Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
415
8.36, on the basis of
AGap,
one would expect the
y-rotor to orient with its most hydrophobic face
away from the two catalytic sites containing
ADP-AIF4 and therefore be directed toward
the pE catalytic site containing ADP-SO4. This
is the orientation of the y-rotor in the structure
in Figure 8.36. Before proceeding to the most
significant aspect of the structures in Figures
8.35 and 8.36, however, additional structures
with different p-subunit occupancies can be
examined to address the consistency of the
expectation that the orientation of hydropho-
bic face of the y-rotor would be toward the least
polar occupancy site.
8.4.4.8 Consistency of Least Apolar-Polar
Repulsion Positioning the Hydrophobic
Face with Three Additional and Different
Binding Site Occupancies
In addition to the structures in Figures 8.30^"*
and 8.36,^^ three more crystal structures of the
Fi-ATPase have been determined by the
Walker group. The first of the three involve
the occupancy states of ATP(A), ATP(B),
ATP(C), ADP(D), P04(E), and ATP(F) for the
protein structure listed in the Protein Data
Bank as Structure File 1H8H.^^ The second of
the three involve the occupancy states of
ANP(A), ANP(B), ANP(C), ADP(D), P04(E),
and ANP(F) where PNP may also be repre-
sented as AMPPNP, that is, a nitrogen replaces
the bridge oxygen of ATP between the p-
and y-phosphates; this structure, shown in
Figure 8.37, is listed in the Protein Data
Bank as Structure File lElQ.^^ The third of
the three additional structures involves occu-
pancy states of ANP(A), ANP(B), ANP(C),
ANP(D), empty(E), and ANP(F) and is listed
in the Protein Data Bank as Structure File
lOHH.^^ In each case the hydrophobic side of
the y-rotor faces the occupancy state of
