Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


Advanced Materials for the Future:
Protein-based Materials with Potential
to Sustain Individual Health and
Societal Development
The industrial revolution of the Nineteenth
Century was born of the water-to-steam phase
transition. As implicit in the last three chapters
and explicit in the Epilogue, biology was born of
the inverse temperature transition. As argued in
this chapter, the advanced biomaterials renais-
sance of the Twenty-first Century will also have
been born of the inverse temperature transition.
9.1 Introduction
9.1.1 The Appeal of New Discoveries
and Their Utilization
"Nothing is more agreeable to men (those)
devoted to a scientific career than to increase
the number of discoveries, but when the result
of their observation is demonstrated by practi-
cal utility their joy is complete."^ Louis Pasteur
penned these words a century and a half ago.
So appropriate is this perspective today to
those "devoted to a scientific career" that one
might only add that further joy comes when
"practical utility" enables further discoveries by
providing the necessary funding. It is in this
vein, fueled by the firm belief in the remark-
able range of applications of protein-based
machines and materials, that we have efforts
underway to develop uses. The potential appli-
cations promise to help society attain a number
of its goals, such as improving health and
quality of life, reducing cost of health care, alle-
viating addiction, and relieving environmental
pollution.
9.1.2 The Unrivaled Opportunity to
Achieve Utility of Advanced
Biomaterials
In Chapter 5, based on an inverse temperature
transition due to hydrophobic association in
water, a set of Axioms were derived from the
phenomenological demonstration that de novo
designed model proteins could efficiently inter-
convert the set of energies interconverted by
living organisms. Then there followed a series
of experimental results and analyses that
defined the comprehensive hydrophobic effect.
The operative component of the comprehen-
sive hydrophobic effect arises from the compe-
tition between charged and oil-like groups. TTiis
was shown to result in a previously unknown
repulsive force embodied within an interaction
energy called an apolar-polar repulsive free
energy of hydration, AGap. During function,
AGap works in conjunction with elastic force
development by the restriction of internal
chain dynamics. These have been called the
hydrophobic and elastic consilient mechanisms.
In Chapters 6,7, and 8, these consilient mecha-
nisms were demonstrated to be fundamental
to understanding the functions of biology's
proteins.
If our advances built over the last three
decades and culminating in the new analyses
and data in Chapter 5 are sound, then we are at
a historic moment in biomaterials development.
This moment opens the door to an unprece-
dented future for biomaterials. The opportunity
is founded on an understanding of the forces
455

456 9. Advanced Materials for the Future
responsible for protein function, demonstrated
in part in the new structure-function analyses in
Chapter 8 and culminating in the Epilogue as
constituting the "vital force" of biology. The
practical utilization of the hydrophobic and con-
silient mechanisms couples with the capacity for
biological production of designed protein-based
polymers of almost unlimited diversity of com-
position and size.
Finally, for medical applications, the extraor-
dinary biocompatibility of these elastic protein-
based materials, we believe, arises from the
specific means whereby these elastic protein-
based polymers exhibit their motion. Being
composed of repeating peptide sequences that
order into regular, nonrandom, dynamic struc-
tures,
these elastic protein-based polymers
exhibit mechanical resonances that present bar-
riers to the approach of antibodies as required
to be identified as foreign. In addition, we also
believe that these mechanical resonances result
in extraordinary absorption properties in the
acoustic frequency range.
9.1.3 The Relationship Between Basic
Science and Its AppUcations
9,13,1 Our Perspective for
Protein-based Polymers
Chapter 5 presents in one place, more exten-
sively and in a more advanced state than pre-
viously, the decades long development of the
comprehensive hydrophobic effect, the under-
pinnings of the hydrophobic consilient mecha-
nism, whereby the control of hydrophobic
association commands diverse energy conver-
sion functions of protein-based polymers.
Chapters 7 and 8 demonstrate the comprehen-
sive hydrophobic effect and its interlinked
elastic consiUent mechanism to be vital aspects
of protein function and dysfunction in biology.
In the present chapter, we utilize this developed
capacity to engineer protein-based polymers to
demonstrate a few of an extraordinary range of
appUcations.
Design of function comes through control of
the Gibbs free energy of hydrophobic associa-
tion,
AGHA-With
regard to medical appUcations,
protein, biology's own workhorse polymer of
choice, provides the most congruent means
with which to restore function and defeat
disease. As noted in section 5.2.5, the nature of
their elasticity and resulting biocompatibility
and their never-ending design potential makes
the family of elastic protein-based polymers,
developed here, the pre-eminent choice of
materials for medical applications.
With regard to nonmedical applications, the
entirely unique opportunity to specify compo-
sition and diverse sequence, so precisely, pro-
vides a profusion of polymers with properties
that bring to the nonmedical world all of the
specificity and sensitivity of which biology's
protein is renown. To the best of our under-
standing, the properties include a remarkable
and unique acoustic absorption capacity. Also
unconstrained by the parameters of living
organisms, but armed with the engineering
capacity provided by knowledge of the hydro-
phobic and elastic consilient mechanisms,
designed protein-based polymers give rise to
materials unknown to living organisms. We now
look toward enumerable challenges where
defined needs stimulate designs of protein-
based polymers to fulfill those needs.
9,1,3,2 Pasteur on the Relationship
Between the Basic and Applied Sciences
Before proceeding to the range and significance
of problems addressable with the diverse prop-
erties of protein-based materials, the relation-
ship between basic research and its derivative
applications warrants further consideration.
Again we turn to Pasteur: 'Wo, a thousand times
no;
there does not exist a category of science to
which one can give the name applied science.
There are science and the applications of science,
bound together as the fruit to the tree which
bears
it,''^
Pasteur knew the relationship well.
