Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.


to stall. How, then, do RNAPs avoid accumulating at dam-
aged or mispaired sites, which, if it occurred on an essential
gene, would be lethal?
RNAPs do not monotonically move forward along the
template DNA. Instead, they frequently backtrack such
that the RNA’s penultimate nucleotide, which was in the
i ⫺ 1 position, has re-entered the i ⫹ 1 position and the 3¿-
nucleotide, now in the i ⫹ 2 position, enters the secondary
channel where it binds in the so-called P (for proofreading)
site. If the forward movement of the RNA is impeded by
damage to the template or by mispairing, further back-
tracking becomes favored so that several more ribonu-
cleotides enter the secondary channel.The backtracking of
only one or a few nucleotides is reversible. Otherwise,tran-
scription is arrested until the RNA is hydrolytically cleaved
at the active site. In E. coli, this requires the assistance of
the homologous proteins GreA and/or GreB, whereas with
RNAP II, this function is carried out by the unrelated
protein TFIIS. These proteins induce the RNAP active site
to hydrolyze the phosphodiester bond between the ribonu-
cleotides in the i ⫹ 1 and i ⫺ 1 positions (a reaction that is
not the reverse of the polymerase reaction since this would
be pyrophosphorolysis). In this way, RNAP can correct its
mistakes and resume RNA synthesis. RNAP I and RNAP
III also efficiently correct their mistakes.
Kornberg determined the X-ray structure of RNAP II
in complex with TFIIS, a 28-nt template DNA, a 14-nt non-
template DNA, and a 13-nt RNA that is complementary to
5¿ end of the template DNA except for the last two residues
at the RNA’s 3¿ end, which are mismatched (Fig. 31-25).
The RNA–DNA hybrid has backtracked such that these
latter residues occupy the i ⫹ 1 and i ⫹ 2 positions. The C-
terminal domain of TFIIS is bound in the RNAP’s funnel
with one of its loops insinuated through the pore to inter-
act with the RNAP’s active site residues. There it presum-
Section 31-2. RNA Polymerase 1281
Figure 31-24 The A and E sites and the trigger loop in RNA
polymerase II. A cutaway view of the transcribing complex
viewed as in Fig. 31-22b. Its bound nucleic acids and nucleotides
are colored differently with template DNA cyan, nontemplate
DNA green, newly transcribed RNA red, GTP in the A site
Figure 31-25 X-ray structure of backtracked RNA polymerase
II in complex with DNA, RNA, and TFIIS. The RNAP, which is
represented by its semitransparent surface diagram, is viewed as
in Figs. 31-22b and 31-24 with its subunits colored as in Fig. 31-
21a.The DNA and RNA are shown in ladder form with the RNA
(13 nt) red, the template DNA (28 nt) green, and the nontem-
plate DNA (14 nt) blue.The base pairs at the i ⫹ 1 and
i ⫹ 2 positions of the RNA ⴢ DNA hybrid helix are mismatched.
An Mg
2⫹
ion, which is represented by a red sphere near the i ⫹ 1
position, marks the RNAP’s active site.TFIIS is drawn in cartoon
form in magenta. Its C-terminal domain is inserted into the
RNAP’s funnel (Fig. 31-22c) with a loop occupying the pore,
where it is in proximity to the RNAP’s active site. [Based on an
X-ray structure by Roger Kornberg, Stanford University. PDBid
3GTM.]
orange, and ATP in the E site blue.The trigger loop is magenta,
the bridge helix is green, and the two Mg
2⫹
ions at the active site
are represented by magenta spheres.The RNAP II surface is
gray. [Courtesy of Dong Wang and Roger Kornberg, Stanford
University. PDBid 2E2H.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1281
ably facilitates the hydrolytic reaction, perhaps by liganding
the active site Mg
2⫹
ion that normally accompanies the in-
coming NTP (Section 31-2Ea) and/or positioning a hy-
drolytic water molecule.The trigger loop in this structure is
in the “open” conformation. Interestingly, the cryo-
EM–based structure of E. coli RNAP in complex with
GreB indicates that GreB likewise inserts an extended
protein finger in the RNAPs active site via its secondary
channel, even though the structures of GreB and TFIIS are
unrelated.
Despite the foregoing,transcription is less accurate than
DNA replication: RNAPs incorporate one incorrect base
for every ⬃10
4
transcribed, whereas, for example, E. coli
Pol I incorporates one incorrect base in ⬃10
7
(Section 30-
2Ab). Cells can tolerate the former rate because most
genes are repeatedly transcribed. In contrast, errors in
DNA synthesis alter all the affected gene’s transcripts in
the cell in which the error occurred and all of its progeny.
d. Mammalian RNA Polymerase I Has
a Bipartite Promoter
Since, as we shall see in Section 31-4B, the numerous
rRNA genes in a given eukaryotic cell have essentially
identical sequences, its RNAP I only recognizes one pro-
moter. Yet, in contrast to the case for RNAPs II and III,
RNAP I promoters are species specific, that is, an RNAP I
only recognizes its own promoter and those of closely re-
lated species. This is because only closely related species
exhibit recognizable sequence identities near the transcrip-
tional start sites of their rRNA genes. RNAP I promoters
were therefore identified by determining how the tran-
scription rate of an rRNA gene is affected by a series of in-
creasingly longer deletions approaching its start site from
either its upstream or its downstream sides. Such studies
have indicated, for example, that mammalian RNAPs I re-
quire the presence of a so-called core promoter element,
which spans positions ⫺31 to ⫹6 and hence overlaps the
transcribed region. However, efficient transcription addi-
tionally requires an upstream promoter element, which is
located between residues ⫺187 and ⫺107. These elements,
which are G ⫹ C-rich and ⬃85% identical, are bound by
specific transcription factors which then recruit RNAP I to
the transcription start site.
e. RNA Polymerase II Promoters Are
Complex and Diverse
The promoters recognized by RNAP II are considerably
longer and more diverse than those of prokaryotic genes
but have not yet been fully described. The structural genes
expressed in all tissues, the so-called housekeeping genes,
which are thought to be constitutively transcribed, have
one or more copies of the sequence GGGCGG or its com-
plement (the GC box) located upstream from their tran-
scription start sites. The analysis of deletion and point mu-
tations in eukaryotic viruses such as SV40 indicates that
GC boxes function analogously to prokaryotic promoters.
On the other hand, structural genes that are selectively ex-
pressed in one or a few types of cells often lack these GC-
rich sequences. Rather, many contain a conserved AT-rich
sequence located 25 to 30 bp upstream from their transcrip-
tion start sites (Fig. 31-26). Note that this so-called TATA
box resembles the ⫺10 region of prokaryotic promoters
(TATAAT), although they differ in their locations relative
to the transcription start site (⫺27 vs ⫺10). The functions
of these two promoter elements are not strictly analogous,
however, since the deletion of the TATA box does not nec-
essarily eliminate transcription. Rather, TATA box dele-
tion or mutation generates heterogeneities in the transcrip-
tional start site, thereby indicating that the TATA box
participates in selecting this site.
