Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

phosphoSer group that it would have to nucleophilically
attack in the religation step and the space between them
is filled with protein. Clearly, the synaptic complex must
undergo a dramatic structural change to accomplish the
religation step.
Section 30-6. Recombination and Mobile Genetic Elements 1241
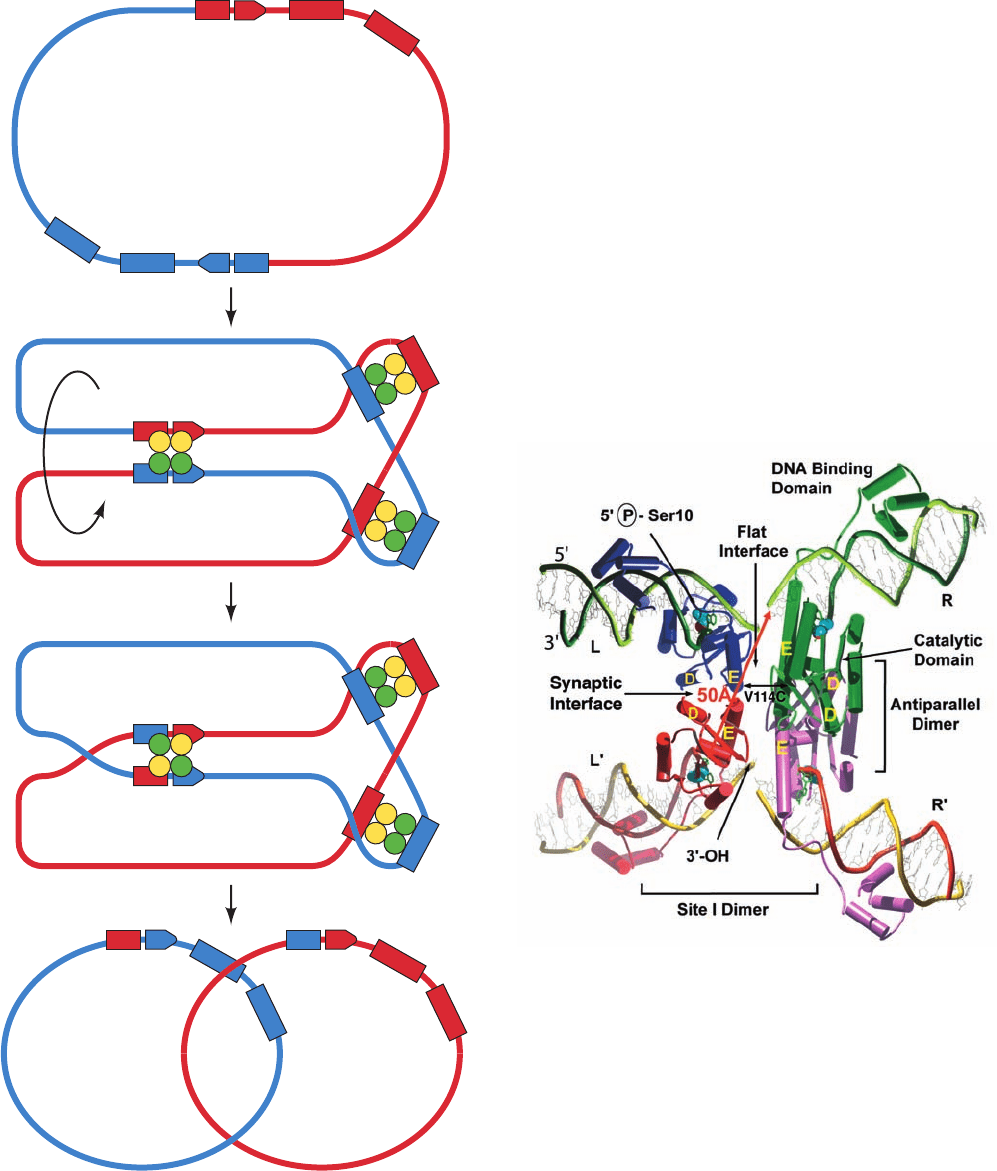
Figure 30-92 A model for the resolution of a cointegrate
containing two ␥␦ transposons to form two catenated dsDNA
circles. (1) The ␥␦ resolvase binds as six homodimers to its
binding sites, I, II, and III, in each of the cointegrate’s two res
sites (yellow and green circles represent the ␥␦ resolvase
monomers initially bound to the red and blue res sites,
respectively), which then associate to form synaptic tetramers.
Although not shown as such, the synaptic tetramers bound to
sites I associate with the synaptic tetramers bound to sites II and
III to form, as seen in the electron microscope, a compact globule
of unknown structure known as a synaptosome. (2) The dsDNA
at sites I both undergo staggered (by 2 bp) double-strand
scissions via the transient formation of phosphoSer bonds
between Ser 10 and the 5¿-phosphates at the cleavage sites.The
cleaved strands then exchange places (cross over) in a process
that apparently requires the rotation of one of the pairs of
resolvase monomers with respect to the other and are then
ligated. (3) The dissociation of the synaptosome yields the
catenated dsDNA circles. [Courtesy of Gregory Mullen,
University of Connecticut Health Center.]
Res
Res
Cointegrate
III
III
III
III
1
2
3
Figure 30-93 X-ray structure of a ␥␦ resolvase synaptic
tetramer in complex with two 34-bp palindromic, site
I–containing dsDNAs. The D
2
-symmetric complex is viewed with
one of its 2-fold axes horizontal (the other 2-fold axes lie in the
vertical plane labeled “Flat interface”).The DNAs (dark and
light green and yellow and orange) have been cleaved into half-
sites labeled L, R, L¿, and R¿ through the nucleophilic attack of
the Ser 10 side chain (drawn in space-filling form with C blue
and O red) on the 5¿-phosphate group of A20 (drawn in stick
form in green).The subunits of the resolvase tetramer, whose
helices are drawn in tube form, are colored blue, green, red, and
pink.The intact DNAs initially bind to the L–R and L¿–R¿
dimers, the so-called site I dimers. The L–L¿ dimer and the
symmetrically equivalent R–R¿ dimer form so-called antiparallel
dimers whose D and E helices associate as four-helix bundles. In
addition, a Val 114 S Cys mutation (V114C) disulfide-cross-links
the E helices of the L–R and L¿–R¿ dimers across the so-called
flat interface. [Courtesy of Thomas Steitz, Yale University. PDBid
1ZR4.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1241
The observation that the interface between the L–L¿ and
R–R¿ dimers is largely hydrophobic and unusually flat (Fig.
30-93) strongly suggests that the religation occurs after a
180° rotation of these dimers with respect to one another
(about the horizontal 2-fold axis in Fig. 30-93), thus exchang-
ing the positions of the R and R¿ subunits with respect to the
L and L¿ subunits. The rotation is presumably driven by the
superhelical tension in the naturally negatively supercoiled
cointegrate. This model is supported by energy calculations
and the observation that mutating Val 114 of the E helix to
Cys, which, under oxidizing conditions, disulfide-links the E
helices on the L and R (and L¿ and R¿) subunits (a mutation
that was present in the foregoing structure), yields a com-
plex that can form the covalent intermediate in Fig. 30-93
but cannot carry out the religation step—presumably be-
cause the disulfide bonds prevent the above rotation. How-
ever, reducing these disulfide bonds restores the complex’s
recombinational activity. Furthermore, mutation of Lys 136,
an E helix residue located at the flat interface, to Cys pre-
vents religation of the cleaved DNA when subjected to oxi-
dizing conditions, even though it requires a 75° rotation of
the L–L¿/R–R¿ interface in the structure in Fig. 30-93 to bring
the Cys 136 side chains on opposing subunits close enough
to form a disulfide bond.Of course, a detailed understanding
of the mechanism of the ␥␦ resolvase reaction will require
the knowledge of how all six ␥␦ resolvase dimers that form
the synaptosome participate in the reaction (Fig. 30-92).
e. Replicative Transposons Are Responsible for
Much Genetic Remodeling in Prokaryotes
In addition to mediating their own insertion into DNA,
replicative transposons promote inversions, deletions, and
rearrangements of the host DNA. Inversions can occur
when the host DNA contains two copies of a transposon in
inverted orientation. The recombination of these trans-
posons inverts the region between them (Fig. 30-94a). If, in-
stead, the two transposons have the same orientation, the
resolution of this cointegrate-like structure deletes the seg-
ment between the two transposons (Fig. 30-94b; if the
deleted segment lacks a replication origin, it will not be
propagated). The deletion of a chromosomal segment in
this manner, followed by its integration into the chromo-
some at a different site by a separate recombinational
event, results in chromosomal rearrangement.
