Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
425
8.5.2.1 Rigor States Substantiate
Hydrophobic Association in Muscle at
the Gross Anatomical Level
As considered in Chapter 7, Borland's Medical
Dictionary has four entries for "rigor."^^ Alpha-
betically listed, they are (1) acid rigor, "coagu-
lation of protein of muscle produced by acids,"
explained by the protonation of carboxylates to
form the uncharged carboxyl, that is, -COO" 4-
H^ = -COOH with the result of contraction
by hydrophobic association; (2) calcium rigor,
"systolic cardiac arrest caused by an excess of
calcium," whereby calcium ion pairing with
paired carboxylates in the presence of hydro-
phobic residues also drives the contraction by
hydrophobic association; (3) heat rigor "rigidity
of muscles induced by heat," explained by the
fundamental property of the consiUent mecha-
nism whereby raising the temperature drives
contraction by hydrophobic association; and (4)
rigor mortis, "the stiffening of a dead body,
accompanying the depletion of adenosine
triphosphate in the muscle fibers," whereby the
suppleness of hydrophobic dissociation in the
presence of the very polar ATP molecule dis-
appears on breakdown of ATP following death
that results in the stiffness of hydrophobic
association.
8.5.2.2 Coherence of Phenomena Relating
to Hydrophobic Association in the
Myofibril and in Elastic-contractile
Model Proteins
8.5.2.2.1
Thermal Activation of
Muscle Contraction
Raising the temperature to drive contraction
by hydrophobic association is the fundamental
property of the consilient mechanism as demon-
strated in Chapter 5 by means of designed
elastic-contractile model proteins. Thermal acti-
vation of muscle contraction also correlates
with contraction by hydrophobic association,
but assisted in this case by the thermal instabil-
ity of phosphoanhydride bonds associated with
ATP,
which on breakdown most dramatically
drive hydrophobic association. In particular,
both muscle and cross-linked elastic protein-
based polymer, (GVGVP)n contract on raising
the temperature over the same temperature
range. Furthermore, there is the corollary of the
release of heat on stretching both muscle
and elastic-contractile model proteins. Both are
exothermic on stretching due to exposure of
hydrophobic groups to water, as may be argued
from the original studies of Butler^^ in 1937.
8.5.2.2.2
Calcium Ion, pH, and Stretch
Activation of Muscle Contraction
At the level of the myofibril, the addition of
calcium ion, the lowering of pH, and stretching
have each been shown to activate muscle con-
traction as well as to drive contraction of suit-
ably designed elastic contractile protein-based
polymers by hydrophobic association (see more
extensive discussion in Chapter 7).
8.5.2.2.3
Dephosphorylation Drives
Contraction, Whereas ATP Binding
Drives Relaxation
Because the energy for contraction comes from
ATP,
original expectations were that ATP and
hydrolysis binding would cause contraction.
TTiis turned out not to be the case. Phosphory-
lation in its many forms causes relaxation by
raising the temperature for the onset of an
inverse temperature transition above physio-
logical temperature, and dephosphorylation
drives contraction exactly in parallel with the
designed elastic-contractile model proteins. In
the latter case, phosphorylation has been shown
to cause relaxation by disrupting hydrophobic
association, and dephosphorylation drives
contraction by allowing re-estabhshment of
hydrophobic association. The very same phe-
nomenological correlations are discussed
below at the molecular level for the myosin II
motor.
8.5.2.2.4 Scenario of Muscle Contraction by
the Inverse Temperature Transition of
Hydrophobic Association
Whether at the anatomical level with the phe-
nomenon of rigor or at the myofibril level with
the variables of physiology, an extensive coher-
ence of phenomena exists. Now we address the
myosin II motor at the molecular level to deter-

426
8. Consilient Mechanisms for Protein-based Machines of Biology
mine if indeed muscle contraction may be
explained in terms of the consilient mechanism
of hydrophobic association under the control of
a competition for hydration between polar (e.g.,
charged) and apolar (hydrophobic) groups. In
this case the common functional groups capable
of existing in different degrees of polarity
would be ADP^- Mg^^ HPOl"; ATP""- Mg'^;
HPOf;
ADP^- Mg'n -COO"; -COO" Me^; and
-COOH, listed in approximate decreasing
order of polarity.
8.5.3 Consideration of Muscle
Contraction at the Molecular Level
8,5,3,1 Approach to the Molecular Level
In the context of relevance of the consilient
mechanism to function of the myosin II motor,
remarkable points are the location and orien-
tation of ATP molecules bound to the cross-
bridge and access to control hydrophobic
associations/dissociations. As considered below
in section 8.5.4.2, narrow clefts function as
conduit through which forces arising from the
polar phosphates are directed at a target site.
