Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

from a number of donors between 0 and 93 years old indi-
cates that there is only a weak correlation between the pro-
liferative capacity of a cell culture and the age of its donor.
There is, however, a strong correlation, valid over the en-
tire donor age range, between the initial telomere length in
a cell culture and its proliferative capacity. Thus, cells that
initially have relatively short telomeres undergo signifi-
cantly fewer doublings than cells with longer telomeres.
Moreover, fibroblasts from individuals with progeria (a
rare disease characterized by rapid and premature aging
resulting in childhood death) have short telomeres, an ob-
servation that is consistent with their known reduced pro-
liferative capacity in culture. In contrast, sperm (which, be-
ing germ cells, are in effect immortal) from donors ranging
in age from 19 to 68 years had telomeres that did not vary
in length with donor age, which indicates that telomerase is
active at some stage of germ cell growth. Likewise, those
few cells in a culture that become immortal (capable of un-
limited proliferation) exhibit an active telomerase and a
telomere of stable length, as do the cells of unicellular eu-
karyotes (which are also immortal). It therefore appears
that telomere erosion is a significant cause of cellular
senescence and hence aging. Indeed transgenic mice that
constitutively (at a constant rate) express telomerase have
increased lifespans (although, in contrast to humans, mice
in which telomerase has been knocked out survive without
significant problems for several generations before they
become infertile).
d. Cancer Cells Have Active Telomerases
What advantage might multicellular organisms gain by
eliminating telomerase activity in their somatic cells? An
intriguing possibility is that cellular senescence is a mecha-
nism that protects multicellular organisms from cancer.
The two defining characteristics of cancer cells are that
they are immortal and grow uncontrollably (Sections 19-
3B and 34-4C). If mammalian cells were normally immor-
tal, the incidence of cancer would probably be far greater
than it is since immortalization, which requires an active
telomerase, is a major step toward malignant transforma-
tion (cancer formation), which requires several independ-
ent genetic changes (Section 19-3B). Indeed, nearly all hu-
man cancers exhibit high telomerase activity. Moreover, as
Robert Weinberg demonstrated, human fibroblasts in cul-
ture can be malignantly transformed by the acquisition of
only three genes, those encoding: (1) TERT (its TER sub-
unit is normally expressed in somatic cells), (2) an onco-
genic variant of H-Ras (an essential participant in intra-
cellular signal transduction pathways; Section 19-3C), and
(3) the SV40 large-T antigen [SV40 is a tumor virus whose
large-T antigen binds and functionally inactivates the tu-
mor suppressor proteins known as Rb and p53 (Section 34-
4C; it also functions as a helicase in viral DNA replica-
tion)]. The age-related decline in telomere length in
humans does not occur in mice, which suggests that telom-
ere loss evolved to suppress tumor formation in long-lived
animals, such as humans, but not in short-lived animals,
Section 30-4. Eukaryotic Replication 1211
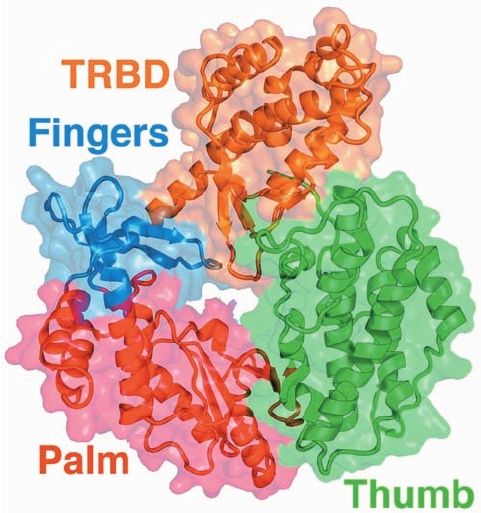
Figure 30-50 X-ray structure of TERT from Tribolium
castaneum. The protein is shown ribbon form with its TRBD,
fingers, palm, and thumb colored orange, blue, red, and green,
respectively, and embedded in its like-colored semitransparent
surface diagram. Compare this structure with that of p66 subunit
of HIV-1 reverse transcriptase (Fig. 30-48a). [Based on an X-ray
structure by Emmanuel Skordalakes,The Wistar Institute,
Philadelphia, Pennsylvania. PDBid 3DU5.]
JWCL281_c30_1173-1259.qxd 9/2/10 9:04 AM Page 1211
1212 Chapter 30. DNA Replication, Repair, and Recombination
such as mice. Thus, telomerase inhibitors may be effective
antitumor agents.
e. Telomeric DNA Can Dimerize via G-Quartets
It has long been known that guanine forms strong
Hoogsteen-type base pairs (Table 29-2) that can further
associate to form cyclic tetramers known as G-quartets
(Fig. 30-51a). Indeed, G-rich polynucleotides are notori-
ously difficult to work with because of their propensity to
aggregate. The G-rich overhanging strands of telomeres
dimerize to form stable complexes in solution, presumably
via the formation of G-quartet–containing structures.
The 3¿-terminal telomeric overhang of the ciliated pro-
tozoan Oxytricha nova has the sequence d(T
4
G
4
)
2
, which
resembles the repeating telomeric sequences of other or-
ganisms. The NMR structure of the dodecamer
d(G
4
T
4
G
4
), determined by Juli Feigon (Fig. 30-51b), re-
veals that each oligonucleotide folds back on itself to form
a hairpin, two of which associate in an antiparallel fashion
to form a structure that contains four stacked G-quartets,
with the T
4
sequences forming the loops at the ends of
each stack.
The telomere end binding protein (TEBP) of O. nova is
a heterodimeric capping protein that binds to and protects
the foregoing 3¿ overhang. The X-ray structure of TEBP in
complex with d(G
4
T
4
G
4
), determined by Steve Schultz,
reveals that the DNA binds in a deep cleft between the pro-
tein’s ␣ and  subunits, where it adopts an irregular nonheli-
cal conformation (Fig. 30-52). In addition, two other
d(G
4
T
4
G
4
) molecules form a G-quartet–linked dimer with
the same conformation they adopt in solution (Fig. 30-51).
