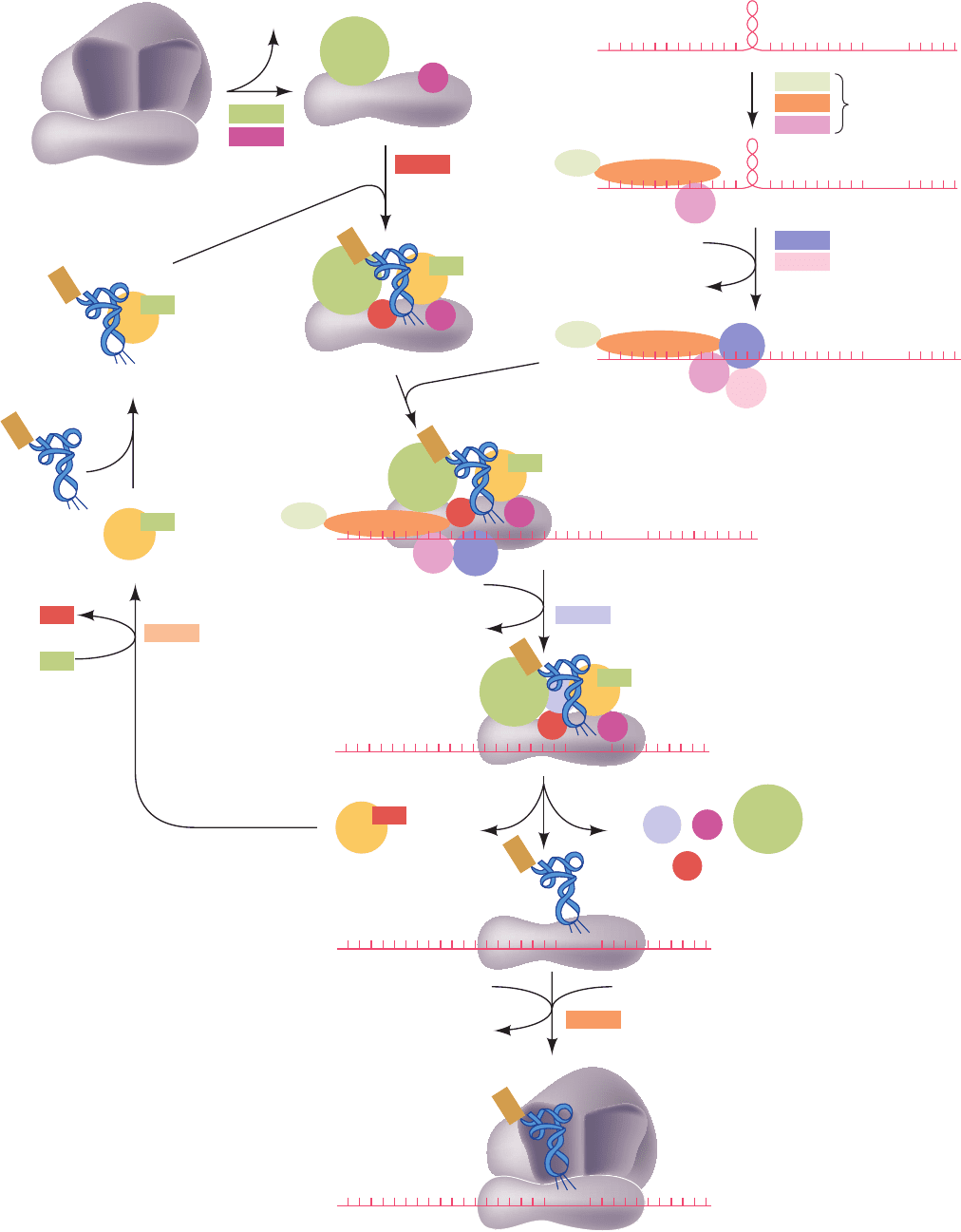
initiation occurs as follows (Fig. 32-44):
1. The process begins with the binding of eIF3 (which in
mammals consists of 13 different subunits) and eIF1A (a
monomer and homolog of bacterial IF-1) to the 40S sub-
unit in the inactive 80S ribosome (which had terminated
elongation in its previous elongation cycle) so that it re-
leases the 60S subunit.
