Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

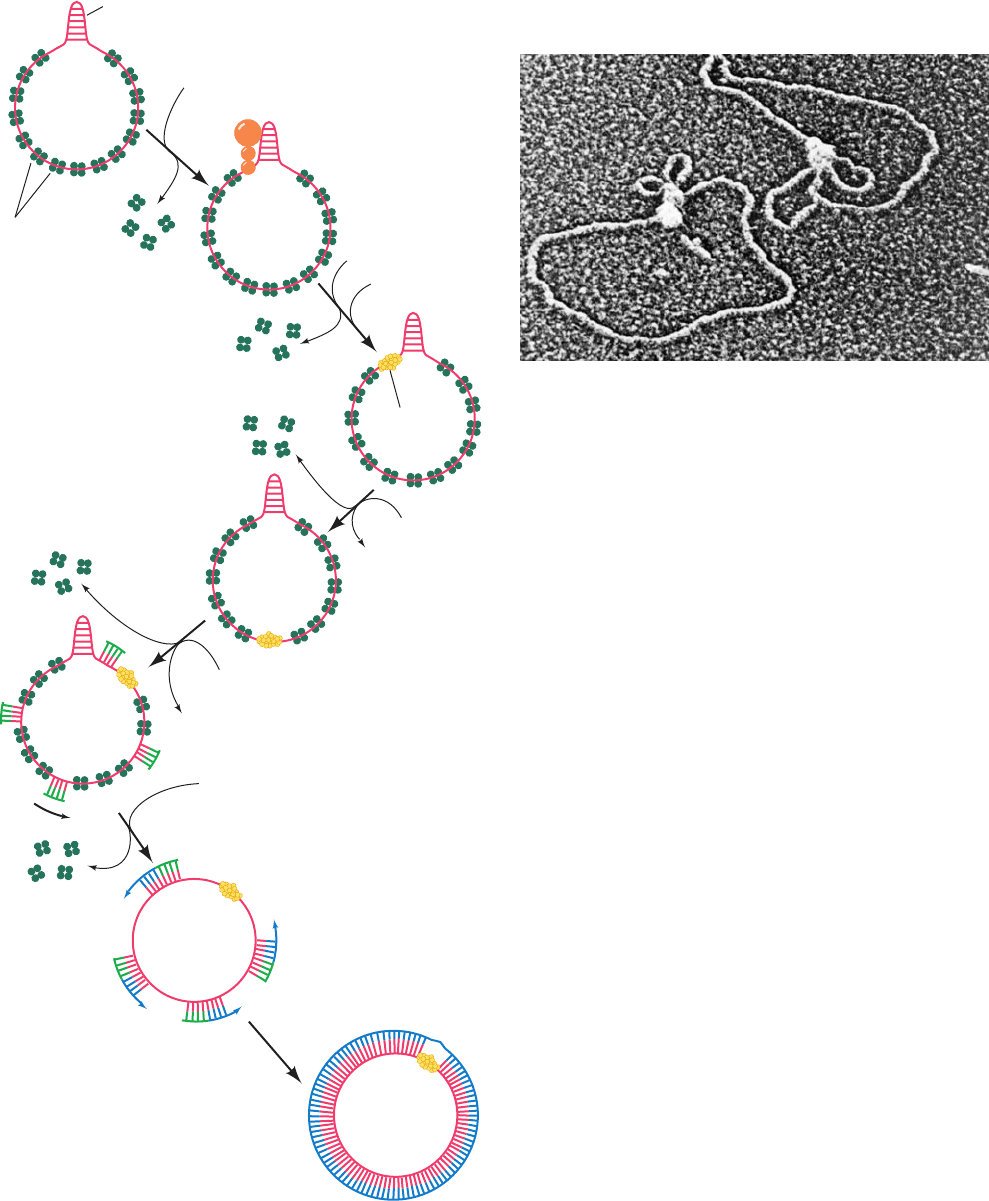
3. The primosome is propelled in the 5¿S3¿ direction
along the (⫹) strand by the PriA and DnaB helicases at the
expense of ATP hydrolysis. This motion, which displaces
the SSB in its path, is opposite in direction to that of tem-
plate reading during DNA chain propagation.
4. At randomly selected sites, the primosome reverses
its migration while primase synthesizes an RNA primer.
The initiation of primer synthesis requires the participation
of DnaB protein which, through concomitant ATP hydrol-
ysis, is thought to alter template DNA conformation in a
manner required by primase.
5. Pol III holoenzyme extends the primers to form
Okazaki fragments.
6. Pol I excises the primers and replaces them by DNA.
The fragments are then joined by DNA ligase and super-
coiled by DNA gyrase to form the X174 RF I.
The primosome remains complexed with the DNA (Fig.30-25)
where it participates in (⫹) strand synthesis (see below).
b. X174 (ⴙ) Strand Replication Serves as a Model
for Leading Strand Synthesis
One strand of a circular duplex DNA may be synthe-
sized via the rolling circle or -replication mode (so called
because of the resemblance of the replicating structure to
the Greek letter sigma; Fig. 30-26). The X174 (⫹) strand is
synthesized on an RF I template by a variation on this
process, the looped rolling circle mode (Fig. 30-27):
1. (⫹) strand synthesis begins with the primosome-
aided binding of the phage-encoded 513-residue enzyme
gene A protein to its ⬃30-bp recognition site. There, gene
A protein cleaves a specific phosphodiester bond on the
(⫹) strand nucleotide (near the beginning of gene A) by
forming a covalent bond between a Tyr residue and the
DNA’s 5¿-phosphoryl group, thereby conserving the
cleaved bond’s energy.
2. Rep helicase (Section 30-2Cb) subsequently attaches
to the (⫺) strand at the gene A protein and, with the aid of
Section 30-3. Prokaryotic Replication 1191
Figure 30-24 The synthesis of the X174 (⫺) strand on a (⫹)
strand template to form X174 RF I DNA. [After Arai, K., Low,
R., Kobori, J., Schlomai, J., and Kornberg, A., J. Biol. Chem. 256,
5280 (1981).]
Figure 30-25 Electron micrograph of a primosome bound to a
X174 RF I DNA. Such complexes always contain a single
primosome with one or two associated small DNA loops.
[Courtesy of Jack Griffith, Lineberger Cancer Research Center,
University of North Carolina.]
Single-strand
binding protein
(SSB)
1. Recognition
+ DnaC
(DnaB)
6
• (DnaC)
6
,
DnaT + ATP
ADP + P
i
ADP
RFII
RFI
ATP
+ 4rNTPs
5'
3'
DNA polymerase III
holoenzyme, 4dNTPs
Pol I, ligase,
gyrase, 4dNTPs,
NAD
+
, ATP
ATP
Primase
Primasome
PriA, PriB, PriC
pas
2. Assembly
3. Migration
4. Priming
5. Elongation
6. Excision,
Gap filling,
ligation,
supercoiling
PriA
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1191
the primosome still associated with the (⫹) strand, com-
mences unwinding the duplex DNA from the (⫹) strand’s
5¿ end. The displaced (⫹) strand is coated with SSB, which
prevents it from reannealing to the (⫺) strand. Rep heli-
case is essential for the replication of X174 DNA, but not
for the E. coli chromosome, as is demonstrated by the in-
ability of X174 to multiply in rep
⫺
E. coli. Pol III holoen-
zyme extends the (⫹) strand from its free 3¿-OH group.