Transposition appears to be important in chromosomal
and plasmid evolution. Indeed, it has been suggested that
transposons are nature’s genetic engineering “tools.” For
example, the rapid evolution, since antibiotics came into
common use, of plasmids that confer resistance to several
antibiotics (Section 5-5Ba) has resulted from the accumu-
lation of the corresponding antibiotic-resistance trans-
posons in these plasmids. Transposon-mediated rearrange-
ments may well have been responsible for organizing
originally distant genes into coordinately regulated oper-
ons (Section 5-4Aa) as well as for forming new proteins by
linking two formerly independent gene segments. More-
over, the occurrence of identical transposons in unrelated
bacteria indicates that the transposon-mediated transfer of
genetic information between organisms is not limited to re-
lated species, in contrast to genetic transfers mediated by ho-
mologous recombination.
f. Phase Variation Is Mediated by Site-Specific
Recombination
Phenotypic expression in bacteria can be regulated by
site-specific recombination. For example, certain strains of
1242 Chapter 30. DNA Replication, Repair, and Recombination
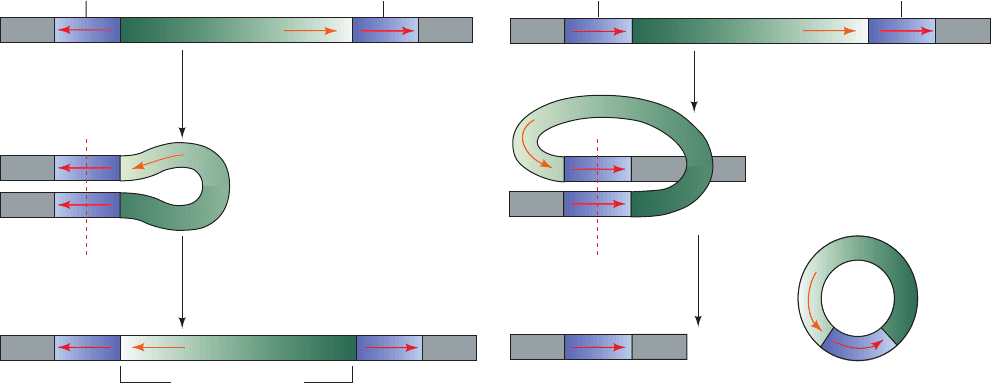
Figure 30-94 Chromosomal rearrangement via recombination.
(a) The inversion of a DNA segment between two identical
transposons with inverted orientations. (b) The deletion of a
Transposons with inverted orientations
(a) (b)
Pairing of inverted repeats
Recombination
Inverted segment
Transposons with the same orientation
Pairing of direct repeats
Recom-
bination
+
Chromosome
containing one
transposon
Deleted segment
containing
one transposon
DNA segment between two identical transposons with the same
orientation.This process parcels one transposon each to the
resulting two DNA segments.
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1242
Salmonella typhimurium make two antigenically distinct
versions of the protein flagellin (the major component of
the whiplike flagella with which bacteria propel them-
selves; Section 35-3I) that are designated H1 and H2. Only
one of these proteins is expressed by any particular cell but
about once every 1000 cell divisions, in a process known as
phase variation, a cell switches the type of flagellin it syn-
thesizes. It is thought that phase variation helps Salmonella
evade its host’s immunological defenses.
What is the mechanism of phase variation? The two fla-
gellin genes reside on different parts of the bacterial chro-
mosome. H2 is linked to the rh1 gene that encodes a repres-
sor of H1 expression (Fig. 30-95; rh1, H2, and H1 are also
known as fljA, fljB, and fljC, respectively). Hence, when the
H2–rh1 transcription unit is expressed, H1 synthesis is
repressed; otherwise H1 is synthesized. Melvin Simon has
shown that the expression of the H2–rh1 unit is controlled
by the orientation of a 995-bp segment that lies upstream of
H2 (Fig. 30-95) and that contains the following elements:
1. A promoter for H2–rh1 expression.
2. The hin gene, which encodes the 190-residue Hin
DNA invertase. Hin,a serine recombinase, mediates the in-
version of the DNA segment in a manner similar to that di-
agrammed in Fig. 30-94a. In fact, Hin is ⬃40% identical in
sequence with the ␥␦ resolvase, which strongly suggests
that these proteins have similar structures.
3. Two closely related 26-bp sites, hixL and hixR, that
form the boundaries of the segment and hence contain its
cleavage sites. They each consist of two imperfect 12-bp in-
verted repeats separated by 2 nt.
In the Phase 2 orientation (Fig. 30-95a), the properly ori-
ented promoter is just upstream of H2, so this gene and rh1
are coordinately expressed, thereby repressing H1 synthe-
sis. In Phase 1 bacteria (Fig. 30-95b), however, this segment
has the opposite orientation. Consequently, neither H2 nor
rh1, which then lacks a promoter, is expressed so that H1 is
synthesized.
g. Cre-Mediated Site-Specific Recombination
Occurs via 3¿-PhosphoTyr Intermediates
Bacteriophages, as we have seen (Fig. 1-31), replicate
themselves within their host bacterial cells which, in most
cases, they then lyse to release the progeny phage, a
lifestyle that is therefore known as the lytic mode. How-
ever, certain bacteriophages can assume an alternative,
nondestructive lifestyle, the lysogenic mode, in which they
install their DNA, usually in the host chromosome via site-
specific recombination, so that the phage DNA is passively
replicated with the host DNA. However, if the bacterial
host encounters conditions in which it is unlikely to sur-
vive, the phage DNA is excised from the bacterial chromo-
some via a reversal of the site-specific recombination reac-
tion and it reenters the lytic mode so as to escape the
doomed host. We discuss the genetic factors that maintain
the balance between the lytic and lysogenic lifestyles in
bacteriophage in Section 33-3.
The enzymes that mediate the foregoing site-specific
recombination reactions are members of the integrase
( Int; alternatively, tyrosine recombinase) family, whose
⬃1000 known members also occur in prokaryotes and eu-
karyotes. These include the XerC and XerD proteins of E.
coli which, operating in concert, function to decatenate the
two linked circular dsDNA products of homologous re-
combination (Fig. 30-69g, left), as well as type IB topoiso-
merases (Section 29-3Cc).