In this section on the myosin II motor, coher-
ence of phenomena with that of the consilient
mechanisms of energy conversion is addressed
at the molecular level. Specifically, the impor-
tance of hydrophobic interactions is noted, as
has been generally appreciated. More to the
point, the presence of the apolar-polar repulsive
free energy of hydration appears as a prominent
factor in the contraction/relaxation cycle, and
this has not been previously appreciated.
We begin with a brief orientation to the
gross structural aspects of striated muscle and
quickly move to the microscopic level where
thick myosin and thin actin filaments are driven
into greater overlap with each other during the
cyclic process of myosin cross-bridge attach-
ment to actin, contraction, and detachment
from actin. Then, in a key surfacing of the con-
silient mechanism, we consider the molecular
detail of ATP orientation in its binding site
within the cross-bridge in relation to the
means whereby ATP binding would bring
about detachment of the cross-bridge from the
actin binding site. Next, we consider geometric
relationships of hydrophobic associations and
dissociations relative to ATP binding, hydroly-
sis to form the most polar state of bound ADP
plus Pi, phosphate release, and finally ADP
release. An effort is made to integrate the role
of the calcium ion trigger for muscle contrac-
tion in relation to changing hydrophobic asso-
ciations attending the contraction/relaxation
cycle. Finally, we consider the thermodynamic
efficiency of the myosin II motor in relation to
the development of elastic forces during con-
traction/relaxation and note the substantial
energy requirement of the essential calcium ion
trigger in the contraction/relaxation cycle.
8.5.3.2 Structure of Striated Muscle and
the Sliding Filament Mechanism of
Muscle Contraction
A striated muscle, such as the biceps, is com-
prised of bundles of muscle fibers. The funda-
mental unit of a muscle fiber is the myofibril
composed of a series of repeating units called
sarcomeres defined by the periodicity of Z fines
(disks) at repeat distances of just over 2|im
(e.g., 2.3|Lim). The structural relationships pro-
ceeding from the anatomical level of the biceps
to the microscopic level of the sarcomere are
shown in Figures 8.43,^^ 8.44,^^ and 8.45.^^
FIGURE 8.44. Drawing of a muscle fiber containing
six myofibrils with each surrounded by the sar-
coplasmic reticulum that releases calcium ion to
trigger contraction and that pumps calcium ion back
out to allow for the relaxation in preparation for the
next contraction/relaxation
cycle.
Just inside the sar-
colemma of the muscle fiber that surrounds the
bundles of myofibrils are the mitochondria that
supply the ATP required to convert the fiber from a
contracted state to a relaxed but energized state in
wait for the next release of calcium ion for trigger-
ing phosphate release for onset of the next contrac-
tion/relaxation cycle. Also shown is the location of
the Z disk that separates sarcomeres (the funda-
mental unit of muscle contraction) and the arrange-
ment of the A and I bands that are defined in detail
in Figure
8.45.)
(From
Fundamental
of Biochemistry,
D Voet, J. Voet & C.W. Pratt,'' Copyright © 1999,
John Wiley & Sons, New York. Copyright © 1999,
John Wiley & Sons, New York. Reprinted with per-
mission of John Wiley & Sons, Inc.)

A
Muscle
B
Bundle
of
muscle
fibers
M \f
D
Myofibril
^ f P *
Nucler
C
Individual
muscle
fiber
FIGURE
8.43. Musculature of a man that highlights
the biceps muscle, perhaps best recognized for per-
forming the mechanical work of lifting a weight, with
a cutaway to an individual muscle fiber, presented as
a bundle of myofibrils that contains the fundamental
contractile element. (From Fundamentals of Bio-
chemistry, D. Voet, J. Voet & C.W. Pratt,^^ Copyright
© 1999, John Wiley & Sons, New York. Below: from
Biochemistry, D. Voet and J. Voet,^^ Copyright ©
1995,
John Wiley & Sons, New York. Reprinted with
permission of John Wiley & Sons, Inc.)