1192 Chapter 30. DNA Replication, Repair, and Recombination
Figure 30-26 The rolling circle mode of DNA replication. The
(⫹) strand being synthesized is extended from a specific cut
made at the replication origin (1) so as to strip away the old (⫹)
strand (2 and 3). The continuous synthesis of the (⫹) strand on a
circular (⫺) strand template produces a series of tandemly
linked (⫹) strands (4), which may later be separated by a specific
endonuclease.
Origin
(+)(–)
Replicative
form
Origin
1
2
3
4
5′
5′
5′
5′
3′
(+)
(+)
(+)
(+)
(–)
(+)
(+)
(–)
(–)
(+)
(–)
RF with
associated primosome
I
Origin
3'
5'
Gene A protein
(–) (+)
1 Gene A protein
2
Rep, SSB,
Pol ,
III
3'
3
SSB
3'
4
(+)
(–)
(+)
etc.
Rep
Figure 30-27 The synthesis of the X174 (⫹)
strand by the looped rolling circle mode. The
numbered steps are described in the text.
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1192
Section 30-3. Prokaryotic Replication 1193
Leading strand
(a)
(b)
DNA polymerase III
holoenzyme
5′
3′
5′
3′
Lagging strand
Parental strand
Primosome
RNA primer
Growing Okazaki
fragment
5′
3′
5′
3′
Primosome making
new RNA primer
5′
3′
RNA primer to be replaced
with DNA by Pol
nick sealed by DNA ligase
I;
Completed Okazaki fragment
5′
3′
(c)
5′
3′
Newly
initiated
Okazaki fragment
Old Okazaki
fragment
5′
3′
5′
3′
DnaB helicase
SSB
Sliding clamp
τ
2
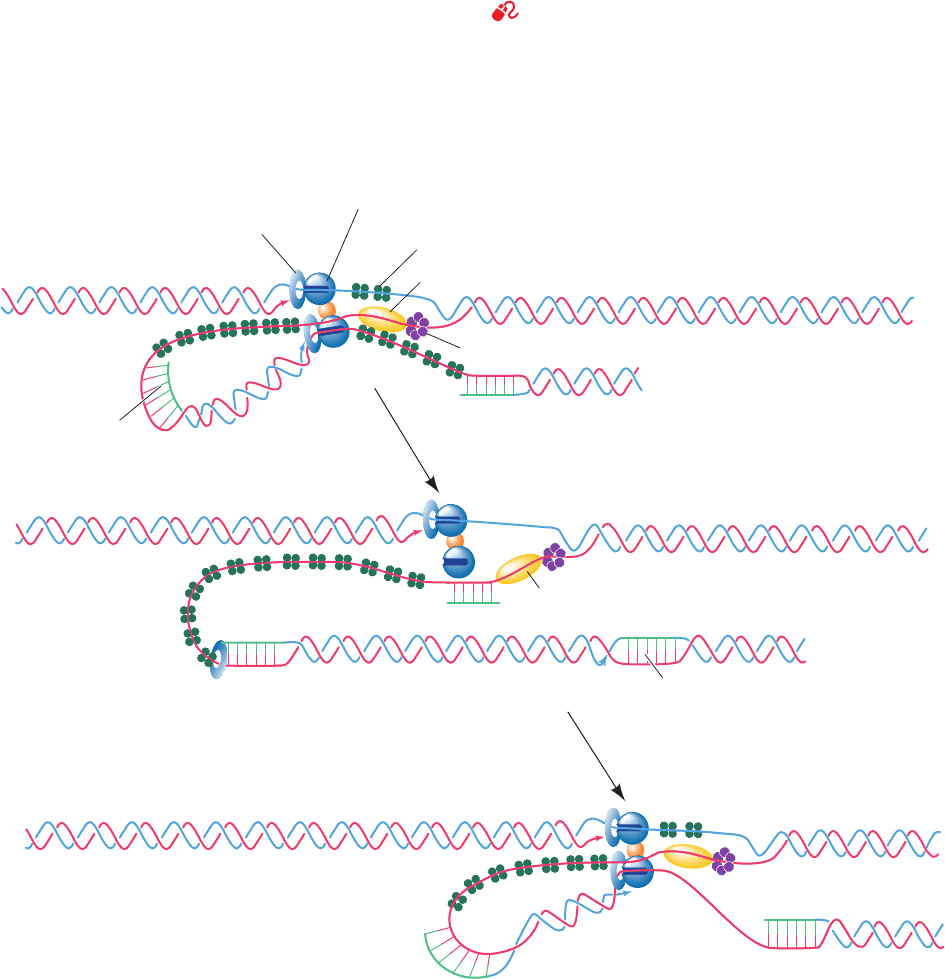
Figure 30-28 The replication of E. coli DNA. (a) The
E. coli DNA replisome, which contains two DNA
polymerase III holoenzyme complexes, synthesizes both
the leading and the lagging strands.The lagging strand
template must loop around to permit the holoenzyme to
extend the primosome-primed lagging strand.Although not
shown here, the DnaB helicase binds to
2
and hence moves with
the replisome. (b) The holoenzyme releases the lagging strand
template when it encounters the previously synthesized Okazaki
fragment.This possibly signals the primosome to initiate the
synthesis of lagging strand RNA primer. (c) The holoenzyme
rebinds the lagging strand template and extends the RNA primer
to form a new Okazaki fragment. Note that in this model, leading
strand synthesis is always ahead of lagging strand synthesis.
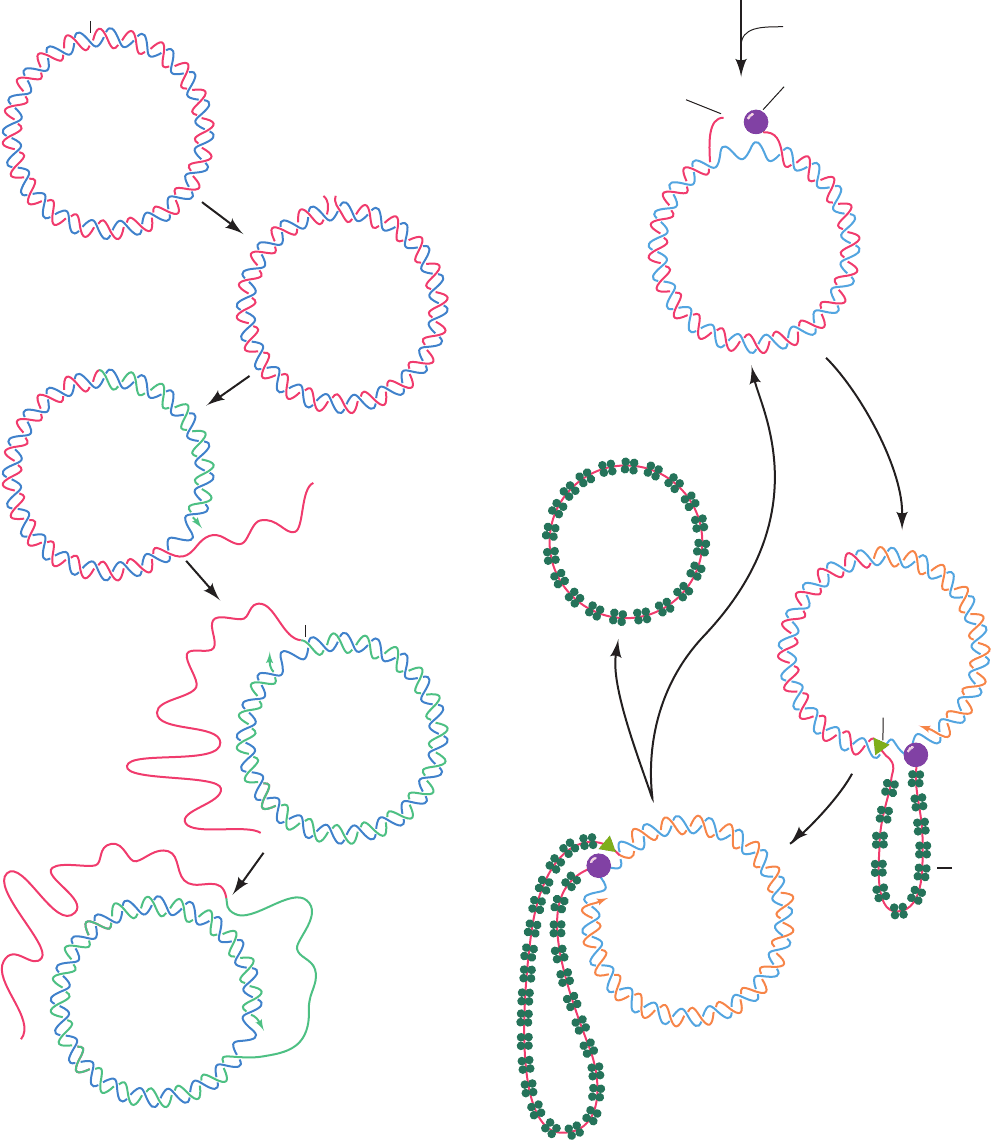
3. The extension process generates a looped rolling cir-
cle structure in which the 5¿ end of the old (⫹) strand re-
mains linked to the gene A protein at the replication fork.
