Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


9.3 Purification
of
Tftype Protein-based Polymers
485
through this continuity identifies residues
i
and
i + 1 of the
sequence. These contacts
are
followed
in the
expanded portion
of the map
shown
in
Figure 9.10,
and
they
are
continuous
along
the
sequence with
the
exception
of an
interruption
at the Pro (P)
residues that have
no
aCH
proton.
The portions
of the
sequence involving
Pro
are obtained
by
proton-proton contacts from
the
aCH
proton
of the
residue preceding
the
Pro residue
to the 8CH
protons
of the Pro
residue
itself.
These contacts
are
designated
as
X'aH-P'^^5H2,
and
they complete
the
missing
step
in the
sequence information obtained from
doN- Additional proton-proton contacts that
sometimes
can be
used
to
fill
in
missing con-
tacts,
dxN
and
dpN,
can
also
act to
confirm pre-
vious sequence assignments. Specifically,
dNN
indicates through space contact between
NN
protons
of
adjacent residues
in the
sequence,
that
is,
residue'NH
to
residue'^^NH. Also,
the
dpN indicates contact between residue'PCH
and
residue'^^NH. The upper part
of
Figure 9.10 lists
the four different types
of
proton-proton con-
tacts that provide sequence information
and
the specific sequence connectivities determined
by each.
The
two-dimensional
NMR
data
in
Figures
9.9 and 9.10
verify
the
repeating
sequence
of
Model Protein
v of
Table
5.5.
This marvelous methodology, due principally
to
the
work
of
Wider
et
al.,^^
has
further
use in
determining
the
three-dimensional conforma-
tion
of
proteins
and was
applied early
in
this
regard
to
poly(GVGVP) and its cyclic analogue
cyclo(GVGVP)3.^^
9.3.2.3 Mass Spectra
to
Determine Size
of
Expressed Polymer
Having verified that
the
repeating sequence
of
the
expressed protein agrees with
the
sequenced monomer gene,
it
is now
to be
deter-
mined how many repeating units constitute
the
expressed protein-based polymer, that
is, the
FIGURE
9.8. Purification by
phase separation
of the
elastic-
contractile protein-based poly-
mer (GVGIP)26o.
(Top)
Pancake
formed
on
phase separation.
(Bottom) Stretching
of
the robust
viscoelastic pancake
out to a
length
of
about
1
meter. (Cour-
tesy of Bioelastics Research, Ltd.)
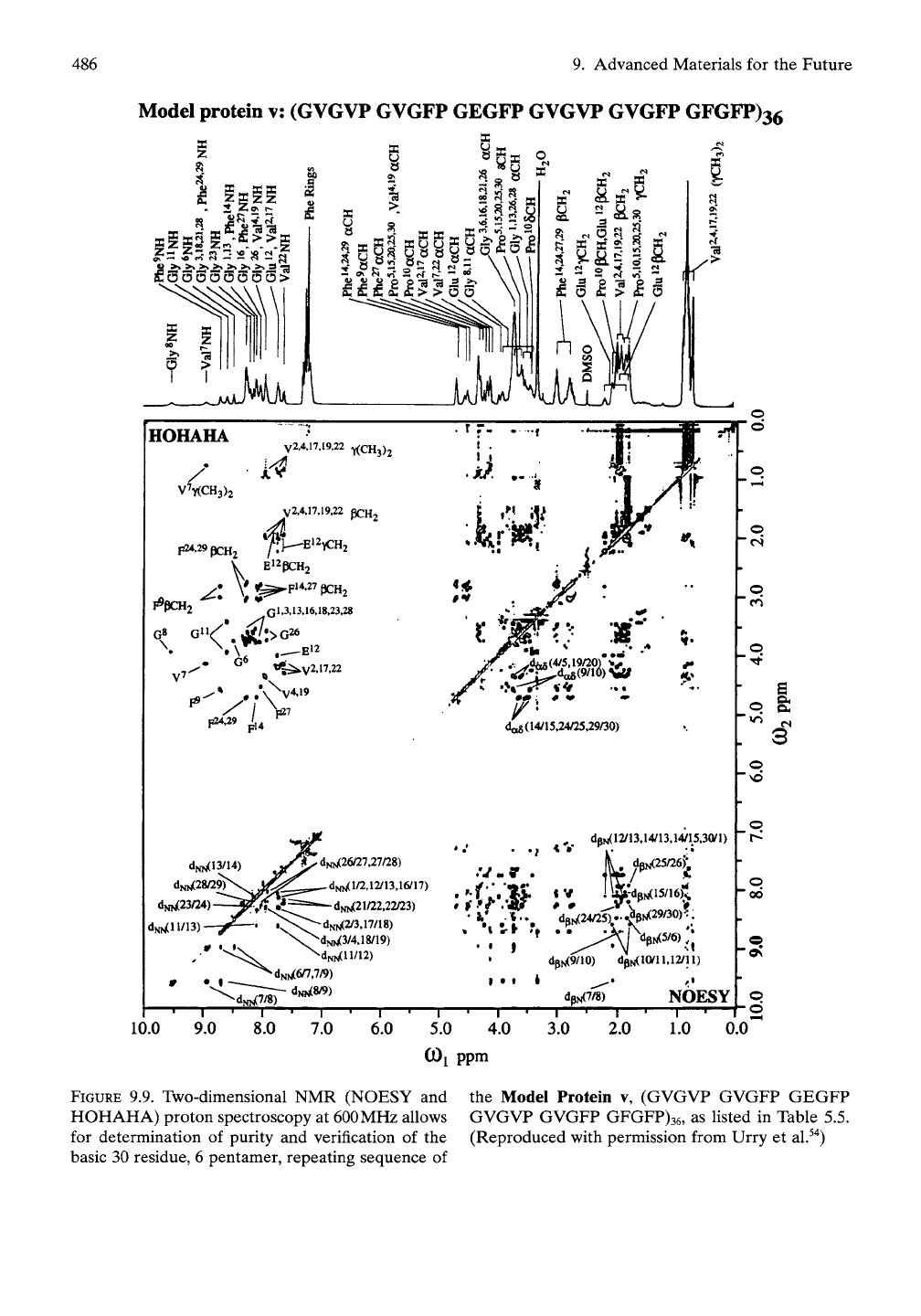
486 9. Advanced Materials for the Future
Model protein v: (GVGVP GVGFP GEGFP GVGVP GVGFP GFGFP)36
i
"^.ii.;
S
i
OS — >0 «*» <
zE3ooo3333;
e
f^ MM
ac
«^'
«o
"T
2
V7Y(CH3)2
F^'29 pcHj
El2pcH2
r pUM2 Gl,3.13.16,18,23,28
,(1^(4/5,19/20) %^
da5(14/15,24/25,29/30)
dNN(13/14)
dN,^28/29^
dNW(23/24)
dN^ll/13)
<v
dNN(26/27,27/28)
dNN(l/2,12/13,16/17)
-dNN(21/22.22/23)
dNN(2/3.17/18)
dNN(3/4.18/19)
'<X^
"^dNN(ll/12)
• • I
dpN(12/13,14/13,14/15.3(yi)
! ia;dpN(15/16)^
VN(24/25^-^M29/30)?.
