Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

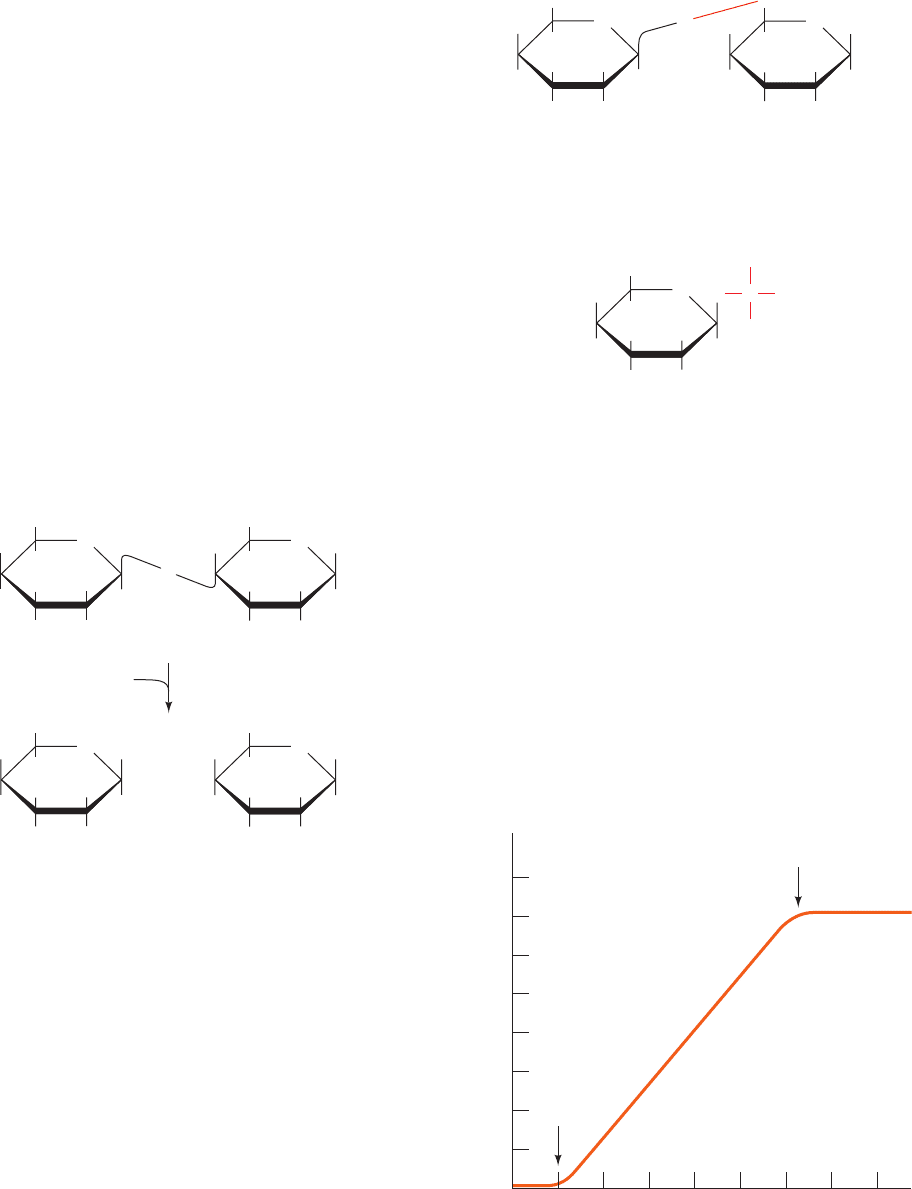
4.0
3.5
β-Galactosidase protein (μg)
Bacterial protein (μg)
Inducer
added
3.0
2.5
2.0
1.5
1.0
0.5
10 20 30 40 50 60 8070
Inducer
removed
A. Enzyme Induction
E. coli can synthesize an estimated ⬃4300 different
polypeptides. There is, however, enormous variation in the
amounts of these different polypeptides that are produced.
For instance, the various ribosomal proteins may each be
present in over 10,000 copies per cell, whereas certain reg-
ulatory proteins (see below) normally occur in ⬍10 copies
per cell. Many enzymes, particularly those involved in basic
cellular “housekeeping” functions, are synthesized at a
more or less constant rate; they are called constitutive
enzymes. Other enzymes, termed adaptive or inducible
enzymes, are synthesized at rates that vary with the cell’s
circumstances.
a. Lactose-Metabolizing Enzymes Are Inducible
Bacteria, as has been recognized since 1900, adapt to
their environments by producing enzymes that metabolize
certain nutrients, for example, lactose, only when those sub-
stances are available. E. coli grown in the absence of lactose
are initially unable to metabolize this disaccharide.To do so
they require the presence of two proteins: -galactosidase,
which catalyzes the hydrolysis of lactose to its component
monosaccharides,
and galactoside permease (also known as lactose perme-
ase; Section 20-4B), which transports lactose into the
cell. E. coli grown in the absence of lactose contain only
a few (⬍5) molecules of these proteins. Yet, a few min-
utes after lactose is introduced into their medium, E. coli
increase the rate at which they synthesize these proteins
by ⬃1000-fold (such that -galactosidase can account for
up to 10% of their soluble protein) and maintain this
pace until lactose is no longer available. The synthesis
rate then returns to its miniscule basal level (Fig. 31-1).
This ability to produce a series of proteins only when the
substances they metabolize are present permits bacteria to
adapt to their environment without the debilitating need to
continuously synthesize large quantities of otherwise un-
necessary substances.
Lactose or one of its metabolic products must somehow
trigger the synthesis of the above proteins. Such a sub-
OH
HO
H
2
O
CH
2
OH
H
OH H
HOH
HH
O
H
OH H
HOH
H
H
O
O
Lactose
OH
HO
CH
2
OH
H
OH H
HOH
HH
O
CH
2
OH
CH
2
OH
H
OH H
HOH
H
H
O
+
Galactose
OH
HO
Glucose
β-galactosidase
stance is known as an inducer. The physiological inducer of
the lactose system, the lactose isomer 1,6-allolactose,
arises from lactose’s occasional transglycosylation by
-galactosidase. Most experimental studies of the lactose
system use isopropylthiogalactoside (IPTG),
a potent inducer that structurally resembles allolactose but
that is not degraded by -galactosidase.
Lactose system inducers also stimulate the synthesis of
thiogalactoside transacetylase, an enzyme that, in vitro,
transfers an acetyl group from acetyl-CoA to the C6-OH
group of a -thiogalactoside such as IPTG. Since lactose
fermentation proceeds normally in the absence of thio-
galactoside transacetylase, however, this enzyme’s physio-
logical role is unknown.