It is thought that as the old (⫹) strand is peeled off the RF,
the primosome synthesizes the primers required for the
later generation of a new (⫺) strand.
4. When it has come full circle around the (⫺) strand,
the gene A protein again makes a specific cut at the repli-
cation origin so as to form a covalent linkage with the new
(⫹) strand’s 5¿ end. Simultaneously, the newly formed 3¿-
terminal OH group of the old, looped-out (⫹) strand nu-
cleophilically attacks its 5¿-phosphoryl attachment to the
gene A protein, thereby liberating a covalently closed (⫹)
strand.This is possible because the gene A protein has two
closely spaced Tyr residues that alternate in their attach-
ment to the 5¿ ends of successively synthesized (⫹) strands.
The replication fork continues its progress about the du-
plex circle, producing new (⫹) strands in a manner reminis-
cent of linked sausages being pulled off a reel.
In the intermediate stages of a X174 infection, each newly
synthesized (⫹) strand directs the synthesis of the (⫺)
strand to form RF I as described above. In the later stages
of infection, however, the newly formed (⫹) strands are
packaged into phage particles.
C. Escherichia coli
See Guided Exploration 25. The replication of DNA in E. coli The
E. coli chromosome replicates by the bidirectional mode from
a single replication origin (Section 30-1Aa).The most plausi-
ble model for events at the E. coli replication fork (Fig. 30-
28) is largely derived from studies on the simpler and more
experimentally accessible DNA replication mechanisms of
coliphages such as M13 and X174. Duplex DNA is un-
wound by DnaB helicase on the lagging strand template,
JWCL281_c30_1173-1259.qxd 8/27/10 7:27 PM Page 1193
1194 Chapter 30. DNA Replication, Repair, and Recombination
where it is joined by the primosome. The separated single
strands are immediately coated by SSB. Leading strand syn-
thesis is catalyzed by Pol III holoenzyme, as is that of the
lagging strand after priming by primosome-associated pri-
mase. Both leading and lagging strand syntheses occur on a
single ⬃900-kD multisubunit particle, the replisome, which
contains two Pol III cores (␣ε) that are joined together by
a dimer of subunits that bridges the ␣ subunits. Hence, the
lagging strand template must be looped around (Fig. 30-28).
The
2
dimer also binds the DnaB helicase (an interaction
that is not indicated in Fig. 30-28), thereby stimulating its
helicase action while holding it to the replication fork.After
completing the synthesis of an Okazaki fragment, the lag-
ging strand holoenzyme relocates to a new primer near the
replication fork, the primer heading the previously synthe-
sized Okazaki fragment is excised by Pol I-catalyzed nick
translation, and the nick is sealed by DNA ligase. Since lag-
ging strand synthesis is more complex and hence more time-
consuming than leading strand synthesis, the replisome func-
tions to coordinate these two processes.
a. E. coli DNA Replication Is Initiated at oriC in a
Process Mediated by DnaA Protein
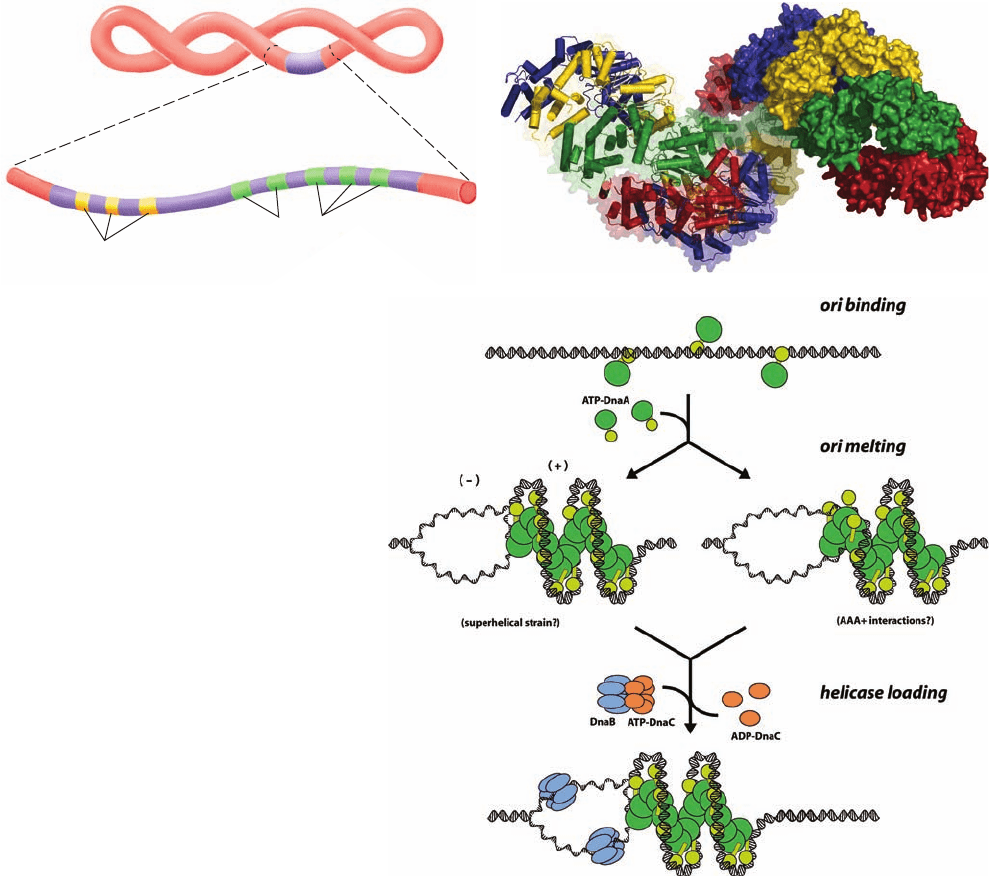
The replication origin of the E. coli chromosome consists
of a unique 250-bp segment known as the oriC locus.This se-
quence, segments of which are highly conserved among
gram-negative bacteria, supports the bidirectional replica-
tion of the various plasmids into which it has been inserted.
