Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

8.4 ATP
Synthase:
The Twofold Rotary Protein Motor of Oxidative Phosphorylation
405
-ATP
6-empt
(E)
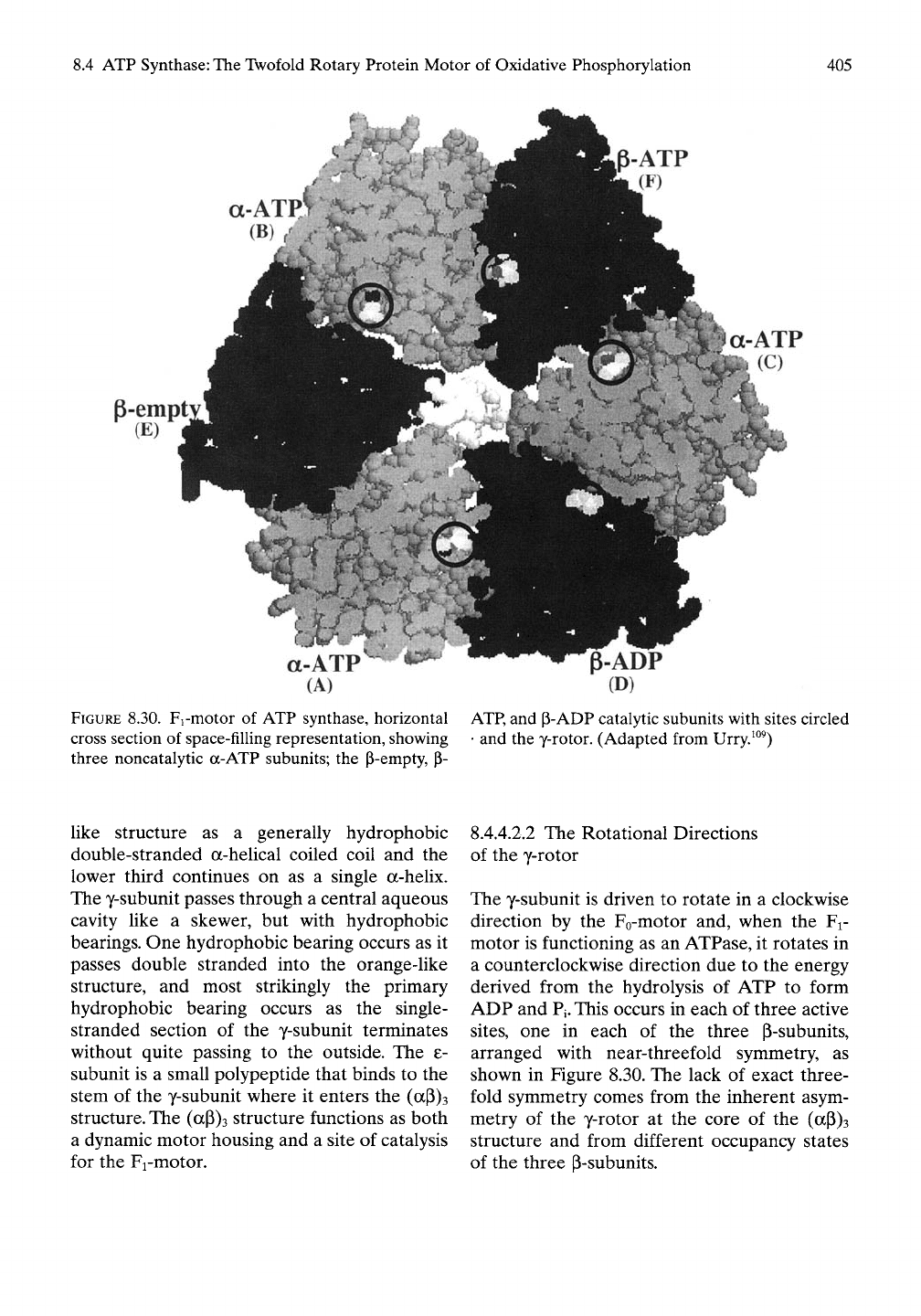
FIGURE 8.30. Fi-motor of ATP synthase, horizontal
cross section of space-filling representation, showing
three noncatalytic a-ATP subunits; the P-empty, p-
P-ADP
(D)
ATP,
and P-ADP catalytic subunits with sites circled
• and the y-rotor. (Adapted from Urry.^°^)
like structure as a generally hydrophobic
double-stranded a-helical coiled coil and the
lower third continues on as a single a-helix.