The gene region extending between about ⫺50 and ⫺110
also contains promoter elements. For instance,many eukary-
otic structural genes, including those encoding the various
globins, have a conserved sequence of consensus CCAAT
(the CCAAT box) located between about ⫺70 and ⫺90
whose alteration greatly reduces the gene’s transcription
rate. Globin genes have, in addition, a conserved CACCC
box upstream from the CCAAT box that has also been im-
plicated in transcriptional initiation. Evidently, the pro-
moter sequences upstream of the TATA box form the initial
DNA-binding sites for RNA polymerase II and the other
proteins involved in transcriptional initiation (see below).
f. Enhancers Are Transcriptional Activators That
Can Have Variable Positions and Orientations
Perhaps the most surprising aspect of eukaryotic tran-
scriptional control elements is that some of them need not
1282 Chapter 31. Transcription
Figure 31-26 The promoter sequences of
selected eukaryotic structural genes. The
homologous segment, the TATA box, is
shaded in red with the base at position ⫺27
underlined and the initial nucleotide to be
transcribed (⫹1) shaded in green.The
bottom row indicates the consensus
sequence of several such promoters with
the subscripts indicating the percent
occurrence of the corresponding base.
[After Gannon, F., et al., Nature 278, 433
(1978).]
Chicken
ovalbumin
Adenovirus
late
Rabbit
β globin
Mouse β
globin major
G
G
T
G
A
G
T
A
G
G
G
G
G
G
G
C
C
C
G
A
T
T
C
T
A
A
A
A
T
T
T
T
A
A
A
T AT
A
A
G
T
G
G
T
C
G
G
G
C
G
C
A
C
G
A
G
C
G
G
G
A
T
A
T
G
G
G
A
G
G
C
G
G
G
A
G
C
G
G
A
T
G
G
T
C
C
G
C
A
G
C
A
G
C
A
G
C
G
G
T
C
T
C
T
A
T
T
G
G
C
G
C
T
G
C
T
G
T
T
C
T
C
G
C
C
C
C
T
T
T
T
C
G
C
A
A
T
A
A
C
A
C
C
A
C
T
A
T
A
C
C
T
T
T
A
A
A
A
A
A
G
T
82
A
97
T
93
A
85
T
37
A
63
A
83
T
37
A
50
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1282

Inactive
chromosome
segment
Active
chromosome
segment
RNA polymerase
Ribosome
mRNA
Direction of
protein
synthesis
(translation)
direction of
RNA synthesis
(transcription)
have fixed positions and orientations relative to their corre-
sponding transcribed sequences. For example, the SV40
genome, in which such elements were first discovered, con-
tains two repeated sequences of 72 bp each that are located
upstream from the promoter for early gene expression.
Transcription is unaffected if one of these repeats is
deleted but is nearly eliminated when both are absent.The
analysis of a series of SV40 mutants containing only one of
these repeats demonstrated that its ability to stimulate
transcription from its corresponding promoter is all but in-
dependent of its position and orientation. Indeed, transcrip-
tion is unimpaired when this segment is several thousand
base pairs upstream or downstream from the transcription
start site. Gene segments with such properties are named
enhancers to indicate that they differ from promoters, with
which they must be associated in order to trigger site-
specific and strand-specific transcription initiation (al-
though the characterization of numerous promoters and
enhancers indicates that their functional properties are
similar). Enhancers occur in both eukaryotic viruses and
cellular genes.
Enhancers are required for the full activities of their cog-
nate promoters. It was originally thought that enhancers
somehow acted as entry points on DNA for RNAP II (per-
haps by altering DNA’s local conformation or through a
lack of binding affinity for the histones that normally coat
eukaryotic DNA; Section 34-1B). However, it is now clear
that enhancers are recognized by specific transcription fac-
tors that stimulate RNA polymerase II to bind to the corre-
sponding but distant promoter. This requires that the DNA
between the enhancer and promoter loop around so that
the transcription factor can simultaneously contact the en-
hancer and the RNAP II and/or its associated proteins at
the promoter. Most cellular enhancers are associated with
genes that are selectively expressed in specific tissues. It
therefore seems, as we discuss in Section 34-3B, that
enhancers mediate much of the selective gene expression in
eukaryotes.
g. RNA Polymerase III Promoters Can Be Located
Downstream from Their Transcription Start Sites
The promoters of genes transcribed by RNAP III can be
located entirely within the genes’ transcribed regions. Don-
ald Brown established this through the construction of a
series of deletion mutants of a Xenopus borealis 5S RNA
gene. Deletions of base sequences that start from outside
one or the other end of the transcribed portion of the 5S
gene only prevent transcription if they extend into the seg-
ment between nucleotides ⫹40 and ⫹80. Indeed, a frag-
ment of the 5S RNA gene consisting of only nucleotides 41
to 87, when cloned in a bacterial plasmid, is sufficient to di-
rect specific initiation by RNAP III at an upstream site.
This is because, as was subsequently demonstrated, the se-
quence contains the binding site for transcription factors
that stimulate the upstream binding of RNAP III. Further
studies have shown, however, that the promoters of other
RNAP III–transcribed genes lie entirely upstream of their
start sites. These upstream sites also bind transcription fac-
tors that recruit RNAP III.
3 CONTROL OF TRANSCRIPTION
IN PROKARYOTES
Prokaryotes respond to sudden environmental changes,
such as the influx of nutrients, by inducing the synthesis of
the appropriate proteins. This process takes only minutes
because transcription and translation in prokaryotes are
closely coupled: Ribosomes commence translation near the
5¿ end of a nascent mRNA soon after it is extruded from
RNA polymerase (Fig. 31-27). Moreover, most prokaryotic
mRNAs are enzymatically degraded within 1 to 3 min of
their synthesis, thereby eliminating the wasteful synthesis
of unneeded proteins after a change in conditions (protein
degradation is discussed in Section 32-6). In fact, the 5¿
ends of some mRNAs are degraded before their 3¿ ends
have been synthesized.
In contrast, the induction of new proteins in eukaryotic
cells frequently takes hours or days, in part because tran-
scription takes place in the nucleus and the resulting
mRNAs must be transported to the cytoplasm, where
translation occurs. However, eukaryotic cells, particularly
those of multicellular organisms, have relatively stable en-
vironments; major changes in their transcription patterns
usually occur only during cell differentiation.