The oriC locus contains five highly conserved 9-bp seg-
ments with consensus sequence 5¿-TTATCCACA-3¿ known as
DnaA boxes because they are specifically bound by DnaA
protein (Fig. 30-29a).These are interspersed with several so-
called I-sites that deviate from this consensus sequence and
are bound by DnaA with lesser affinity. In addition, the
“left” boundary region of oriC contains three tandemly re-
peated, 13-bp, AT-rich segments (consensus sequence 5¿-
GATCTNTTNTTTT-3¿ where N marks nonspecific posi-
tions) that are known as DNA unwinding elements (DUEs).
DnaA (467 residues in E. coli) consists of four domains
that are, from N- to C-terminus, a helicase interaction domain
that mediates interactions with DnaB helicase (see below), a
Figure 30-29 DNA replication initiation at
oriC. (a) Diagram of oriC showing the relative
positions of its DnaA boxes (green) and its DNA
unwinding elements (DUEs; yellow). (b) X-ray
structure of the right-handed helical filament
formed by the two C-terminal domains of
A. aeolicus DnaA. It has eight subunits per turn
and a pitch of 178 Å.Twelve subunits are shown,
from right to left, in the alternating colors red,
green, yellow, and blue.The right subunits are
drawn as surface diagrams and the left subunits
are represented by their polypeptide back bones
with helices in tube form. (c) Model for initiation
at oriC.The green ovals represent the N-terminal
three domains of DnaA and the yellow ovals
represent the associated C-terminal DNA-binding
domains. See the text for an explanation. [Parts b
and c courtesy of James Berger, University of
California at Berkeley. PDBid 2HCB.]
Supercoiled
template
DnaA
boxes
13–bp
segments
oriC
(a)
(b)
(c)
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1194
35 0 min
Division
Initiation
Termination
Multiforked
chromosome
TerminusOrigin
30
25
20 15
10
5
of DnaA. The AAA⫹ domains of DnaA and DnaC interact
in an ATP-dependent manner to recruit and properly position
the DnaB helicases, following which the DnaC is released.
In the presence of SSB and gyrase, DnaB helicase fur-
ther unwinds the DNA in the prepriming complex in both
directions so as to permit the entry of primase and RNA
polymerase. The participation of both these enzymes in
leading strand primer synthesis (Section 30-1D), together
with the limitation of this process to the oriC site, suggests
that the RNA polymerase activates primase to synthesize
the primer. This perhaps explains the similarity of oriC’s
DUEs to RNA polymerase’s transcriptional promoters
(Section 31-2Ba). The stage is thereby set for bidirectional
DNA replication by Pol III holoenzyme as described above.
b. The Initiation of E. coli DNA Replication Is
Strictly Regulated
Chromosome replication in E. coli occurs only once per
cell division, so this process must be tightly controlled.The
doubling (cell generation) time of E. coli at 37°C varies with
growth conditions from ⬍20 min to ⬃10 h.Yet the constant
⬃1000 nt/s rate of movement of each replication fork fixes
the 4.6 ⫻ 10
6
-bp E. coli chromosome’s replication time, C,at
⬃40 min. Moreover,the segregation of cellular components
and the formation of a septum between them, which must
precede cell division, requires a constant time, D ⫽ 20 min,
after the completion of the corresponding round of chro-
mosome replication. Cells with doubling times less than C ⫹
D ⫽ 60 min must consequently initiate chromosome replica-
tion before the end of the preceding cell division cycle. This
results in the formation of multiforked chromosomes as is
diagrammed in Fig. 30-30 for a cell division time of 35 min.
Even in cells that contain multiple oriC sites, DNA
replication is initiated at each such site once and only once
per cell generation. However, after initiation has occurred,
chain elongation proceeds at a uniform, largely uncon-
trolled rate. This suggests that a post-initiation oriC site is
somehow sequestered from (prevented from interacting
with) the replication initiation machinery, a phenomenon
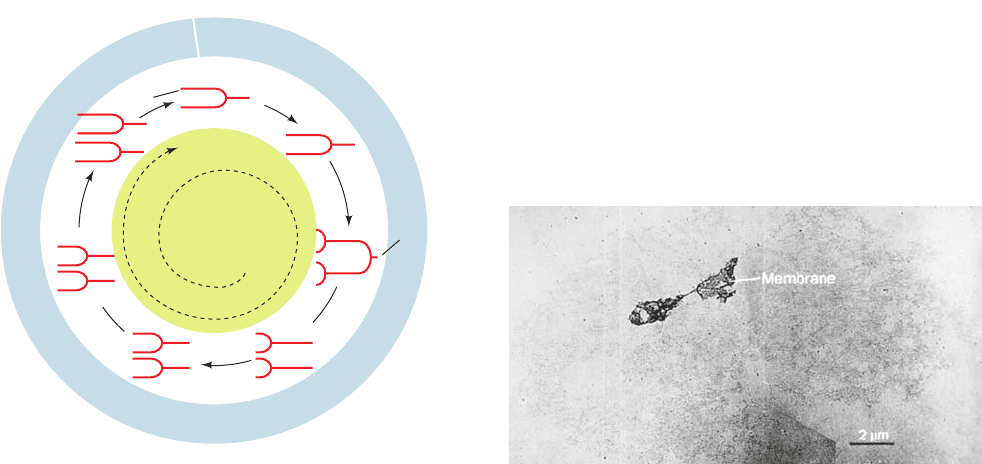
called sequestration. There is extensive morphological evi-
dence, such as shown in Fig. 30-31, that the E. coli chromo-
some is associated with the cell membrane.This attachment
flexible and poorly conserved linker, an ATPase domain that
is a member of the AAA⫹ family (Section 30-2Ca), and a
DNA binding domain.The X-ray structure of the C-terminal
two domains of DnaA from Aquifex aeolicus (a thermophilic
bacterium), determined by Berger, unexpectedly revealed
that it forms a multisubunit right-handed helix (Fig. 30-29b).
Experiments with oriC-containing plasmids, pioneered
by Kornberg, together with the X-ray structure of DnaA,
indicate that replication initiation in E. coli occurs via the
following process (Fig. 30-29c):
1. In the presence of ATP, DnaA, which is normally
bound to three of oriC’s five DnaA boxes throughout
E. coli’s lifetime, recruits additional DnaA subunits to the re-
maining DnaA boxes and to the I-sites so as to form a right-
handed helix of DnaA subunits that is bound to the DNA.
This generates local positive supercoils in the DNA.The su-
perhelical strain resulting from the compensating negative
supercoils (recall that the linking number of a covalently
closed circular DNA such as an E. coli chromosome is invari-
ant; Section 29-3A) melts the DUE-containing segment [Fig.
30-29c,middle left;recall that bacterial chromosomes are nor-
mally already negatively supercoiled (Section 29-2Bb)]. Al-
ternatively, or in addition, the DnaA’s ATPase domains may
actively unwind the DNA (Fig. 30-29c, middle right). This
process is facilitated by two homologous DNA-binding pro-
teins, HU and integration host factor (IHF), that induce
DNA bending (IHF is discussed in Section 33-3Ca).
