Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


E.4 Protein-based Machines as Physical Embodiments of the "Vital Force" That Sustains Life
555
y-rotor rather than behind it in position
to push the rotor in the expected counter-
clockwise direction (see immediately
below).
Prediction 8: AGap drives counterclockwise
rotation of the FrATPase rotor. As was
shown in Figure
8.41,
the AGap-derived repul-
sive force between S04^~ and the y-rotor that
causes movement "in opposite directions" of
the PE-ADP-S04^" and y-subunits in Figures
E.2 through E.4 would provide a counter-
clockwise rotational impulse to the y-rotor, as
demonstrated by Noji et
al.^^
The same con-
clusion was reached by Menz et al.,^^ as indi-
cated by the statement that "the observed
rotation of the y subunit is consistent with the
sense of rotation seen in the direct visualiza-
tion experiments (Noji et al., 1997) "
The success of the above set of predictions
concerning structure and function of the Fj-
ATPase (the Fi-motor of ATP synthase) demon-
strate a dominant role of the hydrophobic and
elastic consilient mechanisms in the function of
this pivotal protein-based machine of biology.
E.4J.3 Use of ATP to Perform
Mechanical Work of the Organism,
Chemo-mechanical Transduction by
the Myosin II Motor
E.4.1.3.1 Perspective of the Hydrophobic
Consilient Mechanism in the Performance
of Mechanical Work
Chemo-mechanical transduction technically
defines the use of chemical energy of ATP to
produce motion, as in muscle contraction. The
fundamental statement of the hydrophobic
consilient mechanism regarding chemo-
mechanical transduction is that the most
charged, polar states disrupt hydrophobic asso-
FiGURE E.3. Cross-eye stereo view in trace repre-
sentation from the top of the Fi-motor of ATP syn-
thase of the superposition of
two
structures, one with
the empty pE-site given in light gray and the second
with the ADP-S04-occupied Pn-subunit in dark gray.
An increase in mean distance between the y-rotor
and the PE-subunit is shown when the empty PE-
subunit is replaced by ADP-SO4 and when the SO4
fills the role of a stable analogue for hydrolyzed y-
phosphate. The doubly charged S04^" acts to free
charged side chains of the PE-subunit from ion
pairing and hydrogen bonding such that they will
also contribute to the total apolar-polar repulsion,
AGap, between the ADP-S04-occupied PE-subunit
and rotor. The resulting
AGap
applies a torque to the
double-stranded a-helical coiled coil section of the
y-rotor (residues 3-35 and 221-250 shown) that
would impart a counterclockwise rotation to the
rotor when functioning as the Fi-ATPase.^^ This is
shown more explicitly in Figure E.4 and especially in
Figures 8.40 and 8.41. (From Menz et al.^^ adapted
for cross-eye view with permission of Elsevier.)
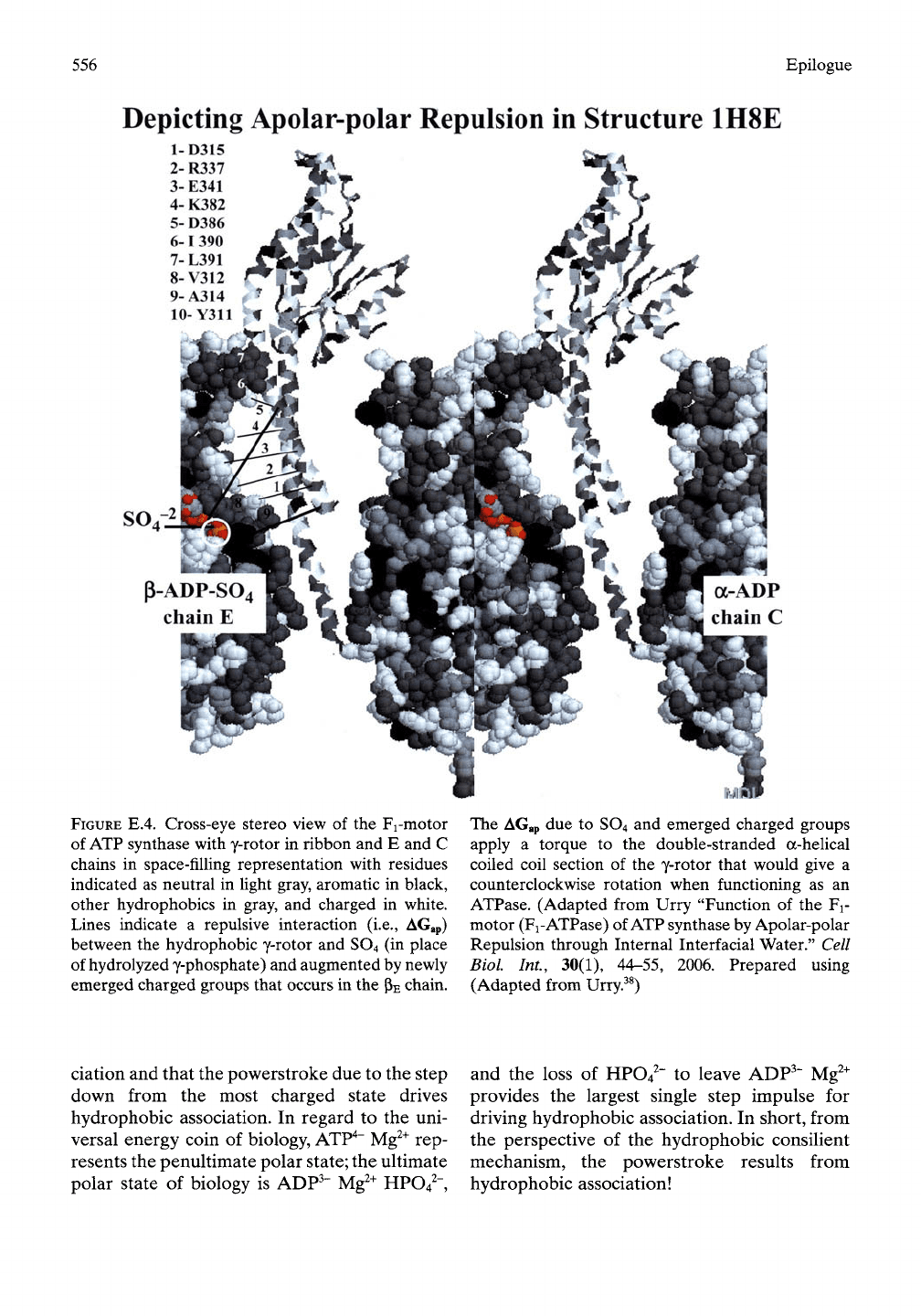
556 Epilogue
Depicting Apolar-polar Repulsion in Structure 1H8E
a-ADP
chain C
FIGURE E.4. Cross-eye stereo view of the Fi-motor
of ATP synthase with y-rotor in ribbon and E and C
chains in space-filhng representation with residues
indicated as neutral in light gray, aromatic in black,
other hydrophobics in gray, and charged in white.
Lines indicate a repulsive interaction (i.e., AGap)
