Venables J. Introduction to Surface and Thin Film Processes
Подождите немного. Документ загружается.


ing. Assuming that the energy channels A, B and C are equally spaced, with A over the
peak, B just above the peak and C an equal distance to higher energy:
(a) Show that (A22B1C)/(2B2C) is the simplest linear measure of the P/B ratio, and
that this reduces to (A2B)/B if the background spectrum has zero slope.
(b) Assuming that the measured counts are limited by electron shot noise, find the
SNR of the peak height (A 22B1 C) and of the peak to background ratio, explain-
ing your reasons carefully.
(c) Compare the SNR of the logarithmic measure (A2B)/(A1B) with that of the
linear measure, and convince yourself that it is typically higher for comparable
values of the numbers of counts.
Student talks related to chapter 3 have included the following
In each case a page of suggestions for narrowing the topic, and suggested references
have been given out. The aim is to give the main features of the techniques clearly, with
adequate visual aids, in about 20 minutes, taking questions from the class.
1. A comparison of XPS (X-ray photoelectron spectroscopy) and AES.
2. Angular resolved AES and/or X-ray Photoelectron diffraction (XPD).
3. ARUPS and inverse photoemission.
4. Electron stimulated desorption (ESD) and the angular distribution of ions
(ESDIAD).
5. High energy ion, or Rutherford back scattering (HEIS-RBS) or medium energy
(MEIS).
6. Low energy ion scattering and ICISS.
7. Scanning tuneling spectroscopy (STS) and microscopy (STM) in UHV.
8. STM in solution.
9. Field ion microscopy (FIM) studies of atomic mobility on surfaces.
10. SIMS and SNMS (sputtered neutral mass spectroscopy).
11. Secondary electron spectroscopy and microscopy in UHV.
12. Low energy and photo-electron microscopy (LEEM/PEEM).
13. RHEED and REM.
14. Optical techniques for monitoring semiconductor crystal growth.
15. Surface magneto-optic Kerr effect (SMOKE).
16. SEM with polarization analysis (SEMPA).
17. Film thickness measurements.
18. Nanoindentation.
19. Reactive ion etching.
Problems, talks and projects for chapter 3 107

4 Surface processes in adsorption
4.1 Chemi- and physisorption
A qualitative distinction is usually made between chemisorption and physisorption, in
terms of the relative binding strengths and mechanisms. In chemisorption, a strong
‘chemical bond’ is formed between the adsorbate atom or molecule and the substrate.
In this case, the adsorption energy, E
a
, of the adatom is likely to be a good fraction of
the sublimation energy of the substrate, and it could be more. For example, in chapter
1, problem 1.2(a), we found that in a nearest neighbor pair bond model, E
a
52 eV for
an adatom on an f.c.c. (100) surface when the sublimation energy L
0
53 eV. In that case
the atoms of the substrate and the ‘adsorbate’ were the same, but the calculation of the
adsorption stay time,
t
a
, would have been valid if they had been different. Energies of
1–10 eV/atom are typical of chemisorption.
Physisorption is weaker, and no chemical interaction in the usual sense is present.
But if there were no attractive interaction, then the atom would not stay on the surface
for any measurable time – it would simply bounce back into the vapor. In physisorp-
tion, the energy of interaction is largely due to the (physical) van der Waals force. This
force arises from fluctuating dipole (and higher order) moments on the interacting
adsorbate and substrate, and is present between closed-shell systems. Typical systems
are rare gases or small molecules on layer compounds or metals, with experiments per-
formed below room temperature. Physisorption energies are ⬃50–500 meV/atom; as
they are small, they can be expressed in kelvin per atom, via 1 eV⬅11604 K, omitting
Boltzmann’s constant in the corresponding equations. These energies are comparable
to the sublimation energies of rare gas solids, as given in section 1.3, table 1.1.
Adsorption of reactive molecules may proceed in two stages, acting either in series or
as alternatives. A first, precursor, stage has all the characteristics of physisorption, but
the resulting state is metastable. In this state the molecule may reevaporate, or it may
stay on the surface long enough to transform irreversibly into a chemisorbed state. This
second stage is rather dramatic, usually resulting in splitting the molecule and adsorb-
ing the individual atoms: dissociative chemisorption. The adsorption energies for the
precursor phase are similar to physisorption of rare gases, but may contain additional
contributions from the dipole, quadrupole, and higher moments, and from the aniso-
tropic shape and polarizability of the molecules. The dissociation stage can be explosive
– literally. The heat of adsorption is given up suddenly, and can be imparted to the
resulting adatoms. Examples are O
2
/Al(111) and O
2
/Pt(111), which will be discussed
briefly in section 4.5. O
2
and N
2
can be condensed at low temperatures as (long-lived)
108

physisorbed molecules on many substrates. Bulk solid F
2
is, however, quite dangerous,
and has an alarming tendency to blow up by reacting dissociatively with its container.