The y-subunit passes through a central aqueous
cavity like a skewer, but with hydrophobic
bearings. One hydrophobic bearing occurs as it
passes double stranded into the orange-like
structure, and most strikingly the primary
hydrophobic bearing occurs as the single-
stranded section of the ysubunit terminates
without quite passing to the outside. The 8-
subunit is a small polypeptide that binds to the
stem of the y-subunit where it enters the (ap)3
structure. The (aP)3 structure functions as both
a dynamic motor housing and a site of catalysis
for the Fi-motor.
8.4.4.2.2
The Rotational Directions
of the y-rotor
The y-subunit is driven to rotate in a clockwise
direction by the Fo-motor and, when the Fi-
motor is functioning as an ATPase, it rotates in
a counterclockwise direction due to the energy
derived from the hydrolysis of ATP to form
ADP and
Pi.
This occurs in each of three active
sites,
one in each of the three P-subunits,
arranged with near-threefold symmetry, as
shown in Figure 8.30. The lack of exact three-
fold symmetry comes from the inherent asym-
metry of the y-rotor at the core of the (aP)3
structure and from different occupancy states
of the three P-subunits.

406
8. Consilient Mechanisms for Protein-based Machines of Biology
FIGURE 8.31. P-(Empty) face of the y-rotor of ATP
synthase identified as the very hydrophobic face
by the calculation shown of Table 8.2 to give
2AGHA(empty face) ~ -20kcal/mole. Shown at right
are the a-ATP and p-ADP ligands, at left the a-ATP
and p-ATP ligands, and the a-ATP ligand behind.
The neutral residues are light gray, the aromatic
residues are black, the other hydrophobic residues
are gray, and the charged residues are white.
(Adapted from Urry.^^^)
8.4.4.2.3
Early Crystal Structure Data
The early crystal structure data, utilized in
Figure 8.30, proved to be particularly interest-
ing as it occurred with noncatalytic ATP bound
in each the three a-subunits, but most interest-
ingly because the three |3-subunits were solved
with ATP bound at one unit, with ADP bound
at the second unit, and with an empty third (3-
subunit. This structure provides many opport-
unities with which to begin analyses of
mechanism. Among other things, this structure
permits definition of three faces of the y-rotor,
as discussed immediately below.
8A.4.3 The Three Faces of the y-rotor
Our perspective of the hydrophobic consilient
mechanism as it would apply to ATP synthase
requires that there be an asymmetric hydro-
phobic rotor and that a variable apolar-polar
repulsion occur between the different occu-
pancy states of the catalytic p-subunits and the
rotor. If the hydrophobic consilient mechanism
is relevant, then the structure utilized in Figure
8.30 should occur with a distinctly and hydro-
phobically asymmetric y-rotor.
8.4.4.3.1
The Hydrophobic Face Defined by
p-Empty Subunit
In the static crystal structure, on the basis of the
hydrophobic consilient mechanism one expects
the side of the y-rotor adjacent to the p-empty
subunit to be the most hydrophobic. By the
apolar-polar repulsive free energy of hydra-
tion, AGap, the polar nucleotide ligands should
repulse the most hydrophobic side of the
y-rotor.
The ligands themselves allow correct identi-
fication of the orientation. By using the labeled
cross section of Figure 8.30 as a guide, posi-
tioning the a-ATP and p-ADP at right, the a-
ATP and P-ATP at the left, and an a-ATP
behind allows direct observation of the P-
empty face of the y-rotor, as shown in Figure
8.31.
The hydrophobicity scale in Table 5.3 lists the
contribution of each amino acid residue to the
Gibbs free energy of hydrophobic association,
AGHA- Table 5.3 also provides the information
required to calculate numbers for the relative
hydrophobicities of the faces of the y-rotor. The
resulting numbers are tabulated and summed in
Table 8.2, where the IlAGnACP-empty face) ~
-20kcal/mole. This is indeed a very hydropho-
bic value.
8.4.4.3.2
The Polar Face Defined by the
p-ADP Subunit
Identification of the face defined by the p-ADP
subunit comes from placing the a-ATP and P-
ATP ligands at right, the a-ATP ligand and P-
empty site at left, and the third a-ATP ligand
behind, as shown in Figure 8.32. With K4, D5,
K260,
E261, and E264 contributing to this face,
it is clearly a polar face. On tabulating the con-
tribution of the visible amino acid residues for
this face and summing, as shown in Table 8.2,
2:AGHA(P - ADP face) « +9kcal/mole. The cal-
culation confirms a quite polar face.
8.4.4.3.3
The Neutral Face Defined by the
p-ATP Subunit
Identification of the face defined by the P-ATP
subunit comes from placing the a-ATP ligand
and p-empty site at right, the a-ATP and p-
ADP ligands at left, and the third a-ATP ligand
behind, as shown in Figure 8.33. Interestingly,
as shown in Table 8.2, tabulation of the amino

TABLE
8.2. Values for ZAGHA(y-rotor faces).^
p-empty
Res.