b. lac System Genes Form an Operon
The genes specifying wild-type -galactosidase, galacto-
side permease, and thiogalactoside transacetylase are des-
ignated Z
⫹
, Y
⫹
, and A
⫹
, respectively. Genetic mapping of
the defective mutants Z
⫺
, Y
⫺
, and A
⫺
indicated that these
SCH
CH
3
CH
3
CH
2
OH
H
OH H
HOH
HH
O
HO
Isopropylthiogalactoside (IPTG)
OH
HO
CH
2
OH
H
OH H
HOH
HH
O
CH
2
H
OH H
HOH
H
H
O
HO
1,6-Allolactose
O
Section 31-1. The Role of RNA in Protein Synthesis 1261
Figure 31-1 The induction kinetics of -galactosidase in
E. coli. [After Cohn, M., Bacteriol. Rev. 21, 156 (1957).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1261
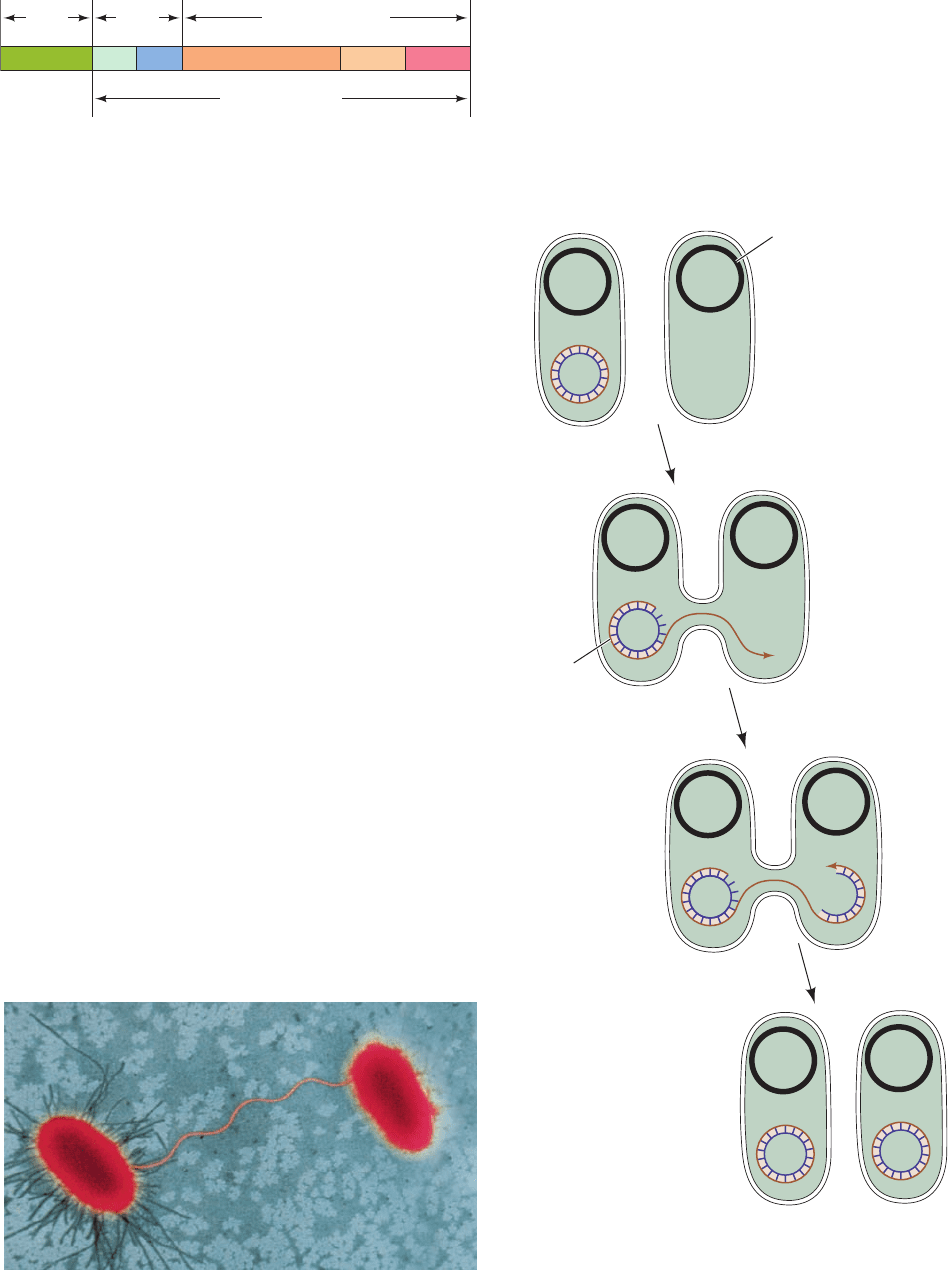
F
+
cell
F
+
cells
F
–
cell
Bacterial
chromosome
F factor
3'
5'
3'
5'
lac structural genes (genes that specify polypeptides) are
contiguously arranged on the E. coli chromosome (Fig. 31-2).
These genes, together with the control elements P and O,
form a genetic unit called an operon, specifically the lac
operon. The nature of the control elements is discussed
below. The role of operons in prokaryotic gene expression
is examined in Section 31-3.
c. Bacteria Can Transmit Genes via Conjugation
An important clue as to how E. coli synthesizes protein
was provided by a mutation that causes the proteins of the
lac operon to be synthesized in large amounts in the absence
of inducer. This so-called constitutive mutation occurs in a
gene, designated I, that is distinct from although closely
linked to the genes specifying the lac enzymes (Fig. 31-2).
What is the nature of the I gene product? This riddle was
solved in 1959 by Arthur Pardee,Fran¸cois Jacob, and Jacques
Monod through an ingenious experiment that is known as
the PaJaMo experiment. To understand this experiment,
however, we must first consider bacterial conjugation.
Bacterial conjugation is a process, discovered in 1946 by
Joshua Lederberg and Edward Tatum, through which some
bacteria can transfer genetic information to others. The
ability to conjugate (“mate”) is conferred on an otherwise
indifferent bacterium by a plasmid named F factor (for fer-
tility). Bacteria that possess an F factor (designated F
⫹
or
male) are covered by hairlike projections known as F pili.
These bind to cell-surface receptors on bacteria that lack
the F factor (F
⫺
or female), which leads to the formation of
a cytoplasmic bridge between these cells (Fig. 31-3). The F
factor then replicates and, as the newly replicated single
strand is formed, it passes through the cytoplasmic bridge to
the F
⫺
cell where the complementary strand is synthesized
(Fig. 31-4). This converts the F
⫺
cell to F
⫹
so that the F fac-
tor is an infectious agent (a bacterial venereal disease?).
On very rare occasions, the F factor spontaneously inte-
grates into the chromosome of the F
⫹
cell. In the resulting
1262 Chapter 31. Transcription
Figure 31-2 Genetic map of the E. coli lac operon. The map
shows the genes encoding the proteins mediating lactose
metabolism and the genetic sites that control their expression.
The Z, Y, and A genes, respectively, encode -galactosidase,
galactoside permease, and thiogalactoside transacetylase.
Figure 31-3 Bacterial conjugation. An electron micrograph in
false color showing an F
⫹
(left) and an F
⫺
(right) E. coli engaged
in sexual conjugation. [Dennis Kunkel/Phototake.]
Figure 31-4 Diagram showing how an F
ⴚ
cell acquires an
F factor from an F
ⴙ
cell. A single strand of the F factor is
replicated, via the rolling circle mode (Section 30-3Bb), and is
transferred to the F
⫺
cell where its complementary strand is
synthesized to form a new F factor.
