Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

dria of trypanosomes (whose DNA encodes only 20 genes),
involve the addition and removal of up to hundreds of U’s
to and from 12 otherwise untranslatable mRNAs. The
process whereby a transcript is altered in this manner is
called RNA editing because it originally seemed that the
required enzymatic reactions occurred without the direc-
tion of a nucleic acid template and hence violated the cen-
tral dogma of molecular biology (Fig. 5-21). Eventually,
however, a new class of trypanosomal mitochondrial tran-
scripts called guide RNAs (gRNAs) was identified.
gRNAs,which consist of 40 to 80 nucleotides,have 3¿ oligo(U)
tails, an internal segment that is precisely complementary
to the edited portion of the pre-edited mRNA (if G ⴢ U
pairs, which are common in RNAs, are taken to be comple-
mentary), and a 10- to 15-nt so-called anchor sequence
near the 5¿ end that is largely complementary in the
Watson–Crick sense to a segment of the mRNA that is not
edited.
An unedited transcript presumably associates with the
corresponding gRNA via its anchor sequence (Fig. 31-69).
Then, in a process mediated by the appropriate enzymatic
machinery in an ⬃20S RNP named the editosome, the
gRNA’s internal segment is used as a template to “correct”
the transcript, thereby yielding the edited mRNA. Inser-
Section 31-4. Post-Transcriptional Processing 1321
Figure 31-68 The sequence
of transesterification reaction
that occurs in trans-splicing.
The chemistry is closely
similar to that of pre-mRNA
cis-splicing (Fig. 31-53).
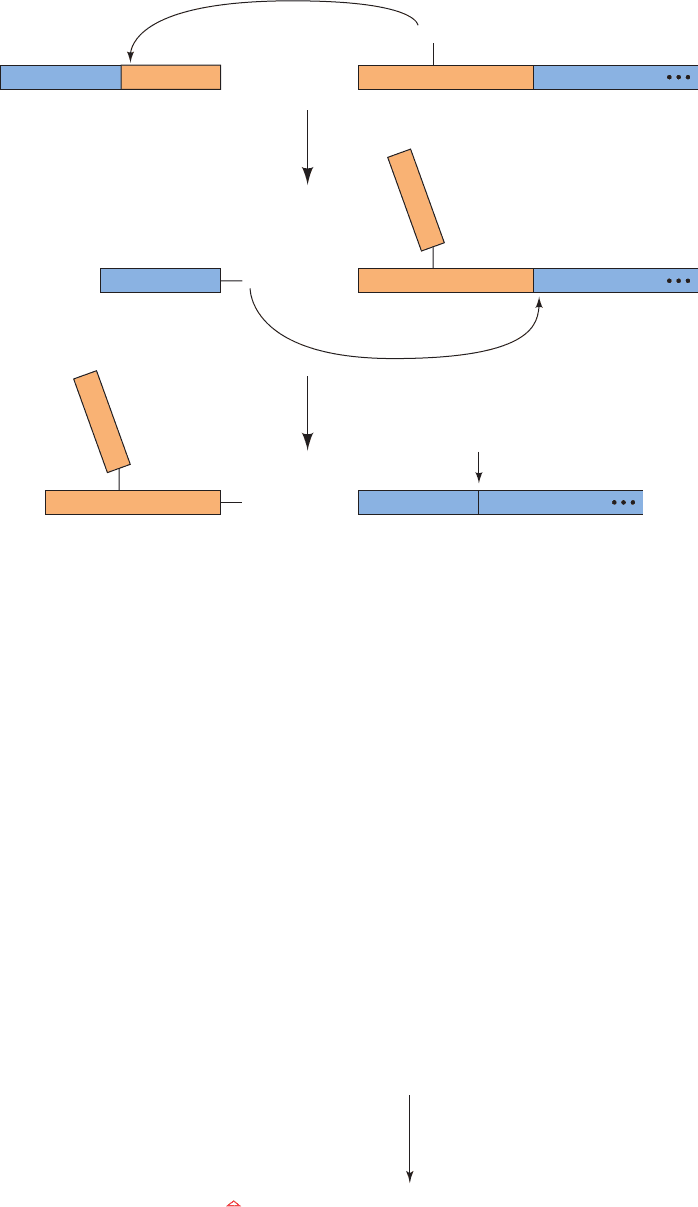
Figure 31-69 A schematic diagram indicating how gRNAs
direct the editing of trypanosomal pre-edited mRNAs. The red
U’s in the edited mRNA are insertions and the triangle (⌬)
marks a deletion. Several gRNAs may be necessary to direct the
1
2
5′ 5′3′
3′
(2′, 5′)
Splice junction
+
+
3′
SL RNA Pre-mRNA
mRNAExcised introns
in Y form
Leader
5′
Leader
ExonpGpUp
(2′, 5′)
3′
pGpUp
ApG p
ApG p
A
OH
2'
OH
3'
5′ 3′
ExonA
5′
ApGA
3′
Exon
pGpUp
5′
Leader
OH
3'
5'
GGGUUUUU U UUUUU UU UUAAA
3' Edited mRN
A
GCCCCAA
– –––––––––– –––––– ––––––––––
3'
CCCGAGGG A GGAGA AA A AUAU
5' gRNA
CGGGGUU
– ––––––––––––––––
5'
G
G
A
CUCAA
A
GG G AUCAU
U
CA
–––
–
–
–
–
–
–
–––––––––––––
3'
3'
5'
Pre-edited mRNA
gRNA
CGAG
G
G
G
UUCC C UAGUAGU
–
–
–
–
G
G
A
G
–
–
A
A
–
–
–
–
––––
–
–
–
––––––––
––––––––––
editing of consecutive segments of a pre-edited mRNA. [After
Bass, B.L., in Gesteland, R.F. and Atkins, J.F. (Eds.), The RNA
World, p. 387, Cold Spring Harbor Laboratory Press (1993).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1321
tion editing requires at least three enzymatic activities that,
somewhat surprisingly, are encoded by nuclear genes
(Fig. 31-70a): (1) an endonuclease at a mismatch between
the gRNA and the pre-edited mRNA to cleave the pre-
edited mRNA on the 5¿ side of the insertion point; (2) ter-
minal uridylyltransferase (TUTase) to insert the new U(s);
and (3) an RNA ligase to reseal the RNA. Deletion re-
quires similar enzymatic apparatus with the exceptions
that the endonuclease cleaves the RNA being edited on
the 3¿ side of the U(s) to be deleted and TUTase is replaced
by 3¿-U-exonuclease (3¿-U-exo), which excises the U(s) at
the deletion site (Fig. 31-70b).A single gRNA mediates the
editing of a block of 1 to 10 sites.Thus, the genetic informa-
tion specifying an edited mRNA is derived from two or
more genes. The functional advantage of this complicated
process, either presently or more likely in some ancestral
organism, is obscure.
r. RNA Can Be Edited by Base Deamination
Humans express two forms of apolipoprotein B (apoB):
apoB-48, which is made only in the small intestine and
functions in chylomicrons to transport triacylglycerols
from the intestine to the liver and peripheral tissues; and
apoB-100, which is made only in the liver and functions in
VLDL, IDL, and LDL to transport cholesterol from the
liver to the peripheral tissues (Sections 12-5A and 12-5B).