The G-quartet assembly fits snugly into a small positively
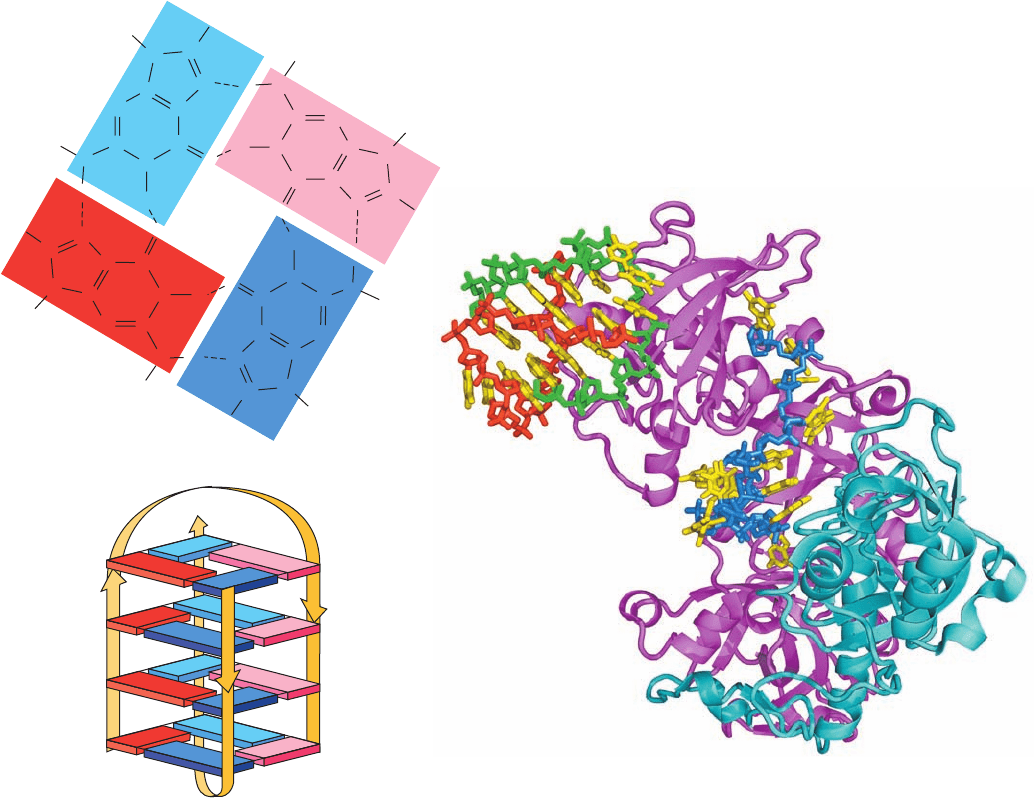
Figure 30-52 X-ray structure of Oxytricha nova telomere end
binding protein (TEBP) in complex with d(G
4
T
4
G
4
). The TEBP
is drawn in ribbon form with its ␣ and  subunits magenta and
cyan.The DNA is drawn in stick form with its bases yellow, the
sugar–phosphate backbone of the single strand that binds in a
cleft between the protein’s ␣ and  subunits blue, and the
backbones of two strands that form a G-quartet–linked dimer
red and green.The G-quartet–linked dimer binds in a cavity
formed by the N-terminal domains of three symmetry-related ␣
chains, although only one of them is shown here. [Based on an
X-ray structure by Steve Schultz, University of Colorado. PDBid
1JB7.]
Figure 30-51 NMR structure of the telomeric oligonucleotide
d(GGGGTTTTGGGG). (a) The base-pairing interactions in the
G-quartet at the end of the quadruplex in solution. (b) Schematic
diagram of the NMR solution structure, in which the strand
directions are indicated by arrows.The nucleotides are numbered
1 to 12 in one strand and 1* to 12* in the symmetry-related
strand. Guanine residues G1 to G4 are represented by dark blue
rectangles, G8 to G12 are light blue, G1* to G4* are red, and
G9* to G12* are pink. [After Schultze, P., Smith, F.W., and
Feigon, J., Structure 2, 227 (1994). PDBid 156D.]
R
H
C
N
(a)
C
C
N
H
H
N
CN
C
C
C
N
N
C
H
H
H
N
C
C
C
C
N
N
H
R
C
N
H
N
C
C
C
O
N
C
H
H
H
N
C
N
H
H
R
N
N
H
R
H
C
O
O
O
C
N
N
N
H
N
4*
9*
12
(b)
1
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1212
charged cavity formed by the N-terminal domains of three
symmetry-related (in the crystal) ␣ subunits at sites distinct
from their ssDNA binding sites. The ␣ subunit (TEBP␣)
contains three so-called OB folds (OB for oligomer bind-
ing), common oligonucleotide/oligosaccharide binding mo-
tifs that each contain a characteristic 5-stranded  barrel.
Two of these OB folds participate in DNA binding and a
third interacts with TEBP, which also contains an OB fold.
The presence of both the ssDNA and the G-quartet assem-
bly in the X-ray structure supports the hypothesis that mul-
tiple DNA structures and, in particular, G-quartets, play a
role in telomere biology.
Although TEBP is not present in yeast or vertebrates,
both humans and fission yeast express a telomere end-
binding protein named POT1 (for protection of telomeres-1)
and its binding partner TPP1 (so named because it had been
previously called TINT1, PTOP, and PIP1), which bind to
the single-stranded overhang at the ends of telomeres. POT1
consists mainly of two OB folds in which the N-terminal OB
fold is homologous to that of TEBP␣.The deletion of POT1
causes rapid loss of telomeric DNA and chromosomal end-
joining.TPP1 is structurally similar to TEBP, despite their
only 11% sequence identity, and hence the POT1–TPP1
complex appears to be a homolog of TEBP.
f. Telomeres Form T-Loops
Mammalian telomeric DNA is also capped by two re-
lated proteins, TRF1 and TRF2 (TRF for telomere repeat-
binding factor). Jack Griffith and Titia de Lange have
shown through electron microscopy (EM) studies that, in
the presence of TRF2, otherwise linear telomeric DNA
forms large duplex end loops named T-loops (T for telom-
ere; Fig. 30-53a). Moreover, the EM of DNA from mam-
malian telomeres, whose strands had been chemically
cross-linked to preserve their structural relationships on
deproteination, likewise revealed the presence of abun-
dant T-loops of varying sizes. These observations suggest
that T-loops are formed by the TRF2-induced invasion of
the 3¿ telomeric overhang into the repeating telomeric
dsDNA (Fig. 30-53b) to form a D-loop (D for displacement;
a segment of dsDNA whose two strands are separated).
T-loops have also been observed in protozoa, suggesting
that T-loops are a conserved feature of eukaryotic telo-
meres. TRF1 is implicated in controlling telomeric length,
presumably by somehow limiting the number of TRF1
molecules that can bind to a telomere.
POT1,which binds to the single-stranded 3¿ extension at
chromosome ends, and TRF1 and TRF2, which bind dou-
ble-stranded telomere DNA, are bridged by TPP1 and
TIN2 (for TRF1 interacting protein 2). A sixth protein,
RAP1 (for repressor activator protein 1), binds mainly to
TRF2. The complex formed by these six proteins, which is
known as shelterin, apparently functions both to protect
the mammalian telomere from being mistaken for a bro-
ken chromosome and hence being subject to DNA repair,
and to limit telomere length by preventing the extension of
its bound telomere by telomerase.
5 REPAIR OF DNA
DNA is by no means the inert substance that might be sup-
posed from naive consideration of genome stability.
Rather, the reactive environment of the cell, the presence
Section 30-5. Repair of DNA 1213
5'
5'
D–loop
TRF2
POT1
3'
3'
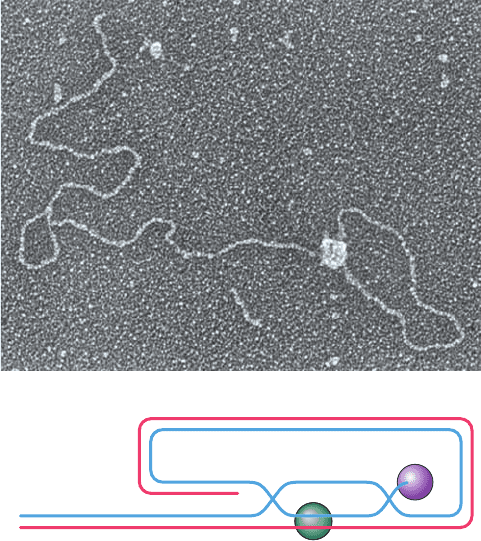
Figure 30-53 The telomeric T-loop. (a) An electron
micrograph of a dsDNA consisting of a 3-kb unique sequence
followed by ⬃2 kb of the repeating sequence TTAGGG on the
strand that ends with a 150- to 200-nt 3¿ overhang.This model
telomeric DNA was then incubated with human TRF2. [Courtesy
of Jack Griffith, University of North Carolina at Chapel Hill.]
(b) The proposed structure of a T-loop. In a process that is
mediated by TRF2, the repeating TTAGGG sequence in the
DNA’s 3¿ overhang displaces a portion of the same strand (blue)
in the double-stranded region of the DNA to form a duplex
segment with the complementary strand (red), thereby
generating a D-loop. The telomere end-binding protein POT1
specifically binds to the end of the 3¿ overhang.