dpN(9/10) dpN(l(yil.l2/ll)
«
V
P 0
dpN(7/8)
NOESY
10.0 9.0 8.0
7.0 6.0
5.0 4.0
COj ppm
3.0
T—
2.0
1—
1.0
o
^ CM
o
'vb
o
o
00
0.0
FIGURE
9.9. Two-dimensional NMR (NOESY and the Model Protein v, (GVGVP GVGFP GEGFP
HOHAHA) proton spectroscopy at 600MHz allows GVGVP GVGFP GFGFP)36, as listed in Table 5.5.
for determination of purity and verification of the (Reproduced with permission from Urry et al.^"^)
basic 30 residue, 6 pentamer, repeating sequence of
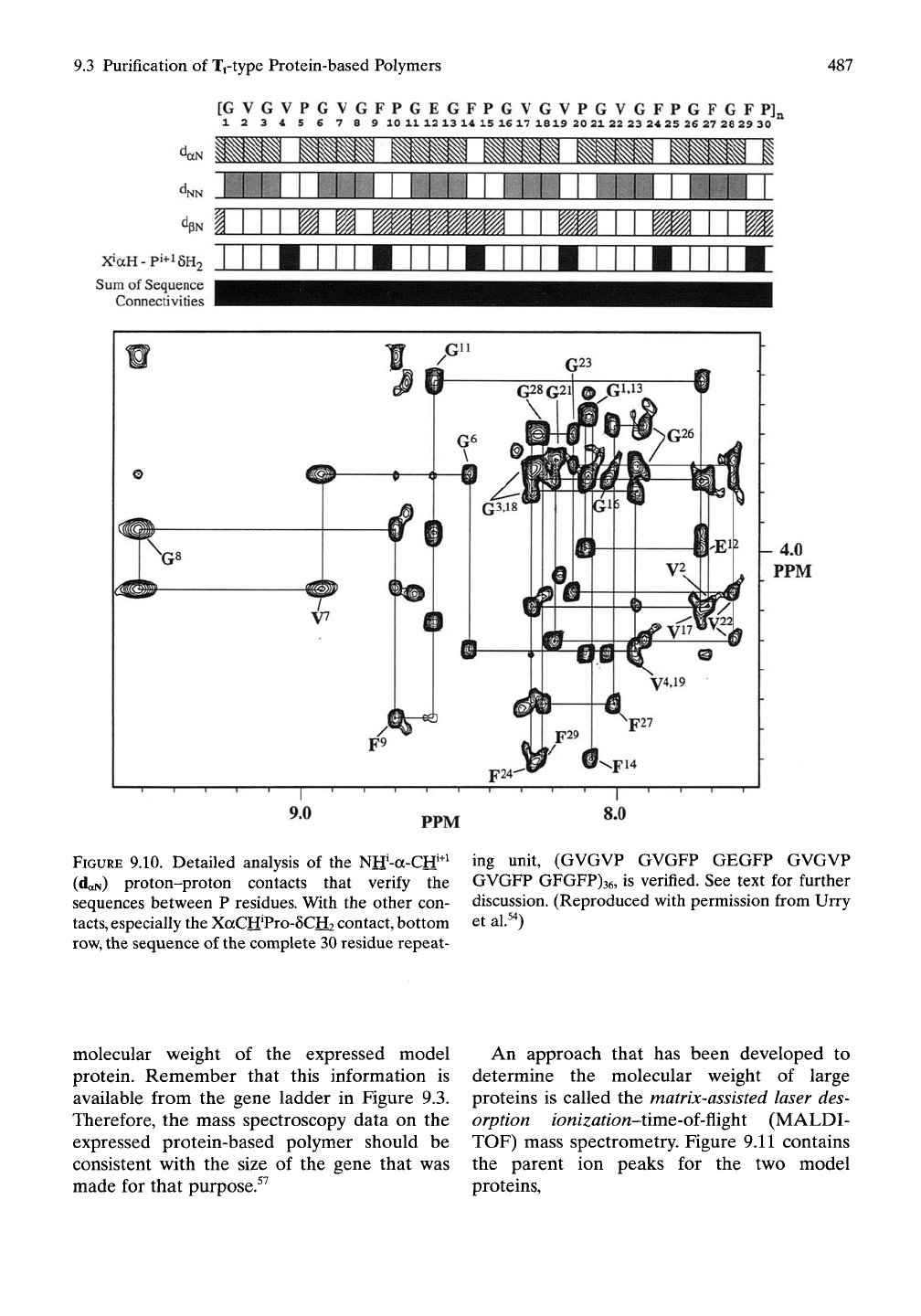
9.3 Purification of Tftype Protein-based Polymers
487
[GVGVPGVGFPGEGFPGVGVPGVGFPGFGFPJa
12345678 9 10 1112 13 14 15 1617 1819 20 2122 23 24 25 26 27 28 29 30
X»aH-pi+i6H2
Sum
of Sequence
Connectivities
PPM
FIGURE
9.10. Detailed analysis of the
Ntf-a-Crf^^
ing unit, (GVGVP GVGFP GEGFP GVGVP
(daN) proton-proton contacts that verify the
sequences between P residues. With the other con-
tacts,
especially the XaCH'Pro-SCH? contact, bottom
row,
the sequence of the complete 30 residue repeat-
GVGFP GFGFP)36, is verified. See text for further
discussion. (Reproduced with permission from Urry
et al.^')
molecular weight of the expressed model
protein. Remember that this information is
available from the gene ladder in Figure 9.3.
