Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


E.2 The Inverse Temperature Transition: The Foundation of the "Vital Force"
545
associates. Also, as shown in Figure 5.25B,
forming charged carboxylates destroys much of
the hydrophobic hydration, as the carboxylate
recruits water for its own hydration. This is
depicted in the leftmost structure in Figure 2.8.
Thus,
indeed the law of physics, called the
second law of thermodynamics, does apply to
living matter. Obeyance occurs, however, under
a special set of circumstances where the water
solvent becomes a key player. Decrease in order
is greater for the change to ordinary water from
structured water surrounding oil-like groups
than is the increase in order of the protein as it
hydrophobically associates to form a more
ordered structure on raising the temperature. In
fact, the changing structure of the protein com-
ponent of the two-component system, acted on
by additional energy inputs, provides function-
ality essential to sustain the living organism.
E.2.7 Biology's ''Vital Force''Arises Out
of Interlinking Thermodynamics and
Molecular Dynamics
Somewhere near its origins, biology reconciled
thermodynamicists' entropy and dynamicists'
reversibility,^"* as it evolved functional protein-
based machines by interlinking the two pro-
cesses that seemed so disparate during the
debates of the 19th century. Energy driven
hydrophobic association with its increase in the
thermodynamicists' entropy on conversion of
hydrophobic solvation to bulk water commonly
couples with a reversible decrease in internal
chain dynamics in the development of a near-
ideal elastic force wherein the force-relaxation
curve exactly follows the same trajectory as
the force-extension curve. As we now stand,
almost paradoxically from the viewpoint of
the early controversy, the entropic contribution
to the elastic force becomes the primary basis
for reversibility and efficiency of energy
conversion.
In our view, the hydrophobic and elastic con-
silient mechanisms comprise the "vital force" of
living matter The forces arising out of inverse
temperature transitions and elastic deforma-
tion,
for example, apolar-polar repulsion and
damping of backbone mobility on deformation,
couple to create biology's "vital force."
E.2.8 The Hydrophobic ConsiHent
Mechanism Derives from the Inverse
Temperature Transition
The energy conversions that produce motion
in living organisms consist of two distinct but
interlinked physical processes of hydrophobic
association and elastic force development, col-
lectively referred to as consilient mechanisms
in that they each provide "a common ground-
work of explanation."^ The association of
oil-like domains, hydrophobic association, has
been characterized in terms of the comprehen-
sive hydrophobic effect (CHE), and elastic
force development has been described in terms
of the damping of internal chain dynamics on
deformation, whether deformation occurs by
extension, compression or solvent-mediated
repulsion (see section E.4.1.2 and Figures E.3
and E.4, below).
In aqueous systems, CHE controls hydropho-
bic association by means of a Gibbs free energy
of hydrophobic association,
AGHA,
which is
quantifiable as a variable-induced change in the
net heat of the endothermic inverse tempera-
ture transition in Figures 7.1 and
8.1.
The mag-
nitude of
AGHA
depends primarily on the
amount of hydrophobic hydration displaced
on hydrophobic association. Fundamentally,
competition for hydration between apolar
(hydrophobic) and polar (e.g., charged) groups
controls the amount of hydrophobic hydration,
as shown in Figure 5.25B, and the competition
is quantifiable as an apolar-polar repulsive
free energy of hydration, AGap. Thus, the
hydrophobic consilient mechanism applies to
all amphiphilic polymers in aqueous systems
regardless of polymer structure.
E.2.9 The Elastic ConsiHent
Mechanism as the Efficient
Mechanical Coupler Within
the "Vital Force"
E.2.9,1 Dynamic Chains Without Random
Chain Networks
The mechanically linked process of entropic
elasticity also constitutes a "common ground-
work of explanation." It applies to all polymer
structures, of whatever composition, that

546
Epilogue
contain a sufficiently kinetically free chain
segment that can exhibit constrained internal
chain
dynamics,
for example, damped backbone
torsional oscillations, on deformation. In par-
ticular, the polymer structure need not be
describable as a random chain network with a
Gaussian distribution of deformable end-to-
end chain lengths as has been characterized'
by the Flory random chain network theory of
elasticity.^^'^^
Importantly, efficient energy conversion
requires the element of entropic (ideal) elastic-
ity where the energy of deformation is recov-
ered on relaxation. Very often deformation/
relaxation curves exhibit significant hysteresis,
where the input energy required to deform a
chain molecule is substantially greater than the
energy recovered on relaxation of the defor-
mation. In
hysteresis,
the energy of deformation
has been lost to chain components that do
not sustain the deformation. Accordingly, the
occurrence of a hysteresis to the deformation
constitutes a loss of energy. Because of
this,
the
occurrence of hysteresis during the structural
changes of a protein-based machine effecting
an energy conversion results in an inefficient
machine.
If the energy of deformation remains in the
backbone of the chain that sustains the deform-
ing force, then it can be recovered on relax-
ation. This occurs when the energy of
deformation results in the damping of internal
chain dynamics. Random chain networks do
this reasonably well, but they do not represent
the only means of doing so. In fact, certain
elastic-contractile model proteins, comprised as
they are of repeating sequences that assemble
into regular dynamic structures, give near-
perfect overlap of force extension and force
relaxation curves. These elastic-contractile
model proteins exhibit mechanical resonances
that have been observed centered at
5
MHz
(just below radio frequency) and centered near
3
kHz (positioned over the acoustic frequency
range).