No.
Thr2/3
Leu
3
Lys
4
Thr7
Leu 10/2
He 263/3
Leu 262/2
Glu 261/2
Thr259
He 258
Val 257/2
Gin 255
Arg 254
Arg 252/2
Asn 251
Phe 250
Leu 248
Thr247
Sum
face
AGHA
-0.20
-4.05
+2.94
-0.60
-2.00
-1.20
-2.00
+1.85
-0.60
-3.65
-1.25
+0.75
+0.70
+0.35
-0.05
-6.15
-4.05
-0.60
-19.8
P-ATP
face
Res.
No.
Alal
Thr2/3
Asp
5
He
6/3
Glu 264
He 263/3
Lys 260
Thr259
He 258/3
Ala 256
Gin 255
Val 257/3
Thr253
Arg 252
Asn 251
Leu 248/2
Thr249
Sum
AGHA
-0.75
-0.20
+3.40
-1.20
+3.72
-1.20
+2.94
-0.60
-1.20
-0.75
+0.75
-0.80
-0.60
+0.70
-0.05
-2.00
-0.60
+0.4
P-ADP
face
Res.
No.
Alal
Thr2
Leu 3/3
Lys
4
Asp
5
Thr7/2
Glu 264
Glu 261
Lys 260
He 258/2
Val 257
Ala 256/3
Arg 254/2
Thr 253/3
Thr 249/3
Sum
AGHA
-0.75
-0.60
-1.30
+2.94
+3.40
-0.30
+3.72
+3.72
+2.94
-1.80
-2.50
-0.25
+0.35
-0.20
-0.20
+9.2
'
IAGHA(p-empty face) « -20kcal/mole;
EAGHA(P-ATP
face) « +Okcal/mole;
5:AGHA(P-ADP
face) « +9kcal/mole.
Source:
Adapted from Urry.^^^
407

408
8. Consilient Mechanisms for Protein-based Machines of Biology
FIGURE 8.32. p-(ADP) face of the y-rotor of ATP
synthase seen from the side of the ADP site with
ADP overlying the ADP face identified as the polar
face by the calculation
in
Table 8.2 to give 2AGHA(P-
ADP face) ^ +9kcal/mole. Shown at right are a-ATP
and P-ATP ligands, at left the a-ATP ligand and the
P-(empty) site, and the a-ATP Ugand behind. The
neutral residues are hght gray, the aromatic residues
are black, the other hydrophobic residues are gray,
and the charged residues are white. (Adapted from
Urry.i^^)
acid residues contributing to this face and sum-
ming gives ZAGHA(P-ADP face) ~ Okcal/mole.
8.4.4.3.4 An Additional Point About the
Relative Hydrophobicities of the Faces of
the y-rotor
Thus,
the calculated hydrophobicities of the
structurally defined rotor delineate the three
faces to give a polar face, a neutral face, and
a hydrophobic face. Although the order of
hydrophobicity allows striking delineation
between empty and nucleotide-containing
sites,
the finer distinction of the decrease in
hydrophobicity of the rotor faces with increase
in polarity from the p-ADP site to the p-ATP
site does not follow in proportion. If one con-
siders the mechanism for the ATP synthase
8.4 ATP
Synthase:
The Twofold Rotary Protein Motor of Oxidative Phosphorylation
409
function, however, the relative order does make
sense. In order to add Pi to an ADP-containing
site,
this site should be in apposition to the most
polar face of the y-rotor. Such a configuration
would exhibit the lesser apolar-polar repulsive
free energy of hydration,
AG^p,
and would make
formation of the most polar ADP plus Pi occu-
pancy state more probable. Accordingly, the
hydrophobically asymmetric y-rotor is as
expected for the hydrophobic consilient mech-
anism to be dominant in the function of ATP
synthase.