(b)
(a)
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1213
N
O
O
...
...
C
C C
C C
N
C
N
C
C
N
O
O
H
O
HCH
3
HCH
3
Cyclobutyl
ring
O
H
P
P
P
1214 Chapter 30. DNA Replication, Repair, and Recombination
of a variety of toxic substances, and exposure to UV or ion-
izing radiation subjects it to numerous chemical insults that
excise or modify bases and alter sugar–phosphate groups
(Fig. 30-54). Indeed, some of these reactions occur at sur-
prisingly high rates. For example, under normal physiologi-
cal conditions, the glycosidic bonds of ⬃10,000 of the 3 bil-
lion purine nucleotides in each human cell hydrolyze
spontaneously each day.
Any DNA damage must be repaired if the genetic mes-
sage is to maintain its integrity. Such repair is possible be-
cause of duplex DNA’s inherent information redundancy.
The biological importance of DNA repair is indicated by
the identification of at least 130 genes in the human
genome that participate in DNA repair and by the great
variety of DNA repair pathways possessed by even rela-
tively simple organisms such as E. coli. In fact, the major
DNA repair processes in eukaryotic cells and E. coli are
chemically quite similar. These processes are outlined in
this section.
The two redundant copies of genetic information carried
by dsDNA ideally suit it for the repair of damage to one of
its strands: The damaged strand can be repaired under the
direction of the undamaged strand. The importance of
dsDNA to the storage of genetic information is indicated by
the fact that only a few small viruses carry single-stranded
DNA or RNA as their genetic material (e.g., X174 and
HIV).The DNA repair systems discussed below do not op-
erate on single-stranded nucleic acids and hence these
viruses have very high rates of mutation. Thus it appears
that only organisms with very small genomes can afford the
economy of not encoding their genomes on dsDNA.
A. Direct Reversal of Damage
a. Pyrimidine Dimers Are Split by DNA Photolyase
UV radiation of 200 to 300 nm promotes the formation
of a cyclobutyl ring between adjacent thymine residues on
the same DNA strand to form an intrastrand thymine
dimer (Fig. 30-55). Similar cytosine and thymine–cytosine
dimers are likewise formed but at lesser rates. Such
cyclobutane pyrimidine dimers (CPDs) locally distort
DNA’s base-paired structure such that it can be neither
transcribed nor replicated. Indeed, a single thymine dimer,
if unrepaired, is sufficient to kill an E. coli.
Pyrimidine dimers may be restored to their monomeric
forms through the action of light-absorbing enzymes named
photoreactivating enzymes or DNA photolyases that are
present in many prokaryotes and eukaryotes (including
goldfish, rattlesnakes, and marsupials, but not placental
mammals).These enzymes are 55- to 65-kD monomers that
bind to a pyrimidine dimer in DNA, a process that can
occur in the dark.A noncovalently bound chromophore, in
Figure 30-55 The cyclobutylthymine dimer that forms on UV
irradiation of two adjacent thymine residues on a DNA strand.
The ⬃1.6-Å-long covalent bonds joining the thymine rings (red)
are much shorter than the normal 3.4-Å spacing between stacked
rings in B-DNA, thereby locally distorting the DNA.
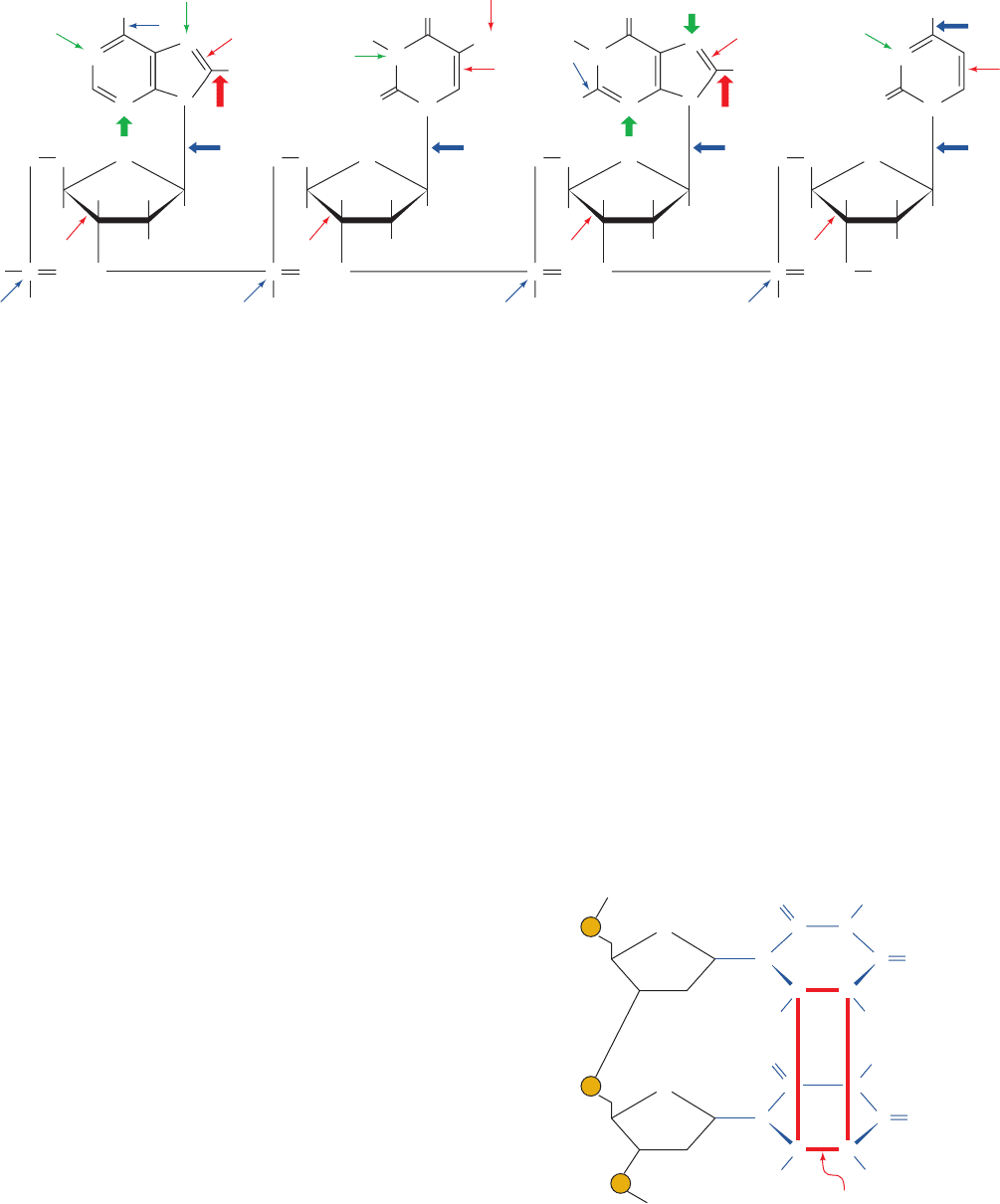
Figure 30-54 The types and sites of chemical damage to which
DNA is normally susceptible in vivo. Red arrows indicate sites
subject to oxidative attack, blue arrows indicate sites subject to
spontaneous hydrolysis, and green arrows indicate sites subject
CH
3
H
2
N
NH
2
CH
2
O
NH
2
N
AT
N
N
N
H
H
O
N
G
N
N
N
O
H
O
N
H
N
C
O
N
N
O
HH
H
H
OO
O
–
P
H
CH
2
O
O
HH
H
H
OO
O
–
P
H
CH
2
O
O
HH
H
H
OO
O
–
P
H
CH
2
O
O
HH
H
H
OO
O
–
P
H
to nonenzymatic methylation by S-adenosylmethionine. The
width of an arrow is indicative of the relative frequency of the
reaction. [After Lindahl, T., Nature 362, 709 (1993).]