Such mechanical resonances arise from
a regularly repeating dynamic structure. These
properties are not comprehensible by the con-
cepts of the random chain network school of
entropic elasticity, but, in fact, they provide
exciting new materials for the future.
E.2.9,2 Hydrophobic Associations Stretch
Interconnecting Chain Segments
Elastic forces come into play as hydrophobic
associations stretch interconnecting chain seg-
ments. Only if the elastic deformation is ideal
does all of the energy of deformation become
recovered on relaxation. To the extent that
hysteresis occurs in the elastic deformation/
relaxation, energy is lost and the protein-based
machine loses efficiency. Thus, the elastic con-
silient mechanism, whereby the force-extension
curve can be found to overlay the force-
relaxation curve becomes the efficient mechan-
ical coupler within the "vital force." The
objective now becomes one of understanding
the age-old problem of a reluctance to discard
past idols.
E.2.9J Bursts of Apolar-Polar Repulsive
Free Energy on Hydrolysis of ATP, by
the Hydrophobic Elastic Consilient
Mechanism, Can Convert to Elastic
Deformation for Efficient
Energy Conversion
As is briefly noted below in section E.4.1.2 and
as was presented in Chapter 8, a burst of
apolar-polar repulsive free energy of hydration
occurs on hydrolysis of ATP to ADP (adeno-
sine diphosphate) plus inorganic phosphate.
This repulsive burst within the structure of Fi-
ATPase causes elastic deformations in the
protein subunit holding the catalytic site and in
the protein subunit functioning as a hydropho-
bically asymmetric rotor. On the basis of the
hydrophobic elastic consilient mechanism, this
repulsion drives the rotor in a highly efficient
conversion of chemical energy into mechanical
work. By driving the rotation of the rotor in the
opposite direction with the use of the chemical
energy of a proton concentration (actually elec-
trochemical) gradient, ATP
is
synthesized in the
protein-based machine called ATP synthase.
An understanding of this process, in the
author's view, requires setting aside past idols
relating to the nature of elasticity and the treat-
ments, or nontreatments, of water structures
surrounding protein subunits of protein-based
machines.

E.3 Bacon's "Idols Which Beset Men's Minds" 547
E,2.9A The Problematic 'Idols Which
Beset Men's Minds'' and Acceptance of
the Consilient Mechanisms
Sir Francis Bacon (1561-1626), known as the
patron saint of the scientific revolution, recog-
nized a set of troublesome idols of the mind
some four centuries ago. Zagorin^^ gave defini-
tion as, "Bacon's ... doctrine of the idols of
the mind [are] those fallacies obstructing the
progress of knowledge...." Then there is the
classic statement of Bacon as conveyed by
Boorstin,^^ " 'Being convinced that the human
intellect makes its own difficulties,' Bacon
offered his vivid catalog of the illusions of
knowledge—'idols which beset men's minds.'"
Considered below is one of Bacon's four idols
of the mind, the idol of the mind of most direct
relevance to advances in understanding the
laws of nature with specific relevance to the
hydrophobic and elastic consilient mechanisms
and the elan vital.
E.3 Bacon's "Idols Which Beset
Men's Minds"
E.3.1 "...The Idols of the
Mind [are] Those Fallacies
Obstructing the Progress of
Knowledge "
Bacon wrote of four "idols of the mind." The
fourth applies to our concerns of the mecha-
nisms or elan vital whereby biology's protein-
based machines function, namely, "idols of
the theater ('idola theatri'): these were the
off-
spring of the false dogmas of philosophers, false
demonstrations in logic, and false principles
and axioms in the sciences, which, like stage
plays,
generated fictions and unreal worlds."^^In
our efforts to understand the forces that domi-
nate function of protein-based machines, two
examples of "the idols of the mind" that con-
stitute "fictions and unreal worlds" spring to
mind. The examples derive from impediments
to the utilization of each of the consilient
mechanisms, to the hydrophobic consilient
mechanism and a most cogent example to the
elastic consilient mechanism.
Each consilient mechanism presents a
"common groundwork of explanation" without
employing "fictions and unreal worlds." In past
practice, fictions were born of limited experi-
mental data and mathematical formaUsm. The
"fictions and unreal worlds" were compelled by
the needs of the time and indeed in their time
represented valuable steps toward utilization.
These once helpful fictions, however, paradoxi-
cally turn into idols that restrain acceptance of
new experimental data and thereby impede
utilization.
E.3.2 Idols That Retard Utilization of
the Elastic Consilient Mechanism in
Understanding and Developing
Protein-based Machines
and Materials
A particularly evident fiction underlies the
classic (random chain network) theory of
rubber elasticity. To perform certain calcula-
tions,
there enters the assumption of phantom
chains. Phantom chains occupy no space, and
one chain or chain segment can pass through
another as though it never existed. Of course,
this is patently fiction, but stunningly it has not
prevented the users of the theory to emerge
from such an "unreal world" and make conclu-
sions about structures in the real world.