Section 31-3. Control of Transcription in Prokaryotes 1283
Figure 31-27 An electron micrograph and its interpretive
drawing showing the simultaneous transcription and translation
of an E. coli gene. RNA polymerase molecules are transcribing
the DNA from right to left while ribosomes are translating the
nascent RNAs (mostly from bottom to top). [Courtesy of Oscar
L. Miller, Jr. and Barbara Hamkalo, University of Virginia.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1283
In this section we examine some of the ways in which
prokaryotic gene expression is regulated through tran-
scriptional control. Eukaryotes, being vastly more complex
creatures than are prokaryotes, have a correspondingly
more complicated transcriptional control system whose
general outlines are beginning to come into focus. We
therefore defer discussion of eukaryotic transcriptional
control until Section 34-3B, where it can be considered in
light of what we know about the structure and organization
of the eukaryotic chromosome.
A. Promoters
In the presence of high concentrations of inducer, the lac
operon (Section 31-1Ab) is rapidly transcribed. In contrast,
the lacI gene is transcribed at such a low rate that a typical
E. coli cell contains ⬍10 molecules of the lac repressor.Yet,
the I gene has no repressor. Rather, it has such an ineffi-
cient promoter (Fig. 31-10) that it is transcribed an average
of about once per bacterial generation. Genes that are tran-
scribed at high rates have efficient promoters. In general, the
more efficient a promoter, the more closely its sequence
resembles that of the corresponding consensus sequence.
a. Gene Expression Can Be Controlled by
a Succession of Factors
The processes of development and differentiation involve
the temporally ordered expression of sets of genes according
to genetically specified programs. Phage infections are
among the simplest examples of developmental processes.
Typically, only a subset of the phage genome, often referred
to as early genes, are expressed in the host immediately af-
ter phage infection.As time passes, middle genes start to be
expressed, and the early genes as well as the bacterial genes
are turned off. In the final stages of phage infection, the
middle genes give way to the late genes. Of course some
phage types express more than three sets of genes and
some genes may be expressed in more than one stage of an
infection.
One way in which families of genes are sequentially ex-
pressed is through “cascades” of factors. In the infection
of Bacillus subtilis by bacteriophage SP01, for example, the
early gene promoters are recognized by the bacterial
RNAP holoenzyme. Among these early genes is gene 28,
whose gene product is a new subunit, designated
gp28
,
that displaces the bacterial subunit from the core en-
zyme. The reconstituted holoenzyme recognizes only the
phage middle gene promoters, which all have similar ⫺35
and ⫺10 regions but bear little resemblance to the corre-
sponding regions of bacterial and phage early genes. The
early genes therefore become inactive once their corre-
sponding mRNAs have been degraded. The phage middle
genes include genes 33 and 34, which together specify yet
another factor,
gp33/34
, which, in turn, permits the tran-
scription of only late phage genes.
Most bacteria, including E. coli and B. subtilis, likewise
have several different factors (E. coli has seven). These
are not necessarily utilized in a sequential manner. Rather,
those that differ from the predominant or primary factor
(
70
in E. coli) control the transcription of coordinately ex-
pressed groups of special purpose genes, whose promoters
are quite different from those recognized by the primary
factor. For example, in E. coli, the alternative factor
32
is
the master regulator of the heat shock response (Section
9–2C), whereas
54
directs the expression of proteins in-
volved in nitrogen assimilation. Likewise, sporulation in B.
subtilis, a process in which the bacterial cell is asymmetri-
cally partitioned into two compartments, the forespore
(which becomes the spore, a germline cell from which sub-
sequent progeny arise) and the mother cell (which synthe-
sizes the spore’s protective cell wall and is eventually dis-
carded), is governed by five factors in addition to that of
the vegetative (nonsporulating) cell: one that is active be-
fore cell partition occurs, two that are sequentially active in
the forespore, and two that are sequentially active in the
mother cell. Cross-regulation of the compartmentalized
factors permits the forespore and mother cell to tightly co-
ordinate this differentiation process.
B. lac Repressor I: Binding
In 1966, Benno Müller-Hill and Walter Gilbert isolated lac
repressor on the basis of its ability to bind
14
C-labeled
IPTG (Section 31-1Aa) and demonstrated that it is a pro-
tein. This was an exceedingly difficult task because lac
repressor comprises only ⬃0.002% of the protein in wild-
type E. coli. Now, however, lac repressor is available in
quantity via molecular cloning techniques (Section 5-5G).
a. lac Repressor Finds Its Operator by
Sliding Along DNA
The lac repressor is a tetramer of identical 360-residue
subunits, each of which binds one IPTG molecule with a
dissociation constant of K ⫽ 10
⫺6
M. In the absence of in-
ducer, the repressor tetramer nonspecifically binds duplex
DNA with a dissociation constant of K ⬇ 10
⫺4
M. How-
ever, it specifically binds to the lac operator with far
greater affinity: K
⬇ 10
⫺13
M. Limited proteolysis of lac
repressor with trypsin reveals that each subunit consists of
two functional domains: Its 58-residue N-terminal peptide
binds DNA but not IPTG, whereas the remaining “core
tetramer” binds only IPTG.
The observed rate constant for the binding of lac repres-
sor to lac operator is k
f
⬇ 10
10
M
⫺1
s
⫺1
.This “on” rate is much
greater than that calculated for the diffusion-controlled process
in solution: k
f
⬇ 10
7
M
⫺1
s
⫺1
for molecules the size of lac re-
pressor. Since it is impossible for a reaction to proceed faster
than its diffusion-controlled rate, the lac repressor must not
encounter operator from solution in a random three-dimen-
sional search. Rather, it appears that lac repressor finds oper-
ator by nonspecifically binding to DNA and diffusing along it
in a far more efficient one-dimensional search.