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1214
some species an N
5
,N
10
-methenyltetrahydrofolate (MTHF;
Fig. 26-49) and in others a 5-deazaflavin,
then absorbs 300- to 500-nm light and transfers the excita-
tion energy to a noncovalently bound FADH
⫺
,which in turn
transfers an electron to the pyrimidine dimer, thereby
splitting it. Finally, the resulting pyrimidine anion re-reduces
the FADHⴢ and the now unblemished DNA is released,
thereby completing the catalytic cycle. DNA photolyases
bind either dsDNA or ssDNA with high affinity but without
regard to base sequence.
Thomas Carell and Lars-Oliver Essen determined the
X-ray structure of the 474-residue DNA photolyase from the
cyanobacterium Anacystis nidulans in complex with a 9-bp
dsDNA containing a synthetic thymine dimer whose bridg-
ing phosphate group was replaced by a ¬O¬CH
2
¬O¬
group (which does not affect the enzyme’s ability to split
the dimer). The DNA binds to a highly positively charged
surface on the protein with its thymine dimer flipped out of
the double helix and bound in a deep cavity (Fig. 30-56).
This flip-out is probably facilitated by the relatively weak
base pairing interactions of the thymine dimer and the dis-
tortions it imposes on the double helix. In the following
discussions we shall see that this so-called base flipping
(really nucleotide flipping since entire nucleotides flip out
of the double helix) is by no means an unusual process for
enzymes that perform chemistry on the bases of dsDNA.
The DNA outside of the thymine dimer assumes the B con-
formation but at the thymine dimer is bent by 50° away
from the protein, thereby unstacking the adenine bases
complementary to the dimerized thymine bases.The “hole”
in the DNA helix left by the flipped-out thymine dimer is
partially occupied by an irregular protein ridge.
In the X-ray structure, the thymine dimer’s C5¬C5 and
C6¬C6 bonds are broken. Yet, in the crystal, the enzyme-
bound thymine dimer is stable in the dark for at least a
year. Apparently, the X-rays used to generate the diffrac-
tion data mimic the effects of the light that normally rup-
tures these bonds. Moreover, the FAD’s isoalloxazine ring
exhibits a 9° “butterfly” bend about its N5–N10 axis (the
isoalloxazine ring’s atomic numbering scheme is givien in
Fig. 16-8), which is indicative that it is in the fully reduced
FADH
⫺
form. The isoalloxazine ring and adenine ring of
the FAD
⫺
, which has a folded conformation, are in van der
Waals contact with one or the other bases of the thymine
dimer and the isoalloxazine ring is ⬃10 Å distant from the
8-Hydroxy-7,8-didemethyl-5-deazariboflavin
CH
2
OH
CH
2
HO
N
10
N
1
(CHOH)
3
O
O
H
N
9
65
4
7
8
3
2
N-Methyl-N'-nitro-N-
nitrosoguanidine (MNNG)
O
6
-Methylguanine residue
H
3
C
H
2
N
NH
H
N
NNO
2
CH
3
O
O
N
N
N
N
N
C
flavin-like ring of the MTHF. This permits the observed ef-
ficient energy transfer in the photolyase reaction (which
has a quantum yield of ⬃0.9).
b. Alkyltransferases Dealkylate Alkylated
Nucleotides
The exposure of DNA to alkylating agents such as N-
methyl-Nⴕ-nitro-N-nitrosoguanidine (MNNG)
yields, among other products, O
6
-alkylguanine residues.
The formation of these derivatives is highly mutagenic be-
cause on replication, they frequently cause the incorpora-
tion of thymine instead of cytosine.
Section 30-5. Repair of DNA 1215
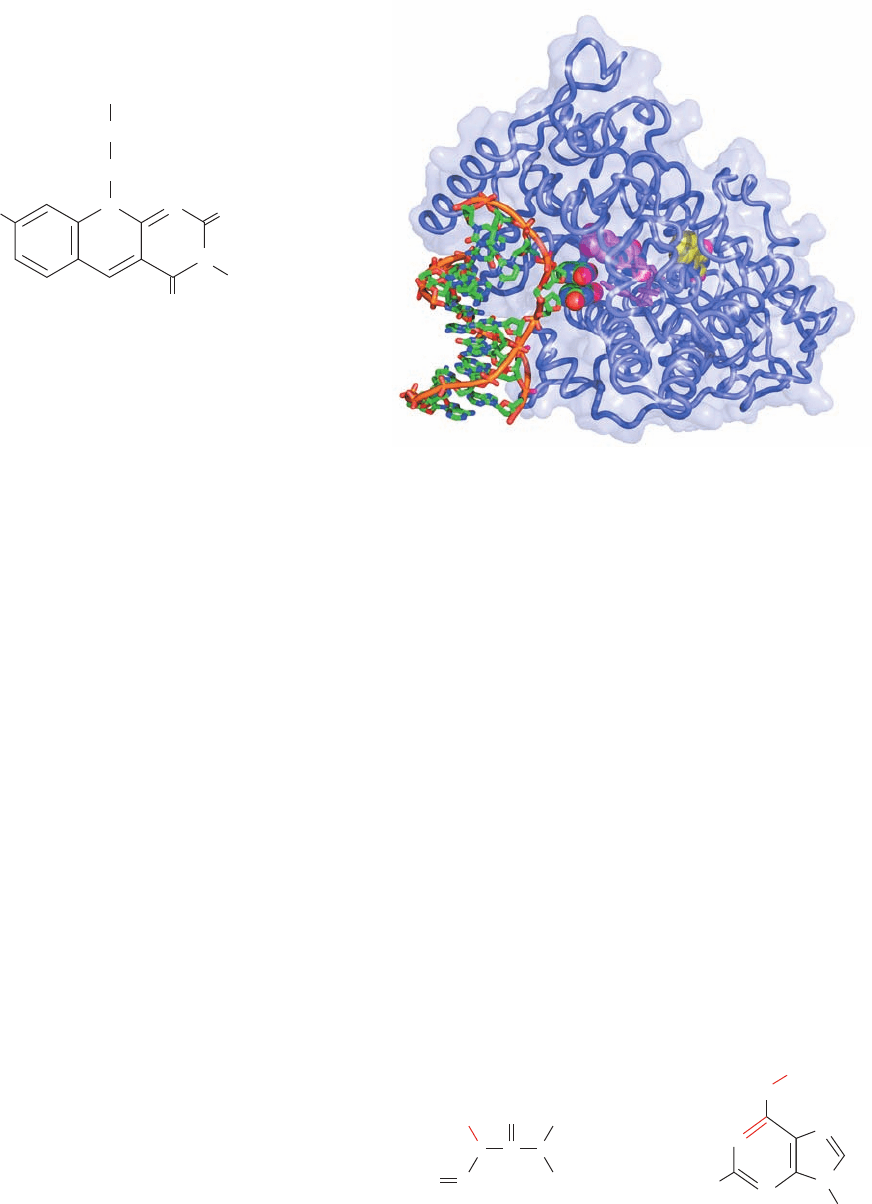
Figure 30-56 X-ray structure of A. nidulans DNA photolyase
in complex with dsDNA containing a synthetic thymine dimer.