The argument that emerges from this partic-
ular fiction is that all good elastic materials
must be completely disordered; they must be
random chain networks. In such an unreal
world there is no acceptance of the possibiUty
of mechanical resonances in elastomers with
dynamic and regularly repeating structural ele-
ments.
Yet experimental data on certain elastic-
contractile model proteins, using different
instrumentation measuring from different
physical bases, show resonances near 5 MHz
and importantly near
3
kHz in the acoustic fre-
quency range. These mechanical resonances
develop on raising the temperature through the
range of the inverse temperature transition.^'^^
In the realm of the elastic consilient mecha-
nism. Bacon's "idols which beset men's minds"
continue their relevance four centuries follow-
ing enlightenment by this "patron saint of the
scientific revolution."

548
Epilogue
E.3.3 Idols That Retard Utilization
of the Hydrophobic Consilient
Mechanism in Understanding and
Developing Protein-based Machines
and Materials
The fiction that blocks recognition of the
primary basis for the "vital force" derives from
ignoring the presence in the real world of
dif-
ferentiated water structures. Section E.2.6.2
briefly reviews unquestioned evidence for the
existence of special water structure surround-
ing hydrophobic groups. All would agree that
structure and thermodynamics of this water
differs from that of bulk water and from that
surrounding charged
groups.
Even the structure
of water surrounding a negatively charged
group differs from that surrounding a positively
charged group. Yet a common approach to
computing the structure and function of
protein-based machines and materials assumes
water to be homogeneous, to be represented by
a mean dielectric constant.
Furthermore, the assumption of a uniform
dielectric constant for all water structures inter-
acting with protein-based machines and mate-
rials conceals the occurrence of competition for
hydration between hydrophobic groups and
charged groups. In the past there has been the
practice of using a dielectric constant of
80
(that
for bulk water) up to the surface of the protein
and then decreasing the dielectric constant to 5
or less when within the protein. However, what
Solomonic wisdom suffices for choice of dielec-
tric constant to be used for ion-pair formation
within the tortuous surfaces with clefts of
varying shapes from acute to
obtuse.
As seen in
Chapter 8, these clefts may reside inside the
protein-based machine, as found in ATP syn-
thase, or outside the protein-based machine, as
occurs for the myosin II motor.
In our view, governing free energy changes
result from the competition for hydration
between hydrophobic groups and charged
groups; they have been determined experimen-
tally as hydrophobically shifted pKa values, for
example. It is competition with hydrophobic
groups for limited hydration, experienced by
charged groups of opposite
sign,
that drives ion-
pair formation. As demonstrated in Chapter 5,
values for AGap, determined from pKa shifts
of acid-base titration data, corroborate corre-
sponding changes in AGHA, measured from
changes in the heat of the inverse temperature
transition. From our experimental experience
with designed elastic-contractile model pro-
teins,
AGap is no fiction; this measure of the
energetics of competition for hydration be-
tween oil-like and vinegar-like groups is part of
the real world. From the analyses of the protein
structures in Chapter
8,
AGap
(if it had not been
derived from model protein studies) would
have to have been invented to describe the
function of key protein-based machines of
biology. In effect, the insightful analogy to mag-
netic forces by Kinosita and coworkers^ in
describing the behavior of Fi-ATPase implici-
itly recognized the existence of such a force.
Both realms, those of the hydrophobic con-
silient mechanism and of the elastic consilient
mechanism, provide clear examples of Bacon's
"idols which beset men's minds'' of four cen-
turies ago that enhance our own personal jour-
neys of
enchantment
today.
Perhaps we can summarize with the follow-
ing perspective:
Useful approximations of the past
become "idols" in the present
that stand as barriers to the future.
E.4 Protein-based Machines as
Physical Embodiments of the
"Vital Force" That Sustains Life
The phenomenological experimental founda-
tion for the challenging new perspectives
presented by the hydrophobic and elastic
consiHent mechanisms stands on the successful
design of model proteins capable of the extra-
ordinarily diverse set of energy conversions
noted above. The mechanistic foundation
derives from the need to obtain explanation for
otherwise inexpHcable experimental results,
such as stretch-induced pKa shifts, hydropho-
bic-induced pKa shifts, hydrophobic-induced
reduction potential shifts, and systematic in-
creases in steepness (positive cooperativity) of
the experimental curves that follow the change

E.4 Protein-based Machines as Physical Embodiments of the "Vital
Force"
That Sustains Life
549
of state of functional groups as the model
proteins are made more oil-like (see Chapter
5).
Furthermore, yet to be computed by any
program
is
the fundamental thermo-mechanical
transduction wherein the cross-linked elastic-
contractile model proteins contract and
perform mechanical work on raising the tem-
perature through their respective inverse
temperature transitions.^^'^^ These results first
appeared in the literature in 1986 and have
repeatedly appeared since that time with
dif-
ferent preparations, compositions, and experi-
mental characterizations. Additionally, the set
of energies converted by moving the tempera-
ture of the inverse temperature transition (as
the result of input energies for which the elastic-
contractile model protein has been designed to
be responsive)^^ have yet to be described by
computations routinely used to explain protein
structure and function.
