Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

9.4 Medical Applications
515
a period of nearly 3 months at the level of
2.2|Lim/day (compared with
1.9|im/day
for
Leu-enkephalin amide). As expected, delivery
shuts off abruptly in about 3 months.
By changing the oil-like nature of groups in
the polymer, different levels of release can be
obtained. By proper design of the protein-
based polymer, different levels of constant
release are obtained, and, for a constant surface
area of an adequately sized depot, the amount
released can be constant for periods of more
than 3 months (see Figure 9.39).
9.4.5.2 Negatively Charged
Pharmaceuticals Delivered by Positively
Charged Polymers: Consideration of
Steroid Phosphates and Oligonucleotides
9.4.5.2.1 Loading Negatively Charged
Dexamethasone-Phosphate and
Betamethasone-Phosphate into a Series of
Positively Charged Model Proteins
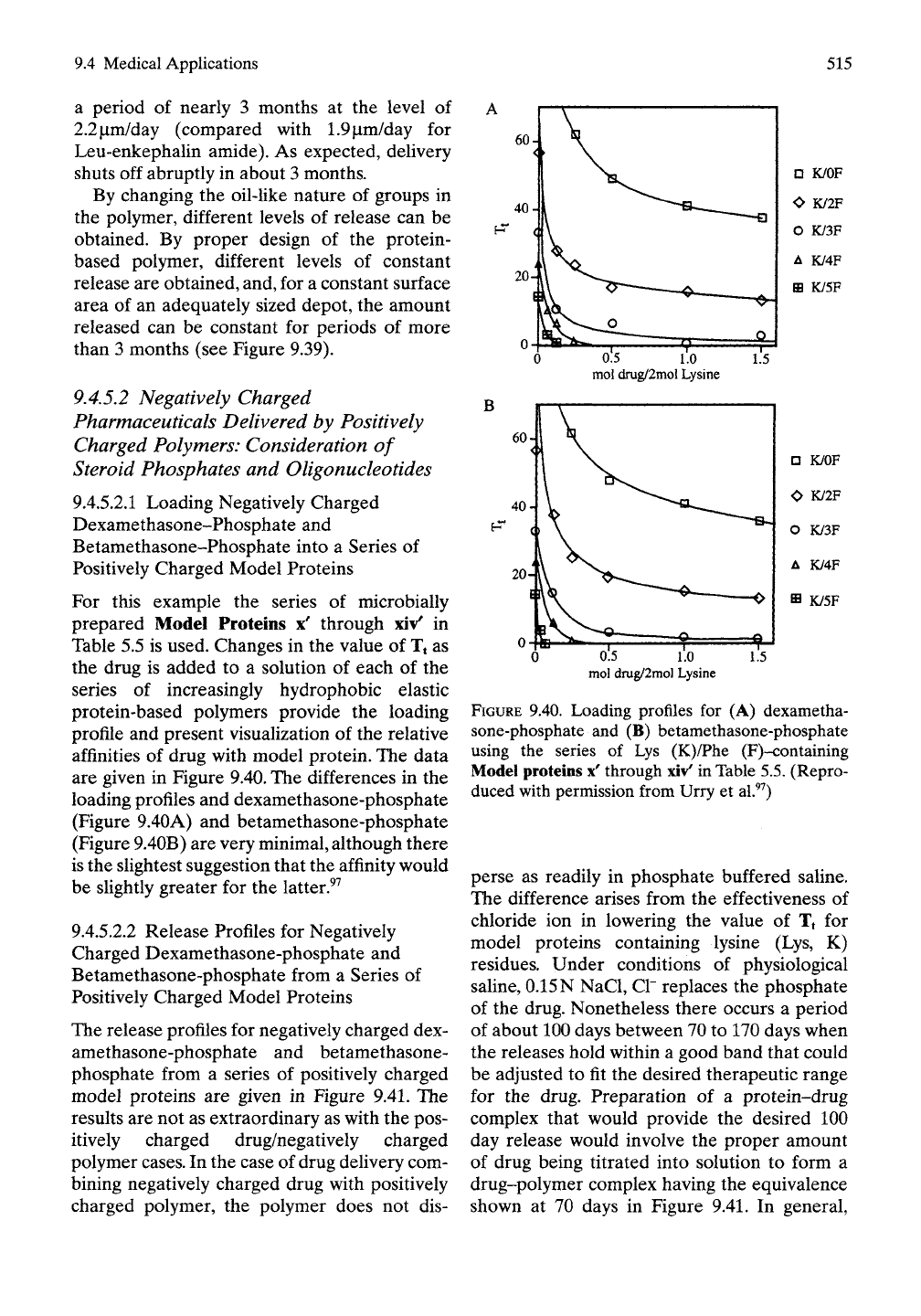
For this example the series of microbially
prepared Model Proteins x' through xiv' in
Table 5.5 is used. Changes in the value of Tt as
the drug is added to a solution of each of the
series of increasingly hydrophobic elastic
protein-based polymers provide the loading
profile and present visualization of the relative
affinities of drug with model protein. The data
are given in Figure
9.40.
The differences in the
loading profiles and dexamethasone-phosphate
(Figure 9.40A) and betamethasone-phosphate
(Figure 9.40B) are very minimal, although there
is the slightest suggestion that the affinity would
be slightly greater for the latter.^^
9.4.5.2.2 Release Profiles for Negatively
Charged Dexamethasone-phosphate and
Betamethasone-phosphate from a Series of
Positively Charged Model Proteins
The release profiles for negatively charged dex-
amethasone-phosphate and betamethasone-
phosphate from a series of positively charged
model proteins are given in Figure 9.41. The
results are not as extraordinary as with the pos-
itively charged drug/negatively charged
polymer
cases.
In the case of drug delivery com-
bining negatively charged drug with positively
charged polymer, the polymer does not dis-
0
0.5 1.0
mol
drug/2mol Lysine
0.5 1.0
mol
drug/2mol Lysine
FIGURE
9.40. Loading profiles for (A) dexametha-
sone-phosphate and (B) betamethasone-phosphate
using the series of Lys (K)/Phe (F)-containing
Model proteins x' through
xiv'
in Table
5.5.
(Repro-
duced with permission from Urry et al.^^)
perse as readily in phosphate buffered saline.