b. lac Operator Has a Nearly Palindromic Sequence
The availability of large quantities of lac repressor made
it possible to characterize the lac operator. E. coli DNA
1284 Chapter 31. Transcription
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1284
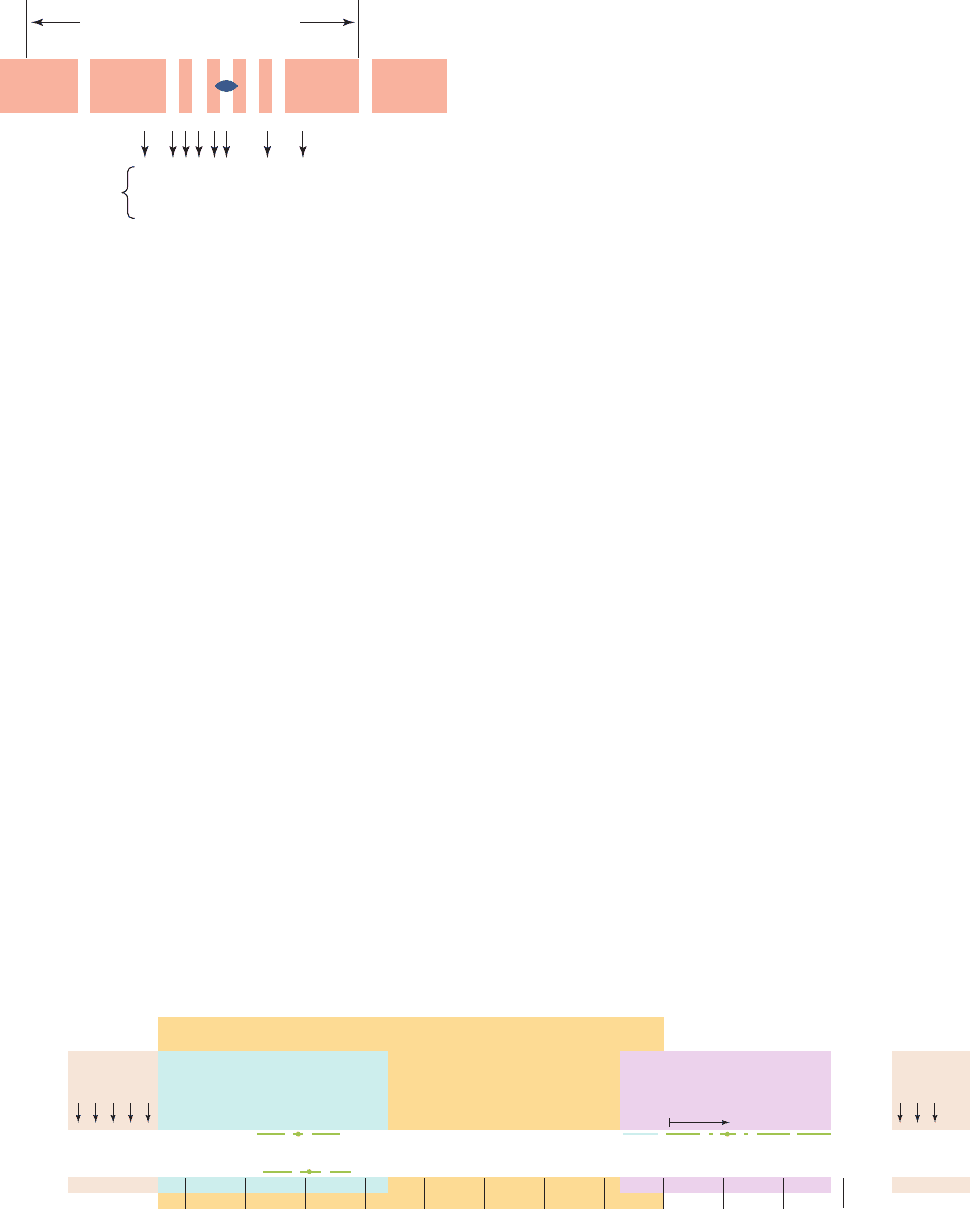
DNA
sequence
CAP-cAMP
binding site
Operator
Z gene
3'
5'
+30+20+10+1–10–20–30–40–50–60–70–80
Promoter
mRNA
fMet
Thr
Met
GAAAGCGGGCAGTGACCGCAACGCAATTAATGTGAGTTAGCTCACTCATTAGGCACCCCAGGCTTTACACTTTATGCTTCCGGCTCGTATGTTGTGTGGAATTGTGAGCGGATAACAATTTCACACAGGAAACAGCTATGACCATG
CTTTCGCCCGTCACTGGCGTTGCGTTAATTACACTCAATCGAGTGAGTAATCCGTGGGGTCCGAAATGTGAAATACGAAGGCCGAGCATACAACACACCTTAACACTCGCCTATTGTTAAAGTGTGTCCT TTGTCGATACTGGTAC
I gene
Glu
Gln
Stop
Ser
Gly
that had been sonicated to small fragments was mixed with
lac repressor and passed through a nitrocellulose filter.
Protein, with or without bound DNA, sticks to nitrocellu-
lose, whereas duplex DNA, by itself, does not. The DNA
was released from the filter-bound protein by washing it
with IPTG solution, recombined with lac repressor, and the
resulting complex treated with DNase I. The DNA frag-
ment that lac repressor protects from nuclease degradation
consists of a run of 26 bp that is embedded in a nearly 2-
fold symmetric sequence of 35 bp (Fig. 31-28, top). Such
palindromic symmetry is a common feature of DNA seg-
ments that are specifically bound by proteins (recall, for
example, that restriction endonuclease recognition sites
are also palindromic; Section 5-5Aa).
Palindromic DNA sequences, as we have seen, bind to
proteins that have matching 2-fold symmetry. However,
methylation protection experiments on the lac repressor–
operator system do not fully support this model: There is
an asymmetric pattern of differences between free and
repressor-bound operator in the susceptibility of its bases
to reaction with DMS (Fig. 31-28). Furthermore, point mu-
tations that render it operator constitutive (O
c
), and that
invariably weaken the binding of repressor to operator,
may increase as well as decrease the operator’s 2-fold
symmetry (Fig. 31-28).
c. lac Repressor Prevents RNA Polymerase from
Forming a Productive Initiation Complex
Operator occupies positions ⫺7 through ⫹28 of the lac
operon relative to the transcription start site (Fig. 31-29).
Nuclease protection studies, it will be recalled, indicate
that, in the initiation complex, RNA polymerase tightly
binds to the DNA between positions ⫺20 and ⫹20 (Sec-
tion 31-2Aa).Thus, the lac operator and promoter sites over-
lap. It was therefore widely assumed for many years that
lac repressor simply physically obstructs the binding of
RNA polymerase to the lac promoter. However, the obser-
vation that lac repressor and RNA polymerase can simul-
taneously bind to the lac operon indicates that lac repres-
sor must act by somehow interfering with the initiation
process. Closer investigation of this phenomenon revealed
that, in the presence of bound lac repressor, RNA poly-
merase holoenzyme still abortively synthesizes oligonu-
cleotides, although they tend to be shorter than those made
in the absence of repressor. Evidently, lac repressor acts by
somehow increasing the already high kinetic barrier for
RNA polymerase to generate the open complex and com-
mence processive elongation.
We discuss the lac repressor structure and further as-
pects of lac operator organization in Section 31-3F.
C. Catabolite Repression: An Example
of Gene Activation
Glucose is E. coli’s metabolite of choice; the availability of
adequate amounts of glucose prevents the full expression of
⬎100 genes that encode proteins involved in the fermentation
Section 31-3. Control of Transcription in Prokaryotes 1285
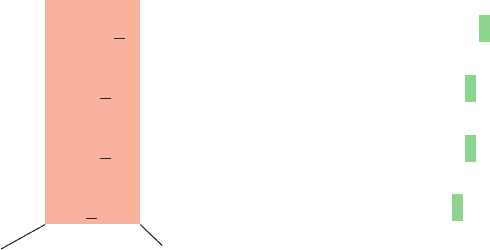
Figure 31-28 The base sequence of the lac operator. The
symmetry related regions (red) comprise 28 of its 35 bp. A “⫹”
denotes positions at which repressor binding enhances
methylation by dimethyl sulfate (which methylates G at N7 and
A at N3) and a “⫺” indicates where this footprinting reaction is
inhibited.The bottom row indicates the positions and identities
of different point mutations that prevent lac repressor binding
(O
c
mutants).Those in red increase the operator’s symmetry.
