Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

participate in ATP binding and are implicated in catalysis.
The Class II synthetases lack the foregoing sequences but
have three other sequences in common. Their X-ray struc-
tures reveal that these sequences occur in a so-called signa-
ture motif, a fold found only in Class II enzymes that con-
sists of a 7-stranded antiparallel sheet with three flanking
helices, which forms the core of their catalytic domains.
Many Class I aaRSs require anticodon recognition to
aminoacylate their cognate tRNAs. In contrast, several
Class II enzymes, including AlaRS and SerRS, do not inter-
act with their bound tRNA’s anticodon. Indeed, several
class II aaRSs accurately aminoacylate “microhelices” de-
rived from only the acceptor stems of their cognate tRNAs.
Another difference between Class I and Class II syn-
thetases is that all Class I enzymes aminoacylate their
bound tRNA’s 3¿-terminal 2¿-OH group, whereas Class II
enzymes, with the exception of PheRS, all charge the 3¿-
OH group. The amino acids for which the Class I syn-
thetases are specific tend to be larger and more hydropho-
bic than those used by Class II synthetases. Finally, as Table
32-4 indicates, Class I aaRSs are mainly monomers,
whereas most Class II aaRSs are homodimers.
LysRS has been classified as a Class II aaRS. However, a
search of the genome sequences of Methanococcus jan-
naschii and Methanobacterium thermoautotrophicum failed
to reveal the presence of such a LysRS.This led to the discov-
ery that the LysRSs expressed by these archaebacteria are
Class I rather than Class II enzymes. This raises the interest-
ing question of how Class I LysRS evolved.
Prokaryotic aaRSs occur as individual protein molecules.
However, in many higher eukaryotes (e.g., Drosophila and
mammals), 9 aaRSs, some of each class, associate to form a
multienzyme particle in which the glutamyl and prolyl syn-
thetase functions are fused into a single polypeptide named
GluProRS. The advantages of these systems are unknown.
b. The Structural Features Recognized by
Aminoacyl–tRNA Synthetases May Be Quite Simple
As we shall see in Section 32-2D, ribosomes select
aminoacyl–tRNAs only via codon–anticodon interactions,
not according to the identities of their aminoacyl groups.
Accurate translation therefore requires not only that each
tRNA be aminoacylated by its cognate aaRS but that it not
be aminoacylated by any of its 19 noncognate aaRSs. More-
over, since most cells express only one aaRS for each
amino acid, each aaRS must aminoacylate all of the sev-
eral, if not many, isoaccepting tRNAs (different tRNAs
that are specific for the same amino acid) in each cell.
Considerable effort has therefore been expended, no-
tably by LaDonne Schulman, Paul Schimmel, Olke Uhlen-
beck, and John Abelson, in elucidating how aaRSs manage
this feat, despite the close structural similarities of nearly
all tRNAs. The experimental methods employed involved
the use of specific tRNA fragments, mutationally altered
tRNAs, chemical cross-linking agents, computerized se-
quence comparisons, and X-ray crystallography. The most
common synthetase contact sites on tRNA occur on the in-
ner (concave) face of the L. Other than that, there appears
to be little regularity in how the various tRNAs are recog-
nized by their cognate synthetases. Indeed, as we shall see,
some aaRSs recognize only their cognate tRNA’s acceptor
stem, whereas others also interact with its anticodon re-
gion.Additional tRNA regions may also be recognized.
Genetic manipulations by Schimmel revealed that the
tRNA features recognized by at least one type of aaRS are
surprisingly simple. Numerous sequence alterations of E.
coli tRNA
Ala
do not appreciably affect its capacity to be
aminoacylated with alanine. Yet, most base substitutions in
the G3 U70 base pair located in the tRNA’s acceptor stem
(Fig. 32-14a) greatly diminish this reaction. Moreover,
the introduction of a G U base pair into the analogous
Section 32-2. Transfer RNA and Its Aminoacylation 1351
Figure 32-14 Major identity elements in four tRNAs. Each
base in the tRNA is represented by a filled circle. Red circles
indicate positions that have been shown to be identity elements
for the recognition of the tRNA by its cognate aminoacyl–tRNA
synthetase. The anticodon bases that are identity elements are
(b)
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•••••
•••••
•
•
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•
•
•
•
••••
••••
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
tRNA
Asp
(yeast)
G73
U35
C36
U25
G3
tRNA
Ala
(a)
G2
G1
•
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•••••
•••••
•
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•
•
•
•
•
••••
••••
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
(E. coli
)
U70
C71
C72
A73
G20
•
•
•
•
•
•
• •
• •
• •
• •
• •
•••••
•••••
•
•
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•
•
•
•
•
••
••
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
(d) tRNA
Ser
G24
C11
G2
C71
UA70
C72
G73
G1
AU3
•
•
•
•
•
•
•
•
•
•
•
••
G10
U35
m
1
G37
C38
G34
•
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•••••
•••••
•
•
•
•
•
•
•
•
• •
• •
• •
• •
• •
•
•
•
•
•
•••
•••
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
(E. coli
)tRNA
Gln
(c)
G36
C34
ψ38
m
2
A37
Um32
U33
G73
G2
G3
C71
C70
•
C25
G10
underlined. In each case, additional identity elements may yet
be discovered.The base at position 73, which is an identity
element in all four tRNAs shown here, is known as the
discriminator base.
JWCL281_c32_1338-1428.qxd 9/7/10 2:22 PM Page 1351
1352 Chapter 32. Translation
ever,this altered tRNA
Ile
is also a much better substrate for
MetRS than it is for IleRS. Thus, both the codon and the
amino acid specificity of this tRNA are changed by a single
post-transcriptional modification. The N
1
-methylation of
G37 in yeast tRNA
Asp
(Fig. 32-14b) provides another ex-
ample of a base modification forming an identity element.
In the absence of this N
1
-methyl group, tRNA
Asp
is recog-
nized by ArgRS, largely via its C36 and G37, whereas
ArgRS normally recognizes only tRNA
Arg
, mainly via its
C35 and U36.
The available experimental evidence has largely located
the various tRNA identifiers in the acceptor stem and the an-
ticodon loop (Fig. 32-16). The X-ray structures of several
aaRS tRNA complexes, which we consider next, have
structurally rationalized some of these observations.
c. The X-Ray Structure of GlnRS tRNA
Gln
, a
Class I Complex
The X-ray structures of all 20 different amino acid–
specific aaRSs from numerous organisms have been deter-
mined, many of which are in complex with ATP, their cog-
nate amino acids, or their analogs. These structures reveal
that the active sites of these enzymes bind the ATP and
target amino acid in optimal positions for in-line nucle-
ophilic displacement (Section 16-2B) during amino acid
position of tRNA
Cys
and tRNA
Phe
causes them to be
aminoacylated with alanine even though there are few
other sequence identities between these mutant tRNAs and
tRNA
Ala
(e.g., Fig. 32-15). In fact, E. coli AlaRS even effi-
ciently aminoacylates a 24-nt “microhelix” derived from
only the G3 U70–containing acceptor stem of E. coli
tRNA
Ala
. Since the only E. coli tRNAs that normally have
a G3 U70 base pair are the tRNA
Ala
, and this base pair is
also present in the tRNA
Ala
from many organisms including
yeast (Fig. 32-8), the foregoing observations strongly sug-
gest that the G3 U70 base pair is a major feature recognized
by AlaRSs. These enzymes presumably recognize the distorted
shape of the G U base pair (Fig. 32-12), a hypothesis corrob-
orated by the observation that base changes at G3 U70 which
least affect the acceptor identity of tRNA
Ala
yield base pairs
that structurally resemble G U.
The elements of three other tRNAs, which are recog-
nized by their cognate tRNA synthetases, are indicated in
Fig. 32-14.As with tRNA
Ala
, these identity elements appear
to comprise only a few bases. Note that the anticodon forms
an identity element in two of these tRNAs. In another
example of an anticodon identifier, the E. coli tRNA
Ile
spe-
cific for the codon AUA has the anticodon LAU, where L is
lysidine, a modified cytosine whose 2-keto group is re-
placed by the amino acid lysine (Fig. 32-10). The L in this
context pairs with A rather than G, a rare instance of base
modification altering base pairing specificity. The replace-
ment of this L with unmodified C, as expected, yields a
tRNA that recognizes the Met codon AUG (codons bind
anticodons in an antiparallel fashion). Surprisingly, how-
Figure 32-15 Three-dimensional model of E. coli tRNA
Ala
.