The difference arises from the effectiveness of
chloride ion in lowering the value of Tj for
model proteins containing lysine (Lys, K)
residues. Under conditions of physiological
saline,
0.15
N
NaCl, CI" replaces the phosphate
of the drug. Nonetheless there occurs a period
of about 100 days between 70 to 170 days when
the releases hold within a good band that could
be adjusted to fit the desired therapeutic range
for the drug. Preparation of a protein-drug
complex that would provide the desired 100
day release would involve the proper amount
of drug being titrated into solution to form a
drug-polymer complex having the equivalence
shown at 70 days in Figure 9.41. In general,
516 9. Advanced Materials for the Future
desired levels of release would be achieved by
the size and shape of the depot and the
hydrophobicity of the model protein.^^
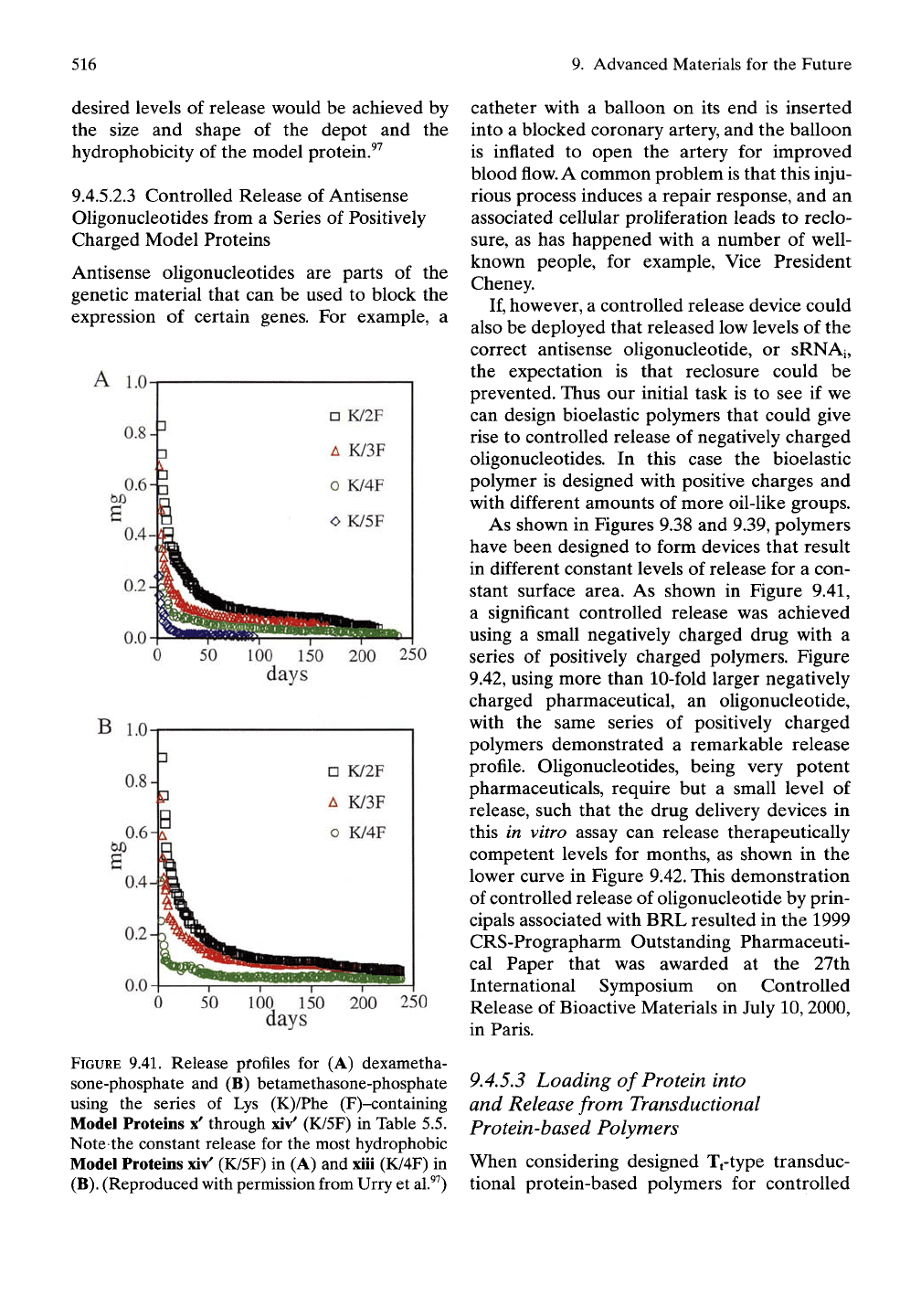
9.4.5.2.3 Controlled Release of Antisense
Oligonucleotides from a Series of Positively
Charged Model Proteins
Antisense oligonucleotides are parts of the
genetic material that can be used to block the
expression of certain genes. For example, a
A 1.0
0 50 100 150 200 250
days
B 1.0-
100 150
days
200 250
catheter with a balloon on its end is inserted
into a blocked coronary artery, and the balloon
is inflated to open the artery for improved
blood flow. A common problem is that this inju-
rious process induces a repair response, and an
associated cellular proliferation leads to reclo-
sure,
as has happened with a number of well-
known people, for example. Vice President
Cheney.
If, however, a controlled release device could
also be deployed that released low levels of the
correct antisense oUgonucleotide, or sRNAi,
the expectation is that reclosure could be
prevented. Thus our initial task is to see if we
can design bioelastic polymers that could give
rise to controlled release of negatively charged
oligonucleotides. In this case the bioelastic
polymer is designed with positive charges and
with different amounts of more oil-like groups.
As shown in Figures 9.38 and 9.39, polymers
have been designed to form devices that result
in different constant levels of release for a con-
stant surface area. As shown in Figure 9.41,
a significant controlled release was achieved
using a small negatively charged drug with a
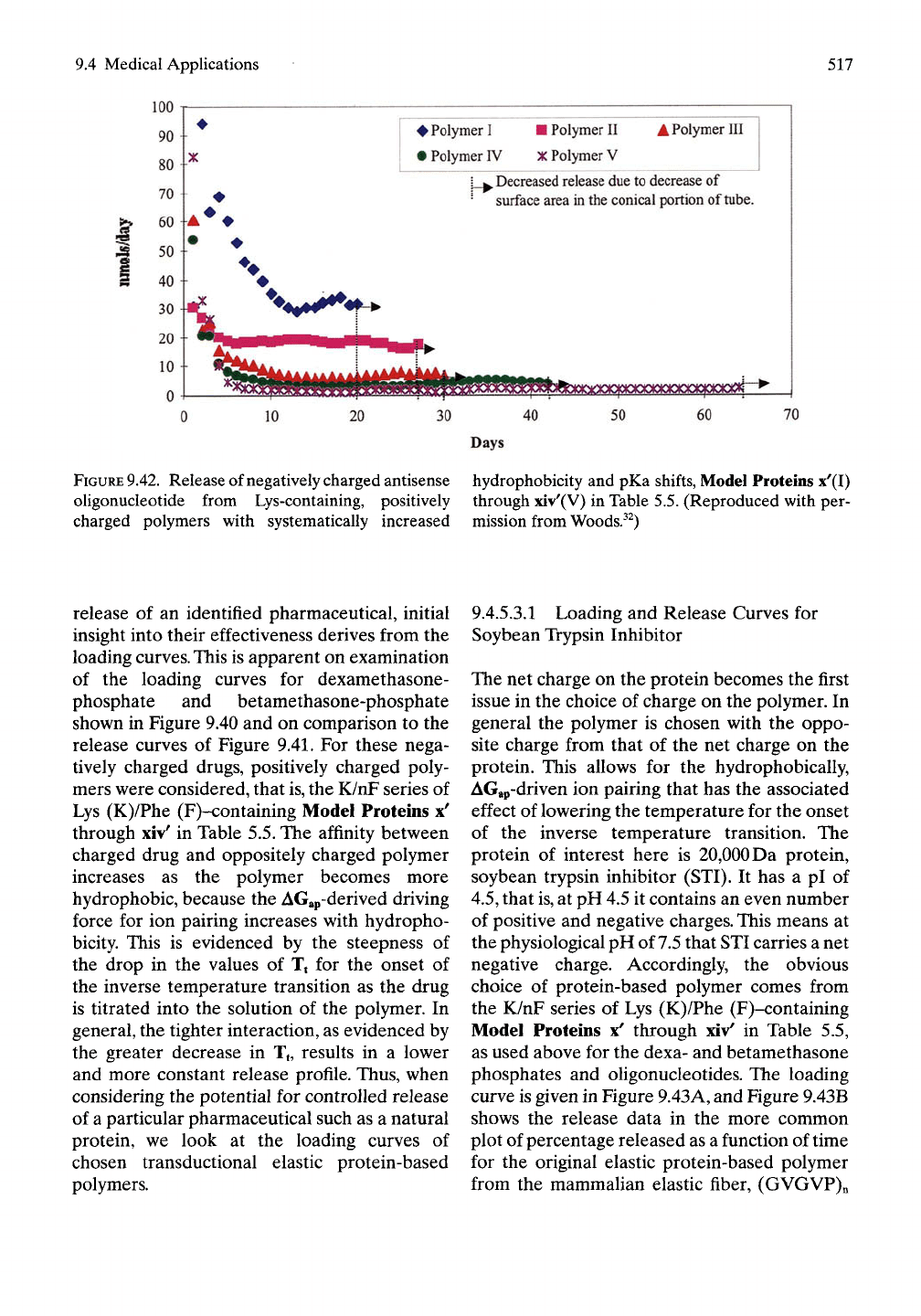
series of positively charged polymers. Figure
9.42, using more than 10-fold larger negatively
charged pharmaceutical, an oUgonucleotide,
with the same series of positively charged
polymers demonstrated a remarkable release
profile. Oligonucleotides, being very potent
pharmaceuticals, require but a small level of
release, such that the drug delivery devices in
this in vitro assay can release therapeutically