This chapter starts by considering adsorption at low coverage, where the statistical
mechanics of adsorption can be worked out precisely in terms of simple models; two of
these limiting models are considered in some detail in section 4.2. The next section 4.3
discusses the application of thermodynamic reasoning to the adsorbed state of matter,
including how to describe phases and phase transitions. The final two sections 4.4 and
4.5 discuss the application of thermodynamic and statistical models to first physisorp-
tion and then chemisorption, with experimental examples and literature references.
4.2 Statistical physics of adsorption at low coverage
4.2.1 General points
We have already discussed, in section 1.3.1, the sublimation of a pure solid at equilib-
rium, given by the condition
m
v
5
m
s
, with
m
v
5
m
0
1kT ln (p), and the standard free energy
m
0
52kT ln (kT/
l
3
). (4.1)
Now we wish to consider adsorbed layers in more detail, with a corresponding chem-
ical potential
m
a
. Thus we have two possible conditions:
m
a
5
m
v
for equilibrium with
the vapor, and
m
a
5
m
s
for equilibrium with the solid. The first case is discussed in the
following sections 4.2.2 and 4.2.3. The second case was the subject of problem 1.2(b)
in chapter 1.
4.2.2 Localized adsorption: the Langmuir adsorption isotherm
In the Langmuir picture, each adatom is adsorbed at a well-defined adsorption site on
the surface. The canonical partition function for the adsorbed atoms is Z
a
5兺
i
exp
(2E
i
/kT), and in general the Helmholtz free energy F52kTln(Z), where E
i
represents
the energies of all the quantized states of the system. For N
a
adsorbed atoms distrib-
uted over N
0
sites, each of which have the same adsorption energy E
a
, Z
a
5
Q(N
a
,N
0
)exp(N
a
E
a
/kT), where Q represents the configurational (and vibrational)
degeneracy. The new element is the configurational entropy, since there are many ways,
at low coverage, to arrange the adatoms on the available adsorption sites. This Q is
given by (e.g. Hill 1960, chapter 7.1) as
Q5N
0
!/(N
a
!)((N
0
2N
a
)!), (4.2)
multiplied by a factor q
N
a
if vibrational effects are included, as discussed below. The
expression for ln(Q) is evaluated using Stirling’s approximation for ln(N!)5N
ln(N)2 N, valid for large N, to give
m
a
5F/N
a
52(kT/N
a
) ln (Z
a
)5kT ln (
u
/(12
u
))2 E
a
2kT ln(q), (4.3)
where
u
5N
a
/N
0
.
4.2 Statistical physics of adsorption at low coverage 109

The first term is the configurational contribution in terms of the adatom coverage
u
,
the second the adsorption energy (measured positive with the vacuum level zero),
and the last term is the (optional) vibrational contribution. We can now see that if
m
a
5
m
s
, the density of adatoms in ML units is determined, in the high temperature
Einstein model, by
m
s
53kT ln(h
n
/kT)2 L
0
5
m
a
. (4.4a)
Using the form of
m
a
in (4.3), and rearranging to find
u
gives, at low coverage,
u
5C exp{(2L
0
1E
a
)/kT}, (4.4b)
where the pre-exponential function C depends on vibrations in both the solid and the
adsorbed layer, and the important exponential term depends on the difference between
the sublimation and the adsorption energy.
The Langmuir adsorption isotherm results from putting
m
a
5
m
v
, using this to calcu-
late the vapor pressure p in equilibrium with the adsorbed layer. We now have
p5C
1
u
/(12
u
) exp (2E
a
/kT), or (4.5a)
u
5
x
(T)p/(11
x
(T)p), with
x
(T)5C
1
21
exp(E
a
/kT); (4.5b)
the constant C
1
can be shown by direct substitution to be kT/q
l
3
. The form of this iso-
therm is shown in figure 4.1(a), using parameters appropriate to xenon adsorbed on
graphite. The coverage starts out linearly proportional to p, but goes to 1 as p → `.