We consider these repeatedly demonstrated
properties, using designed elastic-contractile
model proteins, to be fundamental to the func-
tion of the protein machines of biology, as most
directly examined in Chapter 8. The function
of Complex III of the electron transport chain,
of the Fi-motor of ATP synthase, and of the
myosin II motor of muscle contraction consti-
tute protein-based machines that appear to
have their functions well-described by the
hydrophobic and elastic consilient mechanisms.
These protein-based machines represent three
major classes of energy conversion of biology,
and the relevance of their function to the con-
silient mechanisms, derived using the designed
elastic-contractile model proteins, comes into
focus in the synopses given immediately below.
E.4.1 Three Primary Classes
of Energy Conversion by
Protein-based Machines
E.4.L1 Production of
a
Proton
Concentration Gradient, an Example of
Electro-chemical Transduction
The
flow
of electrons (from reduced nucleotides
of intermediary metabolism through Com-
plexes I, II, III, and finally IV of the electron
transport chain within the inner mitochondrial
membrane) pumps protons from the matrix side
to the cytoplasmic side of the membrane. Using
a
flow
of electrons to pump the chemical, proton
(the acid, H"^), represents electro-chemical
transduction, and the protein-based machine
capable of doing so may be called an electro-
chemical transducer. Of the four complexes, at
this stage, the function of Complex III provides
stunning examples of both hydrophobic and
elastic consilient mechanisms.
E.4.1.1.1 In the Complex III Example
Formation of Negative Charges and of
Positive Charges Individually Open Pathways
to Effect Proton Translocation Across the
Inner Mitochondrial Membrane
On the matrix side of the membrane, two elec-
trons are added to ubiquinone to become neg-
atively charged. By the apolar-polar repulsion
of
AGap,
the negative charge disrupts hydropho-
bic association to open an aqueous channel
allowing ingress of two protons from the matrix
side of the membrane to result in uncharged
and lipid soluble ubiquinol. On the cytoplasmic
side of the membrane hydrophobic association
holds the FeS center of the Rieske Iron Protein
at the ubiquinol site, where ubiquinol gives up
two electrons to carry a double positive charge.
The two positive charges on ubiquinol, again by
means of
AGap,
disrupt the noted hydrophobic
association on the cytoplasmic side of the mem-
brane, and the two positive charges, released as
two protons to the cytoplasmic side, return
ubiquinol to ubiquinone. This completes the
transit of two protons across the inner mito-
chondrial membrane.
E.4.1.1.2 Mechanical Coupling of
Hydrophobic Dissociation and Elastic
Retraction Achieves Electron Transfer on the
Pathway from Complex III to Complex IV
Formation of the hydrophobic association
between the hydrophobic tip of the Rieske Iron
Protein and the hydrophobic ubiquinol-
containing site stretches an interconnecting
chain segment. This extended chain segment
functions as a free-standing tether originating
from an anchor in the membrane and bridging

550
Epilogue
to the globular component of the Rieske Iron
Protein that contains the electron transferring
FeS center on the cytoplasmic side of the mem-
brane. This is depicted in Figures 8.14 through
8.19 and from a somewhat different represen-
tation in Figure E.l. On oxidation to result in
double positive charge on ubiquinol, the hydra-
tion-based repulsive force between charged
and hydrophobic groups, AGap, disrupts the
hydrophobic association holding the Rieske
Iron Protein at the ubiquinol (Qo) site, and the
stretched tether, making use of an aromatic
side chain as a fulcrum and applying force at
the appropriate angle, lifts the FeS center of
the Rieske Iron Protein from the ubiquinol
site over a hydrophobic rim and places it at
the cytochrome Ci site. From the heme of
cytochrome Ci the electron transfers to the
heme of cytochrome c. Cytochrome c then
dif-
fuses to Complex IV in the transfer of an elec-
tron from Complex III to Complex IV. Within
Complex IV the electron completes its transit
from glucose to reach its ultimate goal of reduc-
ing molecular oxygen.
E.4.1.1.3 An Astounding Example of
Coupled Hydrophobic and Elastic
Consilient Mechanisms
The concept of two distinct but interlinked
mechanical processes, expanded here as the
coupling of hydrophobic and elastic consilient
mechanisms, entered the public domain in the
publication of Urry and Parker.^ Experimental
results on elastic-contractile model proteins
forged the concept, and the work of Urry and
Parker^ extended the concept to contraction in
biology. Unexpected in our examination of the
relevance of this perspective to biology was to
find the first clear demonstration of the concept
in biology in a protein-based machine of the
electron transport chain as a transmembrane
protein of the inner mitochondrial membrane.
Unimaginable was the occurrence of the
coupled forces precisely at the nexus at which
electron transfer couples to proton pumping.
The figurative critical crossroad between
electron transfer and proton translocation
exhibits a Uteral crossing of elastic structures in
Figure E.l.^"^ Electron flow couples to proton
pumping by interlinking the physical processes
of charge disruption of hydrophobic associa-
tion and relaxation of damped internal chain
dynamics. Coupled hydrophobic dissociation of
the hydrophobic consiUent mechanism and
elastic retraction of the elastic consilient mech-
anism simultaneously release proton to the
inner membrane space and move the reduced
FeS center within the Rieske Iron Protein for
electron transfer to the cytochrome Ci site for
further transfer to cytochrome c, followed by
diffusional transit to Complex IV.