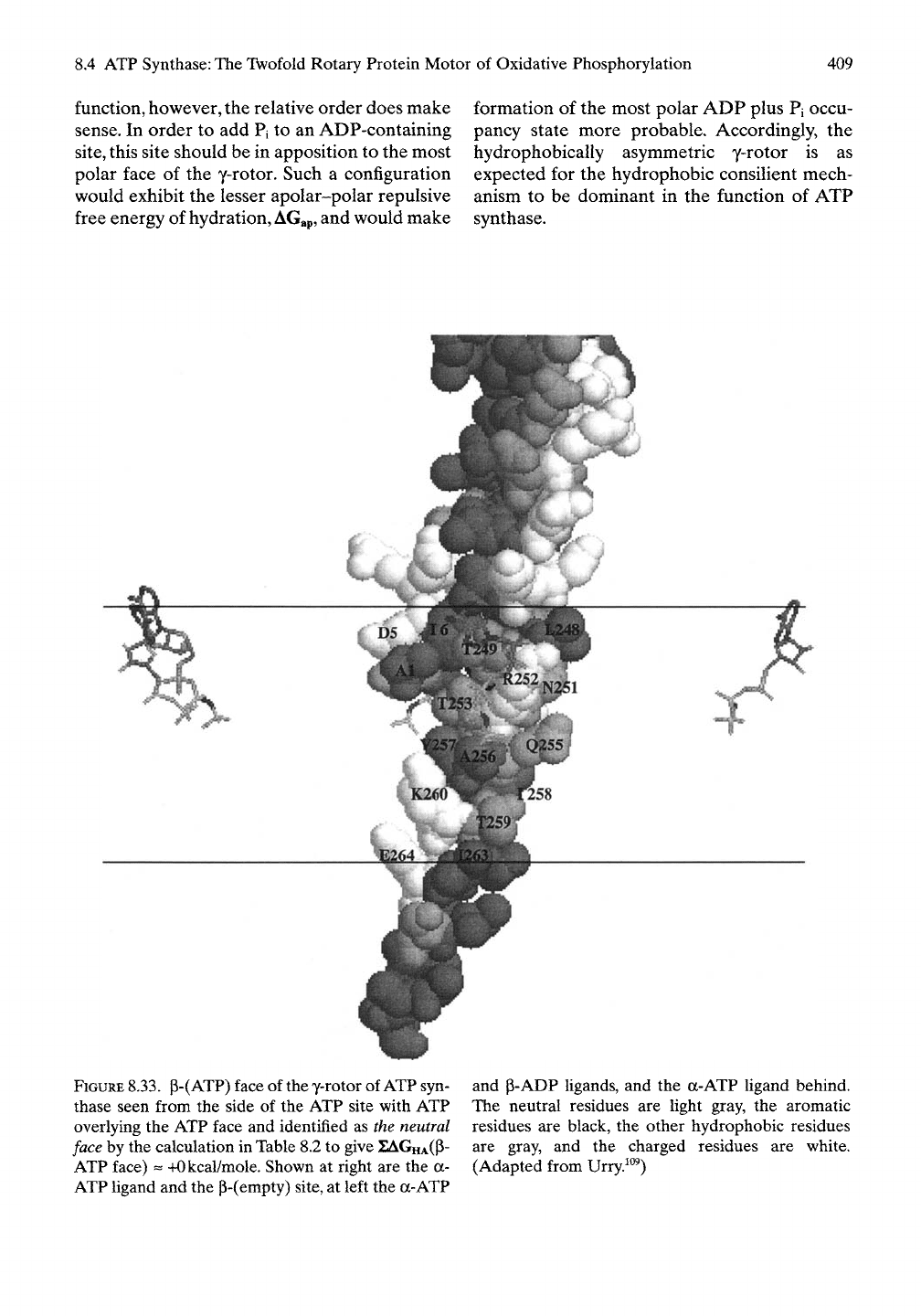
FIGURE
8.33.
p-(ATP) face of the y-rotor of
ATP
syn-
thase seen from the side of the ATP site with ATP
overlying the ATP face and identified as the
neutral
face by the calculation in Table 8.2 to give XAGHA(P-
ATP face)
==
+Okcal/mole. Shown at right are the a-
ATP Hgand and the p-(empty)
site,
at left the a-ATP
and p-ADP ligands, and the a-ATP Ugand behind.
The neutral residues are light gray, the aromatic
residues are black, the other hydrophobic residues
are gray, and the charged residues are white.
(Adapted from Urry.^"^)

410
8. Consilient Mechanisms for Protein-based Machines of Biology
8.4A.4 Side Views (3) of the Interaction of
the Paired Diametrically Opposed
Subunits with the Asymmetric Rotor
Now the question becomes whether the differ-
ent hydrophobicities of the faces of the y-rotor
affect the interaction between rotor and cat-
alytic housing of the Fi-motor. Examination of
side views of the three arrangements of dia-
metrically opposed a-P-subunits in association
with the rotor provides the answer. Using the
chain designations of the crystal structure,
included in Figure 8.30 with the y-rotor
also indicated by its chain designation G, the
three arrangements may be given as (3-
empty(E)-Y-rotor(G)-a-ATP(C), P-ADP(D)-
Y-rotor(G)-a-ATP(B), and p-ATP(F)-Y-rotor
(G)-a-ATP(A).
To examine these interactions in Figure 8.34,
cross-eye stereo views with the protein subunits
in space-filling representation are used in which
the neutral residues are light gray, the aromatic
residues are black, the other hydrophobic
residues are gray, and the charged residues are
white.
This choice of representation and residue
shading allows immediate identification of
dark regions as hydrophobic domains and the
hydrophobic associations between subunits and
rotor, if and when they occur.