This model is based on the X-ray structure of yeast tRNA
Phe
(Fig.
32-11b) in which the nucleotides that are different in E. coli
tRNA
Cys
are highlighted in cyan and the G3 U70 base pair is
highlighted in ivory. [Courtesy of Ya-Ming Hou, MIT.]
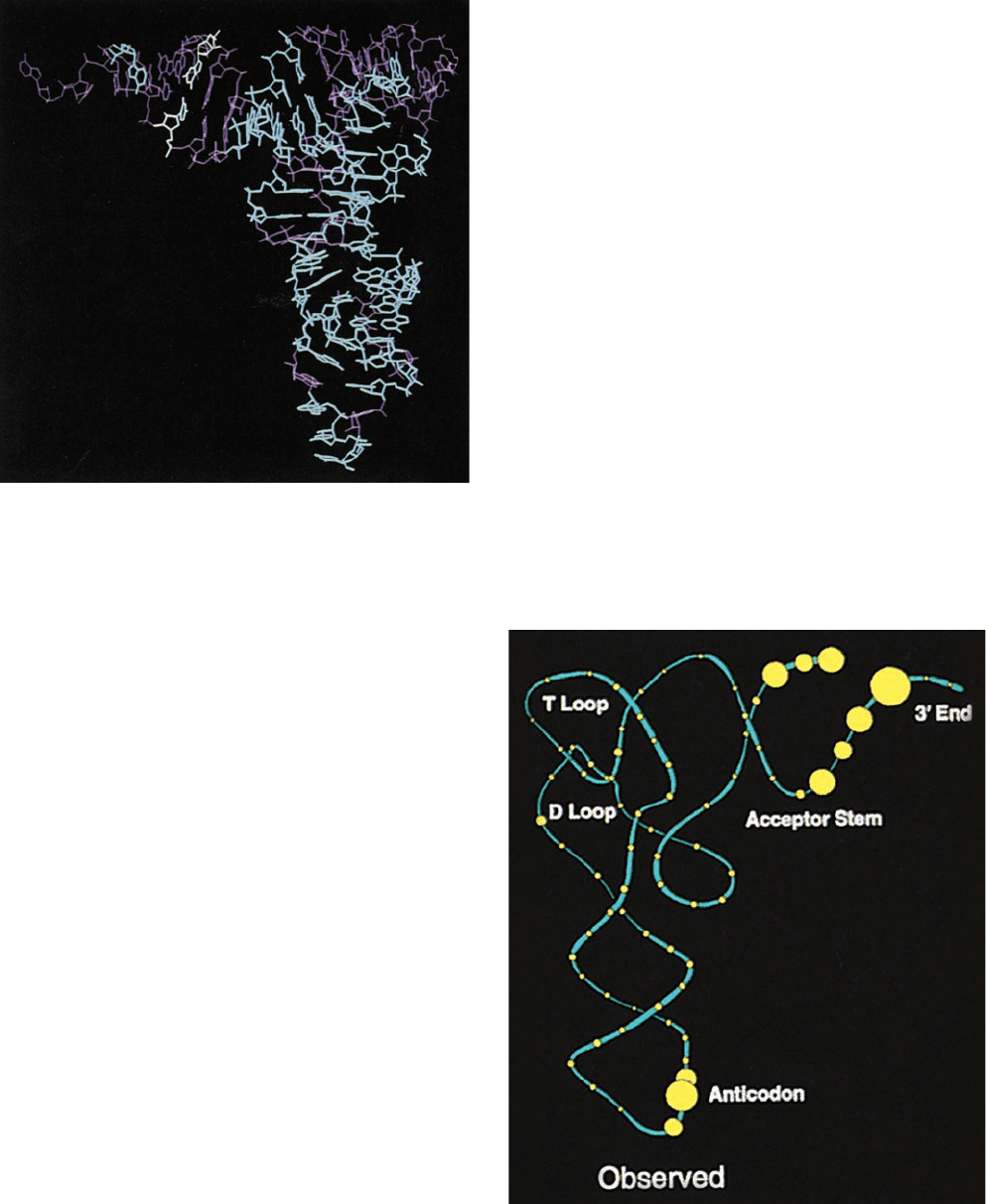
Figure 32-16 Experimentally observed identity elements of
tRNAs. The tRNA backbone is cyan and each of its nucleotides
is represented by a yellow circle whose diameter is proportional
to the fraction of the 20 tRNA acceptor types for which the
nucleotide is an observed determinant. [Courtesy of William
McClain, University of Wisconsin.]
JWCL281_c32_1338-1428.qxd 8/19/10 10:05 PM Page 1352
(a)
activation and that the specificity of an aaRS for its target
amino acid is determined by idiosyncratic contacts with
the side chain of the amino acid.
The X-ray structures of 16 different aaRSs in their com-
plexes with their cognate tRNAs (all but those of Gly,Ala,
Lys, and His) have so far been reported.The first of them to
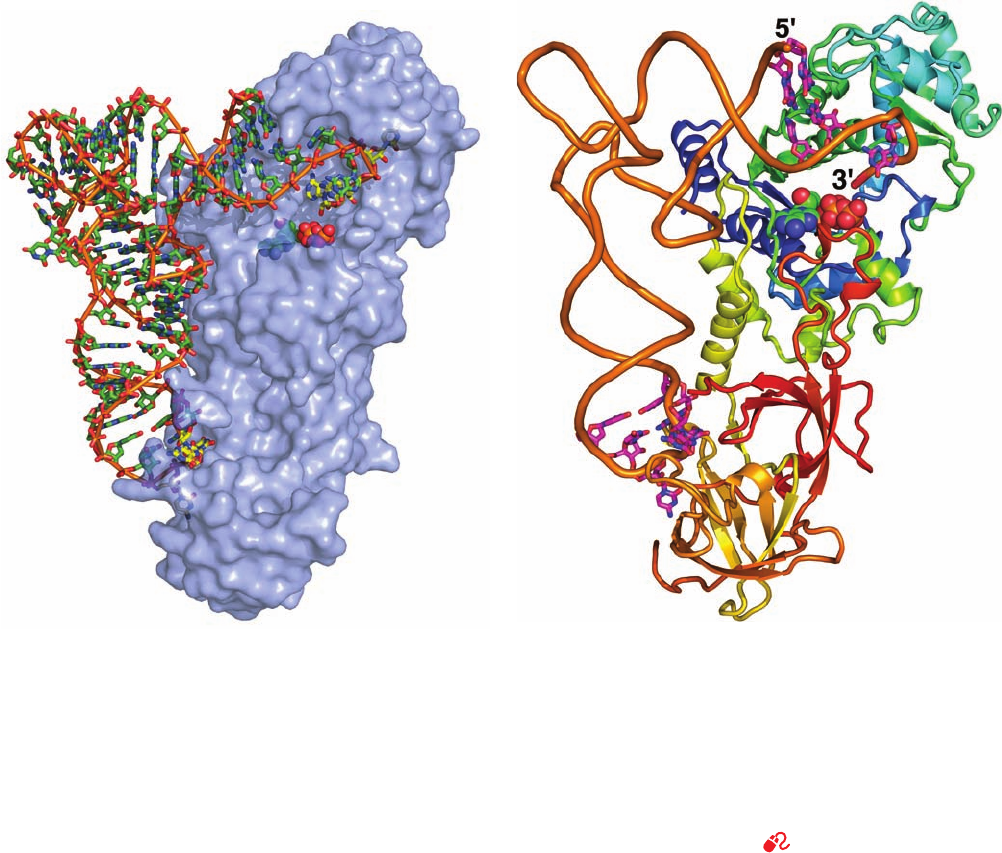
be elucidated, that of E. coli GlnRS, a Class I synthetase, in
its complex with tRNA
Gln
and ATP (Fig. 32-17), was deter-
mined by Thomas Steitz. The tRNA
Gln
assumes an L-
shaped conformation that resembles those of tRNAs of
known structures (e.g., Fig. 32-11b). GlnRS, a 553-residue
monomeric protein that consists of four domains arranged
to form an elongated molecule, interacts with the tRNA
along the entire inside face of the L such that the anticodon
is bound near one end of the protein and the acceptor stem
is bound near its other end.