Therefore, the mass spectroscopy data on the
expressed protein-based polymer should be
consistent with the size of the gene that was
made for that purpose.^^
An approach that has been developed to
determine the molecular weight of large
proteins is called the matrix-assisted laser des-
orption ionization-iivaQ-oi-^ighi (MALDI-
TOF) mass spectrometry. Figure 9.11 contains
the parent ion peaks for the two model
proteins,

488 9. Advanced Materials for the Future
Model protein A
Model protein B
i
3
40000
60000
Mass (m/z)
80000 40000
60000 80000
Mass (m/z)
FIGURE 9.11. Mass spectrometry using
the matrix-assisted laser desorption
ionization time of flight (MALDI-TOF)
method to determine atomic mass for
verification of chain length
(i.e.,
number
of repeating units for the model
proteins: (A) [(GVGVP)ioGVGVP-
GRGDSP(GVGVP)io]7(GVGVP). (B)
[(GVGVP)io(GVGVAP)8(GVGVP)io]5
(GVGVP). See text for further discus-
sion. (Reproduced with permission
from Urry et al.^^)
A: [(GVGVP)ioGVGVPGRGDSP(GVGV
P)io]7(GVGVP) with seven repeats of the
basic repeating unit of 111 residues with a
molecular weight of 64,300 Da
B:
[(GVGVP)io (GVGVAP)8(GVGVP)io]5
(GVGVP) with five repeats of the basic
repeating unit of 148 residues, giving a mole-
cular weight of 60,300 Da
Thus,
the capacity to verify the sequence of
the basic repeating unit and the number of re-
peating units in the expressed protein-based
polymer has been demonstrated.
9.4 Medical Applications
Consideration of potential medical applications
of protein-based polymers will always be
incomplete. The source of any particular listing
will be limited by confidentiality constraints on
its author, by the limits of the author's imagi-
nation, and by the decision to leave out appli-
cations presumed to be out of context. At first
glance, the list of medical applications included
in this section 9.4 may seem long. It
is,
nonethe-
less,
only a beginning.
A major element when considering medical
applications is the potential antigenicity of
protein-based materials and the adequacy of
means of purification, especially when prepared
from sources such as E. coli. Before discussing
the medical applications, however, it is impor-
tant to address immunogenicity and purifica-
tion sufficient for medical applications. Because
of the truly remarkable biocompatibility of
elastic protein-based polymers, a short state-
ment of the considered basis of the biocom-
patibility is given. Then a number of medical
applications are treated.
9.4.1 The Opportunity and the
Challenge for Use of Protein-based
Polymers as Biomaterials
The opportunity for using protein-based poly-
mers as biomaterials for medical applications
comes with demonstration of the biocompati-
bility of the basic hydrogel and elastic and
plastic states of the protein-based polymers,
and it comes with the capacity to produce
protein-based polymers by microbial fermenta-
tion with sufficiently low-cost production for a
broad range of medical applications.
The challenge is to purify microbially pro-
duced protein-based polymers to adequate
levels for use as biomaterials. The pharmaceu-
tical use of microbially prepared insulin
requires that impurities be less than 10 parts
per million (ppm). Repeated use of the phase
separation property for purification results in a
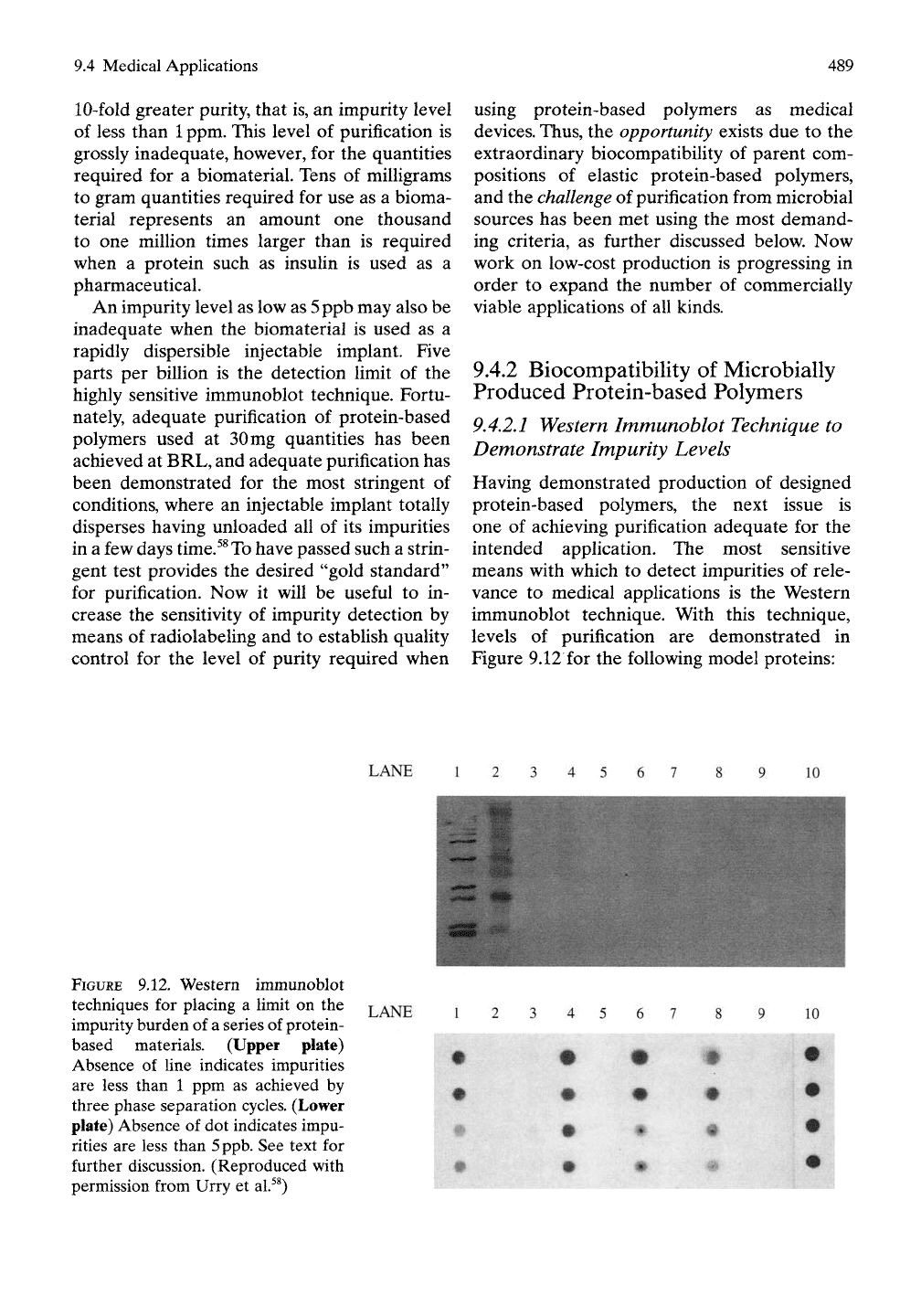
9.4 Medical Applications
489
10-fold greater purity, that is, an impurity level