OPIZYA
Control
sites
Regulatory
gene Structural genes
Lactose operon
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1262
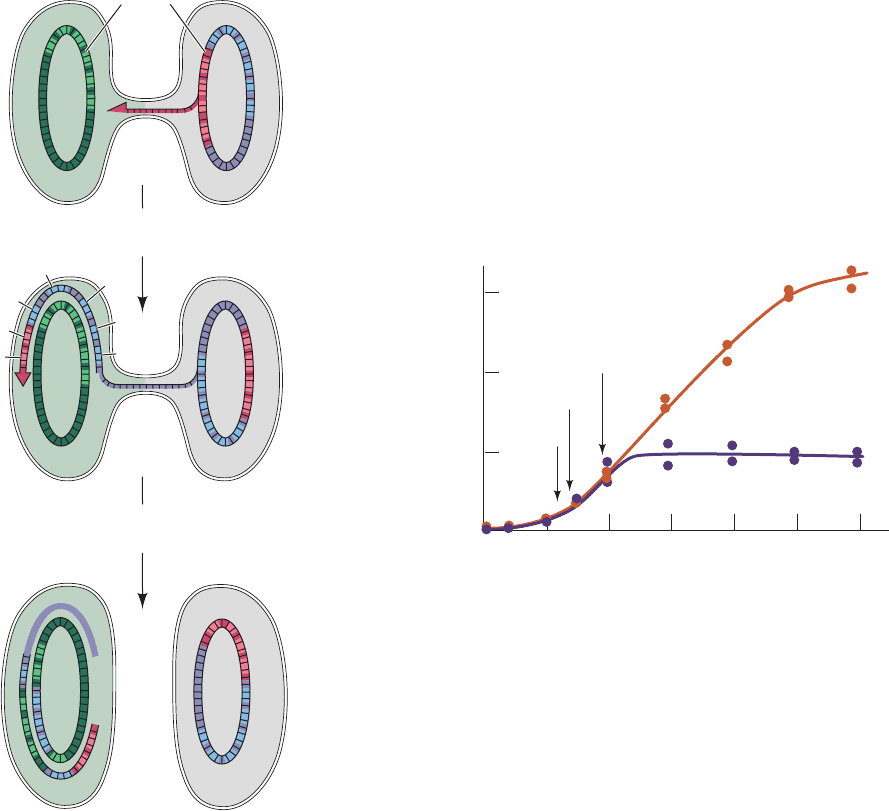
F
–
cell
Bacterial
chromosome
Chromosomal
transfer
Mating interruption
and genetic recombination
Hfr cell
A
a
b
c
d
e
g
B
3'
5'
C
D
E
G
δ
γ
β
α
A
A
A
B
B
C
C
D
D
E
E
G
δ
δ
γ
γ
β
α
A
B
B
d
c
b
a
e
g
C
C
D
+
D
E
E
G
δ
δ
γ
γ
β
α
a
b
c
d
e
g
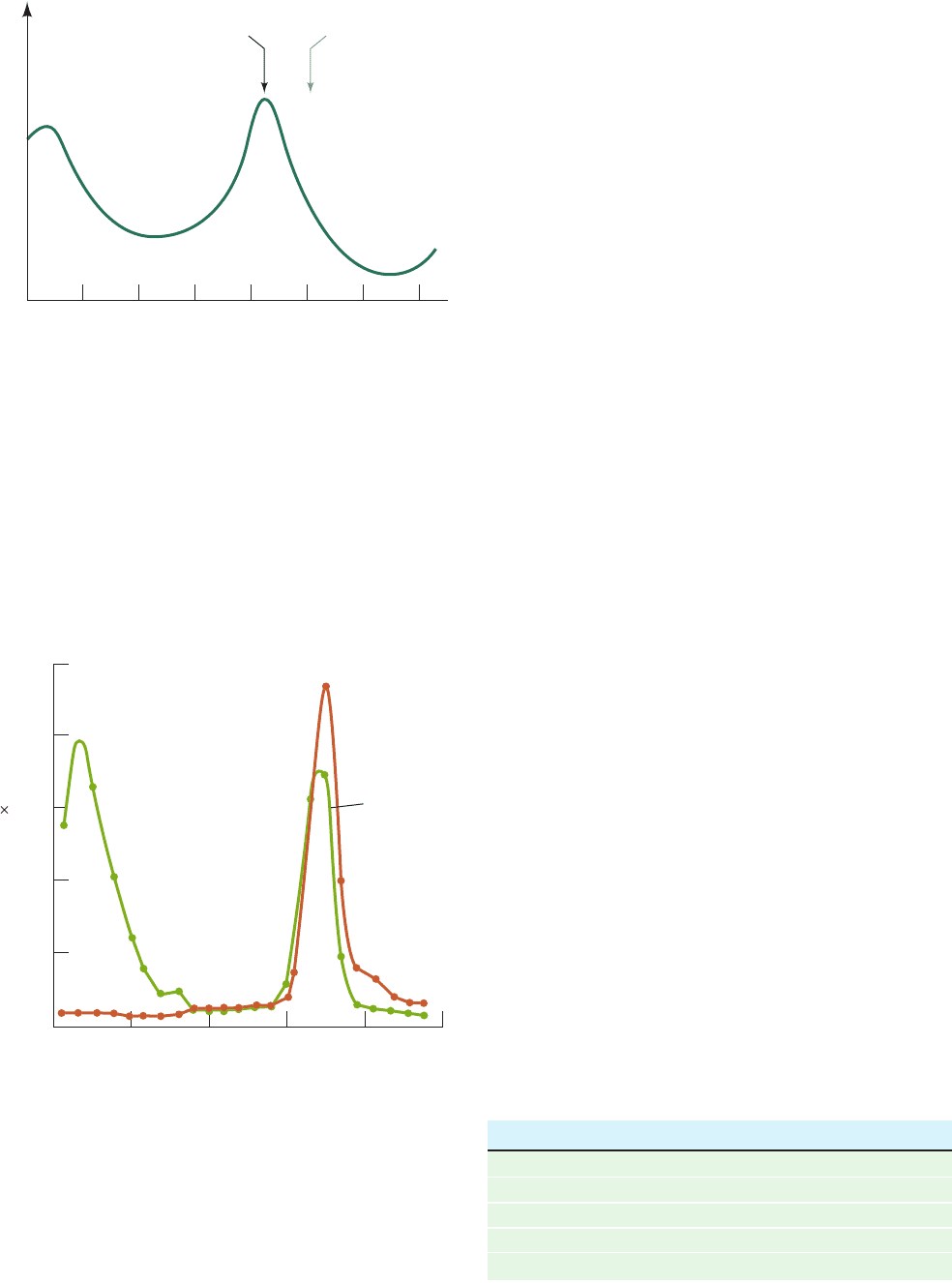
Inducer
added
No induce
r
Inducer
Streptomycin
T6
24613 50
0
5
10
15
Time (h)
β-Galactosidase (units · mL
–1
)
Hfr (for high frequency of recombination) cells, the F fac-
tor behaves much as it does in the autonomous state. Its
replication commences at a specific internal point in the F
factor,and the replicated section passes through a cytoplas-
mic bridge to the F
⫺
cell, where its complementary strand
is synthesized. In this case,however,the replicated chromo-
some of the Hfr cell is also transmitted to the F
⫺
cell
(Fig. 31-5). Bacterial genes are transferred from the Hfr cell
to the F
⫺
cell in fixed order. This is because the F factor in a
given Hfr strain is integrated into the bacterial chromo-
some at a specific site and because only a particular strand
of the Hfr chromosomal DNA is replicated and transferred
to the F
⫺
cell. Usually, only part of the Hfr bacterial chro-
mosome is transferred during sexual conjugation because
the cytoplasmic bridge almost always breaks off sometime
during the ⬃90 min required to complete the transfer
process. In the resulting merozygote (a partially diploid
bacterium), the chromosomal fragment, which lacks a com-
plete F factor, neither transforms the F
⫺
cell to Hfr nor is
subsequently replicated.However,the transferred chromo-
somal fragment recombines with the chromosome of the
F
⫺
cell (Section 30-6A), thereby permanently endowing
the F
⫺
cell with some of the traits of the Hfr strain.
The integrated F factor in an Hfr cell occasionally un-
dergoes spontaneous excision to yield an F
⫹
cell. In rare in-
stances, the F factor is aberrantly excised such that a por-
tion of the adjacent bacterial chromosome is incorporated
in the subsequently autonomously replicating F factor.
Bacteria carrying such a so-called Fⴕ factor are perma-
nently diploid for its bacterial genes.
d. lac Repressor Inhibits the Synthesis of lac
Operon Proteins
In the PaJaMo experiment, Hfr bacteria of genotype
I
⫹
Z
⫹
were mated to an F
⫺
strain of genotype I
⫺
Z
⫺
in the
absence of inducer while the -galactosidase activity of the
culture was monitored (Fig. 31-6). At first, as expected,
Section 31-1. The Role of RNA in Protein Synthesis 1263
Figure 31-5 Transfer of the bacterial chromosome from an
Hfr cell to an F
ⴚ
cell and its subsequent recombination with the
F
ⴚ
chromosome. Here, Greek letters represent F factor genes,
uppercase Roman letters represent bacterial genes from the Hfr
cell, and lowercase Roman letters represent the corresponding
alleles in the F
⫺
cell. Since chromosomal transfer, which begins
within the F factor, is rarely complete, the entire F factor is
seldom transferred. Hence the recipient cell usually remains F
⫺
.