The structurally best characterized member of the in-
tegrase family is the Cre recombinase of E. coli bacterio-
phage P1. In its lysogenic state, bacteriophage P1 is a sin-
gle-copy circular plasmid (rather than being inserted in the
host chromosome as is bacteriophage ), but in the phage
head (the lytic mode), P1 DNA is a linear dsDNA that has
a 34-bp loxP site at each end. The main function of Cre,
Section 30-6. Recombination and Mobile Genetic Elements 1243
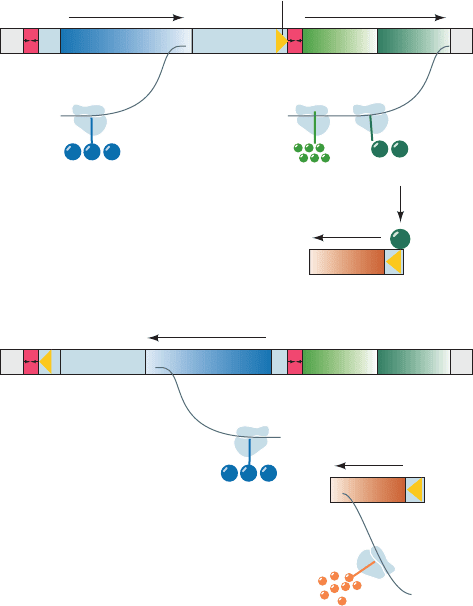
Figure 30-95 The mechanism of phase variation in
Salmonella. (a) In Phase 2 bacteria, the H2–rh1 promoter is
oriented so that H2 flagellin and repressor are synthesized.
Repressor binds to the H1 gene, thereby preventing its expression.
(b) In Phase 1 bacteria, the segment preceding the H2–rh1
transcription unit has been inverted relative to its orientation in
Phase 2 bacteria. Hence this transcription unit cannot be
expressed because it lacks a promoter.This releases H1 from
repression and results in the synthesis of H1 flagellin.The
inversion of the segment preceding the H2–rh1 transcription unit
is mediated by the Hin protein, which is expressed in either
orientation by the hin gene.
hin
Phase 2(a)
rh1H2
hixR
hixR
Hin protein
Hin protein
Repressor
H2–rh1
promoter
Inactive
Phase 1(b)
H1
hin rh1H2
Inactive
H1
H1 flagellin
mRNA
mRNA
mRNA
hixL
hixL
H2 flagellin
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1243

which is encoded by bacteriophage P1, is to mediate the
site-specific recombination between these two loxP sites so
as to circularize the linear DNA (Fig. 30-96).
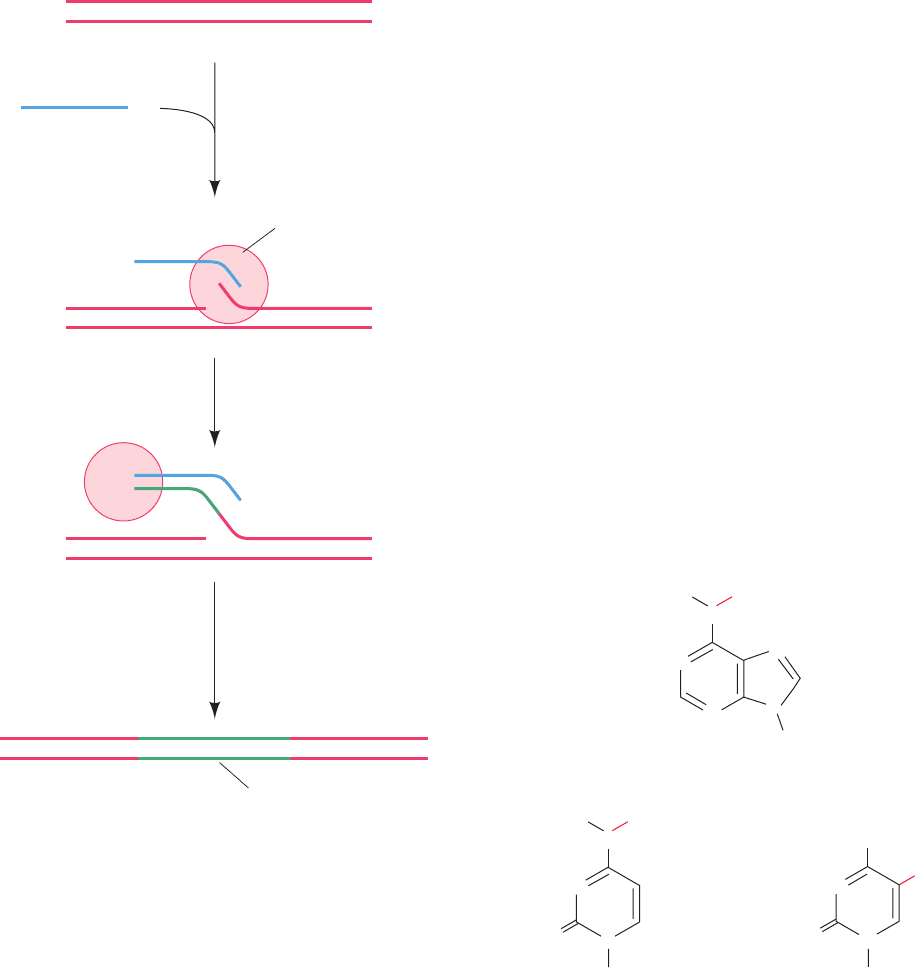
The loxP site is palindromic except for its central 8-bp
crossover region, which confers directionality on the site.
In carrying out the recombination reaction, the 343-residue
Cre subunits form a homotetramer that binds two loxP sites
in an antiparallel orientation, with each Cre subunit binding
half of a loxP site. Then, as is diagrammed in Fig. 30-97,
oppositely located Cre subunits catalyze single-strand scis-
sions on the 5¿ side of the crossover region on one strand of
each of the two dsDNAs. This occurs through the nucle-
ophilic attack of each of these active Cre subunit’s con-
served Tyr 324 residues on the DNA’s scissile phosphoester
bond to yield a 3¿-phosphoTyr intermediate on one side of
the cleaved bond and a free 5¿-OH group on the other side
(as similarly occurs in the reactions catalyzed by type IB
topoisomerases; Section 29-3Cc). Each of the liberated 5¿-
OH groups then nucleophilically attacks the 3¿-phospho-
Tyr group on the opposite duplex to form a Holliday junc-
tion, thereby releasing the Tyr residues. The Holliday
junction is resolved into two recombined dsDNAs when
the two Cre subunits that had not yet participated in the re-
action mediate the same cleavage and strand exchange re-
actions on the two heretofore unreacted single strands.This
latter process must be preceded by a structural rearrange-
ment (isomerization) of the Cre tetramer that positions the
catalytic Tyr residues in the latter pair of subunits to partic-
ipate in the reaction while those in the former pair of sub-
units are similarly removed from the scene of the action.
Note that this mechanism differs from that mediated by
serine recombinases in that the latter cleave all four DNA
strands prior to initiating their exchange and hence do not
have a Holliday junction intermediate (Section 30-6Bd).