Such is our journey of Ionian Enchantment
from de novo design of elastic-contractile model
protein-based machines to enlightenment at a
key juncture of energy conversion within living
matter.
E.4J,2 Use of the Proton Gradient to
Produce ATP, the Energy Coin of Biology,
an Example of Chemo-chemical
Transduction
ATP synthase produces nearly 90% of the ATP
utilized by living organisms. The structure and
phenomenology of ATP synthase gives rise to a
set of predictions that can test the relevance of
the hydrophobic and elastic consilient mecha-
nisms to the function of this central protein-
based motor of biology. ATP, the energy coin of
the biological realm, derives from the coupling
of two rotary motors driven by the chemical
energy of the proton concentration gradient
considered above. The structural coupling
element is a rotor that extends perpendicular
to the inner mitochondrial membrane and is
driven by the membrane-bound Fo-motor.
Return of excess proton from the cytoplasmic
side of the inner mitochondrial membrane
powers the Fo-motor that turns the rotor in a
clockwise direction. The rotor turns within an
extramembranous catalytic motor housing,
collectively called the Fj-motor, to produce
ATP by adding inorganic phosphate. Pi, to
ADP (see Figures 8.26 through 8.41).
By the reverse process of catalyzing the
hydrolysis of ATP to ADP plus Pi, the Fi-motor
can operate in reverse to drive the rotor in a
counterclockwise direction. In this mode the Fi-
motor is called the Fi-ATPase. The predictions
E.4 Protein-based Machines as Physical Embodiments of the "Vital Force" That Sustains Life
551
lipid layer
of inner
mitochond:
membrani
matrix
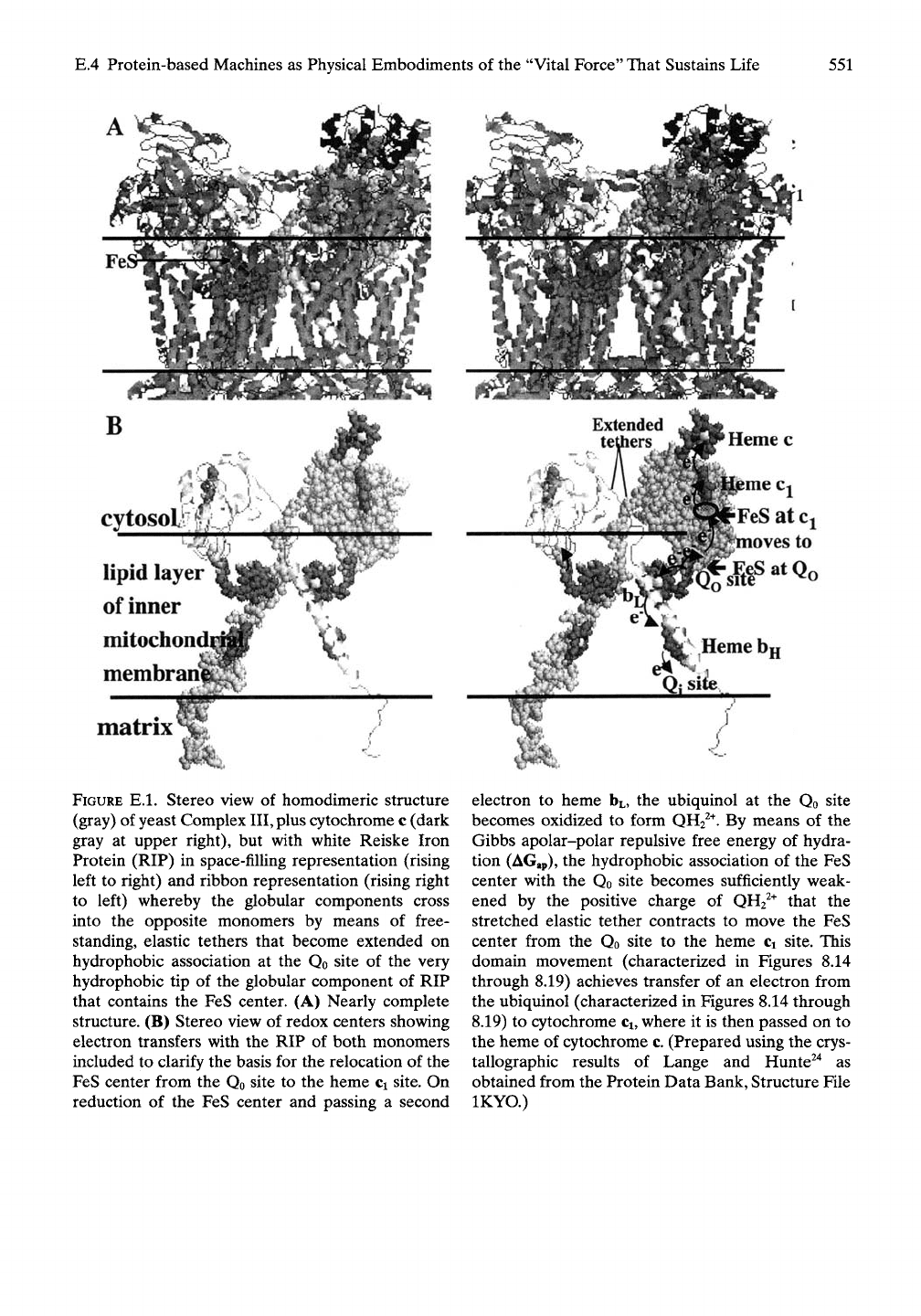
FIGURE E.l. Stereo view of homodimeric structure
(gray) of yeast Complex
III,
plus cytochrome c (dark
gray at upper right), but with white Reiske Iron
Protein (RIP) in space-filling representation (rising
left to right) and ribbon representation (rising right
to left) whereby the globular components cross
into the opposite monomers by means of free-
standing, elastic tethers that become extended on
hydrophobic association at the Qo site of the very
hydrophobic tip of the globular component of RIP
that contains the FeS center. (A) Nearly complete
structure. (B) Stereo view of redox centers showing
electron transfers with the RIP of both monomers
included to clarify the basis for the relocation of the
FeS center from the Qo site to the heme Ci site. On
reduction of the FeS center and passing a second
oves to
electron to heme
HL,
the ubiquinol at the Qo site
becomes oxidized to form QH2^^. By means of the
Gibbs apolar-polar repulsive free energy of hydra-
tion (AGap), the hydrophobic association of the FeS
center with the Qo site becomes sufficiently weak-
ened by the positive charge of QH2^^ that the
stretched elastic tether contracts to move the FeS
center from the Qo site to the heme Ci site. This
domain movement (characterized in Figures 8.14
through 8.19) achieves transfer of an electron from
the ubiquinol (characterized in Figures 8.14 through
8.19) to cytochrome
Ci,
where it is then passed on to
the heme of cytochrome c. (Prepared using the crys-
tallographic results of Lange and Hunte^"* as
obtained from the Protein Data Bank, Structure File
IKYO.)

552
Epilogue
