Voet D., Voet Ju.G. Biochemistry
Подождите немного. Документ загружается.

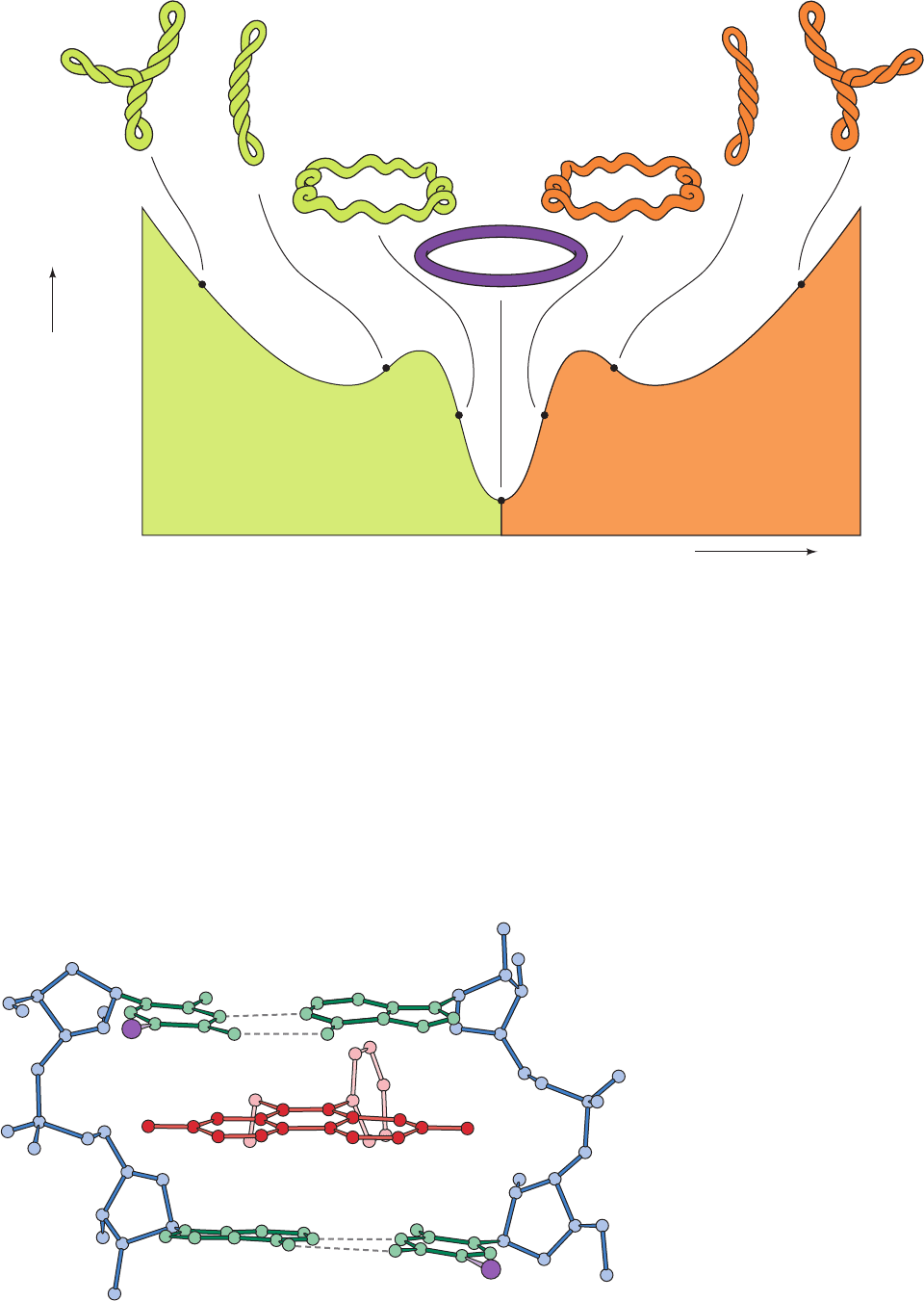
corresponding relaxed circles. This phenomenon has been
established by observing the effect of ethidium ion bind-
ing on the sedimentation rate of circular DNA (Fig. 29-
21). Intercalating agents such as ethidium (a planar aro-
matic cation; Section 6-6Ca) alter a circular DNA’s degree
of superhelicity because they cause the DNA double helix
to unwind (untwist) by ⬃26° at the site of the intercalated
molecule (Fig. 29-22). W 0 in an unconstrained under-
wound circle because of the tendency of a duplex DNA to
maintain its normal twist of 1 turn per 10.5 bp. The titra-
Section 29-3. Supercoiled DNA 1161
W>0W<0
DNA
sedimentation
velocity
Ethidium bromide concentration
Figure 29-21 Sedimentation rate of underwound closed
circular duplex DNA as a function of ethidium bromide
concentration. The intercalation of ethidium between the base
pairs locally untwists the double helix (Fig. 29-22), which, since
the linking number of the circle is constant, is accompanied by an
equivalent increase in the writhing number.As the negatively
coiled superhelix untwists, it becomes less compact and hence
sediments more slowly. At the low point on the curve, the DNA
Figure 29-22 X-ray structure of a
complex of ethidium with 5-iodo-UpA.
Ethidium (red) intercalates between the
base pairs (green with I purple) of the
double helically paired dinucleoside
phosphate and thereby provides a model
for the binding of ethidium to duplex DNA.
[After Tsai, C.-C., Jain, S.C., and Sobell,
H.M., Proc. Natl.Acad. Sci. 72, 629 (1975).]
circles have bound sufficient ethidium to become fully relaxed.
As the ethidium concentration is further increased, the DNA
supercoils in the opposite direction, yielding a positively coiled
superhelix.The supertwisted appearances of the depicted DNAs
have been verified by electron microscopy. [After Bauer, W.R.,
Crick, F.H.C., and White, J.H., Sci. Am. 243(1), 129 (1980).
Copyright © 1981 by Scientific American, Inc.]
JWCL281_c29_1143-1172.qxd 7/8/10 8:39 PM Page 1161
tion of a DNA circle by ethidium unwinds the duplex (de-
creases T), which must be accompanied by a compensat-
ing increase in W. This, at first, lessens the superhelicity of
an underwound circle. However, as the circle binds more
and more ethidium, its value of W passes through zero
(relaxed circles) and then becomes positive, so that the
circle again becomes superhelical.Thus the sedimentation
rate of underwound DNAs, which is a measure of their
compactness and therefore their superhelicity, passes
through a minimum as the ethidium concentration in-
creases. This is what is observed with native DNAs (Fig.
