Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

D. The SOS Response
Agents that damage DNA, such as UV radiation, alkylat-
ing agents, and cross-linking agents, induce a complex sys-
tem of cellular changes in E. coli known as the SOS re-
sponse. E. coli so treated cease dividing and increase their
capacity to repair damaged DNA.
a. LexA Protein Represses the SOS Response
Clues as to the nature of the SOS response were provided
by the observations that E. coli with mutant recA or lexA
genes have their SOS response permanently switched on.
RecA, a 353-residue protein that coats DNA as a multimeric
helical filament, plays a central role, as we shall see, in homol-
ogous recombination (Section 30-6Ab). When E. coli are ex-
posed to agents that damage DNA or inhibit DNA replica-
tion,their RecA specifically mediates the proteolytic cleavage
of LexA (202 residues) between its Asp 84 and Gly 85. RecA
is activated to do so on binding to ssDNA (it was initially as-
sumed that RecA catalyzes the proteolysis of LexA but sub-
sequent experiments by John Little indicate that activated
RecA stimulates LexA to cleave itself).Further investigations
indicated that LexA functions as a repressor of 43 genes that
participate in DNA repair and the control of cell division, in-
cluding recA, lexA, uvrA, and uvrB. DNA sequence analyses
of the LexA-repressible genes revealed that they are all pre-
ceded by a homologous 20-nt sequence, the so-called SOS
box, that has the palindromic symmetry characteristic of op-
erators (control sites to which repressors bind so as to inter-
fere with transcriptional initiation by RNA polymerase; Sec-
tion 5-4A). Indeed, LexA has been shown to specifically bind
the SOS boxes of recA and lexA.
The preceding observations suggest a model for the reg-
ulation of the SOS response (Fig. 30-62). During normal
Section 30-5. Repair of DNA 1221
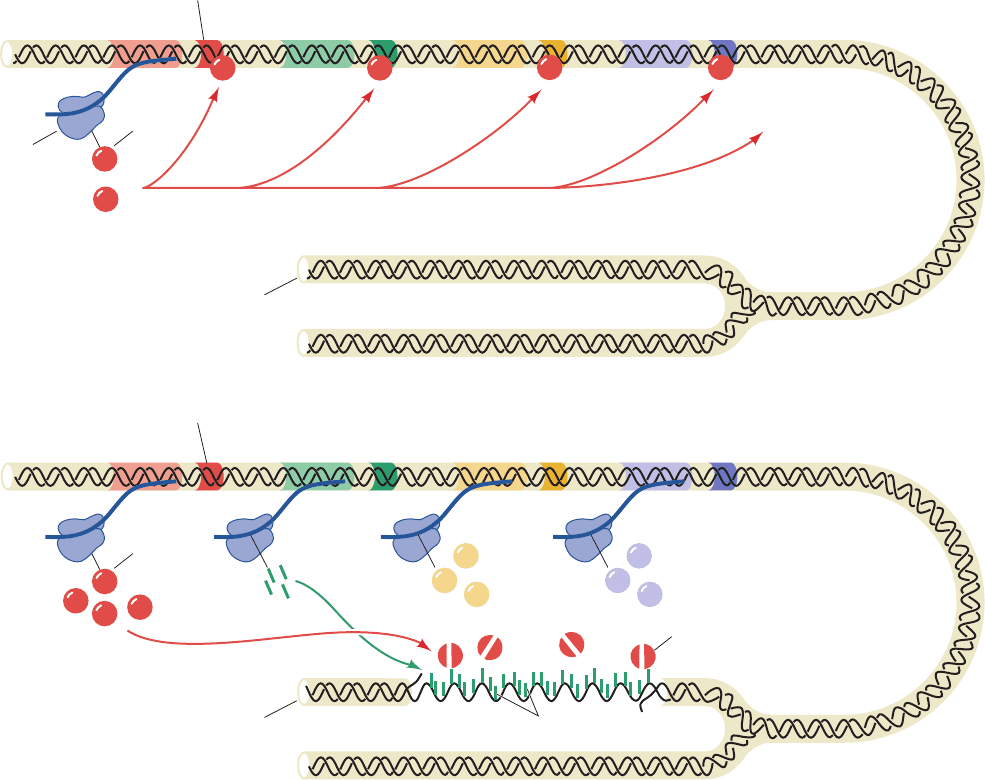
Figure 30-62 Regulation of the SOS response in E. coli. In a
cell with undamaged DNA (above), LexA largely represses the
synthesis of LexA, RecA, UvrA, UvrB, and other proteins
involved in the SOS response. When there has been extensive
Induced state
Damaged DNA
Operator
mRNA
lexA recA uvrA
UvrA
RecA
Activated RecA
Cleaved LexA
UvrB
uvrB
LexA
Operator
To other genes
controlled by LexA
LexA binds to operators,
repressing the expression
of genes involved in the
SOS response
mRNA
ribosome
Uninduced state
Undamaged DNA
lexA recA uvrA uvrB
LexA
DNA damage (below), RecA is activated by binding to the
resulting single-stranded DNA to stimulate LexA self-cleavage.
The consequent synthesis of the SOS proteins results in the
repair of the DNA damage.
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1221
growth, LexA largely represses the expression of the SOS
genes, including the lexA gene, by binding to their SOS
boxes so as to inhibit RNA polymerase from initiating the
transcription of these genes. When DNA damage has been
sufficient to produce postreplication gaps, however, this ss-
DNA binds to RecA so as to stimulate LexA cleavage.The
LexA-repressible genes are consequently released from re-
pression and direct the synthesis of SOS proteins including
that of LexA (although this repressor continues to be
cleaved through the influence of RecA). When the DNA
lesions have been eliminated, RecA ceases stimulating
LexA’s autoproteolysis. The newly synthesized LexA can
then function as a repressor, which permits the cell to re-
turn to normality.
b. SOS Repair Is Error Prone
The E. coli Pol III holoenzyme is unable to replicate
through a variety of lesions such as AP sites and thymine
dimers. On encountering such lesions, the replisome stalls
and disassembles by releasing its Pol III cores, a process
that is called replication fork “collapse.” Cells have two
general modes for restoring collapsed replication forks,
recombination repair and SOS repair. Recombination repair
circumvents the damaged template by using a homologous
chromosome as its template DNA in a process known as
homologous recombination, which also functions to gener-
ate genetic diversity. Hence we shall postpone our discussion
of recombination repair until after our consideration of
homologous recombination in Section 30-6A. In the follow-
ing paragraphs we discuss SOS repair.
In SOS repair, the Pol III core lost from the collapsed
replication fork is replaced by one of two so-called bypass
DNA polymerases, whose synthesis is induced by the SOS
response: DNA polymerase IV (Pol IV, the 336-residue
product of the dinB gene) or DNA polymerase V [Pol V;
the heterotrimeric product of the umuD and umuC genes,
UmuDⴕ
2
C (umu for UV mutagenesis), where UmuD¿ is
produced by the RecA-assisted self-cleavage of the 139-
residue UmuD to remove its N-terminal 24 residues, and
UmuC consists of 422 residues]. Both of these enzymes are
Y-family DNA polymerases, all of whose members lack
3¿S5¿ proofreading exonuclease activity and replicate un-
damaged DNA with poor fidelity and low processivity and
hence are also known as error-prone DNA polymerases.