ApoB-100 is an enormous 4536-residue protein, whereas
apoB-48 consists of apoB-100’s N-terminal 2152 residues
and therefore lacks the C-terminal domain of apoB-100
that mediates LDL receptor binding.
Despite their differences, both apoB-48 and apoB-100
are expressed from the same gene. How does this occur?
Comparison of the mRNAs encoding the two proteins indi-
cates that they differ by a single C S U change: The codon
for Gln 2153 (CAA) in apoB-100 mRNA is, in apoB-48
mRNA, a UAA Stop codon. The activity that catalyzes this
conversion is a protein:It is destroyed by proteases and pro-
tein-specific reagents but not by nucleases. When apoB
mRNA is synthesized with [␣-
32
P]CTP, in vitro editing
yields a [
32
P]UMP residue solely at the editing site. Evi-
dently, the editing activity is a site-specific cytidine deami-
nase. This type of RNA editing differs in character from
that in trypanosomal mitochondria, which inserts and
deletes multiple U’s into mRNAs under the direction of
gRNAs. ApoB mRNA editing therefore falls into a differ-
ent class of RNA editing that is called substitutional editing.
The several other known examples of pre-mRNA substi-
tutional editing all occur on pre-mRNAs that encode ion
channels and G protein–coupled receptors in nerve tissue.
Among them is vertebrate brain glutamate receptor pre-
mRNA, which undergoes an A S I deamination [where I is
inosine (guanosine lacking its 2-amino group), which the
translational apparatus reads as G] that transforms a Gln
codon (CAG) to that of a functionally important Arg (CIG;
normally CGG). The vertebrate enzymes that catalyze such
A S I RNA editing of pre-mRNAs, ADAR1 (1200
residues), ADAR2 (729 residues), and ADAR3 (739
residues;ADAR for adenosine deaminases acting on RNA),
have the curious requirement that their target A residues
must be members of RNA double helices that are formed
between the editing site and a complementary sequence that
is usually located in a downstream intron (Fig. 31-71).
Hence,ADAR-mediated editing must precede splicing.
Substitutional editing may contribute to protein diver-
sity. For example, Drosophila cacophony pre-mRNA that
encodes a voltage-gated Ca
2⫹
channel subunit contains 10
different substitutional editing sites and hence has the po-
tential of generating 1000 different isoforms in the absence
of alternative splicing.
Substitutional editing can also generate alternative
splice sites. For example, rat ADAR2 edits its own pre-
mRNA by converting an intronic AA dinucleotide to AI,
which mimics the AG normally found at 3¿ splice sites (Fig.
31-53). The consequent new splice site adds 47 nucleotides
near the 5¿ end of the ADAR2 mRNA so as to generate a
new translational initiation site. The resulting ADAR2
isozyme is catalytically active but is produced in smaller
amounts than that from unedited transcripts, perhaps due
to a less efficient translational initiation site. Thus, rat
ADAR2 appears to regulate its own rate of expression.
ADAR1 contains an N-terminal Z-DNA–binding do-
main, Zab, that is composed of two subdomains, Z␣ and
Z.We have seen that in the X-ray structure of Z␣ in com-
plex with Z-DNA (Fig. 29-3), Z␣ binds Z-DNA via
1322 Chapter 31. Transcription
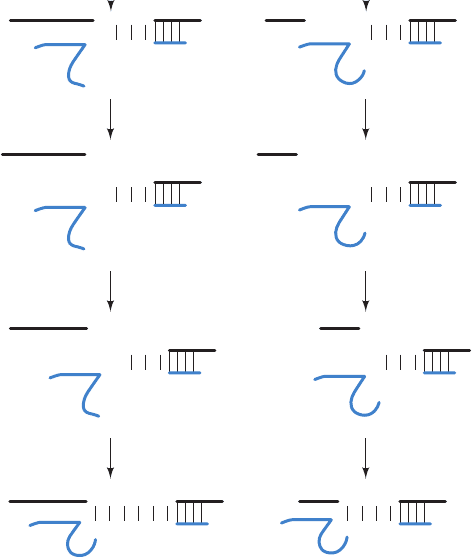
Figure 31-70 Trypanosomal RNA editing pathways. The
RNAs being edited (black) are shown base-paired to the gRNAs
(blue) with the U’s that are (a) inserted by TUTase or (b) deleted
by 3¿-U-exo drawn in red.The arrowheads indicate the positions
that are cleaved by the endonuclease. [After Madison-Antenucci,
S., Grams, J., and Hajduk, S.L., Cell 108, 435 (2002).]
3′
3′5′
5′
C
C
A
A
C
U
A
G
Insertion Deletion
G
G
5′
G
5′ 5′
G
5′
G
5′
3′
C
C
A
A
C
U
A
G
UU
G
3′
G
A
U
GUUUUA
CC
3′
C
C
A
A
C
U
A
G
G
3′
CUCUU
CA
G
AACG
3′5′
5′
G
A
U
GU
CC
3′
5′
G
A
U
GU
C
C
3′
3′
3′
5′
G
A
GUA
CU C
5′
UUUA
5′
A
endonuclease
RNA ligase
TUTase 3′-U-exo
(a)(b)
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1322
Trigger dsRNA
RISC
Guide RNA
Passenger RNA
Argonaute
p
p
3'
3'
1
2
3
Dicer
RISC
Target mRNA
p
p
p
3'
3'
p
p
3'
3'
p
RISC
p
4
mRNA
Cleaved mRNA
+
sequence-independent complementary surfaces (Section
29-1Bb). What is the function of Zab? Alexander Rich has
proposed that since the negative supercoiling of the DNA
immediately behind actively transcribing RNAP (Section
31-2Ca) stimulates the transient formation of Z-DNA (re-
call that Z-DNA has a left-handed helix), Zab targets
ADAR1 to genes that are undergoing transcription. This
would facilitate rapid A S I editing, which must take place
before the next splicing reaction occurs.
s. RNA Interference Degrades mRNAs
Since the 1990s it has become increasingly clear that
noncoding RNAs have important roles in controlling gene
expression. One of the first indications of this phenomenon
occurred in Richard Jorgensen’s attempt to genetically en-
gineer more vividly purple petunias by introducing extra
copies of the gene that directs the synthesis of the purple
pigment. Surprisingly, the resulting transgenic plants had
variegated and often entirely white flowers. Apparently,
the purple-making genes somehow switched each other
off. Similarly, it is well known that antisense RNA (RNA
that is complementary to at least a portion of an mRNA)
prevents the translation of the corresponding mRNA be-
cause ribosomes cannot translate double-stranded RNA.
Yet, injecting sense RNA (RNA with the same sequence as
an mRNA) into the nematode Caenorhabditis elegans also
blocks protein production. Since the added RNA somehow
interferes with gene expression, this phenomenon is known
as RNA interference (RNAi). RNAi is now known to oc-
cur in all eukaryotes investigated except baker’s yeast.