Figure 31-6 The PaJaMo experiment. This experiment
demonstrated the existence of the lac repressor through the
appearance of -galactosidase in the transient merozygotes (partial
diploids) formed by mating I
⫹
Z
⫹
Hfr donors with I
⫺
Z
⫺
F
⫺
recipients.The F
⫺
strain was also resistant to both bacteriophage
T6 and streptomycin, whereas the Hfr strain was sensitive to
these agents. Both types of cells were grown and mated in the
absence of inducer.After sufficient time had passed for the
transfer of the lac genes, the Hfr cells were selectively killed by
the addition of T6 phage and streptomycin. In the absence of
inducer (lower curve), -galactosidase synthesis commenced at
around the time at which the lac genes had entered the F
⫺
cells
but stopped after ⬃1 h. If inducer was added shortly after the
Hfr donors had been killed (upper curve), enzyme synthesis
continued unabated.This demonstrates that the cessation of
-galactosidase synthesis in uninduced cells is not due to the
intrinsic loss of the ability to synthesize this enzyme but to the
production of a repressor specified by the I
⫹
gene. [After Pardee,
A.B., Jacob, F., and Monod, J., J. Mol. Biol. 1, 173 (1959).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1263

there was no -galactosidase activity because the Hfr
donors lacked inducer and the F
⫺
recipients were unable to
produce active enzyme (only DNA passes through the
cytoplasmic bridge connecting mating bacteria).About 1 h
after conjugation began, however, when the I
⫹
Z
⫹
genes
had just entered the F
⫺
cells, -galactosidase synthesis be-
gan and only ceased after about another hour. The expla-
nation for these observations is that the donated Z
⫹
gene,
on entering the cytoplasm of the I
⫺
cell, directs the synthe-
sis of -galactosidase in a constitutive manner. Only after
the donated I
⫹
gene has had sufficient time to be expressed
is it able to repress -galactosidase synthesis. The I
⫹
gene
must therefore give rise to a diffusible product, the lac re-
pressor, which inhibits the synthesis of -galactosidase (and
the other lac proteins). Inducers such as IPTG temporarily
inactivate lac repressor, whereas I
⫺
cells constitutively
synthesize lac enzymes because they lack a functional re-
pressor. Lac repressor, as we shall see in Section 31-3B, is a
protein.
B. Messenger RNA
The nature of the lac repressor’s target molecule was de-
duced in 1961 through a penetrating genetic analysis by Ja-
cob and Monod. A second type of constitutive mutation in
the lactose system, designated O
c
(for operator constitu-
tive), which complementation analysis (Section 1-4Cc) has
shown to be independent of the I gene, maps between the I
and Z genes (Fig. 31-2). In the partially diploid F¿ strain
O
c
Z
⫺
/F O
⫹
Z
⫹
, -galactosidase activity is inducible by
IPTG, whereas the strain O
c
Z
⫹
/F O
⫹
Z
⫺
constitutively syn-
thesizes this enzyme. An O
⫹
gene can therefore only control
the expression of a Z gene on the same chromosome.The
same is true with the Y
⫹
and A
⫹
genes.
Jacob and Monod’s observations led them to conclude
that the proteins are synthesized in a two-stage process:
1. The structural genes on DNA are transcribed onto
complementary strands of messenger RNA (mRNA).
2. The mRNAs transiently associate with ribosomes,
which they direct in polypeptide synthesis.
This hypothesis explains the behavior of the lac system
that we previously outlined in Section 5-4Ab (Fig. 5-25;
See Guided Exploration 2: Regulation of gene expression by the
lac repressor system).
In the absence of inducer, the lac repres-
sor specifically binds to the O gene (the operator) so as to
prevent the enzymatic transcription of mRNA. On binding
inducer, the repressor dissociates from the operator,
thereby permitting the transcription and subsequent trans-
lation of the lac enzymes. The operator–repressor–inducer
system thereby acts as a molecular switch so that the lac
operator can only control the expression of lac enzymes
on the same chromosome. The O
c
mutants constitutively
synthesize lac enzymes because they are unable to bind
repressor. The coordinate (simultaneous) expression of
all three lac enzymes under the control of a single opera-
tor site arises, as Jacob and Monod theorized, from the
transcription of the lac operon as a single polycistronic
mRNA which directs the ribosomal synthesis of each of
these proteins (the term cistron is a somewhat archaic
synonym for gene). This transcriptional control mecha-
nism is further discussed in Section 31-3. [DNA sequences
that are on the same DNA molecule are said to be “in cis”
(Latin: on this side of), whereas those on different DNA
molecules are said to be “in trans” (Latin: across). Control
sequences such as the O gene, which are only active on
the same DNA molecule as the genes they control, are
called cis-acting elements. Genes such as lacI, which spec-
ify the synthesis of diffusible products and can therefore
be located on a different DNA molecule from the genes
they control, are said to direct the synthesis of trans-act-
ing factors.]
a. mRNAs Have Their Predicted Properties
The kinetics of enzyme induction, as indicated, for ex-
ample, in Figs. 31-1 and 31-6, requires that the postulated
mRNA be both rapidly synthesized and rapidly degraded.
An RNA with such quick turnover had, in fact, been ob-
served in T2-infected E. coli. Moreover, the base composi-
tion of this RNA fraction resembles that of the viral DNA
rather than that of the bacterial RNA (keep in mind that
base sequencing techniques would not be formulated for
another ⬃15 years). Ribosomal RNA, which comprises up
to 90% of a cell’s RNA, turns over much more slowly than
mRNA. Ribosomes are therefore not permanently com-
mitted to the synthesis of a particular protein (a once pop-
ular hypothesis). Rather, ribosomes are nonspecific protein
synthesizers that produce the polypeptide specified by the
mRNA with which they are transiently associated. A bac-
terium can therefore respond within a few minutes to
changes in its environment.
Evidence favoring the Jacob and Monod model rapidly
accumulated. Sydney Brenner, Jacob, and Matthew Mesel-
son carried out experiments designed to characterize the
RNA that E. coli synthesized after T4 phage infection. E.
coli were grown in a medium containing
15
N and
13
C so as
to label all cell constituents with these heavy isotopes. The
cells were then infected with T4 phages and immediately
transferred to an unlabeled medium (which contained only
the light isotopes
14
N and
12
C) so that cell components syn-
thesized before and after phage infection could be sepa-
rated by equilibrium density gradient ultracentrifugation
in CsCl solution (Section 6-5Bb). No “light” ribosomes
were observed, which indicates, in agreement with the
above-mentioned T2 phage results, that no new ribosomes
are synthesized after phage infection.
The growth medium also contained either
32
P or
35
S so as
to radioactively label the newly synthesized and presum-
ably phage-specific RNA and protein, respectively. Much of
the
32
P-labeled RNA was associated, as was postulated for
mRNA, with the preexisting “heavy” ribosomes (Fig. 31-7).
Likewise, the
35
S-labeled proteins were transiently associ-
ated with, and therefore synthesized by, these ribosomes.
Sol Spiegelman developed the RNA–DNA hybridiza-
tion technique (Section 5-3Cb) in 1961 to characterize the
1264 Chapter 31. Transcription
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1264
RNA synthesized by T2-infected E. coli. He found that this
phage-derived RNA hybridizes with T2 DNA (Fig. 31-8)
but does not hybridize with DNAs from unrelated phage
nor with the DNA from uninfected E. coli. This RNA must
therefore be complementary to T2 DNA in agreement with
Jacob and Monod’s prediction; that is, the phage-specific
RNA is a messenger RNA. Hybridization studies have
likewise shown that mRNAs from uninfected E. coli are
complementary to portions of E. coli DNA. In fact, other
RNAs, such as transfer RNA and ribosomal RNA, have
corresponding complementary sequences on DNA from
the same organism. Thus, all cellular RNAs are transcribed
from DNA templates.
2 RNA POLYMERASE
RNA polymerase (RNAP), the enzyme responsible for the
DNA-directed synthesis of RNA, was discovered independ-
ently in 1960 by Samuel Weiss and Jerard Hurwitz. The en-
zyme couples together the ribonucleoside triphosphates
ATP, CTP, GTP, and UTP on DNA templates in a reaction
that is driven by the release and subsequent hydrolysis of
PP
i
:
All cells contain RNAP. In bacteria, one species of this
enzyme synthesizes all of the cell’s RNA except the RNA
primers employed in DNA replication (Section 30-1D).
Various bacteriophages encode RNAPs that synthesize
only phage-specific RNAs. Eukaryotic cells contain four
or five RNAPs that each synthesize a different class of
RNA. In this section we first consider the properties of
the bacterial RNAPs and then consider the eukaryotic
enzymes.