29-21). In contrast, the sedimentation rate of an over-
wound circle would only increase with increasing ethid-
ium concentration.
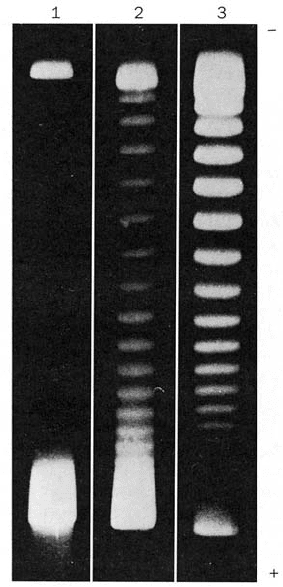
b. DNAs Are Separated According to Their Linking
Number by Gel Electrophoresis
Gel electrophoresis (Sections 6-4 and 6-6C) also sepa-
rates similar molecules on the basis of their compactness,
so that the rate of migration of a circular duplex DNA in-
creases with its degree of superhelicity. The agarose gel
electrophoresis pattern of a population of chemically iden-
tical DNA molecules with different linking numbers there-
fore consists of a series of discrete bands (Fig. 29-23). The
molecules in a given band all have the same linking num-
ber and differ from those in adjacent bands by L 1.
Comparison of the electrophoretic band patterns of
simian virus 40 (SV40) DNA that had been enzymatically
relaxed to varying degrees and then resealed (Fig. 29-23)
reveals that 26 bands separate native from fully relaxed
SV40 DNAs. Native SV40 DNA therefore has W 26
(although it is somewhat heterogeneous in this quantity).
Since SV40 DNA consists of 5243 bp, it has 1 negative su-
perhelical turn per ⬃19 duplex turns. Such a superhelix
density (W/T) is typical of circular DNAs from various bi-
ological sources.
c. DNA in Physiological Solution Has 10.5 Base
Pairs per Turn
The insertion, using genetic engineering techniques
(Section 5-5C), of an additional x base pairs into a super-
helical DNA with a given linking number will increase
the DNA’s twist and hence decrease its writhing number
by x/h°, where h° is the number of base pairs per duplex
turn. Such an insertion shifts the position of each band in
the DNA’s gel electrophoretic pattern by x/h° of the
spacing between bands. By measuring the effects of sev-
eral such insertions, James Wang established that h°
10.5 0.1 bp for B-DNA in solution under physiological
conditions.
C. Topoisomerases
The normal biological functioning of DNA occurs only if it
is in the proper topological state. In such basic biological
processes as RNA transcription and DNA replication, the
recognition of a base sequence requires the local separa-
tion of complementary polynucleotide strands. The nega-
tive supercoiling of naturally occurring DNAs results in a
torsional strain that promotes such separations since it
tends to unwind the duplex helix (an increase in T must be
accompanied by a decrease in W). If DNA lacks the proper
superhelical tension, the above vital processes (which them-
selves supercoil DNA; Sections 30-2C and 31-2Ca) occur
quite slowly, if at all.
The supercoiling of DNA is controlled by a remarkable
group of enzymes known as DNA topoisomerases (or sim-
ply topoisomerases).They are so named because they alter
the topological state (linking number) of circular DNA but
not its covalent structure. There are two classes of topoiso-
merases:
1. Type I topoisomerases, the first of which was discov-
ered by James Wang in 1971, act by creating transient
single-strand breaks in DNA.Type I enzymes are subclassi-
fied into types IA, IB, and IC topoisomerases on the basis
of their amino acid sequences and reaction mechanisms
(see below). Type I topoisomerases are denoted by odd
Roman numerals (e.g., topoisomerase I, III, etc.).
1162 Chapter 29. Nucleic Acid Structures
Figure 29-23 Agarose gel electrophoresis pattern of SV40
DNA. Lane 1 contains the negatively supercoiled native DNA
(lower band; the DNA was applied to the top of the gel). In lanes
2 and 3, the DNA was exposed for 5 and 30 min, respectively, to
an enzyme, known as a type IA topoisomerase (Section 29-3Cb),
that relaxes negative supercoils one at a time by increasing the
DNA’s linking number (L).The DNAs in consecutively higher
bands of a given gel have successively increasing linking numbers
(L 1). [From Keller, W., Proc. Natl. Acad. Sci. 72, 2553
(1975).]
JWCL281_c29_1143-1172.qxd 7/8/10 8:39 PM Page 1162
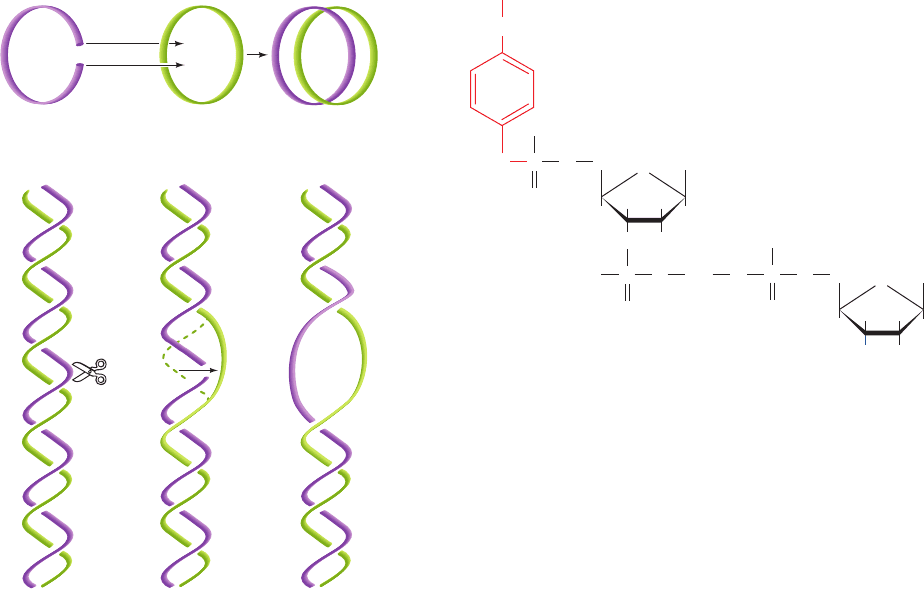
2. Type II topoisomerases, the first of which was discov-
ered by Martin Gellert in 1976, act by making transient
double-strand breaks in DNA with the accompanying hy-
drolysis of ATP to ADP P
i
.Type II enzymes are subclas-
sified into types IIA and IIB topoisomerases on the basis
of their amino acid sequences. Type II topoisomerases are
denoted by even Roman numerals (e.g., topoisomerase II,
IV, etc.).