The protein is drawn in worm form embedded in its
semitransparent surface diagram.The DNA, in which the
phosphate group bridging the nucleotides of the thymine dimer is
replaced by a ¬O¬CH
2
¬O¬ group, is drawn mainly in stick
form but with the bases of the thymine dimer in space-filling
form, all colored according atom type (C green, N blue, O red,
and P orange) and with successive P atoms in each polynucleotide
chain connected by orange rods.The FAD and MTHF are drawn
in stick form with their flavin and flavinlike rings in space-filling
form and with FAD C magenta and MTHF C yellow. [Based on
an X-ray structure by Thomas Carell, Ludwig Maximilians
University, Munich, Germany, and Lars-Oliver Essen, Philipps
University, Marburg, Germany. PDBid 1TEZ.]
JWCL281_c30_1173-1259.qxd 9/2/10 9:05 AM Page 1215
(a)
O
6
-Methylguanine and O
6
-ethylguanine lesions of
DNA in all species tested are repaired by O
6
-alkylgua-
nine–DNA alkyltransferase, which directly transfers the
offending alkyl group to one of its own Cys residues. The
reaction inactivates this protein, which therefore cannot be
strictly classified as an enzyme. The alkyltransferase reac-
tion has elicited considerable attention because carcino-
genesis induced by methylating and ethylating agents is
correlated with deficient repair of O
6
-alkylguanine lesions.
The E. coli O
6
-alkylguanine–DNA alkyltransferase
activity occurs on the 178-residue C-terminal segment of
the 354-residue Ada protein (the product of the ada gene).
Its X-ray structure (Fig. 30-57a), determined by Eleanor
Dodson and Peter Moody, reveals, unexpectedly, that its
active site Cys residue, Cys 321, is buried inside the protein.
Apparently, the protein must undergo a significant confor-
mational change on DNA binding in order to effect the
methyl transfer reaction.
Ada protein’s 92-residue N-terminal segment has an in-
dependent function: It repairs methyl phosphotriesters in
DNA (methylated phosphate groups) by irreversibly trans-
ferring the offending methyl group to its Cys 69.The NMR
structure of Ada’s N-terminal domain (Fig. 30-57b), deter-
mined by Gregory Verdine and Gerhard Wagner, reveals
that Cys 69, together with three other Cys residues, tetrahe-
drally coordinates a Zn
2⫹
ion. This presumably stabilizes
the thiolate form of Cys 69 over its thiol form, thereby fa-
cilitating its nucleophilic attack on the methyl group.
Intact Ada protein that is methylated at its Cys 69 binds to
a specific DNA sequence, which is located upstream of the
ada gene and several other genes encoding DNA repair pro-
teins, thereby inducing their transcription. Evidently, Ada
also functions as a chemosensor of methylation damage.
B. Excision Repair
Cells employ two types of excision repair mechanisms:
(1) nucleotide excision repair (NER), which functions to
repair relatively bulky DNA lesions; and (2) base excision
repair (BER), which repairs nonbulky lesions involving a
single base.
a. Nucleotide Excision Repair
NER is a DNA repair mechanism found in all cells that
eliminates damage to dsDNA by excising an oligonucleotide
containing the lesion and filling in the resulting single-strand
1216 Chapter 30. DNA Replication, Repair, and Recombination
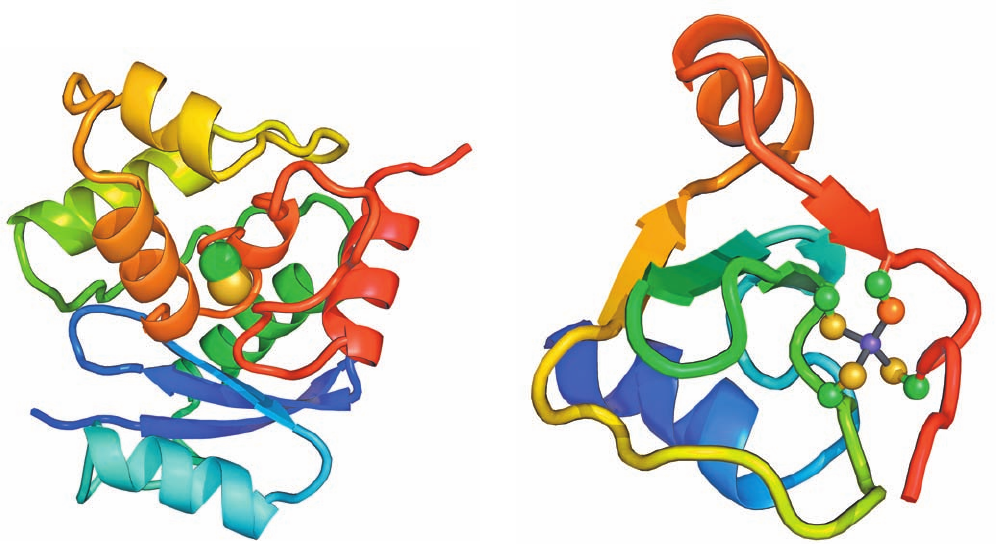
Figure 30-57 Structure of the E. coli Ada protein. (a) The
X-ray structure of Ada’s 178-residue C-terminal segment, which
contains its O
6
-alkylguanine–DNA alkyltransferase function. The
protein is drawn in ribbon form colored in rainbow order from
its N-terminus (blue) to its C-terminus (red).The side chain of
Cys 146 (Cys 321 in the intact protein), to which the methyl
group is irreversibly transferred, is shown in space-filling form
with C green and S yellow. Note that this residue is almost
entirely buried within the protein. [Based on an X-ray structure
by Eleanor Dodson and Peter Moody, University of York, U.K.
PDBid 1SFE.] (b) The NMR structure of Ada’s 92-residue,
(b)
N-terminal segment, which mediates its methyl phosphotriester
repair function.The protein is drawn in ribbon form colored in
rainbow order from its N-terminus (blue) to its C-terminus (red).
Its bound Zn
2⫹
ion is represented by a purple sphere and its four
tetrahedrally coordinating Cys side chains are shown in ball-and-
stick form, with C green and S yellow except for the orange S
atom of Cys 69, which becomes irreversibly methylated when the
protein encounters a methylated phosphate group on DNA. The
coordinate bonds to the Zn
2⫹
ion are represented by gray sticks.
[Based on an NMR structure by Gregory Verdine and Gerhard
Wagner, Harvard University. PDBid 1ADN.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1216
gap. NER repairs lesions that are characterized by the dis-
placement of bases from their normal positions, such as
pyrimidine dimers, or by the addition of a bulky substituent
to a base.This system appears to be activated by a helix dis-
tortion rather than by the recognition of any particular
group. In humans, NER is the major defense against two
important carcinogens, sunlight and cigarette smoke. The
mechanism of NER in prokaryotes is similar to that in eu-
karyotes. However, prokaryotic NER employs 3 subunits,
whereas eukaryotic NER involves the actions of 16 sub-
units. The eukaryotic proteins are conserved from yeast to
humans but none of them exhibit any sequence similarity
to the prokaryotic proteins, suggesting that the two NER
systems arose by convergent evolution.
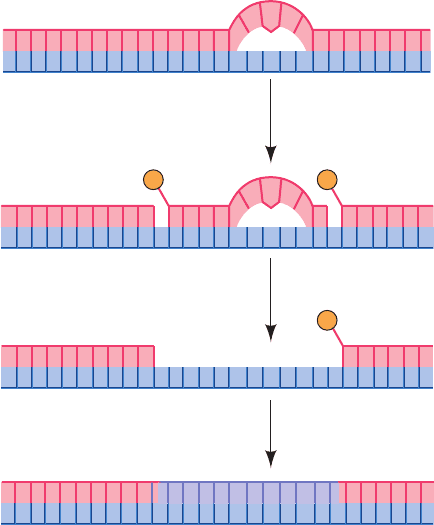
In E. coli, NER is carried out in an ATP-dependent
process through the actions of the UvrA, UvrB, and UvrC
proteins (the products of the uvrA, uvrB, and uvrC genes).