of less than
1
ppm. This level of purification is
grossly inadequate, however, for the quantities
required for a biomaterial. Tens of milligrams
to gram quantities required for use as a bioma-
terial represents an amount one thousand
to one milHon times larger than is required
when a protein such as insulin is used as a
pharmaceutical.
An impurity level as low as
5
ppb may also be
inadequate when the biomaterial is used as a
rapidly dispersible injectable implant. Five
parts per biUion is the detection limit of the
highly sensitive immunoblot technique. Fortu-
nately, adequate purification of protein-based
polymers used at 30 mg quantities has been
achieved at BRL, and adequate purification has
been demonstrated for the most stringent of
conditions, where an injectable implant totally
disperses having unloaded all of its impurities
in a few days time.^^To have passed such a strin-
gent test provides the desired "gold standard"
for purification. Now it will be useful to in-
crease the sensitivity of impurity detection by
means of radiolabeling and to establish quality
control for the level of purity required when
using protein-based polymers as medical
devices. Thus, the opportunity exists due to the
extraordinary biocompatibility of parent com-
positions of elastic protein-based polymers,
and the challenge of purification from microbial
sources has been met using the most demand-
ing criteria, as further discussed below. Now
work on low-cost production is progressing in
order to expand the number of commercially
viable applications of all kinds.
9.4.2 Biocompatibility of Microbially
Produced Protein-based Polymers
9A,2,1 Western Immunoblot Technique to
Demonstrate Impurity Levels
Having demonstrated production of designed
protein-based polymers, the next issue is
one of achieving purification adequate for the
intended application. The most sensitive
means with which to detect impurities of rele-
vance to medical applications is the Western
immunoblot technique. With this technique,
levels of purification are demonstrated in
Figure 9.12 for the following model proteins:
LANE
10
FIGURE 9.12. Western immunoblot
techniques for placing a limit on the
impurity burden of a series of protein-
based materials. (Upper plate)
Absence of line indicates impurities
are less than 1 ppm as achieved by
three phase separation cycles. (Lower
plate) Absence of dot indicates impu-
rities are less than
5
ppb.
See text for
further discussion. (Reproduced with
permission from Urry et al.^^)
LANE
10
•

490
9. Advanced Materials for the Future
Model Protein A': (GVGVP)25i
Model Protein A: [(GVGVP)ioGVGVPGRG
DSP(GVGVP)io]7(GVGVP)
Model Protein B: (GVGVP)io(GVGi^\P)8
(GVGVP)io]5(GVGVP)
Model Protein i: (GVGVP GVGVP GEGVP
GVGVP GVGVP)„(GVGVP)
Model Protein x': (GVGVP GVGVP GKGVP
GVGVP GVGVP)n(GVGVP)
The upper part of Figure 9.12 utiUzes
5|Lig
of
protein per well. Lane 1 is the positive control
obtained from the lysate of the E. coli strain
used to produce the model proteins. Lane 2
contains molecular weight markers. Lanes 3
through 6 are Model Proteins A', x', A, and B,
respectively, and lanes 7 through 10 are nega-
tive controls, in this case unused wells. At the
concentration of
5 jiig
protein per well, no impu-
rities are observed with these model proteins
purified solely by three phase separation cycles.
This allows the statement that the impurities
are less than
1
ppm.