The internal partition function q is the product of vibrational functions for the three
dimensions, i.e. q5q
x
q
y
q
z
. If the Einstein model is chosen, then we can think of the z-
direction, perpendicular to the surface, having a vibrational frequency
n
a
; this is the fre-
quency appropriate to desorption, and in the high temperature limit q
z
5(kT/h
n
a
). The
other two (x,y) frequencies, in the plane of the surface, will be the same on the square
(or triangular, hexagonal) lattice, and correspond to diffusion frequencies
n
d
. Thus q is
inversely proportional to an ‘effective’ value
n
e
3
, for the adsorbed state, namely
n
a
n
d
2
.As
we will see in section 4.4.4, this model is very good for the z-vibrations, but is certainly
not exact for vibrations in the surface plane.
The pre-exponential constant in (4.5) is
C
1
5kT/q
l
3
5(2pm
n
e
2
)
3/2
(kT)
21/2
. (4.6)
It is instructive to note that this is in exactly the same form as that for the vapor pres-
sure in section 1.3.1. Moreover, the value of E
a
includes the zero-point motion, analo-
gously to the sublimation energy L
0
. Inserting reasonably realistic values for the
vibration frequencies in (4.6) gives the full curve of figure 4.1(a), to be compared with
the dashed curve in which vibrational effects are neglected.
4.2.3 The two-dimensional adsorbed gas: Henry law adsorption
If the entropy due to vibrations in the adsorbed layer becomes even more important, the
adsorbate can eventually translate freely in two dimensions. This case is appropriate to
a very smooth substrate, with shallow potential wells, and/or at high temperatures. Thus,
110 4 Surface processes in adsorption
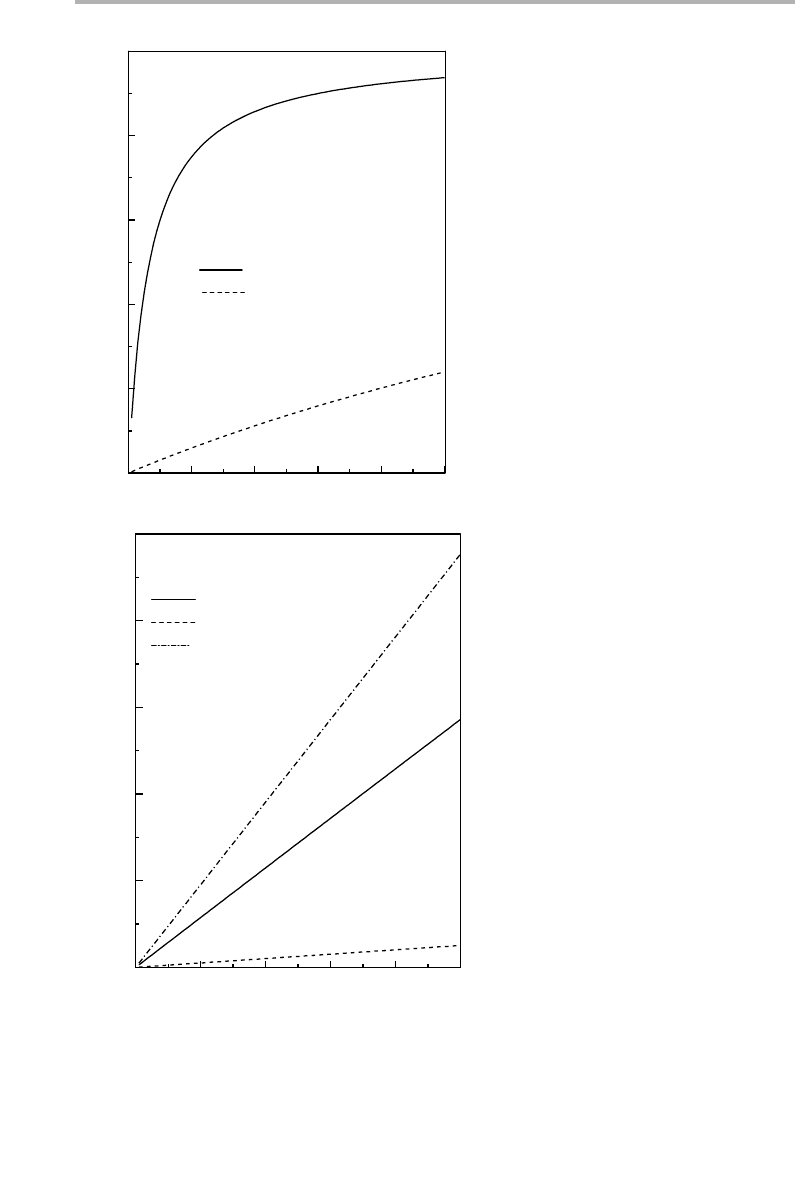
4.2 Statistical physics of adsorption at low coverage 111
Figure 4.1. Vapor pressure isotherms of a monolayer using parameters approximating to