He made major advances in the basic sciences,
for example, in crystal structure and optical
activity and in advancing the germ theory that
put to rest the unproductive concept of sponta-
neous generation of living organisms. He also
made advances resulting in more familiar appli-
cations. He developed the process now known
as pasteurization that saved the wine, vinegar,
and beer industries of France but that we daily
identify with pasteurized milk products; he
solved a threat to the silk industry by alleviat-

9.1 Introduction
457
ing diseases of the silkworm, and he developed
an understanding and successful treatment of
rabies. All of this came about by singular
advances that were often in severe conflict with
the prevailing thought of his day.
9.1.3.3 Cyclical Enabling: The
Relationship Between Science and the
Applications of Science
Development of applications proceeds effec-
tively when a scientific foundation is employed.
On the other hand, development of the scien-
tific foundation becomes empowered by
society's need to solve current problems. This is
what is intended by the term cyclical enabling.
Building upon Pasteur's words, it might further
be said that. To separate the development of
science from the development of applications
would be to separate the growing fruit from its
tree; the immature fruit wither and waste and
yield no new trees, and the barren tree too soon
dies
The development of thermodynamics is one
example of this interdependence. Thermody-
namics, a paradigm of basic science, grew from
society's appetite for better steam engines with
which to relieve humans and horses of mechan-
ical work. Of particular relevance here, the
discipUne of thermodynamics provides the
scientific foundation for energy conversion by
protein machines. Chapter 5 demonstrates this
with consideration of the thermodynamics of
hydrophobic association in model proteins. A
thermodynamic understanding of energy con-
version provides the scientific foundation for
the most effective development of the applica-
tions considered below. The Epilogue inte-
grates the keystone of thermodynamics, the
second law, into the unique adherence by bio-
logical systems.
9.1.3.4 Specific Enablers of the
Development of Elastic
Protein-based Polymers
In general, development of the scientific foun-
dation for protein-based polymers began in an
academic setting and continued very effectively
in a company setting. The primary funding
impetus (the enabler) for the development of
elastic protein-based polymers has been grants
and contracts from the Office of Naval
Research, covering nearly two decades of
support in academic institutions and in a
company setting, also with support from the
Naval Medical Research and Development
Command in the company setting. Develop-
ment of a scientific foundation for elastic
protein-based polymers occurred in order to
provide the most effective approach to devel-
opment of materials applications. The second
most prominent level of support has come from
the National Institutes of Health, primarily in
terms of Small Business Innovation Research
contracts (SBIRs), The SBIRs decidedly focus
on appHcations and draw from the foundations
of the science of the elastic-contractile model
proteins presented in Chapter 5.
The primary advances toward appHcations
occurred in a company setting. In fact, even
much of the foundation of the relevant under-
lying science occurred in the company set-
ting. For example, fundamental knowledge of
hydrophobic association in proteins, of protein
elasticity, of mechanical (e.g., acoustical) reso-
nances, and so forth resulted from progress
made in the company setting. These contribu-
tions to the foundation of the science obviously
relate to the solution of practical problems—
developing rules for correctly folding micro-
bially prepared protein of higher organisms,
designing a new class of sound-absorbing
materials, and developing nanomachines and
nanosensors. Consistent with the Pasteur per-
spective, progress toward applications for
elastic and plastic protein-based polymers
resulted from the cyclical enabling noted
above.
9.1.4 The Incomparable Potential of
Protein-based Polymers
9.1.4.1 General Advantages of
Protein-based Polymers
Many extraordinary advantages of protein-
based polymers exist. Some of the advantages
are briefly listed.
1.
Two modes of synthesis: chemical and
biological: Chemical synthesis allows for rela-
tively rapid screening of physical properties of
hundreds of protein-based polymer composi-

458
9. Advanced Materials for the Future
tions as long as they are not too complex. Even
so,
chemical synthesis has disadvantages of
racemization, of side reactions on protection/
deprotection of functional side chains, of
random incorporation of guest pentamer, of a
distribution of chain lengths, and so forth, all of
which limit quantification of engineering prin-
ciples.
On identification of chemically synthe-
sized model protein compositions of particular
interest, the slower process of recombinant
DNA technology for gene construction and
development of an effective expression system
ultimately provides for rapid, large-scale pro-
duction and other advantages discussed below.
Of importance in the most accurate develop-
ment of protein-based polymers as biomateri-
als,
however, will be to obtain the most accurate
set of controlling interaction energies to arrive
at the desired level of reUance on derived engi-
neering principles.
2.
Diversity of monomers: The biosynthesis
of natural proteins utilizes 20 different
monomeric units, and chemical and enzy-
matic post-translational modifications provide
further diversity of sequence. As discussed in
Chapter 4 and noted below, the availabiUty of
20 different monomers results in an inordinate
number of different protein sequences, even for
a small 100 residue protein, that is,
10^^^.
3.
Precise control of the sequence of amino
acid residues'. Any protein-based polymer
sequence utilizing the 20 naturally occurring
monomers can be specified using recombinant
DNA technology. This allows for equivalent
ease of production of diverse protein-based
polymer sequences, many of which would
otherwise be quite difficult or essentially im-
possible to prepare due to problems of chemi-
cal synthesis and unfavorable energetics in the
final polymer.
4.
Exact control of stereochemistry. Control
of stereochemistry, for example, use of a single
optical isomer as occurs in biology, is essential
for control of physical properties resulting in
unique structures and more precise function.
The occurrence of some racemization is
unavoidable during chemical synthesis of long
protein-based polymers. Some inclusion of D-
amino acid residues, rather than having all L-
amino acid residues (see Figure 3.3C) as occurs
in biology, is unavoidable in chemical synthesis.
