Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


9.2 Production of Protein-based Polymers 475
TABLE
9.2.
Continued
31.
[(GVGIP)io-GVGIPGNGIP]n(GVGVP): n = 30^ 40^
32.
(GVGVP GVGVP GEGVP GVGVP GVGVP GVGVP)n(GVGVP) YGSEFELRRQACGRTRAPP-PPPLPSGC:
n =
44% 36^
33.
(GVGVP GVGVP GKGVP GVGVP GVGVP GVGVP)n(GVGVP): n =
55% 36%
22^
34.
[(FEGFPAEGFP)5]n(GVGVP): n = 19^
35.
[(GVGVP)io-GVGRGYSLGIP-(GVGVP)io)]n: n =
12%
7'
36.
[(GVGVP)io-GVGRGYSLGIP-(GVGIP)io)]n: n = 7'
37.
[(GVGIP)io-FPGVGQKRPSKRSKYLIP-(GVGIP)io)]n: n =
11%
6'
38.
[(GVGVP)io-FPGVGQKRPSKRSKYLIP-(GVGVP)io]n(GVGVP): n =
9%
6^
39.
[(GVGVP)io-FPGVGQKRPSKRSKYLIP-(GVGIP)io]n(GVGVP): n =
9% 7%
5^
40.
GKGKAPGK-(GVGVP GVGVP GEGVP GVGVPGVGVP GVGVP)n(GVGVP): n = 1
41.
[(FEGVP)io]n: n =
28%
15% 11,4
42.
[(GVGVP)io-GVGVPGRGDSP-(GVGVP GVGVP GKGVP GVGVP GVGFP GFGFP)2]n(GVGVP): n =
8%
5
43.
GKGKAPGK-[(GVGVP)io-GVGVPGRGDSP-(GVGVP)io]n: n = 7^
44.
(FEGFP AFGFP)5: n = 33, 29, 26, 25, 23,19,18,16,14,11, 6
45.
[(GVGVP GVGVP GKGVP GVGVP GVGFP GFGFP)2-GVGVPGRGDSP-(GVGVP)io(GVGVAP)8]n: n=
11%
8%
4% 3^
46.
[(GVGVP GVGVP GKGVP GVGVP GVGFP GFGFP)2-(GVGVP)io(GVGVAP)8]n: n =
12% 8%
4^
47.
[(GVGIP)ii-GYGIP-(GVGIP)io]2(GVGIP):Re*: n =
11%
10, 9, 8, 7,
6%
5
48.
[(FVGEP FVGFP)5]n: n = 18 ,17,12, 2,1
49.
(GIGVP GAGVP GIGVP GIGVP GAGVP GIGVP)n: n = 22% IV
50.
[(GVGIP)ll-GYGIP]n(GVGIP)^:
n =
18%
17,16,15,14,13,12,11%
6,
3
51.
[(GVGVP)io]n(GVGVP)^:
n =
28,
26,18,17% 16 ,15,14,13%
12,11,
6,
4
52.
[(GVGIP)io]n(GVGVP)^
n =
32%
25% 21% 15%
9% 6%
4%
2'
53.
{[(GVGIP GEGIP GVGIP)2]2)n: n = 20% 9'
54.
([GVGIP GEGIP (GVGIP)4]2)n: n =
23%
10^
55.
[(GVGIP GEGIP GVGIP GEGIP GVGIP GVGIP)2]n: n = 26% 17% 15^
56.
([(GVGIP GKGIP GVGIP)2]2)n: n = 19,18,17,12
57.
([GVGIP GKGIP (GVGIP)4]2}n: n =
21%
12^
58.
[(GVGIP GKGIP GVGIP GKGIP GVGIP GVGIP)2]n: n = 73,
41,
21,17,14,11
59.
[(GVGIP)8(GYGIP]n: n =
32%
24% 18^
60.
([(GVGIP)5GYGIP]2ln: n = 20% 12% ir
61.
([(GVGIP)2GYGIP]3ln: n = 26,15% 14
62.
[(GVGIP),o]ni-Fn3-[(GVGIP)io]„2: nl = 16 and n2 = 6
63.
(GVGVP GVGVP GEGVP GVGVP GVGVP GVGVP)n(GVGVP): n = 42%
23% 22%
18% 16^
64.
AKKKKKKG-[(GVGIP)io]n-VCCC: n = 15^
65.
AKKKKKKG-[(GVGIP)io]n-VCCC: n = 18^
66.
GKGKAPGK-[(GGAP)i2]n(GVGVP): n = 1
67.
[(GGAP)i2]n: n = 1
68.
[(FVGVP FEGVP)5]n: n = 1
69.
([(GVGVP)2-GFGVP]3)n: n = 1
70.
[(GVGIP)ii-GSGIP-(GVGIP)io]n(GVGIP)'': n = 1
71.
[(GVGIP)n-GTGIP-(GVGIP)io]n(GVGIP)': n = 1
72.
[(FFGEP)io]n: n = 1
73.
[(GVGIP)n-GSGIP]n(GVGIP)*': n = 1
74.
[(GVGIP)n-GTGIP]n(GVGIP)'': n = 1
75.
[(GVGVP GVGVP GKGVP GVGVP GVGFP GFGFP)2-GVGVPGRGDSP-(GVGVP GVGVP GKGVP GVGVP
GVGFP GFGFP)2]n: n = 1
76.
(GIGVP GAGVP GKGVP GIGVP GAGVP GIGVP)n: n = 1
77.
(GIGVP GAGFP GKGFP GIGVP GAGVP GIGVP)n: n = 1
78.
(GIGVP GAGFP GKGFP GIGVP GAGFP GVGFP)^: n = 1
79.
[ACPGCGGVGIPCPGCG-[(GVGIP)26-Fn3(RGYSLG)-(GVGIP)6]-CPGCGGVGIPCPGCG]„: n = r
^ The protein-based polymer was expressed.
^ The gene was made explicitly using the same codon for a repeating residue in the sequence, and then a different codon
was used in the next repeat.
^ Fn3(RGYSLG) stands for the tenth type III domain of fibronectin in which the cell attachment sequence, GRGDSP,
was replaced by the kinase site, RGYSLG, as a site for phosphorylation.

476 9. Advanced Materials for the Future
looking for a market with future growth. Yeast
has the advantages of excreting the protein-
based polymer from the cell, such that harvest-
ing could proceed without cell destruction, and
a protein-based polymer product from yeast
would not be expected to have such purification
demands as occur with E. coli. Production in the
seeds of a plant such as canola has the advan-
tage of being a value added product where the
base product is the healthful canola oil. In this
case the protein-based polymer would reside
in the protein byproduct that is often sold as
feed for livestock at costs of less than a dollar
per pound. Accordingly, purification from the
protein byproduct could be expected to yield
protein-based polymer at a cost of little more
than that of purification. Because the purifica-
tion process uses a water-based phase separa-
tion, even the remainder of the protein
byproduct could retain its value.