The mechanism of RNAi began to come to light in 1998
when Andrew Fire and Craig Mello showed that double-
stranded RNA (dsRNA) was substantially more effective in
causing RNAi in C.elegans than were either of its component
strands alone. RNAi is induced by only a few molecules of
dsRNA per affected cell, suggesting that RNAi is a catalytic
rather than a stoichiometric effect. Further investigations, in
large part in Drosophila, have led to the elucidation of the
following pathway mediating RNAi (Fig. 31-72):
1. The trigger dsRNA, as Phillip Zamore discovered, is
chopped up into ⬃21- to 25-nt-long double-stranded frag-
ments known as small interfering RNAs (siRNAs), each of
Section 31-4. Post-Transcriptional Processing 1323
Figure 31-71 The recognition of ADAR editing sites. Both
ADAR1 and ADAR2 bind to a 9- to 15-bp double-stranded
RNA that is formed between the editing site (orange) on a
pre-mRNA exon and a so-called editing site complementary
sequence (ECS; pink) that is often located in a downstream
intron (brown).A represents the adenosine that the ADAR
(green) converts to inosine. [After Keegan, L.P., Gallo, A., and
O’Connell, M.A., Nature Rev. Genet. 2, 869 (2001).]
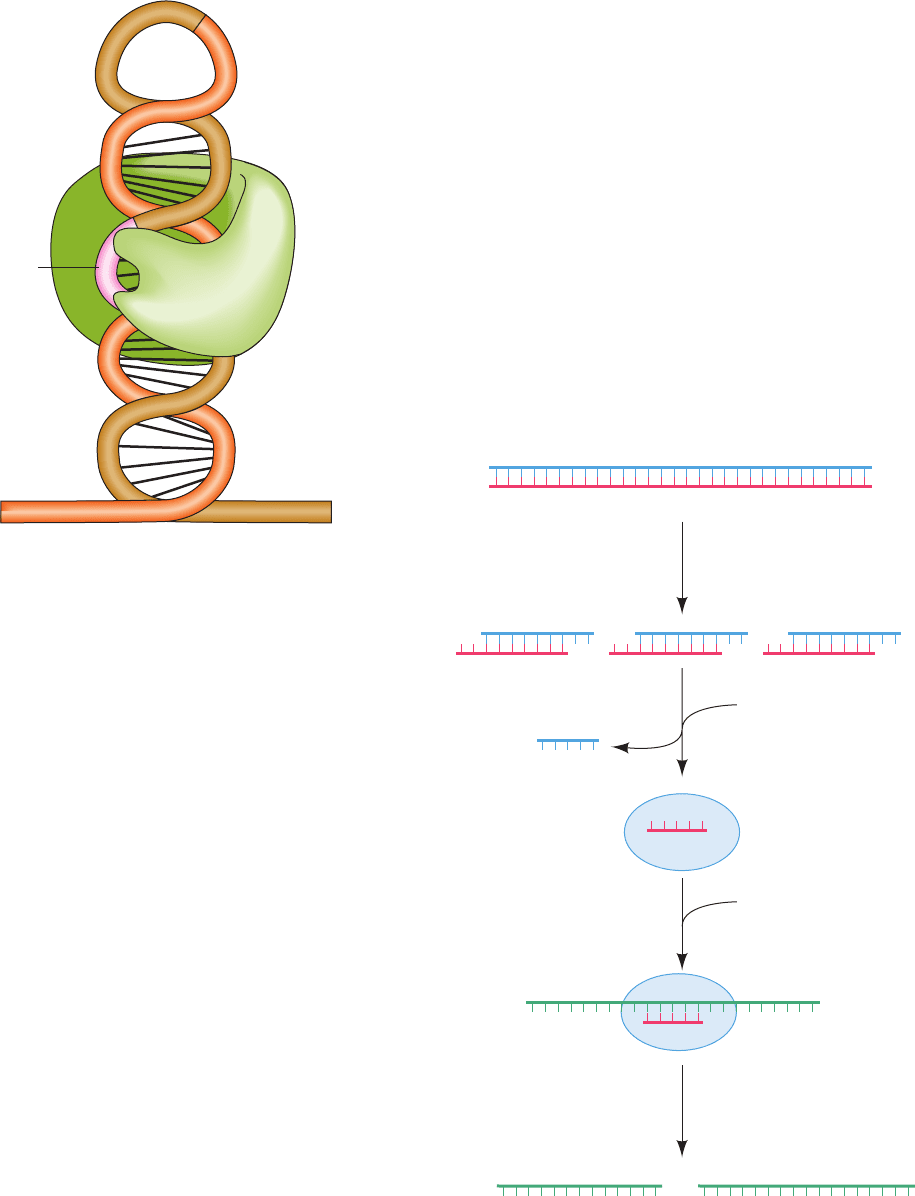
Figure 31-72 A mechanism for RNA interference (RNAi).
See the text for details.ATP is required for Dicer-catalyzed
cleavage of RNA and for RISC-associated helicase unwinding of
double-stranded RNA. Depending on the species, the mRNA
may not be completely degraded.
5′
Exon Intron
ECS
ADAR
A
pre-mRNA
3′
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1323
whose strands has a 2-nt overhang at its 3¿ end and a 5¿
phosphate.This reaction is mediated by an ATP-dependent
RNase named Dicer, a homodimer of ⬃1900-residue sub-
units in animals that is a member of the RNase III family of
double-strand–specific RNA endonucleases.
2. An siRNA is transferred to a 250- to 500-kD multi-
subunit complex known as RNA-induced silencing com-
plex (RISC). RISC has at least four protein components,
one of which is an ATP-dependent RNA helicase that sep-
arates the two strands of the siRNA. The strand whose 5¿
end has the lower free energy of binding,the guide RNA, is
bound by the RISC whereas its complementary strand, the
passenger RNA, is cleaved and discarded. In some species,
but apparently not in humans, the original siRNA signal is
amplified by the action of an RNA-dependent RNA poly-
merase (RdRP).
3. The guide RNA recruits the RISC complex to an
mRNA with the complementary sequence.
4. An RNase III component of RISC known as Arg-
onaute (AGO; also called Slicer) cleaves the mRNA oppo-
site the bound guide RNA.The cleaved mRNA is then fur-
ther degraded by cellular nucleases, thereby preventing its
translation.
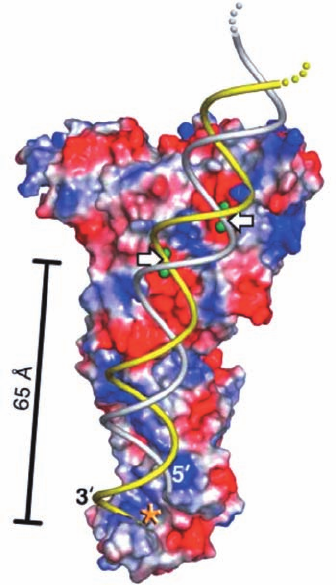
The X-ray structure of Dicer from the parasitic proto-
zoan Giardia intestinalis, determined by Jennifer Doudna,
reveals that its shape resembles that of a hatchet, with its
two RNase III domains forming the blade and its PAZ do-
main (named for three proteins in which it is contained,
PIWI, Argonaute, and Zwille) forming the base of its han-
dle (Fig. 31-73; Dicers from higher eukaryotes additionally
contain an N-terminal DExD/H box helicase domain and a
C-terminal dsRNA-binding domain). The two RNase III
domains form an internal heterodimer that resembles the
homodimeric structure of bacterial RNase III. Four con-
served acidic residues in each RNase III domain bind two
Mg
2⫹
ions and hence are postulated to cleave an RNA
strand via a two-metal-ion mechanism (Section 30-2Af).