Genetic and biochemical data indicate that the identity
elements of tRNA
Gln
are largely clustered in its anticodon
loop and acceptor stem (Fig. 32-14c).The anticodon loop of
tRNA
Gln
is extended by two novel non-Watson–Crick base
pairs (2¿-O-methyl-U32 ⴢ 38 and U33 ⴢ m
2
A37), thereby
causing the bases of the anticodon to unstack and splay
outward in different directions so as to bind in separate
recognition pockets of GlnRS. These structural features
suggest that GlnRS uses all seven bases of the anticodon
loop to discriminate among tRNAs. Indeed, changes to any
one of the bases of residues C34 through 38 yield tRNAs
with decreases in k
cat
/K
M
for aminoacylation by GlnRS by
factors ranging from 70 to 28,000.
The GCCA at the 3¿ end of the tRNA
Gln
makes a hair-
pin turn toward the inside of the L rather than continuing
helically onward (as does the ACCA at the 3¿ end in the X-
ray structure of tRNA
Phe
; Fig. 32-11b). This conformation
change is facilitated by the insinuation of a Leu side chain
between the 5¿ and 3¿ ends of the tRNA so as to disrupt the
first base pair of the acceptor stem (U1 ⴢ A72).The GlnRS
reaction is therefore relatively insensitive to base changes
in these latter two positions except when base pairing is
strengthened by their conversion to G1 ⴢ C72. The GCCA
end of the tRNA
Gln
plunges deeply into a protein pocket
Section 32-2. Transfer RNA and Its Aminoacylation 1353
Figure 32-17 X-ray structure of E. coli GlnRS ⴢ tRNA
Gln
ⴢ
ATP. (a) The tRNA is drawn in stick form colored as the ATP
but with the C atoms of the anticodon (UCG) and the 3¿-CCA
end yellow. An orange rod links its successive P atoms. The ATP
bound in the protein’s active site is drawn in space-filling form
with C green, N blue, O red, and P orange. The protein is
represented by a semitransparent light blue surface diagram that
reveals the buried portions of the tRNA and ATP. Note that both
the 3¿ end of the tRNA (top right) and its anticodon bases
(b)
(bottom) are inserted into deep pockets in the protein. (b) The
complex viewed as in Part a.The tRNA’s sugar–phosphate
backbone is represented by an orange worm and the bases
forming its identity elements (Fig. 32-14c) are drawn in stick form
colored according to atom type with C magenta, N blue, and O
red.The ATP is drawn as in Part a. The protein is shown in ribbon
form colored in rainbow order from its N-terminus (blue) to its
C-terminus (red). [Based on an X-ray structure by Thomas Steitz,
Yale University. PDBid 1GTR.]
See Kinemage Exercise 20
JWCL281_c32_1338-1428.qxd 10/27/10 1:40 PM Page 1353
1354 Chapter 32. Translation
that also binds the enzyme’s ATP and glutamine substrates.
Three protein “fingers” are inserted into the minor groove
of the acceptor stem to make sequence-specific interac-
tions with base pairs G2 C71 and G3 C70 [recall that
double helical RNA has an A-DNA-like structure (Section
29-1Bc) whose wide minor groove readily admits protein
but whose major groove is normally too narrow to do so].
The GlnRS domain that binds glutamine, ATP, and the
GCCA end of tRNA
Gln
, the so-called catalytic domain, con-
tains, as we previously discussed, a dinucleotide-binding
fold. Much of this domain is nearly superimposable with and
thus evolutionarily related to the corresponding domains of
other Class I aaRSs.
d. The X-Ray Structure of AspRS tRNA
Asp
, a
Class II Complex
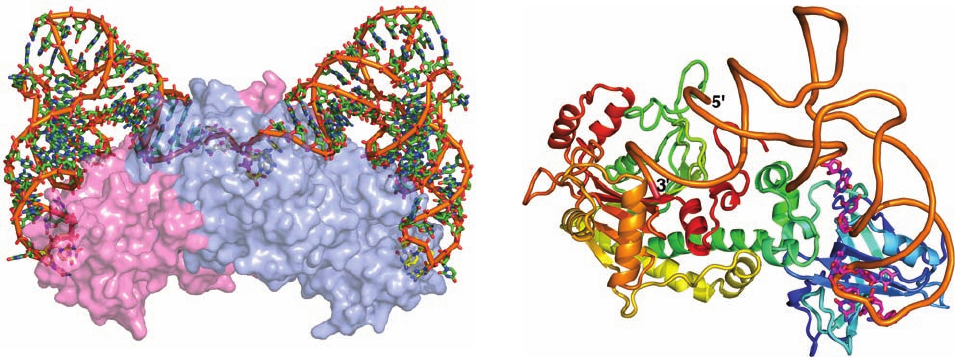
Yeast AspRS, a Class II synthetase, is an
2
dimer of
557-residue subunits. Its X-ray structure in complex with
tRNA
Asp
, determined by Moras, reveals that the protein
symmetrically binds two tRNA molecules (Fig. 32-18).Like
GlnRS, AspRS principally contacts its bound tRNA both
at the end of its acceptor stem and in its anticodon region.
The contacts in these two enzymes are, nevertheless, quite
different in character (Fig. 32-19): Although both tRNAs
approach their cognate synthetases along the inside of
their L shapes, tRNA
Gln
does so toward the direction of the
minor groove of its acceptor stem, whereas tRNA
Asp
does
so toward the direction of its major groove. The GCCA at
the 3¿ end of tRNA
Asp
thereby continues its helical track as
it plunges into AspRS’s catalytic site, whereas, as we saw,
the GCCA end of tRNA
Gln
bends backward into a hairpin
turn that opens up the first base pair (U1 A72) of its
acceptor stem. Although the deep major groove of an
A-RNA helix is normally too narrow to admit groups
larger than water molecules (Section 29-1Bc), the major
groove at the end of the acceptor stem in AspRS tRNA
Asp
is sufficiently widened for its base pairs to interact with a
protein loop.
The anticodon arm of tRNA
Asp
is bent by as much as 20
Å toward the inside of the L relative to that in the X-ray
structure of uncomplexed tRNA
Asp
and its anticodon bases
are unstacked.The hinge point for this bend is a G30 U40
base pair in the anticodon stem which, in nearly all other
species of tRNA, is a Watson–Crick base pair. The anti-
codon bases of tRNA
Gln
are also unstacked in contacting
GlnRS but with a backbone conformation that differs from
that in tRNA
Asp
. Evidently, the conformation of a tRNA in
complex with its cognate synthetase appears to be dictated
more by its interactions with the protein (induced fit) than
by its sequence.
Structural analyses of complexes of AspRS tRNA
Asp
with ATP and aspartic acid, and of GlnRS tRNA
Gln
with
ATP, have permitted models of the aminoacyl–AMP com-
plexes of these enzymes to be independently formulated.
Comparison of these models reveals that the 3¿-terminal A
residues of tRNA
Gln
and tRNA
Asp
(to which the aminoacyl
groups are appended; Fig. 32-13) are positioned on oppo-
site sides of the enzyme-bound aminoacyl–AMP interme-
diate (Fig. 32-20).The 3¿-terminal ribose residues are puck-
ered C2¿-endo for tRNA
Asp
and C3¿-endo for tRNA
Gln
(see
Fig. 29-8) such that the 2¿-hydroxyl group of tRNA
Gln
(Class I) is stereochemically positioned to attack the
Figure 32-18 X-ray structure of yeast AspRS tRNA
Asp
ATP. (a) The homodimeric enzyme with its two symmetrically
bound tRNAs is viewed with its 2-fold axis approximately
vertical.The tRNAs are drawn in skeletal form colored according
to atom type with the C atoms of the anticodon (GUC) and the
3¿-CCA end yellow, the remaining C atoms green, N blue, O red,
and P orange. An orange rod connects its successive P atoms. The
two protein subunits are represented by semitransparent pink
and light blue surface diagrams that reveal the buried portions of
(a)
(b)
the tRNAs. (b) A ribbon diagram of the AspRS tRNA
Asp
protomer.The tRNA’s sugar–phosphate backbone is represented
by an orange worm and the bases forming its identity elements
(Fig. 32-14b) are drawn in stick form colored according to atom
type with C magenta, N blue, and O red. The protein is shown in
ribbon form colored in rainbow order from its N-terminus (blue)
to its C-terminus (red). [Based on an X-ray structure by Dino
Moras, CNRS/INSERM/ULP, Illkirch Cédex, France. PDBid
1ASY.]