Translesion synthesis (TLS) by Pol V, which was charac-
terized in large part by O’Donnell and Myron Goodman,
requires the simultaneous presence of the 
2
sliding clamp,
the ␥ complex (clamp loader), and SSB, together with a
RecA filament in complex with the ssDNA arising from
the action of helicase on the dsDNA ahead of the stalled
replication fork.This so-called Pol V mutasome tends to in-
corporate G about half as often as A opposite thymine
dimers and AP sites, with pyrimidines being installed infre-
quently. This process is, of course, highly mutagenic. But
even in replicating undamaged DNA, Pol V is at least 1000-
fold more error prone than is Pol I or Pol III holoenzyme.
However, after synthesizing ⬃7 nt, the Pol V mutasome is
replaced by Pol III holoenzyme, which commences normal
DNA replication after the now bypassed lesion. Pol II, a
TLS participant that accurately replicates DNA, is also in-
duced by the SOS response but it is synthesized well before
Pol V appears (see below). The role of Pol II appears to be
the mediation of error-free TLS, and only if this process
fails is it replaced by Pol V to carry out error-prone TLS.
There are numerous types of DNA lesions besides AP
sites and thymine dimers that interfere with normal DNA
replication. Depending on the type of lesion, Pol IV, which
is also error prone, may instead be recruited to carry out
TLS. With many lesions,TLS may skip over the altered nu-
cleotide, resulting in deletion of one or two bases in the
daughter strand opposite the lesion (yielding a frameshift
mutation, so called because it would change a structural
gene’s reading frame from that point onward; Section 5-
4Bd). Moreover, Pol IV is prone to generating frameshift
mutations even when replicating undamaged DNA.
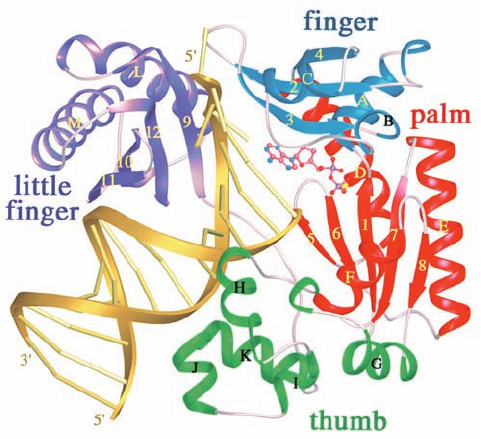
The Y-family DNA polymerase Dpo4 from the archae-
bacterium Sulfolobus solfataricus P2, a homolog of E. coli
Pol IV and Pol V, misincorporates ⬃1 base per 500 repli-
cated nucleotides. The X-ray structure of a complex of
Dpo4 with a primer–template DNA that had been incu-
bated with ddATP (which is complementary to the tem-
plate base), determined by Wei Yang, reveals the struc-
tural basis for this low fidelity (Fig. 30-63).The 352-residue
protein contains the fingers, palm, and thumb domains
1222 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-63 X-ray structure of the bypass DNA polymerase
Dpo4 from Sulfolobus solfataricus P2 in complex with a
primer–template DNA and ddADP. The protein is drawn in
ribbon form with its fingers, palm, thumb, and little finger
domains blue, red, green, and purple, respectively. The DNA is
gold with its backbones drawn as ribbons and its bases
represented by rods.The ddADP, which is base-paired to a
template T in the enzyme’s active site, is shown in ball-and-stick
form colored according to atom type (C pink, N blue, O red, and
P magenta). [Courtesy of Wei Yang, NIH, Bethesda, Maryland.
PDBid 1JX4.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1222
common to all known DNA polymerases (although their
orders differ in the sequences of the different families of
DNA polymerases) and, in addition, has a C-terminal do-
main unique to Y-family DNA polymerases that has been
dubbed the “little finger” domain. The enzyme, as ex-
pected, has incorporated a ddA residue at the 3¿ end of the
primer and, in addition, binds a ddADP in base-paired
complex to the new template T. The little finger domain
binds in the major groove of the DNA. However, the fin-
gers and thumb domains are small and stubby compared
to those of replicative DNA polymerases such as Klentaq1
(Fig. 30-9) and pol ␦ (Fig. 30-41), and the residues that con-
tact the base pair in the active site are all Gly and Ala
rather than the Phe, Tyr, and Arg that mainly do so in the
replicative DNA polymerases. Moreover, the bound DNA
is entirely in the B form rather than in the A form at the
active site as occurs in many replicative DNA poly-
merases. Since the minor groove is more accessible in A-
DNA than in B-DNA (Section 29-1B), this suggests that
error-prone DNA polymerases have relatively little facil-
ity to monitor the base-pairing fidelity of the incoming nu-
cleotide. This accounts for the ability of error-prone DNA
polymerases to accommodate distorted template DNA as
well as non-Watson–Crick base pairs at their active sites.
SOS repair is an error-prone and hence mutagenic
process. It is therefore a process of last resort that is only
initiated ⬃50 min after SOS induction if the DNA has not
already been repaired by other means. Yet, DNA damage
that normally activates the SOS response is nonmutagenic
in the recA
⫺
E. coli that survive.This is, as we saw, because
bypass DNA polymerases will replicate over a DNA lesion
even when there is no information as to which bases were
originally present. Indeed, most mutations in E. coli arise
from the actions of the SOS repair system, which is there-
fore a testimonial to the proposition that survival with a
chance of loss of function (and the possible gain of new
ones) is advantageous, in the Darwinian sense, over death,
although only a small fraction of cells actually survive this
process. It has therefore been suggested that, under condi-
tions of environmental stress, the SOS system functions to
increase the rate of mutation so as to increase the rate at
which the E. coli adapt to the new conditions. Finally, it
should be noted that the eukaryotic pols , , and , all Y-
family members, and pol , an X-family member, are impli-
cated in TLS and that pol , the product of the XPV gene,
is defective in the XPV form of xeroderma pigmentosum
(Section 30-5Bb).
E. Double-Strand Break Repair
Double-strand breaks (DSBs) in DNA are produced when
a replication fork encounters a nick and by the reactive
oxygen species (ROS) by-products of oxidative metabo-
lism and ionizing radiation (which also produces ROS). In
fact, around 5 to 10% of dividing cells in culture exhibit at
least one chromosome break at any given time. Moreover,
DSBs are normal intermediates in certain specialized cel-
lular processes such as recombination during meiosis (Sec-
tion 1-4Ab) and V(D)J recombination in lymphoid cells,
which helps generate the vast diversity of antigen-binding
sites in antibodies and T-cell receptors (Section 35-2C).
Unrepaired or misrepaired DSBs can be lethal to cells or
cause chromosomal aberrations that may lead to cancer.
Hence, the efficient repair of DSBs is essential for cell via-
bility and genomic integrity.