a. Type I Topoisomerases Incrementally Relax
Supercoiled DNA
Type I topoisomerases catalyze the relaxation of super-
coils in DNA by changing their linking number in incre-
ments of one turn until the supercoil is entirely relaxed. In
most organisms, type IA enzymes, which are present in
all cells, relax only negatively supercoiled DNA, whereas
type IB enzymes, which are present in all eukaryotes and
many prokaryotes (but not E. coli), relax both negatively
and positively coiled DNA. However, many hyperther-
mophiles, both eubacteria and archaea, have a type IA
topoisomerase known as reverse gyrase that induces pos-
itive supercoiling in DNA through the ATP-driven action
of a helicase domain that is fused to the N-terminus of
the topoisomerase domain (helicases are discussed in
Section 30-2C). This suggests that positive supercoiling,
which tightens the DNA double helix, protects DNA
from thermal denaturation. Although types IA and IB
topoisomerases are both monomeric, ⬃100-kD enzymes,
they share no apparent sequence or structural similari-
ties and function, as we shall see, via different enzymatic
mechanisms.
A clue to the mechanism of type IA topoisomerase was
provided by the observation that it reversibly catenates
(interlinks) single-stranded circles (Fig. 29-24a). Appar-
ently the enzyme operates by cutting a single strand, pass-
ing a single-strand loop through the resulting gap, and
then resealing the break (Fig. 29-24b), thereby twisting
double helical DNA by one turn. In support of this strand
passage mechanism, the denaturation of type IA enzyme
that has been incubated with single-stranded circular
DNA yields a linear DNA that has its 5¿-terminal phos-
phoryl group linked to the enzyme via a phosphoTyr di-
ester linkage.
In contrast, denatured type IB enzyme is linked to the 3¿
end of DNA via a phosphoTyr linkage.By forming such co-
valent enzyme–DNA intermediates, the free energy of the
cleaved phosphodiester bond is preserved, so that no energy
input is required to reseal the nick.
b. Type IA Topoisomerase Functions via a Strand
Passage Mechanism
Cells of E. coli contain two type IA topoisomerases
named topoisomerase I (also called protein) and topo-
isomerase III. Topoisomerase III’s Tyr 328 is the active site
residue that forms a 5¿-phosphoTyr linkage with the cleaved
DNA. The X-ray structure of the inactive Y328F mutant of
topoisomerase III in complex with the single-stranded oc-
tanucleotide d(CGCAACTT), determined by Alfonso
CH
2
Tyr
Type IA topoisomerase
DNA
O
CH
2
HH
HO
H
Base
H
O
CH
2
HH
HOH
H
Base
H
O
O
PO
O
–
O
O
–
–
O
OO
O
P OP
...
Section 29-3. Supercoiled DNA 1163
Figure 29-24 Type IA topoisomerase action. By cutting a
single-stranded DNA, passing a loop of a second strand through
the break, and then resealing the break, a type IA topoisomerase
can (a) catenate two single-stranded circles or (b) unwind duplex
DNA by one turn.
1
(a)
(b)
23
Duplex DNA
(n turns)
Duplex DNA
(n – 1 turns)
JWCL281_c29_1143-1172.qxd 7/8/10 8:39 PM Page 1163
1164 Chapter 29. Nucleic Acid Structures
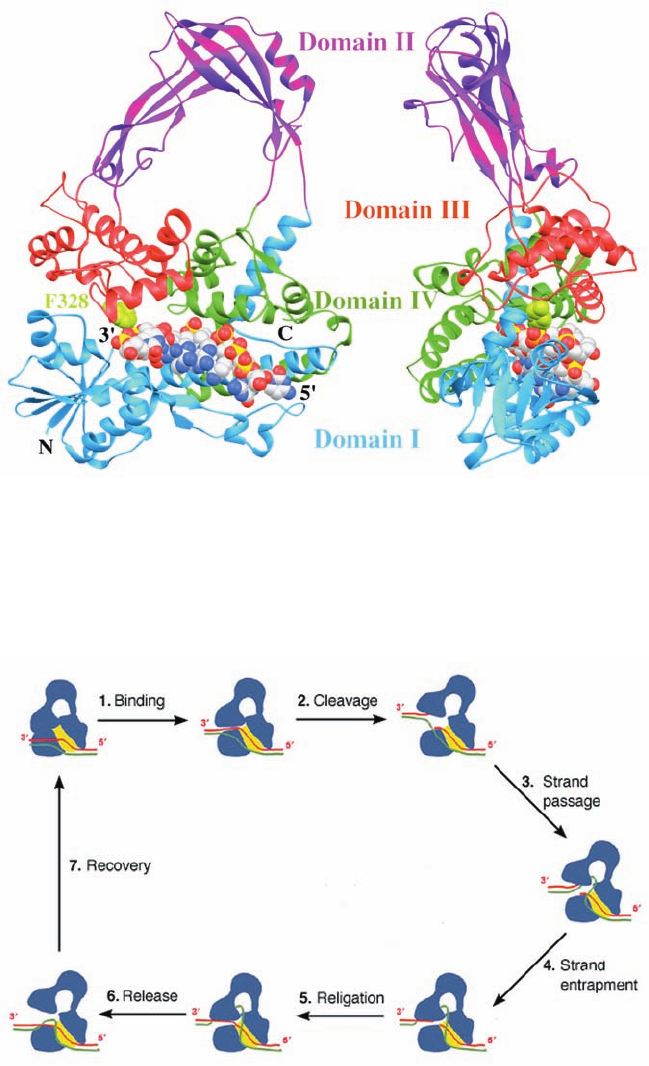
Figure 29-26 Proposed mechanism for the strand passage
reaction catalyzed by type IA topoisomerases. The enzyme is
shown in blue with the yellow patch representing the binding
groove for single-stranded (ss) DNA. The two DNA strands,
which are drawn in red and green, could represent the two
strands of a covalently closed circular duplex or two ss circles.
(1) The protein recognizes a ss region of the DNA, here the red
strand, and binds it in its binding groove. This is followed by or
occurs simultaneously with the opening of a gap between
domains I and III. (2) The DNA is cleaved with the newly formed
5¿ end becoming covalently linked to the active site Tyr and the
segment with the newly formed 3¿ end remaining tightly but
noncovalently bound in the binding groove. (3) The unbroken
(green) strand is passed through the opening or gate formed by
Figure 29-25 X-ray structure of the Y328F mutant of E. coli
topoisomerase III, a type IA topoisomerase, in complex with the
single-stranded octanucleotide d(CGCAACTT). The two views
shown are related by a 90° rotation about a vertical axis.The
protein’s four domains are drawn in different colors. The DNA is
the cleaved (red) strand to enter the protein’s central hole.