between the hydrophobic y-rotor and SO4 (in place
of hydrolyzed y-phosphate) and augmented by newly
emerged charged groups that occurs in the
PE
chain.
The AGap due to SO4 and emerged charged groups
apply a torque to the double-stranded a-helical
coiled coil section of the y-rotor that would give a
counterclockwise rotation when functioning as an
ATPase. (Adapted from Urry "Function of the Fi-
motor (Fi-ATPase) of ATP synthase by Apolar-polar
Repulsion through Internal Interfacial Water." Cell
Biol Int., 30(1), 44-55, 2006. Prepared using
(Adapted from Urry.^^)
ciation and that the powerstroke due to the step
down from the most charged state drives
hydrophobic association. In regard to the uni-
versal energy coin of biology,
ATP"^"
Mg^^ rep-
resents the penultimate polar state; the ultimate
polar state of biology is ADP^" Mg^^ HPO/",
and the loss of HP04^- to leave ADP^" Mg^^
provides the largest single step impulse for
driving hydrophobic association. In short, from
the perspective of the hydrophobic consilient
mechanism, the powerstroke results from
hydrophobic association!

E.4 Protein-based Machines as Physical Embodiments of the "Vital Force" That Sustains Life
557
E.4.1.3.2 Structure of the Myosin II Motor
and the Sliding Filament Model of
Muscle Contraction
The myosin II motor produces the very famil-
iar motion resulting from contraction of skele-
tal muscle. From the gross anatomical
perspective, the motion of contraction derives
from the decrease in length between two sites
of muscle attachment. At the level of the elec-
tron microscopic, shortening results from short-
ening the length of the fundamental repeating
unit of muscle, the sarcomere. As shown in
Figure 8.45, shortening of the sarcomere length
defined by the distance between Z disks would
occur by the sliding into greater overlap of two
classes of filaments (myosin and actin) that
intersperse in hexagonal arrays such that 24
thin actin filaments separate and surround
seven thick myosin filaments. Long myosin mol-
ecules, staggered in their association to make
the thick filament, terminate in cross-bridges
that attach to the thin actin filaments. Each
cross-bridge contains a single nucleotide
binding site that functions as an ATPase, and
the breakdown of ATP to ADP with release of
Pi produces the motion of muscle contraction.
Thus,
contraction by the sliding filament model
of the foregoing construction requires cyclic
attachment/detachment from the actin filament
correlated with cyclic contraction/relaxation.
In our approach we look to the most polar
states associated with the use of ATP for dis-
ruption of hydrophobic association and to the
absence of such polar states as giving rise to
hydrophobic association. For the myosin II
motor, two crystal structures of the cross-bridge
from scallop muscle, due to Szent-Gyorgyi and
Cohen and their coUeagues,^^ provide the
opportunity for such an analysis. One structure
is called a near-rigor state without nucleotide,
but it does contain a polar sulfate, but partially
neutralized as the
Mg^"^
SO/~. At the active site
the other structure contains an analogue of
ATP^-
Mg'% namely, ADP^" Mg^^-BeFx (where
BeFx is to fill the role of the y-phosphate).