9.2.1.5.2 Elements in the Comparison of
Low-cost Production
Chapter 4 emphasized that biological produc-
tion of protein is energetically an extravagant
process. This represents the basic cost of
biology's capacity to place any of 20 different
amino acid residues at each position and to do
so while ensuring the correct optical isomer.
Such diverse sequences, while maintaining the
same optical isomer at each position, consti-
tutes an impossible feat for chemical synthesis,
and the cost of chemical synthesis yet remains
greater by orders of magnitude.
Despite the very low efficiency for conver-
sion of the energy of photons into the energy
represented by protein that was discussed in
Chapter 4, plants provide the most promising
approach to low-cost production of protein-
based polymers. The cost of harvesting photons
from the sun resides within the cost of main-
taining a healthy plant. Of course, when we
speak of seeking low-cost production of
protein-based polymers, the cost-comparison is
between chemical synthesis and biological syn-
thesis.
Even though chemical synthesis loses
badly in this comparison, specific contributions
of chemical synthesis become invaluable, as dis-
cussed below.
9.2.2 Chemical Synthesis
9,2.2.1 Important Contributions of
Chemically Synthesized Polymers
9.2.2.1.1 Chemical Synthesis Achieved Many
Different Protein-based Polymers Quickly
The approximately 100 polymers that provided
the basis for the data in Figure 5.9 and Table 5.1
were chemically synthesized in about 2 years'
time.
Over the last 10 years, some 80 basic
monomer genes were prepared at BRL. In
general, these monomers were then concate-
merized to produce a multimer gene of the
desired
size.
For proper comparison of the poly-
mers in Figure 5.9 and Tables 5.1, 5.2, and 5.3,
the polymers needed to be of a comparable size.
Therefore, it would have taken 10 years instead
of 2 years to move the basic science to the
understandings provided by the data on chem-
ically synthesized polymers. As shown in Figure
5.10, the data from the chemically sythesized
poly[0.8(GVGVP),0.2(GXGVP)], where X is
either the uncharged Lys (K°) or the uncharged
Glu (E°), are quite comparable with those from
biosynthesized Model Proteins i (E°/OF) and x'
(K°/OF) of Table 5.5. Nonetheless, to obtain the
most quantitative understanding of the com-
prehensive hydrophobic effect as represented
in Figure 5.10, the work should be redone with
biosynthesized protein-based polymers. Suc-
cinctly
stated,
chemical synthesis has moved the
most significant utilization of protein-based
polymers forward by about one decade.
9.2.2.1.2 Comparison of Protein-based
Polymers Having a Random Incorporation of
a Set of Repeats with Those Having the
Repeats in a Fixed Sequence
The profound importance of sequence control
and its relevance to the mechanistic basis of
function could not have been demonstrable
without the capacity to compare the pKa
shifts of Glu residues with a fixed sequence
with those of a random mixture of the same
sequences. For example, the pKa of the chemi-
cally synthesized fixed sequence poly(GVGVP
GVGFP GEGFP GVGVP GVGFP GFGFP)
of 8.1 can be compared with the pKa of
5.2 obtained for the chemically synthesized

9.2 Production of Protein-based Polymers
477
polymer having a random mix of the same
composition of pentamers, namely, poly
[(GEGYF),2(GyGFF),(GFGFF)y' Therefore,
sequence has fundamental consequence. No
wonder biology pays such a high price for the
control of sequence, as detailed in Chapter 4.
9.2.2.1.3 Chemical Synthesis Provided Proof
of Biocompatibility as the Product Had No
Microbial Contaminants
Only because the remarkable biocompatibility
of chemically synthesized poly(GVGVP) was
already known was there adequate impetus
to purify microbially prepared (GVGVP)25i.
Otherwise, it would have been presumed, as
had been widely expected, that the toxicity of
inadequately purified (GVGVP)25i was an
inherent property of the protein-based
polymer. To be left in such a state of misunder-
standing would have meant that the dramatic
potential of elastic protein-based polymers for
use in medical appHcations would be neither
appreciated nor realized. The inflammatory
response ehcited by an inadequately purified
biosynthetic elastic protein-based polymer
would have overwhelmed most considered
medical applications.
Our research utilizing chemical synthesis of
repeating peptide sequences is represented
in over 170 scientific publications. It utilized
classic solution synthesis and to a much lesser
extent solid phase methods. The primary focus
in all cases has been development of an under-
standing relevant to structure, function, and
mechanism rather than development of syn-
thetic methodologies, although some of the
latter did indeed occur principally due to the
expert capacities of T. Ohnishi, K. Okamoto,
R. Rapaka, K.U. Prasad, T.R Parker, and D.C.
Gowda. Here we note a few issues relevant to
the production of protein-based polymers, that
is,
of polymers composed of repeating peptide
sequences.
9.2.2.2 Classic Solution Methods
for Chemical Synthesis of
Repeating Sequences
A principal concern during chemical synthesis
is racemization, the formation of some amino
acid residues of the D-configuration rather
than maintaining only the L-configuration. The
result of only L-amino acid residues in the
expressed protein, of course, results from
protein synthesis by means of the genetic code
and recombinant DNA technology. (See Figure
3.3 and the associated text in Chapter 3 to
review the mirror image relationship between
L- and D-amino acid residues and to appreciate
the consequences relating to structure and
function.) In particular, during the chemical
activation of the carboxyl group of an amino
acid and its subsequent reaction with an amino
function of another amino acid, the amino acid
residue with the activated carboxyl can racem-
ize;
some D-amino acid residues become
incorporated into the growing chain with a
consequence of structural disruption like that
schematically represented in Figure 3.3C.
There are two ways to avoid recemization
during chemical synthesis. One way is to rely at
critical steps on the glycine (Gly, G) residue,
which with two hydrogens on the a-carbon
does not form mirror images. Therefore, for G,
there is no consequence of interchanging posi-
tions of the a-carbon substituents during acti-
vation and reaction. The second way is to rely
on the prohne (Pro, P) residue in which the
bridging of the R-group, -CH2-CH2-CH2-,
between a-carbon and nitrogen atom of the
same residue limits racemization. Accordingly,
with repeating units containing G and P, which
are critical residues in the repeating sequences
of elastic protein-based polymers, the strategy
is to build the repeating unit with a G or P at
its carboxyl terminus and to utilize crystalliza-
tion to remove component peptide sequences
that contain racemized residues.
The build-up strategy for the GVGVP or
VPGVG repeating unit is a 2 x 3 strategy. First
the dipeptide VP is synthesized and crystallized
to form pure dimer containing only L-amino
acid residues. Then the GVG tripeptide is syn-
thesized and also crystallized to result in pure
GVG containing only L-valine (Val, V). The
dipeptide can be added to the tripeptide to
form VPGVG, or the tripeptide can be added
to the dipeptide to form the pentamer GVGVP.