Sarcotubuies Mitochondrion
Terminal
Myofibrils
Triad
of the
reticulum
A
band
I
band
428
8. Consilient Mechanisms for Protein-based Machines of Biology
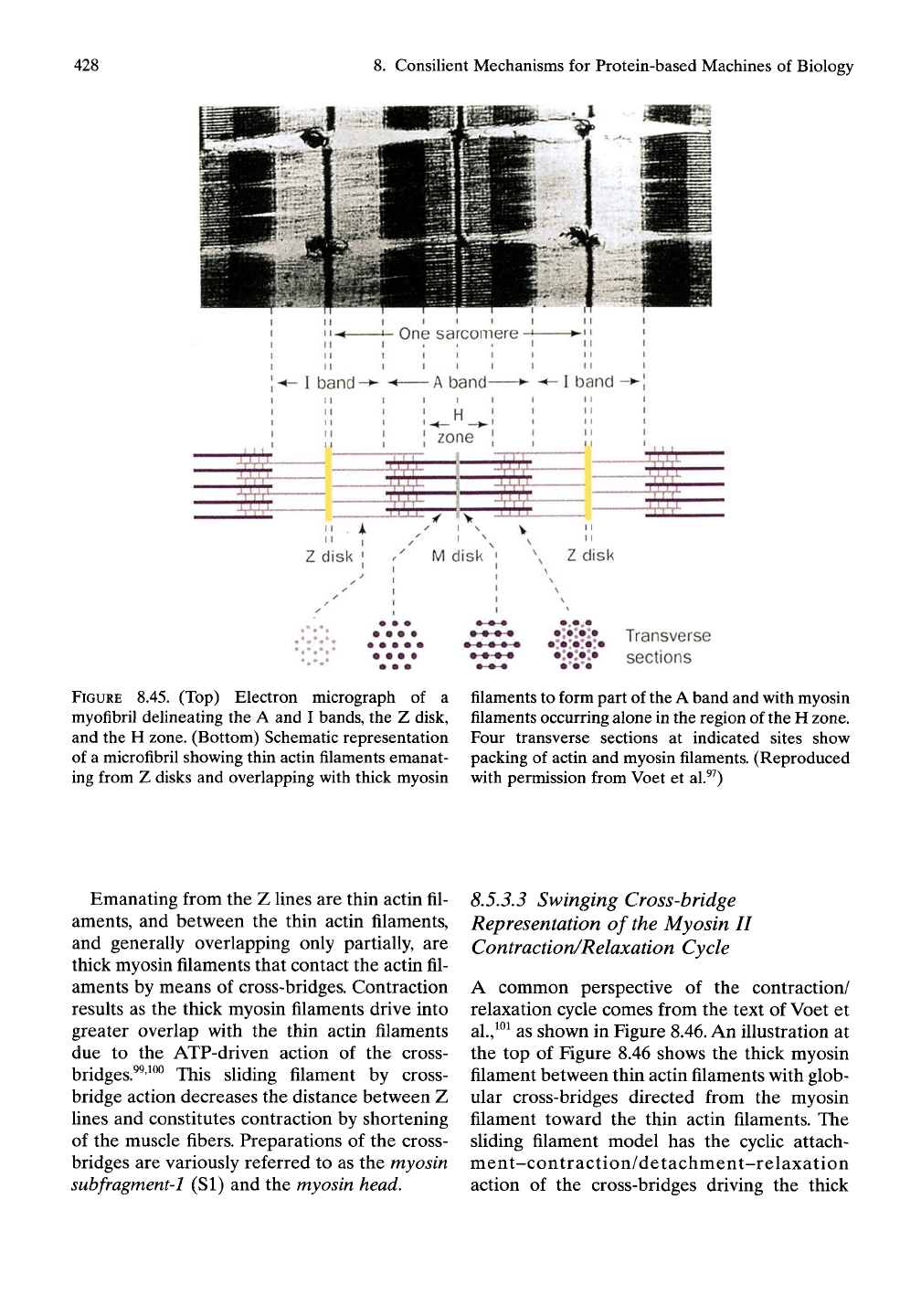
FIGURE 8.45. (Top) Electron micrograph of a
myofibril delineating the A and I bands, the Z disk,
and the H zone. (Bottom) Schematic representation
of a microfibril showing thin actin filaments emanat-
ing from Z disks and overlapping with thick myosin
•*iV4%
Transverse
•:•>:•
sections
filaments to form part of the A band and with myosin
filaments occurring alone in the region of the H zone.
Four transverse sections at indicated sites show
packing of actin and myosin filaments. (Reproduced
with permission from Voet et al.^^)
Emanating from the Z lines are thin actin fil-
aments, and between the thin actin filaments,
and generally overlapping only partially, are
thick myosin filaments that contact the actin fil-
aments by means of cross-bridges. Contraction
results as the thick myosin filaments drive into
greater overlap with the thin actin filaments
due to the ATP-driven action of the cross-
bridges.^^'^^^ This sliding filament by cross-
bridge action decreases the distance between Z
lines and constitutes contraction by shortening
of the muscle fibers. Preparations of the cross-
bridges are variously referred to as the myosin
subfragment'l (SI) and the myosin
head.