Cells have two general modes to repair DSBs: recombi-
nation repair, which only occurs during the late S and G
2
phases of the cell cycle (when sister chromatids are present
to serve as templates), and nonhomologous end-joining
(NHEJ), which functions throughout the cell cycle. Here
we discuss NHEJ, a process which, as its name implies, di-
rectly rejoins DSBs. The recombination repair of DSBs is
discussed in Section 30-6Ag.
In NHEJ, the broken ends of the DSB must be aligned,
its frayed ends trimmed and/or filled in, and their strands
ligated.The core NHEJ machinery in eukaryotes includes
the DNA end-binding protein Ku (a heterodimer of ho-
mologous 70- and 83-kD subunits, Ku70 and Ku80), DNA
ligase IV, and the accessory protein Xrcc4. Ku, an abun-
dant nuclear protein, binds to a DSB, whether blunt or
with an overhang, and hence appears to be the cell’s pri-
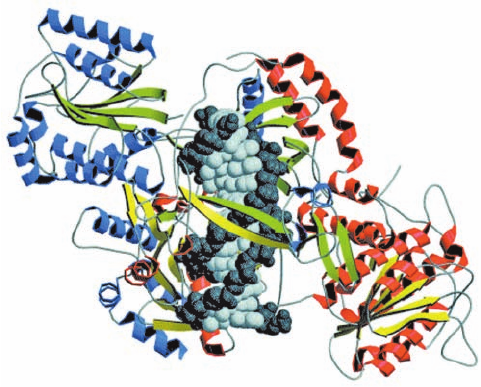
mary DSB sensor. The X-ray structure of Ku in complex
with a 14-bp DNA, determined by Jonathan Goldberg, re-
veals that the protein cradles the dsDNA segment along
its entire length and encircles its central ⬃3 bp segment
(Fig. 30-64). The protein ring is also present in the closely
Section 30-5. Repair of DNA 1223
Figure 30-64 X-ray structure of human Ku protein in complex
with DNA containing 14 bp. The subunits of Ku70 (red helices
and yellow strands) and Ku80 (blue helices and green strands) are
viewed along the pseudo-2-fold axis relating them.The DNA,
viewed with its DSB pointing upward, is drawn in space-filling
form with its sugar–phosphate backbone dark gray and its base
pairs light gray. Note that the DNA is surrounded by a ring of
protein. [Courtesy of John Tainer,The Scripps Research Institute,
La Jolla, California. Based on an X-ray structure by Jonathan
Goldberg, Memorial Sloan-Kettering Cancer Center, New York,
New York. PDBid 1JEY.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1223
similar X-ray structure of Ku alone, thereby explaining
why Ku that is bound to a dsDNA, which is then circular-
ized, becomes permanently associated with it. Ku makes
no specific contacts with the DNA’s bases and few with its
sugar–phosphate backbone, but instead fits snugly into
the DNA’s major and minor grooves so as to precisely ori-
ent it.
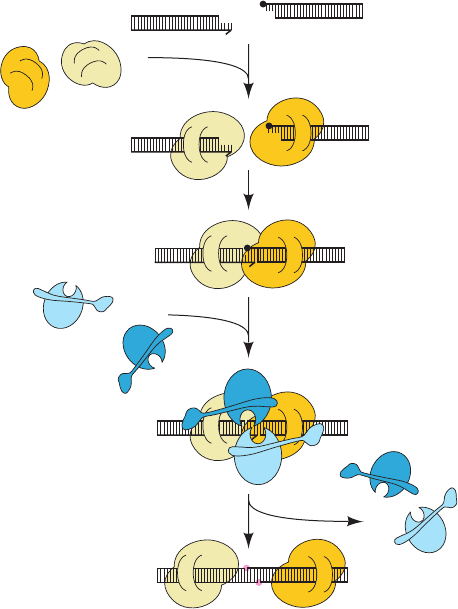
Ku–DNA complexes have been shown to dimerize so
as to align the members of a DSB, both blunt ended and
with short (1–4 bp) complementary single strands, for lig-
ation as is diagrammed in Fig. 30-65. The DNA ends are
exposed along one face of each Ku–DNA complex, pre-
sumably making them accessible to polymerases that fill
in gaps and to nucleases that trim excess and inappropri-
ate ends preparatory for ligation by DNA ligase IV in
complex with Xrcc4. Nucleotide trimming, which of
course generates mutations, appears to be carried out in
an ATP-dependent manner by the evolutionarily con-
served Mre11 complex, which consists of two Mre11 nu-
clease subunits and two Rad50 ATPase subunits. Ku is
eventually released from the rejoined DNA, perhaps by
proteolytic cleavage.
The reason that the mutations generated by NHEJ are
usually not unacceptably deleterious is that only a small
fraction of the mammalian genome is expressed (Section
34-2A). In fact, the genome in a somatic cell of a 70-year-
old human typically contains ⬃2000 “scars” caused by
NHEJ.
F. Identification of Carcinogens
Many forms of cancer are known to be caused by exposure
to certain chemical agents that are therefore known as car-
cinogens. It has been estimated that as much as 80% of hu-
man cancer arises in this fashion. There is considerable ev-
idence that the primary event in carcinogenesis is often
damage to DNA (carcinogenesis is discussed in Section 34-
4C). Carcinogens are consequently also likely to induce the
SOS response in bacteria and thus act as indirect muta-
genic agents. In fact, there is a high correlation between
carcinogenesis and mutagenesis (recall, e.g., the progress of
xeroderma pigmentosum; Section 30-5Bb).
There are presently over 80,000 man-made chemicals of
commercial importance and ⬃1000 new ones are intro-
duced each year.The standard animal tests for carcinogen-
esis, exposing rats or mice to high levels of the suspected
carcinogen and checking for cancer, are expensive and re-
quire ⬃3 years to complete.Thus, relatively few substances
have been tested in this manner.
a. The Ames Test Assays for Probable
Carcinogenicity
Bruce Ames devised a rapid and effective bacterial as-
say for carcinogenicity that is based on the high correla-
tion between carcinogenesis and mutagenesis. He con-
structed special tester strains of Salmonella typhimurium
that are his
⫺
(cannot synthesize histidine so that they are
unable to grow in its absence), have cell envelopes that
lack the lipopolysaccharide coating that renders normal
Salmonella impermeable to many substances (Section 11-
3Bc), and have inactivated excision repair systems. Muta-
genesis in these tester strains is indicated by their rever-
sion to the his
⫹
phenotype.
In the Ames test, ⬃10
9
tester strain bacteria are spread
on a culture plate that contains only a small amount of his-
tidine to permit the bacteria to initially grow and mutate.