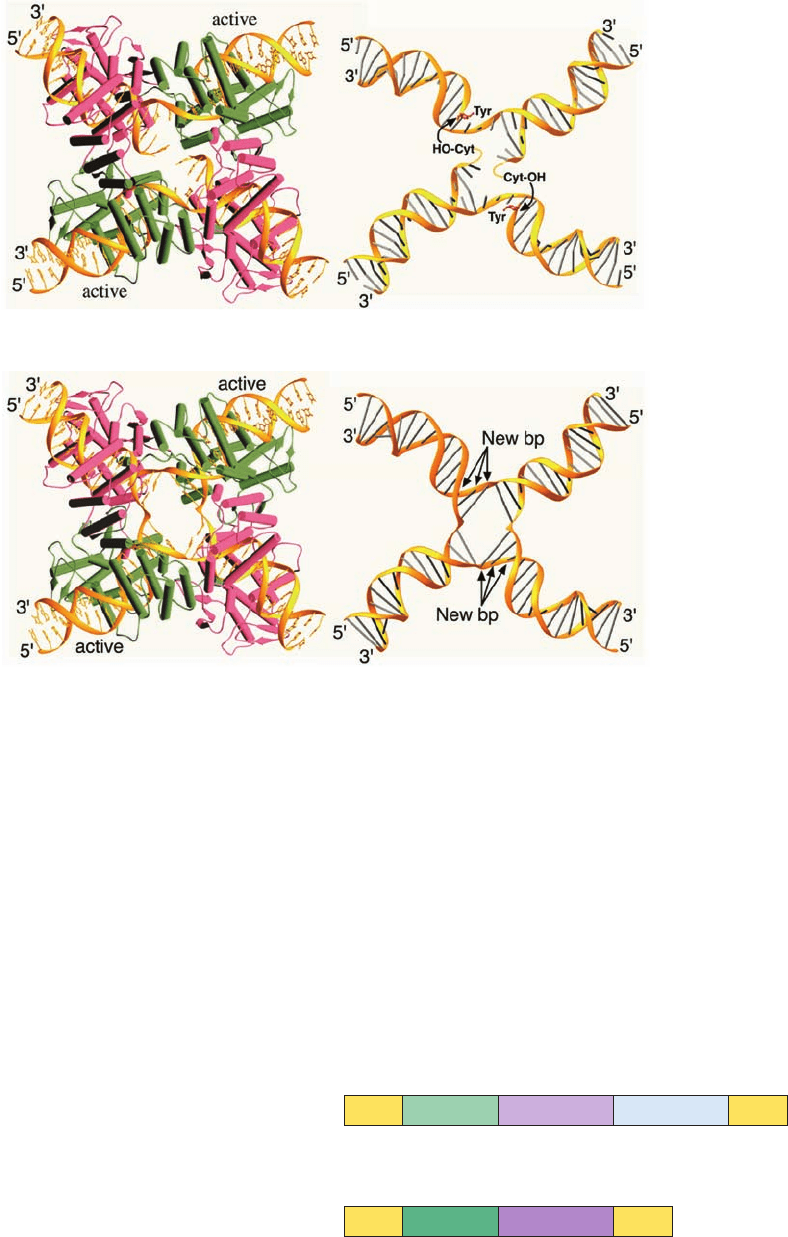
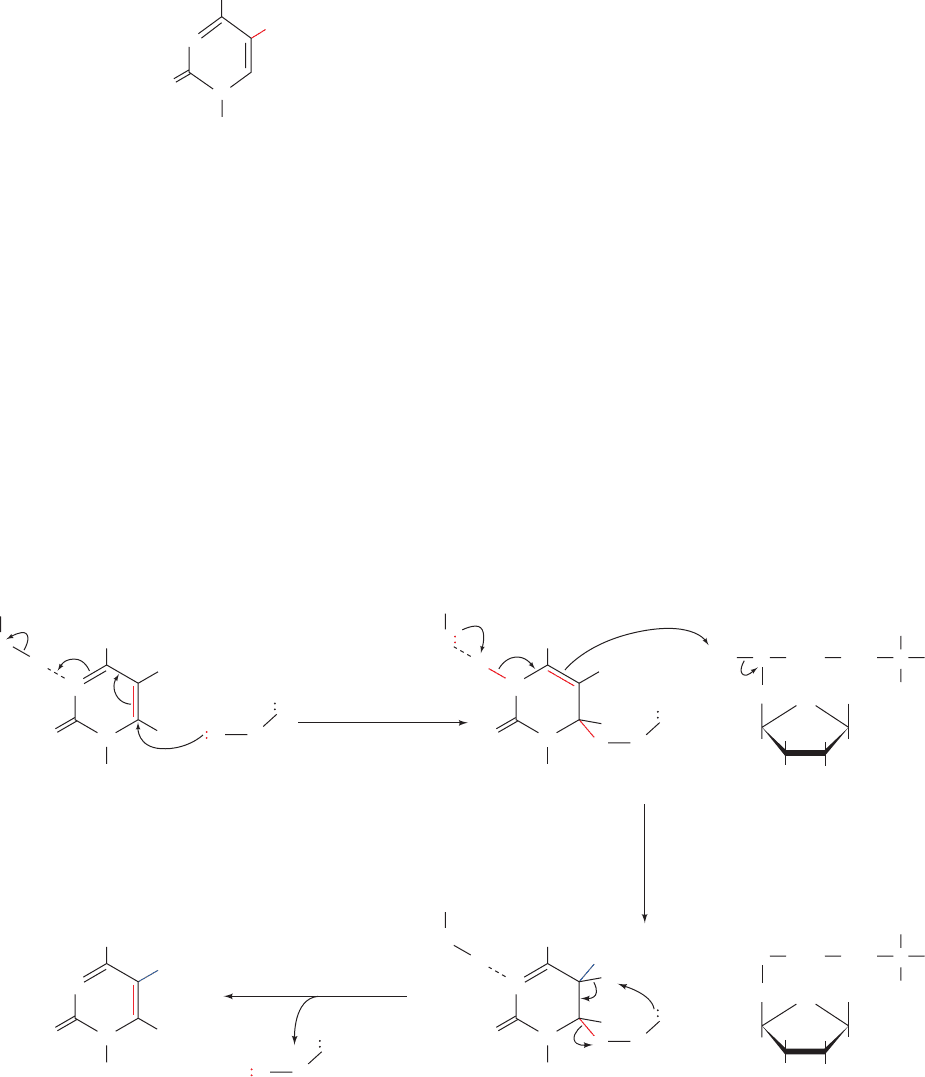
The X-ray structures of Cre tetramers in their complexes
with several loxP model DNAs, determined by Gregory Van
Duyne, have helped elucidate its mechanism.When the DNA
had a single-strand nick past the second nucleotide from the
5¿ end of the crossover region, Cre-catalyzed strand scission
yielded a free nucleotide (a CMP) that diffused away. Since
this nucleotide contained the otherwise reactive 5¿-OH
group, the 3¿-phosphoTyr intermediate was irreversibly
trapped, that is, Cre could not carry out the strand exchange
reaction in Fig. 30-97 (this nicked DNA is a suicide substrate
for Cre; Section 28-3Bc).The X-ray structure of the Cre com-
plex of this nicked DNA confirmed the presence of the 3¿-
phosphoTyr intermediate and indicated, through model
building, that the 5¿-OH group on the missing CMP residue
would be well positioned to nucleophilically attack the 3¿-
phosphoTyr bond on the opposite strand (Fig. 30-98a). Note
that this complex is only 2-fold symmetric although its four
Cre subunits and much of the DNA are related by pseudo-4-
fold symmetry. When the DNA was, instead, an immobile
Holiday junction (Fig. 30-98b),the complex was also pseudo-
4-fold symmetric with the single strands that had crossed
over noticeably kinked at their centers. These structures re-
vealed that the conformational changes necessary to carry
out the strand exchange and isomerization reactions (Fig. 30-
97) required surprisingly small movements on the part of the
Cre subunits and that only the sugar–phosphate backbones
of the strand-exchanged nucleotides needed to move in or-
der to form the Holliday junction.
h. Most Transpositions in Eukaryotes Involve
RNA Intermediates
Transposons similar to those in prokaryotes also occur in
eukaryotes, including yeast, maize, Drosophila, and humans.