xenon on graphite, with E
a
51925 K/atom (166 meV/atom), but ignoring lateral interactions.
(a) Langmuir isotherms for T560 K: full line, Einstein model for vibration frequencies
n
a
51,
n
d
50.2 THz; dashed line, without vibrational effects so that q51. (b) Comparison of
Langmuir with 2D gas isotherm at T580 K: full and dashed lines as (a), dot-dash line, 2D gas
with average adsorption energy E
0
51889 K/atom. Note the lower coverage scale (4100 with
respect to (a)) and the extra factor of 10 for the dashed curve without vibrational effects.
0.0
2.0x10
–7
4.0x10
–7
6.0x10
–7
8.0x10
–7
1.0x10
–6
0.0
0.2
0.4
0.6
0.8
1.0
(b)
E
a
–
E
0
= 36 K
T
=80 K, Einstein vibrations
No vibrations (x10)
2D gas
Coverage (
θ
) x10
–
2
Pressure (Torr)
0
2x10
–7
4x10
–7
6x10
–7
8x10
–7
1x10
–6
0.0
0.2
0.4
0.6
0.8
1.0
(a)
E
a
= 1925 K
ν
a
= 1,
ν
d
= 0.2 THz
T=60 K, Einstein vibrations
No vibrations
Coverage (
θ
)

in contrast to the previous section, the other limit of isolated adatom behavior is the 2D
gas. The mobile adatoms see the average adsorption energy E
0
, rather than the maximum
energy E
a
at the bottom of the potential wells. In compensation, they gain additional
entropy from the gaseous motion. The chemical potential is now
m
a
52E
0
1kT ln(N
a
l
2
/Aq
z
), (4.7)
where this expression is valid at sufficiently low density for the distinction between clas-
sical, Bose–Einstein and Fermi–Dirac statistics not to be important. The derivation
involves evaluating the partition function by summing over 2D momenta, analogously
to a 3D gas, while retaining the z-motion partition function q
z
. The difference between
2D and 3D accounts for
l
2
rather than
l
3
, and the N
a
/A, the number of adsorbed atoms
per unit area, is the 2D version of the 3D density N/V, as in pV5NkT.
In fact, there is a 2D version of the perfect gas law of the form
F
A5N
a
kT, where
F
is known as the spreading pressure. This means that we could write
m
a
52E
0
1kT ln(
Fl
2
/kTq
z
), (4.8a)
52E
0
1
m
2
1kT ln(
F
), (4.8b)
where
m
2
52kT ln (kTq
z
/
l
2
) is the standard free energy of a 2D gas. This makes the
correspondence between 3D gases and 2D adsorption clear: p↔
F
,
m
0
↔
m
2
, and the
energy is lower in the 2D case by E
0
. Note that it is easy to forget the q
z
term, as is often
done, since the various qs are dimensionless: this doesn’t make them unimportant
numerically.
By equating
m
a
5
m
v
we get Henry’s law for 2D gas adsorption:
p5C
2
(N
a
/A)exp(2E
0
/kT), or (4.9a)
(N
a
/A)5
x
9(T)p, (4.9b)
with
x
9(T)5C
21
2
exp(E
0
/kT), and C
2
5kT/q
z
l
.