When the dot blot technique is used, as much
as
1
mg of protein is placed in each well dot, and
the total protein is tested for a visible immuno-
genic reaction. No visible dot means that the
impurities are less than 5ppb. Lanes
1,4,6,
and
8, contain Model Proteins A', A, B, and x',
respectively. In Lanes
1,4,6,
and 8, the top two
dots contain Img, whereas the bottom two
dots contain 0.1 mg; thus the effect of a 10-fold
dilution can be seen. Lanes 2 and 10 are the
negative and the positive controls, respectively,
showing the experiment to be working well. On
further purification of Model Proteins A', A, B,
and x', as shown in lanes 3, 5, 7, and 9, respec-
tively, no spot can be detected. This means
that purification of the model proteins has
been obtained to the level where impurities
detectable by the Western immunodotblot
technique are less than 5ppb.
9.4.2.2 Subcutaneous Injection in
the Guinea Pig
Having established the extraordinary biocom-
patibility of poly (GVGVP), as reviewed above,
it becomes possible to use the chemically
synthesized poly(GVGVP) to determine the
extent of purification required of the micro-
bially prepared polymer (GVGVP)25i. Ac-
cordingly, 30 mg of the product resulting
from three cycles of phase separation to purify
(GVGVP)25i was injected subcutaneously in
the guinea pig. The result shown in Figure
9.13 demonstrates a substantial inflammatory
response. Clearly based on our previous knowl-
edge of the biocompatibility of chemically syn-
thesized poly(GVGVP), three cycles of phase
separation did not sufficiently remove the E.
coli impurities.
Further purification beyond three cycles of
phase separation with Model Protein i, above
and as defined in Table 5.5, resulted in the most
remarkable demonstration of biocompatibility.
As demonstrated in Figure 9.14, the subcuta-
neous injection of 30mg in the guinea pig, when
examined at 2 weeks, left no trace of having
been present. This was the case at four differ-
ent sites.^^ Such a result provides the most
demanding test of purification. All of the impu-
rities in the 30 mg sample had been released to
the host, and yet there was no trace of its having
been present. In fact, based on the formation of
the subcutaneous "bump" and its essential dis-
appearance within several days, it would seem
that all of the impurities were released in a few
days without an inflammatory response having
been elicited. This we take to be the "gold stan-
dard" for demonstrating the desired level of
purification.
9.4.2.3 Entropic Elasticity as a Barrier
to Antigenicity of Elastic
Protein-based Polymers
9.4.2.3.1 The Natural Anticipation of Foreign
Protein as Antigenic
Historically, foreign proteins have been recog-
nized as some of the most potent antigens.
Because of this, it is simply to be expected that
protein-based polymers would be antigenic.
The mammalian elastic fiber, however, has
generally been considered to be of low anti-
genicity. Thus, the expectation for repeating
sequences from the mammalian elastic protein,
such as (GVGVP)n, was that antigenicity would
be low. In pursuing medical uses growing out
of the development of the elastic protein-
based polymers, we had fully expected that
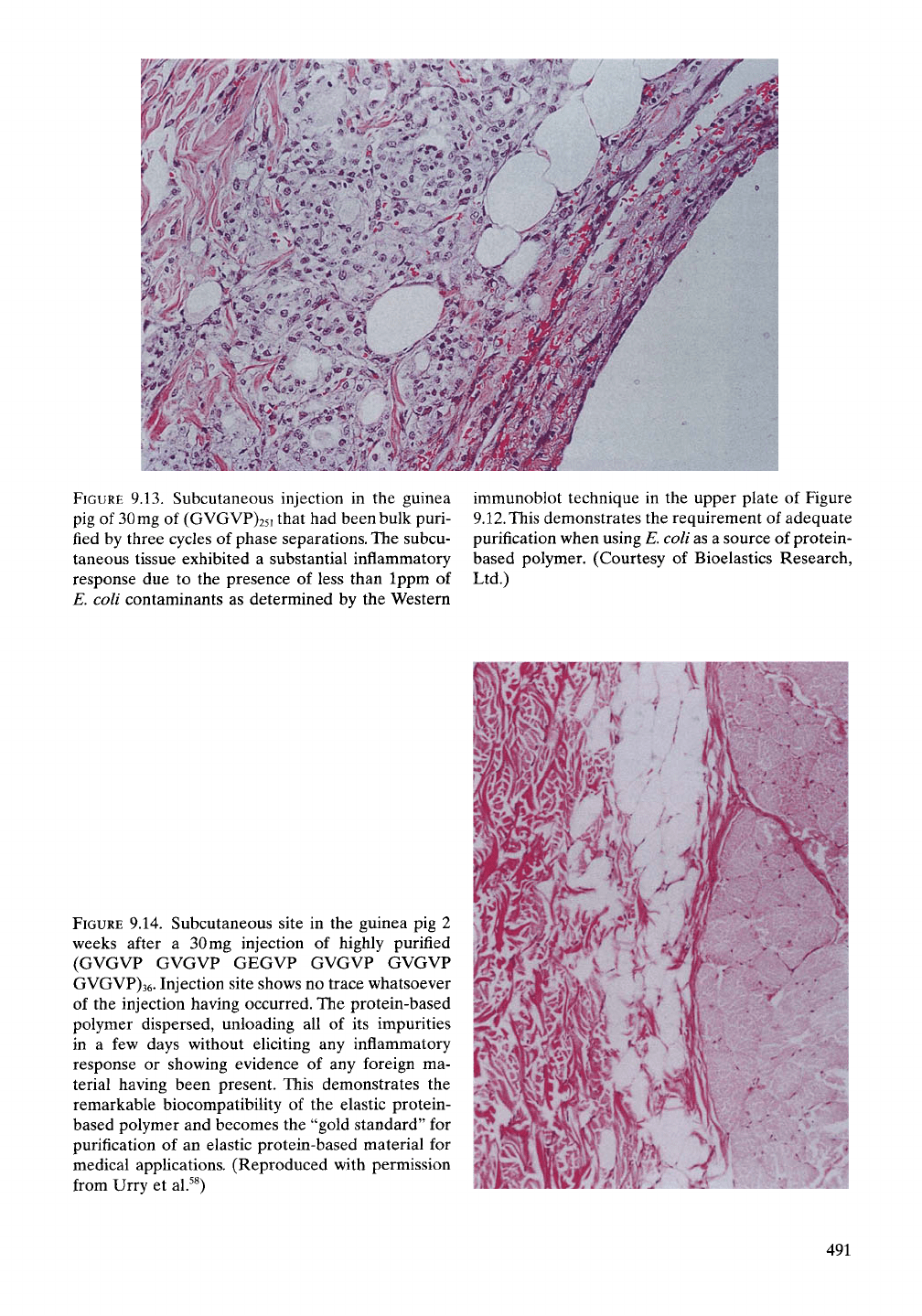
FIGURE
9.13. Subcutaneous injection in the guinea
pig of
30
mg of (GVGVP)25i that had been bulk puri-
fied by three cycles of phase separations. The subcu-
taneous tissue exhibited a substantial inflammatory
response due to the presence of less than Ippm of
E. coli contaminants as determined by the Western
immunoblot technique in the upper plate of Figure
9.12.