chain E.
Certainly, the third structure holds the
hydrophobic side of the y-rotor most tena-
ciously at the p-empty catalytic site, even more
so than the structures in Figures 8.30 through
8.34, because all five additional sites are occu-
pied by the ATP equivalent, namely, ANP. The
other two structures depend on the relative
polarities of ADP(D) and P04(E) in their
respective positions of exposure to the y-rotor.
That the P-P04(E) is least polar from the per-
spective of the y-rotor becomes apparent from
the display of the second structure of the three
in Figure 8.37B, where the P-PO4 of chain E is
positioned so as to be sterically obscured from
the y-rotor. Accordingly, consistency holds for
all five structures of the Fi-ATPase; the most
hydrophobic side of the y-rotor is always found
opposite the least polar occupancy site, where
the apolar-polar repulsive free energy of
hydration, AGap, is the least.
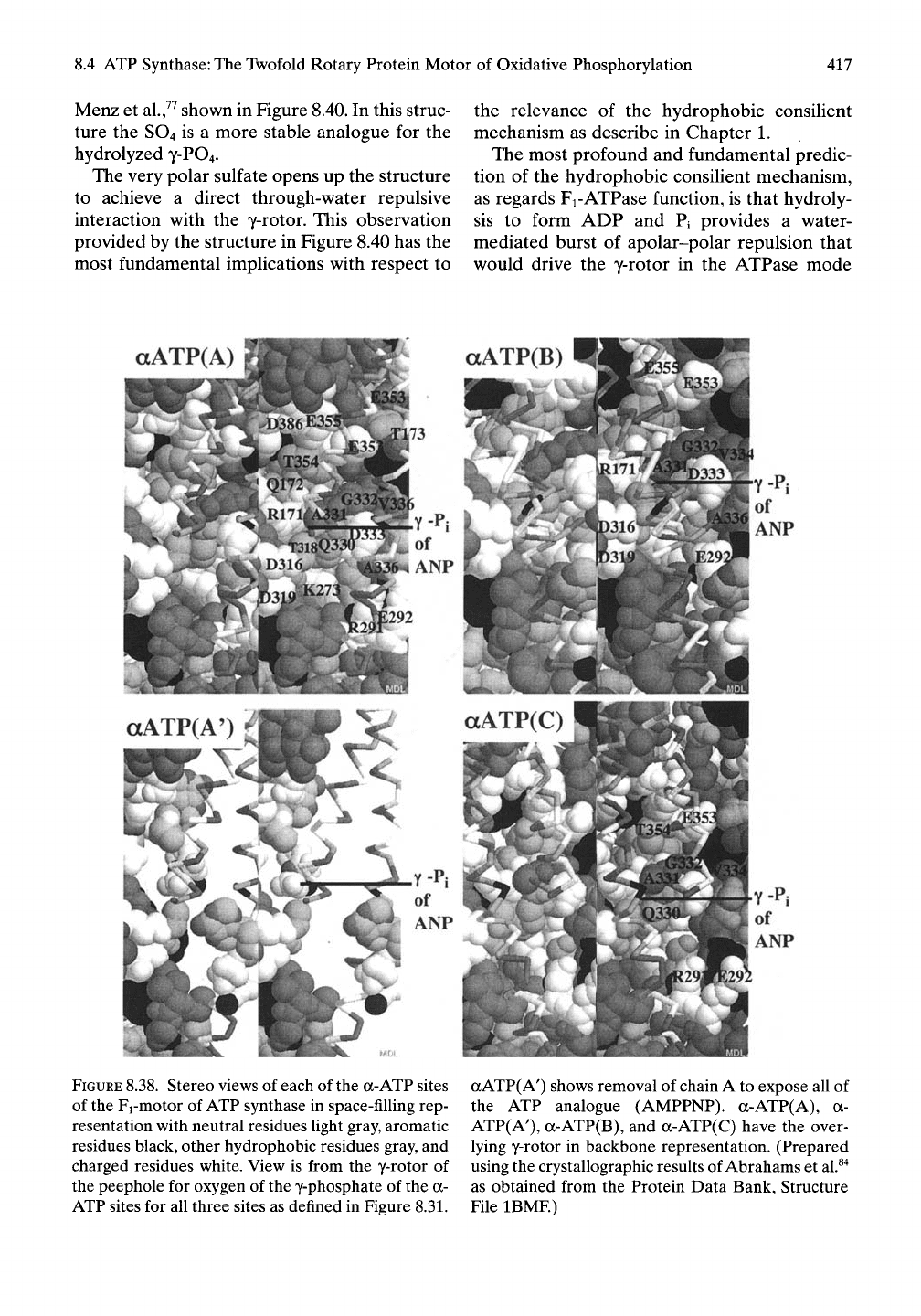
8A.4.9 Role of ATP in the a-ATP
Subunits: Triangulation of
Repulsive Forces
When the p-ATP and a-ATP sites are diamet-
rically opposed, as in Figure 8.34C, the appear-
ance read in terms of AGap is that the P-ATP
site expresses slightly more apolar-polar repul-
sion than does the a-ATP site. From the point
of view of the hydrophobic consilient mecha-
nism, this suggests that the exposure of phos-
phates of the a-ATP sites through water to the
y-rotor is greater for the p-ATP sites. Figure
8.38 shows the exposure of the y-rotor to a-
ATP phosphates of chains A, B, and C of the
structures utilized in Figures 8.30 through 8.34.
In each of the a-ATP only a single oxygen of
the y-phosphate can be seen through a small
peephole. While exposure of the a-ATP phos-
phates to the y-rotor could be larger in the