These two crystal structures do not provide
snapshots of the extremes that would be pre-
ferred, but they do allow useful comparisons of
two structures near the desired limits.
Starting from the prejudice of the hydropho-
bic and elastic consilient mechanisms and uti-
lizing the two noted structures, we proceed
within the framework of the sliding-filament
cross-bridge structure with a single nucleotide
binding site in each cross-bridge. In Chapter 8,
section 8.5.4, this conceptual context brought
to light a set of findings much as would be
expected from the understanding of the
hydrophobic and elastic consilient mechanisms
developed in Chapter 5. The following sum-
marizes these findings for the myosin II motor
in terms of a set of six expectations that we suc-
cinctly examine for extent of realization.
Expectation 1: That ATP binding at the cross-
bridge active site provides a AGap-based repul-
sive force for detachment by hydrophobic
dissociation from the actin filament. On occu-
pancy of an empty active site by the penul-
timate polar state, ATP"^" Mg^"^, disruption
of hydrophobic association becomes the
primary expectation of the hydrophobic con-
silient mechanism. Of course, ATP binding
disrupts cross-bridge binding to the actin fil-
ament, that is, disrupts the hydrophobic
association that provides for attachment of
cross-bridge to the actin filament. The
hydrophobic consilient mechanism requires,
however, that "waters of Thales" occur
between the phosphates of ATP and the site
of hydrophobic association in order that an
apolar-polar repulsive free energy of hydra-
tion, AGap, may exist between the two sites.
Expectation 5 and Figures E.5 and E.6
address with the structural feature whereby
the myosin II motor directs this AGap-based
repulsive force'.
Expectation 2: That the ADP plus Pi state at the
cross-bridge active site effects a repulsive
AGap force for dissociation of hydrophobic
domains within the cross-bridge. Relevance
of the hydrophobic consihent mechanism to
the motion of contraction requires that
formation of the most polar state, ADP^"
Mg^^ HPO/~, effects hydrophobic dissocia-
tion within the cross-bridge. This sets the
stage for the loss of Pi, that is, of HPO/", that
drives the hydrophobic association required
by the hydrophobic consilient mechanism as

558
Epilogue
N-terminal domain
with lever arm tucked
under it by means of
rophobic association
Tip of triangular scale
which overhangs cleft
to site of hydrophobic
association for
the powerstroke
Hydrophobic
hea<
of lever arm used
in powerstroke
FIGURE E.5. Stereo view (cross-eye) in space-filling
representation of the scallop muscle cross-
bridge(Sl) viewed approximately from the side of
the actin binding site on myosin cross-bridge for the
purpose of locating the narrow clefts that direct the
apolar-polar repulsive force. (A) The hydrophobic
association of the near-rigor state. (Prepared using
the crystallographic results of Himmel et al.^ as
obtained from the Protein Data Bank, Structure File
1KK7.)
(B)
The hydrophobic dissociation of the ATP
analogue state. (Prepared using the crystallographic
results of Himmel et al.^ as obtained from the
Protein Data Bank, Structure File 1KK8.)
the basis for the powerstroke. The very