In fact, ⬃3% of the human genome consists of DNA-based
transposons although, in most cases, their sequences have
mutated so as to render them inactive, that is, these trans-
posons are evolutionary fossils. However, many eukaryotic
transposons exhibit little similarity to those of prokaryotes.
Rather, their base sequences resemble those of retroviruses
(see below), which suggests that these transposons are de-
generate retroviruses. The transposition of these so-called
retrotransposons occurs via a pathway that resembles the
replication of retroviral DNA (Section 15-4C): (1) their tran-
scription to RNA, (2) the reverse transcriptase–mediated
copying of this RNA to cDNA (Section 30-4C), and (3) the
largely random insertion of this DNA into the host
1244 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-96 The circularization of linear bacteriophage P1
DNA. This occurs through the Cre-mediated site-specific
recombination between its two terminally located loxP sites (red
and green) to yield its lysogenic plasmid.
Figure 30-97 The mechanism of Cre–loxP site-specific
recombination. The dashed lines represent the nonpalindromic
crossover regions of the loxP sites. The green and magenta Cre
subunits are active for cleavage in the top and bottom parts of
the diagram, respectively, with their roles being switched by the
isomerization step. Note that the mechanism does not require
branch migration of the Holliday junction intermediate.
[Courtesy of Gregory Van Duyne, University of Pennsylvania
School of Medicine.]
Bacteriophage P1
linear
Bacteriophage P1
lysogenic plasmid
Cre
+
LoxP LoxP
LoxP LoxP
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1244
organism’s genome as mediated by enzymes known as
integrases (which catalyze reactions similar to and struc-
turally resemble cut-and-paste DNA transposases).
The involvement of RNA in retrotransposon-mediated
transposition was ingeniously shown by Gerald Fink
through his remodeling of Ty1, the most common transpos-
able element in budding yeast (which has ⬃35 copies of this
6.3-kb element comprising ⬃13% of its 1700 kb genome;Ty
stands for Transposon yeast), so that it contained a yeast in-
tron (a sequence that is excised from an RNA transcript
and hence is absent in the mature RNA; Section 5-4Ac) and
was preceded by a galactose-sensitive yeast promoter. The
transposition rate of this remodeled Ty1 element varied
with the galactose concentration in the medium and the
transposed elements all lacked the intron, thereby demon-
strating the participation of an RNA intermediate.
A retroviral genome (Fig. 30-99a) is flanked by direct
long terminal repeats (LTRs) of 250 to 600 bp and typically
contains the genes encoding three polyproteins:gag, which is
Section 30-6. Recombination and Mobile Genetic Elements 1245
(a)
(b)
Figure 30-98 X-ray structures of the Cre homotetramer in its
complexes with model loxP DNAs. (a) Two identical dsDNAs
that were nicked past the second nucleotide from the 5¿ end of
their crossover regions; and (b) an immobile Holliday junction.
The left panels show the Cre–DNA complexes as viewed along
their exact 2-fold and pseudo-4-fold axes, with the active and
inactive subunits green and magenta, respectively (as in Fig. 30-97),
and with the DNA gold. The right panels show only the DNAs in
the X-ray structures as viewed from below the left panels. In the
right panel of Part a, the active site Tyr that is covalently linked
to the 3¿-OH group of the cleaved DNA strand is shown in stick
form (red) and the modeled-in position of the cleaved CMP’s
5¿-OH group is shown positioned to nucleophilically attack the
3¿-phosphoTyr group on the opposite dsDNA (curved arrows).
In the right panel of Part b, the three base pairs that form as a
consequence of strand exchange are indicated. Note that the
vertical strands in the crossovers but not the horizontal strands
are distinctly kinked at their centers. [Courtesy of Gregory Van
Duyne, University of Pennsylvania School of Medicine. PDBids
2CRX, 3CRX, 4CRX, and 5CRX.]
Figure 30-99 Gene sequences of (a) retroviruses and (b) the
Ty1 retrotransposon.
Ty1
LTRLTR TYA TYB
(b)
Retrovirus
LTRLTR gag pol env
(a)
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1245
cleaved to the proteins comprising the viral core (Fig. 15-34);
pol, which is cleaved to the above-mentioned reverse tran-
scriptase and integrase, as well as the protease that catalyzes
these cleavages; and env, which is cleaved to viral outer en-
velope proteins. Ty1 (Fig. 30-99b) is likewise flanked by
LTRs (of 330 bp) but expresses only two polyproteins: TYA
and TYB, the counterparts of gag and pol. Moreover, TYA
and TYB, together with Ty1 RNA, form viruslike particles
in the yeast cytoplasm. However,Ty1 lacks a counterpart of
the retroviral env gene.Hence Ty1 is an “internal virus” that
can only replicate within a genome, albeit at an extremely
low rate compared to that of real retroviral infections.
Copia (Latin for abundance), the most abundant retro-
transposon in the Drosophila genome (which contains
20–60 copies of copia), resembles Ty1.
The LTRs in retroviruses and retrotransposons such as
Ty1 and copia are essential elements for their transcription
and hence for their transposition. Yet, vertebrate genomes
also contain retrotransposons that lack LTRs and hence
cannot be transcribed analogously to retroviruses. A com-
mon family of these nonviral retrotransposons, the 1- to 7-
kb long interspersed nuclear elements (LINEs), each con-
tain two open reading frames: ORF1, which contains
sequences similar to those in gag; and ORF2, which con-
tains sequences similar to those encoding reverse tran-
scriptase. A proposed mechanism for the transposition of
LINEs is diagrammed in Fig. 30-100.
Different types of transposons, DNA-only, retroviral,
and nonviral, predominate in different organisms.Thus bac-
teria, as we have seen, contain nearly exclusively DNA-only
transposons, yeast have mainly retroviral retrotransposons,
Drosophila have all three types, and in humans LINEs pre-
dominate. In fact, the human genome contains an estimated
1.4 million LINEs or LINE fragments that comprise ⬃20%
of the 3.0-billion-bp human genome (genomic organization
is discussed in Section 34-2). The great majority of these
molecular parasites have mutated to the point of inactivity
but a few still appear capable of further transposition. In-
deed, several hereditary diseases are caused by the inser-
tion of a LINE into a gene. Several other types of retro-
transposons also comprise significant fractions of the
human genome as we shall see in Section 34-2.
7 DNA METHYLATION AND
TRINUCLEOTIDE REPEAT EXPANSIONS
The A and C residues of DNA may be methylated, in a
species-specific pattern, to form N
6
-methyladenine (m
6
A),
N
4
-methylcytosine (m
4
C), and 5-methylcytosine (m
5
C)
residues, respectively.
CH
3
N
N
N
H
CH
3
N
N
N
H
CH
3
N
N
NH
2
O
N
N
N
6
-Methyladenine (m
6
A)
residue
5-Methylcytosine (m
5
C)
residue
N
4
-Methylcytosine (m
4
C)
residue
O
1246 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-100 Proposed mechanism for the transposition of
nonviral retrotransposons. (1) The retrotransposon-encoded
reverse transcriptase/endonuclease nicks one strand of the target
DNA and then recruits the RNA transcript of the retrotransposon
to this site. (2) The DNA-primed reverse transcription of the
retrotransposon RNA. (3) The RNA is degraded, and the second
DNA strand is synthesized using the first strand as its template
(normal reverse transcriptase reactions; Section 30-4C), followed
by the insertion of the resulting nonviral retrotransposon into
the target DNA via a poorly understood process.