Usually a mixture of several his
⫺
strains is used so that mu-
tations due to both base changes and nucleotide insertions
or deletions can be detected. A mutagen placed in the cul-
ture medium causes some of these his
⫺
bacteria to revert to
the his
⫹
phenotype, which is detected by their growth into
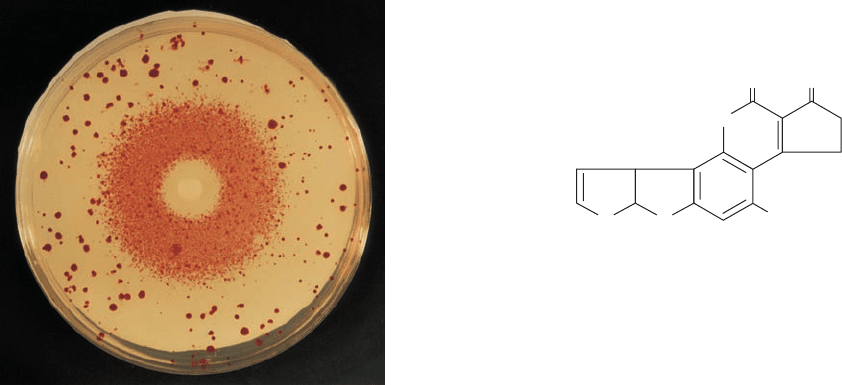
visible colonies after 2 days at 37°C (Fig. 30-66). The muta-
genicity of a substance is scored as the number of such
colonies less the few spontaneously revertant colonies that
occur in the absence of the mutagen.
1224 Chapter 30. DNA Replication, Repair, and Recombination
Processing enzymes
fill gap (not shown)
Xrcc4 – LigIV are recruited
Ku bridges ends
Xrcc4 – LigIV
DNA strands are repaired
Ku binds to ends
DNA with double-strand break
Ku
heterodimers
Figure 30-65 Schematic diagram of nonhomologous end-
joining (NHEJ). The left dsDNA fragment is missing a base and
the right fragment is blocked by a nonligatable group (filled
black circle).The two Ku heterodimers are drawn in two shades
of yellow and the Xrcc4–DNA ligase IV complexes are drawn in
two shades of blue. The newly repaired links in the DNA are
represented by pink circles. [After Jones, J.M., Gellert, M., and
Yang, W., Structure 9, 881 (2001).]
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1224
Many noncarcinogens are converted to carcinogens in
the liver or in other tissues via a variety of detoxification
reactions (e.g., those catalyzed by the cytochromes P450;
Section 15-4Bc).A small amount of rat liver homogenate is
therefore included in the Ames test medium in an effort to
approximate the effects of mammalian metabolism.
b. Both Man-Made and Naturally Occurring
Substances Can Be Carcinogenic
There is an ⬃80% correspondence between the com-
pounds determined to be carcinogenic by animal tests
and those found to be mutagenic by the Ames test. Dose–
response curves, which are generated by testing a given
compound at a number of concentrations, are almost al-
ways linear and extrapolate back to zero, indicating that
there is no threshold concentration for mutagenesis. Sev-
eral compounds to which humans have been extensively
exposed that were found to be mutagenic by the Ames
test were later found to be carcinogenic in animal tests.
These include tris(2,3-dibromopropyl)phosphate, which
was used as a flame retardant on children’s sleepwear in
the mid-1970s and can be absorbed through the skin; and
furylfuramide, which was used in Japan in the 1960s and
1970s as an antibacterial additive in many prepared
foods (and had passed two animal tests before it was
found to be mutagenic). Carcinogens are not confined to
man-made compounds but also occur in nature. For ex-
ample, carcinogens are contained in many plants that are
common in the human diet, including alfalfa sprouts.
Aflatoxin B
1
,
one of the most potent carcinogens known, is produced
by fungi that grow on peanuts and corn. Charred or
browned food, such as occurs on broiled meats and
toasted bread, contains a variety of DNA-damaging
agents. Thus, with respect to carcinogenesis, as Ames has
written, “Nature is not benign.”
6 RECOMBINATION AND MOBILE
GENETIC ELEMENTS
The chromosome is not just a simple repository of genetic
information. If this were so, the unit of mutation would
have to be an entire chromosome rather than a gene be-
cause there would be no means of separating a mutated
gene from the other genes of the same chromosome. Chro-
mosomes would therefore accumulate deleterious muta-
tions until they became nonviable.
It has been known from some of the earliest genetic
studies that pairs of allelic genes may exchange chromoso-
mal locations by a process known as genetic recombination
(Section 1-4Cb). Mutated genes can thereby be individu-
ally tested, since their propagation is then not absolutely
dependent on the propagation of the genes with which they
had been previously associated. In this section, we consider
the mechanisms by which genetic elements can move, both
between chromosomes and within them.
A. Homologous Recombination
Homologous recombination (also called general recombi-
nation) is defined as the exchange of homologous segments
between two DNA molecules. Both genetic and cytological
studies have long indicated that such a crossing-over
process occurs in higher organisms during meiosis (Fig.1-27).
Bacteria, which are normally haploid, likewise have elabo-
rate mechanisms for the interchange of genetic informa-
tion. They can acquire foreign DNA through transforma-
tion (Section 5-2A), through a process called conjugation
(mating) in which DNA is directly transferred from one
cell to another via a cytoplasmic bridge (Section 31-1Ac),
and via transduction in which a defective bacteriophage
that has erroneously acquired a segment of bacterial DNA
rather than the viral chromosome transfers this DNA to
another bacterial cell. In all of these processes, the foreign
DNA is installed in the recipient’s chromosome or plasmid
O
OCH
3
Aflatoxin B
1
O
O
O
O
Section 30-6. Recombination and Mobile Genetic Elements 1225
Figure 30-66 The Ames test for mutagenesis. A filter paper
disk containing a mutagen, in this case the alkylating agent ethyl
methanesulfonate, is centered on a culture plate containing his
⫺
tester strains of Salmonella typhimurium in a medium that
initially contained only a small amount histidine. A dense halo of
revertant bacterial colonies appears around the disk from which
the mutagen diffused.The larger colonies distributed about the
culture plate are spontaneous revertants.The bacteria near the
disk have been killed by the toxic mutagen’s high concentration.
[Courtesy of Raymond Devoret, Institut Curie, Orsay, France.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1225
A B
AB
AB
AB
AB
AB
ab
ab
ab
ab
ab
ab
A
B
b
a
a
b
B
A
A
B
b
a
A
B
b
a
A
B
b
a
i
(l)
AB
ab
A
B
a
b
AB
ab
A
Ba
b
+
–
+
–
(a)
(
b)
(
c)
(
d)
(
e)
(
f)
(
h)
( )
(
j)
(
k)
(g)
through homologous recombination (to be propagated, a
DNA segment must be part of a replicon; that is, be associ-
ated with a replication origin such as occurs in a chromo-
some, a plasmid, or a virus).