competent levels for months, as shown in the
lower curve in Figure 9.42. This demonstration
of controlled release of oligonucleotide by prin-
cipals associated with BRL resulted in the 1999
CRS-Prographarm Outstanding Pharmaceuti-
cal Paper that was awarded at the 27th
International Symposium on Controlled
Release of Bioactive Materials in July 10,2000,
in Paris.
FIGURE 9.41. Release pfofiles for (A) dexametha-
sone-phosphate and (B) betamethasone-phosphate
using the series of Lys (K)/Phe (F)-containing
Model Proteins x' through xiv' (K/5F) in Table 5.5.
Note the constant release for the most hydrophobic
Model Proteins xiv' (K/5F) in (A) and xiii (K/4F) in
(B).
(Reproduced with permission from Urry et al.^^)
9.4.5,3 Loading of Protein into
and Release from Transductional
Protein-based Polymers
When considering designed Tt-type transduc-
tional protein-based polymers for controlled
9.4 Medical Applications
517
t
1
• Polymer
I
• Polymer
IV
• Polymer II
X
Polymer
V
A Polymer III
Decreased
release due to decrease of
surface area
in the
conical portion of
tube.
50 60 70
Days
FIGURE
9.42.
Release of negatively charged antisense hydrophobicity and pKa shifts, Model Proteins x'(I)
oligonucleotide from Lys-containing, positively through xiv'(V) in Table 5.5. (Reproduced with per-
charged polymers with systematically increased mission from Woods.^^)
release of an identified pharmaceutical, initial
insight into their effectiveness derives from the
loading
curves.
This is apparent on examination
of the loading curves for dexamethasone-
phosphate and betamethasone-phosphate
shown in Figure 9.40 and on comparison to the
release curves of Figure 9.41. For these nega-
tively charged drugs, positively charged poly-
mers were considered, that
is,
the K/nF series of
Lys (K)/Phe (F)-containing Model Proteins x'
through xiv' in Table 5.5. The affinity between
charged drug and oppositely charged polymer
increases as the polymer becomes more
hydrophobic, because the AGap-derived driving
force for ion pairing increases with hydropho-
bicity. This is evidenced by the steepness of
the drop in the values of Tt for the onset of
the inverse temperature transition as the drug
is titrated into the solution of the polymer. In
general, the tighter interaction, as evidenced by
the greater decrease in Tt, results in a lower
and more constant release profile. Thus, when
considering the potential for controlled release
of a particular pharmaceutical such as a natural
protein, we look at the loading curves of
chosen transductional elastic protein-based
polymers.
9.4.5.3.1 Loading and Release Curves for
Soybean Trypsin Inhibitor
The net charge on the protein becomes the first
issue in the choice of charge on the polymer. In
general the polymer is chosen with the oppo-