of the hydrophobic and elastic consiUent
mechanisms address the ATPase function, and
the predictions readily reverse when the rotor
is driven by the Fo-motor to describe the func-
tion of ATP synthase.
On the basis of the hydrophobic consilient
mechanism as presented in Chapter 8, function
results from an aqueous-based repulsion, called
an apolar-polar repulsive free energy of hydra-
tion and represented as AGap. The repulsion
occurs between the most hydrophobic side of
the rotor and the most charged occupancy state
(AD? plus Pi) of a catalytic site in a P-subunit.
The repulsion either drives the rotor in a coun-
terclockwise direction on hydrolysis of ATP to
produce ADP plus Pj, or, when the rotor is
driven in the clockwise direction by the Fo-
motor, the repulsion causes ADP plus Pi to
convert to ATP as a means of lowering the free
energy of repulsion.
We complete this synopsis with a list of
substantive predictions and their realizations
that flow from the hydrophobic and elastic
consilient mechanisms when confronted with
the structure and phenomenology of the
Fi-ATPase:
Prediction 1: The rotor must be hydrophobi-
cally asymmetric. Because the catalytic
housing of the Fi-ATPase is essentially three-
fold symmetric but with different occupan-
cies in the three p-catalytic subunits, three
different faces of the y-rotor are identified
from the crystal structure with different
occupancies of the three catalytic subunits.^^
Indeed, the three faces are calculated to
have very different Gibbs free energies for
hydrophobic association,
AGHA,
namely, -20,
0, and +9kcal/mole in order of decreasing
hydrophobicity. Thus, the prediction of a
rotor with hydrophobic asymmetry is strik-
ingly borne out. The order of hydrophobicity,
when considered in terms of the apolar-polar
repulsion between occupancy and direction
of rotation, makes sense with respect to
mechanism.
Prediction 2: In the static state the most
hydrophobic side of the rotor faces the least
polar side of the motor housing. Examination
of five crystal structures with different
occupancy states demonstrates the most
hydrophobic side to always be oriented
toward the least polar portion of the motor
housing, that is, toward the least polar occu-
pancy state of the P-subunits. Thus, the
hydrophobic asymmetry properly responds
to the occupancy state as required for it to be
central to mechanism.
Prediction 3: The role of ATP in the noncat-
alytic a-ATP subunits is one of triangulation
of repulsive forces to lessen viscoelastic drag
between rotor and housing as required for
efficiency. The structure of the catalytic
housing of the Fi-ATPase can be given as
(aP)3,
where a and p are paired protein sub-
units arranged in approximate threefold sym-
metry. While unchanging and noncatalytic,
the a-subunits contain ATP. By the
hydrophobic consilient mechanism, the a-
ATPs apply a triangular apolar-polar repul-
sive force between housing and rotor. This
repulsive force, AGap, hniits hydrophobic
association of rotor with housing that
would result in a viscoelastic drag or could
even lock up rotation by hydrophobic asso-
ciation. Thus, the hydrophobic consilient
mechanism ascribes an essential role of facil-
itating rotation to the three a-ATP nucleo-
tides within the noncatalytic part of the
housing.