Both pentapeptides have either the required
G or the required P to prevent racemization

478
9. Advanced Materials for the Future
during polymerization of the pentamer to make
the poly(GVGVP) or poly(VPGVG). Because
higher molecular weight peptides could be
obtained using GVGVP, this became the poly-
merization of preference. Also a search for the
best activating reagent resulted in molecular
weights higher than had been previously
achieved by chemical synthesis."*^'^^
9.2.2.3 Merrifield Solid Phase Methods
for Chemical Synthesis of
Repeating Sequences
The Merrifield solid phase synthesis
approach'*'^^ constitutes an apparatus that is
constructed and programmed to go through the
many many steps involved in forming a single
peptide bond and to grow the peptide chain
by coupUng each desired amino acid of the
sequence to the growing chain attached to a
solid phase, a so-called resin. No matter how
much care may be taken at each coupUng step,
however, there remains a finite probability of
racemization. For protein-based polymers this
Umitation is overcome by proceeding with
pentamer additions, by adding GVGVP or
VPGVG, for example. This has the great advan-
tages of preparing racemization-free polymers
of essentially a single chain size and of setting
up and programming the apparatus to run con-
tinuously through many couplings."^^ There is
the disadvantage, however, because coupling
efficiency is not 100%, that much pentamer is
required for the synthesis and is not incorpo-
rated into the growing chain.
The advent of recombinant DNA technology,
two decades after the remarkable Merrifield
advance, has overtaken many of the advantages
of the Merrifield approach. After the time-
consuming gene construction and development
of a good expression system, large quantities of
elastic protein-based polymers are produced in
a short time, as shown in Figure 9.5.
9.2.2.4 Practicality of Chemical Synthesis
A major limitation of chemical synthesis is cost,
which is central to the practicality of particular
applications. Cost of production at the labora-
tory bench is on the order of $200/gram. Scale
up could be expected to reduce that cost by an
order of magnitude. For medical applications
that require costly FDA approval, however,
there is the added cost of what is called good
manufacturing practices, which increase costs
severalfold. Accordingly, any product that
would not be competitive at $100/gram would
be challenging to consider by chemical synthe-
sis of protein-based materials, particularly
when there can be strict functional require-
ments for certain elements of purity, optical and
otherwise.
Nonetheless, when carried out with sufficient
care,
chemical synthesis of the polymers can be
the best approach for establishing biocompati-
bility of protein-based polymers. As discussed
below, the chemically synthesized products that
have been carefully purified became primary
standards against which purification of biopro-
duced polymers could be held. The specific
disadvantages of chemical synthesis are its
expense, the inability to be totally free of
racemization, its reproducibility, and the need
to use noxious and toxic chemicals. On the
other hand, the primary challenge of biosyn-
thesis using recombinant DNA technology is
one of removing the toxic components unique
to the producing organism or plant.
9.2.2.5 Biocompatibility of Chemically
Synthesized Protein-based Materials
The biocompatibiUties of the three physical
states of these protein-based polymers, that
is,
hydrogel, elastic, and plastic, were ini-
tially determined using the representative
chemically synthesized products. The polymer
poly(GGAP) may be considered the parent
polymer for the hydrogel state, because it exists
as a hydrogel except under extreme conditions
of high concentrations of multivalent salts.
Poly(GVGVP) is the parent polymer for the
elastomeric state, as it derived from the mam-
malian elastic fiber. It was the beginning
sequence from which many of the other poly-
mers were derived, initially by design and
chemical synthesis of compositional variants.
Poly(AVGVP) was the original sequence for
the plastic state; it derived by simple substitu-
tion of a glycine (G) residue of poly(GVGVP)
by an alanine (A) residue. The initial purpose
9.2 Production of Protein-based Polymers
479
of the synthesis of poly(AVGVP) was to test
the newly proposed basis for near ideal or
entropic elasticity.
Of course, the primary requirement for use
of these polymers as part or all of a medical
device is that the protein-based polymer must
be sufficiently nontoxic, that is, it must exhibit
adequate biocompatibility. As representative
polymers for each of the interesting physical
states,
each of the above three compositions
has been thoroughly examined by the standard
set of 11 tests recommended by the American
Society for the Testing of Materials (ASTM) for
materials in contact with tissue, tissue fluids,
and blood.
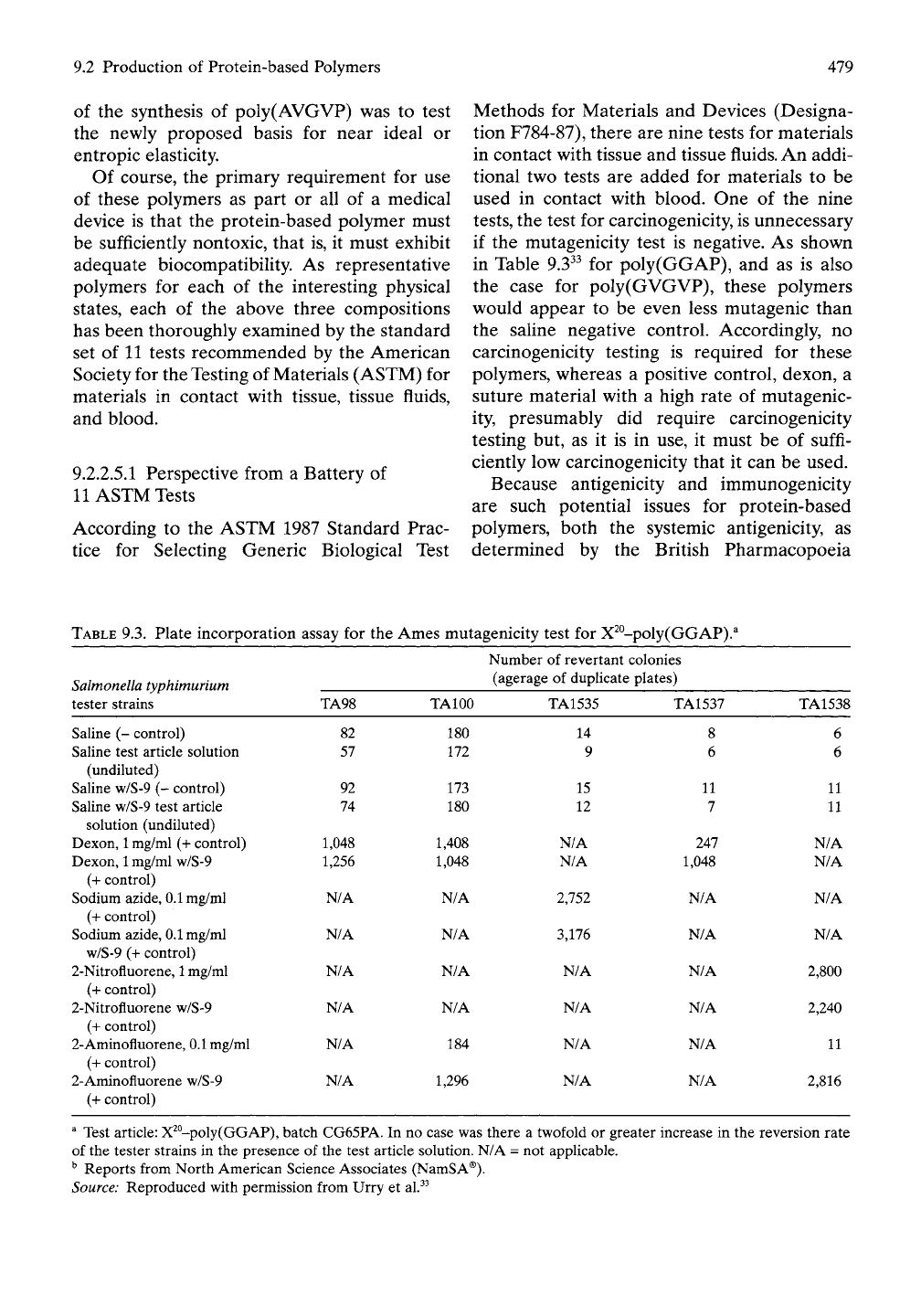
9.2.2.5.1 Perspective from a Battery of
11
ASTM Tests
According to the ASTM 1987 Standard Prac-
tice for Selecting Generic Biological Test
Methods for Materials and Devices (Designa-
tion F784-87), there are nine tests for materials
in contact with tissue and tissue
fluids.