E. coli RNAP’s so-called holoenzyme is an ⬃459-kD
protein with subunit composition ␣
2
¿ (Table 31-1) in
which the  and ¿ subunits contain several colinearly
arranged homologous segments. Once RNA synthesis has
been initiated, however, the subunit (also called factor
or
70
since its molecular mass is 70 kD) dissociates from
the core enzyme, ␣
2
¿, which carries out the actual poly-
merization process (see below).
(RNA)
n residues
⫹ NTP Δ (RNA)
n⫹1 residues
⫹ PP
i
Section 31-2. RNA Polymerase 1265
Table 31-1 Components of E. coli RNA Polymerase
Holoenzyme
Subunit Number of Residues Structural Gene
␣ 329 rpoA
 1342 rpoB
¿ 1407 rpoC
91 rpoZ
70
613 rpsD
Radioactivity
Fraction number
“Light”
ribosomes
“Heavy”
ribosomes
0 1020304050 7060
25
20
15
10
5
0
Counts per min 10
–2
Fraction number
0 5 10 15 20 25
DNA
RNA
RNA • DNA
Figure 31-7 The distribution, in a CsCl density gradient, of
32
P-labeled RNA that had been synthesized by E. coli after T4
phage infection. Free RNA, being relatively dense, bands at the
bottom of the centrifugation cell (left). Much of the RNA,
however, is associated with the
15
N- and
13
C-labeled “heavy”
ribosomes that had been synthesized before the phage infection.
The predicted position of unlabeled “light” ribosomes, which are
not synthesized by phage-infected cells, is also indicated. [After
Brenner, S., Jacob, F., and Meselson, M., Nature 190, 579 (1961).]
Figure 31-8 The hybridization of
32
P-labeled RNA produced
by T2-infected E. coli with
3
H-labeled T2 DNA. On radioactive
decay,
32
P and
3
H emit  particles (electrons) with
characteristically different energies so that these isotopes can be
independently detected.Although free RNA (left) in a CsCl
density gradient is denser than DNA, much of the RNA bands
with the DNA (right). This indicates that the two polynucleotides
have hybridized and are therefore complementary in sequence.
[After Hall, B.D. and Spiegelman, S., Proc. Natl. Acad. Sci. 47,
141 (1961).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1265
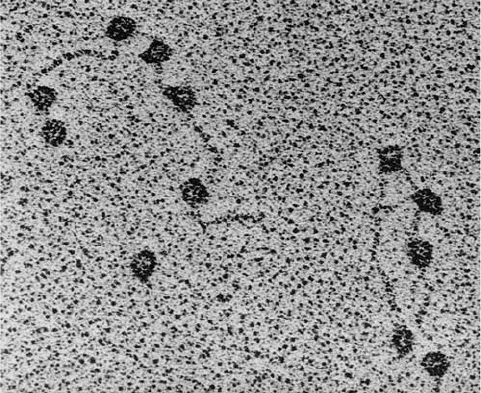
Electron micrographs (Fig. 31-9) clearly indicate that
RNAP, which has a characteristic large size, binds to DNA
as a protomer.This large size is presumably a consequence
of the holoenzyme’s several complex functions including
(1) template binding, (2) RNA chain initiation, (3) chain
elongation, and (4) chain termination. We discuss these
various functions below.
A. Template Binding
RNA synthesis is normally initiated only at specific sites on
the DNA template. This was first demonstrated through
hybridization studies of bacteriophage X174 DNA with
the RNA produced by X174-infected E. coli. Bacterio-
phage X174 carries a single strand of DNA known as the
(⫹) strand. On its injection into E. coli, the (⫹) strand di-
rects the synthesis of the complementary (⫺) strand with
which it combines to form a circular duplex DNA known as
the replicative form (Section 30-3Ba). The RNA produced
by X174-infected E. coli does not hybridize with DNA
from intact phages but does so with the replicative form.
Thus only the (⫺) strand of X174 DNA, the so-called an-
tisense strand, is transcribed, that is, acts as a template; the
(⫹) strand, the sense strand (or coding strand; so called be-
cause it has the same sequence as the transcribed RNA),
does not do so. Similar studies indicate that in larger
phages, such as T4 and , the two viral DNA strands are the
antisense (template) strands for different sets of genes.The
same is true of cellular organisms.
a. Holoenzyme Specifically Binds to Promoters
RNA polymerase binds to its initiation sites through
base sequences known as promoters that are recognized
by the corresponding factor. The existence of promoters
was originally suggested by mutations that enhance or di-
minish the transcription rates of certain genes, including
those of the lac operon. Genetic mapping of such muta-
tions indicated that the promoter consists of an ⬃40-bp se-
quence that is located on the 5¿ side of the transcription
start site. [By convention, the sequence of template DNA
is represented by its sense (nontemplate) strand so that it
will have the same directionality as the transcribed RNA.
A base pair in a promoter region is assigned a negative or
positive number that indicates its position, upstream or
downstream in the direction of RNAP travel, from the
first nucleotide that is transcribed to RNA; this start site
is ⫹1 and there is no 0.] RNA, as we shall see, is synthe-
sized in the 5¿S3¿ direction (Section 31-2C). Conse-
quently, the promoter lies on the “upstream” side of the
RNA’s starting nucleotide. Sequencing studies indicate
that the lac promoter (lacP) overlaps the lac operator
(Fig. 31-2).
The holoenzyme forms tight complexes with promoters
(dissociation constant K ⬇ 10
⫺14
M) and thereby protects
the bound DNA segments from digestion by DNase I. The
region from about ⫺20 to ⫹20 is protected against exhaus-
tive DNase I degradation. The region extending upstream
to about ⫺60 is also protected but to a lesser extent, pre-
sumably because it binds holoenzyme less tightly.
Sequence determinations of the protected regions
from numerous E. coli and phage genes have revealed the
consensus sequence of E. coli promoters (Fig. 31-10).
Their most conserved sequence is a hexamer centered at
about the ⫺10 position, the so-called Pribnow box (named
after David Pribnow, who pointed out its existence in
1975). It has a consensus sequence of TATAAT in which
the leading TA and final T are highly conserved. Up-
stream sequences around position ⫺35 also have a region of
sequence similarity, TTGACA, which is most evident in ef-
ficient promoters. The sequence of the segment between
the ⫺10 and the ⫺35 sites is unimportant but its length is
critical; it ranges from 16 to 19 bp in the great majority of
promoters. The initiating (⫹1) nucleotide, which is nearly
always A or G, is centered in a poorly conserved CAT or
CGT sequence. Most promoter sequences vary consider-
ably from the consensus sequence (Fig. 31-10). Neverthe-
less, a mutation in one of the partially conserved regions
can greatly increase or decrease a promoter’s initiation
efficiency. In addition, Richard Gourse discovered that
certain highly expressed genes contain an A ⫹ T–rich seg-
ment between positions ⫺40 and ⫺60, the upstream pro-
moter (UP) element, which binds to the C-terminal do-
main of RNAP’s ␣ subunits. The UP element-containing
genes include those encoding the ribosomal RNAs, the
rrn genes (e.g., Fig. 31-10), which collectively account for
60% of the RNA synthesized by E. coli. The rates at which
genes are transcribed, which span a range of at least 1000,
vary directly with the rate at which their promoters form
stable initiation complexes with the holoenzyme. Promoter
mutations that increase or decrease the rate at which the
associated gene is transcribed are known as up mutations
and down mutations.
1266 Chapter 31. Transcription
Figure 31-9 An electron micrograph of E. coli RNA
polymerase (RNAP) holoenzyme attached to various promoter
sites on bacteriophage T7 DNA. RNAP is one of the largest
known soluble enzymes. [From Williams, R.C., Proc. Natl.Acad.
Sci. 74, 2313 (1977).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1266
b. Initiation Requires the Formation of
an Open Complex
The promoter regions in contact with the holoenzyme
were identified by determining where the enzyme alters
the susceptibility of the DNA to alkylation by agents such
as dimethyl sulfate (DMS), a procedure named DMS foot-
printing (Section 34-3Bh). These experiments demon-
strated that the holoenzyme contacts the promoter mainly
around its ⫺10 and ⫺35 regions. These protected sites are
both on the same side of the B-DNA double helix as the
initiation site, which suggests that holoenzyme binds to
only one face of the promoter.