8.5.3,3 Swinging Cross-bridge
Representation of the Myosin II
Contraction/Relaxation Cycle
A common perspective of the contraction/
relaxation cycle comes from the text of Voet et
al.,^^^
as shown in Figure 8.46. An illustration at
the top of Figure 8.46 shows the thick myosin
filament between thin actin filaments with glob-
ular cross-bridges directed from the myosin
filament toward the thin actin filaments. The
sliding filament model has the cyclic attach-
ment-contraction/detachment-relaxation
action of the cross-bridges driving the thick
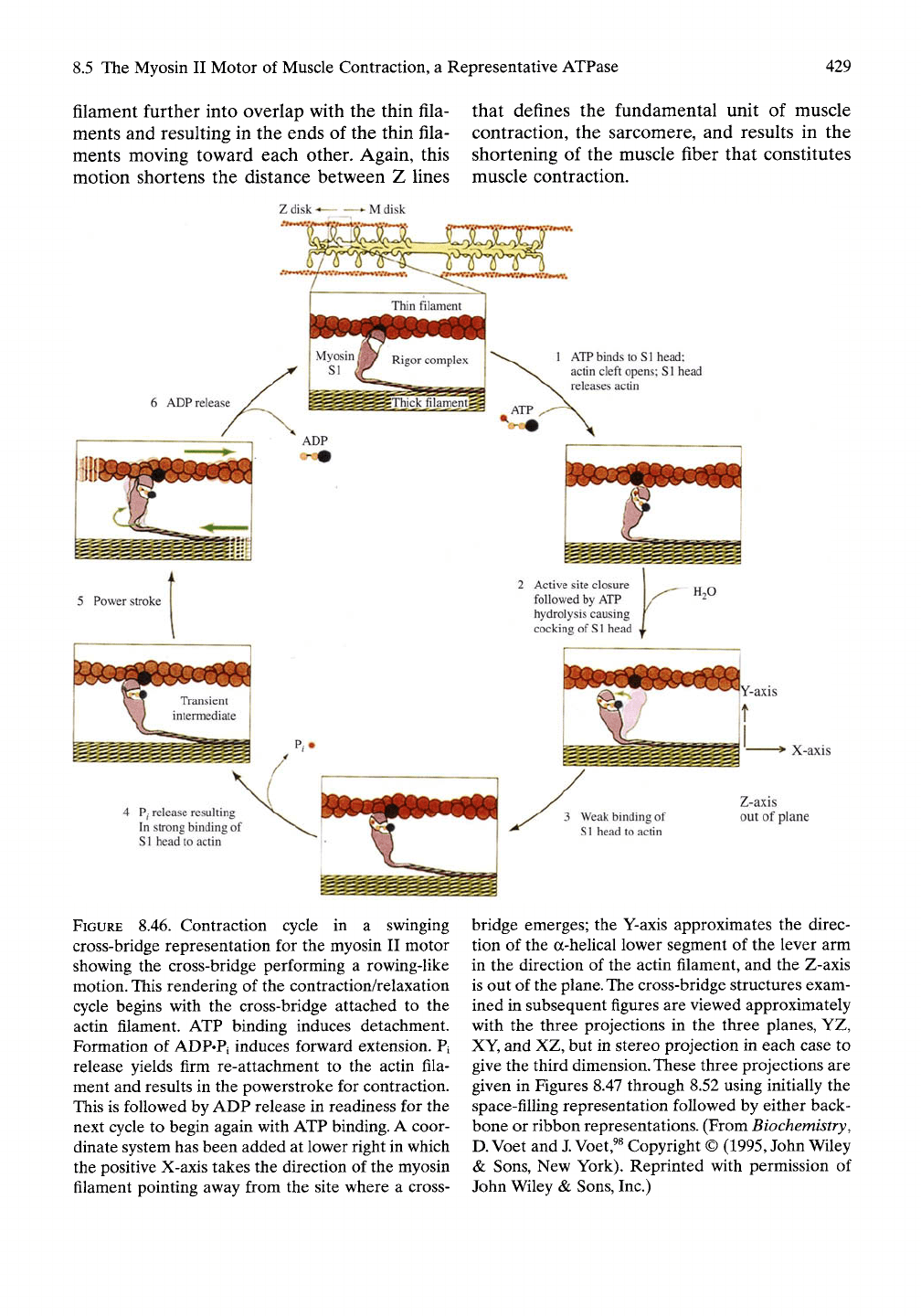
8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase 429
filament further into overlap with the thin fila-
ments and resulting in the ends of the thin fila-
ments moving toward each other. Again, this
motion shortens the distance between Z lines
that defines the fundamental unit of muscle
contraction, the sarcomere, and results in the
shortening of the muscle fiber that constitutes
muscle contraction.
•• M disk
1 ATP binds to Sl head;
actin cleft opens; Sl head
releases actin
4 p. release resulting
In strong binding of
Sl head to actin
FIGURE 8.46. Contraction cycle in a swinging
cross-bridge representation for the myosin II motor
showing the cross-bridge performing a rowing-like
motion. This rendering of the contraction/relaxation
cycle begins with the cross-bridge attached to the
actin filament. ATP binding induces detachment.