Even an amount of racemization as small as
1
%
can critically limit desirable properties in these
nonrandom elastic polymers. For example, lack
of rigorous exclusion of racemization during
chemical synthesis of poly(GVGVP) can cause
the critical onset temperature for the inverse
temperature transition for hydrophobic associ-
ation (Tt) to be raised by 15°C from 25° to 40°C.
As explained in Chapter 5, control of Tj is
central to controlling function.
5.
Precise chain lengths: The gene encoding
for a protein-based polymer occurs, generally,
with a single chain length, and the complete
range of chain lengths, arising from multiples of
a basic repeating gene sequence, are possible in
Escherichia coli, resulting in as many as 4,000
amino acid residues and even more than 1
milHon residues in animals. This allows for fine-
tuning of desirable properties and access to
new capabilities. Several processes occur that
can defeat this possibility of all polymer chains
being of the same chain length. Deletion of
gene sequences can occur under certain cir-
cumstances. The growing protein chain can fall
off the ribosome before completion of transla-
tion, and the expressed protein can be sub-
jected to proteolytic degradation. All of these
complications, however, are generally avoid-
able,
and precise chain lengths are routinely
obtained.
6. Capacity to introduce natural bioactive
peptide sequences (protein mimicry): With an
adequate understanding of protein engineer-
ing, there exists an essentially unlimited capac-
ity to mimic chosen elements of more complex
proteins in terms of both structure and func-
tion. Specific biologically active sequences can
be introduced with ease. Examples are sites at
which selective enzymes can catalyze a desired
reaction, sequences for selective cell attach-
ment, selective attachment to diseased cells for
drug delivery, and so forth.
7.
Circumstances of protein function to
guide approach and analyses: It is possible to
consider functional proteins, for example, with
their natural prosthetic groups and cofactors, to
suggest important variables that relate to func-
tion, and these become tools with which to
achieve protein engineering (see Tables 5.1 and

9.1 Introduction 459
5.2). For example, prosthetic groups and cofac-
tors can be attached to model proteins, and the
role of each as regards function can be quanti-
fied and utilized.
8. Properties and uses beyond those of
known proteins: Once the rules for protein
engineering have been established, protein-
based polymers can be designed with proper-
ties and functions that go beyond what
evolution has called upon proteins to do. For
example, there are model proteins for con-
trolled release of new pharmaceuticals, and
programmable, biodegradable thermoplastic
protein-based polymers that melt for easy
molding or extruding at 150°C, with decompo-
sition not occurring until 250°C.
9. Low cost of bioproduction: As the
designed protein-based polymer becomes more
complex, the cost advantages of bioproduction
become greater. The production of protein-
based polymers by means of recombinant DNA
technology has the potential for at least a
10,000-fold decrease in cost from that of chem-
ical synthesis. It is believed that the cost of
protein-based polymers has the potential to be
competitive with the cost of petroleum-based
polymers, thus relieving, in part, society's
dependence on limited oil reserves. Further-
more, it costs a living organism no more energy
to produce a more efficient protein-based
machine than an inefficient one of the same
size.
10.
Produced from renewable resources:
Living organisms—E. coli, yeast, plants, and
animals—can be designed to produce protein-
based polymers. Protein-based polymers can be
produced with renewable resources. They can
be prepared without resorting to toxic and
noxious chemicals, and they can be pro-
grammed for a desired biodegradation. For
example, they can mean food for the fishes
rather than death to marine life, as occurs with
present plastics. Thus, protein-based polymers
can be environmentally friendly for their com-
plete life cycle, from production to disposal.
11.
Axioms for protein-based polymer engi-
neering: The phenomenology of protein-based
polymer function, categorized in terms of free
energy transduction, is given as a set of five
Axioms in Chapter 5 (see section 5.6.3). These
five Axioms provide the basis for diverse, but
qualitative, designs of polymers capable of
exhibiting inverse temperature transitions.
12.
Quantitative design principles available
for protein-based polymers: The comprehensive
hydrophobic effect, developed in Chapter 5,
provides principles for the quantitative design
of protein-based polymers. The dominant
underlying energetics are embodied in the
Gibbs free energy for hydrophobic association,
AGHA,
as modified by the newly described
Gibbs free energy for an apolar-polar repulsive
free energy of hydration, AGap.
13.
Availability of the most efficient mecha-
nism for achieving function in an aqueous
environment: Comparison of the electrosta-
tic charge-charge repulsion mechanism for
chemo-mechanical transduction with that of
the apolar-polar repulsive free energy of
hydration, AGap, shows the latter to be more
than an order of magnitude more efficient. This
becomes particularly relevant to biomedical
applications of controlled release as required in
drug delivery, but also whenever a sensitive and
responsive (smart) biomaterial is desired.
14.
Remarkable biocompatibility of elastic
protein-based polymers: When considering
medical apphcations of these biomaterials,
utihty depends on biocompatibility. After all,
foreign proteins are generally antigenic and
elicit production of antibodies. This is the basis
for many vaccines. How then can one propose
protein as a biomaterial? The most direct
answer is that many biocompatibility studies
have been carried out on a number of compo-
sitions of elastic protein-based polymers, and
they have been found to exhibit extraordinary
biocompatibility. In fact, very pure preparations
of (GVGVP)n, or equivalently (VPGVG)n,
appear to be entirely ignored by the host. This
we believe is due to the nature of the elasticity.
In our view, ideal or entropic elasticity exhib-
ited by poly(VPGVG) results from the fact that
the repeating conformational unit, (VPGVG),
exhibits mechanical resonances. These are
motions that occur with frequencies localized
near
5
MHz and
3
kHz. Such low-frequency
motions greatly stabilize the structure. Further-
more, the requirement to stop these motions, as
needed to identify an epitope for the develop-

460 9. Advanced Materials for the Future
ment of antibodies to the sequence, presents a
barrier to the approach and identification of the
elastic protein-based polymer as foreign.