The two RNase III active sites are 17.5 Å apart, the width
of dsRNA’s major grove, and thus appear positioned to
cleave the two strands of a bound dsRNA. The PAZ do-
main specifically binds dsRNA ends that have a 3¿ two-nt
overhang. The distance between this binding site and its
closest RNase III domain active site is 65 Å, the length of a
25-bp dsRNA. This explains how Dicer cleaves an ⬃25-bp
segment from the end of dsRNA.
Argonaute proteins consist of four domains: an N-
terminal (N), a PAZ, a middle (Mid), and a PIWI (for
P-element induced wimpy testis) domain. The X-ray struc-
tures of several bacterial Argonaute proteins reveal that
they have a bilobal architecture with the N and PAZ do-
mains forming one lobe and the Mid and PIWI domains
forming the other.The PIWI domain has an RNase H fold
(RNase H cleaves the RNA strand of an RNA ⴢ DNA hy-
brid helix), which strongly suggests that it mediates Arg-
onaute’s “slicer” activity (bacterial Argonautes preferen-
tially bind guide DNA over RNA). The X-ray structure of
T. thermophilus Argonaute in ternary complex with a 21-nt
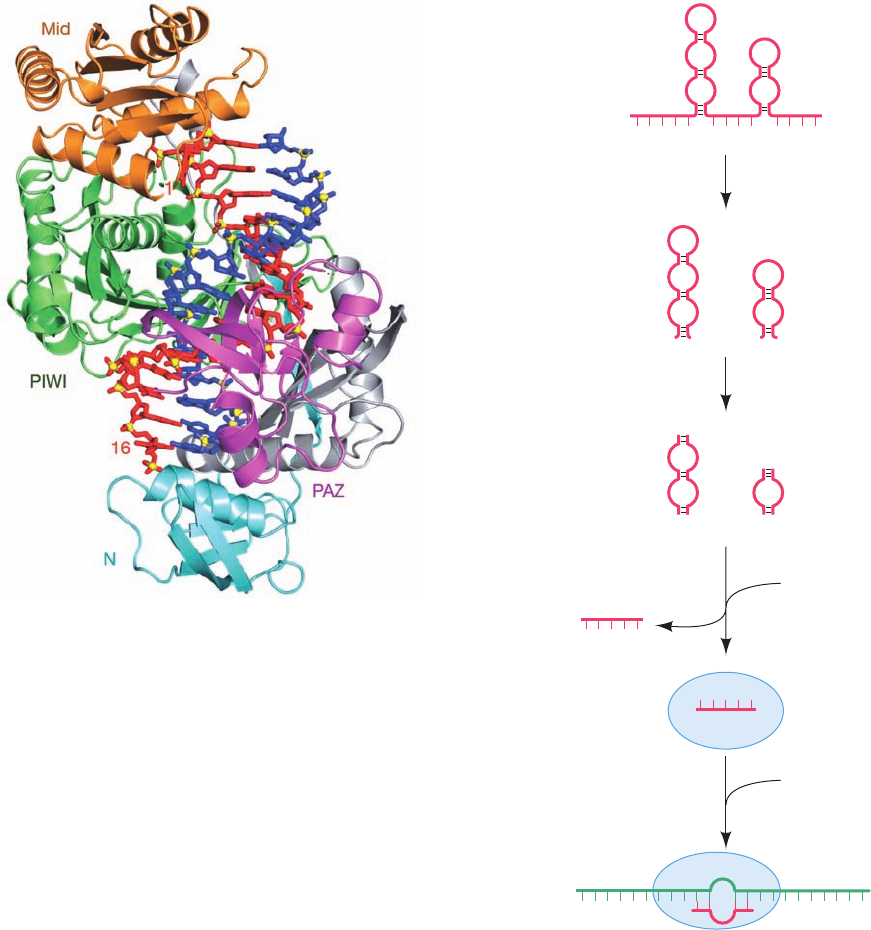
guide DNA and a 19-nt target RNA (Fig. 31-74), deter-
mined by Patel, reveals that the DNA ⴢ RNA hybrid helix
binds in the cleft between Argonaute’s two lobes with the
phosphate group bridging RNA nucleotides 10 and 11 po-
sitioned for cleavage at the PIWI active site. The compari-
son of this structure with those of similar complexes that
lack the target RNA or in which the target RNA has 12 or
15 nucleotides indicates that the guide DNA (and presum-
ably the guide RNA in eukaryotes) initially binds to Arg-
onaute with its 3¿ end in the PAZ binding pocket, but as the
hybrid helix lengthens beyond one turn, this 3¿ end is re-
leased, which facilitates further winding of the hybrid helix.
t. RNAi Defends against Viral Infection and
Regulates Gene Expression
What is the physiological function of RNAi? Since
many eukaryotic viruses store and replicate their genomes
as RNA (Chapter 33), it seems likely that RNAi arose as a
defense against viral infections. Indeed, many plant viruses
contain genes that suppress various steps of RNAi and
which are essential for pathogenesis. RNAi has also been
1324 Chapter 31. Transcription
Figure 31-73 X-ray structure of Dicer from G. intestinalis.
The protein is represented by its molecular surface colored
according to its surface charge with red negative, blue positive,
and white neutral. Bound Mg
2⫹
ions, which are represented by
green spheres, mark the active site of each of the protein’s two
RNase III domains.A dsRNA has been modeled into the
structure with its 3¿ overhang entering the PAZ domain’s binding
pocket (asterisk).The white arrows point to the dsRNA’s scissile
phosphate groups. Note that much of the surface to which the
anionic dsRNA is presumably bound is positively charged (the
calculated surface charge does not take into account the bound
Mg
2⫹
ions). [Courtesy of Jennifer Doudna, University of
California at Berkeley. PDBid 2FFL.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1324
shown to inhibit the intragenomic spread of retrotrans-
posons (Section 30-6Bh).
A wide variety of eukaryotes, including plants, nema-
todes, flies, fish, and mammals, use RNAi to control gene ex-
pression. Certain mRNAs expressed by these organisms
contain ⬃70-nt, imperfectly base-paired, stem–loop struc-
tures that are excised by a 1374-residue RNase III named
Drosha (Fig. 31-75).The stem–loops are exported from the
nucleus to the cytosol where they are cleaved by Dicer to
liberate ⬃22-bp dsRNAs known as microRNAs (miRNAs;
so called to differentiate these endogenous RNAs from ex-
ogenous siRNAs).The transcripts from which miRNAs are
derived are known as pri-miRNAs (pri for primary),
whereas the stem–loops are called a pre-miRNAs (pre for
precursor). Pre-miRNAs can be located within both the in-
trons and, less commonly, the exons of a pri-miRNA. The
miRNAs bind to RISC in which they function to identify the
tens to hundreds of mRNAs containing segments that are
partially complementary to the miRNA.
The RISC-bound miRNA binds to its targets site, which
is usually in the 3¿ untranslated region (3¿UTR) of an
mRNA. A lack of perfect complementarity prevents Arg-
onaute from cleaving the mRNA (Argonaute’s PIWI do-
main catalyzes slicing only if there is perfect complementar-
ity to the miRNA’s so-called seed sequence, which consists
of nucleotides 2–8 from its 5¿ end), and in fact, some species
of Argonaute lack the catalytic residues to do so. Instead,
miRNA-mediated silencing is thought to occur through the
removal of its target mRNA’s poly(A) tail or its m
7
G cap,
which leads to the mRNA’s degradation (Section 31-4Av),
and/or the RISC-mediated repression of the target mRNA’s
translation by interfering with ribosomal initiation (Section
32-3Cd) and sequestering or degrading the mRNA in cyto-
plasmic granules known as P bodies (P for processing).