Prediction 4: There is negative cooperativity
for ATP binding. With three very different
hydrophobic faces to the rotor, ATP would
first bind to the P-catalytic site with minimal
apolar-polar repulsion. The p-catalytic site
exhibiting the least apolar-polar repulsion
would be the one facing the least hydropho-
bic face,
AGHA
~ +9kcal/mole, of the y-rotor.
The second ATP to occupy a catalytic P-
subunit would do so facing the apolar-polar
repulsion of an essentially neutral hydro-
phobic face of the rotor, that is, AGHA ~
0
kcal/mole.
Finally, to achieve complete occu-
pancy of the three catalytic p-subunits, the
third ATP must bind facing the apolar-polar
repulsion of the most hydrophobic face of the
rotor, namely, with AGHA ~ -20 kcal/mole.
Thus,
by the apolar-polar repulsive free
energy of hydration of the hydrophobic con-
siHent mechanism, negative cooperativity of

E.4 Protein-based Machines as Physical Embodiments of the "Vital Force" That Sustains Life
553
ATP binding naturally results from the
hydrophobically asymmetric rotor.
Prediction 5: There is a positive cooperativity
of increased ATP occupancy of catalytic sites
with rate of hydrolysis (rotation). Positive
cooperativity of ATP occupancy with hydro-
lytic rate has the same basis as the role of the
three a-ATPs of Prediction 3. As more sites
become occupied by the very polar ATP mol-
ecule, progressively less viscoelastic drag
occurs between rotor and housing. Less drag
allows a faster rotation of the rotor on which
the rate of hydrolysis depends.
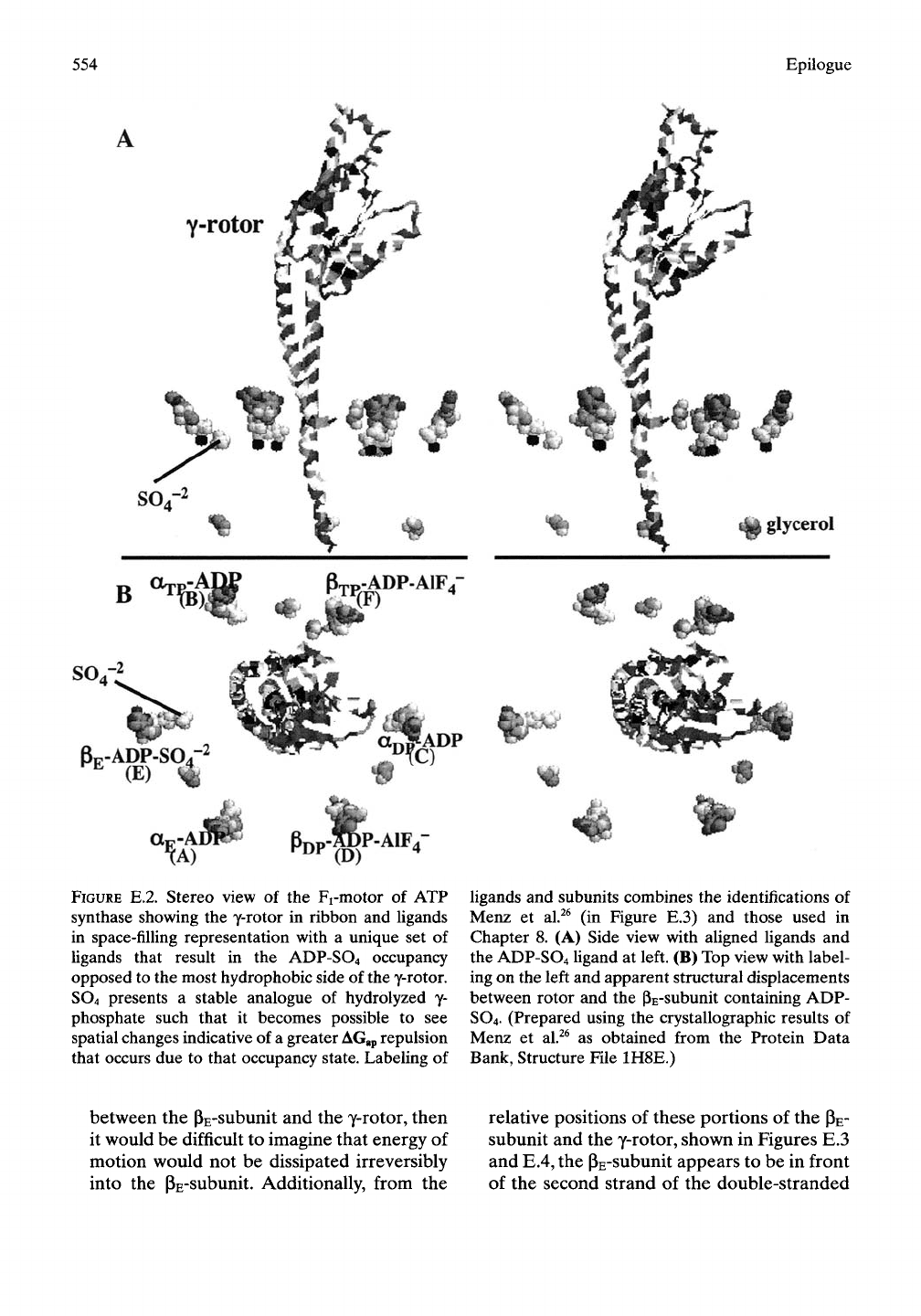
Prediction 6: Increase in distance between rotor
and housing due to AGap repulsion acting
through water between the most hydrophobic
side of the rotor and the ADP-SO4 analogue
of the most polar state. Menz et
al.^^
reported
the particularly interesting crystal structure
with all sites occupied by nucleotide, as
shown in Figure E.2. In the left side of part
Figure E.2B, a distortion in the arrangement
of ligands (nucleotides) is discernible. The pE-
ADP-S04^~ site in chain E is displaced from
the ttTP-ADP site in chain B and at a greater
distance from the y-rotor. By superposition,
Menz et al.^^ in Figure E.3 better represent
the displacement of housing from y-rotor that
occurs on replacing the empty subunit
(PE)
in
the structure of Figures 8.30 through 8.34
with the ADP-S04^" analogue of the most
polar ADP-PO4 state. The crystal structure
containing the ADP-S04^~ analogue was used
in Figures 8.35,8.36,8.40, and
8.41.