15.
In vivo breakdown products: Other poly-
meric biomaterials may also be composed
of natural products, such as polyesters like
poly(glycolic acid) and poly (lactic acid). When
these polymeric biomaterials degrade to the
naturally occurring monomers of glycolic acid
and lactic acid, however, the monomeric car-
boxylic acid ionizes to release acid, H^. The
decreased pH can be a significant irritant to the
tissues. On the other hand, when protein-based
polymers undergo biodegradation, naturally
occurring zwitterionic amino acids are released
without such an effect on the pH and its
ramifications.
When seeking an optimal biomaterial, the
preference is for complete absence of toxicity
on placement in the host. With such a totally
innocuous elastic protein-based biomaterial,
biologically active sequences can be readily
included within the polymer, and the host tissue
can react to the biologically active sequence
without being overwhelmed by unwanted reac-
tions.
Thus, because of the high level of bio-
compatibility, there exists the capacity to elicit
diverse and desired tissue responses.
Hopefully, the foregoing brief Usts provides
some insight into the potential of protein-based
polymers in the marketplace. There simply are
no comparable soft materials for medical appli-
cations. Certain of the above-listed advantages
are discussed in more detail immediately below
and throughout this chapter.
9.1.4.2 A Near-infinite Number of
Different Polymer Compositions
There are 20 different naturally occurring
amino acid residues, and each position in a
biosynthesized protein-based polymer can
contain any one of the 20 amino acids. As con-
sidered in Chapter 4, this means even for a
small 100 residue protein-based polymer that
there are
(20)^^^
=
10^^^
different sequences pos-
sible.
The size of this number is hard to com-
prehend. For example, if the mass of the known
universe were composed of nothing but 100
residue protein-based polymers and if there
were only one molecule of each possible
sequence, the likelihood of the occurrence of a
specific sequence would yet remain extremely
remote.
Capitalizing on the potential of protein-
based polymers with such a near-infinite
number of sequences requires an understand-
ing of the basis for function. The hydrophobic
and elastic consilient mechanisms for design of
protein-based polymer structure and function,
the knowledge for which is developed primar-
ily in Chapter 5, enable utilization of the
extraordinary potential of these incomparable
polymers. Humans utilize fewer than 50,000
dif-
ferent proteins, and, as learned from sequence
data of the human genome and those of lower
and less complex animals, plants, and organ-
isms,
a surprisingly hmited number of the fewer
than 50,000 protein sequences are unique to
humans. Accordingly, the potential for materi-
als that go beyond those known in biology rings
clear.
Obviously, the appUcations of protein-based
polymers are innumerable. The appUcations
discussed in this chapter represent but a few of
very very many, and they are at different stages
of development.
9.1.4.3 Available Fundamental Design
Principles Yield Effective Products
The five phenomenological Axioms, knowledge
of the underlying physical basis and thermody-
namic formaUsm in terms of the Gibbs free
energy of hydrophobic association,
AGHA,
provide a foundation for fundamental design
principles. The efficiency rating, ecM» as pre-
sented in section 5.9.3, recognizes
AGHA
as a
maximal energy output for a given design based
on the consiUent mechanism, that is, the com-
prehensive hydrophobic effect. Given a defined
energy source, which could be a drug, for
example, the issue becomes one of designing
the preferred protein-based polymer that
would most accurately target the desired
outcome. In particular, the drug can be the
energy input for assembly of the drug delivery
vehicle that released drug in the desired
manner as the energy output. In every case with
the hydrophobic and elastic consilient mecha-
nisms, the interlinked physical processes that
achieve the energy conversion are a change in

9.1 Introduction 461
hydrophobic association in conjunction with a
near-ideal polymer elasticity.
9.1.4.4 De novo Design of a Material for
the Intended Application
From the outset, our approach has been to
design materials for an intended function.
In particular, each new energy conversion
described in Chapter 5 was achieved by design
of a new functionality utilizing the perspective
of a common underlying hydrophobic con-
sihent mechanism. Jacob Bronowski^ antici-
pated such an approach three decades ago in
his book Ascent of Man, with the perspective
that, "In effect, the modern problem is no longer
to design a structure from the materials (avail-
able) but to design materials for a structure.''
Knowledge of the Axioms and the principles
underlying the comprehensive hydrophobic
effect, based at the molecular level on the
change in free energy of hydrophobic associa-
tion, makes it possible to "design materials for
a structure." Of course, by structure we gener-
ally mean a unique protein sequence, also
referred to as primary structure or in a more
general way as composition. By materials for a
structure, Bronowski meant a composition
designed for a specific function. This is to be dis-
tinguished from taking a given composition and
coercing a function for which it is not sequence
optimized.
An example of
"^<9
design a structure from the
materials'' would be to achieve the function of
a chemo-mechanical engine using the initial
available model protein composition poly
(GVGVP), cross-linking it by y-irradiation, and
employing the chemical energy of adding
salt(NaCl) to drive contraction.^ In this case,
AGHA[poly(GVGVP)(0.1 N ^ 1.0 N NaCl)] is
-0.5kcal/mole-pentamer/mole-NaCl.