3'
5'
5'
Target DNA
RNA
Transposed nonviral retrotransposon
3'
3'
3'
3'
5'
5'
5'
5'
3'
3'
3'
3'
5'
3'
5'
5'
3'
1
2
3
Strand cleavage by the
endonuclease activity
and binding of transcribed
nonviral retrotransposon RNA
DNA-primed
reverse transcription
Degradation of RNA,
synthesis of second
DNA strand, and insertion
of the dsDNA into the
target DNA
3'
5'
5'
3'
Reverse transcriptase/endonuclease
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1246
Cytosine residue
H
A
E
H S
–
E
O
NH
2
N
N
DNA
5
6
B
S
–
E
B
S-Adenosylmethionine (SAM)
H
E
H
S
A
–
E
O
HH
NH
2
CH
3
CH
2
CH
2
NH
3
+
S
+
C
H
COO
–
N
N
DNA
B +
O
HH
H
H
A
OH
OH
CH
2
m
5
C residue
H
O
NH
2
CH
3
N
N
DNA
S-Adenosylhomocysteine
H
E
H
S
A
E
O
H
NH
2
CH
3
CH
2
CH
2
NH
3
+
H
+
SC
H
COO
–
N
N
DNA
B
+
+
O
HH
H
H
A
OH
OH
CH
2
These are the only types of modifications to which DNA is
subjected in cellular organisms (although all the C residues
of T-even phage DNAs are converted to 5-hydroxymethyl-
cytosine residues,
which may, in turn, be glycosylated). These methyl groups
project into B-DNA’s major groove, where they can inter-
act with DNA-binding proteins. In most cells, only a few
percent of the susceptible bases are methylated, although
this figure rises to ⬎30% of the C residues in some plants.
Bacterial DNAs are methylated at their own particular
restriction sites, thereby preventing the corresponding re-
striction endonucleases from degrading the DNA (Section
5-5A). These restriction–modification systems, however,
account for only part of the methylation of bacterial
DNAs. In E. coli, most DNA methylation is catalyzed by
the products of the dam and dcm genes. The Dam methyl-
transferase (Dam MTase) methylates the A residue in all
GATC sequences, whereas the Dcm MTase methylates
both C residues in GG at their C5 positions. NoteCC
A
T
5-Hydroxymethylcytosine residue
CH
2
OH
NH
2
O
N
N
that both of these sequences are palindromic. We have
seen that E. coli uses Dam Mtase–mediated methylation to
differentiate parental from newly synthesized DNA in mis-
match repair (Section 30-5C) and in limiting oriC-based
DNA replication initiation to once per cell generation via
sequestration (Section 30-3Cb).
a. The MTase Reaction Occurs via a Covalent
Intermediate in Which the Target Base Is Flipped Out
The Dam and Dcm MTases, as do all known DNA
MTases, use S-adenosylmethionine (SAM) as their methyl
donor. Indeed, all m
5
C-MTases share a set of conserved se-
quence motifs. Daniel Santi has proposed that the catalytic
mechanism of these m
5
C-MTases (Fig. 30-101) is similar to
that of thymidylate synthase (Fig. 28-19) in that both types
of enzymes transfer methyl groups to pyrimidine C5 atoms
via a reaction that is initiated by the nucleophilic attack of
a Cys thiolate group on the pyrimidine’s C6 position. The
pyrimidine’s C5 atom is thereby activated as a resonance-
stabilized carbanion that nucleophilically attacks the
methyl donor’s methyl group (which in thymidylate syn-
thase is donated by N
5
,N
10
-methylene-THF rather than
SAM) to yield a covalent intermediate. This intermediate
subsequently decomposes to products through the enzy-
matic abstraction of the proton substituent to C5 and elim-
ination of the enzyme. The Cys thiolate nucleophile is a
component of a Pro-Cys dipeptide that is invariant in all
known m
5
C-MTases and thymidylate synthases.
Section 30-7. DNA Methylation and Trinucleotide Repeat Expansions 1247
Figure 30-101 The catalytic mechanism of 5-methylcytosine
methyltransferases (m
5
C-MTases). The methyl group is supplied
by SAM, which thereby becomes S-adenosylhomocysteine. In
M.HhaI, the DNA MTase from Haemophilus haemolyticus, the
active site thiolate group,
⫺
S¬E, is on Cys 81, the enzyme
general acid, E¬A, is Glu 119, and the enzyme general base,
E¬B, has not been identified. [After Verdine, G.L., Cell 76, 198
(1994).]
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1247
This mechanism is supported by the observation that
the action of m
5
C-MTases on a 5-fluorocytosine (f
5
C)
residue
irreversibly traps the covalent intermediate (and hence in-
activates the enzyme) because the enzyme cannot abstract
fluorine, the most electronegative element, as an F
⫹
ion (5-
fluorodeoxyuridylate is likewise a suicide substrate for
thymidylate synthase; Section 28-3Bc). Stereochemical
principles dictate that the enzyme’s Cys thiolate group can
nucleophilically attack cytosine’s C5 position only from
above or below the ring. This is possible because, as we
shall see below, the enzyme induces its cytosine target to
flip out of the DNA double helix.
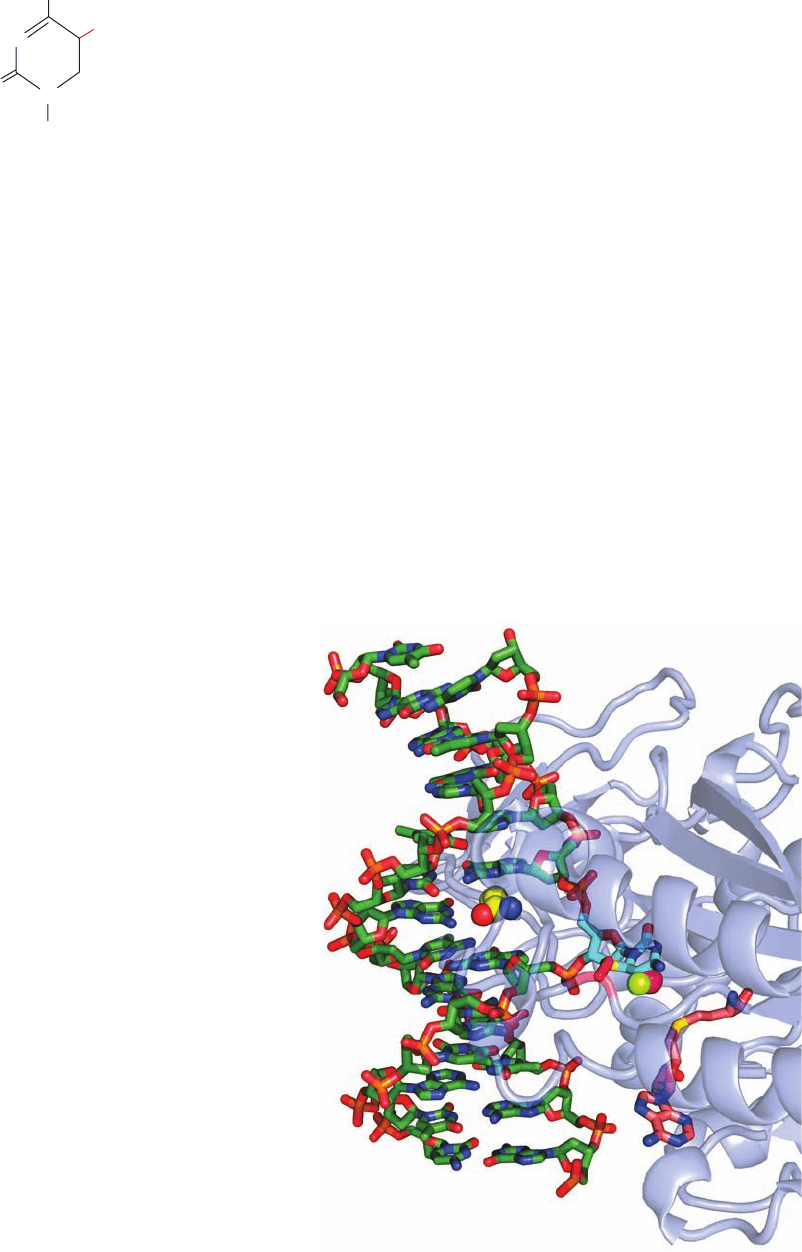
The DNA MTase from Haemophilus haemolyticus
(M.HhaI), a 327-residue monomer, is a component of this
bacterium’s restriction–modification system. M.HhaI
methylates its recognition sequence, 5¿-GCGC-3¿ in double-
stranded DNA, to yield 5¿-G-m
5
C-GC-3¿. Richard Roberts
and Xiaodong Cheng determined the X-ray structure of the
inactivated M.HhaI–DNA complex formed by incubating
the enzyme with the self-complementary sequence
d(TGATAG-f
5
C-GCTATC) (in which the enzyme’s recog-
nition sequence is in bold) in the presence of SAM. The
DNA binds to the enzyme in a large cleft between its two
unequally sized domains (Fig. 30-102). The structure’s most
striking feature is that the f
5
C nucleotide has flipped out of
the minor groove in the otherwise largely undistorted B-
DNA helix and has inserted into the enzyme’s active site.