(4) The unbroken strand is trapped by the partial closing of the
gap. (5) The two cleaved ends of the red strand are rejoined in
what is probably a reversal of the cleavage reaction. (6) The gap
between domains I and III reopens to permit the escape of the
red strand, yielding the reaction product in which the green
strand has been passed through a transient break in the red
strand. (7) The enzyme returns to its initial state. If the two
strands form a negatively supercoiled duplex DNA, its linking
number, L, has increased by 1; if they are separate ss circles, they
have been catenated or decatenated. For duplex DNA, this
process can be repeated until all of its supercoils have been
removed (W 0). [After a drawing by Alfonso Mondragón,
Northwestern University.]
drawn in space-filling form with C white, N blue, O red, and P
yellow. The enzyme’s active site is marked by the side chain of
Phe 328, which is shown in space-filling form in yellow-green.
[Based on an X-ray structure by Alfonso Mondragón,
Northwestern University. PDBid 1I7D.]
JWCL281_c29_1143-1172.qxd 8/2/10 8:06 PM Page 1164
Mondragón (Fig. 29-25), reveals that this 659-residue
monomer folds into four domains which enclose an ⬃20 by
28 Å hole that is large enough to contain a duplex DNA and
which is lined with numerous Arg and Lys side chains. The
octanucleotide binds in a groove that is also lined with Arg
and Lys side chains with its sugar–phosphate backbone in
contact with the protein and with most of its bases exposed
for possible base pairing. Curiously, this single-stranded
DNA assumes a B-DNA-like conformation even though its
complementary strand would be sterically excluded from
the groove. The DNA strand is oriented with its 3¿ end near
the active site, where, if the mutant Phe 328 were the wild-
type Tyr, its side chain would be properly positioned to nu-
cleophilically attack the phosphate group bridging the
DNA’s C6 and T7 to form a 5¿-phosphoTyr linkage with T7
and release C6 with a free 3¿-OH.This structure and that of
the homologous and structurally similar E. coli topoiso-
merase I suggest the mechanism for the type IA topoiso-
merase-catalyzed strand passage reaction that is dia-
grammed in Fig. 29-26.
c. Type IB Topoisomerase Functions via a Controlled
Rotation Mechanism
Human topoisomerase I is a 765-residue type IB topo-
isomerase (and hence is unrelated to E. coli topoisomerase
I). It mediates the transient cleavage of one strand of a du-
plex DNA through the nucleophilic attack of Tyr 723 on a
DNA P atom to yield a 3¿-linked phosphoTyr diester bond
and a free 5¿-OH group on the succeeding nucleotide. Lim-
ited proteolysis studies revealed that topoisomerase I con-
sists of four major regions: its N-terminal, core, linker, and
C-terminal domains. The ⬃210-residue, highly polar, N-
terminal domain, which is poorly conserved, contains
several nuclear targeting signals and is dispensable for en-
zymatic activity.
The X-ray structure of the catalytically inactive Y723F
mutant of topoisomerase I lacking its N-terminal 214
residues and in complex with a 22-bp palindromic duplex
DNA was determined by Wim Hol (Fig. 29-27). The core
domain of this bilobal protein is wrapped around the DNA
in a tight embrace. If the mutant Phe 723 were the wild-
type Tyr, its OH group would be colinear with the scissile
P¬O5¿ bond and hence ideally positioned to nucleophili-
cally attack this P atom so as to form a covalent linkage
with the 3¿ end of the cleaved strand.As expected, the pro-
tein interacts with the DNA in a largely sequence inde-
pendent manner: Of the 41 direct contacts that the protein
makes to the DNA, 37 are protein–phosphate interactions
and only one is base-specific. The protein interacts to a
much greater extent with the five base pairs of the DNA’s
upstream segment (which would contain the cleaved
strand’s newly formed 5¿ end; 29 of the 41 contacts) than it
does with the base pairs of the DNA’s downstream seg-
ment (to which Tyr 723 would be covalently linked; 12 of
the 41 contacts).
Topoisomerase I does not seem sterically capable of un-
winding supercoiled DNA via the strand passage mecha-
nism that type IA topoisomerases appear to follow (Fig.
29-26). Rather, as is diagrammed in Fig. 29-28, it is likely
that topoisomerase I relaxes DNA supercoils by permitting
the cleaved duplex DNA’s loosely held downstream seg-
ment to rotate relative to the tightly held upstream
segment. This rotation can only occur about the
sugar–phosphate bonds in the uncleaved strand (, , , ε,
and in Fig. 29-5) that are opposite the cleavage site be-
cause the cleavage frees these bonds to rotate. In support
of this mechanism, the protein region surrounding the
downstream segment contains 16 conserved, positively
charged residues that form a ring about this duplex DNA,
which would presumably hold the DNA in the ring but not
in any specific orientation. Nevertheless, the downstream
segment is unlikely to rotate freely because the cavity con-
taining it is shaped so as to interact with the downstream
segment during some portions of its rotation. Hence,
Section 29-3. Supercoiled DNA 1165
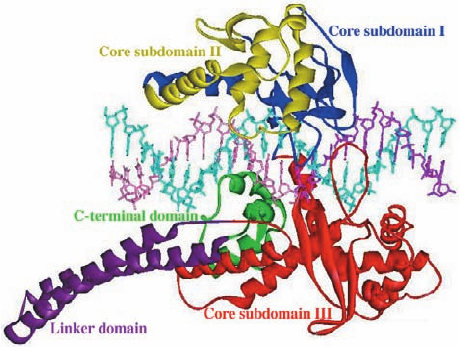
Figure 29-27 X-ray structure of the N-terminally truncated,
Y723F mutant of human topoisomerase I in complex with a
22-bp duplex DNA. The protein’s various domains and
subdomains are drawn in different colors.The DNA’s uncleaved
strand is cyan, and the upstream and downstream portions of the
scissile strand are purple and pink, respectively. [Courtesy of
Wim Hol, University of Washington. PDBid 1A36.]
JWCL281_c29_1143-1172.qxd 8/2/10 8:06 PM Page 1165
type IB topoisomerases are said to mediate a controlled
rotation mechanism in relaxing supercoiled DNA. This
unwinding is driven by the superhelical tension in the
DNA and hence requires no other energy input. Eventu-
ally, the DNA is religated by a reversal of the cleavage re-
action and the now less supercoiled DNA is released.
Type IC topoisomerase, whose only known family mem-
ber, topoisomerase V, occurs exclusively in archaea, resem-
bles type IB topoisomerases (which do not occur in ar-
chaea) in that it forms 3’-phosphoTyr intermediates and
appears to function via a controlled rotation mechanism.