An addi-
tional two tests are added for materials to be
used in contact with blood. One of the nine
tests,
the test for carcinogenicity, is unnecessary
if the mutagenicity test is negative. As shown
in Table 9.3^^ for poly(GGAP), and as is also
the case for poly(GVGVP), these polymers
would appear to be even less mutagenic than
the saline negative control. Accordingly, no
carcinogenicity testing is required for these
polymers, whereas a positive control, dexon, a
suture material with a high rate of mutagenic-
ity, presumably did require carcinogenicity
testing but, as it is in use, it must be of suffi-
ciently low carcinogenicity that it can be used.
Because antigenicity and immunogenicity
are such potential issues for protein-based
polymers, both the systemic antigenicity, as
determined by the British Pharmacopoeia
TABLE
9.3. Plate incorporation assay for the Ames mutagenicity test for X^°-poly(GGAP).^
Salmonella typhimurium
tester
strains
Saline
(-
control)
Saline
test
article
solution
(undiluted)
Saline
w/S-9 (-
control)
Saline
w/S-9
test
article
solution
(undiluted)
Dexon,
1
mg/ml
(+
control)
Dexon,
1
mg/ml
w/S-9
(+
control)
Sodium
azide, 0.1
mg/ml
(+
control)
Sodium
azide, 0.1
mg/ml
w/S-9 (+
control)
2-Nitrofluorene,
1
mg/ml
(+
control)
2-Nitrofluorene
w/S-9
(-1-
control)
2-Aminofluorene,
0.1
mg/ml
(+
control)
2-Aminofluorene
w/S-9
("f-
control)
TA98
82
57
92
74
1,048
1,256
N/A
N/A
N/A
N/A
N/A
N/A
TAIOO
180
172
173
180
1,408
1,048
N/A
N/A
N/A
N/A
184
1,296
Number
of
revertant
colonies
(agerage
of
duplicate
plates)
TA1535
TA1537
14
9
15
12
N/A
N/A
2,752
3,176
N/A
N/A
N/A
N/A
8
6
11
7
247
1,048
N/A
N/A
N/A
N/A
N/A
N/A
TA1538
6
6
11
11
N/A
N/A
N/A
N/A
2,800
2,240
11
2,816
* Test article: X^°-poly(GGAP), batch CG65PA. In no case was there a twofold or greater increase in the reversion rate
of the tester strains in the presence of the test article solution. N/A = not applicable.
^ Reports from North American Science Associates (NamSA®).
Source: Reproduced with permission from Urry et al.^^
480 9. Advanced Materials for the Future
TABLE
9.4. Guinea pig sensitization dermal reac-
tions—challenge with X^°-poly(GGAP), batch
CG65PA.^
Animal
(No.,
Group)
1
Test
2
Test
3
Test
4
Test
5
Test
6
Test
7
Test
8
Test
9
Test
10
Test
11
Control
12
Control
13
Control
14
Control
15
Control
Hours
24
0
0
0
0
0
0.5
0
0
0
0
0
0
0
0
0.5
following patch removal
48
0.5
0.5
0
0
0
0.5
0
0
0
0
0
0
0.5
0
0.5
72
0.5
0.5
0
0
0
0.5
0
0
0
0
0
0
0
0
0.5
96
0
0
0
0
0
0
0
0
0
0
0.5
0
0
0
0
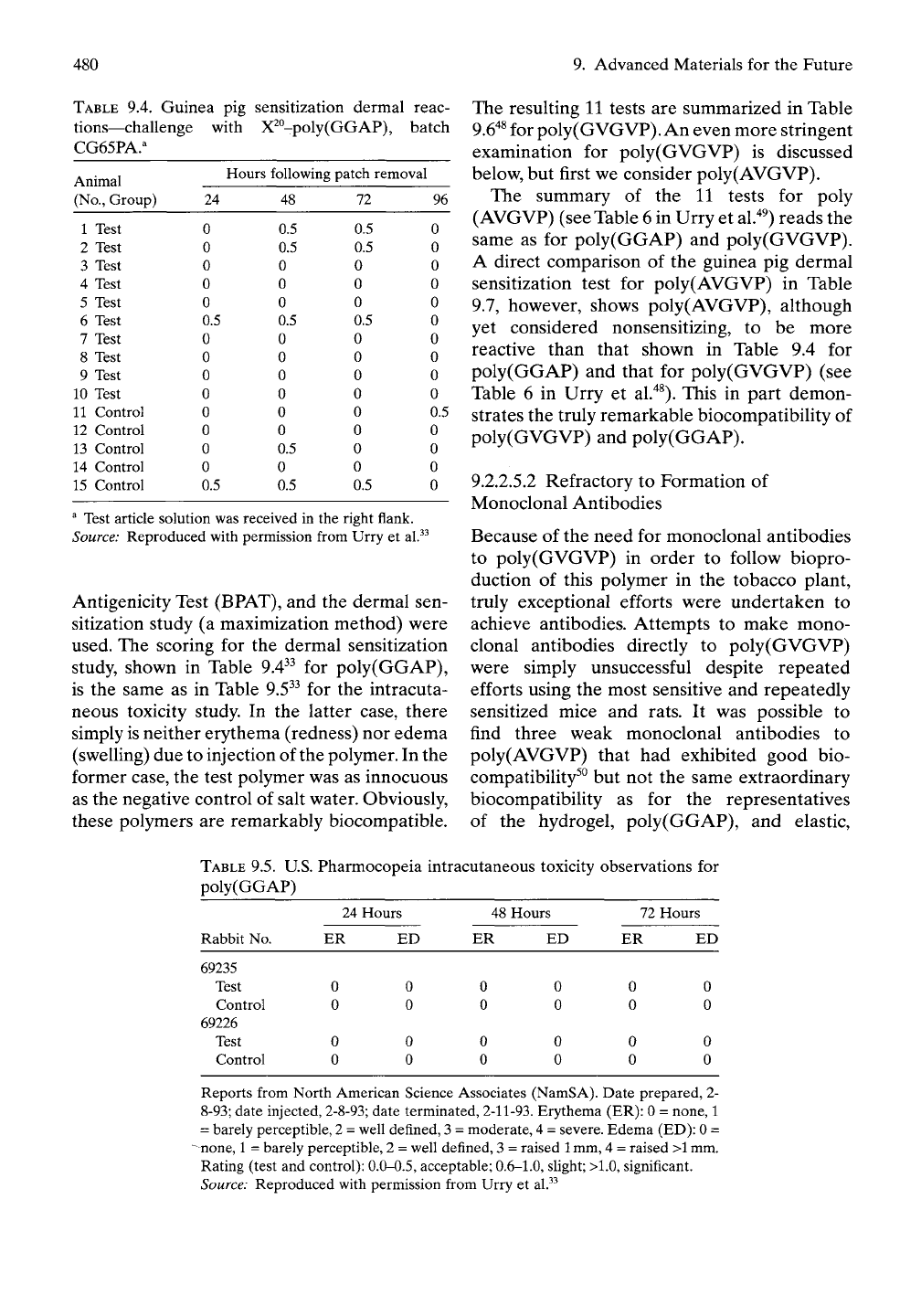
^ Test article solution was received in the right flank.