JWCL281_c32_1338-1428.qxd 8/19/10 10:05 PM Page 1354
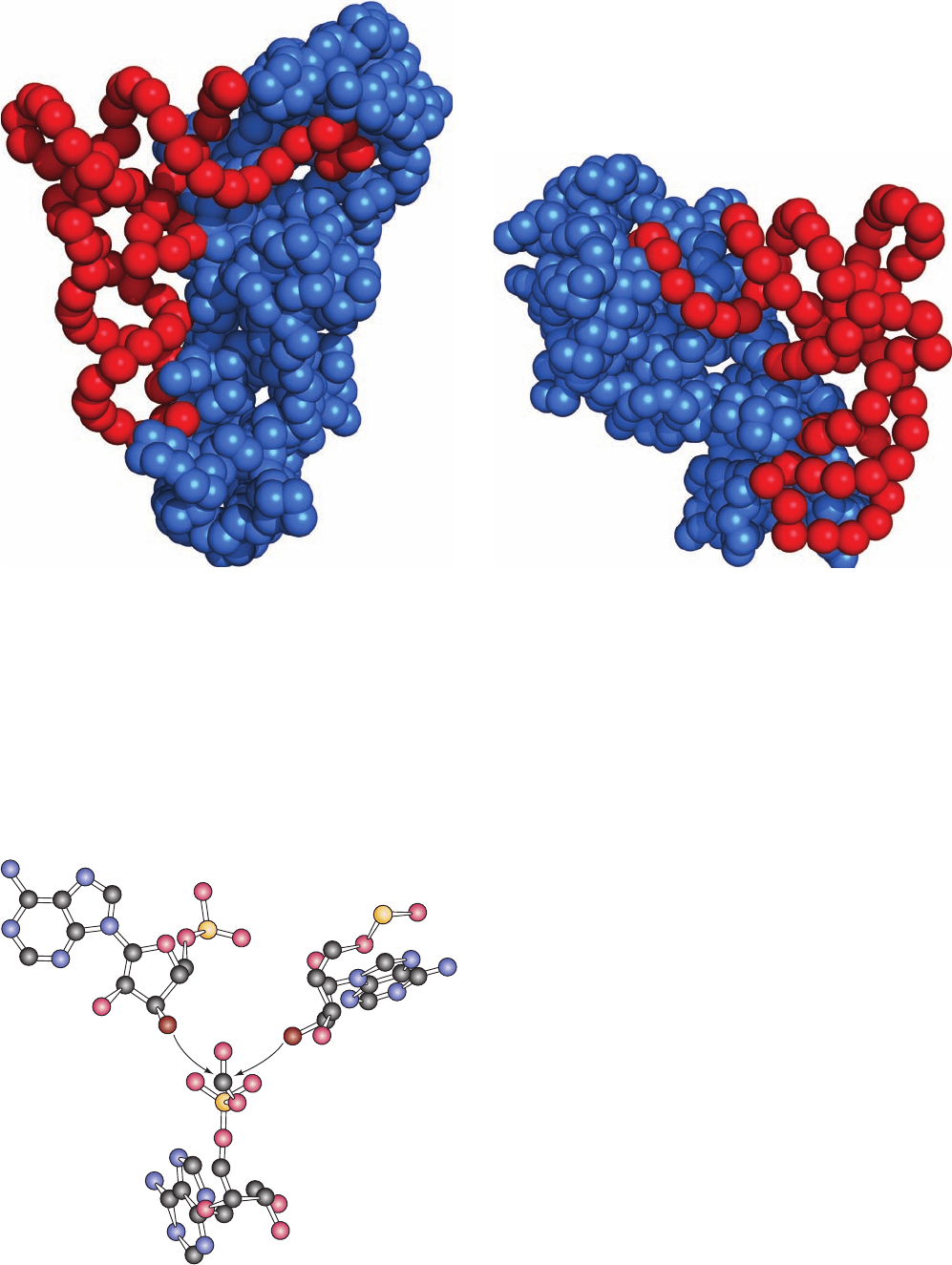
aminoacyl–AMP’s carboxyl group, whereas for tRNA
Asp
(Class II), only the 3¿-hydroxyl group is situated to do so.
This clearly explains the different aminoacylation specifici-
ties of the Class I and Class II aaRSs.
e. Proofreading Enhances the Fidelity of Amino Acid
Attachment to tRNA
The charging of a tRNA with its cognate amino acid is a
remarkably accurate process: aaRSs display an overall er-
ror rate of about 1 in 10,000.We have seen that aaRSs bind
only their cognate tRNAs through an intricate series of
specific contacts. But how do they discriminate among the
various amino acids, some of which are quite similar?
Experimental measurements indicate, for example, that
IleRS transfers as many as 40,000 isoleucines to tRNA
Ile
for every valine it so transfers. Yet, as Linus Pauling first
pointed out, there are insufficient structural differences be-
tween Val and Ile to permit such a high degree of discrimina-
tion in the direct generation of aminoacyl–tRNAs. The X-
ray structure of Thermus thermophilus IleRS, a monomeric
Section 32-2. Transfer RNA and Its Aminoacylation 1355
Figure 32-19 Comparison of the modes by which GlnRS and
AspRS bind their cognate tRNAs. The proteins and tRNAs are
represented by blue and red spheres centered on their C
and P
atom positions. Note how GlnRS (a), a Class I synthetase, binds
tRNA
Gln
from the minor groove side of its acceptor stem so as to
bend its 3¿ end into a hairpin conformation. In contrast, AspRS
(b), a Class II synthetase, binds tRNA
Asp
from the major groove
side of its acceptor stem so that its 3¿ end continues its helical
path on entering the active site. [After drawings by Dino Moras,
CNRS/INSERM/ULP, Illkirch Cédex, France. PDBids 1GTR
abd 1ASY.]
A76
tRNA
Asp
(Class II)
A76
tRNA
Gln
(Class I)
O2
′
O3′
Aminoacyl–AMP
Figure 32-20 Comparison of the stereochemistries of aminoacylation by
Class I and Class II aaRSs. The positions of the 3¿ terminal adenosine residues
(A76) of AspRS (Class II, left) and GlnRS (Class I, right) are drawn relative
to that of the enzyme-bound aminoacyl–AMP (below; only the carbonyl
group of its aminoacyl residue is shown). Note how only O3¿ of tRNA
Asp
and
O2¿ of tRNA
Gln
are suitably positioned to attack the aminoacyl residue’s
carbonyl group and thereby transfer the aminoacyl residue to the tRNA.
[After Cavarelli, J., Eriani, G., Rees, B., Ruff, M., Boeglin, M., Mitschler,A.,
Martin, F., Gangloff, J., Thierry, J.-C., and Moras, D., EMBO J. 13, 335 (1994).]