site charge from that of the net charge on the
protein. This allows for the hydrophobically,
AGap-driven ion pairing that has the associated
effect of lowering the temperature for the onset
of the inverse temperature transition. The
protein of interest here is 20,000 Da protein,
soybean trypsin inhibitor (STI). It has a pi of
4.5,
that
is,
at pH 4.5 it contains an even number
of positive and negative charges. This means at
the physiological pH of
7.5
that STI carries a net
negative charge. Accordingly, the obvious
choice of protein-based polymer comes from
the K/nF series of Lys (K)/Phe (F)-containing
Model Proteins x' through xiv' in Table 5.5,
as used above for the dexa- and betamethasone
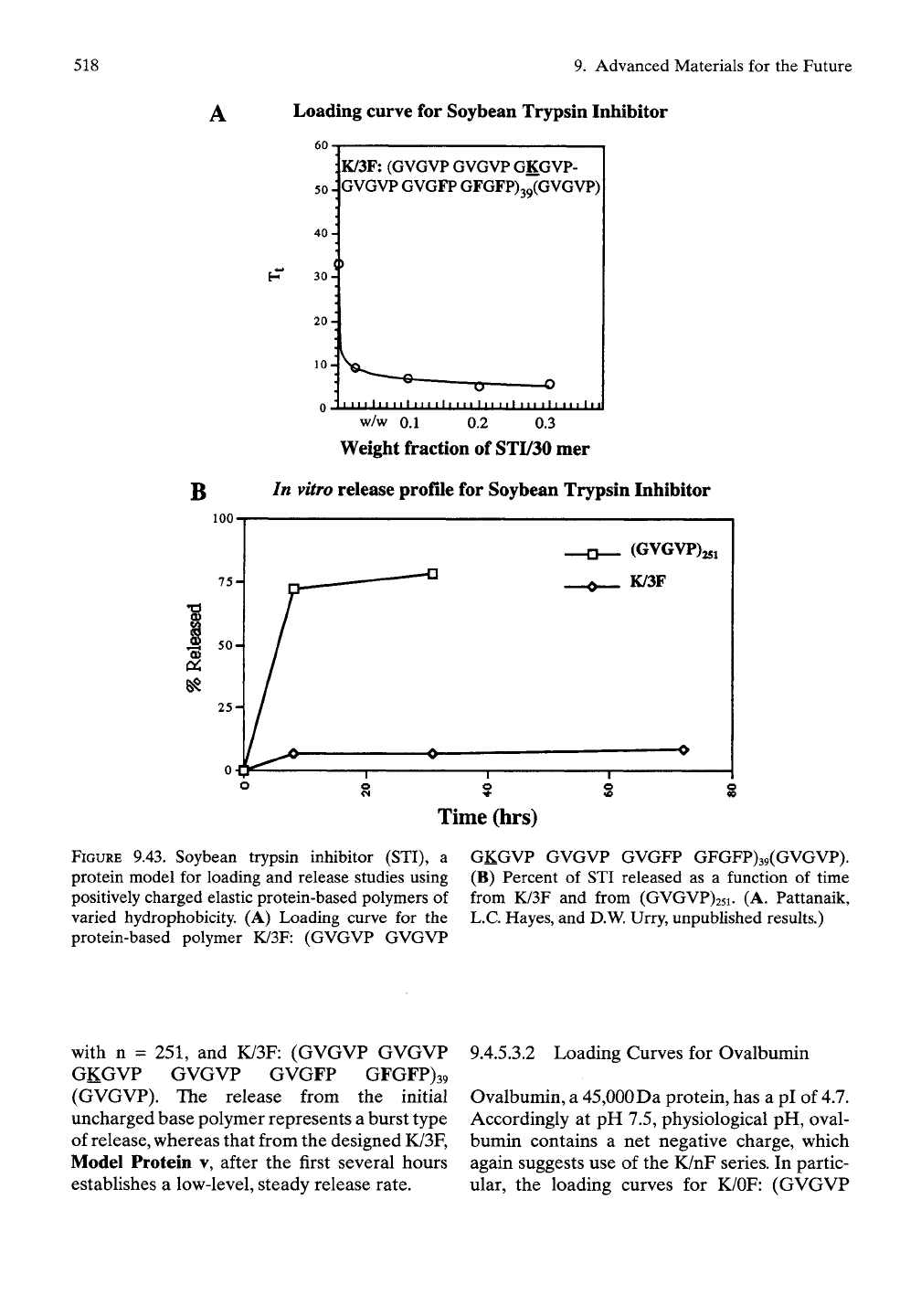
phosphates and oligonucleotides. The loading
curve is given in Figure 9.43 A, and Figure 9.43B
shows the release data in the more common
plot of percentage released as a function of time
for the original elastic protein-based polymer
from the mammalian elastic fiber, (GVGVP)n
518 9. Advanced Materials for the Future
A
Loading curve for Soybean Trypsin Inhibitor
60-
50
H'
40
H
30
H
20 H
lOH
K/3F: (GVGVP GVGVP GKGVP-
GVGVP GVGFP GFGFP)39(GVGVP)
III III III
Ml
ill III III III
I
-o
B
w/w 0.1 0.2 0.3
Weight fraction of STI/30 mer
In vitro release profile for Soybean Trypsin Inhibitor
100
Time (hrs)
FIGURE
9.43. Soybean trypsin inhibitor (STI), a
protein model for loading and release studies using
positively charged elastic protein-based polymers of
varied hydrophobicity. (A) Loading curve for the
protein-based polymer K/3F: (GVGVP GVGVP
GKGVP GVGVP GVGFP GFGFP)39(GVGVP).
(B) Percent of STI released as a function of time
from K/3F and from (GVGVP)25i. (A. Pattanaik,
L.C. Hayes, and D.W. Urry, unpublished results.)
with n = 251, and K/3F: (GVGVP GVGVP
GKGVP GVGVP GVGFP GFGFP)39
(GVGVP). The release from the initial
uncharged base polymer represents a burst type
of release, whereas that from the designed K/3F,
Model Protein v, after the first several hours
establishes a low-level, steady release rate.
9.4.5.3.2 Loading Curves for Ovalbumin
Ovalbumin, a 45,000 Da protein, has a pi of 4.7.
Accordingly at pH 7.5, physiological pH, oval-
bumin contains a net negative charge, which
again suggests use of the K/nF series. In partic-
ular, the loading curves for K/OF: (GVGVP
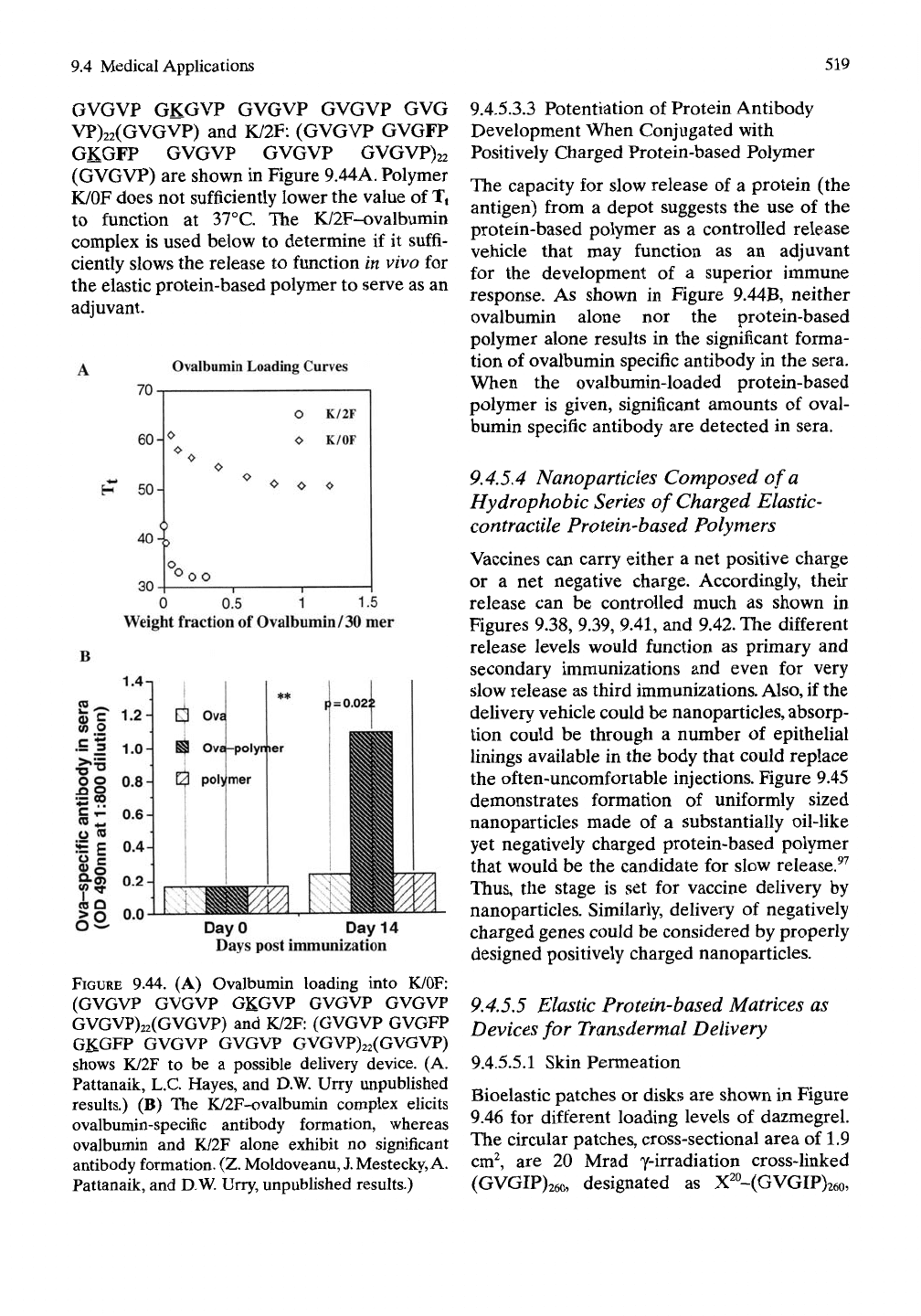
9.4 Medical Applications
519
GVGVP GKGVP GVGVP GVGVP GVG
VP)22(GVGVP) and K/2F: (GVGVP GVGFP
GKGFP GVGVP GVGVP GVGVP)22
(GVGVP) are shown in Figure 9.44A. Polymer
K/OF does not sufficiently lower the value of Tt
to function at 37°C. The K/2F-ovalbumin
complex is used below to determine if it suffi-
ciently slows the release to function in vivo for
the elastic protein-based polymer to serve as an
adjuvant.
