Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

activated expression platform forms an intrinsic transcrip-
tion termination site (Section 31-2Da) so that transcription
beyond this site proceeds only when the effector is absent.
In others, the activated expression platform cleaves itself,
thereby inactivating the mRNA (the ability of RNA to act
as an enzyme is discussed in Section 31-4Ae).
Riboswitches collectively regulate ⬎2% of the genes in
certain bacteria. Plants and fungi also contain riboswitches
(although the above TTP-sensing riboswitch could not
function in eukaryotes because eukaryotic ribosomes do
not bind Shine–Dalgarno sequences; Section 32-3Cd). The
fact that the interaction of riboswitches with their effectors
does not require the participation of proteins suggests that
they are relics of the RNA world (Section 1-5Ca) and
hence among the oldest regulatory systems.
I. Regulation of Ribosomal RNA Synthesis:
The Stringent Response
E. coli cells growing under optimal conditions divide every
20 min. Such cells contain up to 20,000 ribosomes and
hence must synthesize ⬃10,000 ribosomes per cell division
cycle. Yet RNAP can initiate the transcription of an rRNA
gene no faster than about once per second. If E. coli con-
tained only one copy of each of the three types of rRNA
genes (those specifying the so-called 23S, 16S, and 5S
rRNAs; Section 32-3A), fast-growing cells could synthesize
no more than ⬃1200 ribosomes during their cell division
cycle. However, the E. coli genome contains seven sepa-
rately located rRNA operons, all of which contain one
nearly identical copy of each type of rRNA gene. Moreover,
rapidly growing cells contain multiple copies of their repli-
cating chromosomes (Section 30-3Cb), thereby accounting
for the observed rRNA synthesis rate.
Cells have the remarkable ability to coordinate the rates
at which their thousands of components are synthesized.
For example,E. coli adjust their ribosome content to match
the rate at which they can synthesize proteins under the
prevailing growth conditions.The rate of rRNA synthesis is
therefore proportional to the rate of protein synthesis. One
mechanism by which this occurs is known as the stringent
response: A shortage of any species of amino acid–charged
tRNA (usually a result of “stringent” or poor growth condi-
tions) that limits the rate of protein synthesis triggers a
sweeping metabolic readjustment. A major facet of this
change is an abrupt 10- to 20-fold reduction in the rate of
rRNA and tRNA synthesis. This stringent control, more-
over, depresses numerous metabolic processes (including
DNA replication and the biosynthesis of carbohydrates,
lipids, nucleotides, proteoglycans, and glycolytic intermedi-
ates) while stimulating others (such as amino acid biosyn-
thesis). The cell is thereby prepared to withstand nutri-
tional deprivation.
a. (p)ppGpp Mediates the Stringent Response
The stringent response is correlated with a rapid intracel-
lular accumulation of two unusual nucleotides, ppGpp and
pppGpp [known collectively as (p)ppGpp], and their
prompt decay when amino acids become available. The ob-
servation that mutants, designated relA
⫺
, which do not ex-
hibit the stringent response (they are said to have relaxed
control) lack (p)ppGpp suggests that these substances me-
diate the stringent response.This idea was corroborated by
in vitro studies demonstrating, for example, that (p)ppGpp
inhibits the transcription of rRNA genes but stimulates the
transcription of the trp and lac operons as does the strin-
gent response in vivo. Apparently, (p)ppGpp acts by some-
how altering RNAP’s promoter specificity at stringently
controlled operons, a hypothesis that is supported by the
isolation of RNAP mutants that exhibit reduced responses
to (p)ppGpp. In addition, (p)ppGpp causes an increased
frequency of pausing in RNAPs engaged in elongation,
thereby reducing the rate of transcription.
The protein encoded by the wild-type relA gene, named
stringent factor (RelA), catalyzes the reaction
and, to a lesser extent,
However, several ribosomal proteins convert pppGpp to
ppGpp so that ppGpp is the stringent response’s usual effec-
tor. Stringent factor is only active in association with a ribo-
some that is actively engaged in translation. (p)ppGpp syn-
thesis occurs when a ribosome binds its mRNA-specified
but uncharged tRNA (lacking an appended amino acid
residue). The binding of a specified and charged tRNA
greatly reduces the rate of (p)ppGpp synthesis. The ribo-
some apparently signals the shortage of an amino acid by
stimulating the synthesis of (p)ppGpp which,acting as an in-
tracellular messenger, influences the rates at which a great
variety of operons are transcribed.
(p)ppGpp degradation is catalyzed by the spoT gene
product. The spoT
⫺
mutants show a normal increase in
(p)ppGpp level on amino acid starvation but an abnor-
mally slow decay of (p)ppGpp to basal levels when amino
acids again become available.The spoT
⫺
mutants therefore
exhibit a sluggish recovery from the stringent response.
The (p)ppGpp level is apparently regulated by the counter-
vailing activities of stringent factor and the spoT gene
product.
4 POST-TRANSCRIPTIONAL
PROCESSING
The immediate products of transcription, the primary tran-
scripts, are not necessarily functional entities. In order to
acquire biological activity, many of them must be specifi-
cally altered in several ways: (1) by the exo- and endonu-
cleolytic removal of polynucleotide segments; (2) by ap-
pending nucleotide sequences to their 3¿ and 5¿ ends; and
(3) by the modification of specific nucleosides. The three
major classes of RNAs, mRNA, rRNA, and tRNA, are al-
tered in different ways in prokaryotes and in eukaryotes. In
this section we shall outline these post-transcriptional
modification processes.
ATP ⫹ GDP Δ AMP ⫹ ppGpp
ATP ⫹ GTP Δ AMP ⫹ pppGpp
Section 31-4. Post-Transcriptional Processing 1301
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1301
A. Messenger RNA Processing
In prokaryotes, most primary mRNA transcripts function
in translation without further modification. Indeed, as we
have seen, ribosomes in prokaryotes usually commence
translation on nascent mRNAs. In eukaryotes, however,
mRNAs are synthesized in the cell nucleus, whereas trans-
lation occurs in the cytosol. Eukaryotic mRNA transcripts
can therefore undergo extensive post-transcriptional pro-
cessing while still in the nucleus.
a. Eukaryotic mRNAs Are Capped
Eukaryotic mRNAs have a peculiar enzymatically
appended cap structure consisting of a 7-methylguanosine
(m
7
G) residue joined to the transcript’s initial (5¿) nucleo-
side via a 5¿–5¿ triphosphate bridge (Fig. 31-47).This m
7
cap,
which is added to the growing transcript before it is ⬃30
nucleotides long, defines the eukaryotic translational start
site (Section 32-3Cd). A cap may be O
2¿
-methylated at the
transcript’s leading nucleoside (cap-1, the predominant
cap in multicellular organisms), at its first two nucleosides
(cap-2), or at neither of these positions (cap-0, the pre-
dominant cap in unicellular eukaryotes). If the leading
nucleoside is adenosine (it is usually a purine), it may also
be N
6
-methylated.
Capping involves several enzymatic reactions: (1) the
removal of the leading phosphate group from the mRNA’s
5¿ terminal triphosphate group by an RNA triphosphatase;
(2) the guanylation of the mRNA by capping enzyme,
which requires GTP and yields the 5¿–5¿ triphosphate
bridge and PP
i
; (3) the methylation of guanine by guanine-
7-methyltransferase in which the methyl group is supplied
by S-adenosylmethionine (SAM); and possibly (4) the O2¿
methylation of the mRNA’s first and perhaps its second
nucleotide by a SAM-requiring 2ⴕ-O-methyltransferase.