2. The oriC–DnaA complex recruits two DnaB
6
ⴢ DnaC
6
complexes to opposite ends of the melted region to form the
prepriming complex. DnaC, an ATPase that is a homolog of
DnaA, functions to facilitate the loading of the DnaB hexam-
ers onto the DNA. Its, X-ray structure, also determined by
Berger, shows that it forms a helical assembly similar to that
Section 30-3. Prokaryotic Replication 1195
Figure 30-30 Multiforked chromosomes in E. coli. In cells
that are dividing every 35 min, the fixed 60-min interval between
the initiation of replication and cell division results in the
production of multiforked chromosomes. [After Lewin, B., Genes
VII, p. 370, Oxford University Press (2000).]
Figure 30-31 Electron micrograph of an intact and
supercoiled E. coli chromosome attached to two fragments of
the cell membrane. [From Delius, H. and Worcel, A., J. Mol. Biol.
82, 108 (1974).]
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1195
c. The Clamp Loader Loads the Sliding Clamp onto
the DNA
Extensive investigations in many laboratories have led
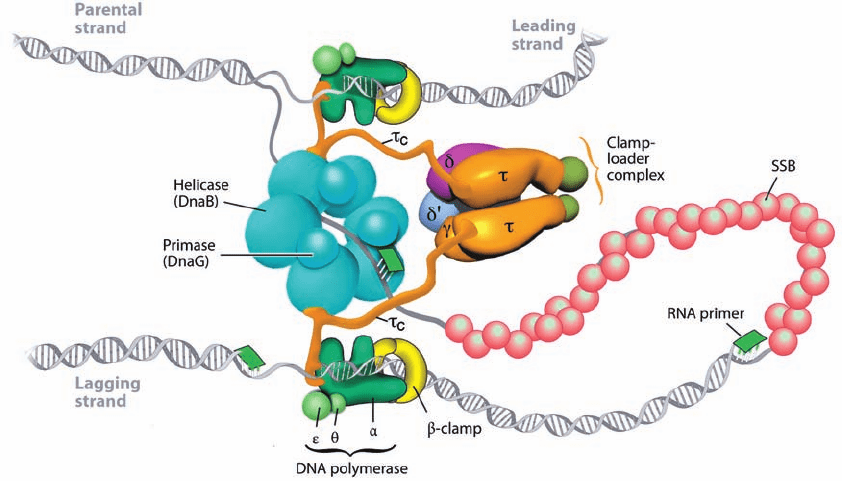
to the model of the E. coli replisome drawn in Fig. 30-32.
The sliding clamp, which is responsible for Pol III’s high
processivity, is a ring-shaped dimer of  subunits through
which the DNA strand being replicated is threaded (Sec-
tion 30-2Bb). The two tightly associated  subunits (K
D
⬍
50 nM) that form the sliding clamp dissociate with a half-
life of ⬃100 min at 37°C.Yet, since each replisome synthe-
sizes around one Okazaki fragment per second, a sliding
clamp must be loaded onto the lagging strand template at
this frequency. This loading function is carried out in an
ATP-dependent process by the ␥ complex (␥
2
␦␦¿⌿). The
and ␥ subunits are both encoded by the dnaX gene with
(643 residues) the full-length product and ␥ (431 residues)
its C-terminally truncated form; the C-terminal 122
residues of are known as
c
.The ␥ complex, of which only
2
is diagrammed in Fig. 30-28, bridges the replisome’s two
Pol III cores via its
c
segments, which also bind the DnaB
helicase (Fig. 30-32). The and ⌿ subunits form a het-
erodimer in which competes with primase for its binding
site on SSB and hence functions to accelerate the dissocia-
tion of primase from the RNA primer it synthesized as well
as link the ␥ complex to SSB. However, and ⌿ are not es-
sential participants in the clamp loading process and, there-
fore, we shall refer to the ␥
2
␦␦¿ complex as the clamp
loader. How does the clamp loader do its job?
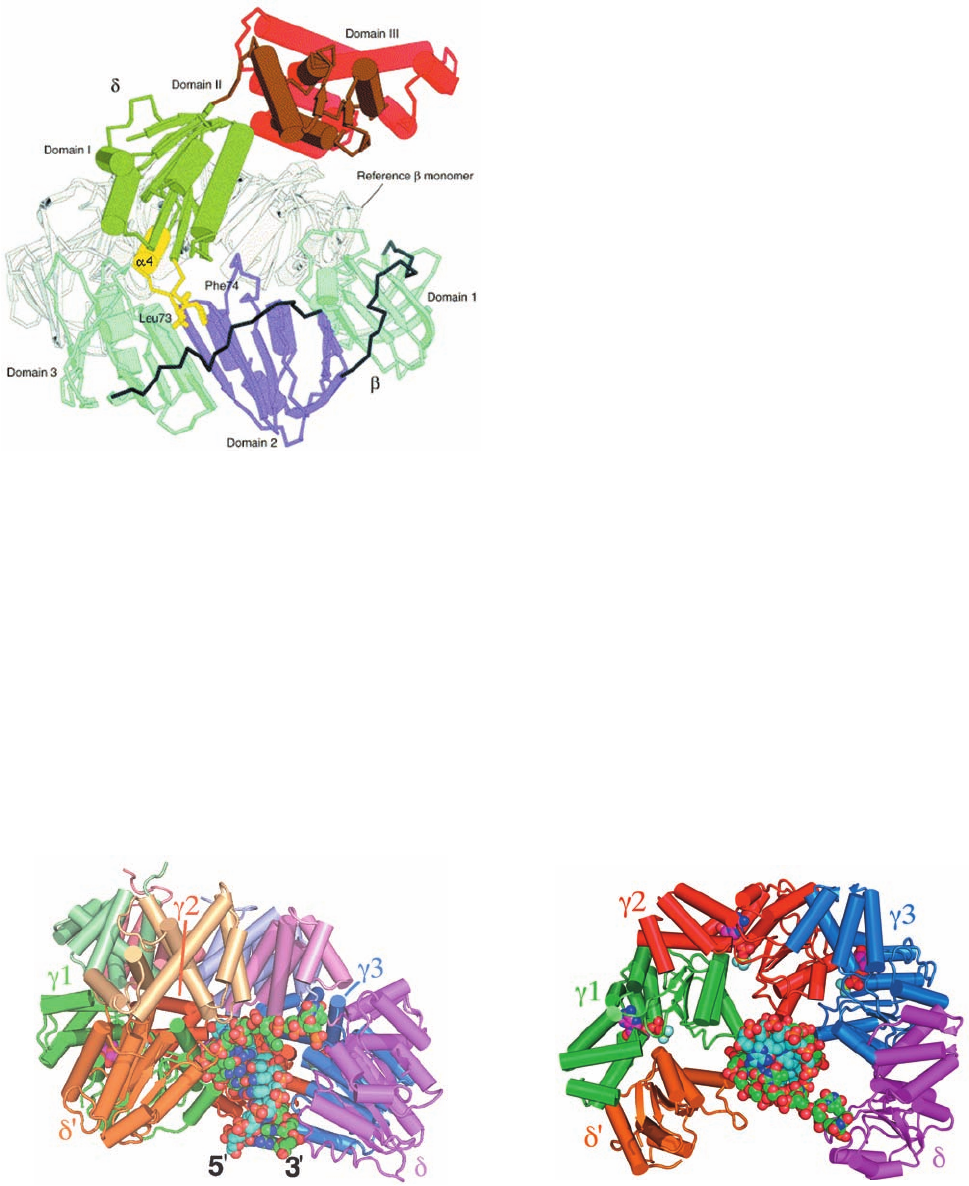
Of the clamp loader’s five subunits, only ␦ is capable of
binding to and opening up the sliding clamp on its own.