a. Recombination Occurs via a Crossed-Over
Intermediate
The prototypical model for homologous recombination
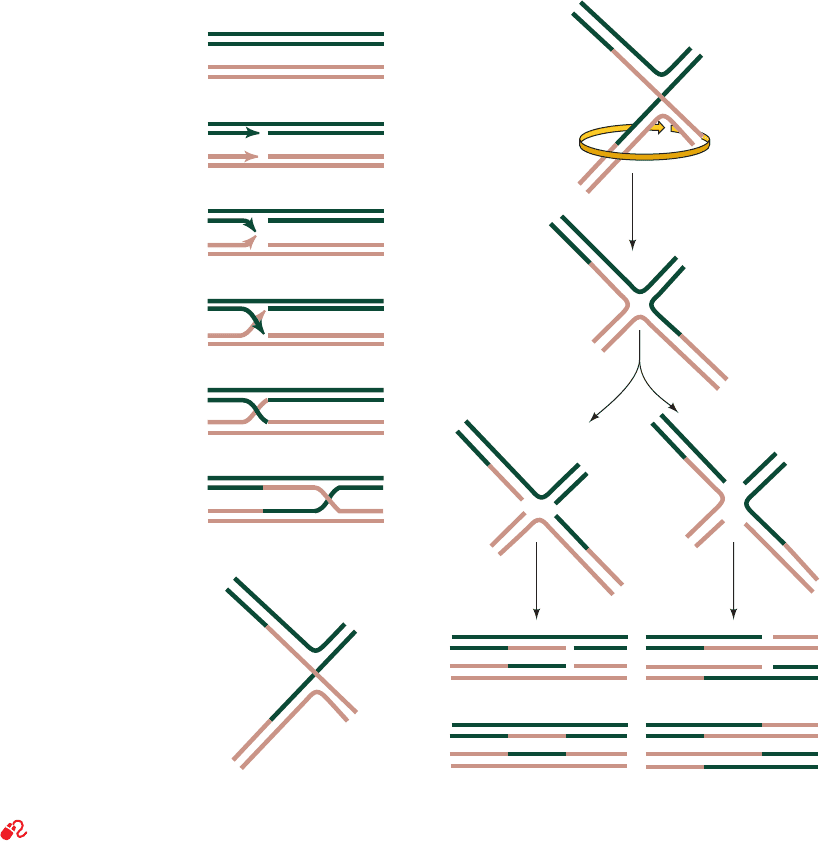
(Fig. 30-67) was proposed by Robin Holliday in 1964 on the
basis of genetic studies on fungi.The corresponding strands
of two aligned homologous DNA duplexes are nicked, and
the nicked strands cross over to pair with the nearly com-
plementary strands of the homologous duplex after which
the nicks are sealed (Fig. 30-67a–e), thereby yielding a four-
way junction known as a Holliday junction (Fig. 30-67e). A
Holliday junction has, in fact, been observed in the X-ray
structure of d(CCGGTACCGG), determined Shing Ho
(Fig. 30-68), in which, perhaps unexpectedly, all the bases
form normal Watson–Crick base pairs without any appar-
ent strain.The crossover point can move in either direction,
often thousands of nucleotides, in a process known as
branch migration (Fig. 30-67e, f ) in which the four strands
exchange base-pairing partners.
A Holliday junction can be resolved into two duplex
DNAs in two equally probable ways (Fig. 30-67g–l ):
1. The cleavage of the strands that did not cross over
(right branch of Fig. 30-67j–l) exchanges the ends of the
original duplexes to form, after nick sealing, the traditional
recombinant DNA (Fig. 1-27b).
2. The cleavage of the strands that crossed over (left
branch of Fig. 30-67j–l) exchanges a pair of homologous
single-stranded segments.
The recombination of circular duplex DNAs results in
the types of structures diagrammed in Fig. 30-69. Electron
microscopic evidence for the existence of the postulated
1226 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-67 The Holliday model of homologous recombination between homologous DNA duplexes.
See the Animated Figures
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1226
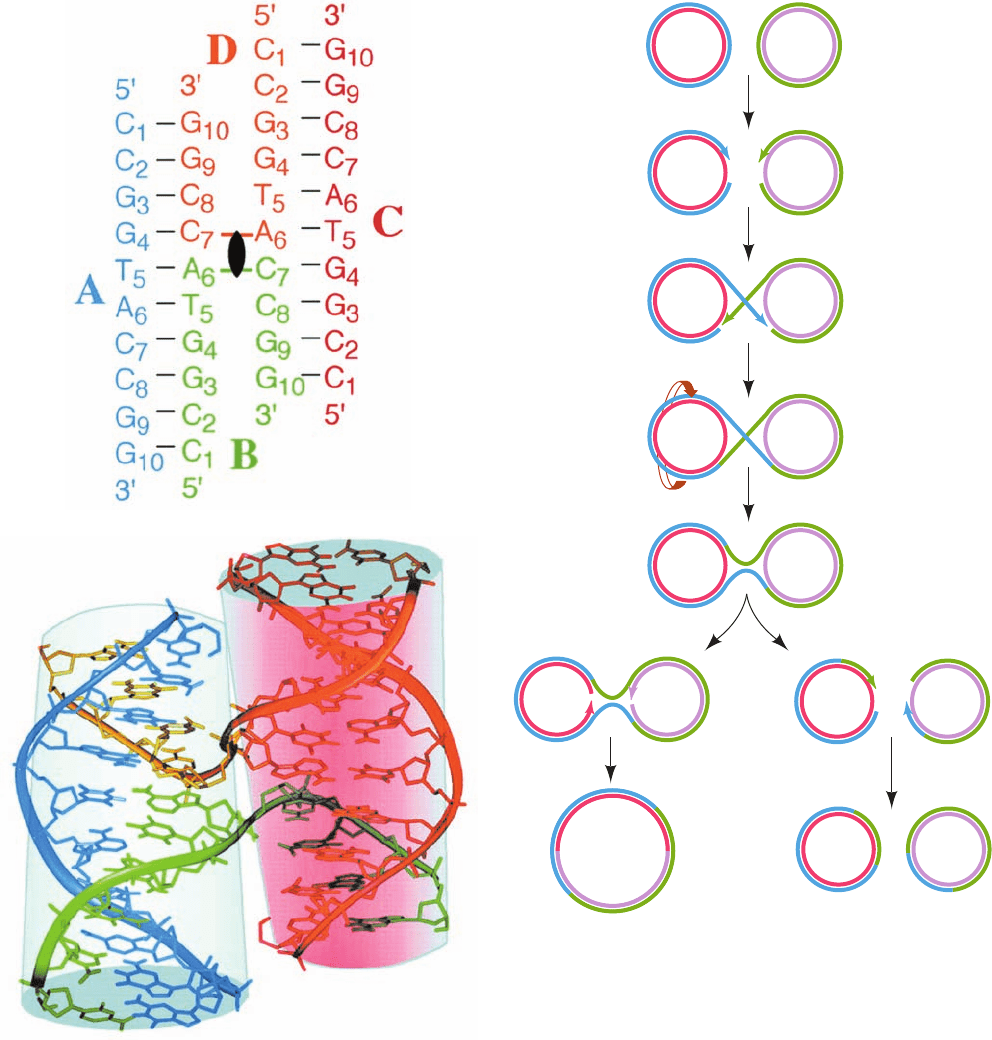
Section 30-6. Recombination and Mobile Genetic Elements 1227
Figure 30-68 X-ray structure of the self-complementary decameric DNA d(CCGGTACCGG).
(a) The secondary structure of the four-stranded Holliday junction formed by this sequence in which
the four strands, A, B, C, and D, are differently colored, their nucleotides are numbered 1 to 10 from
their 5¿ to 3¿ termini, and Watson–Crick base-pairing interactions are represented by black dashes.