On the other hand, to "design materials for a
structure," in this case a better salt (NaCl)-
driven chemo-mechanical engine, would be to
design a protein-based polymer with a few
glutamic acid residues per 100 residues, for
example, poly[4(GVGVP),(GEGVP)]. As
listed in Table 5.4, for the newly designed struc-
ture over its most responsive range to NaCl
from 0.15 N to 0.25 N NaCl, AGeAlpoly
[0.8(GVGVP),0.2(GE-GVP)]}, is -9.4kcal/
mole-pentamer/mole-NaCl; this gives an
improvement for the most effective ranges of
9.35/0.5. Thus, "to design materials for a struc-
ture'' as was done by designing the polymer
poly[4(GVGVP),(GEGVP)], to be an NaCl-
driven chemo-mechanical engine, was to design
a chemo-mechanical engine with an effective-
ness of energy conversion improved by nearly
a factor of 20 when functioning in its most
effective concentration range. Clearly, the
modern problem is "to design materials for a
structure'' and the means to do so comes from
an understanding of the hydrophobic consilient
mechanism's comprehensive hydrophobic ef-
fect, as developed in Chapter 5.
Thus,
poly(GVGVP) was a structure avail-
able from the mammalian elastic fiber. Adding
a carboxyl function resulted in a structure that
was not previously known, and the resulting
design gave rise to a structure that could do 20
times more work for the same chemical energy
input. This exemplifies the modern approach of
designing a material specifically for a chemo-
mechanical structure. Contemplation of any
application of these protein-based polymers
best proceeds with understanding of the under-
lying physical process in order to achieve the
most effective outcome. Most functions can be
analyzed in terms of the efficiency of energy
conversion. This is most readily appreciated for
the controlled release of pharmaceuticals, that
is,
for drug delivery.
9.1.5 Potential of Protein-based
Materials to Improve Health and
Decrease Health Care Costs
Health care costs in the United States exceed a
staggering trilUon dollars per year. Low back
pain, urinary incontinence, pressure ulcers (e.g.,
bed sores), and cardiovascular disease are
major contributors to decreased quality of life
and increased health care
costs.
Apphcations of
protein-based materials, briefly noted in this
section but discussed more extensively below,
have the potential to improve quality of life
while lowering health care costs for these and
additional medical problems. To provide a his-
torical backdrop and a record of the develop-
ment of applications. Table 9.1 provides the set
of patents resulting from our research efforts.
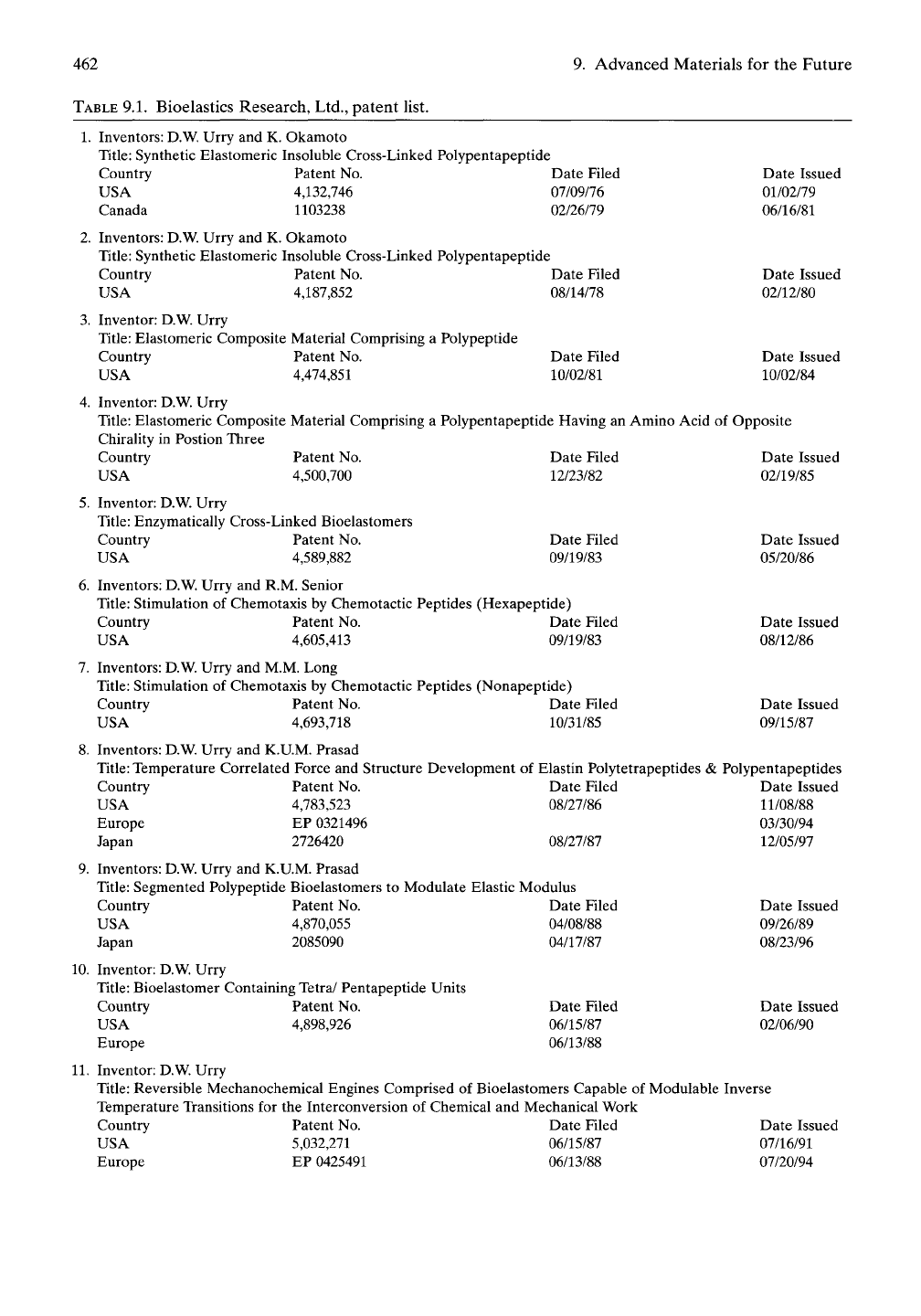
462
9.