Formation of ADP«Pi induces forward extension. Pi
release yields firm re-attachment to the actin fila-
ment and results in the powerstroke for contraction.
This is followed by ADP release in readiness for the
next cycle to begin again with ATP binding. A coor-
dinate system has been added at lower right in which
the positive X-axis takes the direction of the myosin
filament pointing away from the site where a cross-
Active site closure 1 ^^
followed by ATP Y^^
hydrolysis causing f
cocking of
S1
head X
"T*
3 Weak binding of
Sl head to actin
H2O
"Y-axis
i ^-'
Z-axis
out of plane
bridge emerges; the Y-axis approximates the direc-
tion of the a-helical lower segment of the lever arm
in the direction of the actin filament, and the Z-axis
is out of the
plane.
The
cross-bridge structures exam-
ined in subsequent figures are viewed approximately
with the three projections in the three planes, YZ,
XY, and XZ, but in stereo projection in each case to
give the third
dimension.
These three projections are
given in Figures 8.47 through 8.52 using initially the
space-filling representation followed by either back-
bone or ribbon representations. (From Biochemistry,
D.
Voet and
J.
Voet,^^
Copyright ©
(1995,
John Wiley
& Sons, New York). Reprinted with permission of
John Wiley & Sons, Inc.)

430
8. Consilient Mechanisms for Protein-based Machines of Biology
In the expanded view of cross-bridge-actin
interaction of Figure 8.46, step 1, the binding
of ATP to the cross-bridge causes detachment
of cross-bridge from the actin filament. Step 2
is the hydrolysis of bound ATP to yield
bound ADP plus bound phosphate. This occurs
with the forward movement of the cross-bridge
(as in the moving forward of an oar) along
the actin filament and with re-estabUshment of
a weak interaction of the cross-bridge with
actin as step 3. Release of phosphate as step 4
results in the combined strong binding of cross-
bridge to actin immediately followed by the
powerstroke. Step 5 represents the sUding of
the thick filament further into the array of thin
filaments. With release of ADP as step 6, the
cross-bridge-actin interaction returns to the sit-
uation before ATP binding, thereby completing
the cycle. In what follows, these steps will be
considered in terms of detailed molecular inter-
actions with emphasis on the consiUent mecha-
nism involving the apolar-polar repulsive free
energy of interaction and its relationship to
controlling hydrophobic hydration and hydro-
phobic association.
8.5.3.3.1
Analyses of Crystal Structures Below
Do Not Support the Bending Motion of the
Cross-bridge as Depicted in Figure 8.46
As shown below in Figures 8.47 through 8.52,
which compare the different axial projections
of the near-rigor and nucleotide bound states,
there is no bending motion as illustrated in the
results of steps 2 and
5.
The contraction appears
as a more complex motion involving a twisting
within the lower part of the globular portion of
the cross-bridge that changes the direction at
which the single a-helix of the lever arm
leaves the globular component. Rather than
being strengthened by a-helical multistranding
of the lever arm, it would appear that the
essential and regulatory Ught chains fulfill such
a re-enforcement role.
8.5.3.3.2
Absence in Figure 8.46 of Including
Calcium Ion Release as the Event That
Triggers Contraction
Another critical element in the contraction/
relaxation cycle is to position the point of
calcium ion release that provides the in vivo
trigger for the contractile event. This will be
considered in relation to details of an interplay
of hydrophobic dissociations/associations that
attend the contractile process.
8.5.4 Crystal Structure Analyses and
Consilient Mechanisms in the
Myosin II Motor
The crystal structure analyses utilize two crystal
structures of the myosin II motor of scallop
muscle available from the Protein Data Bank
(http://www.rcsb.org/pdb) as Structure Files
1KK7 and 1KK8.^ Structure 1KK7 is a near-
rigor state with a sulfate at the active site, and
structure 1KK8 is a nucleotide-containing state
with ADP-BeFx (where BeFx is an analogue
for the y-phosphate) at the active
site.
The illus-
trations utilize FrontDoor to Protein Explorer
1.982 Beta (Copyright © 2002 by Eric Martz),
which can be obtained at no cost at www.
proteinexplorer.org. All structures are repre-
sented in shades of gray spanning from black
for aromatic residues, gray for other hydro-
phobic residues, light gray for neutral residues,
and white for charged residues, as fits with the
deUneation of polar (charged) as white and
apolar varying from gray to black. In this way
apolar regions can be distinguished at a glance
from polar (charged) regions, with oil-Hke
hydrophobic domains seen as dark regions and
with polar domains seen as white regions. This
allows immediate recognition of the location,
size,
and intensity of hydrophobic domains. The
only exception to this coding scheme is in
Figure 8.47, where the shades of gray are used
to delineate the three different chains of the
cross-bridge.