2. The ternary complex of eIF2 (a heterotrimer), GTP,
and binds to the 40S ribosomal subunit ac-
companied by eIF1 (a monomer) to form the so-called 43S
Met–tRNA
Met
i
tRNA
Met
f
fMet–tRNA
Met
f
fMet–tRNA
Met
f
RNA
Met
f
fMet–tRNA
Met
f
fMet–tRNA
Met
f
fMet–tRNA
Met
f
1376 Chapter 32. Translation
Figure 32-43 Translational initiation pathway in E. coli. The
E site, which is unoccupied during this process, has been omitted
for clarity.
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1376
Section 32-3. Ribosomes and Polypeptide Synthesis 1377
80S Initiation complex
1
3
eIF1A
1A
2
Inactive 80S ribosome
60S
A
Site
P
Site
60S
40S
Met – tRNA
i
Met
43S Preinitiation complex
48S Preinitiation complex
mRNA
3
5
2
9
6
7
3
2
eIF1
1A
1
1
1
elF2B
elF5
elF5B
GTP
2
GTP
GDP
GDP
GTP
3
2
1A
1A
1A
5
5
4A
4G
4A
4H
4A
4G
4G
4E
4E
4E
4B
+ P
i
m
7
G
m
7
G
4B
4B
GTP
4
elF4H
elF4B
elF4A
elF4G elF4F
elF4E
ATP
ADP + P
i
ATP
ADP + P
i
8
GTP 60S subunit
P
i
+ GDP
2
3
3
2
GTP
m
7
G
m
7
G
m
7
G
eIF3
1
AUG
m
7
G
AUG
m
7
G
AUG
AUG
AUG
AUG
AUG
Met
Met
Met
Met
Met
GTP
Met
Met
2
Figure 32-44 Translational initiation
pathway in eukaryotes. Initiation factors
are represented by colored rectangles
where they are first implicated in the
pathway and by circles of the same color
thereafter.The higher order complexes
are hypothetical. Several of the initiation
factors (4A, 4B, 4E, 4G, and 4H) have
been left out of the 48S preinitiation
complex for clarity. [Based on a drawing
by Hershey, J.W.B. and Merrick,W.C., in
Sonenberg, N., Hershey, J.W.B., and
Mathews, M.B. (Eds.), Translational
Control of Gene Expression, Cold Spring
Harbor Laboratory Press (2000).]
JWCL281_c32_1338-1428.qxd 8/5/10 3:16 PM Page 1377
preinitiation complex. Here the subscript “i” on
distinguishes this eukaryotic initiator tRNA, whose ap-
pended Met residue is never N-formylated, from that of
prokaryotes; both species are, nevertheless, readily inter-
changeable in vitro.
3. Eukaryotic mRNAs lack the complementary se-
quences to bind to the 18S rRNA in the Shine–Dalgarno
manner. Rather, they have an entirely different mechanism
for recognizing the mRNA’s initiating AUG codon. Eukary-
otic mRNAs, nearly all of which have an m
7
G cap and a
poly(A) tail (Section 31-4Ab), are invariably monocistronic
and almost always initiate translation at their leading AUG.
This AUG, which occurs at the end of a 5¿-untranslated re-
gion of 50 to 70 nt, is embedded in the consensus sequence
GCCRCCAUGG, with changes in the purine (R) 3 nt be-
fore the AUG and the G immediately following it reducing
translational efficiency by ⬃10-fold each and with other
changes having much smaller effects. In addition, secondary
structure (stem–loops) in the mRNA upstream of the initi-
ation site may affect initiation efficiency.