Kuriyan and O’Donnell determined the X-ray structure of the
␦ subunit in 1:1 complex with a  subunit that had two residues
in its dimerization interface mutated so as to prevent its dimer-
ization.The structure reveals (Fig. 30-33) that ␦, which consists
of three domains, inserts its  interaction element,a hydropho-
1196 Chapter 30. DNA Replication, Repair, and Recombination
would help explain how replicated chromosomes are seg-
regated into different cells during cell division. But what is
the mechanism of sequestration?
The sequence most commonly methylated in E. coli is
the palindrome GATC, which is methylated at N6 of both
its A bases by Dam methyltransferase (Section 30-7).
GATC occurs 11 times in oriC, including at the beginning of
all four of its 13-bp DUEs (see above). Newly replicated
GATC segments are hemimethylated, that is, the GATC
sequences on the newly synthesized strand are unmethyl-
ated. Although Dam methyltransferase begins methylat-
ing most hemimethylated GATC segments immediately
after their synthesis (within ⬃1.5 min), those on oriC re-
main hemimethylated for around one-third of a cell gener-
ation. Consequently, the observation that membranes bind
hemimethylated oriC, but not unmethylated or fully methyl-
ated oriC, suggests that hemimethylated oriC is bound to
the membrane in a way that makes it inaccessible to both
the initiation machinery and Dam methyltransferase.
The association of hemimethylated oriC with mem-
brane requires the presence of the 181-residue SeqA pro-
tein, the product of seqA gene. Thus in seqA
⫺
cells: (1) the
time to fully methylate hemimethylated GATC sites in
oriC is reduced to 5 min, whereas the time to do so for
other GATC sites is unaffected; (2) the synchrony of initia-
tion of multiple oriC sites is lost; and (3) in the absence of
functional Dam methyltransferase, fully methylated oriC-
containing plasmids are replicated numerous times per cell
generation, whereas in the presence of SeqA they are repli-
cated only once. Evidently, sequestration occurs via the
SeqA-mediated binding of hemimethylated oriC to the
membrane. The hemimethylated promoter of the dnaA
gene is similarly sequestered so as to repress its transcrip-
tion, thereby providing an additional mechanism for pre-
venting promiscuous initiation of DNA replication.
Figure 30-32 Architecture of the E. coli replisome. See the text for details. Compare
this to Fig. 30-28. [Courtesy of Charles Richardson, Harvard Medical School.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1196
Section 30-3. Prokaryotic Replication 1197
bic plug that forms the tip of its N-terminal domain, into a hy-
drophobic pocket on one face of . Comparison of ␦ in this
structure with that in the ␥
3
␦␦¿ complex (see below) reveals
that the  interaction element undergoes a dramatic confor-
mational change on binding to  in which its ␣4 helix rotates
by 45° and translates by 5.5 Å. Moreover,in forming the –␦
complex, the  subunit increases its radius of curvature rela-
tive to that in the  dimer (Fig. 30-13) such that the –␦ in-
teraction would induce the opening of one of the sliding
clamp’s – interfaces by ⬃15 Å. Such a gap is large enough
to permit the passage of ssDNA but not dsDNA. Appar-
ently, the clamp loader functions by trapping one  subunit
of the sliding clamp in a conformation that prevents ring clo-
sure rather than actively pulling apart the two halves of the
ring. This is corroborated by molecular dynamics simula-
tions (Section 9-4a) suggesting that a 
2
dimer has a stable
conformation but that an isolated  subunit with the confor-
mation it has in the 
2
dimer rapidly (in ⬃1.5 ns) converts to
a conformation resembling that in the –␦ complex. Thus,
the conformational change of the ␦ subunit’s  interaction
element on binding to a  subunit is reminiscent of the ac-
tion of a plumber’s wrench in unlatching the nearby – in-
terface so as to allow the sliding clamp to spring open.
The X-ray structure of the ␥
3
␦␦¿ complex (the clamp
loader with both its subunits lacking
c
; ␥ and are inter-
changeable in terms of their clamp loading functions) in
complex with a primer–template DNA and ADP ⴢ BeF
3
(an
ATP analog), also determined by Kuriyan and O’Donnell,
suggests how the clamp loader functions. The ␥, ␦, and ␦¿
subunits all have similar folds; their N-terminal domains
are all members of the widely distributed AAA⫹ family
(DnaA and DnaC proteins are also members of this fam-
ily) even though only the ␥ (and ) subunits bind and hy-
drolyze ATP. The conserved regions of AAA⫹ proteins
consist of two domains, an N-terminal ATP-binding do-
main and a smaller domain composed of a 3-helix bundle,
whose relative orientations vary with ATP binding. The
␥
3
␦␦¿ pentamer’s C-terminal domains form a ring-shaped
collar (Fig. 30-34a) in which the subunits are arranged in
clockwise order ␦¿–␥1–␥2–␥3–␦ (Fig. 30-34b). The AAA⫹
domains are arranged in a right-handed spiral that tracks
the minor groove of the dsDNA. Nevertheless, the clamp
Figure 30-33 X-ray structure of the –␦ complex. A second 
subunit taken from the X-ray structure of the sliding clamp (Fig.
30-13), the “Reference  monomer,” is drawn in gray. The view is
along the edge of the  ring.The ␦ subunit’s  interaction
element (yellow) consists largely of the ␣4 helix and two
hydrophobic residues, Leu 73 and Phe 74, whose side chains are
drawn in stick form. [Courtesy of John Kuriyan, University of
California at Berkeley. PDB 1JQJ.]
Figure 30-34 X-ray structure of the ␥
3
␦␦ⴕ clamp loader in
complex with a primer–template DNA and ADP ⴢ BeF
3
. (a)
View between the ␦¿ and ␦ subunits approximately perpendicular
to the dsDNA’s helical axis. The protein is drawn in tube-and-
arrow form with its subunits colored as indicated and with the
C-terminal domain of each subunit a lighter shade. The DNA
consists of 10 bp with a 5¿ overhang of 5 nt and, together with the
ADP ⴢ BeF
3
, is drawn in space-filling form with primer C cyan,
template C green,ADP C magenta, N blue, O red, and P orange,
Be light green, and F light blue. (b) View rotated 90° about the
horizontal axis relative to Part a.The C-terminal domain of each
subunit has been deleted for clarity. Note how the single-
stranded portion of the template strand turns by ⬃90° to avoid
colliding with the collar formed by the clamp loader’s C-terminal
domains.The stiffness of dsDNA makes it unlikely that it could
make such a turn. [Based on an X-ray structure by Mike
O’Donnell,The Rockefeller University, and John Kuriyan,
University of California at Berkeley. PDBid 3GLF.]
(a)
(b)
JWCL281_c30_1173-1259.qxd 9/2/10 9:02 AM Page 1197
1198 Chapter 30. DNA Replication, Repair, and Recombination
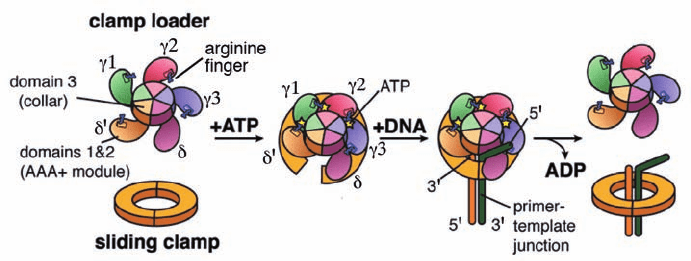
Figure 30-35 Schematic diagram of the clamp loader cycle.