[After Sobell, H.M., in Goldberger, R.F. (Ed.), Biological
Regulation and Development, Vol. 1, p. 193, Plenum Press
(1979).]
Figure 31-29 The nucleotide sequence of the E. coli lac
promoter–operator region. The region extends from the
C-terminal portion of lacI (left) to the N-terminal portion of
lacZ (right).The palindromic sequences of the operator and
Protected by lac repressor
O
c
mutations
AATTTTGC
TTAAAACG
5' TG G G AA A AAAAA A A3'AG G GGGGCCCCCTT T T T TTTT
3' AC C C TT T TTTTT T T5'TC C CCCCGGGGGAA A A A AAAA
+–– –
++ ––––
the CAP-binding site (Section 31-3C) are overscored or
underscored. [After Dickson, R.C.,Abelson, J., Barnes, W.M.,
and Reznikoff, W.A., Science 187, 32 (1975).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1285
of numerous other catabolites, including lactose (Fig. 31-30),
arabinose, and galactose, even when these metabolites are
present in high concentrations. This phenomenon, which is
known as catabolite repression, prevents the wasteful dupli-
cation of energy-producing enzyme systems.
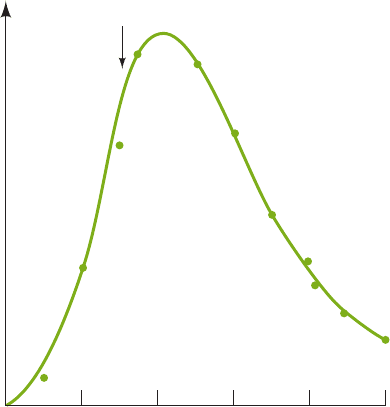
a. cAMP Signals the Lack of Glucose
The first indication of the mechanism of catabolite re-
pression was the observation that, in E. coli, the level of
cAMP, which was known to be a second messenger in ani-
mal cells (Section 18-3Cb), is greatly diminished in the
presence of glucose.This observation led to the finding that
the addition of cAMP to E. coli cultures overcomes catabo-
lite repression by glucose. Recall that, in E. coli, adenylate
cyclase is activated by the phosphorylated enzyme EIIA
glc
(or possibly inactivated by dephospho-EIIA
glc
), which is
dephosphorylated on the transport of glucose across the
cell membrane (Section 20-3D). The presence of glucose,
therefore, normally lowers the cAMP level in E. coli.
b. CAP–cAMP Complex Stimulates the Transcription
of Catabolite Repressed Operons
Certain E. coli mutants, in which the absence of glucose
does not relieve catabolite repression, are missing a cAMP-
binding protein that is synonymously named catabolite
gene activator protein (CAP) and cAMP receptor protein
(CRP). CAP is a homodimer of 209-residue subunits that
undergoes a large conformational change on binding
cAMP. Its function was elucidated by Ira Pastan, who
showed that CAP–cAMP complex, but not CAP itself,
binds to the lac operon (among others) and stimulates tran-
scription from its otherwise low-efficiency promoter in the
absence of lac repressor. CAP is therefore a positive regula-
tor (turns on transcription), in contrast to lac repressor,
which is a negative regulator (turns off transcription).
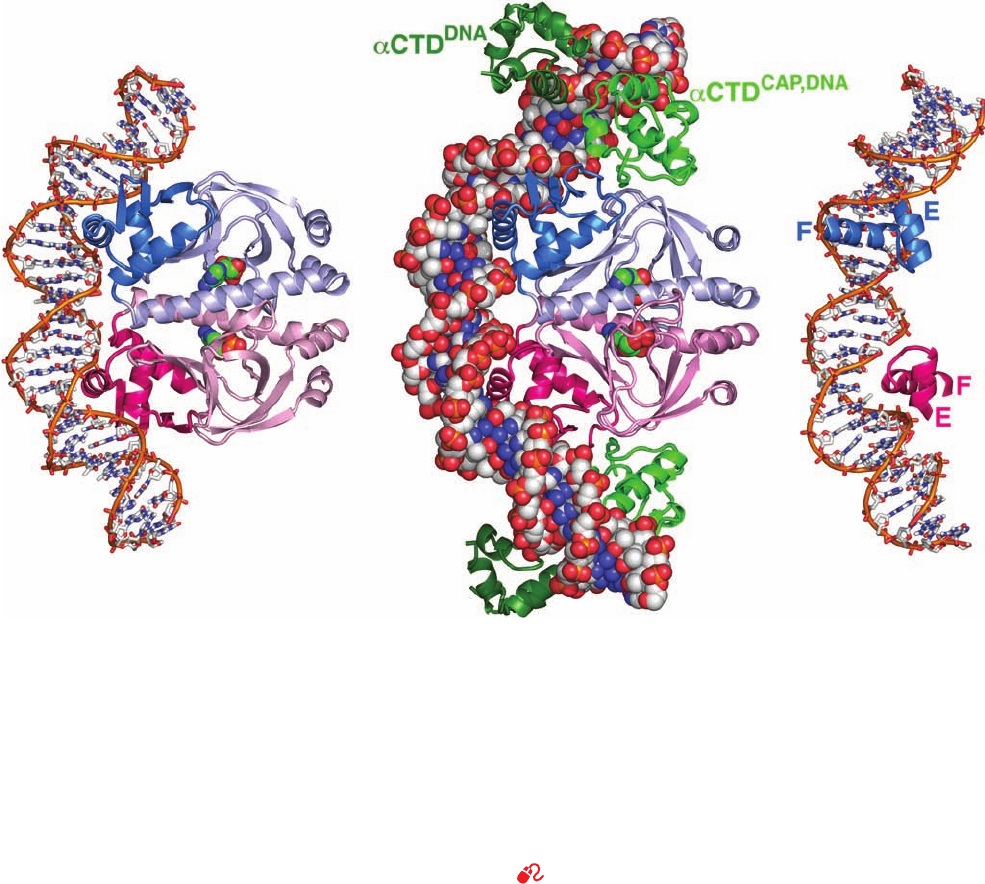
The X-ray structure, by Thomas Steitz, of CAP–cAMP
in complex with a palindromic 30-bp segment of duplex
DNA whose sequence resembles that of the CAP binding
sequence (Fig. 31-29) reveals that the DNA is bent by ⬃90°
around the protein (Fig. 31-31a). The bend arises from two
⬃45° kinks in the DNA between the fifth and sixth bases
out from the complex’s 2-fold axis in both directions. This
distortion results in the closing of the major groove and an
enormous widening of the minor groove at each kink.
Why is the CAP–cAMP complex necessary to stimulate
the transcription of its target operons? And how does it do
so? The lac operon has a weak (low efficiency) promoter;
its ⫺10 and ⫺35 sequences (TATGTT and TTTACA; Fig.