8.4.4.4.1
p-Empty(E)-Y-Rotor(G)-a-ATP(C):
The First of Three Arrangements of
p-Catalytic Subunits with the
Diametrically Opposed
ATP-containing a-Subunits
This configuration, which for simplicity may be
designated as EGG, makes a profound state-
ment. As shown in Figure 8.34A, the association
between the P-empty subunit and the Y-rotor is
profoundly hydrophobic at the levels of both
the nucleotide sites and the interaction as the
Y-rotor enters the (ap)3 construct. The most
hydrophobic face of the Y-rotor hydrophobi-
cally associates extensively with the p-empty
subunit. Interestingly, except at the very tip of
the Y-rotor, there is no interaction with the a-
ATP(C) subunit; instead, there is an aqueous
chasm separating G from C down to but not
including the tip of the Y-rotor. It is difficult to
imagine a more stark contrast between opera-
tive polar and apolar associations.
8.4.4.4.2
P-ADP(D)-Y-Rotor(G)-a-ATP(B):
The Second of Three Arrangements of p-
Catalytic Subunits with the Diametrically
Opposed ATP-containing a-Subunits
In the DGB interaction shown in Figure 8.34B,
the interaction of the Y-rotor with the P-ADP
subunit is significantly hydrophobic, but in a
limited way when compared with the interac-
tion, EG, between p-empty subunit and the
Y-rotor. For this DGB configuration, the
a-ATP(B) interaction with the Y-rotor is partly
re-established.
8.4.4.4.3
p-ATP(F)-Y-Rotor(G)-a-ATP(A):
The Third of Three Arrangements of
p-Catalytic Subunits with the
Diametrically Opposed
ATP-containing a-Subunits
For the FGA configuration shown in Figure
8.34C, both the a- and p-subunits contain ATP,
and strikingly the Y-rotor lies centered between
the two with more nearly similar interactions of
the Y-rotor with the two subunits. This FGA
association is very different from the off-center
positioning of the Y-rotor for the EGG configu-
ration in Figure 8.34A, where the association of
the P-empty subunit with the Y-rotor gives the
impression of being pulled over by the attrac-
tion of hydrophobic domains.
8.4.4.5
Association of Y-Rotor with Fi-motor
Housing Looks Like the "Pull" of
Hydrophobic Association,
AGHA,
Coupled
with the "Push" of Apolar-Polar
Repulsion, AGap
The association of hydrophobic domains be-
tween the Y-rotor and the p-empty subunit in
Figure
8.34A
profoundly dominates the struc-
tural view of the EG interaction. Also strik-
ing is the seeming repulsion between the Y-
rotor(G) and the a-ATP(C) subunit seen as an
aqueous cleft between the G and C chains. The
symmetry is partially restored when the dia-
metrically opposed subunits contain p-ADP
and a-ATP, as shown in Figure 8.34B. The

P-empty
chain E
B
p-ADP
chain D
p-ATP
chain F
a-ATP
H chain C
Isolated
Y-rotor
orientation
Y-L272
a-ATP
i chain B
a-ATP
chain A
FIGURE
8.34. Fi-ATPase: vertical stereo views in
space-filling representation showing the central y-
rotor with diametrically opposed subunits on each
side with the neutral residues light gray, the aromatic
residues black, the other hydrophobic residues gray,
and the charged residues white. The p-subunits are
on the left, and the diametrically opposed a-subunits
are on the right. In terms of crystallographically
defined chains and as defined in Figure 8.30: (A) the
empty P-subunit (chain E) pairs with a-subunit
(chain C) containing ATP; (B) the p-subunit con-
taining ADP (chain D) pairs with the a-subunit
(chain B) containing
ATP;
and (C) the P-subunit con-
taining ATP (chain F) pairs with the a-subunit (chain
A) containing ATP. By this graytone shading
hydrophobic associations are seen as dark regions.
In A, the y-rotor hydrophobically associates so
extensively with the empty p-subunit due to negative
2AGHA(%)
that the two are not visually separable
with the shading scheme. Therefore, the individual y-
rotor is shown separately in position to be seen in
stereo. In
B,
The ADP-bound P-subunit hydrophobi-
cally associates to a lesser extent with the y-rotor. In
C, The ATP-bound P-subunit loses essentially all of
the lower hydrophobic association with the y-rotor
and is very nearly centered between the two ATP-
containing subunits. Very significant in part (A) is the
remaining aqueous chamber between the p-empty
subunit and the y-rotor through which the apolar-
polar repulsive forces can act. See text for further
discussion. (Prepared using the crystallographic
results of Abrahams et al.^"* as obtained from the
Protein Data Bank, Structure File IBMF)
411

412
8. Consilient Mechanisms for Protein-based Machines of Biology
symmetry is nearly entirely restored when the
diametrically opposed subunits both contain
ATP,
as in the configuration of p-ATP(F)-Y-
rotor(G)-a-ATP(A) given in Figure 8.34C.