Advanced Materials
for the
Future
TABLE
9.1. Bioelastics Research, Ltd., patent Ust.
Inventors: D.W. Urry
and K.
Okamoto
Title:
Synthetic Elastomeric Insoluble Cross-Linked Polypentapeptide
Country
USA
Canada
Patent
No.
4,132,746
1103238
Date Filed
07/09/76
02/26/79
2.
Inventors: D.W. Urry
and K.
Okamoto
Title:
Synthetic Elastomeric Insoluble Cross-Linked Polypentapeptide
Country
USA
Patent
No.
4,187,852
3.
Inventor: D.W. Urry
Title:
Elastomeric Composite Material Comprising
a
Polypeptide
Country Patent
No.
USA 4,474,851
Date Filed
08/14/78
Date Filed
10/02/81
Date Issued
01/02/79
06/16/81
Date Issued
02/12/80
Date Issued
10/02/84
4.
Inventor: D.W. Urry
Title:
Elastomeric Composite Material Comprising
a
Polypentapeptide Having
an
Amino Acid
of
Opposite
Chirality
in
Postion Three
Country Patent No. Date Filed Date Issued
USA 4,500,700 12/23/82 02/19/85
Inventor: D.W. Urry
Title:
Enzymatically Cross-Linked Bioelastomers
Country Patent
No.
USA 4,589,882
Date Filed
09/19/83
6. Inventors: D.W. Urry
and
R.M. Senior
Title:
Stimulation
of
Chemotaxis
by
Chemotactic Peptides (Hexapeptide)
Country Patent No. Date Filed
USA 4,605,413 09/19/83
7.
Inventors: D.W. Urry
and
M.M. Long
Title:
Stimulation
of
Chemotaxis
by
Chemotactic Peptides (Nonapeptide)
Country Patent No. Date Filed
USA 4,693,718 10/31/85
Date Issued
05/20/86
Date Issued
08/12/86
Date Issued
09/15/87
8. Inventors: D.W. Urry
and
K.U.M. Prasad
Title:
Temperature Correlated Force
and
Structure Development
of
Elastin Polytetrapeptides
&
Polypentapeptides
Country Patent No. Date Filed Date Issued
USA 4,783,523 08/27/86 11/08/88
Europe
EP
0321496 03/30/94
Japan 2726420 08/27/87 12/05/97
9. Inventors: D.W. Urry
and
K.U.M. Prasad
Title:
Segmented Polypeptide Bioelastomers
to
Modulate Elastic Modulus
Country Patent No. Date Filed Date Issued
USA 4,870,055 04/08/88 09/26/89
Japan 2085090 04/17/87 08/23/96
10.
Inventor: D.W. Urry
Title:
Bioelastomer Containing Tetra/ Pentapeptide Units
Country Patent No. Date Filed Date Issued
USA 4,898,926 06/15/87 02/06/90
Europe 06/13/88
11.
Inventor: D.W. Urry
Title:
Reversible Mechanochemical Engines Comprised
of
Bioelastomers Capable
of
Modulable Inverse
Temperature Transitions
for the
Interconversion
of
Chemical
and
Mechanical Work
Country Patent No. Date Filed Date Issued
USA 5,032,271 06/15/87 07/16/91
Europe
EP
0425491 06/13/88 07/20/94
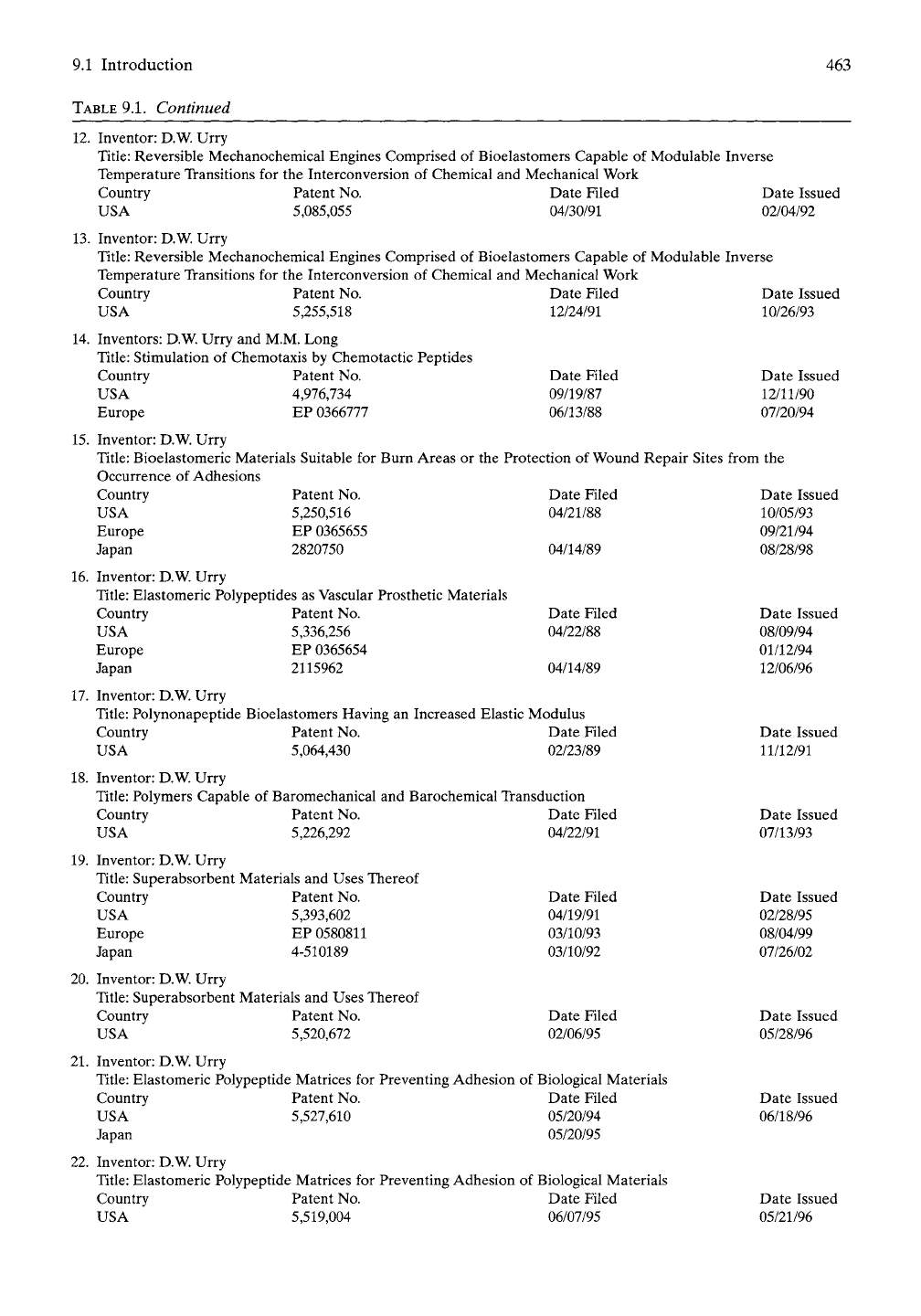
9.1 Introduction
463
TABLE
9.1.