However, it has no sequence or structural resemblance to
type IB topoisomerases.
d. Type II Topoisomerases Function via a Strand
Passage Mechanism
Bacteria have two types of type IIA topoisomerases:
DNA gyrase (or just gyrase) and topoisomerase IV, both of
which are A
2
B
2
heterotetramers. Eukaryotic type IIA
topoisomerases, which are named topoisomerase II, are
homologous to bacterial type IIA topoisomerases but with
their A and B subunits fused so that they are homodimers.
The type IIB topoisomerase family has only one member,
topoisomerase VI, an A
2
B
2
heterotetramer that occurs
mainly in archaea (although some archaea express both
type IIA and IIB topoisomerases).The A subunits of types
IIA and IIB topoisomerases share a common ATPase
module, but their B subunits are unrelated.
Gyrase is unique among topoisomerases in that it gener-
ates negative supercoils in DNA. All other topoisomerases
but reverse gyrase only relax supercoils (DNA supercoiling
in eukaryotes is generated differently from that in prokary-
otes; Section 34-1Ba). It’s A and B subunits are named
GyrA and GyrB.
Type II topoisomerases can also catenate and decate-
nate double-stranded circles as well as tie and untie knots
in them.The importance of this function can be seen as fol-
lows. The ⬃6.1 billion base pairs of DNA in a diploid
human cell have an aggregate length of ⬃ 2 m and are con-
fined to a nucleus that is 5 to 10 m in diameter. Imagine
that the ⬃20-Å-wide DNA was expanded by a factor of
5 million to the width of a 1-cm-diameter rope. It would
1166 Chapter 29. Nucleic Acid Structures
(a)
(b)
(c)
(d)
(f)
(g)
ReleaseBinding
Cleavage Religation
Controlled rotation
Structure
Covalent
intermediate
Covalent
intermediate
Noncovalent
complex
Noncovalent
complex
(e)
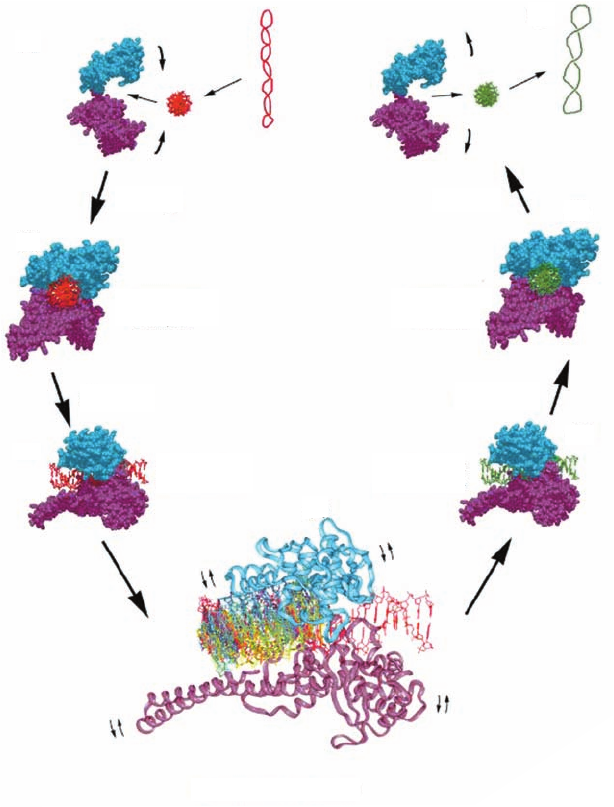
Figure 29-28 Controlled rotation mechanism for
type IB topoisomerases. A highly negatively
supercoiled DNA (red, with a right-handed writhe)
is converted, via stages (a) through (g), to a less
supercoiled form (green).Topoisomerase I is
drawn as a bilobal space-filling structure, in which
the cyan lobe is formed by core subdomains I and
II (Fig. 29-27) and the purple lobe is formed by
core subdomain III, the linker domain, and
the C-terminal domain.The structure shown
in (d), which is expanded by a factor of 2, shows
the downstream portion of the rotating DNA
(that containing the cleaved strand’s new 5¿ end)
at 30° intervals, all differently colored. Since the
enzyme is not always in direct contact with the
rotating DNA, small rocking motions of the
protein (small curved arrows) may accompany
the controlled rotation. [Courtesy of Wim Hol,
University of Washington.]
JWCL281_c29_1143-1172.qxd 8/2/10 8:06 PM Page 1166
Cut duplex
loop and pass
strand through
gap
Reseal
gap
Separate
duplex
strands
lengthwise
Two
turns
then be ⬃10,000 km long (the distance from San Francisco
to Rome) and confined to an expanded nucleus that is 25 to
50 m in diameter. Then imagine trying to manipulate the
ropes in this system without generating tangles or knots.
Yet such difficulties normally occur in cells during DNA
replication, repair, and recombination (Chapter 30), as well
as the segregation of the daughter chromosomes in divid-
ing cells (Section 1-4B). It is the function of type II topoiso-
merases to untangle the DNA during these processes.
Bacterial but not eukaryotic type IIA topoisomerases
are inhibited by a variety of substances including novo-
biocin, a member of the Streptomyces-derived coumarin
family of antibiotics, and ciprofloxacin (trade name Cipro),
a member of the synthetically generated quinolone family
of antibiotics (their coumarin and quinolone groups are
drawn in red):
O
H
CH
3
CH
3
CH
3
H
2
N
H
3
C
H
3
C
H
H
O
O
N
H
Novobiocin
C
O
O
H
O
CO
OH
OH
O
HO
Ciprofloxacin
HN
N
N
F
COOH
O
CH
3
These agents profoundly inhibit bacterial DNA replication
and RNA transcription, thereby demonstrating the impor-
tance of properly supercoiled DNA in these processes. Stud-
ies using E. coli gyrase mutants resistant to these sub-
stances have demonstrated that ciprofloxacin associates
with GyrA and novobiocin binds to GyrB.
The gel electrophoretic pattern of duplex circles that
have been exposed to gyrase shows a band pattern in
which the linking numbers differ by increments of 2 rather
than 1, as occurs with type I topoisomerases. Evidently, gy-
rase acts by cutting both strands of a duplex,passing the du-
plex through the break, and resealing it (Fig. 29-29). This
hypothesis is corroborated by the observation that when
gyrase is incubated with DNA and ciprofloxacin, and sub-
sequently denatured with guanidinium chloride, a GyrA
subunit remains covalently linked to the 5¿ end of each of
the two cut strands through a phosphoTyr linkage. These
cleavage sites are staggered by 4 bp, thereby yielding
sticky ends.