Both the capping enzyme and the guanine-7-methyltrans-
ferase bind to RNAP II’s phosphorylated CTD (Section
31-2E). Hence it is likely that capping marks the comple-
tion of RNAP II’s switch from transcription initiation to
elongation.
b. Eukaryotic mRNAs Have Poly(A) Tails
Eukaryotic mRNAs, unlike those of prokaryotes, are
invariably monocistronic. Moreover, in contrast to the case
in bacteria (Section 31-2D), no eukaryotic transcriptional
termination sequence has been identified (but see below).
In fact, the eukaryotic termination process is imprecise;
that is, the primary transcripts of a given structural gene
have heterogeneous 3¿ sequences. Nevertheless, mature eu-
karyotic mRNAs have well-defined 3¿ ends; almost all of
them in mammals have 3¿-poly(A) tails of ⬃250 nucleotides
(⬃80 in yeast). The poly(A) tails are enzymatically ap-
pended to the primary transcripts in two reactions that are
mediated by a 500- to 1000-kD complex that consists of at
least six proteins:
1. A transcript is cleaved to yield a free 3¿-OH group at
a specific site that is 15 to 25 nucleotides past an
AAUAAA sequence and within 50 nucleotides before a
U-rich or G ⫹ U–rich sequence. The AAUAAA sequence
is highly conserved in higher eukaryotes (but not yeast) in
which its mutation abolishes cleavage and polyadenyla-
tion. The precision of the cleavage reaction has apparently
eliminated the need for accurate transcriptional termina-
tion. Nevertheless, the identity of the endonuclease that
cleaves the RNA is uncertain although cleavage factors I
and II (CFI and CFII) are required for this process.
2. The poly(A) tail is subsequently generated from ATP
through the stepwise action of poly(A) polymerase (PAP).
This enzyme, which by itself only weakly binds RNA, is re-
cruited by cleavage and polyadenylation specificity factor
(CPSF) on this heterotetramer’s recognition of the
AAUAAA sequence, which it does with almost no toler-
ance for sequence variation. The downstream G ⫹ U–rich
element is recognized by the heterotrimeric cleavage stim-
ulation factor (CstF), which increases the affinity with
which CPSF binds the AAUAAA sequence. However,
1302 Chapter 31. Transcription
Figure 31-47 The structure of the 5¿ cap of eukaryotic
mRNAs. It is known as cap-0, cap-1, or cap-2, respectively, if it
has no further modifications, if the leading nucleoside of the
transcript is O
2¿
-methylated, or if its first two nucleosides are
O
2¿
-methylated.
O
HH
H
H
OH OH
CH
2
P
O
O
O
P
–
O
N
O
N
H
H
2
N
N
N
+
H
CH
3
O
O
–
O
P
O
O
–
O
O
P
O
O
–
O
O
P
O
O
–
O
O
HH
H
H
O(CH
3
)
CH
2
Base
2
O
HH
H
H
O(CH
3
)
CH
2
Base
1
...
7-Methyl-G
May be
N
6
-methylated
if A
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1302
once the poly(A) tail has grown to ⬃10 residues, the
AAUAAA sequence is no longer required for further
chain elongation. This suggests that CPSF becomes disen-
gaged from its recognition site in a manner reminiscent of
the way factor is released from the transcriptional initia-
tion site once the elongation of prokaryotic mRNA is un-
der way (Section 31-2B). The final length of the poly(A)
tail is controlled by poly(A)-binding protein II (PAB II),
multiple copies of which bind to successive segments of
poly(A). PAB II also increases the processivity of PAP.
Both CPSF and CstF bind to the phosphorylated RNAP II
CTD (Section 31-2E); deleting the CTD inhibits
polyadenylation. Evidently, the CTD couples polyadenyla-
tion to transcription. The mechanism that controls the
length of a poly(A) tail is unclear.
PAP is a template-independent RNA polymerase that
elongates an mRNA primer with a free 3¿-OH group. The
X-ray structure of the 530-residue D154A mutant form of
yeast PAP (D154 is a catalytically essential active site
residue) in complex with A
5
and ATP, determined by An-
drew Bohm, reveals that this monomeric protein consists
of three domains that form a prominent U-shaped cleft
(Fig. 31-48). Hence it has the handlike domain arrange-
ment of template-directed polymerases (Section 30-2Ad).
Indeed, PAP’s N-terminal domain, which contains the en-
zyme’s active site, is homologous to the palm domain of
DNA polymerase , although it forms the thumb side of
the cleft rather than its base. PAP’s central domain, which
forms the base of the cleft, is functionally but not struc-
turally analogous to the fingers domain of template-
directed polymerases in that it interacts with the  and ␥
phosphates of the incoming ATP. However, the C-terminal
domain shows no resemblance to a fingers domain. Rather,
it is topologically similar to the RNA-recognition motif
[RRM; also known as the RNA-binding domain (RBD)]
that occurs in ⬎200 different RNA-binding proteins (see
below).The A
5
binds in the cleft in an extended conforma-
tion such that, in contrast to the nucleic acids bound to
template-dependent polymerases, its bases are not in con-
tact. However, the 3¿-terminal base of the A
5
stacks on the
base of the ATP.
In comparison to the X-ray structure of yeast PAP in
complex with 3¿-dATP, also determined by Bohm, the N-
terminal domain in the PAP–A
5
–ATP structure has under-
gone an ⬃23⬚ hingelike motion toward the C-terminal do-
main to form a closed conformation resembling that
observed in the structure of Taq DNA polymerase I in
complex with its substrates (Section 30-2Ae). This motion
forms several new interactions, both direct and water-
mediated, between PAP and its ATP substrate that helps
differentiate adenine from other bases. In contrast, in tem-
plate-dependent polymerases, the incoming base only
makes sequence-specific contacts with the template base
(Section 30-2Ae).
In vitro studies indicate that a poly(A) tail is not re-
quired for mRNA translation. Rather, the observations
that an mRNA’s poly(A) tail shortens as it ages in the
cytosol and that unadenylated mRNAs have abbreviated
cytosolic lifetimes suggest that poly(A) tails have a protec-
tive role. In fact, the only mature mRNAs that lack poly(A)
tails, those of histones (which, with few exceptions, lack the
AAUAAA cleavage–polyadenylation signal), have life-
times of ⬍30 min in the cytosol, whereas most other
mRNAs last hours or days.The poly(A) tails are specifically
complexed in the cytosol by poly(A) binding protein
(PABP; not related to PAB II), which organizes poly(A)-
bearing mRNAs into ribonucleoprotein particles. PABP is
thought to protect mRNA from degradation as is sug-
gested, for example, by the observation that the addition of
PABP to a cell-free system containing mRNA and mRNA-
degrading nucleases greatly reduces the rate at which the
mRNAs are degraded and the rate at which their poly(A)
tails are shortened.
All known PABPs contain four tandem and highly con-
served RNA-recognition motifs (RRMs) followed by a less
conserved Pro-rich C-terminal segment of variable length.