The recognition of the initiation site begins by the bind-
ing of eIF4F to the m
7
G cap. eIF4F is a heterotrimeric
complex of eIF4E, eIF4G, and eIF4A (all monomers), in
tRNA
Met
i
which eIF4E (cap-binding protein) recognizes the
mRNA’s m
7
G cap and eIF4G serves as a scaffold to join
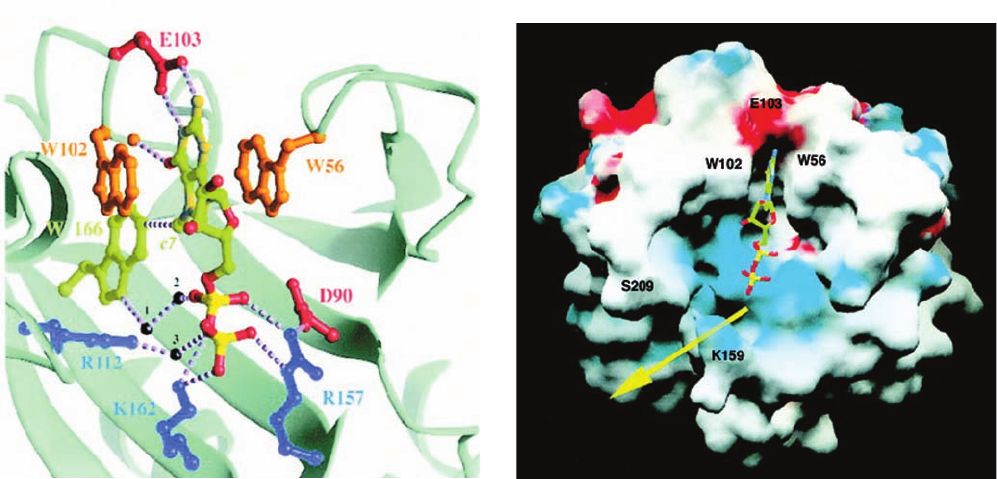
eIF4E with eIF4A. Both the X-ray and NMR structures of
eIF4E in complex with m
7
GDP, determined by Nahum
Sonenberg and Stephen Burley and by Sonenberg and
Gerhard Wagner, reveal that the protein binds the m
7
G
base by intercalating it between two highly conserved
Trp residues (Fig. 32-45a) in a region that is adjacent to a
positively charged cleft that forms the putative mRNA-
binding site (Fig. 32-45b). The m
7
G base is specifically rec-
ognized by hydrogen bonding to protein side chains in a
manner reminiscent of G C base pairing. eIF4G also
binds poly(A)-binding protein (PABP; Section 31-4Ab)
bound to the mRNA’s poly(A) tail, thereby circularizing
the mRNA (not shown in Fig. 32-44). Although this ex-
plains the synergism between an mRNA’s m
7
A cap and its
poly(A) tail in stimulating translational initiation, the
function of this circle is unclear. However, an attractive
hypothesis is that it enables a ribosome that has finished
translating the mRNA to reinitiate translation without
having to disassemble and then reassemble. Another pos-
sibility is that it prevents the translation of incomplete
(broken) mRNAs. This circularization, as we have seen in
Section 31-4Av, also protects the mRNA from degradation
1378 Chapter 32. Translation
Figure 32-45 X-ray structure of murine eIF4E in complex
with the m
7
G cap analog m
7
GDP. (a) The m
7
GDP-binding site
with the m
7
GDP and the side chains that bind it drawn in ball-
and-stick form with the atoms of the m
7
GDP colored according
to type (C green, N dark yellow, O red, and P bright yellow) and
the protein side chains with which the m
7
GDP interacts drawn in
various colors. Hydrogen bonds, salt bridges, and van der Waals
interactions are represented by dashed lines and bridging water
molecules are drawn as black spheres.The m
7
G base is
intercalated between the indole rings of Trp 56 and Trp 102,
where it specifically interacts with protein side chains through
hydrogen bonds and van der Waals interactions. The GDP’s
phosphate groups interact directly and indirectly with three basic
side chains. (b) The solvent-accessible surface of eIF4E colored
according to its electrostatic potential (red negative, blue
positive, and white neutral) and viewed approximately as in Part
a. The m
7
GDP is drawn in ball-and-stick form colored as in Part
a. The mRNA presumably binds in the positively charged cleft
(yellow arrow) that is adjacent to the m
7
G binding site and which
passes between Lys 159 and Ser 209. [Courtesy of Nahum
Sonenberg, McGill University, Montréal, Québec, Canada.