This demonstrates the requirement of adequate
purification when using E. coli as a source of protein-
based polymer. (Courtesy of Bioelastics Research,
Ltd.)
FIGURE
9.14. Subcutaneous site in the guinea pig 2
weeks after a 30mg injection of highly purified
(GVGVP GVGVP GEGVP GVGVP GVGVP
GVGVP)36. Injection site shows no trace whatsoever
of the injection having occurred. The protein-based
polymer dispersed, unloading all of its impurities
in a few days without eliciting any inflammatory
response or showing evidence of any foreign ma-
terial having been present. This demonstrates the
remarkable biocompatibility of the elastic protein-
based polymer and becomes the "gold standard" for
purification of an elastic protein-based material for
medical applications. (Reproduced with permission
from Urry et al.^^)
491

492 9. Advanced Materials for the Future
introduction of more polar groups in combina-
tion with more hydrophobic groups would
produce strong sites for antibody binding, that
is,
would produce antigenic determinants, epi-
topes,
and thereby would markedly increase
antigenicity. It was therefore a pleasant surprise
that the carboxylate-containing Model Proteins
i through v in Table 5.5 did not follow the
expectation of dramatically increasing anti-
genicity as more hydrophobic phenylalanine
(Phe,
F) residues increasingly replaced less
hydrophobic valine (Val, V) residues in a
polymer with glutamic acid (Glu, E) recurring
every 30 residues.
9.4.2.3.2 Greater Immunological Response to
Plastic Than to Elastic Protein-based Polymers
As our studies expanded to consider plastic
protein-based polymers with the parent being
(AVGVP)n, a small but detectable increase in
capacity to elicit formation of monoclonal anti-
bodies was found.^^ Indeed, as A and V was
replaced by E and F, however, ease of forming
monoclonal antibodies increased, which was
consistent with original expectations. Accord-
ingly, the key to the remarkable biocom-
patibility of elastic protein-based polymers
was sought and readily found in experimental
data that determined the nature of their
elasticity.
9.4.2.3.3 Key to Biocompatibility of Elastic
Protein-based Polymers Resides Within the
Nature of the Elasticity
The proposed basis for the nature of the ideal
elasticity exhibited by the family of protein-
based polymers using the generic sequence
(GXGXP)n, where X is a variable L-amino acid
residue, became very controversial. The adher-
ents to the classic (random chain network)
theory of rubber elasticity took great exception
to our proposal that the damping of internal
chain dynamics on extension gave rise to entro-
pic elasticity^^'^ (for more extensive treatment
of this controversy, see Urry and Parker.^^).The
physical basis for the different (even heretical,
to some) mechanism of near-ideal elasticity
provides insight into the remarkable biocom-
patibility of elastic protein-based polymers.
9.4.2.3.4 The Presence of Low Frequency
Mechanical Resonances in Elastic
Protein-based Polymers
Dielectric relaxation studies of the phase
transition of elastic protein-based polymers
demonstrate development of intense relax-
ations centered near
5
MHz and
3
kHz as the
phase separation proceeds.^^"^^ Also, acoustic
absorption measurements demonstrate devel-
opment of a correspondingly intense absorp-
tion near
3
kHz.^^'^^
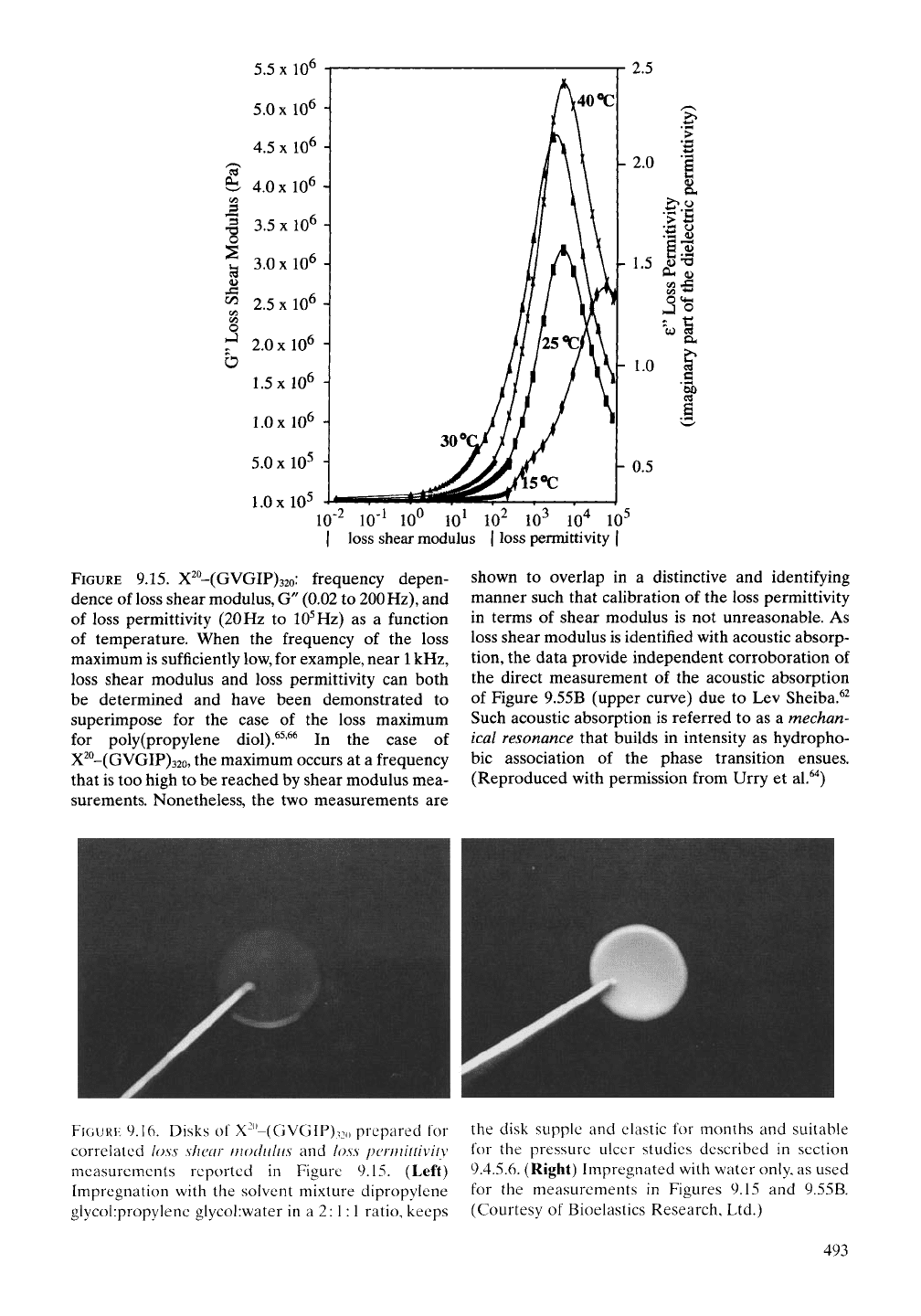
An approximate calibration of the