You may feel that detailed discussion of these constants is rather laboring the point,
but it is instructive if we stick with it for a while. Note that the 2D gas form has (N
a
/A)
proportional to p, whereas the localized form has the coverage
u
5N
a
/N
0
proportional
to p. These can be reconciled if we write (N
a
/A)5
u
(N
0
/A). Here we have defined the
monolayer coverage (N
0
/A), and then defined (N
a
/A), the areal density of adsorbed
atoms in terms of this, rather artificial, constant. (Both N
0
and N
a
are numbers here,
not areal densities, though we can think of them as densities by choosing A51). This
is the identical problem we discussed in section 2.1.4, emphasizing the need for consis-
tency in the definition of the ML unit. If, however, we do make this definition, we can
recast the 2D gas equation as
p5(kTN
0
/Aq
z
l
)
u
exp(2E
0
/kT), (4.10)
which can be compared directly with the corresponding equation for localized adsorp-
tion.
This comparison shows that there is a transition from localized to 2D gas-like behav-
ior as the temperature is raised, because E
a
.E
0
, whereas the pre-exponential (entropic)
term is larger for the 2D gas. The ratio of coverages at a given p for the two states is
112 4 Surface processes in adsorption

(
u
gas
/
u
loc
)5(2pmkT/h
2
q
x
q
y
) (A/N
0
) exp{(E
0
2E
a
)/kT} (4.11a)
5(2pma
2
n
d
2
/kT) exp{(E
0
2E
a
)/kT}, (4.11b)
where the length a is an atomic dimension (a
2
5A/N
0
). The comparison of Langmuir
and 2D gas isotherms is illustrated in figure 4.1(b) for Xe/graphite parameters at
T580K, using the reasonably realistic value of the well depth (E
0
2E
a
)536 K
(Kariotis et al. 1988). Note that in both models the coverage varies linearly with pres-
sure at low coverage. However, as shown here, the 2D gas model is most appropriate,
but if the well depth were much larger, the localized model with vibrations would be a
better description. The model without vibrations is numerically quite poor in all such
situations.
The second equality (4.11b) is only true for the Einstein model at high temperature.
In this limit, where equipartition of energy holds (no term in h), the following argu-
ment can be made. Localized atoms vibrate with amplitude x, and 4p
2
mx
2
n
d
2
is the
energy associated with this 2D oscillation, which is equal to 2kT at high temperature,
if a harmonic approximation is good enough (a big if ). Thus, the pre-exponential is
just a ratio of free areas (a
2
/px
2
), the numerator associated with the 2D gas, and the
denominator with the potential well in which the adatom vibrates. Clearly the vibra-
tional model starts to fail as x increases towards px
2
5a
2
.
4.2.4 Interactions and vibrations in higher density adsorbates
To consider the statistical mechanics of higher density adsorbates, we need both the
interaction potentials and suitable models of the atomic vibrations. In analogy with the
3D case, moderate densities in a 2D fluid phase can be described by virial expansions
(Hill 1960, chapter 15, Bruch et al. 1997, section 4.2.2). The spreading pressure is given
by
F
/kT5(N
a
/A)1B
2
(T)(N
a
/A)
2
1B
3
(T)(N
a
/A)
3
1. . ., (4.12a)
in which the first term in an expansion in powers of the 2D density (N
a
/A) is the second
virial coefficient, B
2
(T), given by
B
2
(T)521/2
兰
A
[exp (2U(r)/kT) – 1]dr, (4.12b)
where the interaction potential U(r) is between two atoms; the 2D integral is performed
over the substrate area A, where for cylindrical symmetry dr ⬅2prdr. In a relatively
low-density gas at high T, this integral is small due to the fact that the atoms spend
most of their time outside the range of influence of U(r).
There is a continuous line of reasoning between the argument leading to (4.11),
(4.12) and the cell model of lattice vibrations. This model was originally introduced by
Lennard-Jones and Devonshire (1937, 1938) as an approximation of the 3D liquid
state, and described e.g. by Hill (1960, chapter 16) and Bruch et al. (1997, chapter 5).
The free area, A
f
5px
2
in the discussion following (4.11), is defined by integrating the
Boltzmann factor over the ‘cell’ in which the atom vibrates, namely
A
f
5
兰
A
exp (2U(r)/kT)dr; (4.13)
4.2 Statistical physics of adsorption at low coverage 113
the corresponding quantity in 3D is a free volume, V
f
. In a high-density adsorbate with
large U(r) at moderate T, the integral is negligible except at positions r close to the equi-
librium spacing. This approximate, classical theory, is very effective in computations
for solids at high temperature, since it includes thermal expansion – the response to the
spreading pressure exerted by the anharmonic vibrations – which many, apparently
more sophisticated models, ignore. For these reasons at least, it deserves to be better
known and more widely used as a teaching aid.