8.5.4.1 Structural Composition of the
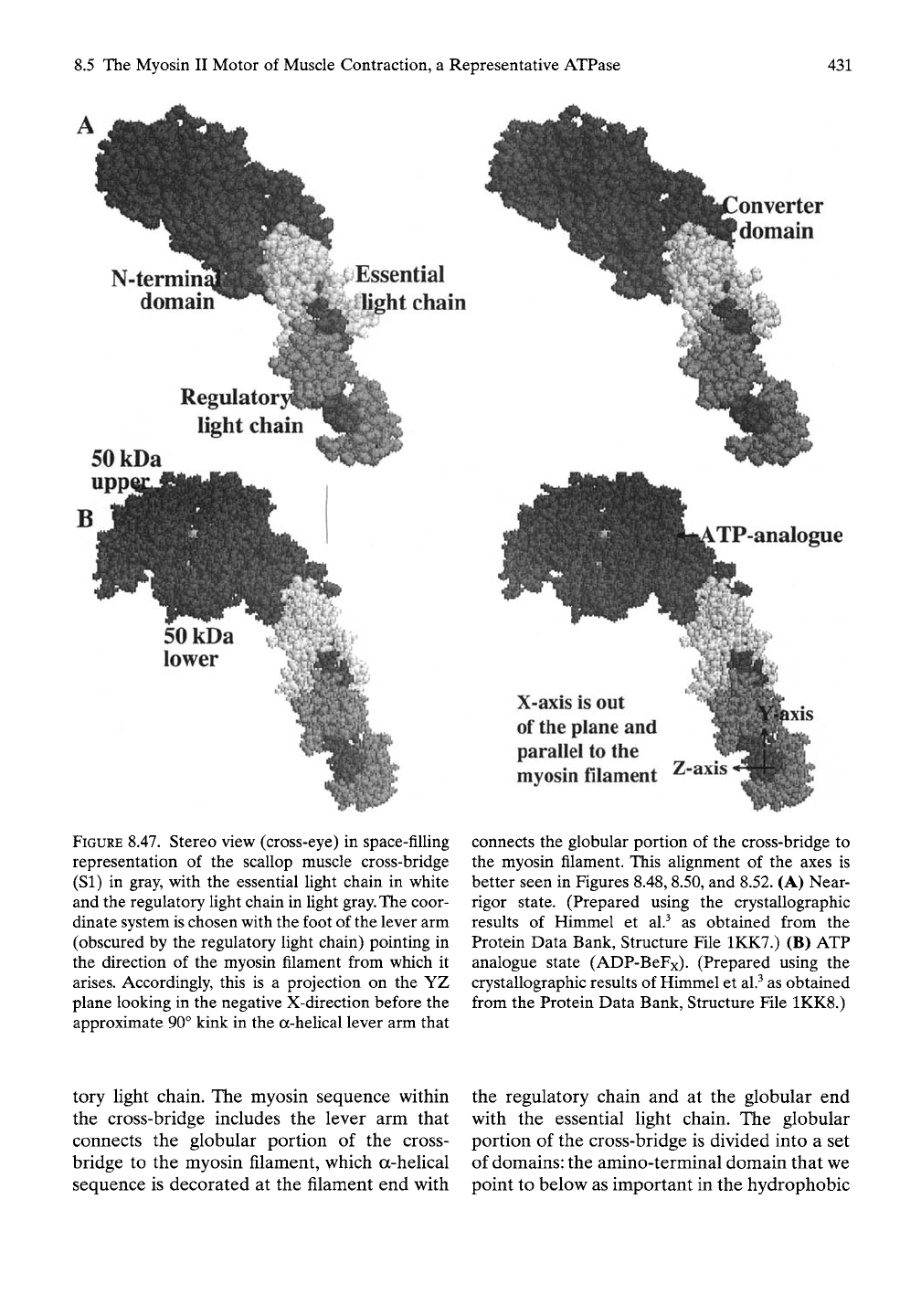
Myosin Cross-bridge
The structures used here contain that part of
the amino terminus of myosin (residues 1 to
835) just sufficient to include the essential and
regulatory light chains. Thus, the cross-bridge is
composed of three chains—approximately 800
residues from the amino terminus of the myosin
chain, the essential light chain, and the regula-
8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
431
onverter
domain
N-termin
domain
^iEssential
Ij^ht chain
Regulator}
light chain
SOkDa
upp
FIGURE 8.47. Stereo view (cross-eye) in space-filling
representation of the scallop muscle cross-bridge
(SI) in gray, with the essential light chain in white
and the regulatory light chain in light
gray.
The coor-
dinate system is chosen with the foot of the lever arm
(obscured by the regulatory light chain) pointing in
the direction of the myosin filament from which it
arises.