dynamic functional state, this situation is com-
pared with a slightly greater exposure of the
phosphates of the P-ATP site immediately
below and to an analogue of the massively
greater exposure on hydrolysis to form ADP
plus Pi.
By the hydrophobic consilient mechanism,
the a-ATP sites provide a critical role of estab-
lishing a triangulation of repulsive forces that
serves to limit the hydrophobic associations
of the y-rotor. This triangulation of repulsive
forces prevents the occurrence of a frictional
drag on rotor rotation that would seriously
limit motor efficiency.
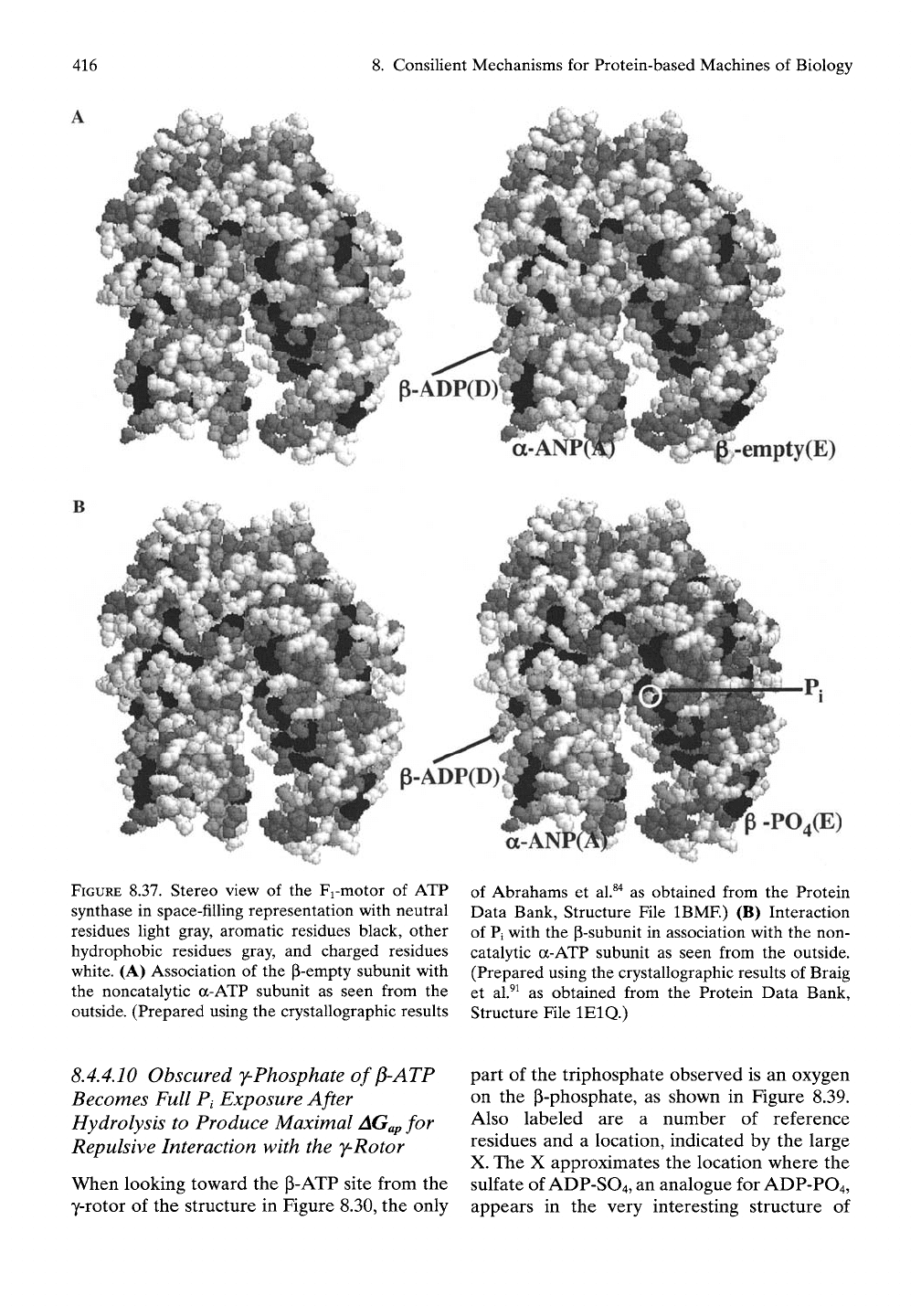
. Consilient Mechanisms
for
Protein-based Machines
of
Biology
empty(E)
FIGURE
8.37.
Stereo view
of the
Fi-motor
of
ATP
synthase
in
space-filling representation with neutral
residues light gray, aromatic residues black, other
hydrophobic residues gray,
and
charged residues
white.
(A)
Association
of the
p-empty subunit with
the noncatalytic a-ATP subunit
as
seen from
the
outside. (Prepared using
the
crystallographic results
PO,(E)
of Abrahams
et
al.^"^
as
obtained from
the
Protein
Data Bank, Structure File IBMF)
(B)
Interaction
of Pi with
the
p-subunit
in
association with
the non-
catalytic a-ATP subunit
as
seen from
the
outside.
(Prepared using the crystallographic results
of
Braig
et
al.^^ as
obtained from
the
Protein Data Bank,
Structure File lElQ.)
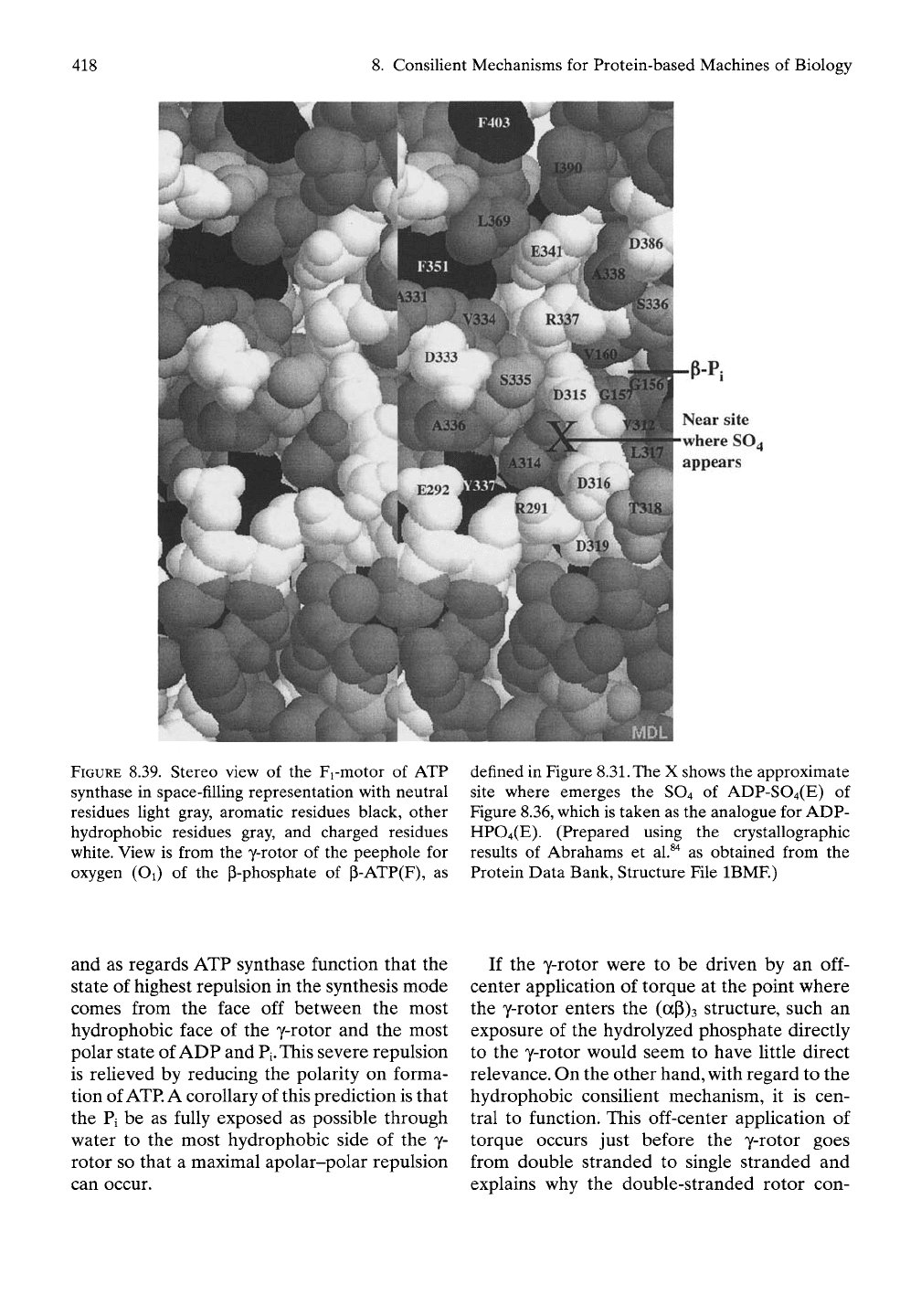
8.4.4.10 Obscured y-Phosphate of P-ATP
Becomes Full Pi Exposure After
Hydrolysis to Produce Maximal
AGap
for
Repulsive Interaction with the y-Rotor
When looking toward the p-ATP site from the
y-rotor of the structure in Figure 8.30, the only
part
of the
triphosphate observed
is an
oxygen
on
the
P-phosphate,
as
shown
in
Figure
8.39.