This system, which is often referred to as the UvrABC en-
donuclease (although, as we shall see, there is no complex
that contains all three subunits), cleaves the damaged
DNA strand at the seventh and at the third or fourth phos-
phodiester bonds from the lesion’s 5¿ and 3¿ sides, respec-
tively (Fig. 30-58; this system is also known as an excinucle-
ase to indicate that it excises a DNA segment rather than
cleaving it in only one place as do most endonucleases).
The excised 11- or 12-nt oligonucleotide is displaced by the
binding of UvrD (also called helicase II) and replaced
through the actions of Pol I and DNA ligase.
The mechanism of prokaryotic NER was elucidated
mainly by Aziz Sancar. It begins with the damage recogni-
tion step in which a (UvrA)
2
UvrB heterotrimer binds
tightly although nonspecifically to dsDNA, which it probes
for damage according to its local propensity for bending
and unwinding.The presence of a lesion activates the heli-
case function of UvrB to unwind 5 bp around the lesion in
an ATP-driven process. This conformation change induces
the dissociation of the UvrA from the complex, which al-
lows the binding of UvrC.UvrB then makes the incision on
the 3¿ side of the lesion following which UvrC makes the
incision on its 5¿ side. UvrD binds to the resulting nicks in
the DNA, which displaces UvrC and the lesion-containing
oligomer.This makes the 5¿ incision site accessible to Pol I,
which fills in the gap and displaces UvrB. Finally, DNA lig-
ase seals the remaining nick yielding refurbished DNA.
b. Xeroderma Pigmentosum and Cockayne
Syndrome Are Caused by Genetically Defective NER
In humans, the rare inherited disease xeroderma pigmen-
tosum (XP; Greek: xeros, dry ⫹ derma, skin) is mainly char-
acterized by the inability of skin cells to repair UV-induced
DNA lesions. Individuals suffering from this autosomal
recessive condition are extremely sensitive to sunlight.
During infancy they develop marked skin changes such as
dryness, excessive freckling, and keratoses (a type of skin tu-
mor; the skin of these children is described as resembling
that of farmers with many years of sun exposure), together
with eye damage, such as opacification and ulceration of the
cornea. Moreover, they develop often fatal skin cancers at a
2000-fold greater rate than normal and internal cancers at a
10 to 20-fold increased rate. Curiously,many individuals with
XP also have a bewildering variety of seemingly unrelated
symptoms including progressive neurological degeneration
and developmental deficits.
Cultured skin fibroblasts from individuals with xero-
derma pigmentosum are defective in the NER of pyrimidine
dimers. Cell-fusion experiments with cultured cells taken
from various patients have demonstrated that this disease
results from defects in any of 8 complementation groups
(Section 1-4Cc), indicating that there must be at least 8
gene products, XPA through XPG and XPV, involved in
this clearly important UV damage repair pathway.
What is the biochemical basis for the diverse group of
symptoms associated with impaired NER? The reactive
oxygen species (ROS; Section 22-4Cg) produced by oxida-
tive metabolism readily damages DNA. Some of these ox-
idative lesions are repaired via NER. Since neurons have
high rates of respiration and are long-lived nondividing
cells, it seems likely that they would be particularly suscep-
tible to oxidative damage in the absence of NER. This ex-
plains the progressive neurological deterioration in XP.
The need to repair a DNA lesion is particularly urgent if
the damaged gene is being transcribed because RNA poly-
merase cannot transcribe through damaged DNA. Cells
therefore recruit their DNA repair machinery to such
genes in a process known as transcription-coupled repair
(TCR), which operates only on the DNA strand that is be-
ing transcribed (lesions on the complementary strand are
repaired at normal rates). For example, pyrimidine dimers
are more rapidly removed from transcribed portions of
DNA than from unexpressed sequences.
Section 30-5. Repair of DNA 1217
Figure 30-58 The mechanism of nucleotide excision repair
(NER) of pyrimidine photodimers.
5′
3′
3′
5′
UvrABC endonuclease
UvrD
Pol I, DNA ligase
P P
P
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1217
Cockayne syndrome (CS) is an inherited disease caused
by defective TCR. Individuals with CS are hypersensitive to
UV radiation (although they have a normal incidence of
skin cancer) and exhibit stunted growth as well as neuro-
logical dysfunction due to neuron demyelination leading to
death in childhood. CS is most often caused by mutations
in two complementation groups, CSA and CSB, although
certain defects in XPB, XPD, and XPG can also cause CS
in addition to XP.
The retarded development typical of XPB and XPD de-
fects and perhaps the demyelination that occurs in CS appear
to be due more to impaired transcription than to defective
NER. This is explained by the fact that the DNA helicases
XPB and XPD are subunits of the ten-subunit eukaryotic
transcription factor TFIIH, whose proper functioning is re-
quired for transcription initiation by RNA polymerase II
(Section 34-3Bb) as well as NER and TCR. A eukaryotic
RNA polymerase that is stalled at a DNA lesion is recog-
nized by CSB, which then recruits the other TCR compo-
nents. CSA interacts with both CSB and the p44 subunit of
TFIIH. Once the damage has been repaired, the RNA poly-
merase resumes transcription.Thus, in CS, RNA polymerase
molecules become permanently stalled on DNA lesions.
TCR also occurs in bacteria. For example, in E. coli, the
protein TCRF (for transcription repair couping factor; also
known as Mfd protein, for mutation frequency decline) is
an ATP-powered DNA translocase that displaces stalled
RNA polymerase from the damaged template strand, fol-
lowing which TCRF recruits the proteins of the UvrABC
system to the damage site. The repaired gene is then tran-
scribed from its beginning. Since prokaryotic genes are
much shorter than eukaryotic genes (most of which con-
tain several long noncoding segments known as introns;
Section 31-4Ac), this is a more efficient use of resources
than generating the complex machinery necessary to
restart transcription as occurs in eukaryotes.
c. Base Excision Repair
DNA bases are modified by reactions that occur under
normal physiological conditions as well as through the action
of environmental agents. For example, adenine and cytosine
residues spontaneously deaminate at finite rates to yield hy-
poxanthine and uracil residues, respectively. S-Adenosylme-
thionine (SAM), a common metabolic methylating agent
(Section 26-3Ea), occasionally nonenzymatically methylates
a base to form derivatives such as 3-methyladenine and 7-
methylguanine residues (Fig. 30-54). Ionizing radiation can
promote ring opening reactions in bases. Such changes mod-
ify or eliminate base pairing properties.