PDBid 1EJ1.]
(a)
(b)
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1378

by preventing the action of decapping enzyme until the
mRNA’s poly(A) tail has been shortened to the point that
it can no longer bind PABP.
4. eIF4B (an RRM-containing homodimer) and eIF4H
(a monomer) join the eIF4F–mRNA complex where they
stimulate the RNA helicase activity of eIF4A to unwind
the mRNA’s helical segments in an ATP-dependent
process. This presumably also strips away the proteins that
are bound to the mRNA (Section 31-4Au). eIF4A is the
prototype of the so-called DEAD-box family of proteins
(also known as DExD/H family proteins; Section 31-4Au),
which is named after one of the sequence motifs shared by
the diverse members of this family, all of which have
NTPase activity.
5. The eIF4F–mRNA–eIF4B–eIF4H complex joins the
43S preinitiation complex through a protein–protein inter-
action between eIF4G and the 40S subunit-bound eIF3.
This differs substantially from the corresponding prokary-
otic process (Fig. 32-43) in which the mRNA is bound to
the 30S ribosomal subunit via associations between RNA
molecules (involving the Shine–Dalgarno sequence and
the codon–anticodon interaction).
6. eIF5 (a monomer) joins the growing assembly. The
43S preinitiation complex then translocates along the
mRNA, an ATP-dependent process called scanning, until it
encounters the mRNA’s AUG initiation codon, which is
optimally in the sequence GCC(A/G)CCAUGG. This
yields the 48S preinitiation complex. The recognition of the
AUG occurs mainly through base pairing with the CUA
anticodon on the bound , as was demon-
strated by the observation that mutating this anticodon re-
sults in the recognition of the new cognate codon instead of
AUG. This explains why the initiator tRNA must bind to
the small subunit before the mRNA.
7. The formation of the 48S preinitiation complex in-
duces eIF2 to hydrolyze its bound GTP to GDP P
i
, which
results in the release of all the initiation factors, thereby
leaving the in the small subunit’s P site. The
hydrolysis reaction is stimulated by eIF5, acting as a GAP
(Section 19-2Ca).
8. The 60S subunit then joins the mRNA-bound
–40S subunit complex in a GTPase reac-
tion mediated by eIF5B (a monomer and homolog of
bacterial IF-2), thereby yielding the 80S ribosomal initia-
tion complex. Thus eukaryotic translation initiation con-
sumes two GTPs versus one for prokaryotic initiation
(Fig. 32-43).
9. What remains is to recycle the eIF2 GDP complex
by exchanging its GDP for GTP. This reaction is mediated
by eIF2B (a heteropentamer), which therefore functions as
eIF2’s GEF (guanine nucleotide exchange factor; Section
19-2Ca).
Many eukaryotic initiation factors are subject to phospho-
rylation/dephosphorylation and are therefore likely to par-
Met–tRNA
Met
i
Met–tRNA
Met
i
Met–tRNA
Met
i
ticipate in the control of eukaryotic translation, a subject
we discuss in Section 32-4.
Although the initiation sites on most eukaryotic
mRNAs are identified by the above-described scanning
mechanism, a few mRNAs have an internal ribosome entry
site (IRES) to which the 40S subunit can directly bind in a
process reminiscent of prokaryotic initiation. However, lit-
tle is yet known about the mechanism of IRES-based ini-
tiation. Indeed, IRESs lack clearly identifiable consensus
sequences.