DMS methylates G residues at N7,A residues at N1 and
N3, and C residues at N3. Since N1 on A and N3 on C par-
ticipate in base pairing interactions, however,they can only
react with DMS in single-stranded DNA. This differential
methylation of single- and double-stranded DNAs pro-
vides a sensitive test for DNA strand separation or “melt-
ing.” Such chemical footprinting studies indicate that the
binding of holoenzyme “melts out” the promoter in a re-
gion of ⬃14 bp extending from the middle of the ⫺10 re-
gion to just past the initiation site, thereby forming a so-
called transcription bubble. The need to form this open
complex explains why promoter efficiency tends to de-
crease with the number of G ⴢ C base pairs in the ⫺10 re-
gion; this presumably increases the difficulty in opening the
double helix as is required for chain initiation (recall that
G ⴢ C pairs are more stable than A ⴢ T pairs).
Core enzyme, which does not specifically bind promoter
(except when it has an UP element), tightly binds duplex
DNA (the complex’s dissociation constant is K ⬇
5 ⫻ 10
⫺12
M and its half-life is ⬃60 min). Holoenzyme, in
contrast, binds to nonpromoter DNA comparatively
loosely (K ⬇ 10
⫺7
M and a half-life ⬎1 s). Evidently, the
subunit allows holoenzyme to move rapidly along a DNA
strand in search of the subunit’s corresponding promoter.
Once transcription has been initiated and the subunit
jettisoned, the tight binding of core enzyme to DNA appar-
ently stabilizes the ternary enzyme–DNA–RNA complex.
B. Chain Initiation
The 5¿-terminal base of prokaryotic RNAs is almost always
a purine with A occurring more often than G.The initiating
reaction of transcription is simply the coupling of two nu-
cleoside triphosphates in the reaction
and hence, unlike DNA replication, does not require a
primer. Bacterial RNAs therefore have 5¿-triphosphate
groups as was demonstrated by the incorporation of
radioactive label into RNA when it was synthesized with
[␥-
32
P]ATP. Only the 5¿ terminus of the RNA can retain the
label because the internal phosphodiester groups of RNA
are derived from the ␣-phosphate groups of nucleoside
triphosphates.
RNAP has a curious behavior: It frequently releases its
newly synthesized RNA after only ⬃10 nt have been poly-
merized, a process known as abortive initiation. When
RNAP initiates transcription, it keeps its grip on the pro-
pppA ⫹ pppN Δ pppApN ⫹ PP
i
Section 31-2. RNA Polymerase 1267
Figure 31-10 The sense (nontemplate) strand sequences of
selected E. coli promoters. A 6-bp region centered around the
⫺10 position (red shading) and a 6-bp sequence around the ⫺35
region (blue shading) are both conserved.The transcription
initiation sites (⫹1), which in most promoters occur at a single
purine nucleotide, are shaded in green.The bottom row shows
A
C
A
G
G
A
T
C
C
C
C
A
C
C
T
G
C
A
T
A
A
A
A
A
C
A
T
A
C
A
C
T
A
A
A
A
C
T
T
T
G
T
C
A
C
A
T
A
C
C
A
C
T
G
A
A
G
A
T
T
A
G
T
C
G
A
A
T
T
A
T
A
G
A
T
T
A
G
A
C
A
A
T
A
G
A
C
A
T
C
A
G
A
A
T
A
C
T
C
C
T
T
C
A
C
T
C
T
T
G
A
C
A
G
G
C
A
A
T
G
T
G
T
T
T
T
T
T
C
C
A
C
T
C
G
G
A
T
G
T
T
T
T
T
A
G
T
A
G
G
T
G
T
T
T
T
C
C
C
C
A
A
T
T
T
G
G
G
A
A
A
G
C
C
T
A
A
T
C
A
C
A
C
C
A
A
T
A
C
G
G
C
T
A
A
T
C
A
T
A
A
C
G
T
T
A
C
T
T
T
T
C
G
A
C
C
T
C
T
T
T
T
G
C
C
A
C
T
A
C
T
T
T
A
T
A
G
A
T
G
T
T
T
T
T
A
T
A
G
A
G
T
G
T
T
A
G
T
G
A
C
A
C
A
C
T
C
T
T
C
T
T
G
A
G
G
T
C
G
C
T
A
T
T
C
T
G
C
T
G
C
G
A
T
A
G
G
T
A
G
C
C
A
C
C
C
A
A
T
G
G
G
C
G
T
A
G
G
T
A
C
G
A
G
G
G
C
A
C
A
T
A
A
G
A
A
G
T
T
C
G
A
C
A
T
A
C
A
C
A
T
T
T
C
G
G
T
T
T
G
T
T
T
C
T
T
G
A
T
C
C
G
C
G
G
T
T
A
T
T
G
C
C
C
G
G
T
C
T
G
G
T
A
C
C
G
T
C
T
T
T
T
T
T
T
T
T
T
A
A
A
A
G
T
A
A
A
A
A
A
T
T
T
C
T
A
G
G
T
T
T
T
G
G
G
T
C
A
A
G
G
A
A
T
T
A
C
G
A
C
C
T
A
A
A
A
T
T
T
T
T
T
T
T
T
T
T
T
G
A
A
T
G
A
T
T
G
G
G
G
T
G
T
T
G
G
G
A
C
C
C
C
G
C
G
C
C
T
T
C
G
G
G
A
T
G
G
T
T
A
A
A
C
C
C
C
G
C
T
C
T
C
A
A
C
C
C
A
G
C
T
C
T
G
A
G
C
T
T
C
A
C
A
A
G
C
C
T
C
C
C
C
A
G
T
T
G
A
C
C
G
C
C
C
T
G
T
A
T
A
T
T
C
G
A
C
T
A
T
C
C
G
A
A
T
T
T
G
G
A
C
C
C
T
A
C
T
T
C
C
T
G
A
C
C
T
A
A
C
G
G
G
G
A
T
G
G
C
T
C
C
A
A
C
A
G
A
T
C
A
C
C
C
G
C
C
G
A
C
T
T
C
T
G
G
A
A
G
C
G
C
T
T
G
T
A
A
C
C
C
G
T
A
T
T
T
T
A
T
G
G
T
G
C
T
T
G
A
T
T
A
A
G
G
Initiation
site (+1)
⫺10 region
(Pribnow box)
⫺35 regionOperon
lac
lacI
g
alP2
araBAD
araC
trp
bioA
bioB
t
rrnD1
rrnE1
rrnA1
Tyr
Consensus
sequence:
T T G A C A
69 79 61 56 54 54
16–19 bp T A T A A T
77 76 60 61 56 82
5–8 bp
CT
42
4855
51
A
G
⫺35 region ⫺10 region
Initiation
site
... ... ... ...
RNA
the consensus sequence of 298 E. coli promoters with the
number below each base indicating its percentage occurrence.
The downstream portions of the rrn genes’ UP elements can be
seen. [After Rosenberg, M. and Court, D., Annu. Rev. Genet. 13,
321–323 (1979). Consensus sequence from Lisser, S. and
Margalit, H., Nucleic Acids Res. 21, 1512 (1993).]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1267
moter (which is on the DNA’s nontemplate/sense strand).