Accordingly, this is a projection on the YZ
plane looking in the negative X-direction before the
approximate 90° kink in the a-heUcal lever arm that
TP-analogue
X-axis is out
of
the
plane and
parallel to the
myosin filament
Z-axis
connects the globular portion of the cross-bridge to
the myosin filament. This alignment of the axes is
better seen in Figures 8.48, 8.50, and 8.52. (A) Near-
rigor state. (Prepared using the crystallographic
results of Himmel et al.^ as obtained from the
Protein Data Bank, Structure File 1KK7.) (B) ATP
analogue state (ADP-BeFx). (Prepared using the
crystallographic results of Himmel et al.^ as obtained
from the Protein Data Bank, Structure File 1KK8.)
tory light chain. The myosin sequence within
the cross-bridge includes the lever arm that
connects the globular portion of the cross-
bridge to the myosin filament, which a-helical
sequence is decorated at the filament end with
the regulatory chain and at the globular end
with the essential light chain. The globular
portion of the cross-bridge is divided into a set
of domains: the amino-terminal domain that we
point to below as important in the hydrophobic

432
8. Consilient Mechanisms for Protein-based Machines of Biology
association associated with the powerstroke, a
50kDa segment divided into upper and lower
domains separated by a cleft that runs from the
nucleotide binding site to the actin binding site,
and a converter domain that resides at the head
of the lever arm that structurally ties to the
lower domain of the 50kDa segment.
8.5.4.2 Early Seminal Contributions of
Rayment and Coworkers and Their
Relationship to the Consilient
Mechanisms
8.5.4.2.1
The Early Insights of Rayment
and Coworkers
In 1993, Rayment and coUeagues^^^'^^^ pub-
lished two seminal papers on the "Three-
dimensional structure of the head portion of
myosin, or subfragment-1, which contains
both the actin and nucleotide-binding sites...."
Thereby, these publications provided key struc-
tural aspects of the contraction/relaxation
cycle. Shortly thereafter in a report of their
structural studies of the myosin head from Dic-
tostelium myosin II, Rayment and coworkers
concluded that "The current structural results
emphasize the importance of the narrow cleft
that splits the 50-kDa segment in the mole-
cular origin of myosin based motility. They
suggest further that it functions not only in
sensing the presence of the y-phosphate of ATP
but is also responsible for transducing the
conformational change that results in the
powerstroke.'"^
8.5.4.2.2
Extensions of the Early Insights of
Rayment and Coworkers by Means of the
Hydrophobic Consilient Mechanism
In what follows, we extend these insights in two
significant ways. One way recognizes that the
"narrow cleft" not only "splits the 50-kDa
segment" in the direction of the binding site
with actin, but also that the "narrow cleft" is
directed toward the junction between the
amino-terminal domain and the head of the
lever arm to, in our view, effect "the conforma-
tional change that results in the powerstroke."
Our second extension is in the nature of the
force emanating from the y-phosphate in both
directions. The force disrupts the hydrophobic
association responsible for attachment to actin
and the hydrophobic association between the
amino-terminal domain and the head of the
lever arm. That force given focus and direction
by the "narrow cleft" derives from the
apolar-polar repulsive free energy of hydra-
tion,
AGap-
AGap
derives from a competition for
hydration between hydrophobic and charged
groups and as such constitutes a repulsive
force that disrupts hydrophobic hydration and
thereby disrupts hydrophobic association.
Hydrolysis of ATP to release the y-phosphate
as
Pi
that leaves the structure, therefore, has two
consequences. Strong hydrophobic association
is re-established between the cross-bridge and
the actin binding site, and strong hydro-
phobic association is re-established between
the amino-terminal domain and the head of the
lever arm to provide the powerstroke. This
perspective resides at the heart of the pro-
posed contribution of the hydrophobic con-
silient mechanism to function of the myosin II
motor. It is considered further below and most
directly in section 8.5.4.7.
8.5.4.2.3
Efficient Energy Transduction
Requires Coupling to Near-ideal Elastic
Force Development
The above perspectives are natural conse-
quences of both the hydrophobic and the
elastic consiHent mechanisms as applied to the
structural data on the myosin II motor. Here we
briefly explore the elastic element. An ideal
elastomer exhibits exactly reversible stress-
strain curves with complete recovery on relax-
ation of the energy of deformation. On the
other hand, an elastomer that exhibits
hysteresis does not recover all of the energy on
relaxation that was expended on deformation.
Accordingly, efficient muscle contraction
should involve the deformation of near-ideal
elastic segments to utilize more efficiently the
energy expended in driving contraction. The
mechanism of elasticity that can provide such
near-ideal elasticity is the damping of internal
chain dynamics on extension.