One anharmonic model which aims to have to have the correct low temperature
limit, and to be useful at higher T, is the quantum (or quantum-corrected) cell model
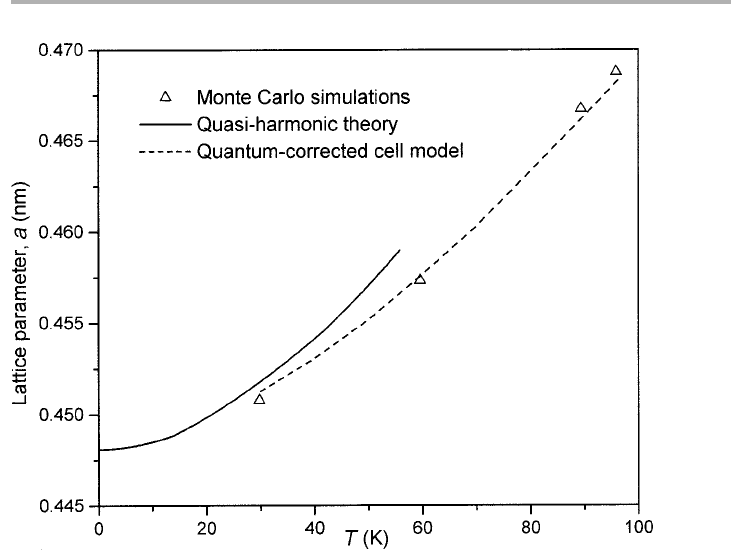
(Holian 1980, Barker et al. 1981, Bruch et al. 1997, chapter 5). A comparison of lattice
dynamical models for a 2D solid monolayer interacting via the approximate Lennard-
Jones potential, with parameters appropriate to xenon, is shown in figure 4.2.
4.3 Phase diagrams and phase transitions
One of the intriguing aspects of both physi- and chemisorption is the large number of
phases that can exist at the surface, and the transitions that occur between these phases.
114 4 Surface processes in adsorption
Figure 4.2. Thermal expansion of a 2D Lennard-Jones triangular monolayer solid on a
smooth substrate, computed for Xe interaction potential parameters (Bruch et al. 1997, after
Phillips et al. 1981, replotted with permission). Results for the quasi-harmonic theory (QHT)
are good at low temperature, with the (quantum-corrected) cell model agreeing closely with
classical Monte Carlo calculations at high T. The triple point of 2D Xe (on graphite) is 99 K.
There is a comparable richness of structure to that displayed in high pressure physics,
where there is both a density
r
, and a corresponding structure, at a given p and T. The
relation
r
5f(p,T ) is called the Equation of State (EOS) in the (3D) physics of bulk
matter, or the (p,V,T ) relation.
4.3.1 Adsorption in equilibrium with the gas phase
The corresponding equilibrium equation for (2D) adsorbed layers is
u
5f(p,T ), and
since we have already used
m
v
5
m
0
1kT ln (p), and
m
v
5
m
a
, we can think of
u
5f(
m
,T)
equivalently. For
u
, read N
a
if we do not define the ML unit in the standard way. So, as
we compress a 2D gas or localized adlayer by increasing the (gas) pressure p, the
adatoms will come within range of their mutual attractive or repulsive forces, and
phase transitions may result, first within the ML, and subsequently from ML to multi-
layer.
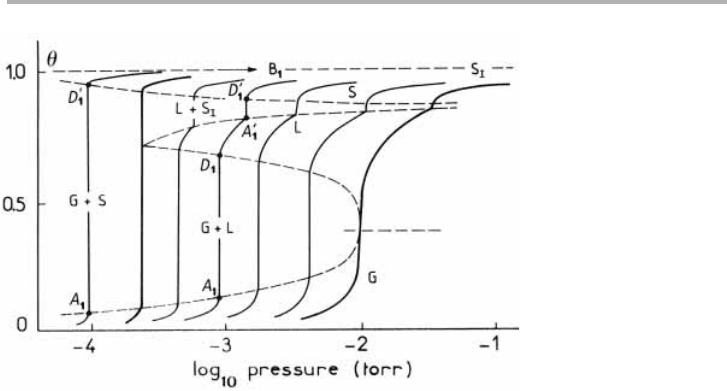
If the substrate and adsorbate are well ordered, the condensation may proceed in
well defined steps, as shown in figures 4.3 and 4.4 for physisorbed Xe and Kr/graphite
respectively at ML and sub-ML coverages. As studied by several French groups espe-
cially (Thomy et al. 1981, Thomy & Duval 1994, Suzanne & Gay 1996), these volumet-
ric studies, using high quality exfoliated graphite, established the existence of 2D solid,
liquid and gaseous layers. The (p,T) positions of the phase transitions (including multi-
layer transitions) and fixed points such as triple points and critical points in these layers
were also accurately measured.