Also labeled
are a
number
of
reference
residues
and a
location, indicated
by the
large
X. The
X
approximates
the
location where
the
sulfate of ADP-SO4,
an
analogue
for
ADP-PO4,
appears
in the
very interesting structure
of
8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
417
Menz et al./^ shown in Figure 8.40. In this struc-
ture the SO4 is a more stable analogue for the
hydrolyzed Y-PO4.
The very polar sulfate opens up the structure
to achieve a direct through-water repulsive
interaction with the y-rotor. This observation
provided by the structure in Figure 8.40 has the
most fundamental implications with respect to
the relevance of the hydrophobic consilient
mechanism as describe in Chapter 1.
The most profound and fundamental predic-
tion of the hydrophobic consihent mechanism,
as regards Fi-ATPase function, is that hydroly-
sis to form ADP and Pi provides a water-
mediated burst of apolar-polar repulsion that
would drive the y-rotor in the ATPase mode
aATP(A)
FIGURE
8.38.
Stereo views of each of
the
a-ATP sites
of the Fi-motor of ATP synthase in space-filling rep-
resentation with neutral residues light gray, aromatic
residues black, other hydrophobic residues gray, and
charged residues white. View is from the y-rotor of
the peephole for oxygen of the y-phosphate of the a-
ATP sites for all three sites as defined in Figure 8.31.
aATP( A') shows removal of chain A to expose all of
the ATP analogue (AMPPNP). a-ATP(A), a-
ATP(AO, a-ATP(B), and a-ATP(C) have the over-
lying y-rotor in backbone representation. (Prepared
using the crystallographic results of Abrahams et
al.^"^
as obtained from the Protein Data Bank, Structure
File IBMF)
418
8. Consilient Mechanisms for Protein-based Machines of Biology
P-Pi
Near site
where SO4
appears
FIGURE 8.39. Stereo view of the Fi-motor of ATP
synthase in space-filling representation with neutral
residues Ught gray, aromatic residues black, other
hydrophobic residues gray, and charged residues
white. View is from the y-rotor of the peephole for
oxygen (Oi) of the p-phosphate of P-ATP(F), as
defined in Figure
8.31.
The X shows the approximate
site where emerges the SO4 of ADP-S04(E) of
Figure 8.36, which is taken as the analogue for ADP-
HP04(E). (Prepared using the crystallographic
results of Abrahams et al.^"^ as obtained from the
Protein Data Bank, Structure File IBMF.)
and as regards ATP synthase function that the
state of highest repulsion in the synthesis mode
comes from the face off between the most
hydrophobic face of the y-rotor and the most
polar state of ADP and
Pi.
This severe repulsion
is relieved by reducing the polarity on forma-
tion of
ATP.
A corollary of this prediction is that
the Pi be as fully exposed as possible through
water to the most hydrophobic side of the y-
rotor so that a maximal apolar-polar repulsion
can occur.
If the y-rotor were to be driven by an off-
center application of torque at the point where
the y-rotor enters the (aP)3 structure, such an
exposure of the hydrolyzed phosphate directly
to the y-rotor would seem to have little direct
relevance. On the other hand, with regard to the
hydrophobic consilient mechanism, it is cen-
tral to function. This off-center application of
torque occurs just before the y-rotor goes
from double stranded to single stranded and
explains why the double-stranded rotor con-

8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
419
tinues as it does beyond entry into the (aP)3
motor housing.
8.4.4.11 Crystal Structure Evidence for a
Significant AGap Between the ADP-
S04-0ccupied pE-subunit and the
y-rotor
Above,
the predicted hydrophobically asym-
metric rotor was found. Furthermore, the
hydrophobically asymmetric rotor associated
hydrophobically with the (ap)3 housing of the
Fi-motor and was appropriately responsive to
the occupancy states of the nucleotide-binding
sites.