apparent hydrophobic dissociation is seen in
Figure 8.51B, 8.52B, 8.53, and 8.54.
Expectation 3: That the rigor-like state demon-
strates a re-established hydrophobic associa-
tion that defined the powerstroke. Tht absence
of highly polar occupancy of the active site,
as in the near-rigor state show^n in Figure
E.5A, illustrates formation of hydrophobic
association between the head of the lever
arm and the underside of the amino-terminal
domain. This involves the very hydrophobic
residues L711, L712, L767, and F716 as well
as F707, F761, and F761, associated with
the head of the lever arm, and the very
hydrophobic residues of L94, Y93,1700, F28,
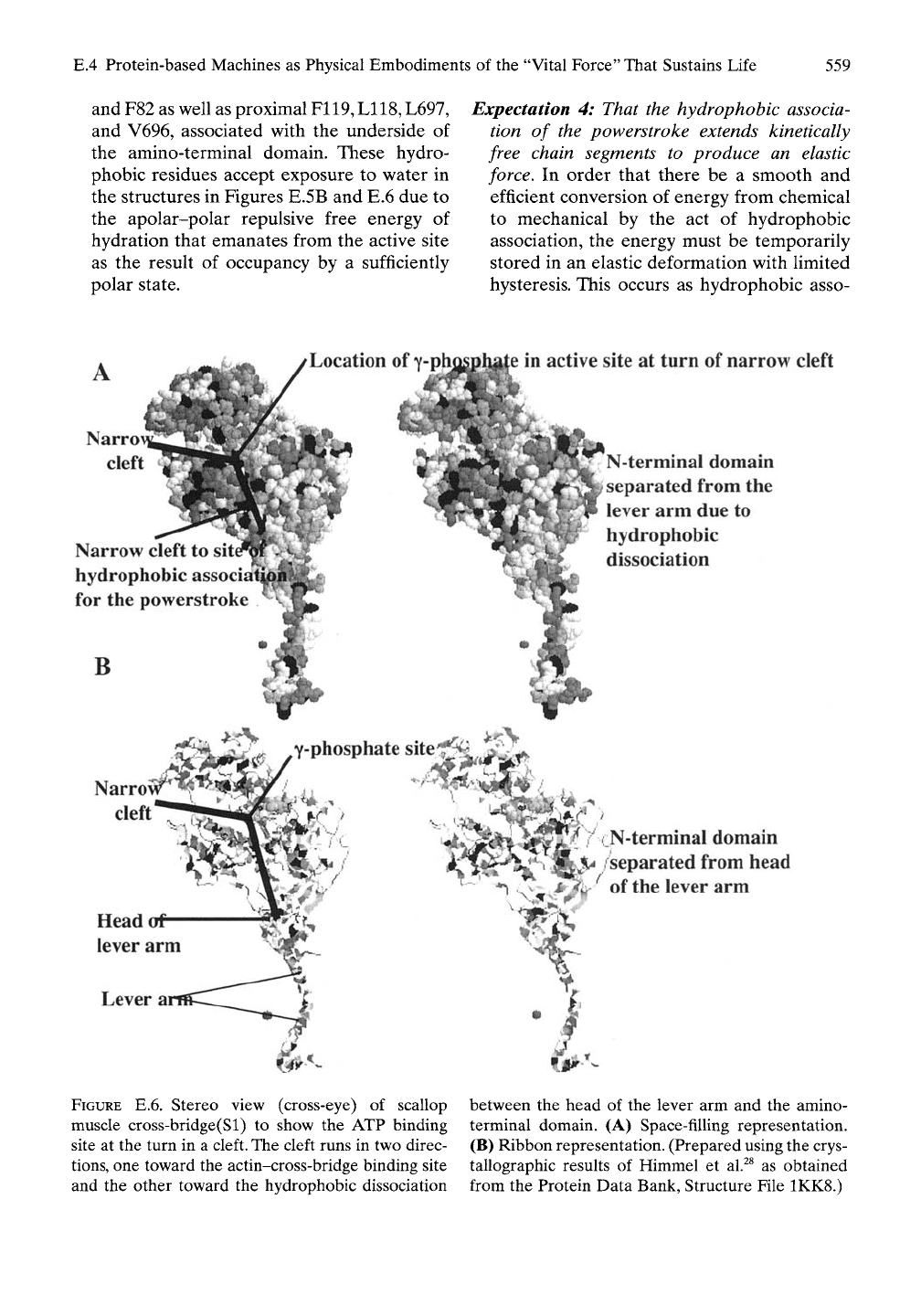
E.4 Protein-based Machines as Physical Embodiments of the "Vital Force" That Sustains Life
559
and F82 as well as proximal F119,
L118,
L697,
and V696, associated with the underside of
the amino-terminal domain. These hydro-
phobic residues accept exposure to water in
the structures in Figures E.5B and E.6 due to
the apolar-polar repulsive free energy of
hydration that emanates from the active site
as the result of occupancy by a sufficiently
polar state.
Expectation 4: That the hydrophobic associa-
tion of the powerstroke extends kinetically
free chain segments to produce an elastic
force. In order that there be a smooth and
efficient conversion of energy from chemical
to mechanical by the act of hydrophobic
association, the energy must be temporarily
stored in an elastic deformation with limited
hysteresis. This occurs as hydrophobic asso-
^.L..^
/Location of
Y*P)U)SliiU^
in active site at turn of
narrov^
cleft
'^ *
Narro
cleft
Narrow cleft to
hydrophobic associ
for the powerstroke
B
Narro^
cleft
' M'^
N-terminal domain
separated from the
lever arm due to
hydrophobic
dissociation
"" Y"Phosphate
sitelf^'-
" •
/' cN-terminal domain
^1i^ /separated from head
"\^^^^' of
the
lever arm
j
FIGURE E.6. Stereo view (cross-eye) of scallop
muscle cross-bridge(Sl) to show the ATP binding
site at the turn in a cleft. The cleft runs in two direc-
tions,
one toward the actin-cross-bridge binding site
and the other toward the hydrophobic dissociation
between the head of the lever arm and the amino-
terminal domain. (A) Space-filling representation.
(B) Ribbon representation. (Prepared using the crys-
tallographic results of Himmel et al.^^ as obtained
from the Protein Data Bank, Structure File 1KK8.)

560
Epilogue
ciation stretches interconnecting chain seg-
ments. A significant search has yet to occur
throughout the cross-bridge. Noted, however,
in Figure 8.52 and featured in Figure 8.55 is
the extension of an interconnecting chain
segment that occurs on going from the
hydrophobically dissociated state with ATP
analogue occupancy of the active site to
the hydrophobic association of the near rigor
state.
Here resides the coupling of the
hydrophobic and elastic consilient mecha-
nisms for the function of protein-based
machines.
As noted by Rayment and coworkers,^^ a
number of flexible loops occur in the cross-
bridge, and we suggest that these are poten-
tial sources for elastic force development
that results from hydrophobic association
of the powerstroke (see Chapter 8, section
8.5.4.5).