31-10) differ significantly from the corresponding consen-
sus sequences of strong (high-efficiency) promoters
(TATAAT and TTGACA; Fig. 31-10). Such weak promot-
ers evidently require some sort of help for efficient tran-
scriptional initiation.
Richard Ebright has shown that CAP interacts directly
with RNAP via the C-terminal domain of its 85-residue ␣
subunit (␣CTD) in a way that stimulates RNAP to initiate
transcription from a nearby promoter. The ␣CTD also
binds dsDNA nonspecifically but does so with higher affin-
ity at A ⫹ T–rich sites such as those of UP elements (Sec-
tion 31-2Aa). It is flexibly linked to the rest of the ␣ subunit
and hence is not seen in the X-ray structure of Tth RNAP
(Fig. 31-11) due to disorder.
Three classes of the over one hundred CAP-dependent
promoters have been characterized:
1. Class I promoters, such as that of the lac operon, re-
quire only CAP–cAMP for transcriptional activation. The
CAP binding site on the DNA can be located at various
distances from the promoter provided that CAP and
RNAP bind to the same face of the DNA helix. Thus,
CAP–cAMP activates the transcription of the lac operon if
its DNA binding site is centered near positions ⫺62 (its
wild-type position; Fig. 31-29), ⫺72, ⫺83, ⫺93, or ⫺103, all
of which are one helical turn apart. For the latter sites, this
requires that the DNA loop around to permit CAP–cAMP
to contact the ␣CTD. Such looping is likely to be facilitated
by the bending of the DNA around CAP–cAMP.
2. Class II promoters also require only CAP–cAMP for
transcriptional activation. However, in class II promoters,
the CAP binding site only occupies a fixed position that
overlaps the RNAP binding site, apparently by replacing
the promoter’s ⫺35 promoter region. CAP then interacts
1286 Chapter 31. Transcription
Minutes after IPTG addition
lac mRNA
0246810
Glucose
Figure 31-30 The kinetics of lac operon mRNA synthesis
following its induction with IPTG, and of its degradation after
glucose addition. E. coli were grown on a medium containing
glycerol as their only carbon-energy source and
3
H-labeled
uridine. IPTG was added to the medium at the beginning of the
experiment to induce the synthesis of the lac enzymes.After
3 min, glucose was added to stop the synthesis. The amount of
3
H-labeled lac RNA was determined by hybridization with DNA
containing the lacZ and lacY genes. [After Adesnik, M. and
Levinthal, C., Cold Spring Harbor Symp. Quant. Biol. 35, 457
(1970).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1286
with RNAP via interactions with both the ␣CTD and the ␣
subunit’s N-terminal domain.
3. Class III promoters require multiple activators to
maximally stimulate transcription. These may be two or
more CAP–cAMP complexes or a CAP–cAMP complex
acting in concert with promoter-specific activators as oc-
curs in the araBAD operon (Section 31-3E).
The X-ray structure of CAP–cAMP in complex with the
E. coli ␣CTD and a 44-bp palindromic DNA containing the
22-bp CAP–cAMP binding site and 5¿-AAAAAA-3¿ at each
end, determined by Helen Berman and Ebright, reveals how
these components interact (Fig.31-31b).The 2-fold symmet-
ric CAP–cAMP–␣CTD complex contains two differently lo-
cated pairs of ␣CTDs. Each member of the pair designated
␣CTD
CAP,DNA
binds to both CAP and to the DNA. CAP and
␣CTD
CAP,DNA
interact over a surprisingly small surface area
involving only six residues on each protein that mutagenesis
experiments had previously implicated. ␣CTD
CAP,DNA
also
interacts with the minor groove of a 6-bp segment of the
DNA (5¿-AAAAAG-3¿) centered 19 bp from the center of
the DNA. Each member of the other pair of ␣CTDs, desig-
nated ␣CTD
DNA
, interacts with the minor groove of an UP
element-like sequence (5¿-GAAAAA-3¿) that is fortu-
itously present in the DNA but it makes no contacts with
other protein molecules. The common portions of the two
CAP complexes pictured in Fig. 31-31a,b are closely super-
imposable, thereby indicating that the conformation of CAP
and its interaction with DNA are not significantly altered by
its association with the ␣CTD. Evidently, CAP–cAMP tran-
scriptionally activates RNAP via a simple “adhesive” mech-
anism that facilitates and/or stabilizes its interaction with the
Section 31-3. Control of Transcription in Prokaryotes 1287
Figure 31-31 X-ray structures of CAP–cAMP–dsDNA
complexes. The dsDNA and cAMP in these 2-fold symmetric
complexes are colored according to atom type with DNA C
white, cAMP green, N blue, O red, and P orange. (a) CAP–cAMP
in complex with a palindromic 30-bp self-complementary DNA
viewed with its 2-fold axis horizontal.The protein is drawn in
ribbon form with its identical subunits pink and blue and with
their C-terminal domains in darker shades.The DNA is shown in
stick form with successive P atoms in the same strand connected
by orange rods and with the cAMP drawn in space-filling form.
(b) CAP–cAMP in complex with a 44-bp palindromic DNA and
four ␣CTD subunits.The DNA, CAP, and cAMP are viewed as in
Part a with the DNA drawn in space-filling form. The ␣CTD
subunits are drawn in ribbon form with the ␣CTD
CAP,DNA
green
and the ␣CTD
DNA
dark green. (c) The same structure as in Part a
showing the binding of the CAP dimer’s two helix–turn–helix
(HTH) motifs in successive major grooves of the DNA. The view
is rotated 45° about the vertical axis relative to Part a. Note how
CAP’s F (recognition) helix is inserted into the DNA’s major
groove, as can also be seen in Parts a and b. [Parts a and c based
on an X-ray structure by Thomas Steitz, Yale University. PDBid
1CGP. Part b based on an X-ray structure by Helen Berman and
Richard Ebright, Rutgers University. PDBid 1LB2.]
See Interactive Exercise 38
(a)
(b)
(c)
JWCL281_c31_1260-1337.qxd 10/19/10 10:37 AM Page 1287

promoter DNA.The structures of ␣CTD
CAP,DNA
and ␣CTD-
CAP
and their interactions with DNA are nearly identical,
thereby suggesting that they are representative of the inter-
action of an ␣CTD with an UP element.