Section 31-4. Post-Transcriptional Processing 1325
Figure 31-74 X-ray structure of T. thermophilus Argonaute in
ternary complex with a 21-nt guide DNA and a 19-nt target
RNA. The protein is shown in ribbon form with its N, PAZ, Mid,
and PIWI domains cyan, magenta, orange, and green, respec-
tively, and with the linkers connecting these domains gray.The
guide DNA and target RNA are drawn in stick form in red and
blue with their P atoms yellow. Only DNA nucleotides 1 to 16
and RNA nucleotides 2 to 16 are visible. [Courtesy of Dinshaw
Patel, Memorial-Sloan Kettering Cancer Center, New York, New
York. PDBid 3HK2.]
Figure 31-75 The generation of miRNAs from pri-miRNAs
and their RISC-mediated binding to target mRNAs. See the text
for details.
RISC
Guide RNA
Passenger RNA
RISC
Target mRNA
RISC
+
+
mRNA
Pri-miRNA
Drosha (in nucleus)
Pre-miRNAs
Dicer (in cytosol)
miRNAs
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1325
In 1993, Victor Ambros discovered the first known
miRNA, which is encoded by the lin-4 gene of C. elegans
(Fig. 31-76a). The lin-4 gene was known to control the tim-
ing of larval development, although it was thought that it
encoded a protein that repressed the expression of the lin-
14 gene. In fact, the lin-4 miRNA is complementary to
seven sites on the 3¿UTR of the lin-14 gene, which had pre-
viously been shown to mediate the repression of lin-14 by
the lin-4 gene product. A puzzling observation at the time
was that this regulation greatly reduces the amount of
LIN-14 protein produced without altering the level of
lin-14 mRNA. These findings were eventually followed by
the discovery that the C. elegans let-7 gene encodes what is
now known to be an miRNA (Fig. 31-76b) that controls the
transition from larval to adult stages of development. Sub-
sequently, let-7 homologs were identified in the Drosophila
and human genomes and let-7 RNA was detected in these
organisms as well as in numerous other animals.
Both the lin-4 and let-7 miRNAs were discovered by
genetic analyses. However most of the nearly 10,000 miRNAs
in plants and animals that are now known, including those in
Fig.31-76c,were identified through bioinformatic approaches
(Section 7-4). The known miRNAs are catalogued in the
miRBase database (http://www.mirbase.org/). Nearly all
miRNAs are conserved among closely related animals (e.g.,
mice and humans) and many are more broadly conserved
throughout animal lineages (e.g., over one-third of the 174 C.
elegans miRNAs have homologs in humans). The significance
of miRNAs is indicated by the fact that humans express over
720 miRNAs that participate in regulating ⬃30% of their
protein-coding genes.
RNAi has become the method of choice for “knocking
out” specific genes in plants and invertebrates. For example,
in C. elegans, RNAi has been used to systematically inacti-
vate over 16,000 of its ⬃19,000 protein-coding genes in an
attempt to assign a function to each gene. C. elegans is par-
ticularly amenable to the RNAi approach, since these
worms eat E. coli cells, and it is relatively easy to genetically
engineer the bacterial cells to express double-stranded
RNA that becomes part of the worms’ diet. One limitation
of the RNAi method is that it permits only the effects of
gene inactivation—not gene activation—to be examined.
RNAi is of similar use in mammalian systems, even
though mammals lack the mechanisms that amplify silenc-
ing in plants and nonvertebrates so that the effects of RNAi
in mammals are transient.The exquisite specificity of RNAi
may make it possible to prevent viral infections and to si-
lence disease-causing mutant genes such as oncogenes. In
fact,experiments have demonstrated that it is possible to use
RNAi to block the liver’s inflammatory response to a hepa-
titis virus, at least in mice, and to prevent HIV replication in
cultured human cells. One challenge for the future is to de-
vise protocols for more specific and longer-lasting gene si-
lencing that would make it possible to prevent viral infec-
tions or to block the effects of disease-causing mutant genes.
u. Mature Eukaryotic mRNAs Are Actively
Transported from the Nucleus to the Cytoplasm
The translation of prokaryotic mRNAs is often initi-
ated, as we have seen in Fig. 31-27, before their synthesis is
complete. This cannot occur in eukaryotes because the
transcription and post-transcriptional processing of eu-
karyotic mRNAs occurs in the nucleus but their translation
takes place in the cytosol. Consequently, mature mRNAs
must be transported from the nucleus to the cytoplasm.
This is a highly selective process because mature mRNAs
comprise only a small fraction of the RNAs present in the
nucleus, the remainder being pre-mRNAs, excised introns
(which are usually much larger than the exons from which
they were liberated), rRNAs, tRNAs, snRNAs, and a vari-
ety of RNAs that participate in the processing of rRNAs
and tRNAs (Sections 31-4B and 31-4C). Indeed, only ⬃5%
of the RNA that is synthesized ever leaves the nucleus.
How are mature mRNAs recognized and transported?
As we have seen, throughout their residency in the nucleus,
pre-mRNAs are continually associated with numerous
proteins, including those that participate in synthesizing
their m
7
G caps and poly(A) tails, and in splicing out their
introns. In addition, the exon junction complex (EJC),
which consists of four core proteins and several transiently
associating proteins, is deposited onto mRNA during the
splicing process at a site that is 20 to 24 nt upstream of the
splice junction without regard to its sequence. The popula-
tion of proteins bound to an mRNA changes as the mRNA
is processed but some of the proteins, including SR pro-
teins, hnRNPs (Section 31-4Ai), and EJCs remain associ-
1326 Chapter 31. Transcription
(a)
(b)
(c)
lin-4 RNA
let-7 RNA
miR-1*
miR-1
A
G
U
G
U
G
U
U
U
C
G
G
G
U
U
U
U
U
G
C
U
U
C
A
C
A
C
U
A
G
A
A
U
G
C
C
G
G
G
A
C
A
C
A
G
A
G
U
C
U
C
C
C
C
G
U
C
C
C
A
G
G
A
C
U
C
U
G
G
G
A
C C
3⬘5⬘
U
U
G
A
U
A
U
G
U
U
G
G
A
U
U
U
A
A
AA
A
G
G
U
C
C
C
U
C
G
G
G
G
A
U
G
G
A
G
U
G
G
C
C
U
C
U
A
C
C
U
U
A
C
C
G
G
A
A
A
C
U
A
U
G
C
A
A
U
U
U
3⬘5⬘
U U
U
U
A
U
C
A
U
G
G
U
C
G
C
C
G
C
C
A
U
G
G
U
C
G
G
A
U
G
G
G
A
A
A
G
A
A
G
U
A
U
G
U
A
G
C
U
U
C
A
U
A
C
G
U
C
3⬘5⬘
C
C
A
U
A
C
C
U
A
U
G
G
G
U
A
C
A
U
U
A
U
G
U
A
A
A
A
A
A
A
A
A
Figure 31-76 The predicted stem–loops of some pre-miRNAs.