The 2-fold axis relating the two helices of this so-called stacked-X conformation is represented by the
black lenticular symbol. (b) The observed three-dimensional structure of the Holliday junction, as
viewed along its 2-fold axis, in which the oligonucleotides are represented in stick form with their
backbones traced by ribbons, all colored as in Part a. With the exception of the backbones of strands
B and D at the crossovers, the two arms of this structure each form an undistorted B-DNA helix,
including the stacking of the base pairs flanking the crossovers. The two helices are inclined to each
other by 41°. Note that Fig. 30-67g is a schematic representation of the stacked-X conformation as
viewed perpendicular to both helices (from the side in this drawing and hence having the projected
appearance of the letter X).A Holliday junction can also assume a so-called open-X conformation,
which is represented by Fig. 30-67i. [Courtesy of Shing Ho, Oregon State University. PDBid 1DCW.]
Figure 30-69 Homologous recombination between two
circular DNA duplexes. This process can result either in two
circles of the original sizes or in a single composite circle.
or
(a)
(b)
(c)
(d)
(e)
(f)
(g)
(b)
(a)
JWCL281_c30_1173-1259.qxd 9/2/10 9:06 AM Page 1227
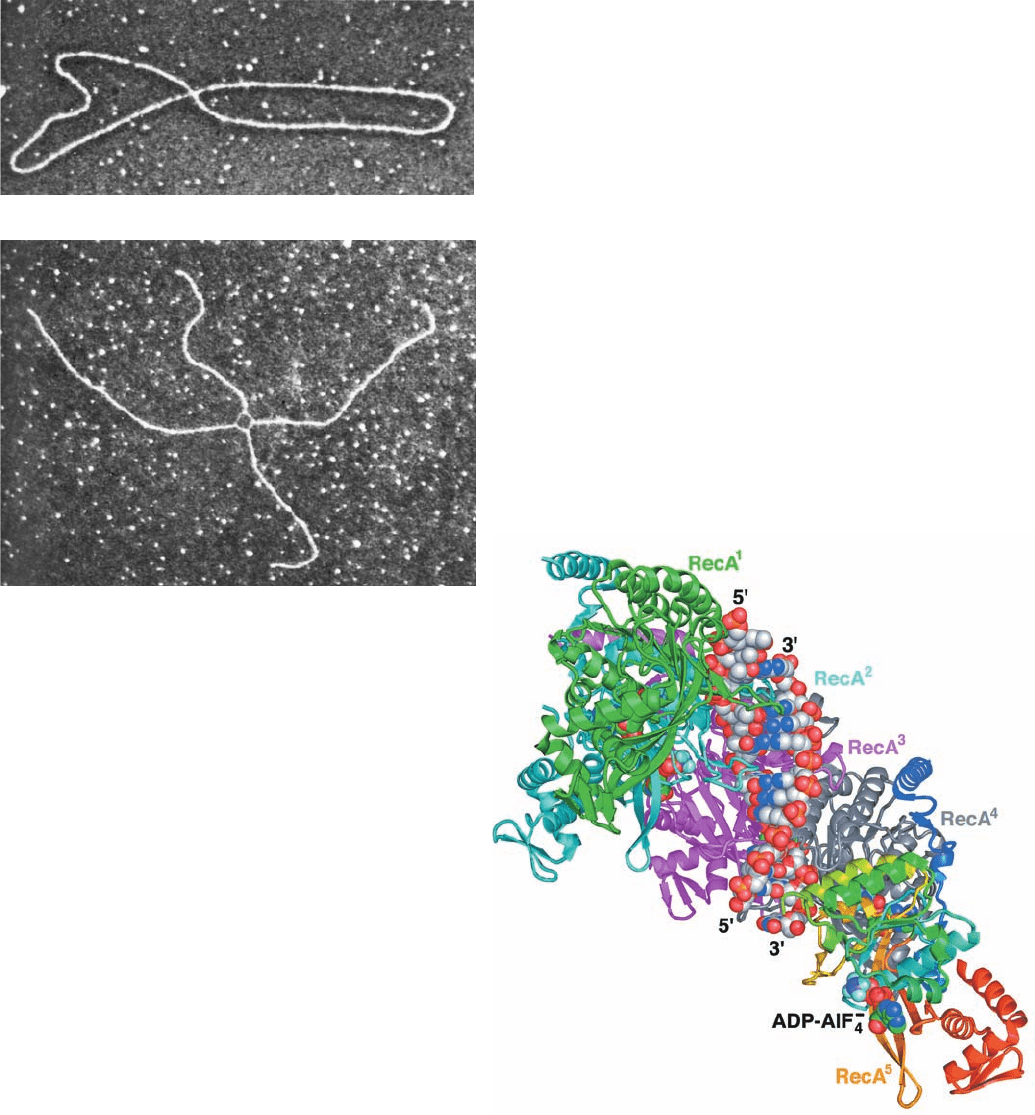
“figure-8” structures is shown in Fig. 30-70a. These figure-8
structures were shown not to be just twisted circles by cut-
ting them with a restriction endonuclease to yield chi struc-
tures (after their resemblance to the Greek letter ) such
as that pictured in Fig. 30-70b.
b. Homologous Recombination in E. coli Is
Catalyzed by RecA
The observation that recA
⫺
E. coli have a 10
4
-fold lower
recombination rate than the wild-type indicates that RecA
protein has an important function in recombination. In-
deed, RecA greatly increases the rate at which comple-
mentary strands renature in vitro. This versatile protein
(recall it also stimulates the autoproteolysis of LexA to
trigger the SOS response and is an essential participant in
the translesion synthesis of DNA; Section 30-5D) polymer-
izes cooperatively without regard to base sequence on
ssDNA or on dsDNA that has a single-stranded gap. The
resulting filaments, which may contain up to several thou-
sand RecA monomers, specifically bind the homologous
dsDNA, and, in an ATP-dependent reaction, catalyze
strand exchange.
EM studies by Edward Egelman revealed that RecA fil-
aments bound to ssDNA or dsDNA form a right-handed
helix with ⬃6.2 RecA monomers per turn and a pitch (rise
per turn) of 95 Å.The DNA in these filaments binds to the
protein with 3 nt (or bp) per RecA monomer and hence is
underwound with ⬃18.5 nt (or bp) per turn (vs 10 bp per
turn for cannonical B-DNA).
The formation of RecA–DNA filaments is highly coop-
erative; it requires five or six RecA protomers to form a
stable assembly. Consequently, attempts to crystallize
RecA–DNA filaments over many years were unsuccessful.
Nikola Pavletich ingeniously solved this conundrum by
linking five or six E. coli RecA genes (each corresponding
to residues 1–335 of this 353-residue protein) in tandem via
14-residue linkers and mutating the first and last RecA so
as to prevent them from forming longer filaments. These
fusion proteins, which had DNA-dependent ATPase and
strand-exchange activities comparable to that of
monomeric RecA, formed crystals containing both ssDNA
and dsDNA.