A variety of evidence suggests that PABP’s first two RRMs
support most of the biochemical functions of full-length
PABP. The X-ray structure of the first two RRMs of human
PABP (RRM1/2; the N-terminal 190 residues of this 636-
residue protein) in complex with A
11
, determined by
Stephen Burley, reveals that RRM1/2 forms a continuous
trough-shaped surface in which the poly(A) binds in an
extended conformation via interactions with conserved
Section 31-4. Post-Transcriptional Processing 1303
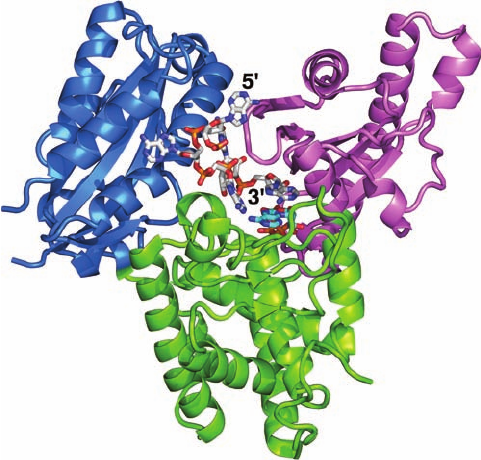
Figure 31-48 X-ray structure of the D154A mutant of yeast
poly(A) polymerase (PAP) in complex with A
5
and ATP. PAP is
drawn in ribbon form with its N-terminal domain lavender, its
central domain yellow-green, and its C-terminal domain light
blue. The A
5
and ATP are drawn in stick form with A
5
C white,
ATP C cyan, N blue, O red, and P orange. [Based on an X-ray
structure by Andrew Bohm,Tufts University School of Medicine.
PDBid 2Q66.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1303
3′
5′
DNA
poly A
1
2
L
3
5
4
6
7
I
II
III
IV
VI
VII
V
mRNA
residues (Fig. 31-49). Each RRM, as also seen in the struc-
tures of a variety of other RNA-binding proteins, consists
of a compact globule made of a 4-stranded antiparallel
sheet that forms the RNA-binding surface backed by two
helices.
The cleavage of a transcript past its AAUAAA se-
quence does not, in itself, terminate transcription. How-
ever, in yeast, the protein Rtt103, which binds to the phos-
phorylated CTD of RNAP II, recognizes the AAUAAA
sequence and recruits the 5¿S3¿ exonuclease known as
Rat1 (Xrn2 in humans). Then, in what is termed the tor-
pedo model, the highly processive Rat1/Xrn2 loads onto
the newly liberated 5¿ end of the still nascent RNA and rap-
idly degrades it until it intercepts the RNAP and induces it
to terminate RNA synthesis. It has been hypothesized that
this occurs in much the same way as Rho factor terminates
bacterial transcription (Section 31-2Db). This frees the
RNAP to initiate a new round of transcription.
c. Eukaryotic Genes Consist of Alternating
Expressed and Unexpressed Sequences
The most striking difference between eukaryotic and
prokaryotic structural genes is that the coding sequences of
most eukaryotic genes are interspersed with unexpressed re-
gions. Early investigations of eukaryotic structural gene
transcription found, quite surprisingly, that primary tran-
scripts are highly heterogeneous in length (from ⬃2000 to
well over 20,000 nucleotides) and are much larger than was
expected from the known sizes of eukaryotic proteins.
Rapid labeling experiments demonstrated that little of this
so-called heterogeneous nuclear RNA (hnRNA) is ever
transported to the cytosol; most of it is quickly turned over
(degraded) in the nucleus. Yet, the hnRNA’s 5¿ caps and 3¿
tails eventually appear in cytosolic mRNAs. The straight-
forward explanation of these observations, that pre-mRNAs
are processed by the excision of internal sequences, seemed
so bizarre that it came as a great surprise in 1977 when
Phillip Sharp and Richard Roberts independently demon-
strated that this is actually the case. In fact, mammalian pre-
mRNAs typically contain eight noncoding intervening se-
quences (introns) whose aggregate length averages 4 to 10
times that of their flanking expressed sequences (exons).
This situation is graphically illustrated in Fig. 31-50, which
is an electron micrograph of chicken ovalbumin mRNA hy-
bridized to the antisense strand of the ovalbumin gene
(ovalbumin is the major protein component of egg white).
1304 Chapter 31. Transcription
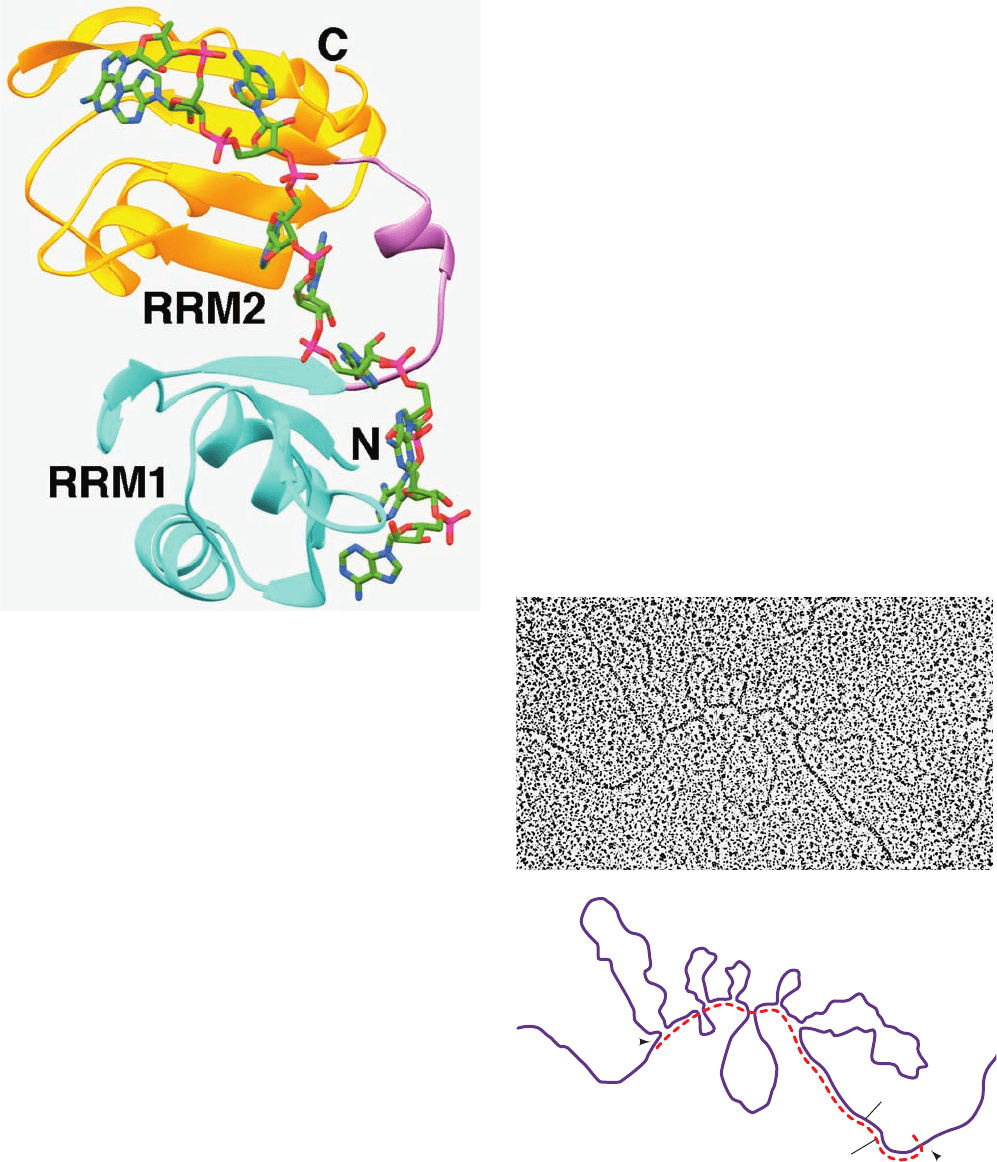
Figure 31-49 X-ray structure of the N-terminal two
RNA-recognition motifs (RRMs) of human PABP in complex
with A
11
. RRM1 is cyan, RRM2 is gold, and their linking segment
is lavender.The poly(A), only nine of whose nucleotides are
observed, is drawn in stick form with C green, N blue, O red, and
P magenta. [Based on an X-ray structure by Stephen Burley, The
Rockefeller University. PDBid 1CVJ.]