Consequently, conformational tension builds up as the
template/antisense strand is pulled through the RNAP’s
active site, a process called scrunching because the result-
ing increased size of the transcription bubble in the down-
stream direction must somehow be accommodated within
the RNAP. In abortive initiation, the RNAP fails to escape
the promoter and instead relieves the conformational ten-
sion by releasing the newly synthesized RNA fragment,
thereby letting the transcription bubble relax to its normal
size. The RNAP then reinitiates transcription from the ⫹1
position. In successful initiation, the strain eventually pro-
vides sufficient energy to strip the promoter from the
RNAP, which then commences the processive (continuous)
transcription of the template. This process requires the
dissociation of the factor from the core–DNA–RNA
complex to form the elongation complex, although recent
experiments indicate that this process often occurs stochas-
tically (randomly) over several nucleotide additions. The
factor can then join with another core to form a new initia-
tion complex as was demonstrated by a burst of RNA syn-
thesis on addition of core enzyme to a transcribing reaction
mixture that initially contained only holoenzyme.
a. Bacterial RNAP Has a Highly Complex Structure
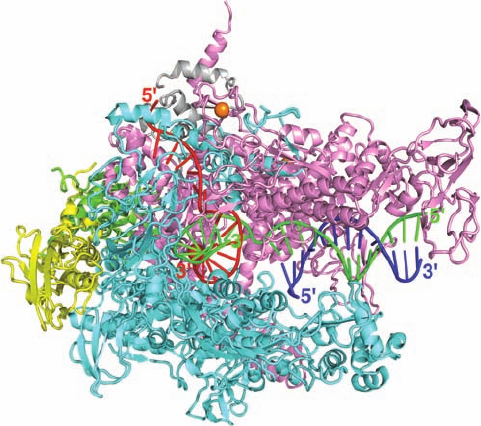
The X-ray structure of E. coli RNAP has not been deter-
mined. However, Seth Darst and Dmitry Vassylyev independ-
ently determined the X-ray structures of the closely similar
Thermus aquaticus (Taq) and Thermus thermophilus (Tth)
RNAP core enzymes and holoenzymes. The structure of the
Tth core enzyme in complex with DNA and RNA, in agree-
ment with EM studies of E. coli RNAP, has the overall shape
of a crab claw whose two “pincers” are formed by the  and ¿
subunits (Fig. 31-11). The protein is ⬃150 Å long (parallel to
the pincers), ⬃115 Å high, and ⬃110 Å deep, with the tunnel
between the two pincers ⬃27 Å wide. The  and ¿ subunits
extensively interact with one another, particularly at the base
of the tunnel (also called the main channel) where an active
site Mg
2⫹
ion is located, which is also where their homologous
segments converge. The ¿ subunit binds two Zn
2⫹
ions, each
via four Cys residues that are invariant in prokaryotes but not
in eukaryotes. The outer surface of the RNAP is almost uni-
formly negatively charged, whereas those surfaces that inter-
act with nucleic acids are positively charged.
The downstream dsDNA occupies the main channel,
which directs the template strand to the active site.There it
base-pairs with the incoming NTP (not present in this
structure) at the so-called i ⫹ 1 site near the Mg
2⫹
ion.The
3¿ end of the RNA forms a 9-bp hybrid helix with the 5¿ end
of the template DNA strand and then exits the protein
through a channel between the  and ¿ subunits (the RNA
exit channel) in which it adopts a conformation similar to
that of a single strand within an RNA double helix. Thus
the structure resembles that of a post-translocated elonga-
tion complex, although the paths taken by the template
and nontemplate DNA strands to rejoin at the end of the
transcription bubble are unclear.
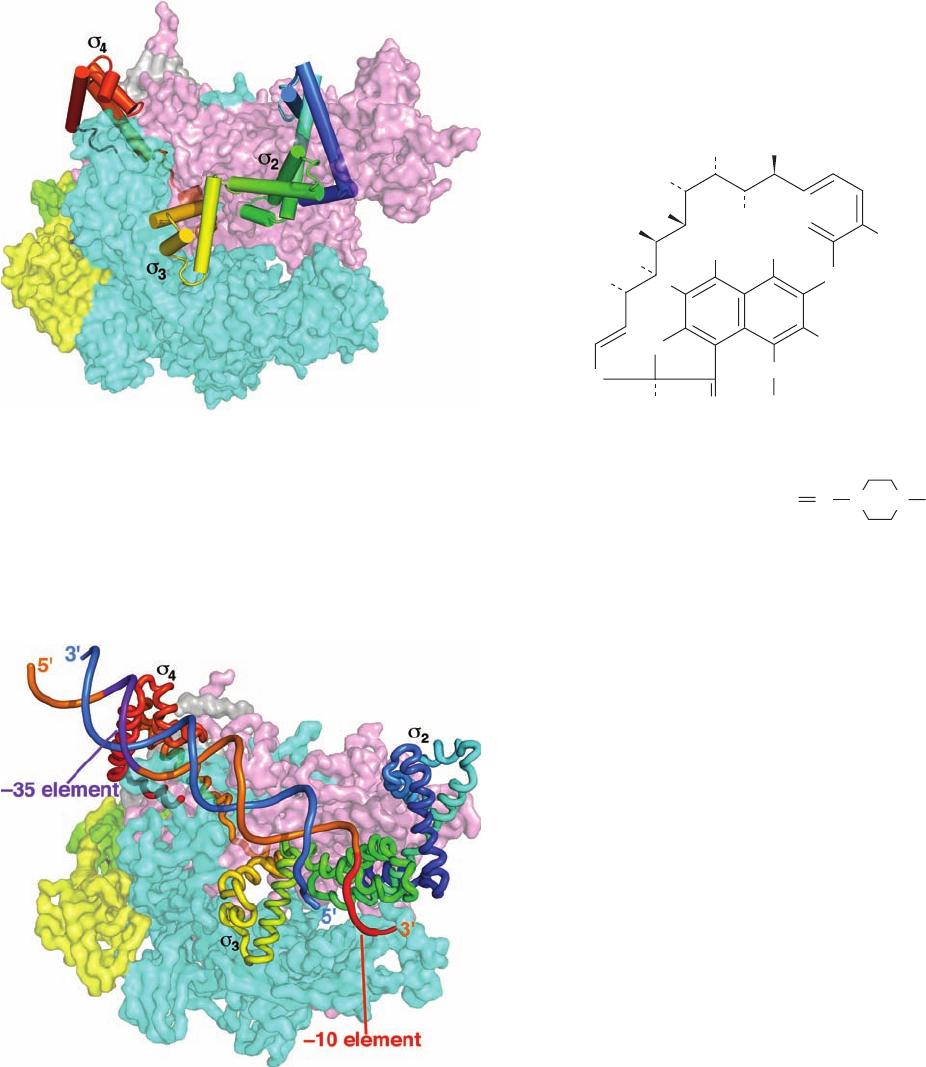
The X-ray structure of the Tth holoenzyme indicates
that its subunit (
70
) has three flexibly linked, largely ␣
helical domains,
2
,
3
, and
4
, that extend across the top of
the holoenzyme (Fig. 31-12;
1
is not visible). The holoen-
zyme’s pincers are ⬃10 Å farther apart than in the elonga-
tion complex. The binding cavities for the downstream
dsDNA and the transcription bubble are partially occupied
by the subunit’s
4
domain and its
3–4
linker.This partially
accounts for the above-described nucleic acid scrunching
that precedes the transition from the initiation complex to
the elongation complex.Thus, the release of only these seg-
ments of the subunit from RNAP is compatible with the
formation of an elongation complex, thereby accounting for
the above-mentioned stochastic release of the subunit
from a successfully initiated RNAP complex.
A low (6.5 Å) resolution X-ray structure of Taq holoen-
zyme in complex with a dsDNA segment containing the pro-
moter’s ⫺10 and ⫺35 elements reveals that the DNA lies
across one face of the holoenzyme, completely outside of the
main channel (Fig. 31-13). All sequence-specific contacts
that the holoenzyme makes with the ⫺10 and ⫺35 elements
as well as with the so-called extended ⫺10 region just up-
stream of the ⫺10 element are mediated by the subunit via
conserved residues.This structure presumably resembles the
so-called closed complex in which the DNA has not yet en-
tered the main channel to form a transcription bubble. The
mechanism through which this occurs is largely unknown.