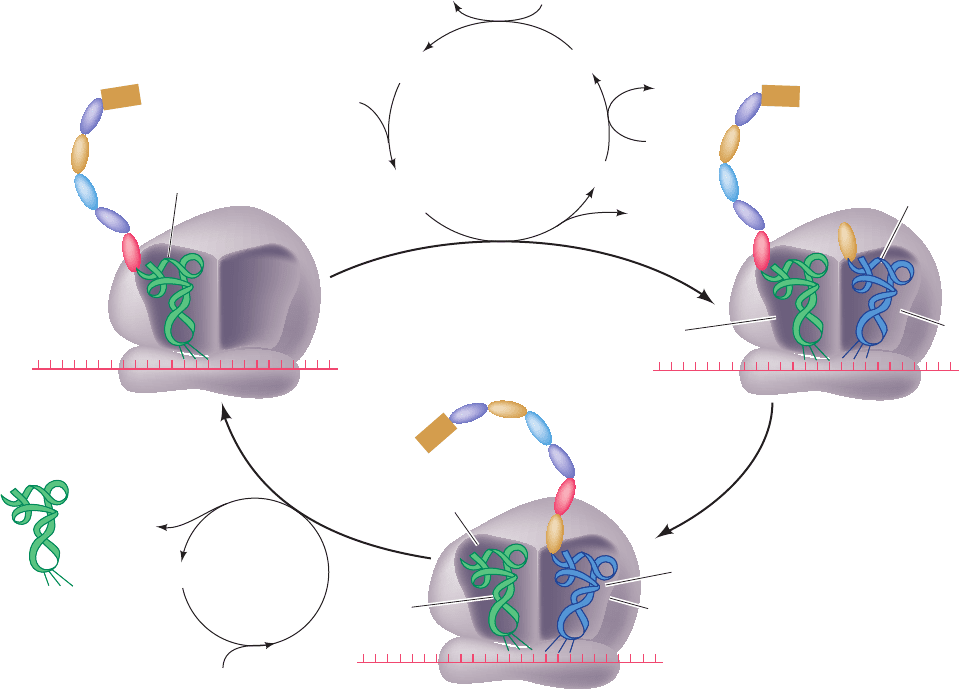
D. Chain Elongation
See Guided Exploration 29: Translational elongation Ribo-
somes elongate polypeptide chains in a three-stage reaction
cycle that adds amino acid residues to a growing polypep-
tide’s C-terminus (Fig. 32-46):
1. Decoding, in which the ribosome selects and binds an
aminoacyl–tRNA, whose anticodon is complementary to
the mRNA codon in the A site.
2. Transpeptidation, in which the peptidyl group on the
P-site tRNA is transferred to the aminoacyl group in the A
site through the formation of a peptide bond (Fig. 32-39).
3. Translocation, in which A-site and P-site tRNAs are
respectively transferred to the P site and E site accompa-
nied by their bound mRNA; that is, the mRNA, together
with its base paired tRNAs, is ratcheted through the ribo-
some by one codon.
Translational elongation, which occurs at a rate of 10 to 20
residues/s, involves the participation of several nonriboso-
mal proteins known as elongation factors (Table 32-9). We
describe these processes in the following paragraphs.
a. Decoding
In the decoding stage of the E. coli elongation cycle, a
binary complex of GTP with the elongation factor EF-Tu
(also called EF1A) combines with an aminoacyl–tRNA.The
resulting ternary complex binds to the ribosome, and,in a re-
action that hydrolyzes the GTP to GDP P
i
, the amino-
acyl–tRNA is bound in a codon–anticodon complex to the
ribosomal A site and EF-Tu GDP P
i
is released. In the
remainder of this stage, the bound GDP is replaced by GTP
in a reaction mediated the elongation factor EF-Ts (also
called EF1B). EF-Tu,as are several other GTP-binding ribo-
somal factors, is a G-protein, and hence the ribosome func-
tions as its GAP and EF-Ts is its GEF.