(a)
(b)
JWCL281_c32_1338-1428.qxd 8/4/10 4:44 PM Page 1355
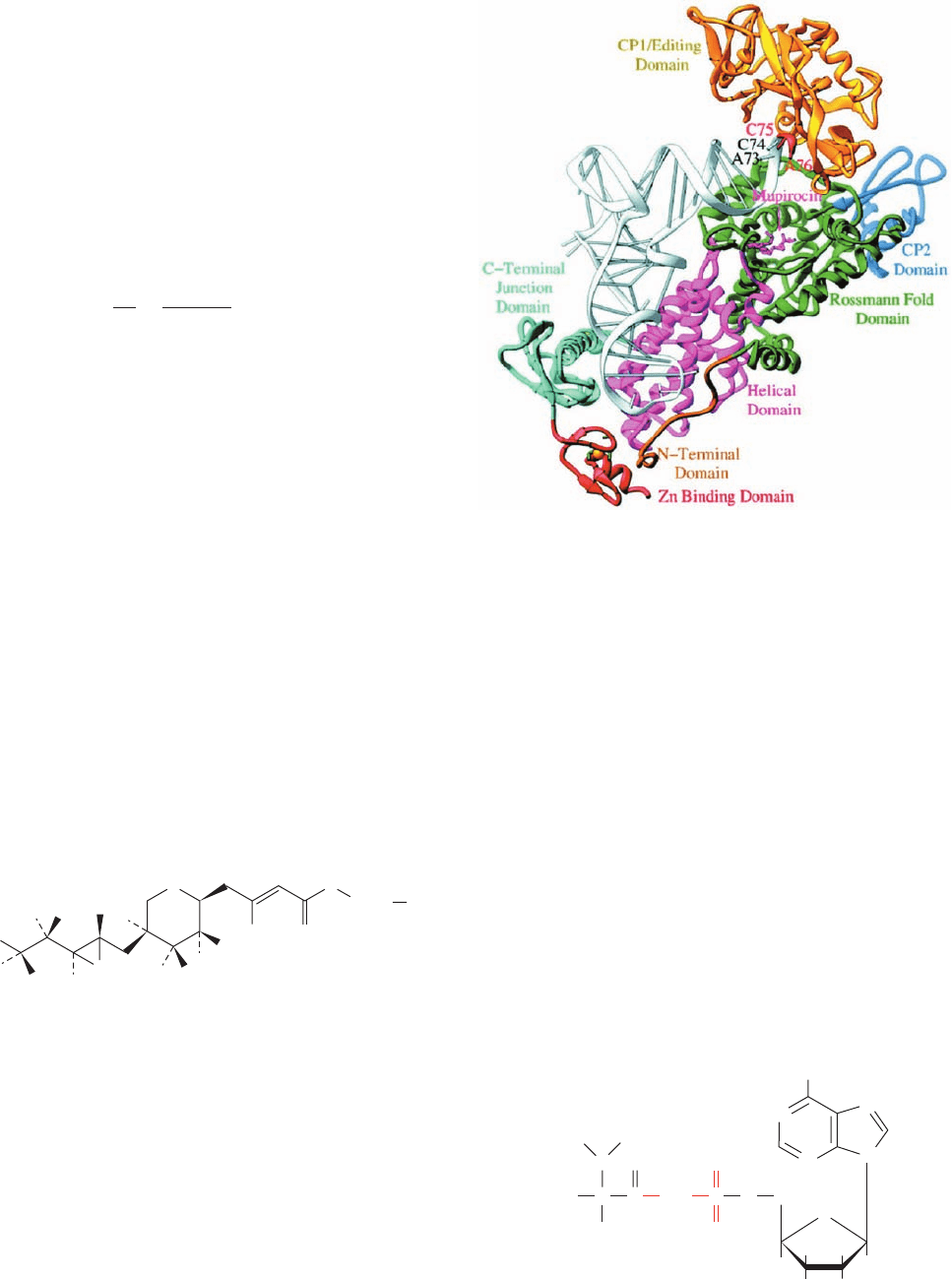
(a product of Pseudomonas fluorescens that acts by specif-
ically binding to bacterial IleRS so as to inhibit bacterial
protein synthesis), determined by Steitz, suggests how
IleRS carries out its editing process. The X-ray struc-
ture (Fig. 32-21) reveals that this complex resembles the
GlnRS tRNA
Gln
ATP complex (Fig. 32-17) but with IleRS
having an additional editing domain (also called CP1 for
connective peptide 1) inserted in its Rossmann fold domain.
The two 3¿ terminal residues of the tRNA
Ile
, C75 and A76,
are disordered but, when modeled so as to continue the ac-
ceptor stem’s stacked A-form helix, extend into a cleft in
the editing domain that has been implicated as its hy-
drolytic site (Fig. 32-22a, left).Thus, this IleRS complex ap-
pears to resemble an “editing complex” instead of a “trans-
fer complex” as seen in the GlnRS structure. However, a
transfer complex would form if the 3¿ ending segment of
the tRNA
Ile
assumes a hairpin conformation (Fig. 32-22a,
right) similar to that in the GlnRS structure (Figs. 32-17b
and 32-19a; recall that IleRS and GlnRS are both Class I
aaRSs). Steitz has therefore postulated that the aminoacyl
group is shuttled between the IleRS’s aminoacylation site
and its editing site by such a conformational change (Fig.
32-22b). This process functionally resembles the way in
which DNA polymerase I edits its newly synthesized
strand (Section 30-2Ag), which Steitz also elucidated.
ValRS is a monomeric Class I aaRS that resembles IleRS.
The X-ray structure of the complex of T. thermophilus
ValRS, tRNA
Va l
, and the nonhydrolyzable valyl–adenylate
analog 5-O-[N-(
L-valyl)sulfamoyl]adenosine (Val-AMS),
5-O-[N-(L-Valyl)sulfamoyl]adenosine (Val-AMS)
O
H
H
H
N
N
N
N
H
OH OH
H
3
C
CH
CH
3
CC
NH S
O
O
O
O
H
H
3
+
NCH
2
NH
2
1356 Chapter 32. Translation
Class I aaRS, in complex with isoleucine, determined by
Shigeyuki Yokoyama and Schimmel, indicates that
isoleucine fits snugly into its binding site in the enzyme’s
Rossmann fold domain and hence that this binding site
would sterically exclude leucine as well as larger amino
acids. However, valine, which differs from isoleucine by
only the lack of a single methylene group, fits into this
isoleucine-binding site. The binding free energy of a meth-
ylene group is estimated to be ⬃12 kJ mol
1
. Equation
[3.17] indicates that the ratio f of the equilibrium constants,
K
1
and K
2
, with which two substances bind to a given bind-
ing site is given by
[32.1]
where G°G
1
G
2
is the difference between the
free energies of binding of the two substances. It is there-
fore estimated that isoleucyl–tRNA synthetase could dis-
criminate between isoleucine and valine by no more than a
factor of ⬃100.
Berg resolved this apparent paradox by demonstrating
that, in the presence of tRNA
Ile
, IleRS catalyzes the nearly
quantitative hydrolysis of valyl–adenylate to valine AMP
rather than forming Val–tRNA
Ile
. Moreover, the few
Val–tRNA
Ile
molecules that do form are hydrolyzed to va-
line tRNA
Ile
.Thus, IleRS subjects both aminoacyl–adeny-
late and aminoacyl–tRNA
Ile
to a proofreading or editing
step that occurs at a separate catalytic site.This site binds Val
residues but excludes the larger Ile residues. The enzyme’s
overall selectivity is therefore the product of the selectivities
of its synthesis and proofreading steps, thereby accounting
for the high fidelity of aminoacylation. Note that in this so-
called double-sieve mechanism, editing occurs at the ex-
pense of ATP hydrolysis, the thermodynamic price of high
fidelity (increased order).
The X-ray structure of Staphylococcus aureus IleRS in
complex with tRNA
Ile
and the clinically useful antibiotic
mupirocin
f
K
1
K
2
e
¢G
1
°¿
RT
e
¢G
2
°¿
RT
e
¢¢G°¿
RT
H
H
H
H
H
HO
CO
2
H
CH
3
(CH
2
)
8
H
3
C
H
O
OH
OH
O
O
O
H
3
C
H
Mupirocin
Figure 32-21 X-ray structure of S. Aureus isoleucyl–tRNA
synthetase in complex with tRNA
Ile
and mupirocin. The tRNA is
white, the protein is colored by domain, and the mupirocin is
shown in stick form in pink. [Courtesy of Thomas Steitz,Yale
University. PDBid 1QU2.]