Expectation 5: That narrow clefts direct the
repulsive AGap force in two directions to
disrupt hydrophobic association of cross-
bridge-actin binding and to disrupt the
hydrophobic association responsible for the
powerstroke. As shown on Figure E.6, a
narrow cleft runs from the site of the cross-
bridge-actin binding to the active site where
it makes a sharp turn and runs directly to the
site for hydrophobic association between the
head of the lever arm and the hydrophobic
underside of the amino-terminal domain. In
terms of the hydrophobic consiUent mecha-
nism these clefts direct the thirst for hydra-
tion of the polar species in the active site
toward the sites of hydrophobic association.
The narrow clefts function as conduits to
direct the repulsive force due to the apolar-
polar repulsive free energy of hydration,
AGap, to interaction with sites for hydropho-
bic association. In Figure E.5, the cleft, con-
necting the y-phosphate of ATP to the
hydrophobic association between the head of
the lever arm and the underside of the
amino-terminal domain, runs under an over-
hang of a triangular surface scale identified
by residues P571-R560-F539 at the three
apices of the triangle. Figure E.6A allows a
look directly into this section of the cleft.
Expectation 6: That calcium ion triggers con-
traction by opening a hydrophobic site on
actin that hydrophobically associates with the
cross-bridge and increases the repulsive AGap
force on ADP plus Pi to expel P, that relieves
repulsion to drive the hydrophobic associa-
tion of the powerstroke. Introduction of the
process whereby calcium ion triggers con-
traction by the hydrophobic consilient
mechanism requires knowledge of the state
of ATP in the active site of the dissociated
cross-bridge and the state that binds to the
actin filament. In the ATP occupied state of
the detached cross-bridge, an active ATP <-^
ADP Pi interchange occurs that appears to
be more toward the hydrolyzed state.^^ Fur-
thermore, as noted by Lymn and Taylor^^
quite early, "actin binds with the myosin ADP
P complex and displaces products of the
hydrolysis reaction. It is concluded that this
step is responsible for activation of myosin
ATPase by actin." Thus the state of
nucleotide in the cross-bridge on binding to
the actin site would be the most polar state,
ADP^-
Mg^^ HPO/-. The description of the
process whereby calcium ion triggers con-
traction by binding to troponin C on the
actin filament is particularly well-suited to
description by the force resulting from the
apolar-polar repulsive free energy of hydra-
tion, AGap, of the hydrophobic consiUent
mechanism.
The essential result of calcium ion binding
is that it displaces tropomyosin to open a
strong hydrophobic binding site on actin for
cross-bridge attachment. Hie attachment to
actin would close off a major source of hydra-
tion for the most polar state, ADP^" Mg^^
HPO/",
that was provided by the narrow cleft
connecting the active site to the cross-bridge-
actin binding site. This could raise the free
energy of the most polar state to such an
extent that lowering the free energy would be
achieved by expulsion of HPO/". With this
loss of phosphate, the apolar-polar repulsion
maintaining the hydrophobic dissociation at
the other end of the narrow cleft is lost such
that the hydrophobic association of the
powerstroke results.

E.5 Extraordinary Biomaterials Opportunities Arise
561
The above expectations that describe the
structure and function of the myosin II motor
suggest a dominant role for the hydrophobic
and elastic consilient mechanisms in the function
of this representative motor for producing
motion in living organisms.
E.4.1.4 Concluding Comment for Physical
Embodiments of the ''Vital Force''
We have discussed the nexus of hydrophobic
and elastic consihent mechanisms in Complex
III at the intersection of electron flow and
proton translocation (electro-chemical trans-
duction) that was unimaginable, absent the
detailed analysis of structure. The hydrophobic
and elastic consilient mechanisms, however,
when applied to the general structure and
phenomenology of the Fi-motor of ATP
synthase (chemo-chemical transduction), gave
rise to a host of successful predictions, and,
when applied to the structure and phenome-
nology of the myosin II motor (chemo-
mechanical transduction), resulted in a
half-
dozen realized expectations. These findings do
much to substantiate the relevance of the
hydrophobic and elastic consilient mechanisms
to the protein-based machines of biology.
The above three discussed protein-based
machines—Complex III of the electron trans-
port chain, ATP synthase/Fj-ATPase, and the
myosin II motor of muscle contraction—repre-
sent the three major classes of energy conversion
that sustain Life. Therefore, the facility with
which the consilient mechanisms explain their
function indeed support the thesis that biology's
"vital force" arises from the coupled hydropho-
bic and elastic consilient mechanisms.
When taken together, the successes of the
hydrophobic and consilient mechanisms in
describing the functions of these three represen-
tative classes of biological energy conversion
constitute a significant statement in favor of the
roles of these mechanisms as "vital forces" that
impart function to the protein-based machines
of biology.
E.5 Extraordinary Biomaterials
Opportunities Arise from
Identified Vital Forces,
Biocompatibility, and
Biosynthesis
E.5.1 An Enabling Triumvirate:
Knowledge of Vital Forces, Capacity
for Biosynthesis, and Elastic
Protein-based Polymers with
Unique BiocompatibiUty
E.5.1.1 An Enabling Triumvirate
The above-detailed successes of the hydro-
phobic and elastic consilient mechanisms in
describing the functions of multifaceted pro-
teins of biology herald an historic naissance for
biomaterials innovation. Singularity arises from
the near-simultaneous emergence of three
developments: (1) the deepened understanding
of the forces that provide the basis for protein
function, (2) the unparalleled capacity to
produce diverse protein-based materials by
recombinant DNA technology, and (3) the
remarkable biocompatibility of elastic protein-
based polymers. Achieving an emerging
command of the vital forces that underpin
living organisms points to the design of protein-
based polymers with the capacity to perform
the desired tasks of biomaterials with the speci-
ficity, selectivity, and efficiency that attend
biology's own protein function.