The super-
position of Figure E.3 clearly shows the
double-stranded a-heUcal coiled coil portion
of the y-rotor and the pE-subunit to be
repulsed from each other on occupancy of
the pE-subunit by the very polar ADP-S04^~
analogue. Again, we see AGap in action.
Menz et al.^^ describe their findings: "the
coiled coil region has moved significantly ...
resulting in an overall rmsd of 2.9A in a-
carbon positions for the entire y subunit.
The two conformations of the y-subunit are
related by a rotation that varies in magnitude
along the length of the C-terminal helix,
ranging from less than 1° for the final
residues (y259-272) to a maximum of about
20° for residues y234-244 (which form the
coiled coil with y20-10)." In their figure
legend, Menz et al.^^ state: "Note that inter-
acting residues in the PE-subunit and the y-
subunit move in opposite directions." This is
exactly as expected for the occurrence of
an apolar-polar repulsion, a AGap, acting
through water between the most hydrophobic
side of the rotor and the ADP-SO4 analogue
of the most polar state that constitutes Pre-
diction 6.
There is also the expectation that the polar
sulfate would tip the competition for hydra-
tion between apolar and polar groups toward
polar groups. Charged groups, previously
driven to ion pair and to hydrogen bond due
to lack of hydration, as shown in Figure 8.39,
assisted by the highly charged ADP-S04^"
occupancy state now access more hydration
and no longer require these associations, as
shown in Figure 8.40. Figure E.4 illustrates
with straight Hues the arc of apolar-polar
repulsion emanating from the sulfate mole-
cule to the hydrophobic side of the y-rotor.
The repulsion due to the emergent charged
residues, for example, D315, D352, D349,
K382,
D386, and R337 in chain E and E399
in chain A, supplement the repulsion due to
the
PE-ADP-S04^~
subunit, as indicated by
the finer straight lines. All charged species
compete for hydration with the hydrophobic
side of the y-rotor. The series of straight inter-
connecting lines represents the lines of force
due to AGap causing the
PE
and y subunits to
move in opposite directions in Figure E.4.
Prediction 7: A repulsive force acting through
"waters of Thales" to store energy in elastic
deformation provides the opportunity for
high efficiencies of energy conversion by Fj-
ATPase. The movement in opposite direc-
tions observed in Figure E.4 of the pE-subunit
from the y-rotor on occupancy of the pE-
subunit by ADP-S04^" constitutes an elastic
deformation. The elastic deformation results
from AGap-based repulsive forces acting
through water, that is, through the "waters
of Thales." Under such circumstances as the
ATPase the deforming forces relax efficiently
into rotational motion of the rotor.
If the application of force for rotation were
instead to occur above the aqueous chamber
554
Epilogue
Y-rotor .jX^;^''^
4|l
glycerol
B
%,^ ^ fe^""*'^^-
6P.-ADP-SO4-2
»'E (E) ^
<*^
FIGURE E.2. Stereo view of the Fi-motor of ATP
synthase showing the y-rotor in ribbon and Ugands
in space-filling representation with a unique set of
Ugands that result in the ADP-SO4 occupancy
opposed to the most hydrophobic side of the y-rotor.
SO4 presents a stable analogue of hydrolyzed y-
phosphate such that it becomes possible to see
spatial changes indicative of a greater
AGgp
repulsion
that occurs due to that occupancy state. Labeling of
Ugands and subunits combines the identifications of
Menz et al.^^ (in Figure E.3) and those used in
Chapter 8. (A) Side view with aUgned Ugands and
the ADP-SO4 ligand at left. (B) Top view with label-
ing on the left and apparent structural displacements
between rotor and the PE-subunit containing ADP-
SO4.
(Prepared using the crystallographic results of
Menz et al.^^ as obtained from the Protein Data
Bank, Structure File 1H8E.)
between the pE-subunit and the y-rotor, then
it would be difficult to imagine that energy of
motion would not be dissipated irreversibly
into the PE-subunit. Additionally, from the
relative positions of these portions of the PE-
subunit and the y-rotor, shown in Figures E.3
and E.4, the PE-subunit appears to be in front
of the second strand of the double-stranded