The X-ray structure of the RecA
5
–(ADP–AlF
4
⫺
)
5
–
(dT)
15
–(dA)
12
complex (Fig. 30-71;ADP–AlF
4
⫺
is a nonhy-
1228 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-70 Electron micrographs of intermediates in the
homologous recombination of two plasmids. (a) A figure-8
structure. This corresponds to Fig. 30-69d.(b) A chi structure that
results from the treatment of a figure-8 structure with a
restriction endonuclease. Note the thinner single-stranded
connections in the crossover region. [Courtesy of Huntington
Potter, University of South Florida, and David Dressler, Oxford
University, U.K.]
Figure 30-71 X-ray structure of the RecA
5
–(ADP–AlF
4
⫺
)
5
–
(dT)
15
–(dA)
12
complex viewed with its filament axis vertical. The
RecA units RecA
1
(the N-terminal unit) through RecA
4
are
colored green, cyan, magenta, and gray, respectively, with the
C-terminal unit, RecA
5
, colored in rainbow order from its
N-terminus (blue) to its C-terminus (red).The DNA and
ADP–AlF
4
⫺
are drawn in space-filling form with DNA C gray,
ADP C green, N blue, O red, P orange, F light blue, and Al
purple. [Based on an X-ray structure by Nikola Pavletich,
Memorial Sloan-Kettering Cancer Center, New York,
New York. PDBid 3CMX.]
(a)
(b)
JWCL281_c30_1173-1259.qxd 9/2/10 9:06 AM Page 1228
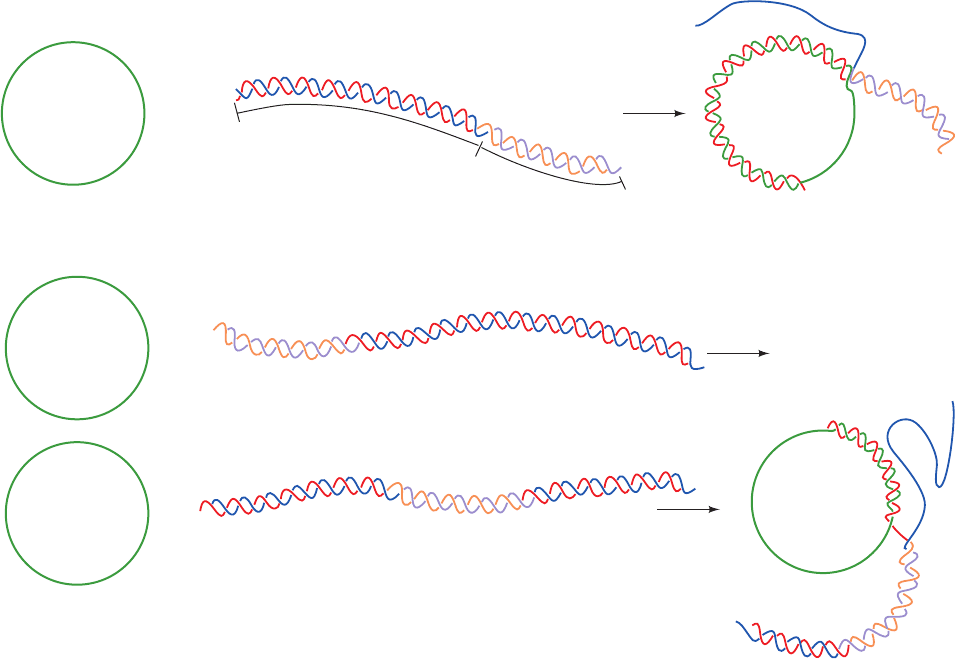
drolyzable ATP analog) exhibits a straight filament axis
with overall helical parameters that are closely similar to
those derived from EM studies. Each RecA unit consists of
a largely helical 30-residue N-terminal segment, a 240-
residue ␣/ ATPase core, and a 64-residue C-terminal glob-
ular domain. The linkers connecting adjacent RecA units
are disordered. Each RecA unit makes extensive contacts
with its nearest neighbors so as to form a filament with a
deep helical groove that exposes the DNA bound in its in-
terior (Fig. 30-71 is viewed looking into this groove).
The two DNA strands, which lie close to the filament
axis, form a complete set of Watson–Crick base pairs. How-
ever, rather than being smoothly stretched out, as had been
expected, the dsDNA assumes an irregular conformation in
which each 3-bp segment that is bound to a RecA unit
closely resembles B-DNA with the steps between succes-
sive base pairs in this triplet having an axial rise of ⬃3.4 Å
and a helical twist of ⬃30° (vs 3.4 Å and 36° for canonical B-
DNA; Table 29-1). In contrast, the step between successive
base pair triplets has an axial rise of 8.4 Å and a helical twist
of ⫺4°, thereby forming a 5-Å-high gap between successive
triplets that is partially filled by the side chain of the con-
served Ile 199.The sugar–phosphate backbone of the DNA
strand furthest from the viewer in Fig. 30-71 [the (dT)
15
]
makes extensive contacts with RecA. In contrast, the other
strand [the (dA)
12
] makes few contacts with the protein; it is
held in place almost entirely by base pairing with the first
strand. The ADP–AlF
4
⫺
is sandwiched between adjacent
␣/ ATPase cores, where it is completely buried.
The X-ray structure of the ssDNA-containing
RecA
6
–(ADP–AlF
4
⫺
)
6
–(dT)
18
complex closely resembles
that of the foregoing dsDNA-containing complex but with
the absence of the DNA strand closest to the viewer in Fig.
30-71. Thus, each RecA unit binds a (dT)
3
segment that is
held in a B-DNA-like conformation with successive (dT)
3
segments separated by a 7.8-Å axial rise.
How does RecA mediate DNA strand exchange between
single-stranded and duplex DNAs? On encountering a
dsDNA with a strand that is complementary to its bound
ssDNA, RecA partially unwinds the duplex and, in a reac-
tion driven by RecA-catalyzed ATP hydrolysis, exchanges
the ssDNA with the corresponding strand on the duplex.
This process tolerates only a limited degree of mispairing and
requires that one of the participating DNA strands have a free
end. The assimilation (exchange) of a single-stranded circle
with a strand on a linear duplex (Fig. 30-72) cannot proceed
past the 3¿ end of a highly mismatched segment in the com-
plementary strand. The invasion of the single strand must
Section 30-6. Recombination and Mobile Genetic Elements 1229
No assimilation of noncomplementary DNA
No assimilation
3'
5'
Assimilation stops at
heterologous
segment
Assimilation of 3' end of homologous DNA
Homologous
DNA
Heterologous
DNA
5'
3'
Figure 30-72 The RecA-catalyzed assimilation of a
single-stranded circle by a dsDNA can occur only if the dsDNA
has a 3ⴕ end that can base-pair with the circle (red strand).
Strand assimilation cannot proceed through a noncomplemen-
tary segment (purple and orange strands).