These findings all support the role of the
Gibbs free energy of hydrophobic association,
AGHA,
and of its operative apolar-polar repul-
sive free energy of hydration,
AGap,
in the func-
tion of the Fi-motor. Now by comparison of two
crystal structures, one with the pE-catalytic site
empty and the second with the PE-subunit con-
E399/A
FIGURE
8.40. Stereo view of the Fi-motor of ATP
synthase in space-filling representation with neutral
residues light gray, aromatic residues black, other
hydrophobic residues gray, and charged residues
white. View is of the more stable SO4 in place of the
more polar PO4 (the hydrolyzed y-P of ATP) that
occurs in chain E and is shown in Figure 8.41 with
the overlying y-rotor in gray ribbon representation.
The polar SO4 (encircled) opens up the protein struc-
ture of chain E (when compared with the p-ATP(F)
site in Figure 8.39) to expose the SO4 to the
hydrophobic side of the y-rotor and thereby to
express a large AGap repulsive force through the
intervening waters of Thales. (Prepared using the
crystallographic results of Menz et aP as obtained
from the Protein Data Bank, Structure File 1H8E.)

420
8. Consilient Mechanisms for Protein-based Machines of Biology
taining ADP-SO4, a
AGap
repulsion between the
ADP-S04-containing (JE-subunit and the y-
rotor is found.
To quote from the legend of Figure 5 of Menz
et al./^ "Note that interacting residues in the
PE-
subunit and the y-subunit move in opposite
directions." This movement in opposite direc-
tions of the very polar ADP-S04-containing
PE-
subunit and the hydrophobic side of the y-rotor,
in our view, is a direct result of the AGap repul-
sion between the apolar and polar subunits.The
separation of housing and rotor identifies a
repulsion that introduces an elastic displace-
ment and twist in the y-rotor. Because of the rel-
ative stiffness of the rotor and housing, this
elastic deformation (from otherwise preferred
torsion angles) would have a large elastic
modulus. Accordingly, the reported mean dis-
placement of 2.9 A and twist of up to 20 would
reflect the application of a substantial torque to
the y-rotor. This occurrence again unites the
hydrophobic and elastic consilient mechanisms
in a way that would result in high efficiencies of
energy conversion. The question now becomes
whether or not this applied torque, due to the
AGap
repulsion between the exposed sulfate and
the hydrophobic side of the y-rotor, can predict
the direction of rotation of the Fi-ATPase.
8.4.4.12 Predicted Direction of Rotation
for Fj-ATPase
AGap[sulfate
<->
hydrophobic rotor] provides a
water-mediated off-center "push" on the y-
rotor like an off-center push on a camshaft. The
direction of the repulsion between exposed
sulfate and the hydrophobic side of the y-rotor,
written as AGapfsulfate
<->
hydrophobic rotor],
can be deduced from the structural relation-
ships of Figure
8.41.
The apolar-polar repulsion
between sulfate and y-rotor appUes toward the
lower part of the double-stranded section of
the y-rotor above the amino-terminus of the
y-subunit. As evidenced by the structural
relationships showing the overlay of the y-rotor
on the ADP-S04-containing pE-subunit, the