Seen grossly, these relationships are as one
might expect if the hydrophobic consilient
mechanism provided the dominant interaction
energies. Additional crystal structures, exam-
ined below, allow this possibiHty to be explored
further. The result is most encouraging for the
consilient mechanisms that constitute the major
message of this volume.
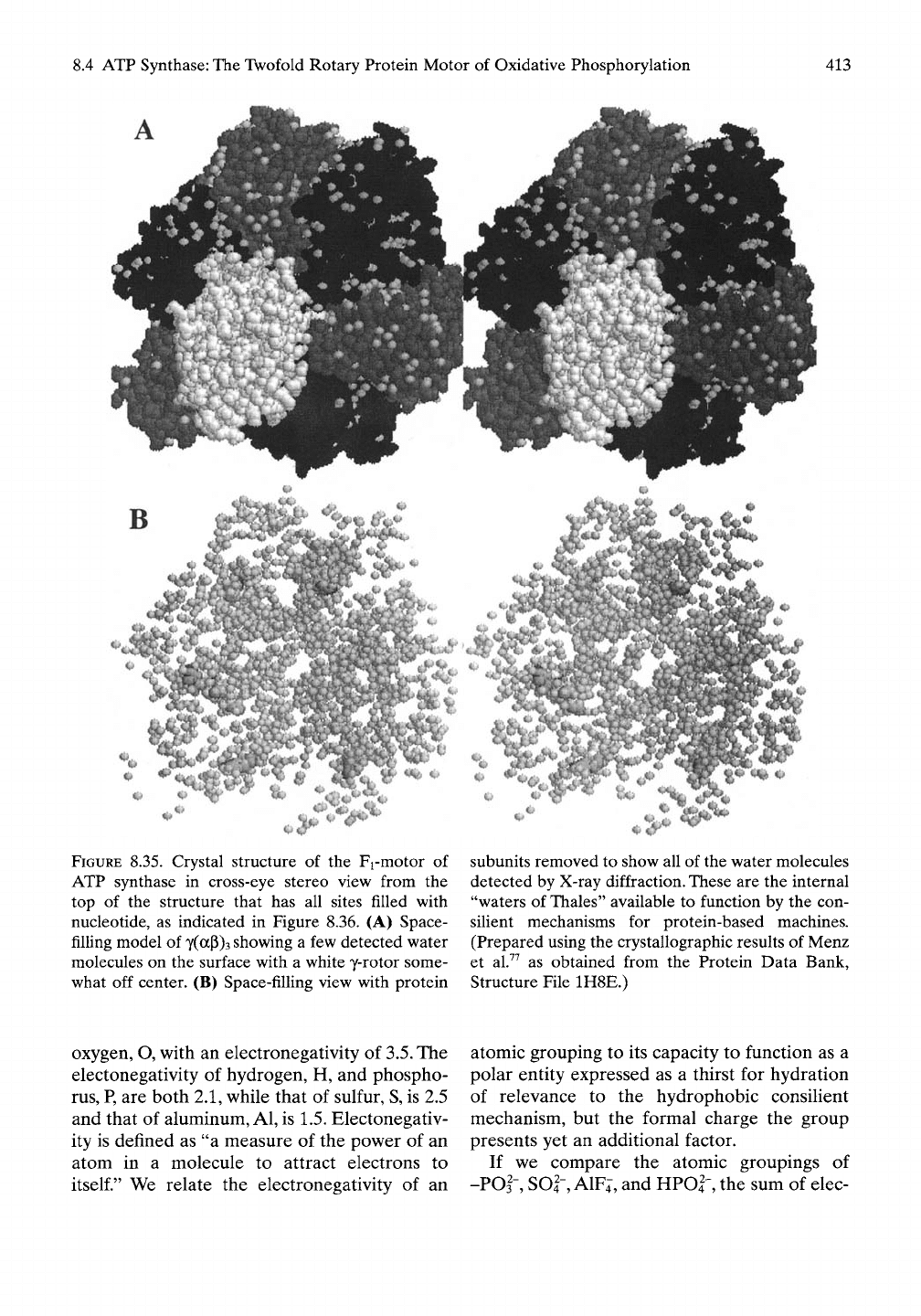
8.4.4.6 Stereo Top View of Assembled Fj-
ATPase in Which All Sites Are Occupied
by Nucleotides
As appreciated by Boyer,^^ a more recent struc-
ture of the Fi-ATPase due to Menz et al.^^
makes major strides in giving further insight
into the function of ATP synthase. The top
perspective of this structure is shown in stereo
view in Figure 8.35 using space-filling repre-
sentation. Included are the water molecules
detectable by X-ray diffraction.