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1229
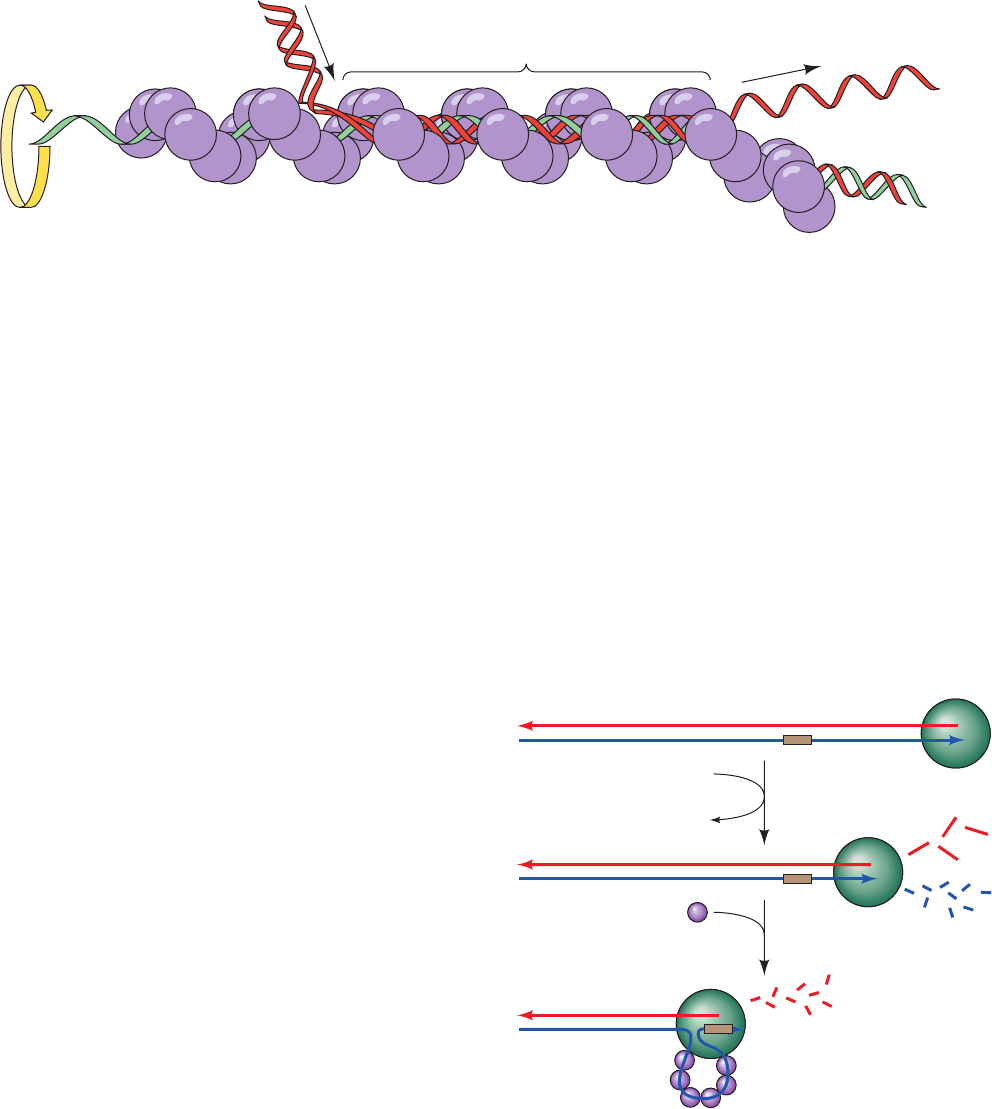
therefore begin at its 5¿ end. A model for the consequent
branch migration process is diagrammed in Fig. 30-73. Of
course, two such strand exchange processes must occur si-
multaneously in a Holliday junction (Figs. 30-67 and 30-69).
The above structures suggest that the fidelity of homol-
ogous recombination arises from the B-DNA-like confor-
mation that RecA imposes on the otherwise flexible bound
ssDNA strand, which would exclude non-Watson–Crick
base pairs. Strand exchange, of course, requires the separa-
tion of the two strands of the incoming dsDNA to permit
one of its strands to sample base pairing with the ssDNA
substrate. The above structures suggest that this is facili-
tated by the disruption of base stacking between base pair
triplets in the RecA–DNA complex. However, the struc-
ture of the triple helical DNA intermediate in the strand
exchange reaction is, as yet, unknown.
c. Eukaryotes Have RecA-Like Proteins
Yeast RAD51 (339 residues) functions in the ATP-
dependent repair and recombination of DNA in much the
same way as does the 30% homologous E. coli RecA pro-
tein. The electron micrograph–based image reconstruction
of RAD51 in complex with double-stranded DNA is nearly
identical to that of RecA at low resolution: Both com-
plexes form helical filaments in which the DNA has an
⬃5.1-Å rise per base pair and 18.6 bp per turn. Since
RAD51 homologs occur in chickens, mice,and humans, it is
very likely that such filaments universally mediate DNA
repair and recombination.
d. RecBCD Initiates Recombination by Making
Single-Strand Nicks
The single-strand nicks to which RecA binds are made
by the RecBCD protein, the 330-kD heterotrimeric prod-
uct of the SOS genes recB, recC, and recD. RecB is both a
3¿S5¿ helicase and a nuclease, whereas RecD is a 5¿S3¿
helicase. The formation of a RecA binding site begins with
RecBCD binding to the end of a dsDNA and then unwind-
ing it via its two ATP-driven helicase functions (Fig. 30-74).
As it does so, RecB nucleolytically degrades the unwound
single strands behind it, with the 3¿-ending strand being
cleaved more often and hence broken down to smaller
fragments than the 5¿-ending strand. However, on RecC
encountering the sequence GCTGGTGG from its 3¿ end
(the so-called Chi sequence, which occurs about every
⬃5 kb in the E. coli genome), the enzyme pauses and
ceases its cleavage of the 3¿-ending strand but increases the
rate at which it cleaves the 5¿-ending strand. RecBCD then
helps load RecA onto the 3¿-ending strand before dissoci-
ating from the DNA.
1230 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-73 Hypothetical model for the RecA-mediated
strand exchange reaction. Homologous DNA molecules are
paired in advance of strand exchange in a three-stranded helix.
The ATP-driven rotation of the RecA filament about its helix
Figure 30-74 The generation of a 3ⴕ-ending single-strand
DNA segment by RecBCD to initiate recombination. (1)
RecBCD binds to a free end of a dsDNA and, in an ATP-driven
process, advances along the helix, unwinding the DNA and
degrading the resulting single strands behind it, with the
3¿-ending strand cleaved more often than the 5¿-ending strand.
(2) When RecBCD encounters a properly oriented Chi
sequence, it binds it and thus stops cleaving the 3¿-ending strand
but increases the frequency at which it cleaves the 5¿-ending
strand.This generates the potentially invasive 3¿-ending strand
segment to which RecA binds.
Spooling
out
Spooling
in
5′
5′
3′
3′
Three-stranded DNA
1
RecBCD
RecA
ATP
ADP + P
i
2
Chi
3'
3'
5'
5'
3'
5'
axis would cause duplex DNA to be “spooled in” to the filament,
right to left as drawn. [After West, S.C., Annu. Rev. Biochem. 61,
617 (1992).]
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1230