AGap[sulfate <-> hydrophobic rotor] repul-
sive interaction would provide an impulse to
rotate the y-rotor in a counterclockwise
direction.
8.4.4.13 Demonstrated Direction of
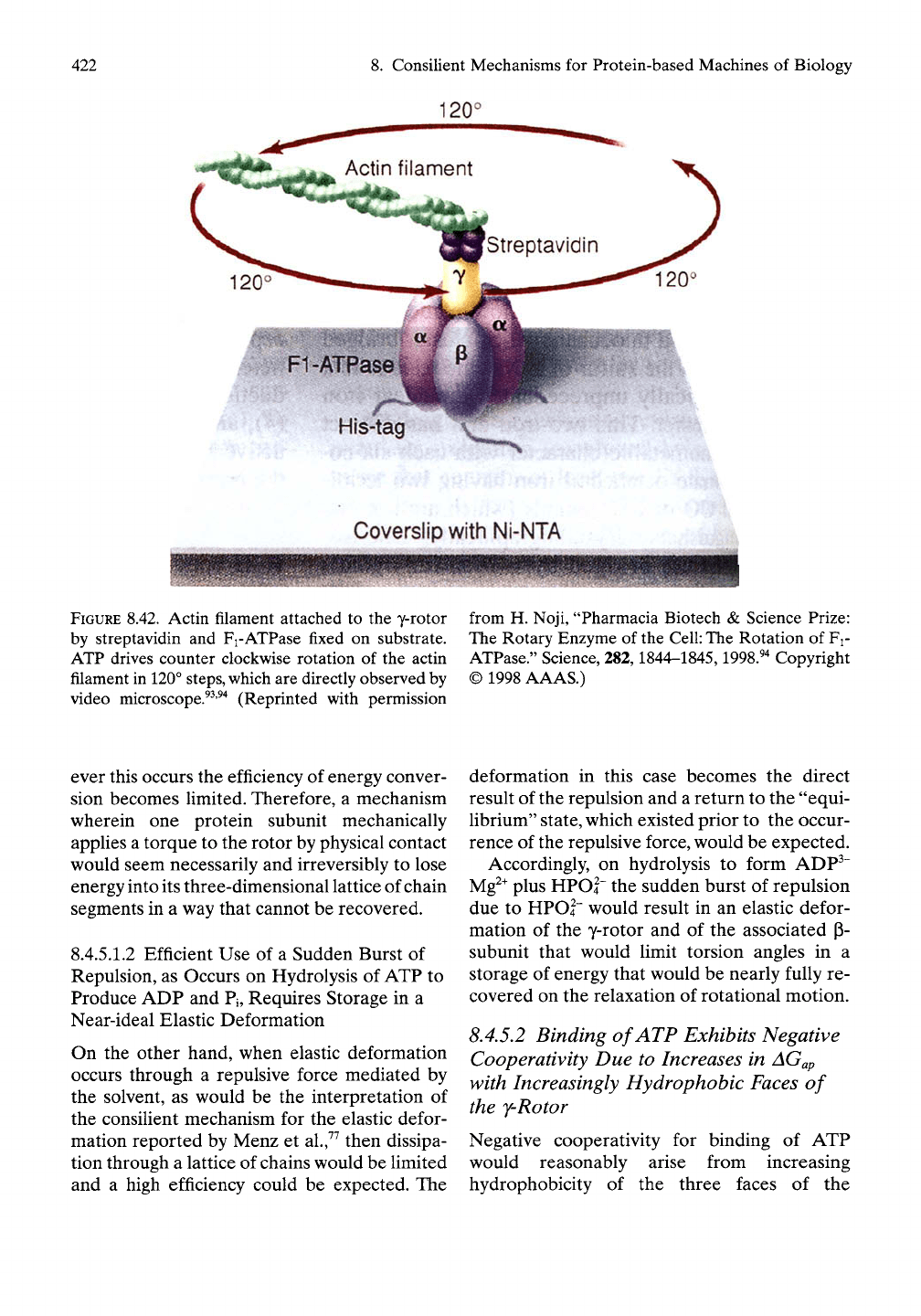
Rotation of the y-rotor
As seen from the landmark study of Noji et
al.^^'^"^
and represented in Figure 8.42, the direc-
tion of rotation for the Fi-ATPase is indeed in
the counterclockwise direction. Thus, the foun-
dation for the hydrophobic and elastic consilient
mechanisms in the function of ATP synthase and
Fj-ATPase becomes increasingly compelling.
8.4.5 Consideration of the Efficiency
and Cooperativities Exhibited by
Fi-ATPase
By analysis of crystal structures, we have just
demonstrated the success of a significant num-
ber of predictions fundamental to the bio-
physics of the hydrophobic and elastic con-
silient mechanisms. Now we address the unique
and fundamental properties of efficiency of
energy conversion and of negative cooperativ-
ity of ATP binding and yet of positive cooper-
ativity of the effect of ATP binding on catalytic
rate.
Can an understanding of these challenging
phenomena exhibited by Fi-ATPase also fall to
the comprehension flowing from the hydropho-
bic consilient mechanism?
8.4.5.1 High Efficiency for Performance
of Mechanical Work
8.4.5.1.1
Near 100% Efficiency of the
Fi-ATPase
With the capacity to attach an actin filament to
the y-rotor and to observe microscopically the
rotation of the actin filament driven by the
Fi-ATPase,^^'^"^ estimates of work performed on
rotation of the actin filament could be esti-
mated as could the energy consumed in the
performance of that work in terms of
the hydrolysis of ATP to form ADP and Pj.
The resulting estimate was remarkably close
to 100%.^^ Such a result stands as a severe
challenge to any proposed mechanism.
Insight comes from considering elastic defor-
mation as a machine for storing energy. By
means of atomic force microscopy in the single-
chain force extension mode, a single chain of
(GVGVP)502 could be observed to exhibit an

8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
421
F(F)
FIGURE
8.41. Inside stereo view of chains A, E, and
B and their ligands of the F,-motor of ATP synthase
in space-filling representation with neutral residues
light gray, aromatic residues black, other hydropho-
bic residues gray, and charged residues white and
with the ligands for chains D, C, and F in the fore-
ground. With this perspective, the overlying y-rotor
in gray ribbon representation properly overlays the
catalytic site of the pE-subunit containing the
exposed sulfate. The large repulsive AGap force due
to SO4 apphes a torque just above the amino-
terminal sequence of the y-rotor that would give a
counterclockwise rotation when functioning as the
ATPase. This has analogy to repulsion between like
poles of two magnets that made such an effective
analogue model for the Fi-motor, as presented by
Kinosita et al.^^ The experimentally determined
direction of rotation is counterclockwise, as shown in
Figure 8.42. (Adapted from Urry^^^)
ideal elasticity in which all of the energy
expended in deformation was recovered on
relaxation (see Figure 5A of Urry and Parker^).