This speculative model is based on a combination of structural
and biochemical information.The “arginine finger,” an Arg side
chain that interacts with the ␥-phosphate group of an ATP bound
tacts between the core and the DNA). Thus once the slid-
ing clamp has been loaded onto the primer–template, the
clamp loader is replaced by the Pol III core, which thereby
blocks the clamp loader from unloading the clamp. Instead,
the clamp loader loads a new clamp onto the lagging strand
template in association with the primer that the primo-
some had synthesized in preparation for the next round of
Okazaki fragment synthesis (Fig. 30-28b).
The X-ray structure of the sliding clamp in complex with
primer–template DNA (Fig. 30-13) indicates that its
ssDNA segment binds to the same site as do the ␣ and ␦
subunits. This may serve to attract the primer–template
DNA to the inside of the open clamp, which in turn may
facilitate the release of the clamp loader and hence the clo-
sure of the sliding clamp. The binding of ssDNA to the
sliding clamp may also prevent it from sliding away before
it can be bound by the ␣ subunit.
When the Pol III core has completed its synthesis of the
Okazaki fragment, that is, when the gap between the two
successively synthesized Okazaki fragments has been re-
duced to a nick, it releases the DNA and the sliding clamp.
The Pol III core then binds to the newly primed template
and its associated clamp (displacing the clamp loader),
where it commences the synthesis of the next Okazaki
fragment. Thus, a series of switches that are activated by
ATP and DNA structure ensure the vectorial progression
of lagging strand replication. Throughout this process, the
Pol III holoenzyme is held at the replication fork by the
leading strand Pol III core, which remains tethered to
the DNA by its associated sliding clamp.
The sliding clamp that remains around the completed
Okazaki fragment probably functions to recruit Pol I and
DNA ligase so as to replace the RNA primer on the previ-
ously synthesized Okazaki fragment with DNA and seal
the remaining nick. However, the sliding clamp must even-
tually be recycled. It was initially assumed that this was the
job of the clamp loader. However, it is now clear that the
release of the sliding clamp from its associated DNA is
largely carried out by free ␦ subunit (the “wrench” in the
clamp loader that cracks apart the  subunits forming the
sliding clamp), which is synthesized in 5-fold excess over
that required to populate the cell’s few clamp loaders.
loader associates with the DNA almost entirely through
contacts with the phosphate groups of the template strand
alone. Thus, this structure is reminiscent of that of the E1
helicase in complex with ssDNA (Section 30-2Ca) with one
of its six subunits missing.
The clamp loader must tightly bind the sliding clamp
prior to its loading on the template DNA but must subse-
quently release the clamp to avoid interfering with its
binding to the Pol III core (␣ε). The structures of the
clamp loader and the –␦ complex, together with a variety
of biochemical evidence, suggest a model of how this
might occur (Fig. 30-35): The binding of ATP to ␥1 (the ␥
subunit that contacts ␦¿) results in a conformational
change that exposes the otherwise occluded ATP-binding
site of ␥2;ATP binding to ␥2 likewise exposes ␥3; and ATP
binding to ␥3 exposes the ␦ subunit’s  interaction ele-
ment, thereby permitting it to bind to a  subunit so as to
spring open the sliding clamp. Primer–template DNA then
inserts itself through the resulting gap in the sliding clamp.
This process is facilitated by the gap between the AAA⫹
domains of ␦ and ␦¿ subunits, which permits the clamp
loader to track the template strand while avoiding contact
with the primer strand. Eventually, - and DNA-stimu-
lated hydrolysis of the bound ATPs releases the  subunit
from the clamp loader, whereon the sliding clamp closes
around the DNA.
The departure of the clamp loader permits the Pol III
core to bind to the sliding clamp. However, when the syn-
thesis of an Okazaki fragment has been completed, the Pol
III core must dissociate from the sliding clamp so that it
can initiate the synthesis of the next Okazaki fragment.
How does this occur?
Pol III’s ␣ subunit binds to the same hydrophobic
pocket on the 
2
sliding clamp as does the ␦ subunit. This
was shown by the observations that the phosphorylation of
a kinase recognition sequence that had been engineered
into the C-terminal segment of  is inhibited by both ␣ and
␦. The  subunit has an ⬃30-fold greater affinity for the ␥
complex in the presence of ATP than it has for the Pol III
core. However, when primer–template DNA is also pres-
ent, this order of affinity is reversed with  preferring to
bind to the Pol III core (possibly due to the additional con-
to a neighboring subunit, is a common feature of AAA⫹
ATPases that form ringlike structures. [Modified from a drawing
by Mike O’Donnell,The Rockefeller University, and John
Kuriyan, University of California at Berkeley.]
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1198
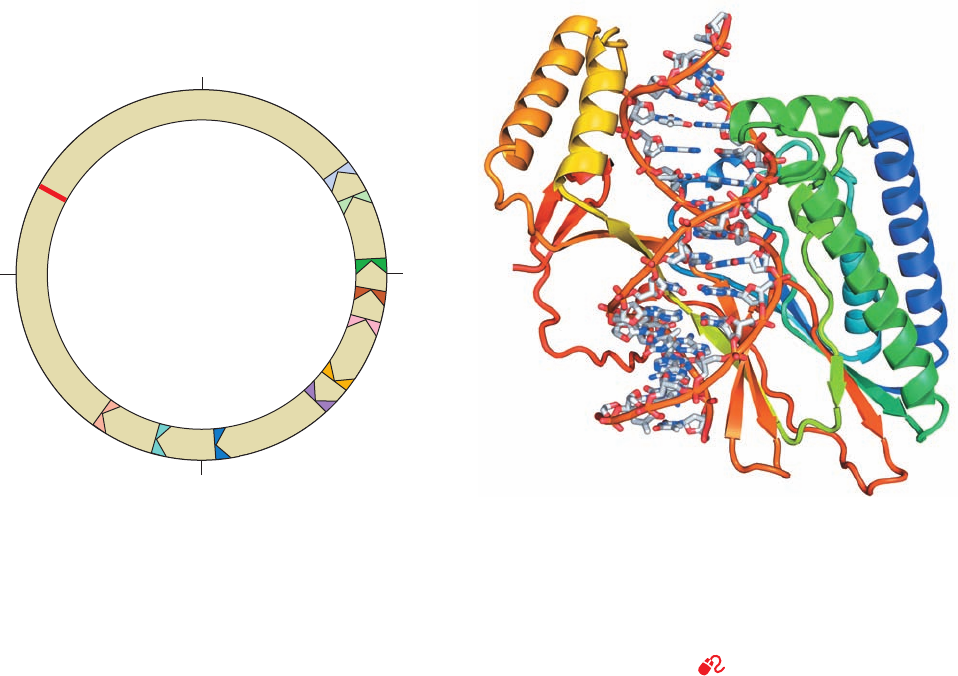
form a deep positively charged cleft that largely envelops
the bound DNA (Fig. 30-37). A 5-bp segment of the DNA
near the side of Tus that permits the passage of the replica-
tion fork (the lower side of Fig. 30-37) is deformed and un-
derwound relative to canonical (ideal) B-DNA such that its
major groove becomes deeper and its minor groove is sig-
nificantly expanded.The protein makes polar contacts with
more than two-thirds of the phosphate groups in a 13-bp re-
gion and its interdomain  sheet penetrates the deepened
major groove to make sequence-specific contacts with the
exposed bases. The importance of this interdomain region
for Tus function is demonstrated by the observation that
most single residue mutations that reduce the ability of Tus
to arrest replication occur in this interdomain region.