E.5.1.2 Each Application Warrants a
Specifically Optimized Sequence
This brings us in an unprecedented way to the
circumstance envisioned by Jacob Bronowski
in his 1973 book. Ascent of Man,^^ where he
stated, "In effect, the modern problem is no
longer to design a structure from the materials
(available) but to design materials for a struc-
ture." We are now in the position to design
protein-based polymers for each specific appli-

562
Epilogue
cation. The past has seen coercion of function
by chemical treatments and physical manipula-
tion of a single starting polymer composition.
Now, whenever an application is defined,
response can be the design of a unique
protein-based polymer with a specified and
application-optimized sequence. Often the
basic consideration becomes the apolar-polar
repulsive free energy of hydration,
AGap,
within
the chain that results from the repulsive inter-
action of the charged and hydrophobic groups.
E.5.1.3 Mechanical Resonances Are Key
to Biocompatibility and Certain Medical
and Nonmedical Applications
For medical applications, in our view, mechan-
ical resonances (see Section 9.4.2.3)^^ present
barriers to antibody interaction such that these
soft elastic biomaterials exhibit a remarkable
biocompatibility otherwise considered impossi-
ble for "foreign proteins." As a specific example
for medical and nonmedical applications, the
author beUeves that the finding of mechanical
resonances, so innovative as to be denounced as
artifact by those constrained by the "idols of the
present,"^^ constitutes opportunities for the
future ranging from biosensors capable of
single molecule detection to hearing protection
and underwater sound absorption.
E.5.2 Medical Devices Using
Biology's Own Mechanisms
and Materials
E.5.2,1 The
Consilient
Approach to
Medical Devices
Here again the adjective consilient becomes
relevant. In the present context there occurs
"a common groundwork of explanation"^ for
function within the medical device, within the
patient for whom support is being designed,
and between device and patient. The consilient
approach, therefore, employs the same hydro-
phobic and elastic consilient mechanisms in the
design of the medical device that operate at the
site of appUcation, as demonstrated in Chapters
6, 7, 8, and 9 and above in section E.4.
E.5.2.2 The Consilient Approach to Soft
Tissue Engineering
E.5.2.2.1 The Concept of the Consilient
Approach to Soft Tissue Engineering
As stated in Chapter 9, "The consilient
approach to tissue engineering utilizes
biology's own materials and mechanisms, con-
cerned with tissue structure and function, to
achieve tissue restoration." The key materials
elements are three: the capacity of the elastic
protein-based material to match the elastic
modulus of the tissue to be restored, a remark-
able biocompatibility of the pure elastic
protein-based material, and the facility to
design into the protein-based polymer
sequence any desired biologically active
sequence, which, by virtue of the innocuousness
of the elastic protein-based material, allows
proper expression of the incorporated biologi-
cally active sequence. This provides the oppor-
tunity for the proper functional relationship of
protein-based material to the cells and the
extracellular matrix of the tissue to be restored.
E.5.2.2.2 The Concept of Temporary
Functional Scaffoldings
It is our thesis that materials and tissue (extra-
cellular matrix and cells) function by the
hydrophobic and elastic consiUent mechanisms.
Importantly, the material can be designed to
contain the appropriate cell attachment
sequences for the natural cells of the tissue to
be restored. The natural cells of the tissue to be
restored can migrate into the material, attach,
and sense the tensional force changes that
occur during functioning of the prosthesis. As
cells are themselves mechano-chemical trans-
ducers, the magnitude and periodicity of the
tensional force changes to which the cells are
subjected induce the cells to produce the extra-
cellular matrix sufficient to sustain those force
changes. The concept continues; in the normal
ongoing remodeling process the natural cells
within the prosthesis remodel it into a natural
tissue as the protein-based prosthesis is
replaced by an appropriate rate of degradation.
The concept of a temporary functional
scaf-
folding for soft tissue restoration is temporary

E.5 Extraordinary Biomaterials Opportunities Arise
563
in that it can be set for an appropriate rate of
degradation and
is
functional because it has the
appropriate elastic modulus with which to fill
the mechanical role of prosthesis, and it con-
tains cell attachment sequences whereby the
cells interact and sense the demand on the
tissue that they remodel into the natural tissue.
E.5.2.3 The Central Role of AGap in the
Controlled Release of Pharmaceuticals
E.5.2.3.1 AGap Sequence Energizes
Protein-based Polymers for Function
The competition for hydration between
charged and hydrophobic groups constrained
by sequence to coexist in a chain molecule
energizes the chain molecule as these disparate
groups reach out for hydration spheres unper-
turbed by the other. This gives rise to an
apolar-polar repulsive free energy of hydra-
tion,
AGap,
that energizes a particular sequence.