Ovalbumin Loading Curves
70
60
H*
50-1
40
30
O
K/2F
O
K/OF
O O O
i
'OO
0 0.5 1 1.5
Weight fraction of Ovalbumin/30 mer
(B
$ O
£-5
^=3
S
CD
C
1-
O
(0
5 F
«j)5
sa
1.4
1.2
1.0
0.8
0.6
0.4
0.2
0.0
DayO Day 14
Days post immunization
FIGURE
9.44. (A) Ovalbumin loading into K/OF:
(GVGVP GVGVP GKGVP GVGVP GVGVP
GVGVP)22(GVGVP) and K/2F: (GVGVP GVGFP
GKGFP GVGVP GVGVP GVGVP)22(GVGVP)
shows K/2F to be a possible delivery device. (A.
Pattanaik, L.C. Hayes, and D.W. Urry unpublished
results.) (B) The K/2F-ovalbumin complex elicits
ovalbumin-specific antibody formation, whereas
ovalbumin and K/2F alone exhibit no significant
antibody formation. (Z. Moldoveanu,
J.
Mestecky, A.
Pattanaik, and D.W. Urry, unpubhshed results.)
9.4.5.3.3 Potentiation of Protein Antibody
Development When Conjugated with
Positively Charged Protein-based Polymer
The capacity for slow release of a protein (the
antigen) from a depot suggests the use of the
protein-based polymer as a controlled release
vehicle that may function as an adjuvant
for the development of a superior immune
response. As shown in Figure 9.44B, neither
ovalbumin alone nor the protein-based
polymer alone results in the significant forma-
tion of ovalbumin specific antibody in the sera.
When the ovalbumin-loaded protein-based
polymer is given, significant amounts of oval-
bumin specific antibody are detected in sera.
9.4.5.4 Nanoparticles Composed of a
Hydrophobic Series of Charged Elastic-
contractile Protein-based Polymers
Vaccines can carry either a net positive charge
or a net negative charge. Accordingly, their
release can be controlled much as shown in
Figures 9.38, 9.39, 9.41, and 9.42. The different
release levels would function as primary and
secondary immunizations and even for very
slow release as third immunizations.
Also,
if the
delivery vehicle could be nanoparticles, absorp-
tion could be through a number of epithelial
linings available in the body that could replace
the often-uncomfortable injections. Figure 9.45
demonstrates formation of uniformly sized
nanoparticles made of a substantially oil-like
yet negatively charged protein-based polymer
that would be the candidate for slow release,^^
Thus,
the stage is set for vaccine delivery by
nanoparticles. Similarly, delivery of negatively
charged genes could be considered by properly