Source: Reproduced with permission from Urry et al.^-
Antigenicity Test (BPAT), and the dermal sen-
sitization study (a maximization method) were
used. The scoring for the dermal sensitization
study, shown in Table 9.4^^ for poly(GGAP),
is the same as in Table 9.5^^ for the intracuta-
neous toxicity study. In the latter case, there
simply
is
neither erythema (redness) nor edema
(swelling) due to injection of the polymer. In the
former case, the test polymer was as innocuous
as the negative control of salt water. Obviously,
these polymers are remarkably biocompatible.
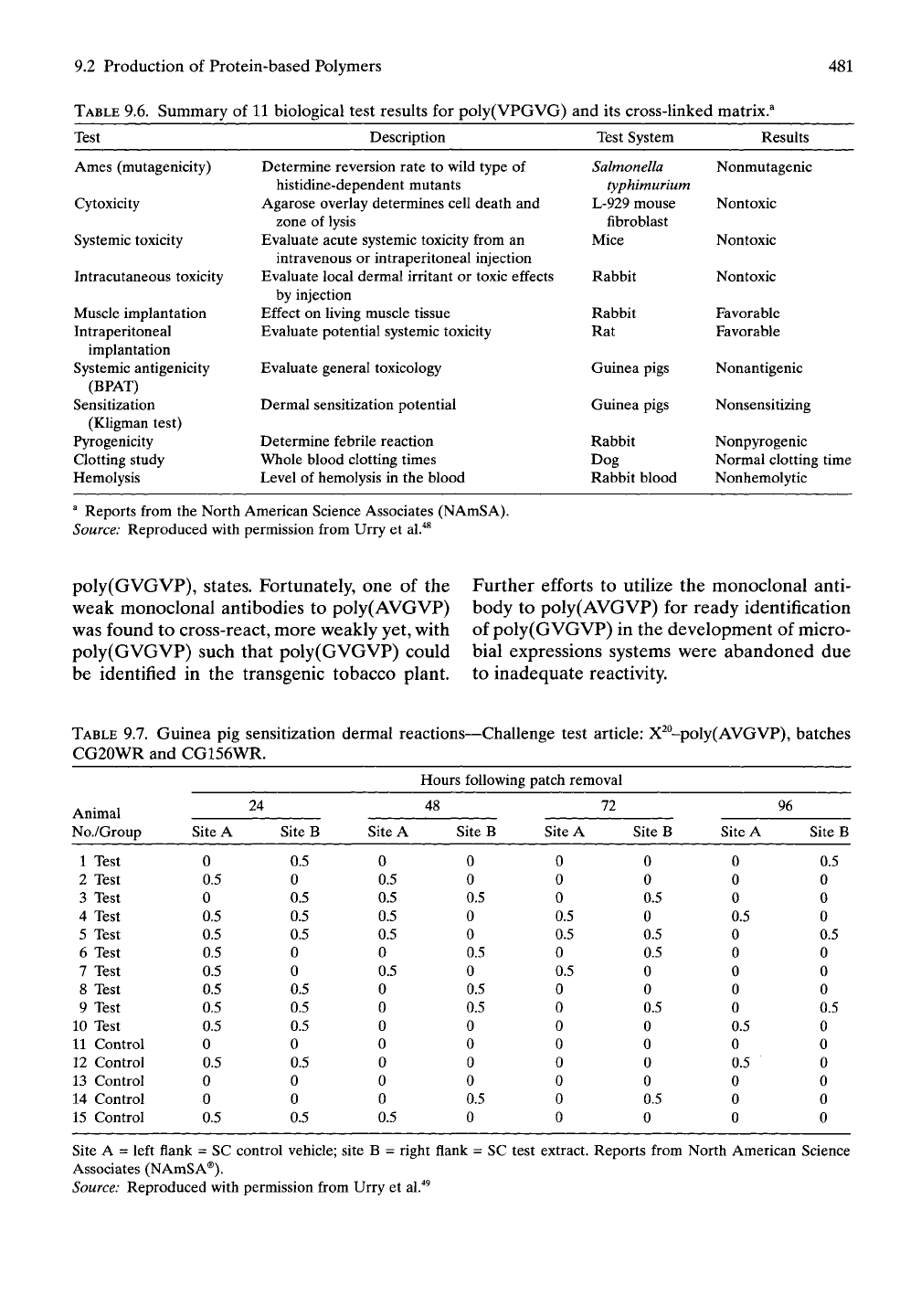
The resulting 11 tests are summarized in Table
9.6"^^
for poly(GVGVP). An even more stringent
examination for poly(GVGVP) is discussed
below, but first we consider poly(AVGVP).
The summary of the 11 tests for poly
(AVGVP) (see Table
6
in Urry et al."') reads the
same as for poly(GGAP) and poly(GVGVP).
A direct comparison of the guinea pig dermal
sensitization test for poly(AVGVP) in Table
9.7, however, shows poly(AVGVP), although
yet considered nonsensitizing, to be more
reactive than that shown in Table 9.4 for
poly(GGAP) and that for poly(GVGVP) (see
Table 6 in Urry et
al."^^).
TTiis in part demon-
strates the truly remarkable biocompatibility of
poly(GVGVP) and poly(GGAP).
9.2.2.5.2 Refractory to Formation of
Monoclonal Antibodies
Because of the need for monoclonal antibodies
to poly(GVGVP) in order to follow biopro-
duction of this polymer in the tobacco plant,
truly exceptional efforts were undertaken to
achieve antibodies. Attempts to make mono-
clonal antibodies directly to poly(GVGVP)
were simply unsuccessful despite repeated
efforts using the most sensitive and repeatedly
sensitized mice and rats. It was possible to
find three weak monoclonal antibodies to
poly(AVGVP) that had exhibited good bio-
compatibility^^ but not the same extraordinary
biocompatibility as for the representatives
of the hydrogel, poly(GGAP), and elastic.