Figure 31-50 An electron micrograph and its interpretive
drawing of a hybrid between the antisense strand of the
chicken ovalbumin gene and its corresponding mRNA. The
complementary segments of the DNA (purple line in the
drawing) and mRNA (red dashed line) have annealed to reveal
the exon positions (L, 1–7). The looped-out segments (I–VII),
which have no complementary sequences in the mRNA, are the
introns. [From Chambon, P., Sci. Am. 244(5), 61 (1981).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1304
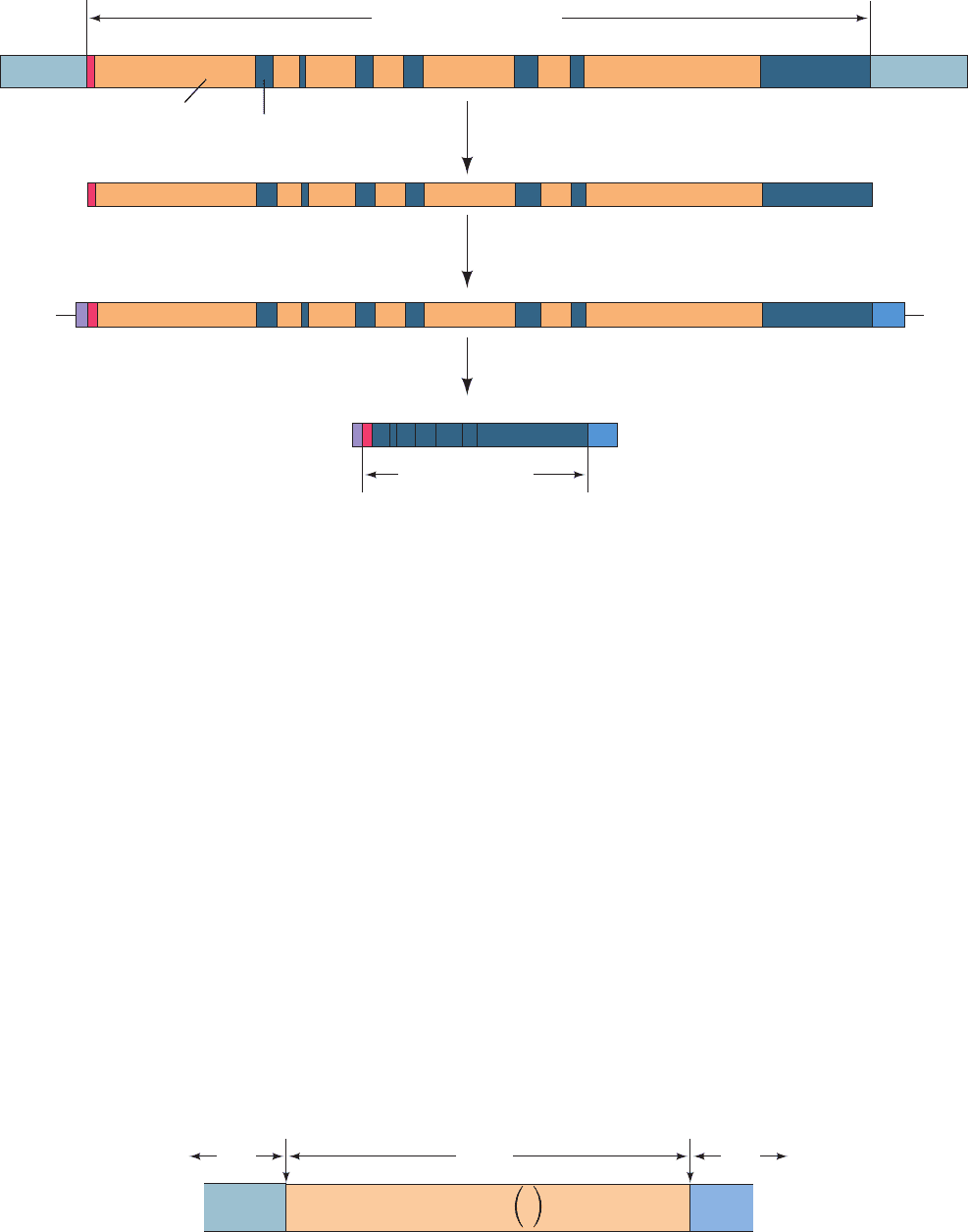
Exons have lengths that range up to 17,106 nt (in the
gene encoding the 34,350-residue muscle protein titin, the
largest known single-chain protein; Section 35-3Ae) but
with most ⬍300 nt (and averaging 150 nt in humans). In-
trons, in contrast, are usually much longer, with lengths av-
eraging ⬃3500 nt and as high as ⬃800,000 nt [in the gene
encoding the muscle protein dystrophin (Section 35-3Ae),
whose length is ⬃2400 kb and hence is the largest human
gene] with no obvious periodicity. Moreover, the corre-
sponding introns from genes in two vertebrate species can
vary extensively in both length and sequence so as to bear
little resemblance to one another.The number of introns in
a gene averages 7.8 in the human genome and varies from
none to 364 (with the latter number occurring in the gene
encoding titin).
The formation of eukaryotic mRNA begins with the
transcription of an entire structural gene, including its in-
trons, to form pre-mRNA (Fig. 31-51).Then, following cap-
ping, the introns are excised and their flanking exons are
connected, a process called gene splicing or just splicing,
that often occurs cotranscriptionally. The most striking as-
pect of gene splicing is its precision; if one nucleotide too few
or too many were excised, the resulting mRNA could not be
translated properly (Section 32-1B). Moreover, exons are
never shuffled; their order in the mature mRNA is exactly
the same as that in the gene from which it is transcribed.
d. Exons Are Spliced in a Two-Stage Reaction
Sequence comparisons of exon–intron junctions from a
diverse group of eukaryotes indicate that they have a high
degree of homology (Fig. 31-52), including, as Richard
Breathnach and Pierre Chambon first pointed out, an in-
variant GU at the intron’s 5¿ boundary and an invariant AG
at its 3¿ boundary. These sequences are necessary and suffi-
cient to define a splice junction: Mutations that alter the se-
quences interfere with splicing, whereas mutations that
change a nonjunction to a consensus-like sequence can
generate a new splice junction.
Section 31-4. Post-Transcriptional Processing 1305
Figure 31-51 The sequence of steps in the production of
mature eukaryotic mRNA as shown for the chicken ovalbumin
gene. Following transcription, the primary transcript is capped
Figure 31-52 The consensus sequence at the exon–intron
junctions of vertebrate pre-mRNAs. The subscripts indicate the
percentage of pre-mRNAs in which the specified base(s) occurs.