3
kHz
dielectric relaxation, called a loss permittivity,
in terms of elastic modulus is shown in Figure
9.15 using mechanical shear modulus measure-
ments to obtain what is called the loss shear
modulus,^ Shear modulus measurements of
interest here begin with a disk of cross-linked
(GVGIP)32o, shown in Figure 9.16, placed
between similarly sized disk-shaped plates. One
of the disk-shaped plates oscillates at frequen-
cies from very low values to a maximum of
about 200 Hz to measure as a function of
frequency the absorption by the elastic disk
of the energy of the mechanical oscillation. The
results are given over the lower frequency
range of Figure 9.15, up to about 200 Hz.
Now, it has been shown for materials such as
poly(propylene diol) (wherein both the absorp-
tion maximum for loss shear modulus and
loss permittivity overlap near the frequency of
IHz) that their normalized curves perfectly
superimpose over their frequency band
width.^^'^^ As shown in Figure 9.15, the lower
frequency loss shear modulus curves uniquely
overlap with the loss permittivity data at higher
frequency. As such the former is melded to cal-
ibrate the loss permittivity data to obtain a
coarse estimate of the elastic modulus values.
This provides an independent demonstration of
the mechanical resonance near
3
kHz and also
allows reference to the
5
MHz dielectric relax-
ation as a mechanical resonance. Thus, as the
folding and assembly of the elastic protein-
based polymers proceed through the phase
(inverse temperature) transition, the pentamers
wrap up into a structurally repeating helical
arrangement like that represented in Figure
9.17.
o
CO
o
10"^
10'' 10'' 10^ 10^ 10^ lO"^ 10^
I loss shear modulus | loss permittivity |
FIGURE
9.15. X^^-(GVGlF)32o: frequency depen-
dence of loss shear modulus, G" (0.02 to 200
Hz),
and
of loss permittivity
(20
Hz to
10^
Hz) as a function
of temperature. When the frequency of the loss
maximum is sufficiently low, for example, near
1
kHz,
loss shear modulus and loss permittivity can both
be determined and have been demonstrated to
superimpose for the case of the loss maximum
for poly (propylene diol).^^'^^ In the case of
X^°-(GVGIP)32o, the maximum occurs at a frequency
that is too high to be reached by shear modulus mea-
surements. Nonetheless, the two measurements are
shown to overlap in a distinctive and identifying
manner such that calibration of the loss permittivity
in terms of shear modulus is not unreasonable. As
loss shear modulus is identified with acoustic absorp-
tion,
the data provide independent corroboration of
the direct measurement of the acoustic absorption
of Figure 9.55B (upper curve) due to Lev Sheiba.^^
Such acoustic absorption is referred to as a mechan-
ical resonance that builds in intensity as hydropho-
bic association of the phase transition ensues.
(Reproduced with permission from Urry et al.^'^)
FIGURE
9.16. Disks of X-"-(GVGIP)32() prepared for
correlated loss shear modulus and loss permittivity
measurements reported in Figure 9.15. (Left)
Impregnation with the solvent mixture dipropylene
glycol:propylene glycol:water in a 2:1:1 ratio, keeps
the disk supple and elastic for months and suitable
for the pressure ulcer studies described in section
9.4.5.6. (Right) Impregnated with water only, as used
for the measurements in Figures 9.15 and 9.55B.
(Courtesy of Bioelastics Research, Ltd.)