Continued
12.
Inventor: D.W. Urry
Title:
Reversible Mechanochemical Engines Comprised of Bioelastomers Capable of Modulable Inverse
Temperature Transitions for the Interconversion of Chemical and Mechanical Work
Country Patent No. Date Filed Date Issued
USA 5,085,055 04/30/91 02/04/92
13.
Inventor: D.W. Urry
Title:
Reversible Mechanochemical Engines Comprised of Bioelastomers Capable of Modulable Inverse
Temperature Transitions for the Interconversion of Chemical and Mechanical Work
Country Patent No. Date Filed Date Issued
USA 5,255,518 12/24/91 10/26/93
14.
Inventors: D.W. Urry and M.M. Long
Title:
Stimulation of Chemotaxis by Chemotactic Peptides
Country Patent No. Date Filed Date Issued
USA 4,976,734 09/19/87 12/11/90
Europe EP 0366777 06/13/88 07/20/94
15.
Inventor: D.W. Urry
Title:
Bioelastomeric Materials Suitable for Burn Areas or the Protection of Wound Repair Sites from the
Occurrence of Adhesions
Country Patent No. Date Filed Date Issued
USA 5,250,516 04/21/88 10/05/93
Europe EP 0365655 09/21/94
Japan 2820750 04/14/89 08/28/98
16.
Inventor: D.W. Urry
Title:
Elastomeric Polypeptides as Vascular Prosthetic Materials
Country Patent No. Date Filed
USA 5,336,256 04/22/88
Europe EP 0365654
Japan 2115962 04/14/89
17.
Inventor: D.W. Urry
Title:
Polynonapeptide Bioelastomers Having an Increased Elastic Modulus
Country Patent No. Date Filed
USA 5,064,430 02/23/89
18.
Inventor: D.W Urry
Title:
Polymers Capable of Baromechanical and Barochemical Transduction
Country Patent No. Date Filed
USA 5,226,292 04/22/91
19.
Inventor: D.W. Urry
Title:
Superabsorbent Materials and Uses Thereof
Country Patent No. Date Filed
USA 5,393,602 04/19/91
Europe EP 0580811 03/10/93
Japan 4-510189 03/10/92
20.
Inventor: D.W. Urry
Title:
Superabsorbent Materials and Uses Thereof
Country Patent No. Date Filed
USA 5,520,672 02/06/95
21.
Inventor: D.W. Urry
Title:
Elastomeric Polypeptide Matrices for Preventing Adhesion of Biological Materials
Country Patent No. Date Filed
USA 5,527,610 05/20/94
Japan 05/20/95
22.