JWCL281_c32_1338-1428.qxd 8/4/10 4:44 PM Page 1356
determined by Yokoyama, reveals that the Val-AMS is
bound in the aminoacylation pocket in the Rossmann fold
domain, which accommodates the isosteric Val and Thr
moieties but sterically excludes Ile. Modeling studies based
on the IleRS tRNA
Ile
mupirocin structure indicate that
Valyl-adenylate
H
3
+
N
O
H
H
H
N
H
OH OH
H
3
C
CH
CH
3
CC
O P
O
O
O
_
O
H
CH
2
N
N
N
NH
2
the Thr side chain would fit into the ValRS editing pocket
with its side chain hydroxyl group hydrogen bonded to the
side chain of Asp 279 of ValRS, which protrudes into the
pocket in contrast to the corresponding Asp 328 of IleRS,
which does not. Consequently, a Val side chain would be
excluded from the ValRS editing pocket because it cannot
form such a hydrogen bond, thereby explaining why this
editing pocket hydrolyzes threonyl–adenylate and Thr–
tRNA
Va l
but not the corresponding Val derivatives. The
ValRS tRNA
Val
structure also indicates that ValRS and
tRNA
Va l
together form a tunnel connecting the ValRS’s
aminoacylation pocket with its editing pocket. Improperly
formed threonyl–adenylate is proposed to be channeled
through this tunnel for hydrolysis in the editing pocket,
thereby explaining why tRNA
Va l
must be bound to ValRS for
this pretransfer editing reaction to occur.Valyl–adenylate is
presumably channeled through the similar IleRS tRNA
Ile
complex for its hydrolysis.
Section 32-2. Transfer RNA and Its Aminoacylation 1357
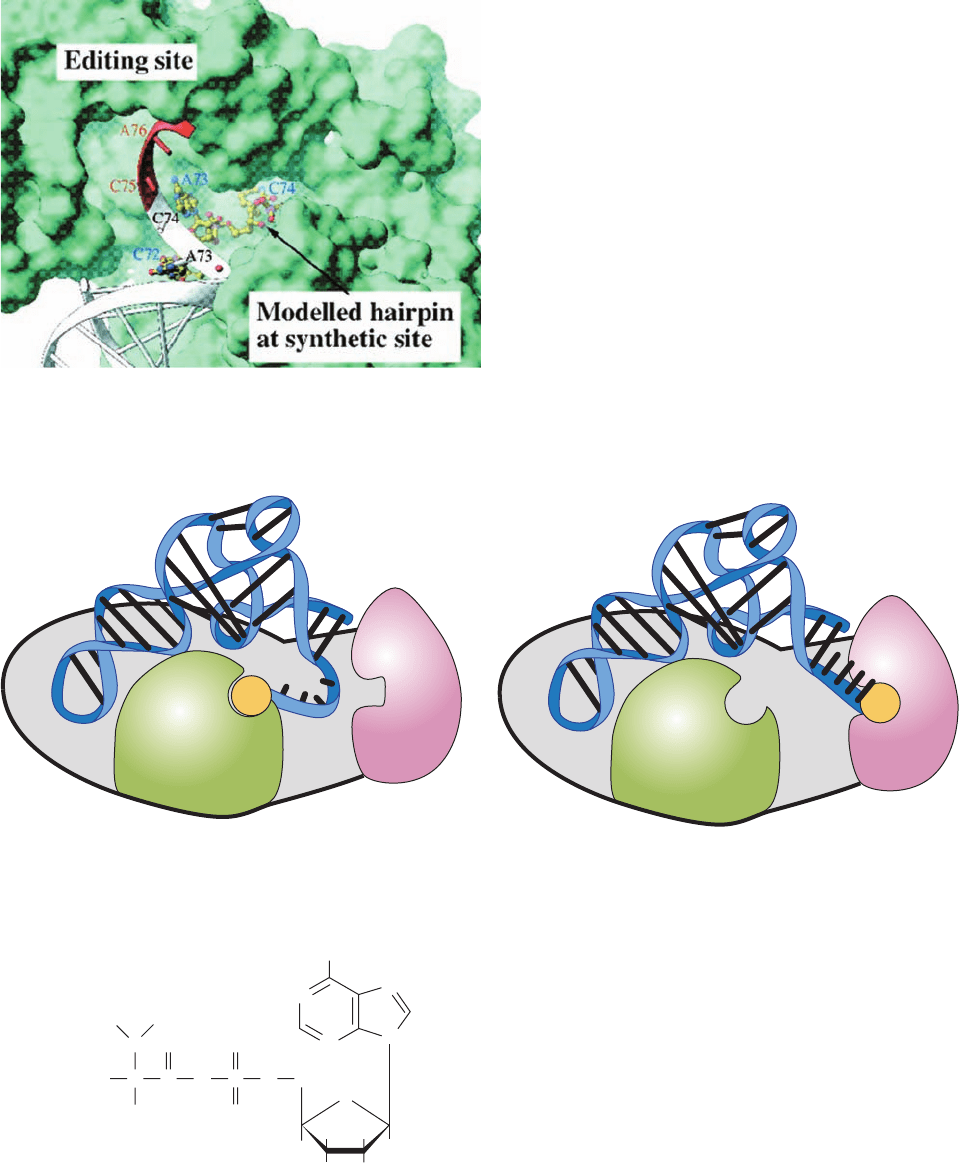
Figure 32-22 Comparison of the putative aminoacylation and
editing modes of IleRS tRNA
Ile
. (a) The superposition of
tRNA
Ile
in these two binding modes on the solvent-accessible
surface of IleRS (green).The acceptor strand of tRNA
Ile
in the
editing mode observed in the X-ray structure of IleRS
tRNA
Ile
mupirocin (Fig. 32–21) is drawn in ribbon form in white
with the modeled positions of C75 and A76 in red.This places
the tRNA’s 3¿ end in the editing site. In contrast, the three 3¿
terminal residues of tRNA
Ile
, as positioned through homology
modeling based on the X-ray structure of GlnRS tRNA
Gln
ATP (Fig. 32-17) and drawn in ball-and-stick form with C yellow,
N blue, O red, and P magenta, places the tRNA’s 3¿ end in the
synthetic (aminoacylation) site, 34 Å distant from its position in
the editing site. Note that there is a cleft running between the
editing and synthetic sites and that the 3¿ end of the tRNA
continues its A-form helical path in the editing mode but
assumes a hairpin conformation in the synthetic mode. (b) A
cartoon comparing the positions of the 3¿ end of tRNA
Ile
in its
complex with IleRS in its synthetic mode (left) and in its editing
mode (right). [Part a courtesy of and Part b based on a drawing
by Thomas Steitz, Yale University.]
Synthetic
domain
Synthetic mode Editing mode
Editing
domain
5′
3′
3′
5′
aa
aa
(a)
(b)
JWCL281_c32_1338-1428.qxd 8/4/10 4:44 PM Page 1357
Class I
Class II
Catalytic
domain
Catalytic
domain
Editing
domain
Editing
domain
tRNA acceptor stem
tRNA acceptor stem
ThrRS, a Class II homodimer, has the opposite problem
of ValRS: It must synthesize Thr–tRNA
Thr
but not Val–
tRNA
Thr
.The X-ray structure of E. coli ThrRS that lacks its
N-terminal domain but remains catalytically active in a
complex with either threonine or the threonyl–adenylate
analog Thr-AMS, determined by Moras, reveals that
ThrRS’s aminoacylation pocket contains a Zn
2
ion that is
coordinated by the side chain hydroxyl and amino groups
of the threonyl group as well as by three protein side
chains. The isosteric valine could not coordinate the Zn
2
ion in this way and hence does not undergo adenylylation
by ThrRS. However, what prevents ThrRS from synthesiz-
ing Ser–tRNA
Thr
? In fact, the truncated ThrRS synthesizes
Ser–tRNA
Thr
at more than half the rate it synthesizes
Thr–tRNA
Thr
, thereby indicating that the N-terminal do-
main of wild-type ThrRS contains the enzyme’s editing site.