DNA containing a damaged base may be restored to its
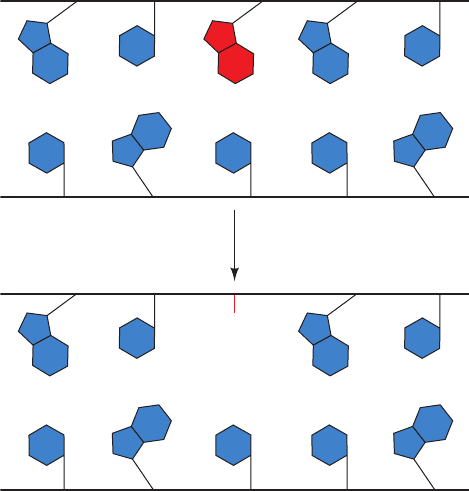
native state through base excision repair (BER). Cells con-
tain a variety of DNA glycosylases that each cleave the gly-
cosidic bond of a corresponding specific type of altered nu-
cleotide (Fig. 30-59), thereby leaving a deoxyribose residue
in the backbone. Such apurinic or apyrimidinic (AP) sites
(also called abasic sites) are also generated under normal
physiological conditions by the spontaneous hydrolysis of a
glycosidic bond. The deoxyribose residue is then cleaved
on one side by an AP endonuclease, the deoxyribose and
several adjacent residues are removed by the action of a
cellular exonuclease (possibly associated with a DNA poly-
merase), and the gap is filled in and sealed by a DNA poly-
merase and DNA ligase.
d. Uracil in DNA Would Be Highly Mutagenic
For some time after the essential functions of nucleic
acids had been elucidated, there seemed no apparent reason
for nature to go to the considerable metabolic effort of using
thymine in DNA and uracil in RNA when these substances
have virtually identical base pairing properties. This enigma
was solved by the discovery of cytosine’s penchant for con-
version to uracil by deamination, either via spontaneous hy-
drolysis (Fig. 30-54), which is estimated to occur ⬃120 times
per day in each human cell, or by reaction with nitrites (Sec-
tion 32-1Aa). If U were the normal DNA base, the deamina-
tion of C would be highly mutagenic because there would be
no indication of whether the resulting mismatched G ⴢ U
base pair had originally been G ⴢ C or A ⴢ U. Since T is DNA’s
normal base, however, any U in DNA is almost certainly a
deaminated C. U’s that occur in DNA are efficiently excised
by uracil–DNA glycosylase [UDG; also called uracil N-gly-
cosylase (UNG)] and then replaced by C through BER.
UDG also has an important function in DNA replica-
tion. dUTP, an intermediate in dTTP synthesis, is present in
all cells in small amounts (Section 28-3Ba). DNA poly-
merases do not discriminate well between dUTP and dTTP
(recall that DNA polymerases select a base for incorpora-
tion into DNA according to its ability to form a
Watson–Crick-shaped base pair with the template; Section
30-2Aa) so that, despite the low dUTP level that cells main-
tain, newly synthesized DNA contains an occasional U.
These U’s are rapidly replaced by T through BER. How-
ever, since excision occurs more rapidly than repair, all
1218 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-59 Action of DNA glycosylases. These enzymes
hydrolyze the glycosidic bond of their corresponding altered
base (red) to yield an AP site.
OH
DNA glycosylase
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1218
newly synthesized DNA is fragmented.When Okazaki frag-
ments were first discovered (Section 30-1C), it therefore
seemed that all DNA was synthesized discontinuously.This
ambiguity was resolved with the discovery of E. coli defec-
tive in UDG. In these ung
⫺
mutants, only about half of the
newly synthesized DNA is fragmented, strongly suggesting
that DNA’s leading strand is synthesized continuously.
e. Uracil–DNA Glycosylase Induces Uridine
Nucleotides to Flip Out
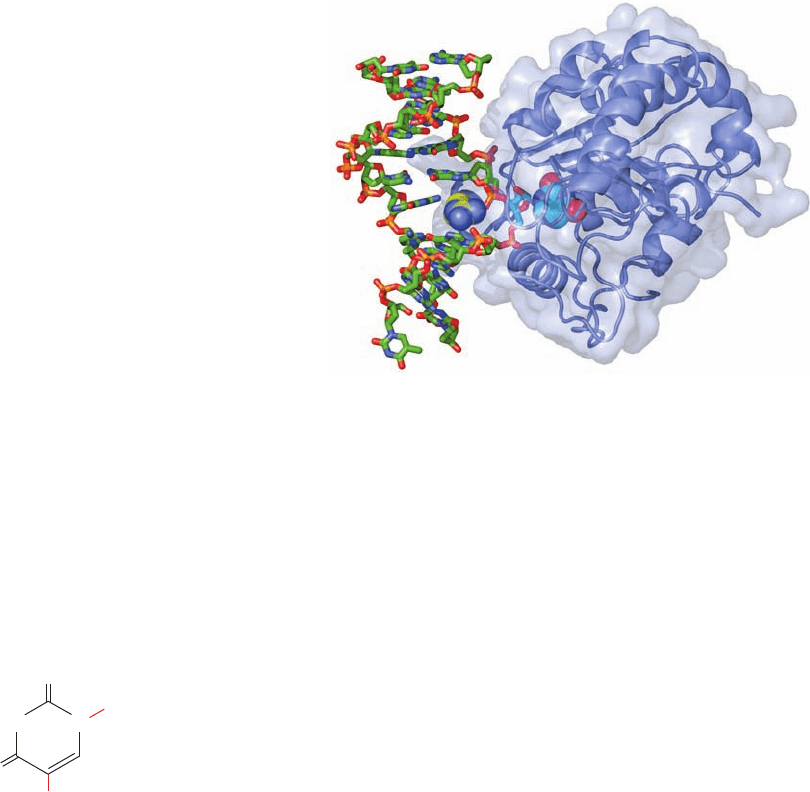
The X-ray structure of human UDG in complex with a
10-bp DNA containing a U ⴢ G mismatch (which can form a
doubly hydrogen-bonded base pair whose shape differs
from that of Watson–Crick base pairs; Section 32-2Db),
determined by John Tainer,reveals that the UDG has bound
the DNA with its uridine nucleotide flipped out of the ds-
DNA (Fig. 30-60). Moreover, the enzyme has hydrolyzed
uridine’s glycosidic bond yielding the free uracil base and an
AP site on the DNA, although both remain bound to the en-
zyme. The cavity in the DNA’s base stack that would other-
wise be occupied by the flipped-out uracil is filled by the side
chain of Arg 272, which intercalates into the DNA from its
minor groove side. The X-ray structure of a similar complex
in which the U ⴢ G mismatch was replaced by a U ⴢ A base
pair contained essentially identical features. However, when
the U in the U ⴢ A-containing complex was replaced by
pseudouridine (in which the “glycosidic” bond is made to
uracil’s C5 atom rather than to N1),
the uracil remained covalently linked to the DNA because the
UDG could not hydrolyze its now C—C “glycosidic” bond.
How does UDG detect a base-paired uracil in the center
of DNA and how does it discriminate so acutely between
uracil and other bases, particularly the closely similar
thymine? The above X-ray structures indicate that the phos-
phate groups flanking the flipped-out nucleotide are 4 Å
closer together than they are in B-DNA (8 Å vs 12 Å), which
causes the DNA to kink by ⬃45° in the direction parallel to
the view in Fig. 30-60.These distortions arise from the bind-
ing of three rigid protein loops to the DNA, which would be
unable to simultaneously bind to undistorted B-DNA. This
led Tainer to formulate the “pinch–push–pull” mechanism
for uracil detection in which he postulated that UDG rap-
idly scans a DNA for uracil by periodically binding to it so as
to compress and thereby slightly bend the DNA’s backbone
(pinch).The DNA’s presumed low resistance to bending at a
uracil-containing site (a U ⴢ G base pair is smaller than C ⴢ G
and hence leaves a space in the base stack, whereas a U ⴢ A
base pair is even weaker than T ⴢ A) permits the enzyme to
flip out the uracil by intercalating Arg 272 into the minor
groove (push), thereby fully bending and kinking the DNA.
O
Pseudouridine
H
O
Ribose
NN
This process is aided by the tight binding of the flipped-out
uracil to the enzyme (pull). The exquisite specificity of this
binding pocket for uracil prevents the binding and hence hy-
drolysis of any other base that the enzyme may have induced
to flip out. Thus the overall shapes of adenine and guanine
exclude them from this pocket, whereas thymine’s 5-methyl
group is sterically blocked by the rigidly held side chain of
Tyr 147. Cytosine, which has approximately the same shape
as uracil, is excluded through a set of hydrogen bonds ema-
nating from the protein that mimic those made by adenine in
a Watson–Crick A ⴢ U base pair.