As the repulsion becomes greater, the charges
become driven toward charge neutralization
by ion pairing. The hydrophobic induced pKa
shift provides a measure of the extent of the
repulsion, that is, of AGap.
When the pH is not suitable, instead of neu-
tralization by protonation of a carboxylate, for
example, ion pairing becomes the possible
means of neutralization. Ion pairing, however,
only lowers the free energy about half as well
as protonation. Nonetheless, ion pairing pro-
vides a most useful force as in the process of
aligning complementary chains and in loading
charged drug into interaction with an op-
positely charged polymer. With regard to
the charged-drug-oppositely-charged-polymer
interaction, increased hydrophobicity of the
polymer enhances drug affinity and decreases
release rate of drug.
E.5.2.3.2 Drug Delivery by Trtype
(Transductional) Protein-based Polymers
Another aspect of ion pairing to lower AGap is
lowering the temperature of the inverse tem-
perature transition. This means that the oppo-
sitely charged polymer can be soluble, because
the onset temperature for the transition, Tt, is
above physiological temperature. The effect of
ion pairing with the pharmaceutical, as part of
lowering AGap, is to lower the value of Tt such
that ion pairing drives the phase separation.
Therefore, the phase-separated state
becomes the drug delivery vehicle, and one of
such a nature that on release of the drug the
vehicle itself disperses. This means for a con-
stant surface area that there occurs a zero order
release of drug, as shown in Figure E.7 for
positively charged Leu-enkephalin amide ion
pairing with carboxylate-containing protein-
based polymers. By polymer design the number
of ion pairing sites determines the density of
drug in the drug delivery vehicle, and the level
of drug release is inversely proportional to the
intensity and number of hydrophobic groups.
Thus,
as shown in Figure E.7, a remarkable
control of pharmaceutical release is possible.
Furthermore, the pharmaceutical can vary all
the way from a simple bare cation or anion
to a protein or nucleic acid. Under favorable
circumstances, as with carboxylate groups,
the vehicle disappears as the pharmaceutical
releases. With a cationic polymer such as a
lysine-containing protein-based polymer, the
chloride ion can displace the pharmaceutical to
lessen the zero order release. Significantly, with
elastic protein-based polymers, no fibrous
capsule forms around the adequately purified
polymer such that this does not affect the
release process.
This enabling triumvirate—knowledge of the
vital forces (e.g., AGap), the capacity for precise
control of desired sequence, and extraordinary
biocompatibility—provides controlled release of
pharmaceuticals to an extent that has only now
become possible.
E.5.2.4 The Central Role of AGap for
Production of Fibers with Superior
Physical Properties
The issue of communication between mole-
cules enters into the consideration of "order
out of chaos" as discussed below. The specific
aspect of communication between molecules
here involves the association of complementary
chains to achieve aligned and cross-linked poly-
mers.