5-Fluorocytosine (f
5
C) residue
F
NH
2
N
O
N
There, the f
5
C has reacted with SAM so as to yield adeno-
sylhomocysteine (SAM without its methyl group) and the
methylated intermediate covalently linked to Cys 81. The
side chain of Gln 237 fills the cavity in the DNA double he-
lix left by the departure of the f
5
C by hydrogen-bonding to
the opposing G base. Comparison of this structure with that
of M.HhaI in complex only with SAM indicates that on
binding the DNA the protein’s so-called active site loop
(residues 80–99) swings around to contact the DNA, a
movement of up to 25 Å. Nearly all base-specific interac-
tions are made in the major groove by two Gly-rich loops
(residues 233–240 and 250–257), the so-called recognition
loops. The protein also makes extensive sequence-nonspe-
cific contacts with DNA phosphate groups.
Base flipping was first observed in the above X-ray
structure. However, as is now clear from the structures of
other MTases as well as those of variety of DNA repair en-
zymes (e.g., Sections 30-5Aa and 30-5Be), base flipping is a
common mechanism through which enzymes gain access to
the bases in dsDNA on which they perform chemistry.
b. DNA Methylation in Eukaryotes Functions
in Gene Regulation
5-Methylcytosine is the only methylated base in most
eukaryotic DNAs, including those of vertebrates. This
modification occurs largely in the CG dinucleotide of vari-
ous palindromic sequences. CG is present in the vertebrate
genome at only about one-fifth its randomly expected
1248 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-102 X-ray structure of the M.HhaI DNA methyltransferase
in complex with S-adenosylhomocysteine and a dsDNA containing a
methylated 5-fluorocytosine base at the enzyme’s target site. The
protein is represented by a semitransparent ribbon.The DNA is
drawn in stick form colored according to atom type (C green,
N blue, O red, and P orange). Its methylated 5-fluorocytosine
residue (C atoms cyan) has swung out of the DNA helix into the
enzyme’s active site pocket, where its C6 forms a covalent bond with
the S atom of an enzyme Cys residue (C atoms magenta and S yellow).
The methyl group and a fluorine atom at C5 (which prevents the
methylation reaction from going to completion) are represented
by magenta and yellow-green spheres, respectively. The position of
the flipped-out cytosine base in the DNA double helix is occupied
by the side chain of a Gln residue (shown in space-filling form
with C yellow), which hydrogen bonds to the “orphaned” guanine
base. The S-adenosylmethionine, which has given up its methyl group,
is drawn in stick form with its C atoms pink. [Based on an X-ray structure
by Richard Roberts, New England Biolabs, Beverly, Massachusetts, and Xiaodong
Cheng, Cold Spring Harbor Laboratory, New York. PDBid 1MHT.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1248
CG
GC CH
3
CG
GC
replication
GC
CG
CH
3
CH
3
CH
3
CG
GC CH
3
CG
GC
GC
CG
CH
3
CH
3
CH
3
CG
GC CH
3
CG
GC
GC
CG
CH
3
CH
3
CH
3
CG
GC
CG
GC
GC +
+
CGCH
3
CH
3
CG
GC CH
3
CG
GC
GC
CG
CH
3
maintenance
methylation
frequency. The upstream regions of many genes, however,
have normal CG frequencies and are therefore known as
CpG islands.
The degree of eukaryotic DNA methylation and its pat-
tern are conveniently assessed by comparing the Southern
blots (Section 5-5D) of DNA cleaved by the restriction en-
donucleases HpaII (which cleaves CCGG but not C-m
5
C-
GG) and MspI (which cleaves both). Such studies indicate
that eukaryotic DNA methylation varies with the species,
the tissue, and the position along a chromosome.
The m
5
C residues in a given DNA segment can be iden-
tified through bisulfite sequencing, in which the DNA is re-
acted with bisulfite ion which selectively deami-
nates C (but not m
5
C) residues to U, followed by PCR
amplification (Section 5-5F), which copies these U’s to T’s
and the m
5
C’s to C’s. Comparison of the sequences of the
amplified DNA with that of untreated DNA reveals which
C’s in the untreated DNA are methylated.
There is clear evidence that DNA methylation switches
off eukaryotic gene expression, particularly when it occurs
in the promoter regions upstream of a gene’s transcribed
sequences. For example, globin genes are less methylated
in erythroid cells than they are in nonerythroid cells and,
in fact, the specific methylation of the control region in a
recombinant globin gene inhibits its transcription in
transfected cells. In further support of the inhibitory ef-
fect of DNA methylation is the observation that 5-aza-
cytosine (5-azaC),
a base analog that cannot be methylated at its N5 position
and that inhibits DNA MTases, stimulates the synthesis of
several proteins and changes the cellular differentiation
patterns of cultured eukaryotic cells. The observation that
repetitive intragenic parasites such as LINEs are highly
methylated in somatic tissues has led to the hypothesis that
CpG methylation in mammals arose to prevent the spuri-
ous transcriptional initiation of these retrotransposons.
The way in which DNA methylation prevents gene ex-
pression is poorly understood. One possibility is that DNA
methylation can directly block the binding of transcrip-
tional activators to their target sequences. However, in
many cases, DNA methylation is recognized by a family of
proteins that contain a conserved methyl-CpG binding
domain (MBD). Since the methyl groups of m
5
C residues
extend into dsDNA’s major groove, MBDs can bind to
them without perturbing DNA’s double helical structure.