Saccharomyces cerevisiae (baker’s yeast) topoisomerase
II is a homodimer of 1428-residue subunits whose N- and
C-terminal segments are homologous to E. coli’s GyrB
(804 residues) and GyrA (878 residues) subunits, respec-
tively. The breakage/reunion domain, which encompasses
residues 410 to 1202, can, by itself, cleave duplex DNA but
cannot transport it through the break without the action of
the enzyme’s ATPase domain (residues 1–409). However,
the C-terminal segment (residues 1203–1428), which is
poorly conserved, appears to be dispensable.
Although the structure of a full length type IIA topoiso-
merase is as yet unknown, those of its ATPase and break-
age/reunion domains from both E. coli gyrase and yeast
topoisomerase II have been determined. The X-ray struc-
ture of the homodimeric topoisomerase II ATPase in com-
plex with the nonhydrolyzable ATP analog AMPPNP,
determined by James Berger, consists of two domains (Fig.
Section 29-3. Supercoiled DNA 1167
Figure 29-29 A demonstration, in which DNA is represented
by a ribbon, that cutting a duplex circle, passing the double helix
through the resulting gap, and then resealing the break changes
the linking number by 2. Separating the resulting single strands
(slitting the ribbon along its length; right) indicates that one
single strand makes two complete revolutions about the other.
JWCL281_c29_1143-1172.qxd 8/2/10 8:06 PM Page 1167
29-30a). The N-terminal domain binds AMPPNP and the
C-terminal domains form the walls of a large hole through
the dimer, which in the X-ray structure of the structurally
similar E. coli GyrB is 20 Å across, the same width as the B-
DNA double helix.
Berger also determined the X-ray structure of the yeast
topoisomerase breakage/reunion domain in complex a 15-
bp DNA that has a self-complementary 4-nucleotide over-
hang on the 5¿ end of one its strands. The DNA thereby
forms a 2-fold symmetric 34-bp duplex with nicks on oppo-
site strands separated by 4 bp (Fig. 29-30b,c).These are pre-
cisely the sites at which the enzyme would cleave an intact
DNA by linking its newly formed 5¿-ending strands to the
active site Tyr 782 residues.The protein binds the DNA in a
positively charged groove that spans the width of the dimer
and, in doing so, bends it through an arc of 150° (we shall
see in later chapters that DNA-binding proteins often de-
form their bound DNA, although such an extreme defor-
mation is unusual). Interestingly, the DNA between the two
cleavage sites is essentially in the A form. There are almost
no direct contacts between the protein and the DNA bases,
as is expected for a protein with little sequence specificity.
Note also that the C-terminal portions of the protein come
together to enclose a large centrally located empty space.
Consideration of the foregoing two structures and those
of the corresponding portions of E. coli gyrase suggests a
1168 Chapter 29. Nucleic Acid Structures
(a)
(b)
(c)
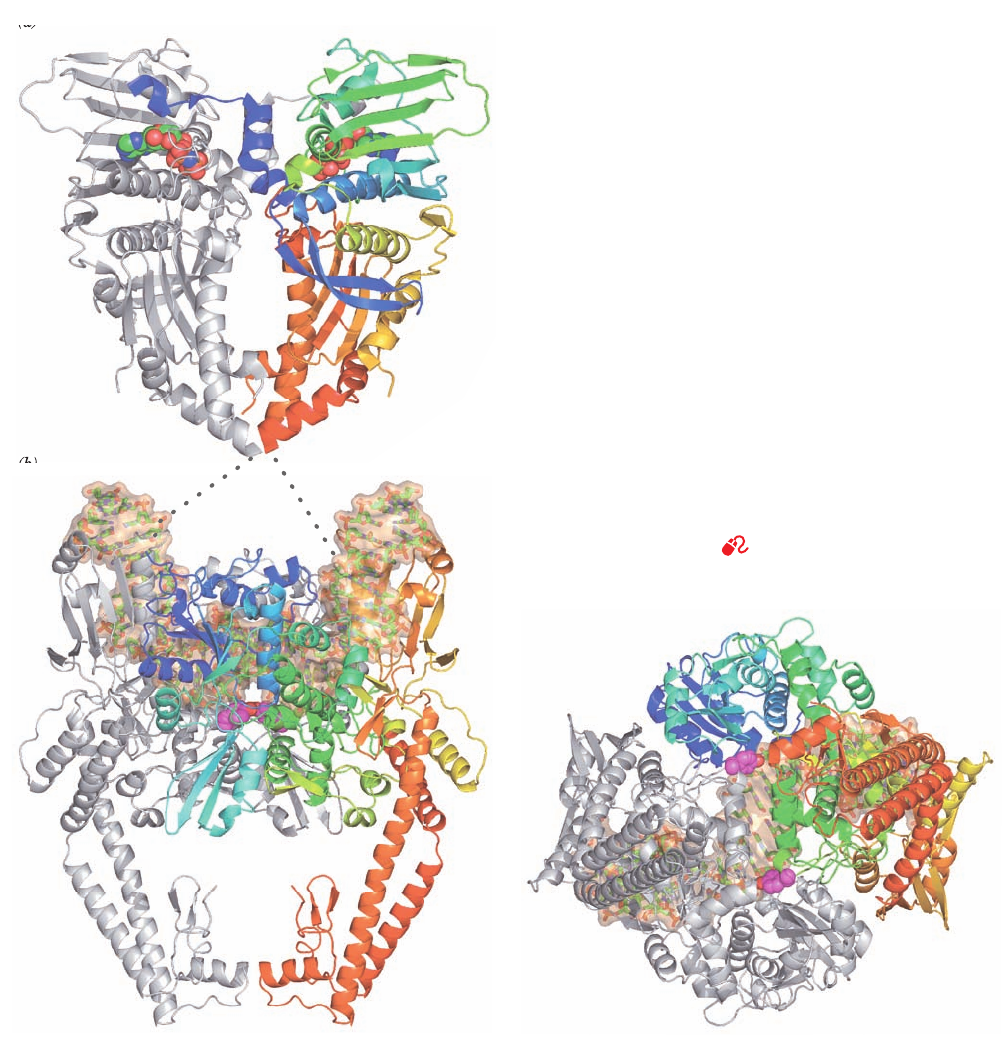
Figure 29-30 X-ray structures of S. cerevisiae topoisomerase
II. (a) Structure of a homodimer of the N-terminal ATPase do-
main (residues 7–406) in complex with AMPPNP as viewed with
its 2-fold axis vertical.The protein is displayed in ribbon form
with one subunit gray and the other colored in rainbow order
from its N-terminus (blue) to its C-terminus (red).The bound
AMPPNP molecules are shown in space-filling form with C
green, N blue, O red, and P orange. (b) Structure of a homodimer
of the DNA breakage/reunion domain (residues 419–1177) in
complex with a doubly nicked 34-bp DNA. The structure is viewed
with its 2-fold axis vertical.The DNA, which describes a 150° arc,
is drawn in stick form with C green, N blue, O red, and P orange
embedded in its semitransparent molecular surface (orange).The
protein is displayed in ribbon form with one subunit gray and the
other colored in rainbow order from its N-terminus (blue) to its
C-terminus (red).The side chains of the two active site Tyr
residues (Y782) are shown in space-filling form with C magenta
and O red.The polypeptides in Parts a and b, which in the intact
protein are joined by an 11-residue linker (represented by the
dotted lines), presumably share the same 2-fold axis as drawn,
although their relative orientation about this axis is unknown.