D. Sequence-Specific Protein–DNA Interactions
Since genetic expression is controlled by proteins such as
CAP and lac repressor, an important issue in the study of
gene regulation is how these proteins recognize their target
base sequences on DNA. Sequence-specific DNA-binding
proteins generally do not disrupt the base pairs of the
duplex DNA to which they bind. Consequently, these
proteins can only discriminate among the four base pairs
(A ⴢ T, T ⴢ A, G ⴢ C, and C ⴢ G) according to the functional
groups of these base pairs that project into DNA’s major
and minor grooves. An inspection of Fig. 5-12 reveals that
the groups exposed in the major groove have a greater
variation in their types and arrangements than do those
that are exposed in the minor groove. Indeed, the positions
of the hydrogen bonding acceptors in the major groove
vary with both the identity and orientation of the base pair,
whereas in the minor groove they are largely sequence
independent. Moreover, the ⬃5-Å-wide and ⬃8-Å-deep
minor groove of canonical (ideal) B-DNA is too narrow to
admit protein structural elements such as an ␣ helix,
whereas its ⬃12-Å-wide and ⬃8-Å-deep major groove can
do so. Thus, in the absence of major conformational
changes to B-DNA, it would be expected that proteins
could more readily differentiate base sequences from its
major groove than from its minor groove. We shall see
below that this is, in fact, the case.
a. The Helix–Turn–Helix Motif Is a Common DNA
Recognition Element in Prokaryotes
See Guided Exploration 30: Transcription factor–DNA interactions
The CAP dimer’s two symmetrically disposed F helices
protrude from the protein surface in such a way that they
fit into successive major grooves of B-DNA (Fig. 31-31).
CAP’s E and F helices form a helix–turn–helix (HTH)
motif (supersecondary structure) that conformationally re-
sembles analogous HTH motifs in numerous other prokary-
otic repressors of known X-ray and NMR structure, includ-
ing the lac repressor, the E. coli trp repressor (Section
31-3G), and the cI repressors and Cro proteins from bac-
teriophages and 434 (Section 33-3D). HTH motifs are
⬃20-residue polypeptide segments that form two ␣ helices
which cross at ⬃120° (Fig. 31-31c). They occur as compo-
nents of domains that otherwise have widely varying struc-
tures, although all of them bind DNA. Note that HTH mo-
tifs are structurally stable only when they are components
of larger proteins.
The X-ray and NMR structures of a number of
protein–DNA complexes (see below) indicate that DNA-
binding proteins containing an HTH motif associate with
their target base pairs mainly via the side chains extending
from the second helix of the HTH motif, the so-called recog-
nition helix (helix F in CAP, E in trp repressor, and ␣3 in
the phage proteins). Indeed, replacing the outward-facing
residues of the 434 repressor’s recognition helix with the
corresponding residues of the related bacteriophage P22
yields a hybrid repressor that binds to P22 operators but
not to those of 434. Moreover, the HTH motifs in all these
proteins have amino acid sequences that are similar to each
other and to polypeptide segments in numerous other
prokaryotic DNA-binding proteins, including lac repres-
sor. Evidently, these proteins are evolutionarily related and
bind their target DNAs in a similar manner.
How does the recognition helix recognize its target se-
quence? Since each base pair presents a different and pre-
sumably readily differentiated constellation of hydrogen
bonding groups in DNA’s major groove, it seemed likely
that there would be a simple correspondence, analogous to
Watson–Crick base pairing, between the amino acid
residues of the recognition helix and the bases they contact
in forming sequence-specific associations.The above X-ray
structures, however, indicate this idea to be incorrect.
Rather, base sequence recognition arises from complex
structural interactions. For instance:
1. The X-ray structures of the 48% identical N-termi-
nal domain of 434 repressor (residues 1–69) and the en-
tire 71-residue 434 Cro protein in their complexes with
the identical 20-bp target DNA (the expression of phage
434 is regulated through the differential binding of these
proteins to the same DNA segments; Section 33-3Db)
were both determined by Stephen Harrison. Both ho-
modimeric proteins, as seen for CAP (Fig. 31-31), associ-
ate with the DNA in a 2-fold symmetric manner with their
recognition helices bound in successive turns of the
DNA’s major groove (Figs. 31-32 and 31-33). In both com-
plexes, the protein closely conforms to the DNA surface
and interacts with its paired bases and sugar–phosphate
chains through elaborate networks of hydrogen bonds, salt
bridges, and van der Waals contacts. Nevertheless, the de-
tailed geometries of these associations are significantly
different. In the repressor–DNA complex (Fig. 31-32), the
DNA bends around the protein in an arc of radius ⬃65 Å
which compresses the minor groove by ⬃2.5 Å near its
center (between the two protein monomers) and widens
it by ⬃2.5 Å toward its ends. In contrast, the DNA in com-
plex with Cro (Fig. 31-33), although also bent, is nearly
straight at its center and has a less compressed minor
groove (compare Figs. 31-32a and 31-33a). This explains
why the simultaneous replacement of three residues in
the repressor’s recognition helix with those occurring in
Cro does not cause the resulting hybrid protein to bind
DNA with Cro-like affinity: The different conformations
of the DNA in the repressor and Cro complexes prevents
any particular side chain from interacting identically with
the DNA in the two complexes.
2. Paul Sigler determined the X-ray structure of E. coli
trp repressor in complex with a DNA containing an 18-bp
palindrome (TGTA
CTAGTTAACTAGTAC, where the
trp repressor’s target sequence is underlined) that closely
resembles the trp operator (Section 31-3G). The dimeric
protein’s recognition helices bind, as expected, in succes-
sive major grooves of the DNA, each in contact with an
1288 Chapter 31. Transcription
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1288
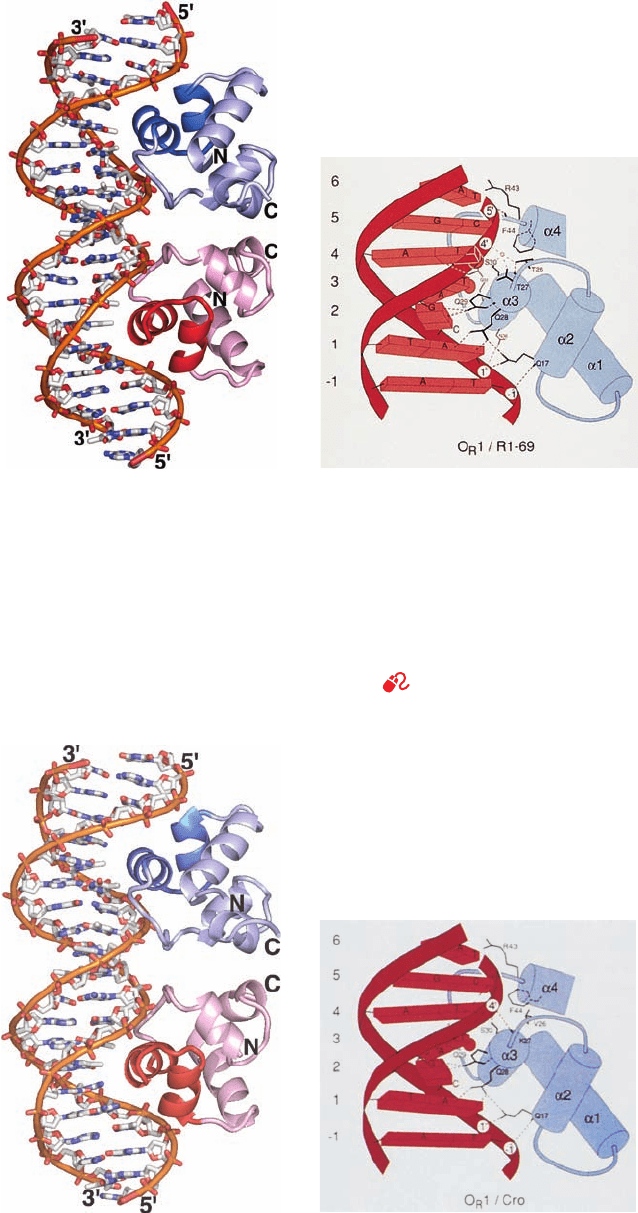
Section 31-3. Control of Transcription in Prokaryotes 1289
Figure 31-32 X-ray structure of the 69-residue N-terminal
domain of 434 phage repressor in complex with a 20-bp dsDNA
containing its target sequence. One strand of the DNA has the
sequence d(TATACAAGAAAGTTTGTACT). (a) The complex
viewed with the homodimeric protein’s 2-fold axis horizontal.