Aminoacyl–tRNAs can bind to the ribosomal A site
without the mediation of EF-Tu but at a rate too slow to
support cell growth.The importance of EF-Tu is indicated
by the fact that it is the most abundant E. coli protein; it is
present in ⬃100,000 copies per cell (5% of the cell’s
protein), which is approximately the number of tRNA
molecules in the cell. Consequently, the cell’s entire com-
plement of aminoacyl–tRNAs is essentially sequestered by
EF-Tu.
Section 32-3. Ribosomes and Polypeptide Synthesis 1379
JWCL281_c32_1338-1428.qxd 8/19/10 10:05 PM Page 1379
b. EF-Tu Is Sterically Prevented from Binding
Initiator tRNA
The X-ray structure of the Phe–tRNA
Phe
EF-Tu
GMPPNP ternary complex (GMPPNP is a nonhydrolyz-
able GTP analog; Section 19-3Ch), determined by Brian
Clark and Jens Nyborg, reveals that these two macromole-
cules associate to form a corkscrew-shaped complex in
which the EF-Tu and the tRNA’s acceptor stem form a
knoblike handle and the tRNA’s anticodon helix forms the
screw (Fig. 32-47). The conformation of the tRNA
Phe
closely resembles that of the uncomplexed molecule (Fig.
32-11b). The EF-Tu folds into three distinct domains that
are connected by flexible peptides, rather like beads on a
string. The N-terminal domain 1, which binds guanine nu-
cleotides and catalyzes GTP hydrolysis, structurally resem-
bles other known G-proteins.
The two macromolecules associate rather tenuously via
three major regions: (1) the CCA—Phe segment at the 3¿
end of the Phe–tRNA
Phe
binds in a cleft between EF-Tu’s
domains 1 and 2 (the blue and green mainly helical domain
and the yellow sheet domain in Fig. 32-47) that ends in a
pocket large enough to accommodate all amino acid
residues; (2) the 5¿-phosphate of the tRNA binds in a de-
pression at the junction of EF-Tu’s three domains; and (3)
one side of the tRNA’s TC stem contacts the exposed
main chain and side chains of EF-Tu’s C-terminal domain 3
(the orange and red barrel–containing domain in Fig. 32-
47). The tight association of the aminoacyl group with EF-
Tu appears to greatly increase the affinity of EF-Tu for the
otherwise loosely bound tRNA, which explains why EF-Tu
does not bind uncharged elongator tRNAs.
EF-Tu binds neither formylated aminoacyl–tRNAs nor
unformylated , which is why the initiator
tRNA never reads internal AUG or GUG codons.The first
base pair of is mismatched (C A; Fig. 32-40) and
hence this initiator tRNA has a 3¿ overhang of 5 nt vs 4 nt
for an elongator tRNA. It seems likely that this mismatch,
together with the formyl group, prevents
from binding to EF-Tu. Indeed, EF-Tu binds to E. coli
whose 5¿-terminal C residue has been deaminatedtRNA
Met
f
fMet–tRNA
Met
f
tRNA
Met
f
Met–tRNA
Met
f
1380 Chapter 32. Translation
Figure 32-46 Elongation cycle in E. coli ribosomes. The E
site, to which discharged tRNAs are transferred before being
released to solution, is not shown. Eukaryotic elongation follows
EF-G
5′ 3′
5′
5′ 3′
Nascent
polypeptide
A site
Empty
3
Translocation
GTP
+ GDP + P
i
tRNA
mRNA
Peptidyl–tRNA
2
Transpeptidation
P site
Decoding
1
A site
3
′
Aminoacyl–
tRNA
P
i
EF-Tu • GDP
EF-Tu
• EF-Ts
GTPEF-Ts
EF-Tu • GTP
Aminoacyl–tRNA
Aminoacyl–tRNA
•
EF-Tu
• GTP
GDP
EF-Ts
P site
A site
Peptidyl–tRNA
fMet
fMet
fMet
Uncharged
tRNA
a similar cycle but EF-Tu and EF-Ts are replaced by a single
multisubunit protein, eEF1, and EF-G is replaced by eEF2.
JWCL281_c32_1338-1428.qxd 8/4/10 4:45 PM Page 1380