(c) Same as Part b but viewed from below. Note that the Y782
side chain O atoms are positioned to link to opposite DNA
strands at sites separated by 4 base pairs. [Based on X-ray struc-
tures by James Berger, University of California at Berkeley.
PDBids 1PVC and 2RGR.]
See Interactive Exercise 32
JWCL281_c29_1143-1172.qxd 10/19/10 10:23 AM Page 1168
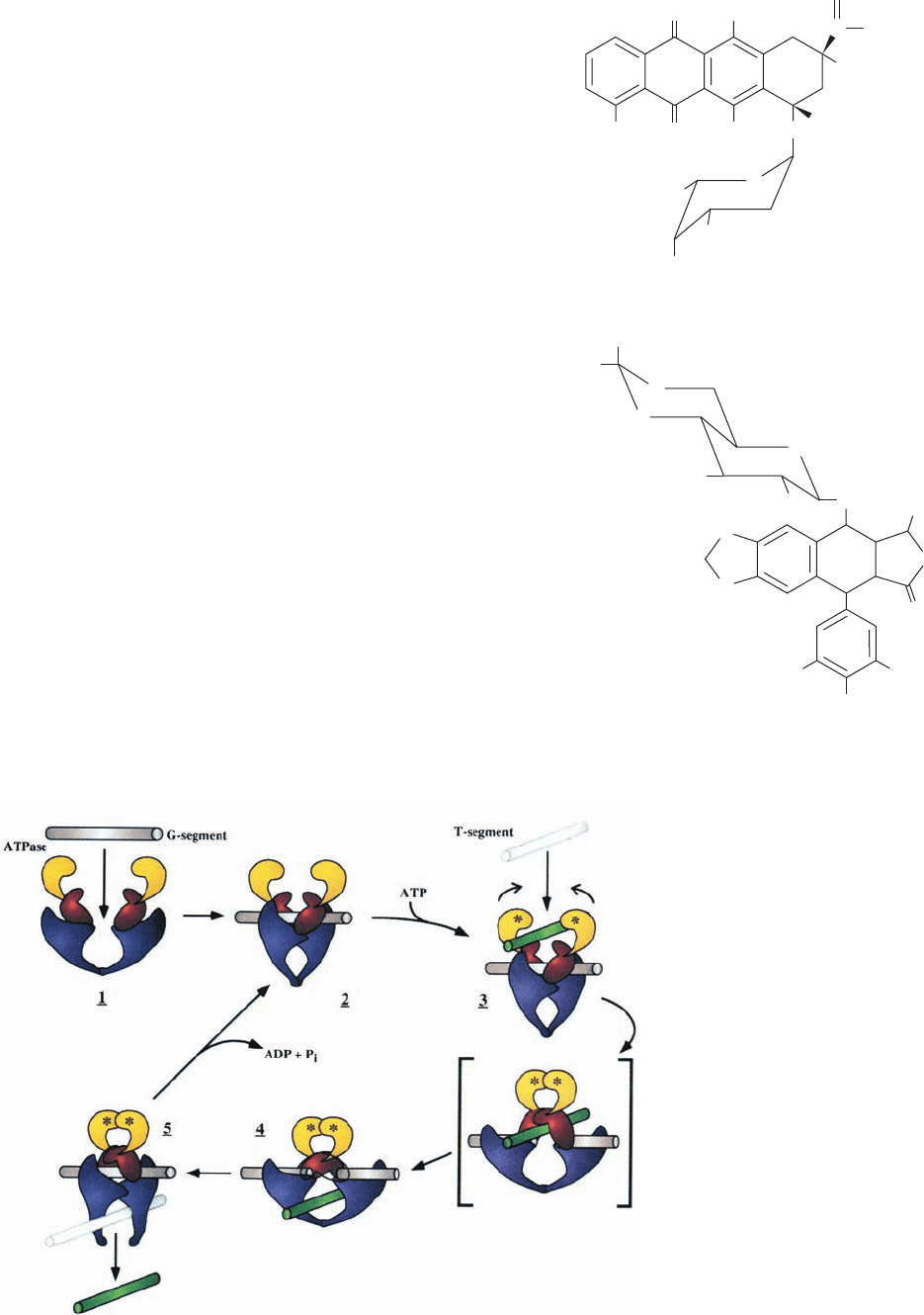
type of strand passage model for the mechanism of type IIA
topoisomerases (Fig. 29-31) in which the DNA duplex to be
cleaved, the so-called G-segment (G for gate). binds in the
above-described groove across the top of the cleavage/re-
union domain. ATP binding to the ATP-binding domain
then induces a sequence of conformational changes in which
the G-segment is cleaved and the resulting two fragments
are spread apart by at least 20 Å through the action of the
protein. This permits the passage of the DNA’s so-called T-
segment (T for transported) through the break in the DNA
and breakage/reunion domain’s upper gate (which may also
contain portions of the ATPase domain) into its central hole,
thereby incrementing the DNA’s linking number by 2.Then,
in a process that is accompanied by ATP hydrolysis, the up-
per gate closes to reseal the cleaved DNA, and the T-seg-
ment passes through the breakage/reunion domain’s bottom
gate. Finally, the resulting ADP and P
i
are released and the
bottom gate closes to yield recycled enzyme. Many of these
enzymatic states have been observed in the several known
X-ray structures of type IIA topoisomerase components.