On the other hand, when there are adherent
chains that do not sustain the deformation as
occurs with (GVGIP)52o, there occurs a marked
hysteresis; the energy of deformation is not all
recovered on relaxation, as shown in Figure 5B
of Urry and Parker.^ Accordingly, chains that
take up energy during deformation but do not
directly bear and store the force of deformation
cannot return the energy on relaxation. When-
422
8. Consilient Mechanisms for Protein-based Machines of Biology
120°
HIs-tag
Coverslip with Ni-NTA
FIGURE 8.42. Actin filament attached to the y-rotor
by streptavidin and Fi-ATPase fixed on substrate.
ATP drives counter clockwise rotation of the actin
filament in 120°
steps,
which are directly observed by
video microscope.^^'^"* (Reprinted with permission
from H. Noji, "Pharmacia Biotech & Science Prize:
The Rotary Enzyme of the
Cell:
The Rotation of Fi-
ATPase." Science, 282,1844-1845,1998.^^ Copyright
© 1998 AAAS.)
ever this occurs the efficiency of energy conver-
sion becomes limited. Therefore, a mechanism
wherein one protein subunit mechanically
applies a torque to the rotor by physical contact
would seem necessarily and irreversibly to lose
energy into its three-dimensional lattice of chain
segments in a way that cannot be recovered.
8.4.5.1.2
Efficient Use of a Sudden Burst of
Repulsion, as Occurs on Hydrolysis of ATP to
Produce ADP and Pi, Requires Storage in a
Near-ideal Elastic Deformation
On the other hand, when elastic deformation
occurs through a repulsive force mediated by
the solvent, as would be the interpretation of
the consilient mechanism for the elastic defor-
mation reported by Menz et al.,^^ then dissipa-
tion through a lattice of chains would be limited
and a high efficiency could be expected. The
deformation in this case becomes the direct
result of the repulsion and a return to the "equi-
librium" state, which existed prior to the occur-
rence of the repulsive force, would be expected.
Accordingly, on hydrolysis to form ADP^"
Mg^^ plus
HPOj"
the sudden burst of repulsion
due to HPO4" would result in an elastic defor-
mation of the y-rotor and of the associated P-
subunit that would limit torsion angles in a
storage of energy that would be nearly fully re-
covered on the relaxation of rotational motion.
8.4.5.2 Binding of ATP Exhibits Negative
Cooperativity Due to Increases in AGap
with Increasingly Hydrophobic Faces of
the y-Rotor
Negative cooperativity for binding of ATP
would reasonably arise from increasing
hydrophobicity of the three faces of the

8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
423
asymmetrically hydrophobic rotor, that is,
an increase in the apolar-polar repulsive free
energy of hydration,
AGap,
with each addition of
ATP.
The first ATP would be expected to bind
adjacent to the most polar face (AGHA ~ +9kcal/
mole),
the second to the essentially neutral face
(AGHA
~ Okcal/mole), and the third opposite
the most hydrophobic face of the y-rotor (AGHA
« -20kcal/mole). The presence of the first ATP
in the most favorable site would leave a less
favorable site for the second ATP binding.
Similarly the presence of the two ATP mole-
cules bound to the more favorable sites would
leave the site with the most repulsion. Thus,
given the structure of the y-rotor with its
hydrophobic asymmetry, negative cooperativity
is the necessary result. While a small contribu-
tion to the negative cooperativity could arise
from direct charge-charge repulsion between
multivalent ATP anions, acting through the
aqueous compartment, this is expected to be
small compared with the increasing apolar-
polar repulsive free energy of hydration,
AGap, that each subsequent ATP molecule
would experience as the result of being con-
fronted with a more hydrophobic side of the
y-rotor.
8.4.5.3 ATP Hydrolytic Rate Exhibits
Positive Cooperativity as Each Additional
Bound ATP Facilitates Hydrophobic
Dissociation of y-Rotor from the Inner
Wall
of
(ap)3
On the basis of the hydrophobic consilient
mechanism, the three ATP molecules in the
noncatalytic a-subunits are expected to apply a
triangular array of repulsive thrusts that would
limit the viscous drag resulting from hydro-
phobic association between the y-rotor and
the (aP)3 housing of the Fi-motor. Even so,
when there is an empty P-subunit, prominent
hydrophobic association
occurs.
Thus,
as each p-
catalytic site is filled, the hydrophobic associa-
tion would decrease until the binding of ATP to
the third P-catalytic site would dramatically
lower the viscous drag arising from hydropho-
bic association of the (ocp)3 housing of the Fi-
motor with the y-rotor.
8.4.6 Review of Correlations Between
the Hydrophobic Elastic Consihent
Mechanism and the Properties of ATP
Synthase/Fi-ATPase
8.4.6.1 Nine Major Points
of Correlation
Below we list nine major points of correlation
of the ATP synthase/Fi-ATPase. It is not a com-
plete Hsting of the correlations, as there are
many more details that should be included in a
more exhausting consideration. Nonetheless, in
our view the nine points are compelling.
1.
As required for the hydrophobic con-
silient mechanism to be operative for an ATP
synthase/Fi-ATPase protein-based machine,
the three faces of the y-rotor exhibit very
different hydrophobicities, that is, different
AGHA
values, namely, approximately -20,0, and
-i-9kcal/mole.