AP sites in DNA are highly cytotoxic because they irre-
versibly trap mammalian topoisomerase I in its covalent
complex with DNA (Section 29-3Ca). Moreover, since the
ribose at the AP site lacks a glycosidic bond, it can readily
convert to its linear form (Section 11-1B), whose reactive
aldehyde group can cross-link to other cell components.
This rationalizes why AP sites remain tightly bound to
UDG in solution as well as in crystals. UDG activity is en-
hanced by AP endonuclease, the next enzyme in the BER
pathway, but the two enzymes do not interact in the absence
of DNA. This suggests that UDG remains bound to an AP
site it generated until it is displaced by the more tightly
binding AP endonuclease, thereby protecting the cell from
the AP site’s cytotoxic effects. It seems likely that other
damage-specific DNA glycosylases function similarly.
Section 30-5. Repair of DNA 1219
Figure 30-60 X-ray structure of human uracil–DNA glycosylase
(UDG) in complex with a 10-bp DNA containing a U ⴢ G base
pair. The protein (the C-terminal 223 residues of the 304-residue
monomer) is represented by its ribbon diagram embedded in its
transparent molecular surface. The DNA, viewed looking into its
major groove, is drawn in stick form colored according to atom
type (C green, N blue, O red, P orange). The U ⴢ G base pair’s
uridine nucleotide has flipped out of the double helix (to the right
of the DNA) and has been hydrolyzed to yield an AP nucleotide
(stick form with C cyan) and uracil (space-filling form with
C cyan), which remains bound in the enzyme’s binding pocket.
The side chain of Arg 272 (space-filling form with C yellow) has
intercalated into the DNA base stack to fill the space vacated by
the flipped-out uracil base. [Based on an X-ray structure by John
Tainer,The Scripps Research Institute, La Jolla, California. PDBid
4SKN.]
JWCL281_c30_1173-1259.qxd 9/2/10 9:05 AM Page 1219
C. Mismatch Repair
Any replicational mispairing that has eluded the editing
functions of the various participating DNA polymerases
may still be corrected by a process known as mismatch re-
pair (MMR). For example, E. coli Pol I and Pol III have er-
ror rates of 10
⫺6
to 10
⫺7
per base pair replicated but the ob-
served mutational rates in E. coli are 10
⫺9
to 10
⫺10
per base
pair replicated. In addition, the MMR system can correct
insertions or deletions of up to 4 nt (which arise from the
slippage of one strand relative to the other in the active site
of DNA polymerase). The importance of MMR is indi-
cated by the fact that defects in the human MMR system
result in a high incidence of cancer, most notably heredi-
tary nonpolyposis colorectal cancer (HNPCC; which af-
fects several organs and may be the most common inher-
ited predisposition to cancer).
If an MMR system is to correct errors in replication
rather than perpetuate them, it must distinguish the
parental DNA, which has the correct base, from the daugh-
ter strand, which has an incorrect although normal base. In
E. coli, as we have seen (Section 30-3C), this is possible be-
cause newly replicated GATC palindromes remain
hemimethylated until the Dam methyltransferase has had
sufficient time to methylate the daughter strand.
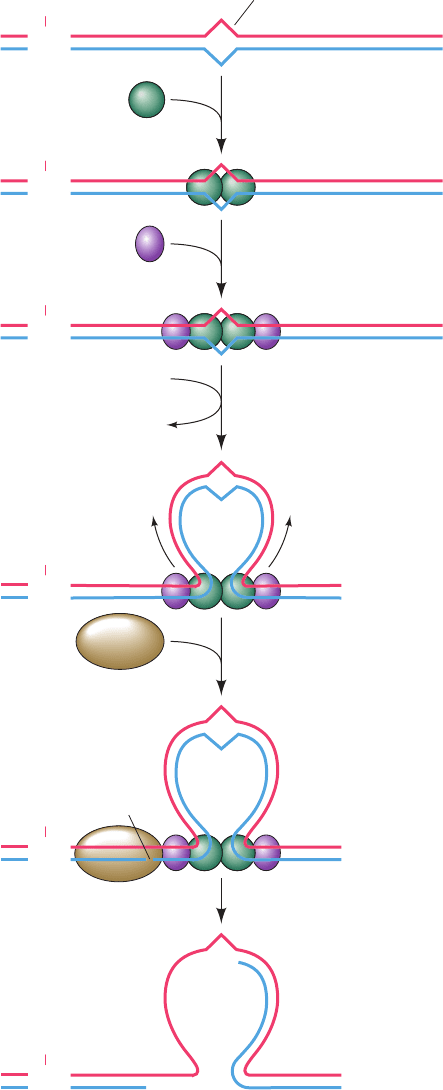
E. coli mismatch repair, which was elucidated in large
part by Paul Modrich, requires the participation of three
proteins and occurs as follows (Fig. 30-61):
1. MutS (853 residues) binds to a mismatched base pair
or unpaired bases as a homodimer.
2. The MutS–DNA complex binds MutL (615 residues),
also as a homodimer.
3. The MutS–MutL complex translocates along the
DNA in both directions, thereby forming a loop in the
DNA.The translocation appears to be driven by the ATPase
function of MutS.
4. On encountering a hemimethylated GATC palin-
drome, the MutS–MutL complex recruits MutH (228
residues) and activates this single-strand endonuclease to
make a nick on the 5¿ side of the unmethylated GATC. This
GATC may be located on either side of the mismatch and
over 1000 bp distant from it, although repair efficiency de-
creases with the distance between the nick and the mismatch.
5. MutS–MutL recruits UvrD helicase, which in concert
with an exonuclease separates the strands and degrades the
nicked strand from the nick to beyond the mismatch. If the
nick is on the 3¿ side of the mismatch as shown, the exonucle-
ase is exonuclease I (a 3¿S5¿ exonuclease), whereas if the
nick is on the 5¿ side of the mismatch, the exonuclease can be
either RecJ or exonuclease VII (both 5¿S3¿ exonucleases).
The resulting gap is filled in by Pol III and sealed by DNA
ligase, thereby correcting the mismatch. MutL is also an
ATPase, which, it is postulated, functions to coordinate the
various steps of mismatch repair.
Eukaryotic MMR systems are, not surprisingly, more
complicated than those of E. coli. Eukaryotes express six
homologs of MutS and five homologs of MutL that form
heterodimers on mismatched DNA. However,homologs of
MutH only occur in gram-negative bacteria. Eukaryotes
must have some other way of differentiating the parental
and daughter DNA strands. Perhaps a newly synthesized
daughter strand is identified by its as-yet unsealed nicks.
DNA resynthesis is probably mediated by pol ␦.
1220 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-61 The mechanism of mismatch repair in E. coli.
Mismatch
Parental DNA
Daughter DNA
GATC
CH
3
CTAG
5'
3'
MutS
3'
5'
Cleavage
GATC
CH
3
CTAG
5'
3'
3'
5'
GATC
CH
3
CTAG
5'
3'
3'
5'
GATC
CH
3
CTAG
5'
3'
3'
5'
1
MutL
2
MutH
4
5
3
ATP
UvrD + exonuclease
ATP + P
i
GATC
CH
3
CTAG
5'
3'
3'
5'
GATC
CH
3
CTAG
5'
3'
3'
3'
5'5'
JWCL281_c30_1173-1259.qxd 8/10/10 9:12 PM Page 1220