Note that the 3¿ splice site is preceded by a tract of 11
Splicing
Capping and polyadenylation
Transcription
Primary transcript (hnRNA)
7156
Ovalbumin mRNA
23 4
1872 nucleotides
3
5′ 3′
1L 24
Ovalbumin gene, 7700 bp
7531642L
VIIVIIV VII IIII
I IIIII IV
576
V VI VII
Exon
Intron
3
Cap
1L 24
I IIIII IV
576
V VI VII Poly(A)
tail
pre-mRNA
mRNA
DNA
5′ A
62
G
77
G
100
U
100
A
60
A
74
G
84
U
50
11
U
C
77–91
NC
78
A
100
G
100
G
55
3′
Intron ExonExon
3′ splice
site
5′ splice
site
... ... ...
and polyadenylated.The introns are then excised and the exons
spliced together to form the mature mRNA. However, splicing
may also occur cotranscriptionally.
predominantly pyrimidine nucleotides. [Based on data from
Padgett, R.A., Grabowski, P.J., Konarska, M.M., Seiler, S.S., and
Sharp, P.A., Annu. Rev. Biochem. 55, 1123 (1986).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1305
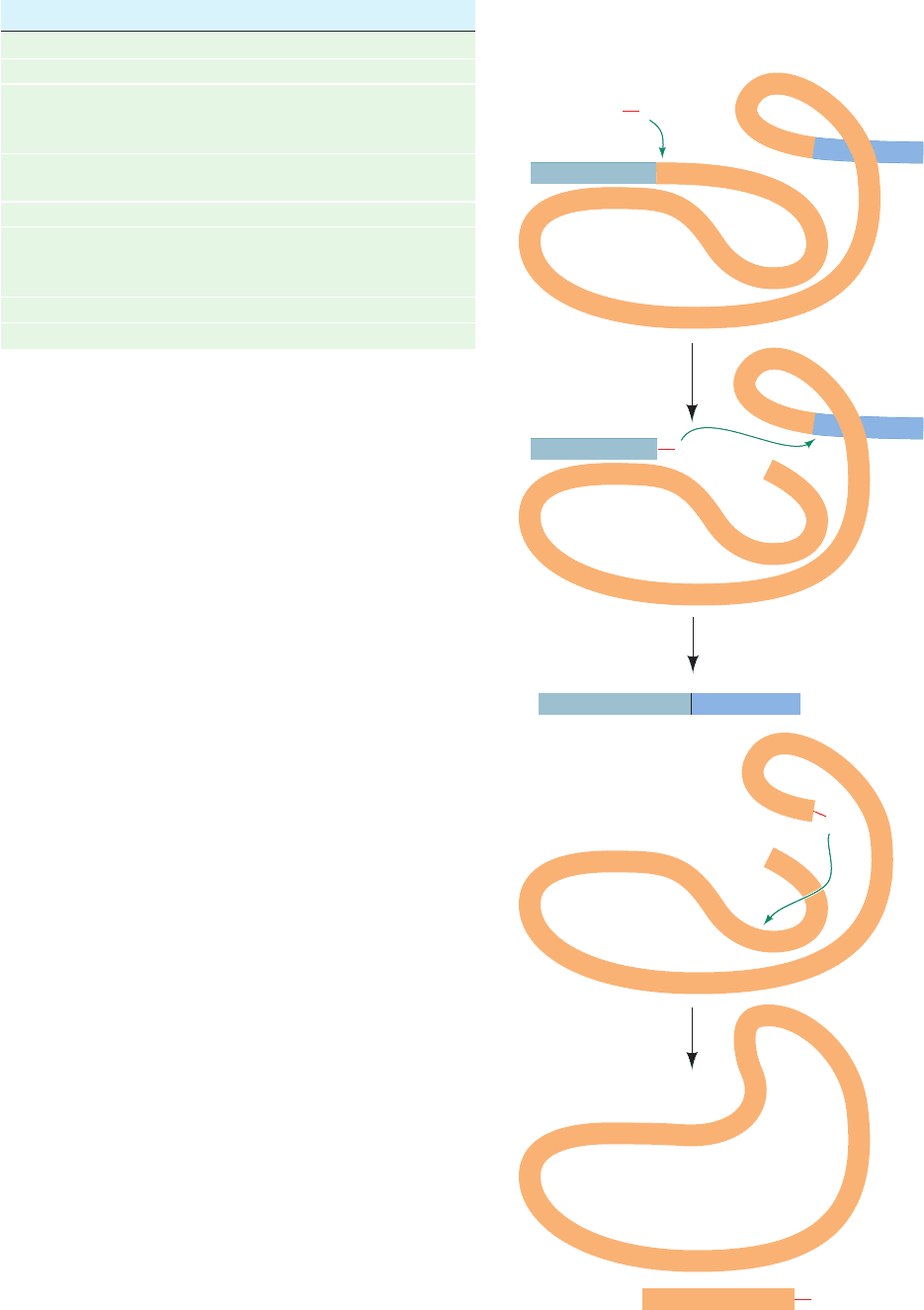
Investigations of both cell-free and in vivo splicing sys-
tems by Argiris Efstratiadis, Tom Maniatis, Michael Ros-
bash, and Sharp established that intron excision occurs via
two transesterification reactions that are remarkably simi-
lar from yeast to humans (Fig. 31-53):
1. The formation of a 2¿,5¿-phosphodiester bond be-
tween an intron adenosine residue and its 5¿-terminal phos-
phate group with the concomitant liberation of the 5¿
exon’s 3¿-OH group. The intron thereby assumes a novel
lariat structure. The adenosine residue at the lariat branch
has been identified in yeast as the last A in the highly con-
served sequence UACUAAC and in vertebrates as the A in
the equivalent but more permissive sequence YNCURAY
[where R represents purines (A or G),Y represents pyrim-
idines (C or U), and N represents any nucleotide]. In yeast
and vertebrates, the branch point A occurs ⬃50 and 18 to
40 residues upstream of the associated 3¿ splice site, respec-
tively. In yeast, which have relatively few introns, mutations
that change this branch point A residue abolish splicing at
that site. However, in higher eukaryotes, the mutation or
deletion of a branch site often activates a so-called cryptic
branch site that is also near the 3¿ splice site. Evidently, the
branch site functions to identify the nearest 3¿ splice site as
a target for linkage to the 5¿ splice site.
2. The now free 3¿-OH group of the 5¿ exon forms a
phosphodiester bond with the 5¿-terminal phosphate of the
3¿ exon yielding the spliced product and releasing the in-
tron lariat with a free 3¿-OH group.The intron lariat is then
debranched (linearized) and, in vivo, is rapidly degraded.
Mutations that alter the conserved AG at the 3¿ splice site
block this second step, although they do not interfere with
lariat formation.
Note that the splicing process proceeds without free en-
ergy input; its transesterification reactions preserve the
free energy of each cleaved phosphodiester bond through
the concomitant formation of a new one.
The sequences required for splicing are the short con-
sensus sequences at the 3¿ and 5¿ splice sites and at the
branch site. Nevertheless, these sequences are poorly con-
served. However, other short sequence elements within ex-
ons and introns that are known as exonic and intronic splic-
ing enhancers (ESEs and ISEs) and silencers (ESSs and
ISSs) also play important roles in splice site selection al-
though their characteristics are poorly understood (even
highly sophisticated computer programs are only ⬃50%
successful in predicting actual splice sites over apparently
equally good candidates that are not). In contrast, large
portions of most introns can be deleted without impeding
splicing.
e. Some Eukaryotic Genes Are Self-Splicing
It is now recognized that there are eight distinct types of
introns, seven of which occur in eukaryotes (Table 31-4).
Group I introns occur in the nuclei, mitochondria, and
chloroplasts of diverse eukaryotes (but not vertebrates),
and even in some bacteria. Thomas Cech’s study of how
1306 Chapter 31. Transcription
Figure 31-53 The sequence of
transesterification reactions that splice
together the exons of eukaryotic
pre-mRNAs. The exons and introns are
drawn in blue and orange, and R and Y
represent purine and pyrimidine residues.
(1) The 2¿-OH group of a specific intron A
residue nucleophilically attacks the
5¿-phosphate at the 5¿ intron boundary to
yield an unusual 2¿,5¿-phosphodiester bond
and thus form a lariat structure. (2) The
liberated 3¿-OH group forms a 3¿,5¿-
phosphodiester bond with the 5¿ terminal
residue of the 3¿ exon, thereby splicing the
two exons together and releasing the
intron in lariat form with a free 3¿-OH.
5′
...
ApG OH
3′
5′
...
ApG pGpUp YpNpCpUpRpApYp ApG pGp
...