designed positively charged nanoparticles.
9.4.5.5 Elastic Protein-based Matrices as
Devices for Transdermal Delivery
9.4.5.5.1 Skin Permeation
Bioelastic patches or disks are shown in Figure
9.46 for different loading levels of dazmegrel.
The circular patches, cross-sectional area of 1.9
cm^, are 20 Mrad y-irradiation cross-linked
(GVGIP)26o, designated as X2°-(GVGIP)26o,

520 9. Advanced Materials for the Future
FIGURE
9.45. Electron microscopy of nanoparticles
comprised of Model Protein v in Table 5.5, namely,
(GVGVP GVGFP GEGFP GVGVP GVGFP
GFGFP)42(GVGVP). (Reproduced with permission
from Urry et al.^^)
that have been equilibrated in dipropylene
glycol/propylene glycol/water (2:1:1). Pro-
gressing from left to right, the dazmegrel levels
are 0, 0.1, 1, and lOmg/cm^. When placed on
dog skin or human skin, these disks release
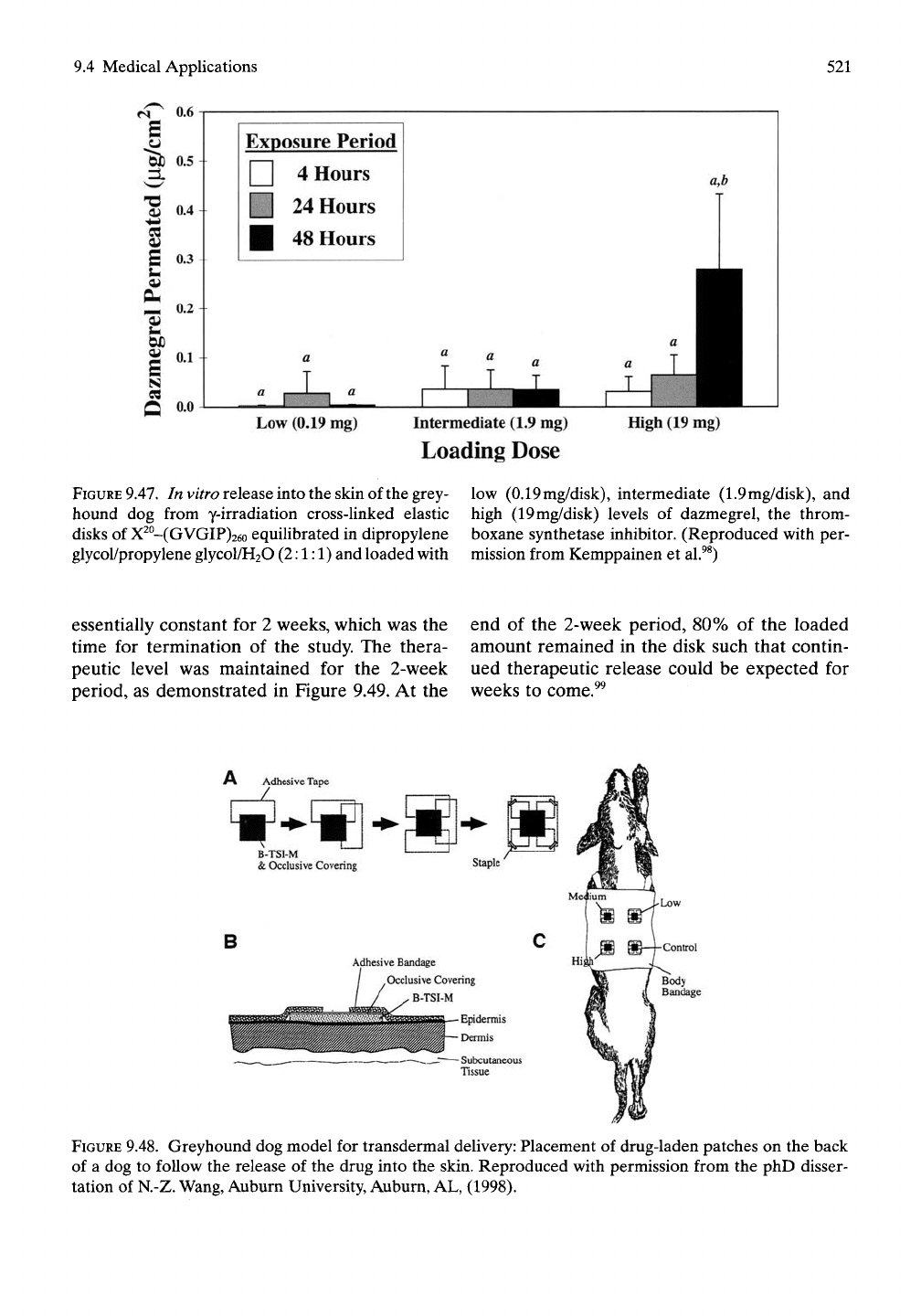
dazmegrel into the skin as shown in Figure 9.47.
Penetration into greyhound dog skin was five
times greater than into human breast skin. For
in vitro greyhound skin permeation studies, the
intermediate dose of
1.9mg/disk
yielded a con-
stant release of dazmegrel from 4 to 48 hours,
as measured by recovery from the receptor and
dermis. The total recovery of dazmegrel was
88 ± 13%, and the retention by the bioelastic
membrane averaged
67
± 9.0%.Thus, it appears
that the intermediate level could be maintained
for a significantly longer period of time, as
demonstrated by the in vivo delivery study
immediately below.^^
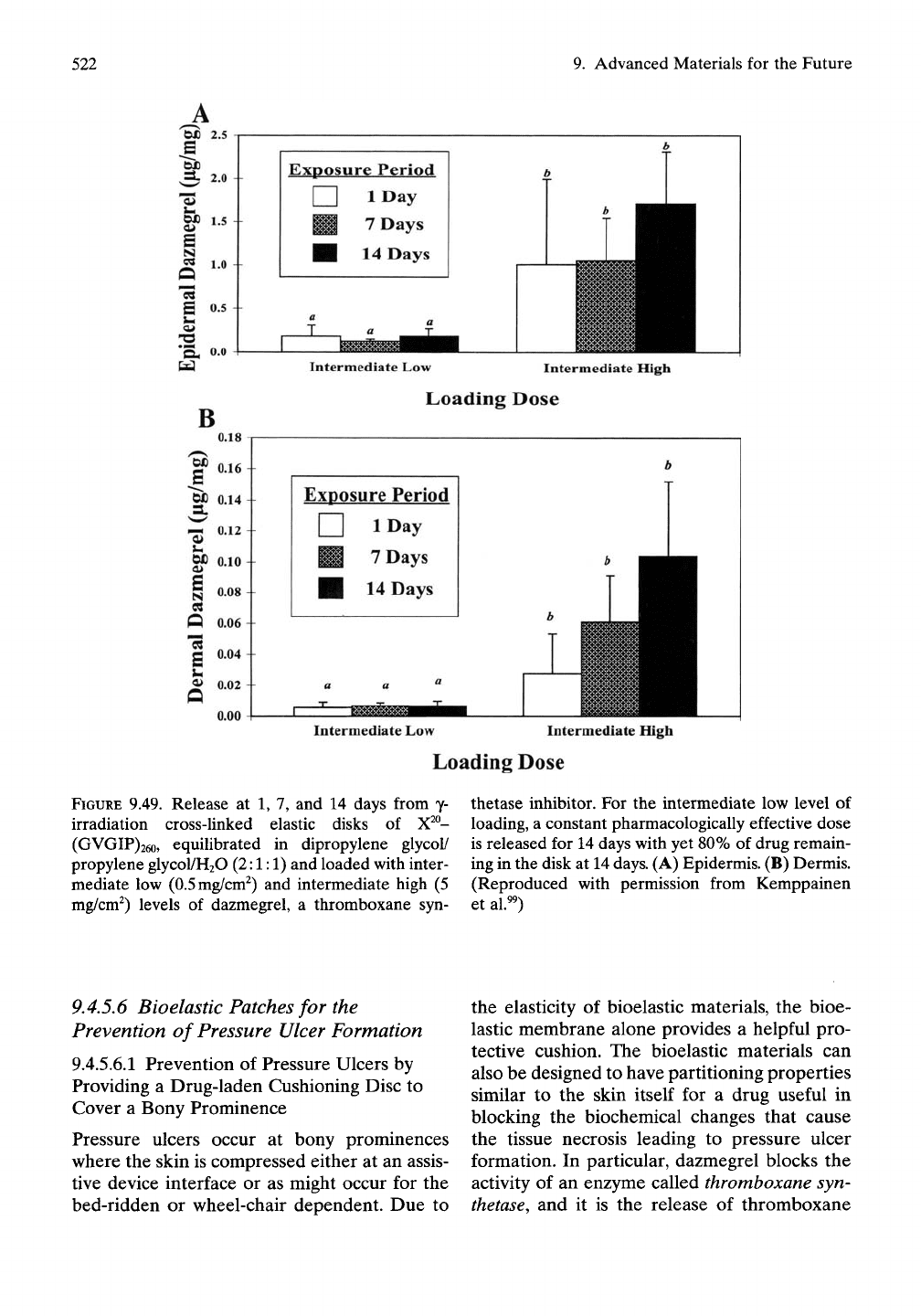
9.4.5.5.2 In vivo Skin Permeation
In narrowing down the concentration range for
loading of the bioelastic membrane, an inter-
mediate low (0.5mg/cm^) and an intermediate
high (5.0mg/cm^) were used. In the greyhound
dog model as shown in Figure 9.48, by 24 hours
the level of dazmegrel in the epidermis and
dermis had reached a constant level with the
intermediate low level of loading, and that
therapeutically competent level remained
FIGURE
9.46. y-Irradiation cross-linked elastic disks
of X^°-(GVGIP)26o, equilibrated in dipropylene
glycol/propylene glycol/H20 (2:1:1) and loaded
with
0,0.1,1,
and lOmg/cm^ dazmegrel, a thrombox-
ane synthetase inhibitor for preventing capillary
constriction and the subsequent necrosis that leads
to a pressure ulcer. (Courtesy of Bioelastics research,
Ltd.)