2.
Furthermore, the most hydrophobic face
of the y-rotor functions as such; it associates
hydrophobically with the P-empty subunit,
which becomes the most hydrophobic p-
subunit due to the absence of polar (charged)
nucleotides.
3.
The Fi-ATPase exhibits extensive "waters
of Thales"; as required for the existence of an
apolar-polar repulsive free energy of hydra-
tion, water-filled clefts exist between the y-rotor
and polar nucleotide filled sites.
4.
Negative cooperativity for binding of ATP
occurs because each ATP addition is con-
fronted through the "waters of Thales" with a
progressively more hydrophobic face of the
rotor; the consequence of increases in the
apolar-polar repulsive free energy of hydra-
tion, AGap, constitute increasing barriers to the
approach of polar ATP molecules and result in
stepwise decreases in binding affinity.
5.
The hydrophobic consihent mechanism
gives a functional role to the a-ATP noncat-
alytic sites; these sites provide a fixed triangu-
lation of repulsive AGap that helps to center the
rotor and to prevent locking up due to the vis-
coelastic drag of hydrophobic association.
6. As each of the p-catalytic sites fills with
ATP,
the rate of hydrolysis would increase due
to the decrease in viscoelastic drag that would

424
8. Consilient Mechanisms for Protein-based Machines of Biology
result in a progressively increased rotational
rate of the y-rotor, that is, there exists a positive
cooperativity in the catalytic rate as all P-
catalytic sites become occupied with ATP.
7.
Because assembly of the Fi-ATPase is
dominated by an inverse temperature transi-
tion of hydrophobic association, the structure
disassembles on lowering the temperature, that
is,
it exhibits cold denaturation.
8. The maximal apolar-polar repulsive free
energy of hydration, AGap, acting through the
"waters of Thales," occurs between the most
hydrophobic side of the y-rotor and the most
polar state of the p-catalytic site, ADP^" Mg^"^
plus hydrolyzed phosphate, HPO4". The struc-
tural relationship between the sulfate analogue
of the hydrolyzed phosphate and the most
hydrophobic side of the y-rotor predicts the
direction of rotation for the Fi-ATPase to be
counterclockwise. Furthermore, this decisive
repulsive thrust indicates why the second chain
of the two-stranded a-helical coiled coil con-
tinues to the level of the y-phosphate of ATP
and is no longer and no shorter than it needs to
be for this function.
9. The remarkably high efficiency of the Fi-
ATPase in converting the chemical energy of
ATP hydrolysis into the performance of
mechanical work occurs because the repulsive
force acts through solvent to produce a
reversible elastic deformation of y-rotor and
the ((xP)3 housing, rather than a mechanical
mechanism where the steps of binding ATP and
its hydrolysis could be expected to cause con-
formational changes in the apical portions of
the p subunit that mechanically drove the
eccentric cam-like strucure of the y-rotor as
it enters the (aP)3 structure as an off-center
double-stranded helical coiled coil.
8,4,6,2 Restatement of the Critical
Synthesis Step of ATP Synthase
In the eighth point of correlation of the
hydrophobic elastic consilient mechanism given
above, the maximal stage of apolar-polar
repulsion occurred when the most polar occu-
pancy state, ADP^- Mg^^ plus
HPOf,
faced off
against the most hydrophobic side of the y-rotor
to provide the thrust for a counterclockwise
rotation. When the y-rotor is driven in a clock-
wise direction by the Fo-motor of ATP synthase,
the most hydrophobic face of the y-rotor is
driven into a face-off with the most polar occu-
pancy
state,
ADP^"
Mg^^
plus
HPOj",
to result in
a maximal apolar-polar repulsion that is
relieved by the formation of ATP. This we
beUeve is the mechanism whereby this pivotal
protein-based machine of living organisms
achieves its essential function of producing
nearly 90% of biology's energy for performing
the many functions required in the process of
sustaining Life.
8.5 The Myosin II Motor of
Muscle Contraction, a
Representative ATPase
8.5.1 Hypothesis: Efficient Production
of Motion by Muscle Contraction
Derives from the Hydrophobic and
Elastic Consilient Mechanisms,
Whereby Dephosphorylation Results
in Hydrophobic Association Coupled
to Near-ideal Elastic Force
Development
8.5.2 Coherence of Phenomena
Between Contraction of Muscle
and Contraction of Model Proteins
Using the Inverse Temperature
Transition
As reviewed in Chapter 7 with a focus on the
issue of insolubiUty, extensive phenomenologi-
cal correlations exist between muscle contrac-
tion and contraction by model proteins capable
of inverse temperature transitions of hydropho-
bic association. As we proceed to examination
of muscle contraction at the molecular level, a
brief restatement of those correlations follows
with observations of rigor at the gross ana-
tomical level and with related physiological
phenomena at the myofibril level. Each of the
phenomena, seen in the elastic-contractile
model proteins as an integral part of the com-
prehensive hydrophobic effect, reappear in the
properties and behavior of muscle. More com-
plete descriptions with references are given in
Chapter 7, sections 7.2.2, and 7.2.3.