MBD-containing proteins inhibit the transcription of their
bound promoter-methylated genes by recruiting protein
complexes that induce the alteration of the local chromo-
5-Azacytosine
(5-azaC)
O
N N
NH
2
N
(HSO
⫺
3
),
some structure in a way that prevents the transcription of
the associated gene (eukaryotic chromosome structure is
discussed in Section 34-1). Another possibility has been
raised by the observation that the methylation of synthetic
poly(GC) stabilizes its Z-DNA conformation. Quite possi-
bly, the formation of Z-DNA, which has been detected
in vivo (Section 29-1Bb), acts as a conformational switch to
turn off local gene expression.
c. DNA Methylation in Eukaryotes
Is Self-Perpetuating
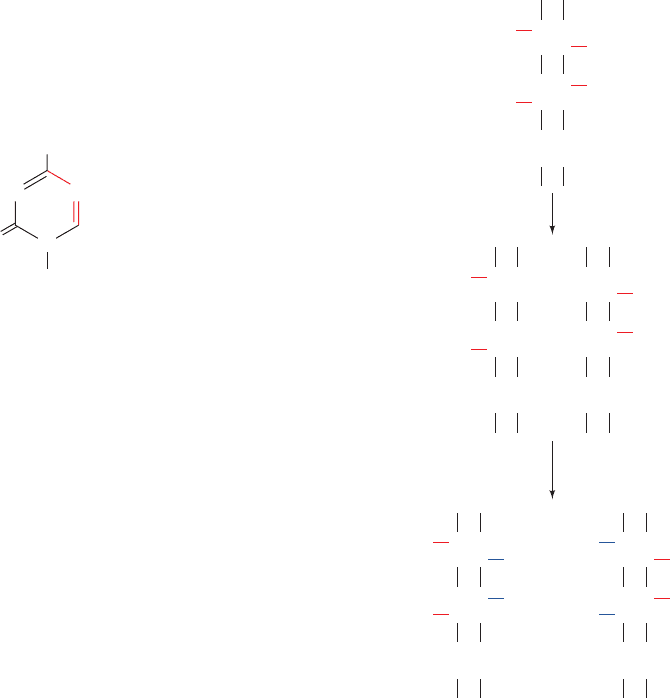
The palindromic nature of DNA methylation sites in eu-
karyotes permits the methylation pattern on a parental
DNA strand to direct the generation of the same pattern in
its daughter strand (Fig. 30-103).This maintenance methyl-
ation would result in the stable “inheritance” of a methyla-
tion pattern in a cell line and hence cause these cells to all
have the same differentiated phenotype. Such changes to
the genome are described as being epigenetic (Greek: epi,
Section 30-7. DNA Methylation and Trinucleotide Repeat Expansions 1249
Figure 30-103 Maintenance methylation. The pattern of
methylation on a parental DNA strand induces the
corresponding methylation pattern in the complementary strand.
In this way, a stable methylation pattern may be maintained in a
cell line.
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1249
upon or beside) because they provide an additional layer
of information that specifies when and where specific por-
tions of the otherwise fixed genome are expressed (an epi-
genetic change that we have already encountered is the
lengthening of telomeres in germ cells; Section 30-4D).
Epigenetic characteristics, as we shall see, are not bound by
the laws of Mendelian inheritance.
There is considerable experimental evidence favoring
the existence of maintenance methylation, including the
observation that artificially methylated viral DNA, on
transfection into eukaryotic cells, maintains its methylation
pattern for at least 30 cell generations. Maintenance
methylation in mammals appears to be mediated mainly by
the protein DNMT1 (for DNA methyltransferase 1), which
has a strong preference for methylating hemimethylated
substrate DNAs. In contrast, prokaryotic DNA MTases
such as M.HhaI do not differentiate between hemimethy-
lated and unmethylated substrate DNAs. The importance
of maintenance methylation is demonstrated by the obser-
vation that mice homozygous for deletion of the DNMT1
gene die early in embryonic development.
The interaction energy provided a single methyl group
in the major groove of DNA seems insufficient to permit
a protein to reliably differentiate between an m
5
C and a
C residue. Nevertheless, the SRA (for SET and RING-as-
sociated) domain of the protein UHRF1 (for ubiquitin-
like, containing PHD and RING finger domains 1) does
so and thereon recruits DNMT1 the site. How does the
SRA domain distinguish a single m
5
C residue from un-
methylated C?
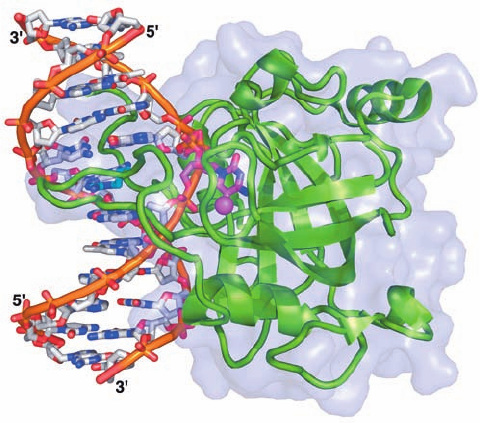
The X-ray structure of the 210-residue SRA domain in
complex with a 12-bp DNA containing a centrally located
hemimethylated CpG was independently determined by
Masahiro Shirakawa and Sirano Dhe-Paganon. It reveals
that the DNA’s m
5
C residue has flipped out from the minor
groove side of the largely straight B-form DNA to tightly
bind deep within in a protein pocket (Fig. 30-104). Two
loops reach around the resulting gap in the DNA helix
from the major and minor grooves to interact with the
other three bases of the hemimethylated CpG site. The
m
5
C base is replaced in the double helix by the side chain
of an Arg residue extending from the minor groove loop,
which hydrogen-bonds to the “orphaned” G residue with-
out greatly disturbing its conformation.
DNMT1 has both a catalytic domain and a UHRF1-
binding domain. The catalytic domain is thought to have
a similar structure and mode of DNA binding as bacter-
ial MTases such as M.HhaI (Fig. 30-102). However, a
model of the DNMT1 catalytic domain–DNA–SRA do-
main complex based on the structures of the
M.HhaI–DNA and SRA–DNA complexes indicates that
it is unlikely that both the m
5
C and C residues of a dou-
ble helical CpG island could be simultaneously flipped
out of the DNA. This suggests that the binding of the
UHRF1-binding domain of DNMT1 to the SRA–DNA
complex causes the m
5
C to flip back into the DNA dou-
ble helix while the C to be methylated flips out to bind to
the catalytic domain of DNMT1.
The pattern of DNA methylation in mammals varies in
early embryological development. DNA methylation lev-
els are high in mature gametes (sperm and ova) but are
nearly eliminated by the time a fertilized ovum has become
a blastocyst (a hollow ball of cells, the stage at which the
embryo implants into the uterine wall; embryonic develop-
ment is discussed in Section 34-4A). After this stage, how-
ever, the embryo’s DNA methylation levels globally rise
until, by the time the embryo has reached the developmen-
tal stage known as a gastrula, its DNA methylation levels
have risen to adult levels, where they remain for the life-
time of the animal. This de novo (new) methylation ap-
pears to be mediated by two DNA MTases distinct from
DNMT1 named DNMT3a and DNMT3b. An important
exception to this remethylation process is that the CpG is-
lands of germline cells (cells that give rise to sperm or ova)
remain unmethylated. This ensures the faithful transmis-
sion of the CpG islands to the succeeding generation in the
face of the strong mutagenic pressure of m
5
C deamination
(which yields T, a mutation that mismatch repair occasion-
ally fails to correct).
The change in DNA methylation levels (epigenetic re-
programming) during embryonic development suggests
that the pattern of genetic expression differs in embryonic
and somatic cells. This explains the observed high failure
1250 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-104 X-ray structure of the SRA domain of mouse
UHRF1 in complex with a hemimethylated 12-bp DNA. The
protein is drawn in ribbon form (green) embedded in its
semitransparent molecular surface. The DNA is shown in stick
form colored according to atom type (DNA C gray except for
the m
5
C residue C which is magenta, N blue, O red, and P orange)
with successive P atoms in the same strand connected by orange
rods.The methyl group of the m
5
C residue is represented by a
magenta sphere. An Arg side chain (C cyan) fills the space in the
DNA double helix vacated by the flipped-out m
5
C. [Based on an
X-ray structure by Masahiro Shirakawa, Kyoto University, Japan.
PDBid 2ZDK.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:13 PM Page 1250