9.4 Medical Applications
521
ri 0.6
WD 0.5
S 0.4
Exposure Period
I I 4 Hours
I 24 Hours
• 48 Hours
a, 6
Low (0.19 mg) Intermediate (1.9 mg)
Loading Dose
High (19 mg)
FIGURE
9.47.
In
vitro
release into the skin of the grey-
hound dog from y-irradiation cross-linked elastic
disks of X^°-(GVGIP)26o equilibrated in dipropylene
glycol/propylene glycol/HaO (2:1:1) and loaded with
low (0.19mg/disk), intermediate (1.9mg/disk), and
high (19mg/disk) levels of dazmegrel, the throm-
boxane synthetase inhibitor. (Reproduced with per-
mission from Kemppainen et al.^^)
essentially constant for 2 weeks, which was the
time for termination of the study. The thera-
peutic level was maintained for the 2-week
period, as demonstrated in Figure 9.49. At the
end of the 2-week period, 80% of the loaded
amount remained in the disk such that contin-
ued therapeutic release could be expected for
weeks to come.^^
Adhesive Tape
B-TSI-M ' ^ ^ A ^
& Occlusive Covering Staple
B
Adhesive Bandage
Occlusive Covering
B-TSI-M
Epidermis
Dennis
_-:: Subcutaneous
Tissue
FIGURE
9.48. Greyhound dog model for transdermal delivery: Placement of drug-laden patches on the back
of a dog to follow the release of the drug into the skin. Reproduced with permission from the phD disser-
tation of N.-Z. Wang, Auburn University, Auburn, AL, (1998).
522
9. Advanced Materials for the Future
on
2.5
I
go 1.5
S
a 0.5
-a
*a 0.0
1.0
0.00
JL
Expo^prg Period
• IDay
B 7 Days
• 14 Days
Intermediate Low Intermediate High
Loading Dose
Intermediate Low Intermediate High
Loading Dose
FIGURE
9.49. Release at 1, 7, and 14 days from y-
irradiation cross-linked elastic disks of X^°-
(GVGIP)26o, equilibrated in dipropylene glycol/
propylene glycol/H20 (2:1:1) and loaded with inter-
mediate low (0.5mg/cm^) and intermediate high (5
mg/cm^) levels of dazmegrel, a thromboxane syn-
thetase inhibitor. For the intermediate low level of
loading, a constant pharmacologically effective dose
is released for 14 days with yet 80% of drug remain-
ing in the disk at 14 days. (A) Epidermis. (B) Dermis.
(Reproduced with permission from Kemppainen
et al.^^)
9,4,5,6 Bioelastic Patches for the
Prevention of Pressure Ulcer Formation
9.4.5.6.1 Prevention of Pressure Ulcers by
Providing a Drug-laden Cushioning Disc to
Cover a Bony Prominence
Pressure ulcers occur at bony prominences
where the skin is compressed either at an assis-
tive device interface or as might occur for the
bed-ridden or wheel-chair dependent. Due to
the elasticity of bioelastic materials, the bioe-
lastic membrane alone provides a helpful pro-
tective cushion. The bioelastic materials can
also be designed to have partitioning properties
similar to the skin itself for a drug useful in
blocking the biochemical changes that cause
the tissue necrosis leading to pressure ulcer
formation. In particular, dazmegrel blocks the
activity of an enzyme called thromboxane syn-
thetase, and it is the release of thromboxane
9.4 Medical Applications
523
that is thought to lead to vascular constriction
and poor circulation that results in tissue break-
down and pressure ulcer formation. Dazmegrel,
therefore, is identified as a thromboxane syn-
thetase inhibitor. ^^
9.4.5.6.2
Preliminary Studies Show
Drug-laden Bioelastic Disc to Prevent
the Tissue Necrosis Resulting in Pressure
Ulcer Formation
The bioelastic disks were then used in a dog
model for pressure ulcer formation to deter-
mine efficacy. The coaptation walking cast
model of Swaim et
al}^^'^^
was utilized in the
greyhound dog. In this model, severe pressure
ulcers develop at the medial malleolus (the
inner ankle bone), as evaluated in Table 9.8.
When a bioelastic disk without drug (B-M) is
fixed in place over the anklebone, the occur-
rence and severity of ulcers is significantly
decreased, as shown as the control (unloaded)
data in Table 9.9. In the limited studies to
date,
the presence of a loaded intact disk, the
bioelastic-thromboxane synthetase inhibitor-
membrane (B-TSI-M) prevented ulcer forma-
TABLE
9.8. Pressure ulcer study: Subjective observa-
tions of skin over the medial malleolus with no bioe-
lastic matrix patch.
Dog^
DP-SN"
DP-9N
DP-ION
DP-llN
Appearance of skin
Hyperemia
Not noted
Small
Large
Not noted
Epidermal
abrasion/
ulceration**
Present
Present
Present (3)
Present (2)
Days in cast
7
5^
5'
1
^ DP-#N = No bioelastic patch.
^ These lesions were deeper into the dermis than the super-
ficial abrasions noted when bioelastic matrix patches were
present.
'^
It was necessary to remove the casts from these dogs
before the scheduled 7 days. The casts had considerable
"strike through" on the surface overlying bony promi-
nences, indicating a significant underlying dermal lesion.
Source: Reproduced from Kemppainen et al.^^
tion, as shown as the treatment (loaded) data in
Table 9.9.
The one limitation at this medial malleolus
site of high shear pressure is an occasional (one
in four of the test cases, DP-7T) splitting or
tearing of the
disk,
with early signs for ulcer for-
TABLE
9.9. Pressure ulcer study: Subjective observations of unloaded and loaded bioelastic matrix patches
and skin over the medial malleolus (casts in place 7 days).
Dog/treatment^
Controls (unloaded)
DP-IC
DP-2C
DP-3C
Treatment (loaded)
DP-4T
DP-5T
DP-6T
DP-7T
Patch appearance
Intact, cloudy white
area
Intact, clear
Cloudy white area,
triangular tear distal
Intact, clear
Intact, clear
Intact, clear
Clear, triangular tear
cranial—distal
Appearance of skin under or immediately adjacent to patch
Hyperemia
Slight over entire
med. mal.
None
Present distal, not
traceable
None
Small cranial—distal
Small central and
proximal,^ slight,
not traceable
Slight distal, not
traceable
Superficial epidermal
abrasion/ulceration
2 small
None
Present distal
None
None
None
Small distal
Skin adjacent to patch
Nothing notable
Nothing notable
Nothing notable
Nothing notable
Hyperemia and
epidermal
abrasion/ulcer—distal.