There are many aspects of the hydrophobic
and elastic consilient mechanisms that warrant

8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
433
discussion in relation to the mechanism of
muscle contraction. Limitations of time and
space, however, necessarily restrict considera-
tion of many important aspects of muscle con-
traction that naturally flow from the insight of
these consilient mechanisms. In what follows,
we emphasize the role of the narrow cleft in
terms of the presence and absence of nucleo-
tide phosphates controlling hydrophobic
associations/dissociations by means of the
apolar-polar repulsive free energy of hydra-
tion,
AGap.
Specifically, the hydrophobic associ-
ations/dissociations involve the binding of the
cross-bridge to the actin filament and the
interaction of the amino-terminal domain with
the head of the lever arm to achieve the
powerstroke.
8.5.4.3 The Near-rigor and ATP
(Analogue) Bound States of the
Cross-bridge Compared in Three
Planes Defined by Axes Set at the
Myosin Filament
In setting up the coordinate system, the X-axis
is taken parallel to the axis of the myosin fila-
ment; the Y-axis is taken perpendicular to the
myosin filament in the direction of the actin
filament to which the cross-bridge would
attach, and the Z-axis is approximated by
sighting through the center of the a-helical
sequence of the lever arm involving residues
825 to 800 of scallop muscle.
8.5.4.3.1
View of Complete Cross-bridge
in the YZ Plane Perpendicular to the
Myosin Filament
A space-filling representation of the complete
cross-bridge is shown in Figure 8.47, with the
near-rigor state in A and the state containing
the ATP analogue in B. The 50kDa upper and
lower domains and other domains of the
myosin chain segment, for example, the amino-
terminal domain and the converter domain, are
in gray. Also shown and labeled are the essen-
tial light chain in white and the regulatory light
chain in light gray. The globular head of the
myosin chain, including in particular the amino-
terminal domain, appears to be twisted in a
clockwise direction as seen from the top.
The structural rearrangements appear more
obvious in Figure 8.48, which is the same view
given in backbone representation. The a-helical
lever arm is shown in its entirety from foot to
head with a knee bend at the junction of the
essential and regulatory light chains and with
an essentially unchanged orientation on going
from the near-rigor state to an analogue
representative of the ATP bound state. In
this view the relocation of the amino-terminal
domain is apparent, but becomes clearer in
subsequent perspectives below in Figures 8.50
and 8.52.
8.5.4.3.2
View of the Cross-bridge in the XY
Plane Demonstrates Absence of the Bending
Motion at the Myosin Filament End of
the Lever Arm
The cross-eye stereo view of the myosin cross-
bridge of scallop muscle is given for the near-
rigor state in Figure
8.49A
and in the ATP
analogue state in Figure 8.49B. In this perspec-
tive the amino-terminal domain is shown to
have shifted from the left side of the head of
the lever arm to the righthand side, while the
essential light chain seems to have shifted very
little.
Again, the globular head of the myosin
chain follows the same reorientation as the
amino-terminal domain, exhibiting a clockwise
rotation when seen from above.
Exactly the same perspectives of Figure 8.49
in space-filling representation are given in
ribbon representation in Figure 8.50, which
allows for a clearer view of any structural
rearrangements that occur between near-rigor
and ATP bound states. Again, it appears that
the essential Ught chain changes little but that
the amino-terminal domain, the leading edge of
which is identified by residue G53, undergoes a
large relocation on conversion to the near-rigor
state.
Although there may be a slight change in
the bend at the knee of the a-helical lever arm,
there is no detectable change in the approxi-
mately right angle turn on going from myosin
filament segment to the lever arm. The bending
motions indicated in steps 2 and 5 of Figure 8.46
do not occur.

8. Consilient Mechanisms for Protein-based Machines of Biology
Converter
domain
N-termini
domain
a-helical^
lever arm
^H''-'^^-
50kDa
ATP-analogue
^Regulatory
flight chain
FIGURE 8.48. Stereo view (cross-eye) in backbone
representation of scallop muscle cross-bridge (SI) in
gray,
with the essential and regulatory Ught chains in
Ught gray. Projection on the YZ plane looking in the
negative X-direction before the 90° kink, forming a
foot-like structure where the a-helical lever arm con-
nects to the myosin filament. The a-helical lever arm
exhibits a knee-like bend at the function of the
essential and regulatory light chains midway
between a foot section and the head portion that
Z-axis<—^^j
X-axis is out yv^"^
of
the
plane
Y-aids
nestles between the converter domain and the
amino-terminal domain in A. (A) Near-rigor state
that contains a single sulfate at the active site. (Pre-
pared using the crystallographic results of Himmel et
al.^
as obtained from the Protein Data Bank, Struc-
ture File 1KK7.) (B) ATP state with ATP analogue
(ADP-BeFx) shown in space-filling representation.
(Prepared using the crystallographic results of
Himmel et al.^ as obtained from the Protein Data
Bank, Structure File 1KK8.)