The protein is drawn in ribbon form with one of its two identical
subunits blue and the other red and with their helix–turn–helix
(HTH) motifs in darker shades.The DNA is drawn in stick form
with C white, N blue, O red, and P orange, and with successive P
atoms in the same chain connected by orange rods. (b) A
(a)
(b)
Figure 31-33 X-ray structure of the 71-residue 434 Cro
protein in complex with the same 20-bp DNA shown in
Fig. 31-32. Parts a and b correspond to those in Fig. 31-32. Note
the close but not identical correspondence between the two
structures and, in particular, the difference in the widths of the
schematic drawing indicating how the HTH motif, which
encompasses helices ␣2 and ␣3, interacts with its target DNA.
Short bars emanating from the polypeptide chain represent
peptide NH groups, hydrogen bonds are represented by dashed
lines, and DNA phosphates are represented by numbered circles.
The small circle is a water molecule. [Part a based on an X-ray
structure by and Part b courtesy of Aneel Aggarwal, John
Anderson, and Stephen Harrison, Harvard University. PDBid
2OR1.]
See Interactive Exercise 39 and Kinemage
Exercise 18-1
minor groove between the two subunits in each structure. [Part a
based on an X-ray structure by and Part b courtesy of Alfonso
Mondragón, Cynthia Wolberger, and Stephen Harrison, Harvard
University. PDBid 3CRO.]
(b)
(a)
JWCL281_c31_1260-1337.qxd 10/27/10 1:34 PM Page 1289

operator half-site (A
CTAGT; Fig. 31-34). There are nu-
merous hydrogen bonding contacts between the trp re-
pressor and its bound DNA’s nonesterified phosphate
oxygens. Astoundingly, however, there are no direct hydro-
gen bonds or nonpolar contacts that can explain the repres-
sor’s specificity for its operator. Rather, all but one of the
side chain–base hydrogen bonding interactions are medi-
ated by bridging water molecules (the one direct interac-
tion involves a base that can be mutated without greatly
affecting repressor binding affinity). Such buried water
molecules have therefore been described as “honorary”
protein side chains. In addition, the operator contains sev-
eral base pairs that are not in contact with the repressor
but whose mutation nevertheless greatly decreases repres-
sor binding affinity. This suggests that the operator as-
sumes a sequence-specific conformation that makes favor-
able contacts with the repressor. Indeed, comparison of
the X-ray structure of an uncomplexed 10-bp self-comple-
mentary DNA containing the trp operator’s half-site
(CCA
CTAGTGG) with that of the DNA in the trp repres-
sor–operator complex reveals that the ACTAGT half-site
assumes nearly identical idiosyncratic conformations and
patterns of hydration in both structures. However, the B-
DNA helix, which is straight in the DNA 10-mer, is bent by
15° toward the major groove in each operator half-site of
the repressor–operator complex. Other DNA sequences
could conceivably assume the repressor-bound operator’s
conformation but at too high an energy cost to form a sta-
ble complex with repressor (trp repressor’s measured 10
4
-
fold preference for its operator over other DNAs implies
an ⬃23 kJ ⴢ mol
⫺1
difference in their binding free ener-
gies).This phenomenon,in which a protein senses the base
sequence of DNA through the DNA’s backbone confor-
mation and/or flexibility, is referred to as indirect readout.
434 repressor apparently also employs indirect readout:
Replacing the central A ⴢ T base pair of the operator
shown in Fig. 31-32 with G ⴢ C reduces repressor binding
affinity by 50-fold even though 434 repressor does not
contact this region of the DNA.
It therefore appears that there are no simple rules govern-
ing how particular amino acid residues interact with bases.
Rather, sequence specificity results from an ensemble of mu-
tually favorable interactions between a protein and its target
DNA.
b. The met Repressor Contains a Two-Stranded
Antiparallel  Sheet That Binds in Its Target
DNA’s Major Groove
The E. coli met repressor (MetJ), when complexed
with S-adenosylmethionine (SAM; Fig. 26-18), represses
the transcription of its own gene and those encoding en-
zymes involved in the synthesis of methionine (Fig. 26-
60) and SAM. The X-ray structure of the met repres-
sor–SAM–operator complex (Fig. 31-35), determined by
Simon Phillips, reveals a symmetric dimer of intertwined
homodimers that lacks an HTH motif. Rather, met re-
pressor binds to its palindromic target DNA sequence
through two symmetry-related pairs of symmetrical two-
stranded antiparallel  sheets (called  ribbons) that are
inserted in successive major grooves of the DNA. Each
 ribbon makes sequence-specific contacts with its tar-
get DNA sequence via hydrogen bonding and, probably,
indirect readout.
1290 Chapter 31. Transcription
Figure 31-34 X-ray structure of an E. coli trp repressor–
operator–tryptophan complex. The complex is viewed with its
molecular 2-fold axis horizontal.The protein’s two identical
subunits are drawn in ribbon form colored pink and blue with
their HTH motifs (helices D and E) more deeply colored.The
18-bp-containing self-complementary dsDNA is shown in stick
form with C white, N blue, O red, P orange, and with successive
P atoms in the same chain connected by orange rods.The trp
repressor binds its operator only when
L-tryptophan, drawn in
space-filling form with C green, is simultaneously bound. Note
that the protein’s recognition helices (E) bind, as expected, in
successive major grooves of the DNA but extend approximately
perpendicular to the DNA duplex axis. In contrast, the
recognition helices of 434 repressor and Cro proteins are nearly
parallel to the major grooves of their bound DNAs (Figs. 31-32
and 31-33), whereas those of CAP assume an intermediate
orientation (Fig. 31-31). [Based on an X-ray structure by Paul
Sigler,Yale University. PDBid 1TRO.]
See Interactive Exercise 40
JWCL281_c31_1260-1337.qxd 10/19/10 10:54 AM Page 1290