Ecchymosis—proximal
Nothing notable
Nothing notable
" DP-#C = Unloaded bioelastic patch; DP-#T = loaded bioelastic patch; DP-#N = no bioelastic patch.
^ This proximal small area of hyperemia was noted before the cast was placed. It was covered by the patch and had not
gotten worse over the 7 day cast period.
Source: Reproduced from Kemppainen et al.^^
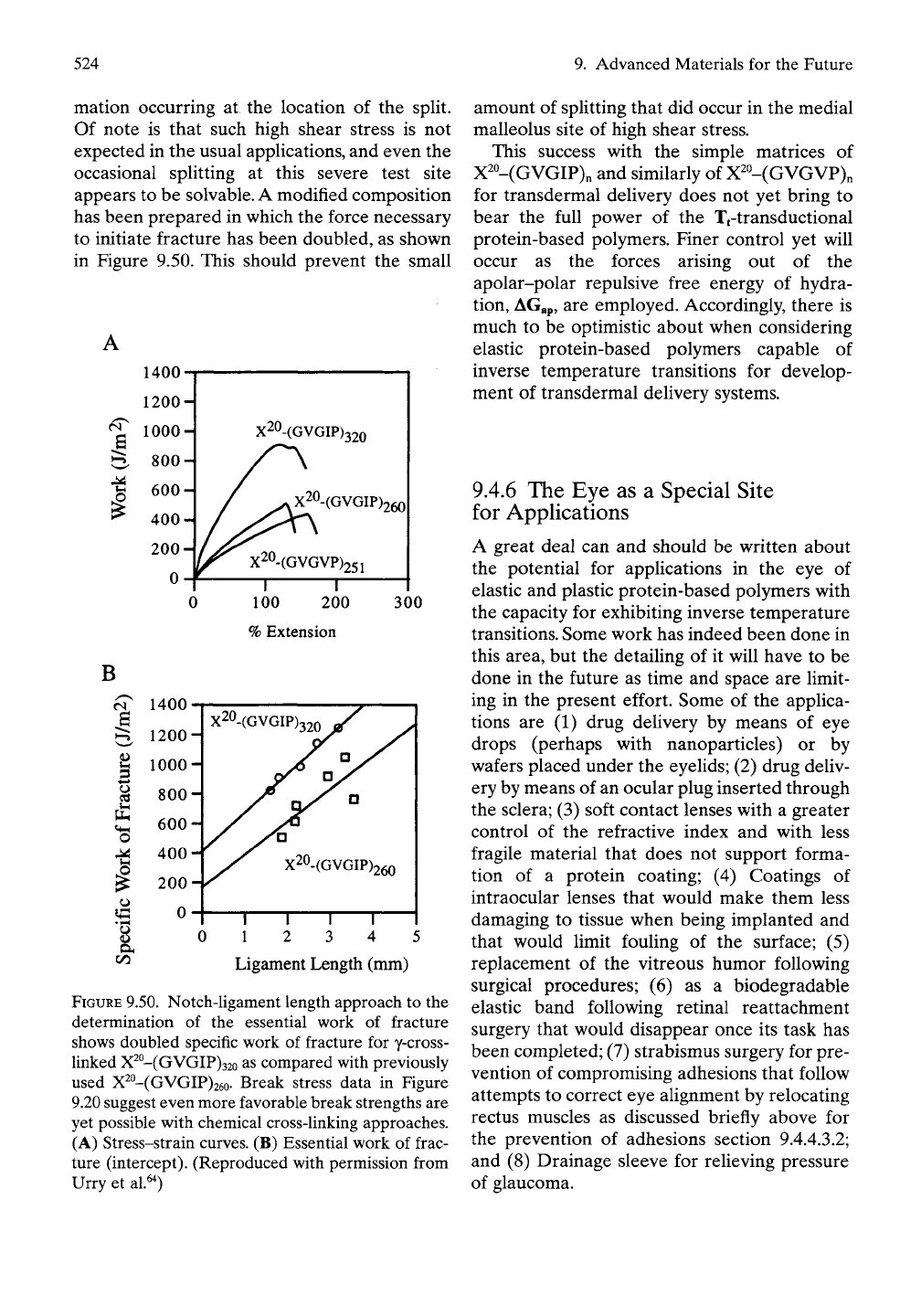
524
9. Advanced Materials for the Future
mation occurring at the location of the split.
Of note is that such high shear stress is not
expected in the usual appUcations, and even the
occasional splitting at this severe test site
appears to be solvable. A modified composition
has been prepared in which the force necessary
to initiate fracture has been doubled, as shown
in Figure 9.50. This should prevent the small
1400-
s
X20-(GVGIP)320
X20.(GVGIP)260
x20-(GVGVP)25i
300
% Extension
B
3
s
1400-
X20-(GVGIP)260
1
12 3 4 5
Ligament Length (nmi)
FIGURE
9.50. Notch-ligament length approach to the
determination of the essential work of fracture
shows doubled specific work of fracture for y-cross-
linked X^°-(GVGIP)32o as compared with previously
used X^°-(GVGIP)26o- Break stress data in Figure
9.20 suggest even more favorable break strengths are
yet possible with chemical cross-linking approaches.
(A) Stress-strain curves. (B) Essential work of frac-
ture (intercept). (Reproduced with permission from
Urry et al.^^
amount of splitting that did occur in the medial
malleolus site of high shear stress.
This success with the simple matrices of
X'°-(GVGIP)n and similarly of X2°-(GVGVP)n
for transdermal delivery does not yet bring to
bear the full power of the Tftransductional
protein-based polymers. Finer control yet will
occur as the forces arising out of the
apolar-polar repulsive free energy of hydra-
tion, AGap, are employed. Accordingly, there is
much to be optimistic about when considering
elastic protein-based polymers capable of
inverse temperature transitions for develop-
ment of transdermal delivery systems.
9.4.6 The Eye as a Special Site
for Applications
A great deal can and should be written about
the potential for applications in the eye of
elastic and plastic protein-based polymers with
the capacity for exhibiting inverse temperature
transitions. Some work has indeed been done in
this area, but the detaiUng of it will have to be
done in the future as time and space are limit-
ing in the present effort. Some of the applica-
tions are (1) drug delivery by means of eye
drops (perhaps with nanoparticles) or by
wafers placed under the eyelids; (2) drug deliv-
ery by means of an ocular plug inserted through
the sclera; (3) soft contact lenses with a greater
control of the refractive index and with less
fragile material that does not support forma-
tion of a protein coating; (4) Coatings of
intraocular lenses that would make them less
damaging to tissue when being implanted and
that would limit fouling of the surface; (5)
replacement of the vitreous humor following
surgical procedures; (6) as a biodegradable
elastic band following retinal reattachment
surgery that would disappear once its task has
been completed; (7) strabismus surgery for pre-
vention of compromising adhesions that follow
attempts to correct eye alignment by relocating
rectus muscles as discussed briefly above for
the prevention of adhesions section 9.4.4.3.2;
and (8) Drainage sleeve for reUeving pressure
of glaucoma.