The few water molecule observed on the
surface in Figure
8.35A
tend to occur in
crevices where the likelihood is greater that
they would be sufficiently fixed in position to
be detectable by the long time periods required
for determination of structure by X-ray
dif-
fraction. On removal of the protein subunits in
Figure 8.35B, it becomes possible to see the
water molecules internal to the structure as well
as the ligands. Almost surprising is the very
large number of detectable water molecules.
This water must contain a clue to function and,
as such, we call them the waters of Thales. As
water is essential for the hydrophobic consilient
mechanism to be relevant, this observation
bodes well for such a mechanism. However,
this structure with its particular set of ligands
becomes especially informative when viewed in
the Ught of the AGap element of the hydro-
phobic consihent mechanism, as considered
below.
8.4.4.7 Stereo View of y-Rotor Surrounded
with Nucleotides in All Sites (3 ADP in the
a-Subunits, Plus One ADP-SO4, Plus Two
ADP'AIF4 Bound in the pSubunit Sites)
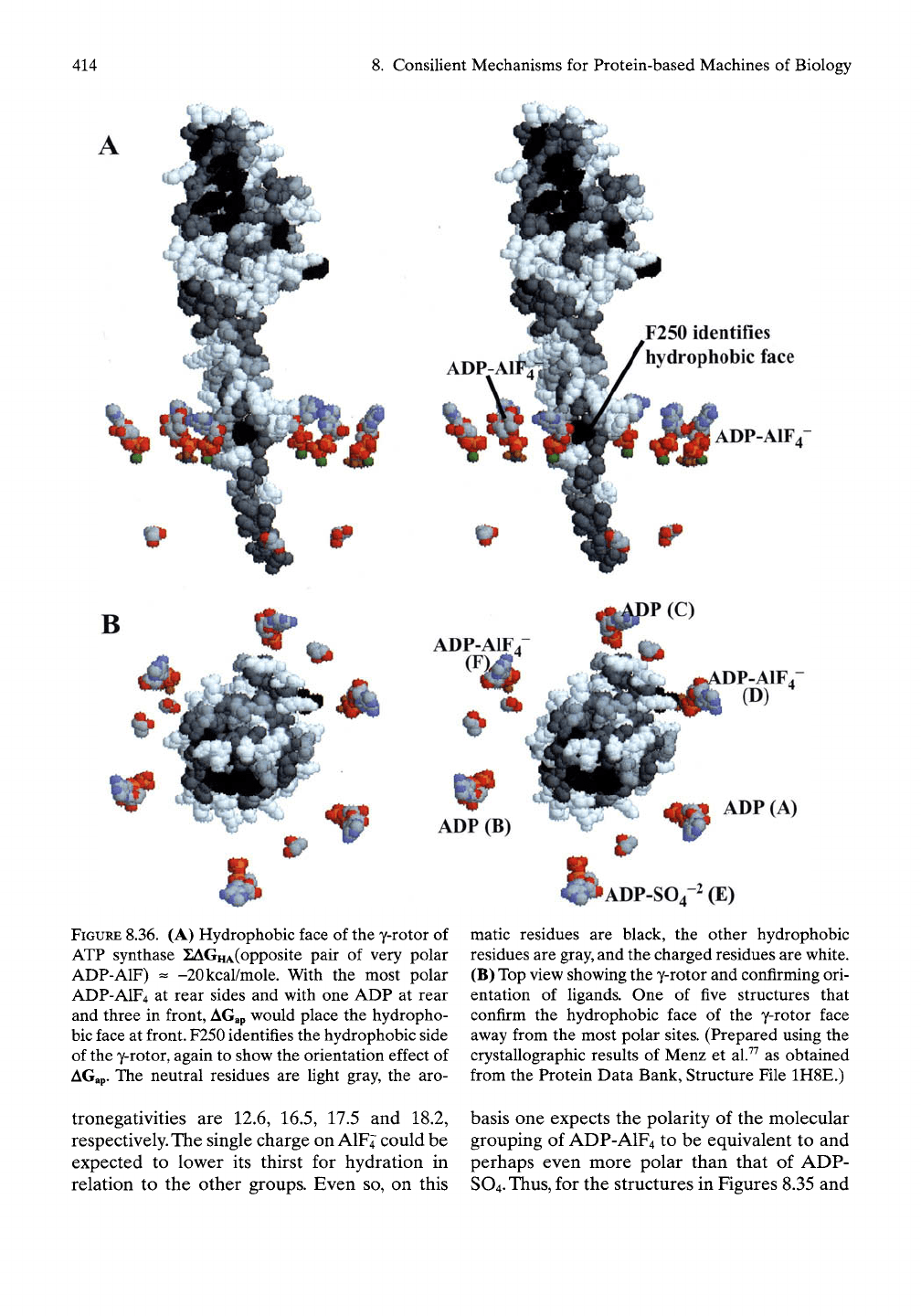
8.4.4.7.1
Side View of y-Rotor Surrounded
by Ligands
The cross-eye stereo perspective of the y-rotor
in side view and in space-filling representation
is shown in Figure
8.36A
surrounded by six
nucleotides with two inhibitory nucleotides,
ADP-AIF4, labeled. Unlabeled are four smaller
glycerol molecules around the y-rotor. The
labeled F250 residue identifies the hydrophobic
side of the rotor, as may be seen by comparison
with Figure 8.31.