493
494
9. Advanced Materials for the Future
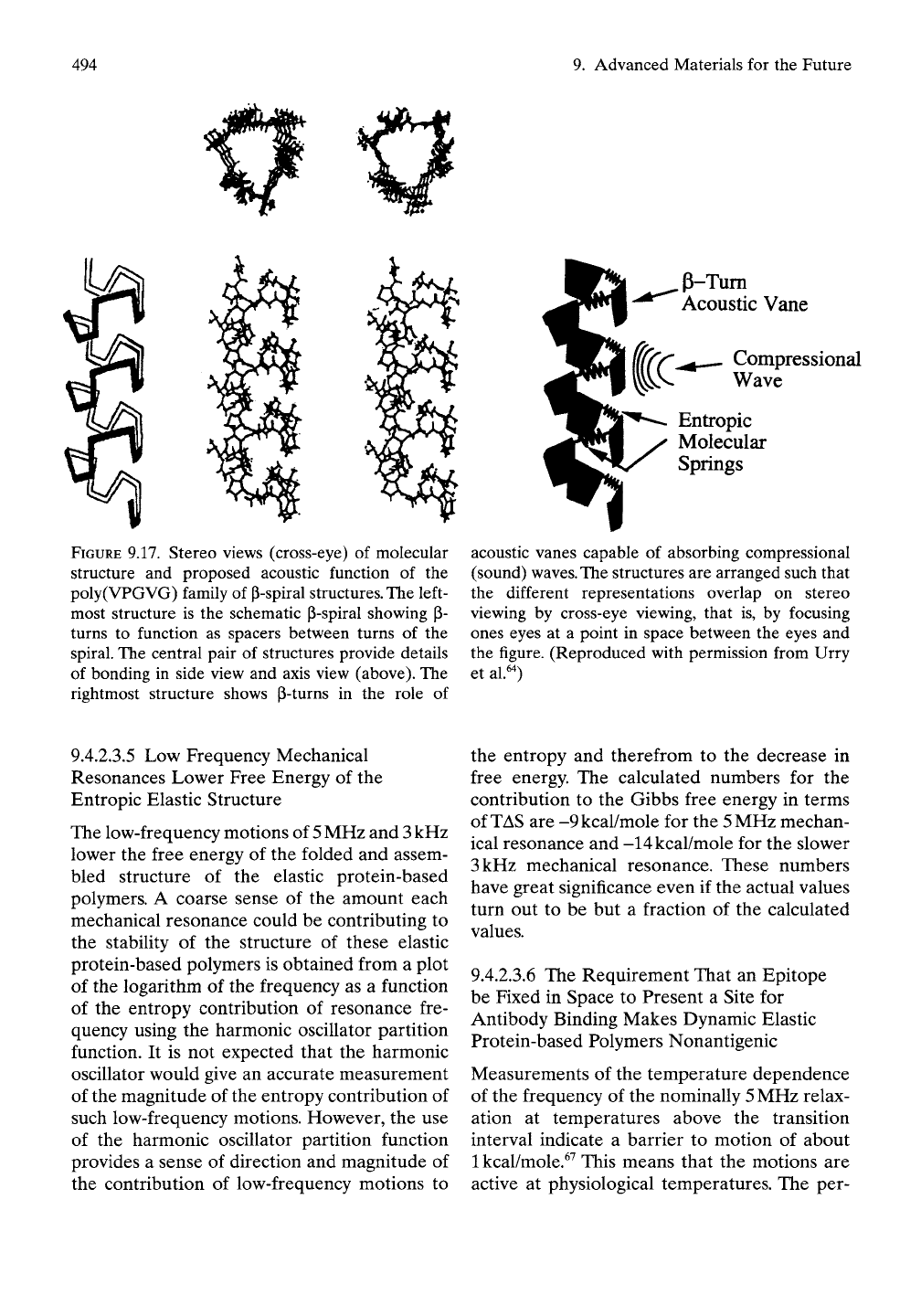
FIGURE 9.17. Stereo views (cross-eye) of molecular
structure and proposed acoustic function of the
poly(VPGVG) family of P-spiral
structures.
The left-
most structure is the schematic P-spiral showing p-
turns to function as spacers between turns of the
spiral. The central pair of structures provide details
of bonding in side view and axis view (above). The
rightmost structure shows P-turns in the role of
p-Tum
Acoustic Vane
Compressional
Wave
Entropic
Molecular
Springs
acoustic vanes capable of absorbing compressional
(sound)
waves.
The
structures are arranged such that
the different representations overlap on stereo
viewing by cross-eye viewing, that is, by focusing
ones eyes at a point in space between the eyes and
the figure. (Reproduced with permission from Urry
et al.'O
9.4.2.3.5 Low Frequency Mechanical
Resonances Lower Free Energy of the
Entropic Elastic Structure
The low-frequency motions of
5
MHz and
3
kHz
lower the free energy of the folded and assem-
bled structure of the elastic protein-based
polymers. A coarse sense of the amount each
mechanical resonance could be contributing to
the stability of the structure of these elastic
protein-based polymers is obtained from a plot
of the logarithm of the frequency as a function
of the entropy contribution of resonance fre-
quency using the harmonic oscillator partition
function. It is not expected that the harmonic
oscillator would give an accurate measurement
of the magnitude of the entropy contribution of
such low-frequency motions. However, the use
of the harmonic oscillator partition function
provides a sense of direction and magnitude of
the contribution of low-frequency motions to
the entropy and therefrom to the decrease in
free energy. The calculated numbers for the
contribution to the Gibbs free energy in terms
of
TAS
are -9kcal/mole for the
5
MHz mechan-
ical resonance and -14kcal/mole for the slower
3 kHz mechanical resonance. These numbers
have great significance even if the actual values
turn out to be but a fraction of the calculated
values.
9.4.2.3.6 The Requirement That an Epitope
be Fixed in Space to Present a Site for
Antibody Binding Makes Dynamic Elastic
Protein-based Polymers Nonantigenic
Measurements of the temperature dependence
of the frequency of the nominally
5
MHz relax-
ation at temperatures above the transition
interval indicate a barrier to motion of about
1 kcal/mole.^^ This means that the motions are
active at physiological temperatures. The per-