Inventor: D.W. Urry
Title:
Elastomeric Polypeptide Matrices for Preventing Adhesion of Biological Materials
Country Patent No. Date Filed
USA 5,519,004 06/07/95
Date Issued
08/09/94
01/12/94
12/06/96
Date Issued
11/12/91
Date Issued
07/13/93
Date Issued
02/28/95
08/04/99
07/26/02
Date Issued
05/28/96
Date Issued
06/18/96
Date Issued
05/21/96
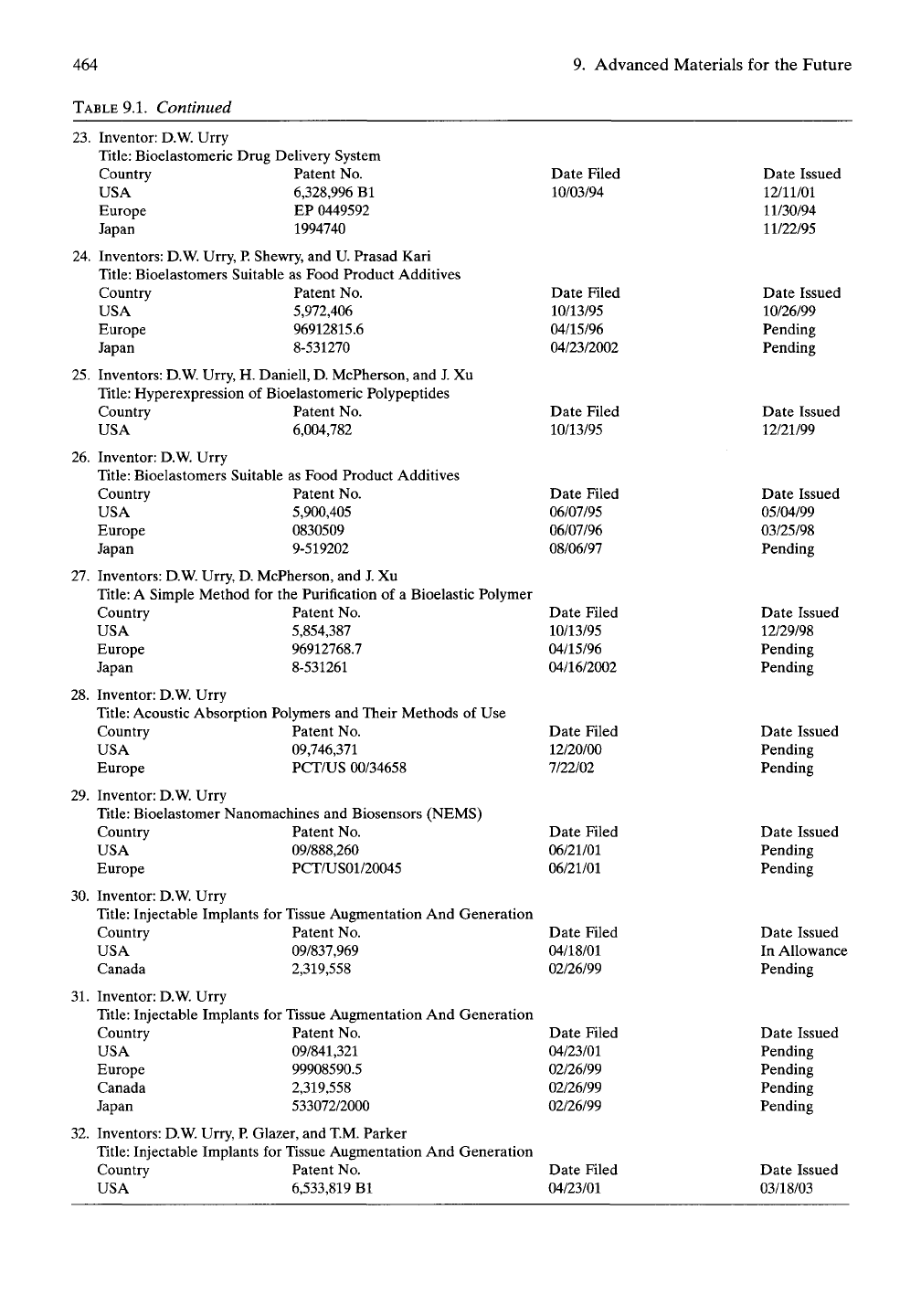
464 9. Advanced Materials for the Future
TABLE
9.1.
Continued
23.
Inventor: D.W. Urry
Title:
Bioelastomeric Drug Delivery System
Country Patent No. Date Filed
USA 6,328,996 Bl 10/03/94
Europe EP 0449592
Japan 1994740
24.
Inventors: D.W. Urry, P. Shewry, and U. Prasad Kari
Title:
Bioelastomers Suitable as Food Product Additives
Country Patent No. Date Filed
USA 5,972,406 10/13/95
Europe 96912815.6 04/15/96
Japan 8-531270 04/23/2002
25.
Inventors: D.W. Urry, H. Daniell, D. McPherson, and J. Xu
Title:
Hyperexpression of Bioelastomeric Polypeptides
Country Patent No. Date Filed
USA 6,004,782 10/13/95
26.
Inventor: D.W. Urry
Title:
Bioelastomers Suitable as Food Product Additives
Country Patent No. Date Filed
USA 5,900,405 06/07/95
Europe 0830509 06/07/96
Japan 9-519202 08/06/97
27.
Inventors: D.W. Urry, D. McPherson, and J. Xu
Title:
A Simple Method for the Purification of a Bioelastic Polymer
Country Patent No. Date Filed
USA 5,854,387 10/13/95
Europe 96912768.7 04/15/96
Japan 8-531261 04/16/2002
28.
Inventor: D.W. Urry
Title:
Acoustic Absorption Polymers and Their Methods of Use
Country Patent No. Date Filed
USA 09,746,371 12/20/00
Europe PCT/US 00/34658 7/22/02
29.
Inventor: D.W. Urry
Title:
Bioelastomer Nanomachines and Biosensors (NEMS)
Country Patent No. Date Filed
USA 09/888,260 06/21/01
Europe PCT/USOl/20045 06/21/01
30.
Inventor: D.W. Urry
Title:
Injectable Implants for Tissue Augmentation And Generation
Country Patent No. Date Filed
USA 09/837,969 04/18/01
Canada 2,319,558 02/26/99
31.
Inventor: D.W. Urry
Title:
Injectable Implants for Tissue Augmentation And Generation
Country Patent No. Date Filed
USA 09/841,321 04/23/01
Europe 99908590.5 02/26/99
Canada 2,319,558 02/26/99
Japan 533072/2000 02/26/99
32.
Inventors: D.W. Urry, P Glazer, and T.M. Parker
Title:
Injectable Implants for Tissue Augmentation And Generation
Patent No. Date Filed
6,533,819 Bl 04/23/01
Country
USA
Date Issued
12/11/01
11/30/94
11/22/95
Date Issued
10/26/99
Pending
Pending
Date Issued
12/21/99
Date Issued
05/04/99
03/25/98
Pending
Date Issued
12/29/98
Pending
Pending
Date Issued
Pending
Pending
Date Issued
Pending
Pending
Date Issued
In Allowance
Pending
Date Issued
Pending
Pending
Pending
Pending
Date Issued
03/18/03