The miRNAs contained in these pre-mRNAs, all of which are
from C. elegans, are red. (a) lin-4, (b) let-7, and (c) miR-1 and
miR-1* (in blue), which are largely complementary to each other.
JWCL281_c31_1260-1337.qxd 8/26/10 10:21 PM Page 1326
ated with mature mRNAs in the nucleus. However, it ap-
pears that it is its entire collection of bound proteins rather
than any individual protein that serves to identify an
mRNA to the nuclear export machinery.
The eukaryotic nucleus (Fig. 1-5) is a double membrane-
enveloped organelle that in animals is penetrated by an
average of ⬃3000 pores. These are formed by nuclear pore
complexes (NPCs), which are massive (⬃120,000 kD),
8-fold symmetric assemblies of ⬃30 different proteins
known as nucleoporins. NPCs, which have inner diameters
of ⬃90 Å (although this may be expandable to as much as
260 Å), allow the free diffusion of molecules of up to 50 kD,
but most macromolecules, including mRNAs in their com-
plexes with proteins, require an active transport process to
pass through an NPC. Some of the proteins associated with
mature mRNAs bear nuclear export signals that are recog-
nized by a protein receptor that in yeast is named Dbp5. This
482-residue DExD/H box protein (Dbp5 stands for
DExD/H box protein 5) is an ATP-driven RNA helicase that
also binds to the NPC.This permits Dbp5 to pull the mRNA
out into the cytosol while simultaneously stripping away
many of its bound proteins.These proteins are later recycled
by returning them to the nucleus through the NPCs.
v. mRNA Degradation Is Elaborately Controlled
The synthesis and maturation of mRNAs, as we have
seen, are subject to multiple controls. The same is true of
their degradation. Indeed, the range of mRNA stability in
eukaryotic cells, measured in half-lives, varies from a few
minutes to many hours or days. The mRNA molecules
themselves contain elements that dictate their decay rates.
These elements include the 3¿ poly(A) tail and the 5¿ m
7
G
cap, which protect against exonucleases, as well as se-
quences that are located within the coding region.
A major route for mRNA degradation begins with the
progressive removal of its poly(A) tail,a process catalyzed by
deadenylases that are located throughout the cytosol. When
the residual poly(A) tail is less than ⬃10 nt long and hence no
longer capable of binding poly(A) binding protein (Section
31-4Ab),the mRNA becomes a substrate for a decapping en-
zyme, which hydrolytically excises the mRNA’s m
7
G cap.
This is possible because the translational initiation factor
eIF4G interacts with both poly(A) binding protein and cap
binding protein (Section 32-3Cd), thereby circularizing the
mRNA so that events at its 3¿ end can be coupled to events at
its 5¿ end.The decapped and deadenylated mRNA is then de-
graded by exonucleases, mainly the 1706-residue 5¿S3¿ ex-
onuclease Xrn1 and the 3¿S5¿ exonuclease complex named
the exosome. A decapping enzyme,5¿S3¿ exonucleases, and
accessory proteins form P bodies (Section 31-4At) that func-
tion to either degrade mRNA or store it in an inactive form.
Proteins that bind to AU-rich elements (AREs) in the 3¿
untranslated region of mRNAs also appear to increase or
decrease the rate of mRNA degradation, although their ex-
act action is poorly understood. RNA secondary structure
and RNA-binding proteins, which may be susceptible to
modification by cellular signaling pathways, are thought to
play a role in regulating mRNA stability.
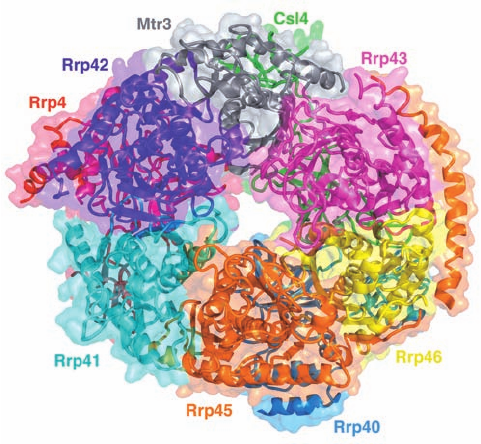
The eukaryotic core exosome consists of single copies of
nine different subunits. Its X-ray structure (Fig. 31-77), de-
termined by Christopher Lima, reveals that six of these
subunits, Rpr41 (Rpr for rRNA processing; the exosome
was discovered as an activity that processed the 3¿ ends of
rRNAs), Rrp42, Mtr3, Rrp43, Rrp46, and Rrp45, form a
six-membered ring with the remaining three subunits,
Rrp4, Csl4, and Rrp40, bound to the same face of this ring.
These subunits are arranged such that the core exosome
contains an ⬃9-Å-wide central channel that allows the en-
trance of only single-stranded RNAs.
The archeal exosome appears to be a simpler version of
the eukaryotic core exosome. Its six-membered ring consists
of only two types of subunits, Rrp41 and Rrp42, that alter-
nate around the ring, with three copies of Rrp4 bound to
the same face of the ring. Only Rrp41 contains an active
site although Rrp42 is required for activity. Not surpris-
ingly, eukaryotic Rrp4, Mtr3, and Rrp46 are homologs of
archeal Rrp41, eukaryotic Rrp42, Rrp43, and Rrp45 are
homologs of archeal Rrp42, and eukaryotic Rrp4, Csl4, and
Rrp40 are homologs of archeal Rrp4. Nevertheless, despite
the fact that each of its core subunits are essential for via-
bility, eukaryotic core exosomes, from yeast to humans, are
catalytically inactive due to changes in active site residues
relative to their archeal homologs. However, the core exo-
some associates with two 3¿-exonucleases, Rrp6 and Rrp44,
whose catalytically inactive mutants are individually viable
in yeast but lethal in combination. Moreover, the core exo-
some interacts with numerous mostly multisubunit cofac-
tors that carry out a variety of RNA processing activities in
both the nucleus and the cytosol.Thus, the eukaryotic core
exosome appears to be a structural platform upon which
many RNA processing enzymes can be mounted.
Section 31-4. Post-Transcriptional Processing 1327
Figure 31-77 X-ray structure of the human core exosome. The
protein complex is drawn in ribbon form embedded in its
semitransparent molecular surface, with each of its nine different
subunits separately colored.The view is toward the face of the
six-membered ring of subunits opposite that to which the three
other subunits bind. [Based on an X-ray structure by Christopher
Lima, Sloan-Kettering Institute, New York, New York. PDBid
2NN6.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1327
B. Ribosomal RNA Processing
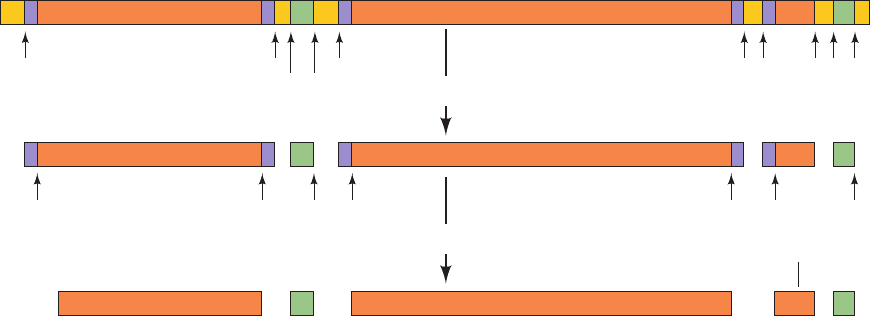
The seven E. coli rRNA operons all contain one (nearly iden-
tical) copy of each of the three types of rRNA genes (Section
32-3A). Their polycistronic primary transcripts, which are
⬃5500 nucleotides in length, contain 16S rRNA at their 5¿
ends followed by the transcripts for 1 or 2 tRNAs, 23S rRNA,
5S rRNA, and, in some rRNA operons, 1 or 2 more tRNAs at
the 3¿ end (Fig. 31-78). The steps in processing these primary
transcripts to mature rRNAs were elucidated with the aid of
mutants defective in one or more of the processing enzymes.