One area where this has application is in
fiber formation. In this case, one chain mole-
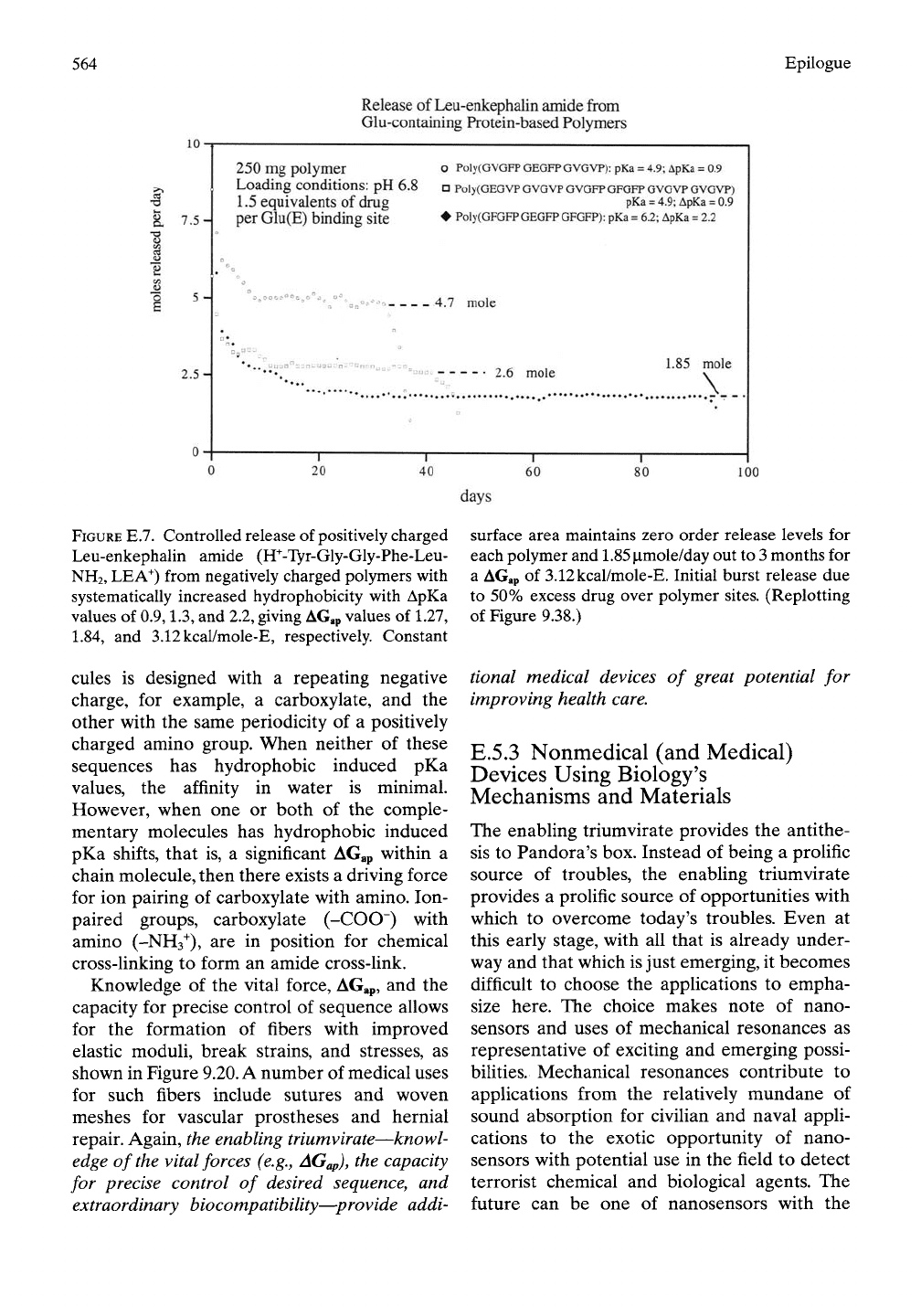
564
Epilogue
Release of Leu-enkephalin amide from
Glu-containing Protein-based Polymers
10
t
7.5-
I "^
2.5-
250 mg polymer
Loading conditions: pH
6.8
1.5 equivalents of drug
per Glu(E) binding site
O PolyCGVGFP GEGFP
GVGVP):
pKa = 4.9; ApKa = 0.9
D Poly(GEGVP GVGVP GVGFP GFGFP GVGVP GVGVP)
pKa =
4.9;
ApKa =
0.9
• Poly(GFGFP GEGFP
GFGFP):
pKa = 6.2; ApKa = 2.2
4.7 mole
2.6 mole
1.85
mole
\
"T"
20
40
FIGURE E.7. Controlled release of positively charged
Leu-enkephalin amide (ff-Tyr-Gly-Gly-Phe-Leu-
NH2,
LEA^) from negatively charged polymers with
systematically increased hydrophobicity with ApKa
values
of
0.9,1.3,
and
2.2,
giving AG.p values
of
L27,
L84,
and
3.12kcal/mole-E, respectively. Constant
cules
is
designed with
a
repeating negative
charge,
for
example,
a
carboxylate,
and the
other with
the
same periodicity
of a
positively
charged amino group. When neither
of
these
sequences
has
hydrophobic induced
pKa
values,
the
affinity
in
water
is
minimal.
However, when
one or
both
of the
comple-
mentary molecules
has
hydrophobic induced
pKa shifts, that
is, a
significant AGap within
a
chain molecule, then there exists
a
driving force
for
ion
pairing
of
carboxylate with amino.
Ion-
paired groups, carboxylate
(-COO~)
with
amino (-NHs^),
are in
position
for
chemical
cross-linking
to
form
an
amide cross-link.
Knowledge
of the
vital force, AGap,
and the
capacity
for
precise control
of
sequence allows
for
the
formation
of
fibers with improved
elastic moduli, break strains,
and
stresses,
as
shown
in
Figure
9.20.
A
number
of
medical uses
for such fibers include sutures
and
woven
meshes
for
vascular prostheses
and
hernial
repair. Again,
the
enabling triumvirate—knowl-
edge
of
the vital forces
(e.g.,
AGap),
the
capacity
for precise control
of
desired sequence,
and
extraordinary biocompatibility—provide addi-
60 80
100
days
surface area maintains zero order release levels
for
each polymer and 1.85 p.mole/day out to
3
months
for
a AGap
of
3.12kcal/mole-E. Initial burst release
due
to 50% excess drug over polymer sites. (Replotting
of Figure
9.38.)
tional medical devices of great potential for
improving health care.
E.5.3 Nonmedical
(and
Medical)
Devices Using Biology's
Mechanisms
and
Materials
The enabling triumvirate provides the antithe-
sis to Pandora's box. Instead of being a prolific
source of troubles, the enabling triumvirate
provides a prolific source of opportunities with
which to overcome today's troubles. Even at
this early stage, with all that is already under-
way and that which is just emerging, it becomes
difficult to choose the applications to empha-
size here. The choice makes note of nano-
sensors and uses of mechanical resonances as
representative of exciting and emerging possi-
bilities. Mechanical resonances contribute to
applications from the relatively mundane of
sound absorption for civilian and naval appli-
cations to the exotic opportunity of nano-
sensors with potential use in the field to detect
terrorist chemical and biological agents. The
future can be one of nanosensors with the