TABLE
9.5. U.S. Pharmocopeia intracutaneous toxicity observations for
poly(GGAP)
Rabbit
No.
69235
Test
Control
69226
Test
Control
24
Hours
ER
0
0
0
0
ED
0
0
0
0
48
Hours
ER
0
0
0
0
ED
0
0
0
0
72
Hours
ER ED
0 0
0 0
0 0
0 0
Reports from North American Science Associates (NamSA). Date prepared, 2-
8-93;
date injected,
2-8-93;
date terminated,
2-11-93.
Erythema (ER): 0 = none, 1
= barely perceptible,
2
= well defined,
3
= moderate, 4 = severe. Edema (ED): 0 =
none,
1
= barely perceptible, 2 = well defined, 3 = raised
1
mm,
4 = raised
>1
mm.
Rating (test and control): 0.0-0.5, acceptable; 0.6-1.0, slight; >1.0, significant.
Source: Reproduced with permission from Urry et al.^^
9.2 Production of Protein-based Polymers
481
TABLE
9.6. Summary of 11 biological test results for poly(VPGVG) and its cross-linked matrix.^
Test
Ames (mutagenicity)
Cytoxicity
Systemic toxicity
Intracutaneous toxicity
Muscle implantation
Intraperitoneal
implantation
Systemic antigenicity
(BPAT)
Sensitization
(Kligman test)
Pyrogenicity
Clotting study
Hemolysis
Description
Determine reversion rate
to
wild type
of
histidine-dependent mutants
Agarose overlay determines cell death
and
zone
of
lysis
Evaluate acute systemic toxicity from
an
intravenous
or
intraperitoneal injection
Evaluate local dermal irritant
or
toxic effects
by injection
Effect
on
living muscle tissue
Evaluate potential systemic toxicity
Evaluate general toxicology
Dermal sensitization potential
Determine febrile reaction
Whole blood clotting times
Level
of
hemolysis
in the
blood
Test System
Salmonella
typhimurium
L-929
mouse
fibroblast
Mice
Rabbit
Rabbit
Rat
Guinea pigs
Guinea pigs
Rabbit
Dog
Rabbit blood
Results
Nonmutagenic
Nontoxic
Nontoxic
Nontoxic
Favorable
Favorable
Nonantigenic
Nonsensitizing
Nonpyrogenic
Normal clotting time
Nonhemolytic
^
Reports from the North American Science Associates (NAmSA).
Source: Reproduced with permission from Urry et al."^^
poly(GVGVP),
states.
Fortunately,
one
of the
weak monoclonal antibodies
to
poly(AVGVP)
was
found
to
cross-react,
more weakly
yet,
with
poly(GVGVP) such that poly(GVGVP) could
be
identified
in the
transgenic tobacco
plant.
Further efforts
to
utilize
the
monoclonal
anti-
body
to
poly(AVGVP)
for
ready identification
of
poly(GVGVP)
in the
development
of
micro-
bial expressions systems were abandoned
due
to
inadequate
reactivity.
TABLE
9.7. Guinea pig sensitization dermal reactions—Challenge test article: X^°-poly(AVGVP), batches
CG20WR and CG156WR.
Animal
No./Group
1 Test
2 Test
3 Test
4 Test
5 Test
6 Test
7 Test
8 Test
9 Test
10 Test
11 Control
12 Control
13 Control
14 Control
15 Control
24
Site
A
0
0.5
0
0.5
0.5
0.5
0.5
0.5
0.5
0.5
0
0.5
0
0
0.5
SiteB
0.5
0
0.5
0.5
0.5
0
0
0.5
0.5
0.5
0
0.5
0
0
0.5
Site
A
0
0.5
0.5
0.5
0.5
0
0.5
0
0
0
0
0
0
0
0.5
Hours following patch removal
48
SiteB
0
0
0.5
0
0
0.5
0
0.5
0.5
0
0
0
0
0.5
0
72
Site
A
0
0
0
0.5
0.5
0
0.5
0
0
0
0
0
0
0
0
SiteB
0
0
0.5
0
0.5
0.5
0
0
0.5
0
0
0
0
0.5
0
96
Site
A
0
0
0
0.5
0
0
0
0
0
0.5
0
0.5
0
0
0
SiteB
0.5
0
0
0
0.5
0
0
0
0.5
0
0
0
0
0
0
Site A = left flank = SC control vehicle; site B = right flank = SC test extract. Reports from North American Science
Associates (NAmSA®).
Source: Reproduced with permission from Urry et al."^^

482
9. Advanced Materials for the Future
9.2.2.5.3 Response of Human Monocytes to
Elastic Protein-based Polymers
There is one further telling study that bears
on the nature of the interaction, or absence
thereof,
of poly(GVGVP) with human cells,
monocytes, whose role it is to identify and
destroy foreign materials in the body. Grace
Picciolo and coworkers at the Center for
Devices and Radiological Health of the FDA,
using instrumentation designed to evaluate the
reaction of human monocytes to biomaterials,
found poly(GVGVP) and the related poly-
mer containing cell attachment sequences
poly[40(GVGVP),(GRGDSP)] to subdue the
background activity of the monocytes.^^ This
innocuous, and possibly even passivating,
response of human monocytes to the basic
polymer poly(GVGVP) means that it becomes
possible to add different biological activities
very selectively. The capacity will be to elicit
selectively the desired biological response to
the added sequence without having to tolerate
background activities of the carrier polymer.
This becomes especially useful when the carrier
protein-based material has an elasticity that can
match that of the natural tissues. One of the
results of this combination of biocompatibility
and biological elasticity is a group of appUca-
tions referred to below as soft tissue restoration.
First, however, the importance of purification
from E. coli fermentation is emphasized, as this
is necessary before the remarkable biocompat-
ibility of elastic protein-based polymers can be
utilized.
9.3 Purification of Tj-type
Protein-based Polymers
By Tftype, we mean polymers that exhibit
inverse temperature transitions in which the
protein-based polymers hydrophobically asso-
ciate on raising the temperature. Tt represents
the onset temperature for the transition. For
the elastic-contractile model proteins of inter-
est here, the inverse temperature transition is
seen as a phase separation resulting from both
intermolecular and intramolecular hydropho-
bic association.^^ On raising the temperature
above that of the onset temperature for the
transition, Tj, Tt-type protein-based polymers
go from being dissolved in solution to being
separated from solution. There exists another
very important means of achieving the phase
separation of Tt-type protein-based polymers;
it is to lower the temperature for the onset of
the phase transition, Tt, from above to below
the operating temperature. When concerned
with the physiological operating temperature of
37° C, to lower the value of Tt from 3T to 25° C
causes the Tt-type protein-based polymer to
separate out of solution. As treated extensively
in Chapter 5, the process can be one of
self-
assembly by hydrophobic association.