3′
Exon 1 Intron
Exon 2
3′ splice
site
5′ splice
site
(2′,5′)
Exon 2
ApG pGp
Intron
Exon 1
+
1
2
pGpUp
(2′,5′)
ApG
OH
3′
+5′ ApG pGp 3′
Exon 2Exon 1
Splice junction
Excised intron in lariat form
......
+ Spliced exons
pre-mRNA
3′
...
OH
2′
pGpUp
Y
p
N
p
CpUpRpApYp
Y
p
N
p
CpUpRpApYp
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1306
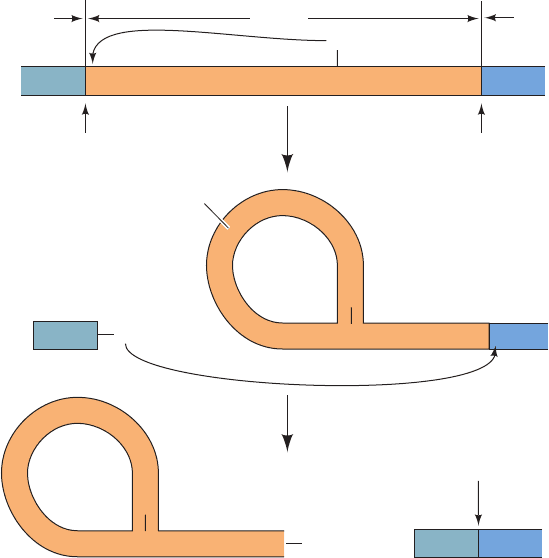
group I introns are spliced in the ciliated protozoan
Tetrahymena thermophila led to an astonishing discovery:
RNA can act as an enzyme. When the isolated pre-rRNA of
this organism is incubated with guanosine or a free guanine
nucleotide (GMP, GDP, or GTP), but in the absence of pro-
tein, its single 421-nucleotide intron excises itself and splices
together its flanking exons; that is, this pre-rRNA is self-
splicing. The three-step reaction sequence of this process
(Fig. 31-54) resembles that of mRNA splicing:
1. The 3¿-OH group of the guanosine forms a phospho-
diester bond with the intron’s 5¿ end, liberating the 5¿ exon.
2. The 3¿-terminal OH group of the newly liberated 5¿
exon forms a phosphodiester bond with the 5¿-terminal
phosphate of the 3¿ exon, thereby splicing together the two
exons and releasing the intron.
3. The 3¿-terminal OH group of the intron forms a phos-
phodiester bond with the phosphate of the nucleotide 15
residues from the intron’s 5¿ end, yielding the 5¿-terminal
fragment with the remainder of the intron in cyclic form.
This self-splicing process consists of a series of transesteri-
fications and therefore does not require free energy input.
Cech further established the enzymatic properties of the
Section 31-4. Post-Transcriptional Processing 1307
Figure 31-54 The sequence of reactions in the self-splicing of
Tetrahymena group I intron. (1) The 3¿-OH group of a guanine
nucleotide attacks the intron’s 5¿-terminal phosphate so as to
form a phosphodiester bond and release the 5¿ exon. (2) The
newly generated 3¿-OH group of the 5¿ exon attacks the
5¿-terminal phosphate of the 3¿ exon, thereby splicing the two
exons and releasing the intron. (3) The 3¿-OH group of the intron
attacks the phosphate of the nucleotide that is 15 residues from
the 5¿ end so as to cyclize the intron and release its 5¿-terminal
fragment.Throughout this process, the RNA maintains a folded,
internally hydrogen bonded conformation that permits the
precise excision of the intron.
Table 31-4 Types of Introns
Intron Type Where Found
GU–AG introns Eukaryotic nuclear pre-mRNA
AU–AC introns Eukaryotic nuclear pre-mRNA
Group I Eukaryotic nuclear pre-mRNA,
organelle RNAs, a few
bacterial RNAs
Group II Organelle RNAs, a few
prokaryotic RNAs
Group III Organelle RNAs
Twintrons (composites Organelle RNAs
of two and/or more
group II or III introns)
Pre-tRNA introns Eukaryotic nuclear pre-tRNAs
Archaeal introns Various RNAs
Source: Brown,T.A., Genomes (3rd ed.), Garland Science, p. 355 (2007).
OH
3′
C
G
.
U
G
.
C
G
.
U
A
.
C
G
.
U
G
.
pA
G
pU
U
U
U
p
A
Left exon
Right
exon
Intron
1
C
G
.
U
G
.
C
G
.
U
A
.
C
G
.
U
G
.
G
pU
5′
5′
OH
3′
G
3′
3′
pA
5′
G
U
U
U
p
A
2
Left exon Right exon
5′ 3′
CUCUCU pU
Spliced exons
OH
3′
G
GGGAGG
U
U
U
p
A
3
GGGAGG
A
p
G
Cyclized intron
pA UUU
+
OH
3′5′
G
5′
G
pA
+
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1307
Tetrahymena intron, which stem from its three-dimensional
structure, by demonstrating that it catalyzes the in vitro
cleavage of poly(C) with an enhancement factor of 10
10
over the rate of spontaneous hydrolysis. Indeed, this RNA
catalyst even exhibits Michaelis–Menten kinetics (K
M
⫽ 42
M and k
cat
⫽ 0.033 s
⫺1
for C
5
). Such RNA enzymes have
been named ribozymes.
Although the idea that an RNA can have enzymatic
properties may seem unorthodox, there is no fundamental
reason why an RNA, or any other macromolecule, cannot
have catalytic activity (recall that it was likewise once gen-
erally accepted that nucleic acids lack the complexity to
carry hereditary information; Section 5-2). Of course, in or-
der to be an efficient catalyst, a macromolecule must be
able to assume a stable structure but, as we shall see below
and in Sections 32-2B and 32-3Ae, RNAs, including tRNAs
and rRNAs, can do so. In fact, the Tetrahymena intron un-
dergoes a series of well-defined conformational changes
during its reaction sequence. [Synthetic ssDNAs are also
known to have catalytic properties although such deoxyri-
bozymes are unknown in biology.]
The group II introns, which occur in the mitochondria of
fungi and plants and comprise the majority of the introns in
chloroplasts, are also self-splicing. They generally employ
an internal A residue as their initial attacking nucleophile
(instead of an external G) to form a lariat intermediate, a
process that resembles the splicing of nuclear pre-mRNAs
(Fig. 31-53). We shall see below that nuclear pre-mRNA
splicing is mediated by complex ribonucleoprotein parti-
cles known as spliceosomes. The chemical similarities of
the pre-mRNA and group II intron splicing reactions
therefore suggest that spliceosomes are ribozymal systems
whose RNA components have evolved from primordial self-
splicing RNAs and that their protein components serve
mainly to fine-tune ribozymal structure and function. Simi-
larly, the RNA components of ribosomes, which are two-
thirds RNA and one-third protein, clearly have a catalytic
function in addition to the structural and recognition roles
traditionally attributed to them (Section 32-3). Thus, the
observations that nucleic acids but not proteins can direct
their own synthesis, that cells contain batteries of protein-
based enzymes for manipulating DNA but relatively few
for processing RNA, and that many coenzymes are ribonu-
cleotides (e.g., ATP, NAD
⫹
, and CoA), led to the hypothe-
sis that RNAs were the original biological catalysts in
precellular times (the RNA world) and that the chemically
more versatile proteins were relative latecomers in macro-
molecular evolution (Section 1-5Ca).