The initial processing, which yields products known as
pre-rRNAs, commences while the primary transcript is still
being synthesized. It consists of specific endonucleolytic
cleavages by RNase III, RNase P, RNase E, and RNase F
at the sites indicated in Fig. 31-78.The base sequence of the
primary transcript suggests the existence of several base-
paired stems. The RNase III cleavages occur in a stem con-
sisting of complementary sequences flanking the 5¿ and 3¿
ends of the 23S segment (Fig. 31-79) as well as that of the
16S segment. Presumably, certain features of these stems
constitute the RNase III recognition site.
The 5¿ and 3¿ ends of the pre-rRNAs are trimmed away
in secondary processing steps (Fig. 31-78) through the ac-
tion of RNases D, M16, M23, and M5 to produce the
mature rRNAs. These final cleavages only occur after the
pre-rRNAs become associated with ribosomal proteins.
a. Ribosomal RNAs Are Methylated
During ribosomal assembly, the 16S and 23S rRNAs are
methylated at a total of 24 specific nucleosides. The methyla-
tion reactions, which employ S-adenosylmethionine (Section
26-3Ea) as a methyl donor, yield N
6
,N
6
-dimethyladenine and
O
2¿
-methylribose residues. O
2¿
-methyl groups may protect ad-
jacent phosphodiester bonds from degradation by intracellu-
lar RNases (the mechanism of RNase hydrolysis involves uti-
lization of the free 2¿-OH group of ribose to eliminate the
substituent on the 3¿-phosphoryl group via the formation of a
2¿,3¿-cyclic phosphate intermediate; Figs. 5-3 and 15-3). How-
ever, the function of base methylation is unknown.
b. Eukaryotic rRNA Processing Is Guided
by snoRNAs
The eukaryotic genome typically has several hundred
tandemly repeated copies of rRNA genes that are contained
in small, dark-staining nuclear bodies known as nucleoli (the
site of rRNA transcription and processing and ribosomal
subunit assembly; Fig. 1-5; note that nucleoli are not mem-
brane enveloped). The primary rRNA transcript is an
⬃7500-nucleotide 45S RNA that contains,starting from its 5¿
end, the 18S, 5.8S, and 28S rRNAs separated by spacer se-
quences (Fig. 31-80). In the first stage of its processing, 45S
RNA is specifically methylated at numerous sites (106 in hu-
mans) that occur mostly in its rRNA sequences.About 80%
of these modifications yield O
2¿
-methylribose residues and
the remainder form methylated bases such as N
6
,N
6
-di-
methyladenine and 2-methylguanine. In addition, many pre-
rRNA U’s (95 in humans) are converted to pseudouridines
(⌿’s) (Section 30-5Be), which may contribute to the rRNA’s
tertiary stability through hydrogen bonding involving its
newly acquired ring NH group. The subsequent cleavage
and trimming of the 45S RNA superficially resembles that of
prokaryotic rRNAs. In fact, enzymes exhibiting RNase
III–and RNase P–like activities occur in eukaryotes. The 5S
eukaryotic rRNA is separately processed in a manner re-
sembling that of tRNA (Section 31-4C).
The methylation sites in eukaryotic rRNAs occur exclu-
sively within conserved domains that are therefore likely
to participate in fundamental ribosomal processes. Indeed,
the methylation sites generally occur in invariant se-
quences among yeast and vertebrates although the meth-
ylations themselves are not always conserved. These meth-
ylation sites do not appear to have a consensus structure
1328 Chapter 31. Transcription
Figure 31-78 The post-transcriptional processing of E. coli
rRNA. The transcriptional map is shown approximately to scale.
The labeled arrows indicate the positions of the various
nucleolytic cuts and the nucleases that generate them. [After
Number
of bases:
5′
180
1700 150 200 2920 300
3′
RNase: III III
PF
III III P F P E
RNase:
M16
5′ 3′
M16 D M23
Primary processing
Primary transcript
Secondary processing
M23 M5 D
Number
of bases:
16S rRNA tRNA(s) 23S rRNA
5S rRNA
tRNA(s)
3′5′
1541 2904 120
Pre-16S rRNA Pre-23S rRNA
Pre-5S
rRNA
Apiron, D., Ghora, B.K., Plantz, G., Misra, T.K., and
Gegenheimer, P., in Söll, D., Abelson, J.N., and Schimmel P.R.
(Eds.), Transfer RNA: Biological Aspects, p. 148, Cold Spring
Harbor Laboratory Press (1980).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1328
that might be recognized by a single methyltransferase.
How, then, are these methylation sites targeted?
An important clue as to how the methylation sites on
rRNA are selected came from the observation that pre-
rRNA interacts with the members of a large family of small
nucleolar RNAs (snoRNAs; ⬃100 in yeast and ⬃200 in
mammals). The snoRNAs, whose lengths vary from 70 to
100 nt, contain segments of 10 to 21 nt that are precisely
complementary to segments of the mature rRNAs that con-
tain the O2¿-methylation sites. These snoRNA sequences
are located between the conserved sequence motifs known
as box C (RUGAUGA) and box D (CUGA), which are re-
spectively located on the 5¿ and 3¿ sides of the complemen-
tary segments. In intron-rich organisms such as vertebrates,
most snoRNAs are encoded by the introns of structural
genes so that not all excised introns are discarded.
The snoRNA nucleotide that pairs with the nucleotide
to be O2¿-methylated always precedes box D by exactly 5
nt. Evidently, each of these so-called box C/D snoRNAs act
to guide the methylation of a single site. In fact, in those
cases in which two adjacent ribose residues are methylated,
two box C/D snoRNAs with overlapping sequences occur.
The methylation is mediated by a complex of at least four
nucleolar proteins, including fibrillarin (⬃325 residues; so
called because it is located in the dense fibrillar region of
the nucleolus), the likely methyltransferase,which together
with a box C/D snoRNA form snoRNPs. The conversion of
specific rRNA U’s to ⌿’s is similarly mediated by a differ-
ent subgroup of snoRNAs, the box H/ACA snoRNAs, so
called because they contain the sequence motifs ACANNN
at the snoRNA’s 3¿ end and box H (ANANNA) at its 5¿
end, so as to flank a sequence that partially base-pairs to
the pre-rRNA segment containing the U to be converted
to ⌿. Archaea also modify their rRNAs via RNA-guided
methylations and U to ⌿ conversions but, interestingly, the
analogous reactions in eubacteria are mediated by protein
enzymes that lack RNA.