When Tus is fused to another DNA-binding protein,
replication is inhibited at the other protein’s binding site.
This suggests that Tus does not act as a simple DNA-bind-
ing clamp, but interacts with DnaB helicase, the leading
component of a replication fork (Fig. 30-32), to inhibit its
helicase action. Apparently, Tus prevents the progress of
DnaB in unwinding DNA from one side of Tus but not the
other. Indeed, the encounter of DnaB with a Tus–Ter com-
plex in the permissive direction causes Tus to rapidly disso-
ciate from the DNA, whereas such an encounter from the
nonpermissive direction generates a so-called locked
Tus–Ter complex. Nevertheless, the way Tus and DnaB in-
teract is unknown. Curiously, however, this termination sys-
tem is not essential for termination. When the replication
Section 30-3. Prokaryotic Replication 1199
Figure 30-36 Map of the E. coli chromosome showing the
positions of the Ter sites and the oriC site. The TerJ, TerG, TerF,
TerB, and TerC sites, in combination with Tus protein, allow a
counterclockwise-moving replisome to pass but not a clockwise-
moving replisome. The opposite is true of the TerH,TerI, TerE,
TerD, and TerA sites. Consequently, two replication forks that
initiate bidirectional DNA replication at oriC will meet between
the oppositely facing Ter sites.
Figure 30-37 X-ray structure of E. coli Tus protein in complex
with a 15-bp Ter-containing DNA. The protein is drawn in ribbon
form colored in rainbow order from its N-terminus (blue) to its
C-terminus (red).The DNA is shown in stick form with C gray,
N blue, O red, and P orange and with successive P atoms in the
same strand joined by orange rods. [Based on an X-ray structure
by Kosuke Morikawa, Protein Engineering Research Institute,
Osaka, Japan. PDBid 1ECR.]
See Interactive Exercise 34
d. Replication Termination Is Facilitated
by Tus Protein
The E. coli replication terminus is a large (350 kb) re-
gion flanked by ten nearly identical nonpalindromic ⬃23-
bp terminator sites, TerH, TerI, TerE, TerD, and TerA on
one side and TerJ, TerG, TerF, TerB, and TerC on the other
(Fig. 30-36; note that oriC is directly opposite the terminus
region on the E. coli chromosome).A replication fork trav-
eling counterclockwise as drawn in Fig. 30-36 passes
through TerJ, TerG, TerF, TerB, and TerC but stops on en-
countering either TerA, TerD, TerE, TerI, or TerH (TerD,
TerE, TerI, and TerH are presumably backup sites for
TerA). Similarly, a clockwise-traveling replication fork
transits TerH, TerI, TerE, TerD, and TerA but halts at TerC
or, failing that, TerB or TerF or TerG or TerI. Thus, these
termination sites act as one-way valves that allow replica-
tion forks to enter the termination region but not to leave
it. This arrangement guarantees that the two replication
forks generated by bidirectional initiation at oriC will meet
in the replication terminus even if one of them arrives
there well ahead of its counterpart.
The arrest of replication fork motion at Ter sites requires
the action of Tus protein,a 309-residue monomer that is the
product of the tus gene (for terminator utilization sub-
stance).Tus specifically binds to a Ter site, where it prevents
strand displacement by DnaB helicase, thereby arresting
replication fork motion. The X-ray structure of Tus in com-
plex with a 15-bp Ter sequence-containing DNA with a sin-
gle T overhang at each 5¿ end, determined by Kosuke
Morikawa, reveals that Tus consists of two domains that
100/0
50
75
TerFTerG
oriC
TerB
TerE
TerH
TerI
TerD
TerA
TerC
TerJ
JWCL281_c30_1173-1259.qxd 10/19/10 10:29 AM Page 1199
1200 Chapter 30. DNA Replication, Repair, and Recombination
D. Fidelity of Replication
Since a single polypeptide as small as the Pol I Klenow
fragment can replicate DNA by itself, why does E. coli
maintain a battery of ⬎20 intricately coordinated proteins
to replicate its chromosome? The answer apparently is to
ensure the nearly perfect fidelity of DNA replication re-
quired to preserve the genetic message’s integrity from gen-
eration to generation.
The rates of reversion of mutant E. coli or T4 phage to
the wild type indicates that only one mispairing occurs per
10
8
to 10
10
base pairs replicated. This corresponds to ⬃1
error per 1000 bacteria per generation. Such high replica-
tion accuracy arises from four sources:
1. Cells maintain balanced levels of dNTPs through the
mechanism discussed in Section 28-3Ad. This is an impor-
tant aspect of replication fidelity because a dNTP present
terminus is deleted, replication simply stops, apparently
through the collision of opposing replication forks. Never-
theless, this termination system is highly conserved in
gram-negative bacteria.
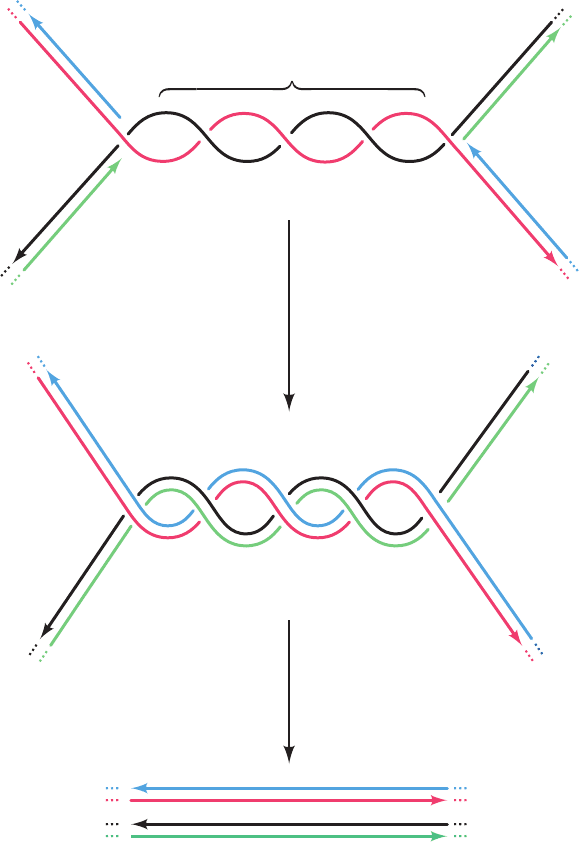
As two oppositely moving replication forks collide at
the termination site, the newly synthesized strands become
covalently linked to yield two covalently closed double-
stranded chromosomes. However, since the parental DNA
strands remain wound about each other by several turns
(presumably, DNA gyrase cannot gain access to the DNA
when the colliding replication forks closely approach each
other), the product dsDNA strands must be wound about
each other by the same number of turns (Fig. 30-38). The
resulting catenated circular dsDNAs must be separated so
that each can be passed to a different daughter cell. This is
the job of the type II topoisomerase named topoisomerase
IV (Section 29-3Cd).
Termination region
Replication without
unwinding
Catenated chromosomes
DNA topoisomerase IV
+
Figure 30-38 The formation and separation of catenated
dsDNAs at the replication termination site. The parental strands
are red and black and the daughter strands are green and blue.
For clarity, the double helical character of the newly formed
dsDNA molecules is not shown.
JWCL281_c30_1173-1259.qxd 8/10/10 9:11 PM Page 1200