As demonstrated below, this property, funda-
mental to the diverse protein functions dis-
cussed in Chapters 5,7, and 8, provides the basis
for purification.^^
9.3.1 Purification by Phase Separation
(Inverse Temperature Transition)
The purpose below is not to describe method-
ologies for evaluating purity, but rather it is
to use selected methodologies to demonstrate or
to monitor the effectiveness of purification
based on phase separation.The utilized method-
ologies are SDS PAGE (sodium dodecyl
sulfate polyacrylamide gel electrophoresis) and
radiolabeling of contaminants. The lysed or
ruptured cells of E. coli, transformed to produce
the specific Tftype protein-based polymer,
provide the impure reference state against
which subsequent purification steps may be
compared.
93.1.1 SDS PAGE Demonstration of
Purification by Phase Separation
Figure 9.6 represents a negative staining tech-
nique involving a CUCI2 stained gel to demon-
strate purification at different steps and for
different fractions during the purification by
phase separation of (GVGVP)i4i. Lane 1
contains all of the components of the gross
lysed cells of E. coli transformed to produce
(GVGVP)i4i; as such it shows all of the protein
bands of the transformed E. coli. As will be dis-
cussed, the bulging band of lane 1 is the product
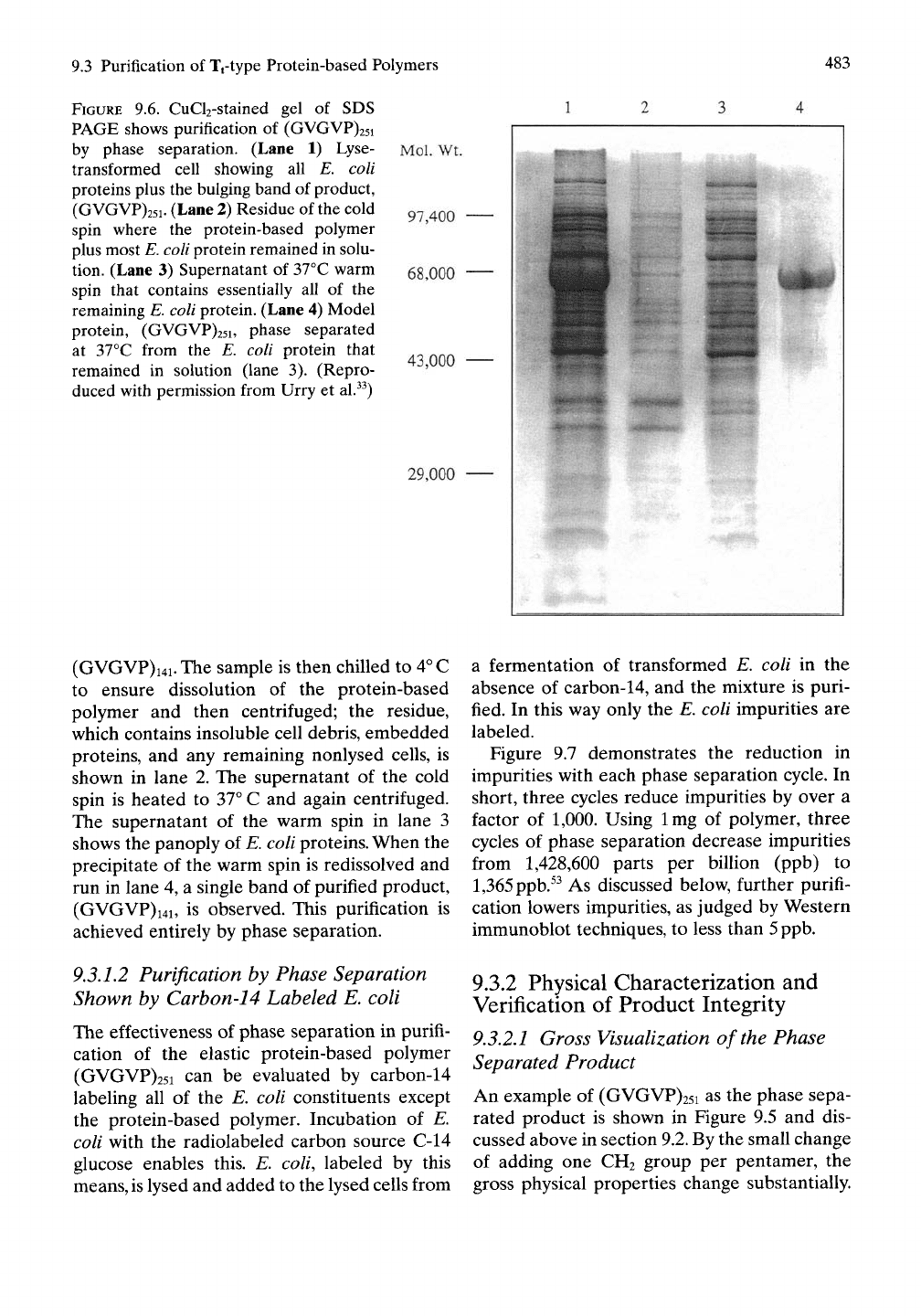
9.3 Purification
of
Trtype Protein-based Polymers
483
FIGURE
9.6. CuCl2-stained gel of SDS
PAGE shows purification
of
(GVGVP)25i
by phase separation. (Lane
1)
Lyse-
transformed cell showing
all E.
coli
proteins plus the bulging band
of
product,
(GVGVP)25i. (Lane 2) Residue
of
the cold
spin where
the
protein-based polymer
plus most E.
coli
protein remained in solu-
tion. (Lane
3)
Supernatant
of
37°C warm
spin that contains essentially
all of the
remaining E.
coli
protein. (Lane 4) Model
protein, (GVGVP)25i, phase separated
at 37°C from
the E.
coli protein that
remained
in
solution (lane
3).
(Repro-
duced with permission from Urry
et
al.^^)
Mol. Wt.
97,400
68,000
43,000
29,000
(GVGVP)i4i. The sample
is
then chilled
to
4^
C
to ensure dissolution
of the
protein-based
polymer
and
then centrifuged;
the
residue,
which contains insoluble cell debris, embedded
proteins,
and any
remaining nonlysed cells,
is
shown
in
lane
2. The
supernatant
of the
cold
spin
is
heated
to
37°
C and
again centrifuged.
The supernatant
of the
warm spin
in
lane
3
shows
the
panoply
of
E, coli proteins. When
the
precipitate
of the
warm spin
is
redissolved
and
run
in
lane
4, a
single band
of
purified product,
(GVGVP)i4i,
is
observed. This purification
is
achieved entirely
by
phase separation.
9.3.1.2 Purification
by
Phase Separation
Shown
by
Carbon-14 Labeled
E.
coli
The effectiveness
of
phase separation
in
purifi-
cation
of the
elastic protein-based polymer
(GVGVP)25i
can be
evaluated
by
carbon-14
labeling
all of the E.
coli constituents except
the protein-based polymer. Incubation
of E.
coli with
the
radiolabeled carbon source
C-14
glucose enables this.
E.
coli, labeled
by
this
means, is lysed and added
to
the lysed cells from
a fermentation
of
transformed
E.
coli
in the
absence
of
carbon-14,
and the
mixture
is
puri-
fied. In this
way
only
the E.
coli impurities
are
labeled.