C. Transfer RNA Processing
tRNAs, as we discuss in Section 32-2A, consist of ⬃80 nu-
cleotides that assume a secondary structure with four base-
paired stems known as the cloverleaf structure (Fig. 31-81).
All tRNAs have a large fraction of modified bases (whose
structures and functions are discussed in Section 32-2Aa)
and each has the 3¿-terminal sequence ¬CCA to which the
Section 31-4. Post-Transcriptional Processing 1329
Figure 31-79 The stem-and-giant-loop secondary structure in the 23S region of the
E. coli primary rRNA transcript. The RNase III cleavage sites are indicated. [After Young,
R.R., Bram, R.J., and Steitz, J.A., in Söll, D., Abelson, J.N., and Schimmel, P.R. (Eds.),
Transfer RNA: Biological Aspects, p. 102, Cold Spring Harbor Laboratory Press (1980).]
Figure 31-80 The organization of the 45S primary transcript
of eukaryotic rRNA.
U
U
U
U
U
U
U
U
U
G–20
G
G
G
G
G
G
G
G
C
C
C
C
A
A
A
A
A
A
A
AA
AG
AC
G
G
A
A
A
A
A
A
A
U
C
C
G
C
C
U
C
C
C
CG
G
GG
G
G
G
G
U
U
U
U
U
U
U
U
23S rRNA
U
20
–10
10
–1
RNase III
RNase III
1
(~2900 nucleotides)
3⬘5⬘
5′ 3′
45S RNA
28S5.8S18S
Figure 31-81 A schematic diagram of the tRNA cloverleaf
secondary structure. Each dot indicates a base pair in the
hydrogen bonded stems.The position of the anticodon triplet
and the 3¿-terminal ¬CCA are indicated.
5′ p
3′OHA
C
C
X
-
Y
-
Z
Anticodon
JWCL281_c31_1260-1337.qxd 8/26/10 10:22 PM Page 1329
corresponding amino acid is appended in the amino
acid–charged tRNA. The anticodon (which is complemen-
tary to the codon specifying the tRNA’s corresponding
amino acid) occurs in the loop of the cloverleaf structure
opposite the stem containing the terminal nucleotides.
The E. coli chromosome contains ⬃60 tRNA genes.
Some of them are components of rRNA operons (Section
31-4B); the others are distributed, often in clusters,
throughout the chromosome. The primary tRNA tran-
scripts, which contain from one to as many as four or five
identical tRNA copies, have extra nucleotides at the 3¿ and
5¿ ends of each tRNA sequence.The excision and trimming
of these tRNA sequences resemble those for E. coli rRNAs
(Section 31-4B) in that both processes employ some of the
same nucleases.
a. RNase P Is a Ribozyme
RNase P, which generates the 5¿ ends of tRNAs (Fig. 31-
78), is a particularly interesting enzyme because it has, in E.
coli, a 377-nucleotide RNA component (⬃125 kD vs 14 kD
for its 119-residue protein subunit) that is essential for its
enzymatic activity. The enzyme’s RNA was, quite under-
standably, first proposed to function in recognizing the sub-
strate RNA through base pairing and to thereby guide the
protein subunit, which was presumed to be the actual nu-
clease, to the cleavage site. However, Sidney Altman
demonstrated that the RNA component of RNase P is, in
fact, the enzyme’s catalytic subunit by showing that protein-
free RNase P RNA catalyzes the cleavage of substrate
RNA at high salt concentrations. RNase P protein, which is
basic, evidently functions at physiological salt concentra-
tions to electrostatically reduce the repulsions between the
polyanionic ribozyme and substrate RNAs. The argument
that trace quantities of RNase P protein are really respon-
sible for the RNase P reaction was disposed of by showing
that catalytic activity is exhibited by RNase P RNA that
has been transcribed in a cell-free system. RNase P activity
occurs in eukaryotes (nuclei, mitochondria, and chloro-
plasts) as well as in prokaryotes although eukaryotic nu-
clear RNase P’s have 9 or 10 protein subunits, none of
which are related to the bacterial protein. Indeed, RNase P
1330 Chapter 31. Transcription
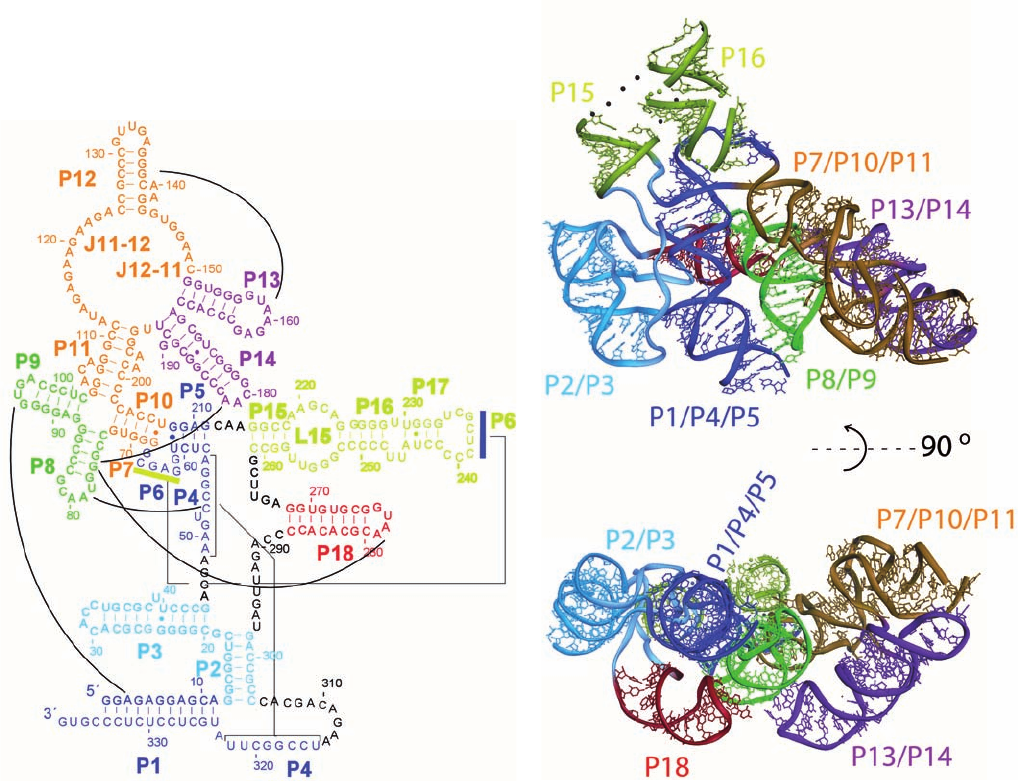
Figure 31-82 Structure of the RNA component of
T. maritima RNase P. (a) Its sequence and secondary structure.
The various segments (P for paired region, J for joining region,
and L for loop) are shown in different colors.The black lines
indicate major interactions that are observed in the X-ray
structure, dashes indicate Watson–Crick base pairs, and small
filled circles represent non-Watson–Crick base pairs. (b) Its
X-ray structure, which is colored as in Part a. Of its 338
nucleotides, 309 are visible.The lower view is related to the
upper view by a 90° rotation about the horizontal axis. [Courtesy
of Alfonso Mondragón, Northwestern University. PDBid 2A2E.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:49 PM Page 1330