Mutational analysis of ThrRS has localized this editing site
to a cleft in the N-terminal domain of wild-type ThrRS,
whose X-ray structure in complex with tRNA
Thr
was also
determined by Moras. In this latter structure, the tRNA’s 3¿
end follows a regular helical path similar to that seen in the
X-ray structure of AspRS tRNA
Asp
ATP (Fig. 32-18) so
as to enter the aminoacylation site. However, if the 3¿ end
of the bound tRNA
Thr
assumed a hairpin conformation
similar to that seen in X-ray structure of tRNA
Gln
in com-
plex with the Class I enzyme GlnRS and ATP (Fig. 32-17),
its covalently linked aminoacyl group would enter the edit-
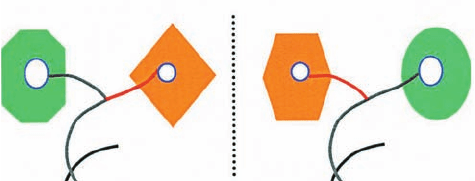
ing site. This indicates an intriguing “mirror symmetry”
(Fig. 32-23): In Class I aaRSs that mediate a double-sieve
editing mechanism, the 3¿ end of the bound cognate tRNA
assumes a hairpin conformation when it enters the aminoa-
cylation site and a helical conformation when it enters the
editing site, whereas the converse holds for Class II aaRSs.
Finally, ThrRS does not appear to mediate pretransfer ed-
iting (does not hydrolyze seryl–adenylate), and, in fact, the
ThrRS tRNA
Thr
complex lacks a channel connecting its
aminoacylation and editing sites such as is seen in the
ValRS tRNA
Va l
complex.
Synthetases that have adequate selectivity for their cor-
responding amino acid lack editing functions. Thus, for ex-
ample, the TyrRS aminoadenylylation site discriminates
between tyrosine and phenylalanine through hydrogen
bonding with the tyrosine ¬OH group. The cell’s other
amino acids, standard as well as nonstandard, have even
less resemblance to tyrosine, which rationalizes why TyrRS
lacks an editing site.
f. Gln–tRNA
Gln
May Be Formed via an
Alternative Pathway
Although it was long believed that each of the 20 stan-
dard amino acids is covalently linked to a tRNA by its corre-
sponding aaRS, it is now clear that gram-positive bacteria,
archaebacteria, cyanobacteria, mitochondria, and chloro-
plasts all lack GlnRS. Rather glutamate is linked to tRNA
Gln
by the same GluRS that synthesizes Glu–tRNA
Glu
. The re-
sulting Glu–tRNA
Gln
is then transamidated to Gln–tRNA
Gln
by the enzyme Glu–tRNA
Gln
amidotransferase (Glu-AdT)
in an ATP-requiring reaction in which glutamine is the
amide donor. Some microorganisms use a similar transami-
dation pathway for the synthesis of Asn–tRNA
Asn
from
Asp–tRNA
Asn
.
The overall reaction catalyzed by Glu-AdT occurs in
three stages (Fig. 32-24): (1) Glutamine is hydrolyzed to
glutamate and the resulting NH
3
sequestered; (2) ATP re-
acts with the Glu side chain of Glu–tRNA
Gln
to yield an ac-
tivated acylphosphate intermediate and ADP; and (3) the
acylphosphate intermediate reacts with the NH
3
to yield
Gln–tRNA
Gln
P
i
. Glu-AdT from Bacillus subtilis, which
was characterized by Dieter Söll, is a heterotrimeric pro-
tein, none of whose subunits exhibit significant sequence
similarity to GlnRS. The genes encoding these subunits,
gatA, gatB, and gatC, form a single operon whose disrup-
tion is lethal, thereby demonstrating that B. subtilis has no
alternative pathway for Gln–tRNA
Gln
production. The
GatA subunit of Glu-AdT appears to catalyze the activa-
tion of the side chain carboxyl of glutamic acid via a reac-
tion resembling that catalyzed by carbamoyl phosphate
synthetase (Section 26-2A). Nevertheless, GatA exhibits
no sequence similarity with other known glutamine amido-
transferases (members of the triad or Ntn families; Section
26-5Aa). The GatB subunit may be used to select the cor-
rect tRNA substrate. The role of the GatC subunit is un-
clear, although the observation that its presence is neces-
sary for the expression of GatA in E. coli suggests that it
participates in the modification, folding, and/or stabiliza-
tion of GatA.
Since Glu is not misincorporated into B. subtilis proteins
in place of Gln, the Glu–tRNA
Gln
product of the above
aminoacylation reaction must not be transported to the ri-
bosome in the same way as Gln–tRNA
Gln
. It is likely that
this occurs because, as has been shown in chloroplasts, EF-
Tu, the elongation factor that binds and transports most
bacterial aminoacyl–tRNAs to the ribosome in a GTP-
dependent process (Section 32-3D), does not bind
Glu–tRNA
Gln
. It is unclear why two independent routes
have evolved for the synthesis of Gln–tRNA
Gln
.
1358 Chapter 32. Translation
Figure 32-23 Schematic diagram of the aminoacylation and
editing mechanisms of Class I and Class II aaRSs emphasizing
the “mirror symmetry” of their overall mechanisms. With Class I
aaRSs (left; e.g., IleRS), the 3¿ end of the bound tRNA’s acceptor
stem assumes a hairpin conformation in the synthetic mode and
a helical conformation in the editing mode, whereas the converse
occurs with Class II aaRSs (right; e.g.,ThrRS). [Courtesy of Dino
Moras, CNRS/INSERM/ULP, Illkirch Cedex, France.]
JWCL281_c32_1338-1428.qxd 8/4/10 4:44 PM Page 1358
g. Some Archaebacteria Lack a Separate CysRS
The genomes of certain archaebacteria such as M. jan-
naschii lack an identifiable gene for CysRS. This is because
the enzyme responsible for synthesizing Pro–tRNA
Pro
in
these organisms also synthesizes Cys–tRNA
Cys
. Interest-
ingly, this enzyme, which is named ProCysRS, does not syn-
thesize Pro–tRNA
Cys
or Cys–tRNA
Pro
.Although ProCysRS
synthesizes cysteinyl–adenylate only in the presence of
tRNA
Cys
, it synthesizes prolyl–adenylate in the absence of
tRNA
Pro
.The binding of tRNA
Cys
to ProCysRS blocks the
activation of proline so that only cysteine can be activated.
Conversely, the activation of proline facilitates the binding
of tRNA
Pro
while preventing the binding of tRNA
Cys
. How-
ever, the mechanism through which ProCysRS carries out
these mutually exclusive syntheses is unknown. In any case,
it appears that some organisms can get by with as few as 17
different aaRSs; they may lack GlnRS, AspRS, and a sepa-
rate CysRS.
D. Codon–Anticodon Interactions
In protein synthesis, the proper tRNA is selected only
through codon–anticodon interactions; the aminoacyl group
does not participate in this process. This phenomenon was
demonstrated as follows. Cys–tRNA
Cys
, in which the Cys
residue was
14
C labeled, was reductively desulfurized with
Raney nickel so as to convert the Cys residue to Ala:
The resulting
14
C-labeled hybrid, Ala–tRNA
Cys
, was added
to a cell-free protein synthesizing system extracted from
rabbit reticulocytes. The product hemoglobin chain’s
only radioactive tryptic peptide was the one that normally
contains the subunit’s only Cys. No radioactivity was found
in the peptides that normally contain Ala but no Cys.
HS
CH
2
H
O
O
C C
NH
3
+
NH
3
+
tRNA
Cys
+
Ni(H)
x
Cys–tRNA
Cys
H
CH
2
H
O
O
C C tRNA
Cys
++
Ala–tRNA
Cys
Raney nickel
H
2
S Ni
Section 32-2. Transfer RNA and Its Aminoacylation 1359
O
CC
CH
2
CH
2
H
2
N
C
H
2
N
H
COO
–
O
CC
CH
2
CH
2
–
O
H
COO
–
Glutamine
Glutamate
O
O
O
CC
CH
2
CH
2
–
O
H
C
Glu–tRNA
Gln
1
2
3
H
2
O
+
NH
3
tRNA
Gln
O
C
CH
2
CH
2
H
C
Gln–tRNA
Gln
tRNA
Gln
C
O
O
C
CH
2
CH
2
H
C
O
Carboxyphosphate intermediate
tRNA
Gln
ATP
ADP
2–
O
3
P
P
i
+
NH
3
+
NH
3
+
NH
3
+
NH
3
+
NH
3
Figure 32-24 The Glu-AdT–mediated synthesis of
Gln–tRNA
Gln
from Glu–tRNA
Gln
. The reaction involves the
ATP-activated transfer of a glutamine-derived NH
3
to the
glutamate moiety of Glu–tRNA
Gln
.