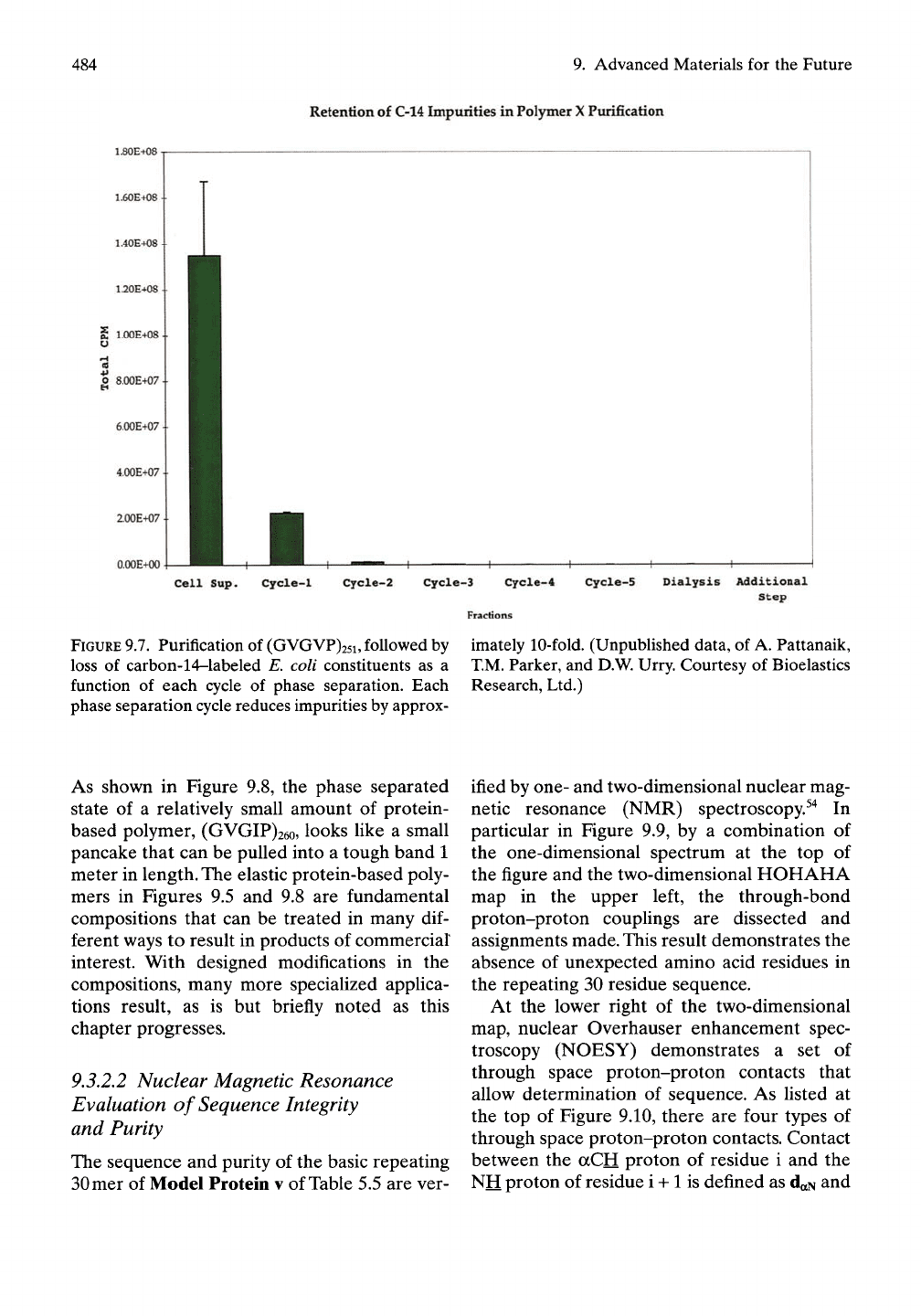
Figure
9.7
demonstrates
the
reduction
in
impurities with each phase separation cycle.
In
short, three cycles reduce impurities
by
over
a
factor
of
1,000.
Using
Img of
polymer, three
cycles
of
phase separation decrease impurities
from
1,428,600
parts
per
billion
(ppb) to
1,365
ppb.^^
As
discussed below, further purifi-
cation lowers impurities,
as
judged
by
Western
immunoblot techniques,
to
less than
5
ppb.
9.3.2 Physical Characterization
and
Verification
of
Product Integrity
9.3.2.1 Gross Visualization of the Phase
Separated Product
An example
of
(GVGVP)25i
as the
phase sepa-
rated product
is
shown
in
Figure
9.5 and
dis-
cussed above
in
section 9.2. By the small change
of adding
one CH2
group
per
pentamer,
the
gross physical properties change substantially.
484
9. Advanced Materials for the Future
Retention of C-14 Impurities in Polymer
X
Purification
1.60E+08
1.40E+08
1.20E+08
S l.OOE+08
0 8.00E+07
6.00E+07
4.00E+07
2.00E+07
O.OOE+00
1
1
1
1
1
1
J
1
1
1
1
1
1
H—1—^^M—1—liiMiai—1 1 1 1 1
Cell Sup. C7cla-1 Cycle-2 Cycle-a Cycle-4 Cycle-S Dialysis Additional
Step
Fractions
FIGURE
9.7.
Purification of
(GVGVP)25i,
followed by imately 10-fold. (Unpublished data, of
A.
Pattanaik,
loss of carbon-14-labeled E. coli constituents as a T.M. Parker, and D.W. Urry. Courtesy of Bioelastics
function of each cycle of phase separation. Each Research, Ltd.)
phase separation cycle reduces impurities by approx-
As shown in Figure 9.8, the phase separated
state of a relatively small amount of protein-
based polymer, (GVGIP)26o, looks like a small
pancake that can be pulled into a tough band 1
meter in length. The elastic protein-based poly-
mers in Figures 9.5 and 9.8 are fundamental
compositions that can be treated in many
dif-
ferent ways to result in products of commerciar
interest. With designed modifications in the
compositions, many more specialized appUca-
tions result, as is but briefly noted as this
chapter progresses.
9.3.2.2 Nuclear Magnetic Resonance
Evaluation of Sequence Integrity
and Purity
The sequence and purity of the basic repeating
30mer of Model Protein v of Table 5.5 are ver-
ified by one- and two-dimensional nuclear mag-
netic resonance (NMR) spectroscopy.^"^ In
particular in Figure 9.9, by a combination of
the one-dimensional spectrum at the top of
the figure and the two-dimensional HOHAHA
map in the upper left, the through-bond
proton-proton couplings are dissected and
assignments made.
TTiis
result demonstrates the
absence of unexpected amino acid residues in
the repeating 30 residue sequence.
At the lower right of the two-dimensional
map,
nuclear Overhauser enhancement spec-
troscopy (NOESY) demonstrates a set of
through space proton-proton contacts that
allow determination of sequence. As Usted at
the top of Figure 9.10, there are four types of
through space proton-proton contacts. Contact
between the aCH proton of residue i and the
NH proton of residue i + 1 is defined as dox and