f. The X-Ray Structures of a Group I Ribozyme
Group I introns are the most abundant self-splicing in-
trons, with ⬎2000 such sequences known. The sequence of
the 413-nt Tetrahymena group I intron, together with phy-
logenetic comparisons, indicates that it contains nine dou-
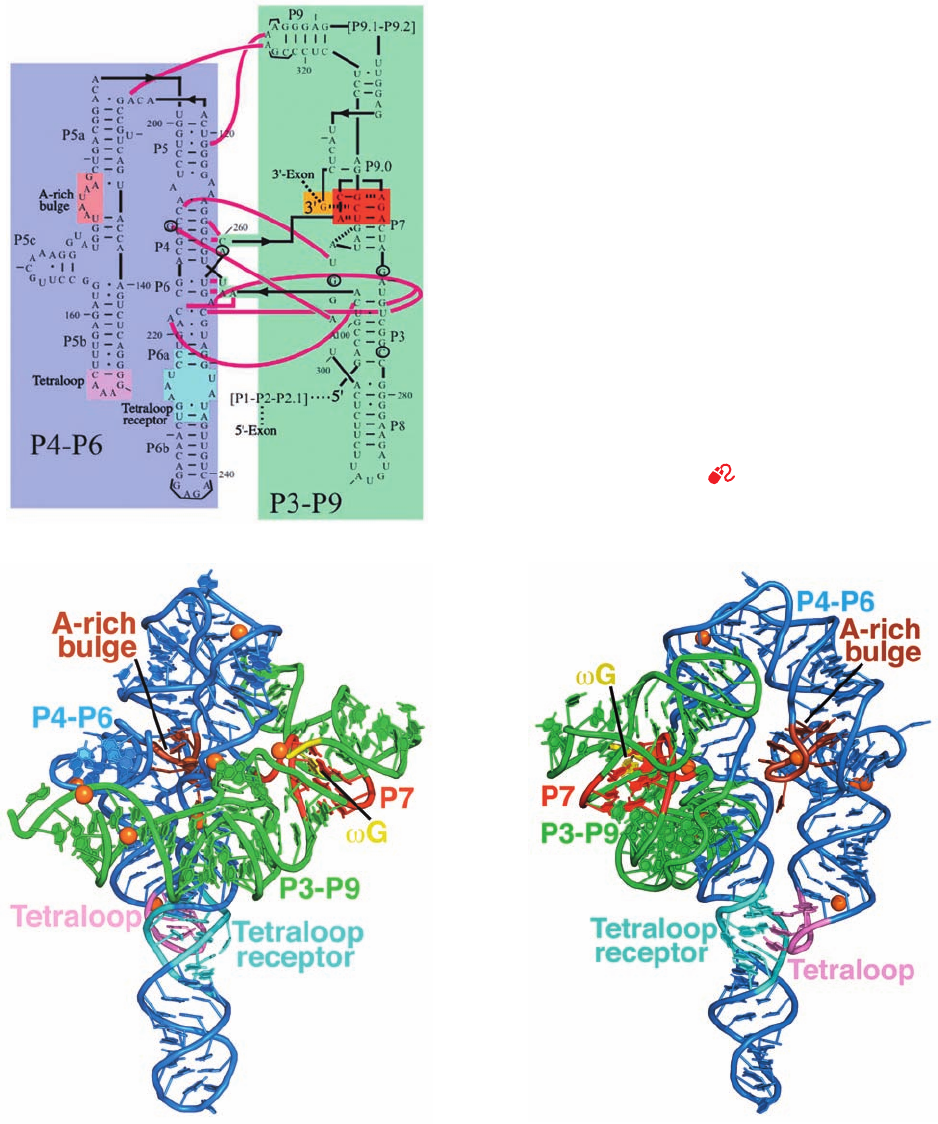
ble helical segments that are designated P1 through P9
(Fig. 31-55a; P for base-paired segment). Such analysis fur-
ther indicates that the conserved catalytic core of group I
introns consists of sets of coaxially stacked helices inter-
spersed with internal loops that are organized into two
domains, the P4-P5-P6 domain (also called P4-P6) and the
P3-P7-P8-P9 domain (also called P3-P9).
Cech designed a 247-nt RNA (Fig. 31-55a) that encom-
passes both the P4-P6 and P3-P9 domains of the Tetrahy-
mena group I intron (it lacks the P1-P2 domain and the at-
tached exons), with the addition of a 3¿ G (G), which
functions as an internal guanosine nucleophile. This RNA
is catalytically active; it binds the P1-P2 domain via tertiary
interactions and, with the assistance of G, cleaves P1 in a
manner similar to the intact intron.
The X-ray structure of this RNA (Fig. 31-55b,c) reveals
that it is largely composed of three coaxially stacked sets of
A-RNA-like helices with P4-P6 consisting of two pseudo-
continous and straight parallel helices connected by a
sharp bend and P3-P9 consisting of a curved helix that
wraps around one side of P4-P6 through extensive interdo-
main interactions that form the ribozyme’s active site. Of
particular note are its so-called A-rich bulge, a 7-nt se-
quence about halfway along the short arm of the U-shaped
P4-P6, and the 6-nt sequence at the tip of the short arm of
the U, whose central GAAA assumes a characteristic con-
formation known as a tetraloop. In both of these substruc-
tures, the bases are splayed outward so as to stack on each
other and to associate in the minor groove of specific seg-
ments of the long arm of the U via hydrogen bonding inter-
actions involving ribose residues as well as bases. In many
such interactions, the close packing of phosphate groups is
mediated by hydrated Mg
2⫹
ions. Throughout this struc-
ture, the defining characteristic of RNA, its 2¿-OH group, is
both a donor and an acceptor of hydrogen bonds to phos-
phates, bases, and other 2¿-OH groups. Interestingly, al-
though this overall fold is highly conserved among group I
introns, their sequences are poorly conserved with the
exception of a few crucial active site residues.
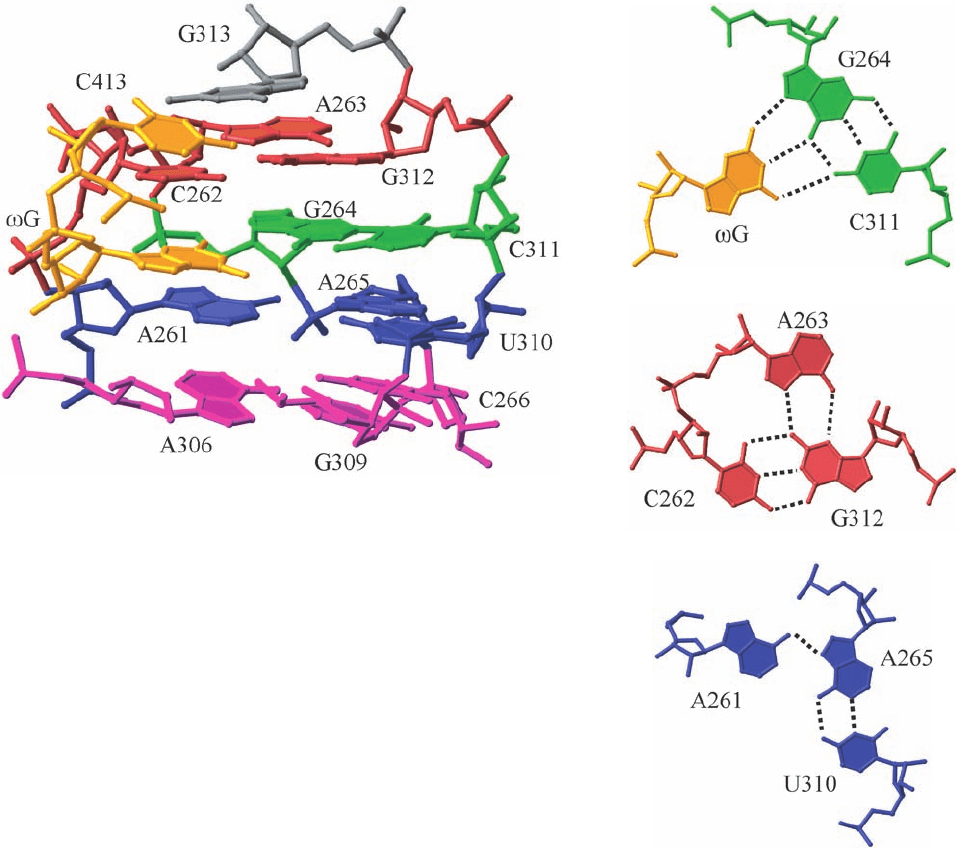
In the initial reaction catalyzed by group I ribozymes,
the 3¿-OH group of G nucleophilically attacks the phos-
phate group linking the 5¿ exon to the ribozyme (Fig. 31-54).