JWCL281_c32_1338-1428.qxd 8/4/10 4:44 PM Page 1359
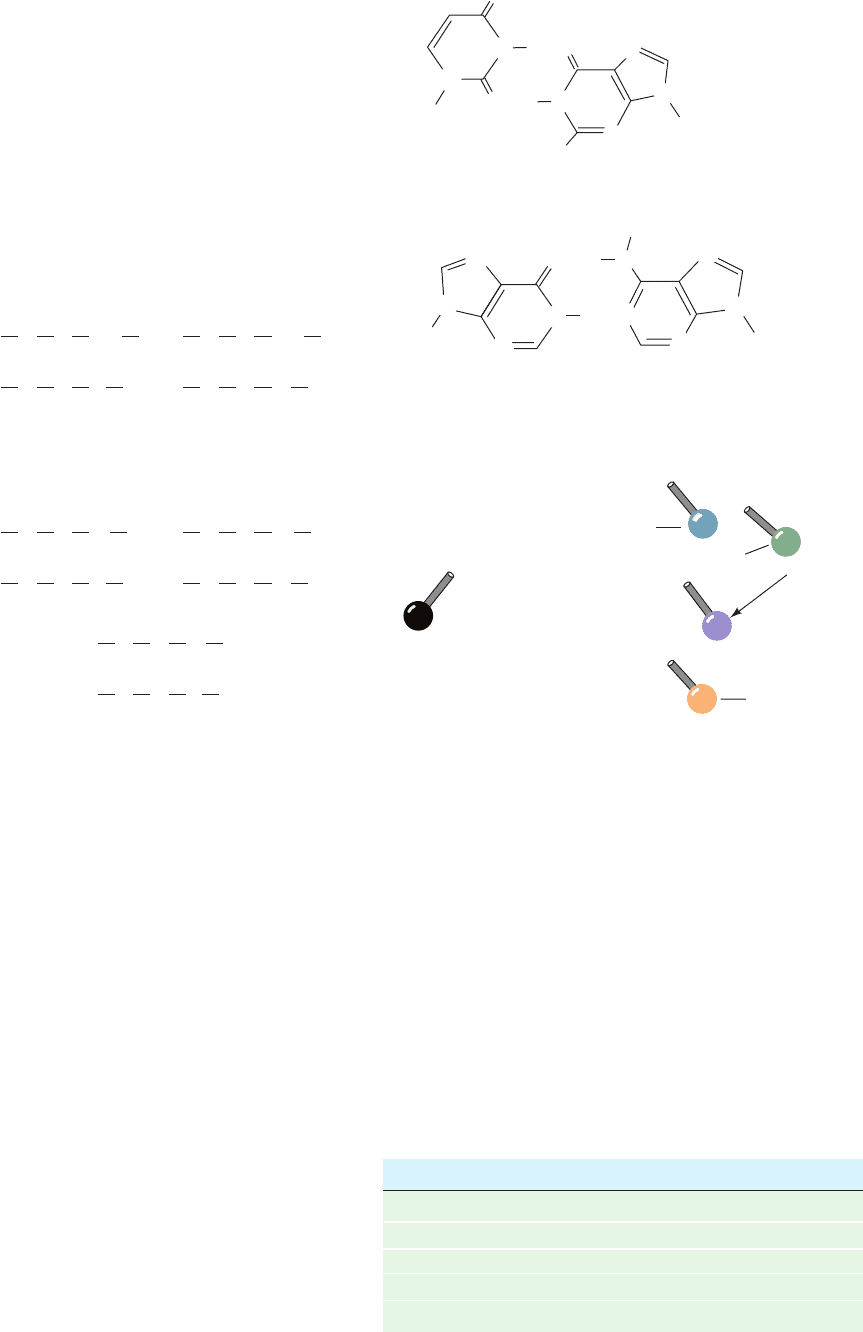
Evidently, only the anticodons of aminoacyl–tRNAs partic-
ipate in codon recognition.
a. Genetic Code Degeneracy Is Largely Due to
Variable Third Position Codon–Anticodon Interactions
One might naively guess that each of the 61 codons
specifying an amino acid would be read by a different
tRNA. Yet, even though most cells contain several groups
of isoaccepting tRNAs, many tRNAs bind to two or three of
the codons specifying their cognate amino acids. For exam-
ple, yeast tRNA
Phe
,which has the anticodon GmAA, recog-
nizes the codons UUC and UUU (remember that the anti-
codon pairs with the codon in an antiparallel fashion),
and yeast tRNA
Ala
, which has the anticodon IGC, recog-
nizes the codons GCU, GCC, and GCA.
It therefore seems that non-Watson–Crick base pairing can
occur at the third codon–anticodon position (the anti-
codon’s first position is defined as its 3¿ nucleotide), the site
of most codon degeneracy (Table 32-2). Note also that the
third (5¿) anticodon position commonly contains a modi-
fied base such as Gm or I.
b. The Wobble Hypothesis Structurally Accounts for
Codon Degeneracy
By combining structural insight with logical deduction,
Crick proposed, in what he named the wobble hypothesis,
how a tRNA can recognize several degenerate codons. He
assumed that the first two codon–anticodon pairings have
normal Watson–Crick geometry. The structural constraints
that this places on the third codon–anticodon pairing en-
sure that its conformation does not drastically differ from
that of a Watson–Crick pair. Crick then proposed that
there could be a small amount of play or “wobble” in the
third codon position which allows limited conformational
adjustments in its pairing geometry. This permits the for-
mation of several non-Watson–Crick pairs such as U G
and I A (Fig. 32-25a). The allowed “wobble” pairings are
indicated in Fig. 32-25b. Then, by analyzing the known pat-
tern of codon–anticodon pairing, Crick deduced the most
plausible sets of pairing combinations in the third
codon–anticodon position (Table 32-5).Thus, an anticodon
...
...
...
...
...
...
3 3 55
A
nticodon: C G I C GI
5 5 33
Codon: G C U
...
...
...
3 5
Anticodon: C G I
5 3
Codon: G C A
G C C
...
...
...
...
...
...
3 3 55
A
nticodon: A A Gm A AGm
5 5 33
Codon: U U C U U U
with C or A in its third position can only pair with its Wat-
son–Crick complementary codon. If U, G, or I occupies the
third anticodon position, two, two, or three codons are rec-
ognized, respectively.
1360 Chapter 32. Translation
Table 32-5 Allowed Wobble Pairing Combinations in the
Third Codon–Anticodon Position
5¿-Anticodon Base 3¿-Codon Base
CG
AU
U A or G
G U or C
I U,C,or A
Figure 32-25 Wobble pairing. (a) U G and I A wobble
pairs. Both have been observed in X-ray structures. (b) The
geometry of wobble pairing.The spheres and their attached
bonds represent the positions of ribose C1¿ atoms with their
accompanying glycosidic bonds. X (left) designates the
nucleoside at the 5¿ end of the anticodon (tRNA). The positions
on the right are those of the 3¿ nucleoside of the codon (mRNA)
in the indicated wobble pairings. [After Crick, F.H.C., J. Mol.
Biol. 19, 552 (1966).]
ON
N
N
N
N
N
C1'
C1'
C1'
C1'
O
H
H
2
N
H
O
U
.
G
O
H
H
H
I
.
A
(a)
...
...
...
...
I
G
X
...
...
U
U
I
...
A
G
...
U
Anticodon
A
U
G
C
C
...
...
...
...
...
U
A
C
G
I
Standard:
Codon
(b)
N
N
N
N
N
N
N
NN
JWCL281_c32_1338-1428.qxd 8/4/10 4:44 PM Page 1360