More recent experimental thermodynamic work has automated the measurement
process, and has achieved very high accuracy for quantities which depend on the slope
4.3 Phase diagrams and phase transitions 115
Figure 4.3. Sub-ML isotherms for Xe/graphite between 97 and 117 K. The isotherms, from left
to right, are at 97.4, 100.1, 102.4, 105.4, 108.3, 112.6 and 117.0 K. Between 110.1 and 117 K,
the layer undergoes two first order phase transitions, showing 2D gas (G), liquid (L) and solid
(S) phases, whereas at 97.4 K only the G to L transition occurs; the 2D triple point is at 99 K
(after Thomy et al. 1981, reproduced with permission).
of the isotherm, such as the isothermal compressibility. Examples for Kr in the multi-
layer region are given by Gangwar & Suter (1990) and for Xe near ML melting by
Gangwar et al. (1989) and Jin et al. (1989). An example showing the much improved pre-
cision of these data is given in figure 4.5 which can be directly compared with figure 4.3.
Before we examine these results, we can note the different forms of graphs and phase
diagrams that can be plotted. The problem arises because we have three variables, T,
ln(p)or
m
, and
u
, but we typically want to output onto paper, so that one of these three
is not plotted; the corresponding (third piece of) information may either not be known,
or may be discarded, or it may be given as a parameter.
An isotherm is a graph of
u
against ln(p) or
m
, with T as the parameter, as used in
the previous section. A phase diagram using log(p) and 1/T as axes is very convenient
for (physisorption) experimentalists, because the pressure can be varied over many
orders of magnitude, and this plot results in straight lines for phase transitions (e.g.
gas–solid or monolayer–bilayer transitions) which show an Arrhenius behavior – the
slope of these lines give the corresponding energies. But typically the coverage infor-
mation is lost. Theorists are fond of phase diagrams as a function of T and
m
: this gives
them the chance to investigate the adsorbed phase, and ignore the 3D gas phase, which
provides the value of
m
, typically D
m
with respect to the bulk 3D phase, when the com-
parison with experiment is made later.
116 4 Surface processes in adsorption
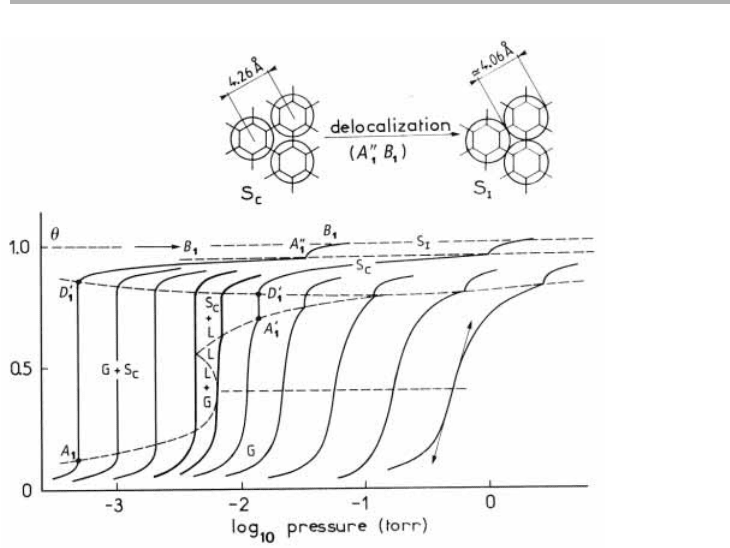
Figure 4.4. Sub-ML isotherms for Kr/graphite between 77 and 110 K. The isotherms are at
77.3, 79.8, 82.3, 84.8, 86.0, 88.0, 91.8, 96.6, 102.6 and 109.5 K. These show 2D gas, liquid
(maybe) and two solid phases, with a presumed solid-solid phase transition at point A
1
0, which
is not first order (after Thomy et al. 1981, reproduced with permission).