8.4.4.7.2
Top View of y-Rotor Surrounded
by Ligands
Figure
8.36B
gives the top view of the y-rotor
with surrounding ligands, labeled with respect
to both ligand structure and crystallographi-
cally defined chain of residence. Three ADP
ligands are in the a-subunits, chains A, B, and
C. The p-catalytic subunits are filled with two
nucleotides that inhibit catalysis, ADP-AlFj, in
chains D and F, and with ADP-SO4" in the third
catalytic subunit in chain E. Based on the argu-
ment of an apolar-polar repulsion between
ligands in the nucleotide-binding sites and the
y-rotor, the observation of the hydrophobic face
opposite the ADP-SOl" occupied site would
suggest that this site is less polar than the effect
of the two opposing ADP-AlFj-occupied sites.
The Pauling electronegativity scale provides
helpful insight with which to evaluate the rela-
tive polarities of these ligands.
8.4.4.7.3
Evaluation of Relative Polarity
of Ligands Using the Pauling
Electronegativity Scale
The Pauling electronegativity scale^^'^^ places
fluorine,
F,
with an electronegativity of 4.0 as
the most electronegative atom, followed by
8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
413
FIGURE
8.35. Crystal structure of the Fi-motor of
ATP synthase in cross-eye stereo view from the
top of the structure that has all sites filled with
nucleotide, as indicated in Figure 8.36. (A) Space-
filHng model of
y(ocp)3
showing a few detected water
molecules on the surface with a white y-rotor some-
what off center. (B) Space-filling view with protein
subunits removed to show all of the water molecules
detected by X-ray diffraction. These are the internal
"waters of
Thales"
available to function by the con-
silient mechanisms for protein-based machines.
(Prepared using the crystallographic results of Menz
et al.^^ as obtained from the Protein Data Bank,
Structure File 1H8E.)
oxygen, O, with an electronegativity of
3.5.
The
electonegativity of hydrogen, H, and phospho-
rus,
P, are both
2.1,
while that of sulfur, S, is 2.5
and that of aluminum, Al, is 1.5. Electonegativ-
ity is defined as "a measure of the power of an
atom in a molecule to attract electrons to
itself."
We relate the electronegativity of an
atomic grouping to its capacity to function as a
polar entity expressed as a thirst for hydration
of relevance to the hydrophobic consilient
mechanism, but the formal charge the group
presents yet an additional factor.
If we compare the atomic groupings of
-PO|-,
SOf, AIF4, and
HPOf,
the sum of elec-
414
8. Consilient Mechanisms for Protein-based Machines of Biology
F250 identifies
hydrophobic face
H
ADP-AIF4-
ADP-AIF4
DP-A1F4-
(D)
If _^
i^l i(F ADP-SO4-2 (E)
ADP (A)
FIGURE 8.36. (A) Hydrophobic face of the y-rotor of
ATP synthase 21AGHA(opposite pair of very polar
ADP-AIF) ^ -20kcal/mole. With the most polar
ADP-AIF4 at rear sides and with one ADP at rear
and three in front,
AGap
would place the hydropho-
bic face at front. F250 identifies the hydrophobic side
of the y-rotor, again to show the orientation effect of
AGap. The neutral residues are Ught gray, the aro-
tronegativities are 12.6, 16.5, 17.5 and 18.2,
respectively. The single charge on AIF4 could be
expected to low^er its thirst for hydration in
relation to the other groups. Even so, on this
matic residues are black, the other hydrophobic
residues are
gray,
and the charged residues are white.
(B) Top view showing the y-rotor and confirming ori-
entation of ligands. One of five structures that
confirm the hydrophobic face of the y-rotor face
away from the most polar sites. (Prepared using the
crystallographic results of Menz et al.^^ as obtained
from the Protein Data Bank, Structure File 1H8E.)
basis one expects the polarity of the molecular
grouping of ADP-AIF4 to be equivalent to and
perhaps even more polar than that of ADP-
SO4.
Thus, for the structures in Figures 8.35 and