e. Topoisomerase Inhibitors Are Effective Antibiotics
and Cancer Chemotherapy Agents
Coumarin derivatives such as novobiocin, and
quinolone derivatives such as ciprofloxacin, specifically
inhibit gyrase and are therefore antibiotics. In fact,
ciprofloxacin is the most efficacious oral antibiotic against
gram-negative bacteria presently in clinical use (novo-
biocin’s adverse side effects and the rapid generation of
bacterial resistance to it have resulted in the discontinua-
tion of its use in the treatment of human infections). A
number of substances, including doxorubicin (also called
adriamycin; a product of Streptomyces peucetius) and
etoposide (a synthetic derivative),
Etoposide
H
3
CO
OCH
3
H
3
C
O
O
O
O
H
HO
OH
H
OH
O
O
O
O
Doxorubicin (Adriamycin)
H
3
CO
H
3
C
O
OH
OH
OH
NH
3
OH
O
CCH
2
OH
O
H
+
O
O
Section 29-3. Supercoiled DNA 1169
Figure 29-31 Model for the enzymatic
mechanism of type II topoisomerases. The
protein’s ATPase domain and the upper and
lower portions of the breakage/reunion
domain are colored yellow, red, and purple,
respectively, and the DNA’s G- and T-
segments are colored gray and green,
respectively. In 1, the G-segment binds to the
enzyme, thereby inducing the conformational
change drawn in 2. The binding of ATP
(represented by asterisks) and a T-segment
(3) induces a series of conformational changes
in which the G-segment is cleaved as the
upper gate opens.The ATPase domains
dimerize, and the T-segment is transported
through the break into the central hole (4).
The DNA transport step is shown as
proceeding through the hypothetical
intermediate in square brackets.The G-
segments are then resealed and the T-segment
is released through the lower gate (5).This
gate then closes as the ATP is hydrolyzed and
the resulting ADP and P
i
released to yield the
enzyme in its starting state (2). [Courtesy of
James Wang, Harvard University.]
JWCL281_c29_1143-1172.qxd 8/2/10 8:06 PM Page 1169
inhibit eukaryotic type IIA topoisomerases and are there-
fore widely used in cancer chemotherapy. Indeed, ⬃50% of
cancer chemotherapy regimens contain at least one drug
targeted to type IIA topoisomerases.
Type IIA topoisomerase inhibitors act in either of two
ways. Many of them, including novobiocin, inhibit their tar-
get enzyme’s ATPase activity (novobiocin is a competitive
inhibitor of ATP because it tightly binds to GyrB in a way
that prevents the binding of ATP’s adenine ring). They
therefore kill cells by blocking topoisomerase activity,
which results in the arrest of DNA replication and RNA
transcription. However, other substances, including
ciprofloxacin, doxorubicin, and etoposide,enhance the rate
at which their target type IIA topoisomerases cleave
double-stranded DNA and/or reduce the rate at which
these enzymes reseal these breaks. Consequently, these
agents induce higher than normal levels of transient
protein-bridged breaks in the DNA of treated cells. These
protein bridges are easily ruptured by the passage of the
replication and transcription machinery, thereby rendering
the breaks permanent. Although all cells have elaborate
enzymatic systems to repair damaged DNA (Section 30-5),
a sufficiently high level of DNA damage overwhelms these
systems and hence results in cell death. Consequently, since
rapidly replicating cells such as cancer cells have elevated
levels of type IIA topoisomerases, they are far more likely
to incur lethal DNA damage through the poisoning of their
type IIA topoisomerases than are slow-growing or quies-
cent cells.
Type IB topoisomerases are specifically inhibited by the
quinoline-based alkaloid camptothecin
(a product of the Chinese yew tree, Camptotheca acumi-
nata) and its derivatives, which act by stabilizing the cova-
lent topoisomerase I–DNA complex. These compounds,
the only known naturally occurring topoisomerase IB in-
hibitors, are potent anticancer agents.
OH
N
NO
O
Camptothecin
CH
2
O
CH
3
1170 Chapter 29. Nucleic Acid Structures
1 Double Helical Structures B-DNA consists of a right-
handed double helix of antiparallel sugar–phosphate chains
with ⬃10 bp per turn of 34 Å and with its bases nearly perpen-
dicular to the helix axis. Bases on opposite strands hydrogen-
bond in a geometrically complementary manner to form A ⴢ T
and G ⴢ C Watson–Crick base pairs. At low humidity, B-DNA
undergoes a reversible transformation to a wider, flatter right-
handed double helix known as A-DNA. Z-DNA, which is
formed at high salt concentrations by polynucleotides of alter-
nating purine and pyrimidine base sequences, is a left-handed
double helix. Double helical RNA and RNA ⴢ DNA hybrids
have A-DNA-like structures. The conformation of DNA, par-
ticularly that of B-DNA, varies with its base sequence largely
because DNA’s flexibility varies with its base sequence.
2 Forces Stabilizing Nucleic Acid Structures The orien-
tations about the glycosidic bond and the various torsion an-
gles in the sugar–phosphate chain are sterically constrained in
nucleic acids. Likewise, only a few of the possible sugar pucker
conformations are commonly observed. Watson–Crick base
pairing is both geometrically and electronically complemen-
tary. Yet hydrogen bonding interactions do not greatly stabi-
lize nucleic acid structures. Rather, the structures are largely
stabilized by hydrophobic interactions. Nevertheless, the hy-
drophobic forces in nucleic acids are qualitatively different in
character from those that stabilize proteins. Electrostatic in-
teractions between charged phosphate groups are also impor-
tant structural determinants of nucleic acids.
CHAPTER SUMMARY
3 Supercoiled DNA The linking number (L) of a cova-
lently closed circular DNA is topologically invariant. Conse-
quently, any change in the twist (T) of a circular duplex must
be balanced by an equal and opposite change in its writhing
number (W), which indicates its degree of supercoiling.Super-
coiling can be induced by intercalation agents. The gel elec-
trophoretic mobility of DNA increases with its degree of su-
perhelicity. Naturally occurring DNAs are all negatively
supercoiled and must be so in order to participate in DNA
replication and RNA transcription.
Type IA topoisomerases relax negatively supercoiled
DNAs via a strand passage mechanism in which they cleave a
single strand of DNA to form a 5¿-phosphoTyr bond, pass a
single-strand DNA segment through the gap, and then reseal
the gap. Type IB topoisomerases relax both negatively and
positively supercoiled DNAs via a controlled rotation mecha-
nism involving a single-strand cleavage in which a transient
phosphoTyr bond is formed with the newly generated 3¿ end.
Type II topoisomerases relax duplex DNA in increments of
two supertwists at the expense of ATP hydrolysis by making a
double-strand scission in the DNA so as to form two transient
5¿-phosphoTyr linkages, passing the duplex through the break,
and resealing it. DNA gyrase also generates negative super-
twists in an ATP-dependent manner. Topoisomerases are the
targets of several antibiotics and chemotherapeutic agents.
JWCL281_c29_1143-1172.qxd 8/2/10 8:06 PM Page 1170