1268 Chapter 31. Transcription
Figure 31-11 X-ray structure of Tth core RNAP in complex
with a 23-nt template DNA, a 14-nt nontemplate DNA, and a
16-nt RNA. The protein is drawn in ribbon form with its two ␣
subunits yellow and green, its  subunit cyan, its ¿ subunit pink,
and its subunit gray. The bound Mg
2⫹
and Zn
2⫹
ions are
represented by red and orange spheres, respectively. The DNA
and RNA are shown in ladder form with template DNA green,
nontemplate DNA blue, and RNA red. Note that residues 208
to 390 of the ¿ subunit, which extend from the tip of its pincer,
are disordered and hence not visible, as are the 86-residue
C-terminal domains of both ␣ subunits. [Based on an X-ray
structure by Dmitry Vassylyev, University of Alabama at
Birmingham. PDBid 5O5I.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1268
b. Rifamycins Inhibit Prokaryotic
Transcription Initiation
Two related antibiotics, rifamycin B, which is produced by
Streptomyces mediterranei, and its semisynthetic derivative
rifampicin,
specifically inhibit transcription by prokaryotic,but not eu-
karyotic, RNAPs. This selectivity and their high potency
(bacterial RNAP is 50% inhibited by 2 ⫻ 10
⫺8
M
rifampicin) made them medically useful bacteriocidal
agents against gram-positive bacteria and tuberculosis. In-
deed, few other antibiotics are effective against tuberculo-
sis, which has reached epidemic levels in some parts of the
world.
The finding that the  subunits of rifamycin-resistant
mutants have altered electrophoretic mobilities first
demonstrated that this subunit contains the rifamycin-
binding site. Rifamycins inhibit neither the binding of
RNAP to the promoter nor the formation of the first phos-
phodiester bond, but they prevent further chain elonga-
tion. The inactivated RNAP remains bound to the pro-
moter, thereby blocking its initiation by uninhibited
enzymes. Once RNA chain initiation has occurred, how-
ever, rifamycins have no effect on the subsequent elonga-
tion process. The rifamycins are therefore useful research
tools because they permit the transcription process to be
dissected into its initiation and its elongation phases.
The X-ray structure of Taq core enzyme in complex with
rifampicin reveals how this antibiotic inhibits RNAP.
Rifampicin binds with close complementary fit but little
conformational change in a pocket in the  subunit that is
located within the main channel, ⬃12 Å distant from the
active site Mg
2⫹
ion. Model building indicates that the
bound rifampicin would sterically interfere with the RNA
transcript at positions ⫺2 to ⫺5 in the transcription bubble.
Thus, as is observed, rifampicin would not interfere with
the initiation of transcription but would mechanically
block the extension of the RNA transcript. The residues
lining the pocket in which rifampicin binds are highly con-
served among prokaryotes but not in eukaryotes, thereby
explaining why rifamycins inhibit only bacterial RNAPs.
HO
H
3
C
H
3
C
CH
3
CH
3
CH
3
CH
3
CH
3
CH
3
OH
OH
OH
R
2
O
O
O
O
R
1
CH
3
COO
CH
3
O
O
NH
Rifamycin B
Rifampicin
R
1
= CH
2
COO
–
; R
2
= H
R
1
= H; R
2
= CH
NN
N
Section 31-2. RNA Polymerase 1269
Figure 31-12 X-ray structure of Tth RNAP holoenzyme
viewed similarly to Fig. 31-11. The subunits of the core enzyme
are represented by their partially transparent molecular surface
colored as in Fig. 31-11. The subunit is drawn with its ␣ helices
as cylinders and colored in rainbow order from its N-terminus
(blue) to its C-terminus (red). [Based on an X-ray structure by
Dmitry Vassylyev, University of Alabama at Birmingham.
PDBid 1IW7.]
Figure 31-13 Low (6.5 Å) resolution X-ray structure of Taq
RNAP holoenzyme in complex with a promoter-containing
dsDNA viewed as in Fig. 31-12. The subunits of the core enzyme
are represented by their partially transparent molecular surface
colored as in Fig. 31-11 (which appears striated due to the
structure’s low resolution, which permits only the polypeptide
backbones to be visualized).The subunit is drawn in worm
form colored in rainbow order from its N-terminus (blue) to its
C-terminus (red).The DNA’s sugar–phosphate backbone is
drawn in cartoon form with the template DNA strand blue and
the nontemplate DNA’s ⫺10 element red, its ⫺35 element
purple, and its remaining portions orange. [Based on an X-ray
structure by Seth Darst,The Rockefeller University. PDBid 1L9Z.]
JWCL281_c31_1260-1337.qxd 8/11/10 9:47 PM Page 1269
*ppp p
OH
p
...
OH OH
OH
+
NN
2
N
*ppp
OH
OH
N
*ppp
OH
N
pp
...
OHOH
OH
NN
2
p
N
1
+ *PP
i
3' 5' growth
(b)
+1 1x x +1x x
*ppp
OH
OH
N
+1
*ppp p
OH
p ...
OH OH
+
OH
OH
N
1
N
2
N N
+ *PP
i
5' 3' growth
*ppp p
OH
p
...
OH OH
OH
N
1
N
2
N
x
(a)
x +1x x
p
C. Chain Elongation
The direction of RNA chain elongation; that is, whether it
occurs by the addition of incoming nucleotides to the 3¿ end
of the nascent (growing) RNA chain (5¿S3¿ growth;Fig.31-
14a) or by their addition to its 5¿ terminus (3¿S5¿ growth;
Fig. 31-14b), was established by determining the rate at
which the radioactive label from [␥-
32
P]GTP is incorporated
into RNA. For 5¿S3¿ elongation, the 5¿ ␥-P is permanently
labeled and, hence, the chain’s level of radioactivity would
not change on replacement of the labeled GTP with unla-
beled GTP. However, for 3¿S5¿ elongation, the 5¿ ␥-P is re-
placed with the addition of every new nucleotide so that, on
replacement of labeled with unlabeled GTP, the nascent
RNA chains would lose their radioactivity. The former was
observed. Chain growth must therefore occur in the 5¿S3¿
direction (Fig.31-14a), the same direction as DNA is synthe-
sized. This conclusion is corroborated by the observation
that the antibiotic cordycepin,
an adenosine analog that lacks a 3¿-OH group, inhibits bac-
terial RNA synthesis. Its addition to the 3¿ end of RNA, as
is expected for 5¿S3¿ growth, prevents the RNA chain’s
Cordycepin
(3'-deoxyadenosine)
O
HH
HH
H OH
HOCH
2
NH
2
N
N
N
N
further elongation. Cordycepin would not have this effect
if chain growth occurred in the opposite direction because
it could not be appended to an RNA’s 5¿ end.
a. Transcription Supercoils DNA
RNA chain elongation requires that the double-stranded
DNA template be opened up at the point of RNA synthesis
so that the template strand can be transcribed to its comple-
mentary RNA strand. In doing so, the RNA chain only tran-
siently forms a short length of RNA–DNA hybrid duplex,as
is indicated by the observation that transcription leaves the
template duplex intact and yields single-stranded RNA.The
unpaired transcription bubble of the DNA in the open initi-
ation complex apparently travels along the DNA with the
RNAP. There are two ways this might occur (Fig. 31-15):
1. If the RNAP followed the template strand in its heli-
cal path around the DNA, the DNA would build up little
supercoiling because the DNA duplex would never be un-
wound by more than about a turn. However, the RNA
transcript would wrap around the DNA, once per duplex
turn. This model is implausible since it is unlikely that its
DNA and RNA could be readily untangled: The RNA
would not spontaneously unwind from the long and often
circular DNA in any reasonable time, and no known topoi-
somerase can accelerate this process.
2. If the RNAP moves in a straight line while the DNA
rotates, the RNA and DNA will not become entangled.
Rather, the DNA’s helical turns are pushed ahead of the ad-
vancing transcription bubble so as to more tightly wind the
DNA ahead of the bubble (which promotes positive super-
coiling), and the DNA behind the bubble becomes equiva-
lently unwound (which promotes negative supercoiling, al-
though note that the linking number of the entire DNA
remains unchanged).This model is supported by the observa-
1270 Chapter 31. Transcription
Figure 31-14 The two possible modes of RNA chain growth.
Growth may occur (a) by the addition of nucleotides to the 3¿
end and (b) by the addition of nucleotides to the 5¿ end. RNA
polymerase catalyzes the former reaction.
JWCL281_c31_1260-1337.qxd 8/11/10 9:48 PM Page 1270