But how is only this 3¿-OH group activated as a nucle-
ophile? The binding site for the G substrate is composed
of four coplanar base triples with the G–G264–C311
triple sandwiched by three other base triples (Fig. 31-56).
Consequently, the base of G is stacked between those of
A261 and C262 (Fig. 31-56a), which stabilizes the binding
of G to this site through base stacking.
Divalent metal ions, usually Mg
2⫹
, are often required for
both the structural stability and the catalytic activity of ri-
bozymes. Unfortunately, the relatively low (3.8 Å) resolu-
tion of the ribozyme X-ray structure precluded the direct
observation of Mg
2⫹
ions (which have the same number of
electrons as water molecules). However, there is good evi-
dence that the heavy metal ions (Eu and Ir) used to solve
the X-ray structure occupied many of the same positions in
the ribozyme as do Mg
2⫹
ions and moreover, several of
these sites were observed to contain Mg
2⫹
ions in other
group I introns whose X-ray structures are known. In par-
ticular, an Mg
2⫹
ion is in contact with the 2¿-OH group of
G as well as being liganded by the phosphate groups of
1308 Chapter 31. Transcription
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1308
three surrounding nucleotides. This both orients the ribose
group of G and nucleophilically activates its 3¿-OH
group. Biochemical studies indicate that a second Mg
2⫹
ion, which accompanies the phosphate group of the RNA
substrate, also participates in the catalytic reaction. Note
that two Mg
2⫹
ions similarly participate in the phosphoryl-
transfer reactions catalyzed by protein enzymes such as
DNA polymerase (Section 30-2Af).
Section 31-4. Post-Transcriptional Processing 1309
Figure 31-55 The group I intron from Tetrahymena
thermophila. (a) The secondary structure of the 414-nt ribozyme.
Its P4-P6 and P3-P9 domains are shaded in blue and green,
respectively, with the catalytically active 3¿ G residue shaded in
yellow, the base triples of the P7 domain shaded in red, and the
A-rich bulge, the tetraloop, and the tetraloop receptor on the
P4-P6 domain shaded in brown, pink, and cyan, respectively.
Watson–Crick and non-Watson–Crick base pairing interactions
are represented by short horizontal lines and small filled circles,
whereas interdomain interactions are indicated by magenta lines.
Every tenth residue is marked by an outwardly pointing dash.
The positions of five residues that have been mutated to stabilize
the ribozyme structure are circled and those of the seven
mutations that facilitated crystallization are bracketed (this
mutant form retains its catalytic activity). (b) The X-ray structure
of the ribozyme, drawn in cartoon form with its bases shown as
paddles, and colored as in Part a. The inferred positions of Mg
2⫹
ions are represented by orange spheres. (c) As in Part b but
rotated 140° about the vertical axis to better show the A-rich
bulge and the interaction between the tetraloop and the
tetraloop receptor. [Part a modified from a drawing by and Parts
b and c based on an X-ray structure by Thomas Cech, University
of Colorado. PDBid 1X8W.]
See Interactive Exercise 42
(a)
(b)
(c)
JWCL281_c31_1260-1337.qxd 10/19/10 11:26 AM Page 1309
g. Hammerhead Ribozymes Catalyze
an In-Line Nucleophilic Attack
One of the best-characterized types of ribozymes is the
hammerhead ribozyme, so called because of the superficial
resemblance of its secondary structure, as it was originally
laid out, to a hammer.This minimally ⬃40-nt RNA partici-
pates in the replication of certain viruslike RNAs that in-
fect plants and also occurs in schistosomes (species of par-
asitic flatworms).The hammerhead ribozyme catalyzes the
site-specific cleavage of one of its own phosphodiester
bonds with an ⬃10
7
-fold rate enhancement. However, it is
not a true catalyst because it cannot return to its original
state.
The secondary structure of the 63-nt hammerhead ri-
bozyme from Schistosoma mansoni has three duplex
stems and an active site core of two nonhelical segments
(Fig. 31-57a). This ribozyme cleaves itself between its C-17
and C-1.1 nucleotides to yield a cyclic 2¿,3¿-phosphodiester
on C-17 with inversion of configuration about the P atom,
together with a free 5¿-OH on C-1.1, much like the interme-
diate product in the RNA hydrolysis reaction catalyzed by
RNase A (Section 15-1Ab). This suggests that the reaction
proceeds via an “in-line” mechanism such as that dia-
grammed in Fig. 16-6b with the transition state forming a
trigonal bipyramidal intermediate in which the attacking
nucleophile, the 2¿-OH group (Y in Fig. 16-6b), and the
leaving group, which forms the free 5¿-OH group (X in Fig.
16-6b), occupy the axial positions.
The X-ray structure of the S. mansoni hammerhead ri-
bozyme, determined by William Scott, reveals that its stem
II, stem III and the terminal end of stem I are coaxially
stacked.The remainder of stem I curves around in a manner
that more closely resembles the handle of a suitcase than
that of a hammer to form a junction with stems II and III
that contains the ribozyme’s active site core (Fig. 31-57b).
The nucleotides in the helical stems mainly form normal
Watson–Crick base pairs, whereas the nucleotides of the
active site core participate in non-Watson–Crick base pairs.
This explains the observations that most helical positions
can be occupied by any Watson–Crick base pair but that
few core bases can be changed without significantly reduc-
ing ribozymal activity.
The bases of the active site core participate in a hydro-
gen bonded network (Fig. 31-57c). This helps position C-17
such that its O2¿ atom is properly oriented for an in-line
1310 Chapter 31. Transcription
Figure 31-56 Structure of the guanosine binding site in the Tetrahymena
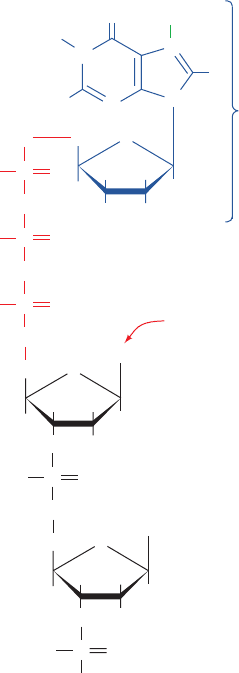
group I intron. (a) Side view of the four coplanar base triples at the
ribozymal active site in P7 (colored in red in Fig. 31-55). The sugar–phosphate
backbone is drawn in stick form and the bases are drawn in paddle form.The
nucleotides at each level have the same color with the exception of the
C413–G dinucleotide, which is yellow. (b) Top view of the base triple
interactions between G and the G264 ⴢ C311 base pair. (c) Top view of the
base triple of C262,A263, and G312, which is located above that containing
G. (d) Top view of the base triple of A261, A265, and U310, which is located
below that containing G. [Courtesy of Thomas Cech, University of
Colorado. PDBid 1X8W.]
(a)
(b)
(c)
(d)
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1310
