Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
435
N-terminal
domain
B
Y-axis
t
' • X-axis
Z-axis is out
of
the
plane
terminal
domain
Essential
light chain
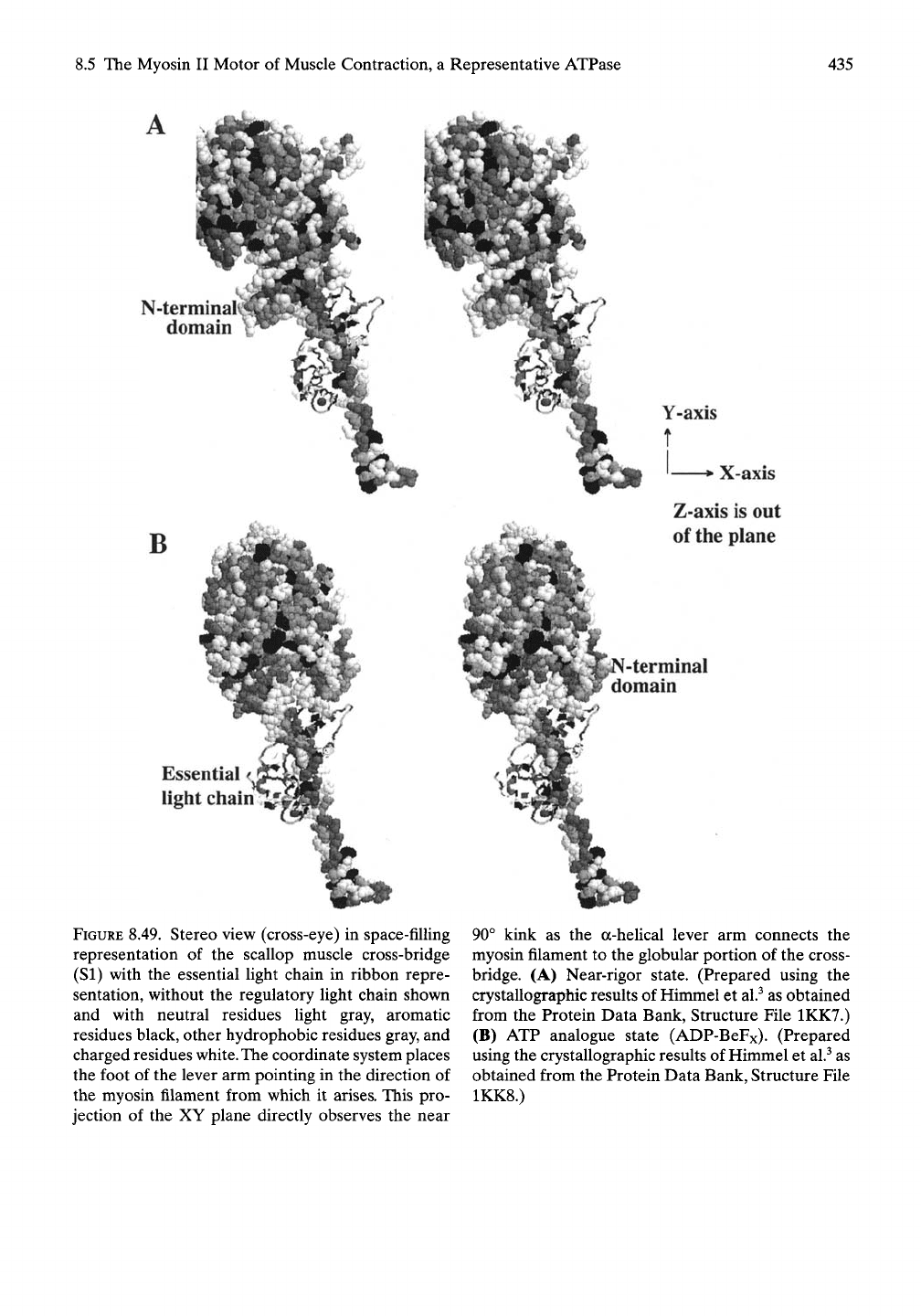
FIGURE 8.49. Stereo view (cross-eye) in space-filling
representation of the scallop muscle cross-bridge
(SI) with the essential light chain in ribbon repre-
sentation, without the regulatory light chain shown
and with neutral residues light gray, aromatic
residues black, other hydrophobic residues gray, and
charged residues white. The coordinate system places
the foot of the lever arm pointing in the direction of
the myosin filament from which it arises. This pro-
jection of the XY plane directly observes the near
90° kink as the a-heUcal lever arm connects the
myosin filament to the globular portion of the cross-
bridge. (A) Near-rigor state. (Prepared using the
crystallographic results of Himmel et al.^ as obtained
from the Protein Data Bank, Structure File 1KK7.)
(B) ATP analogue state (ADP-BeFx). (Prepared
using the crystallographic results of Himmel et al.^ as
obtained from the Protein Data Bank, Structure File
1KK8.)
436
8. Consilient Mechanisms for Protein-based Machines of Biology
B
Z-axis is out
of
the
plane
->
X-axis
AN-terminal
vJ domain
Essentia]
light chai
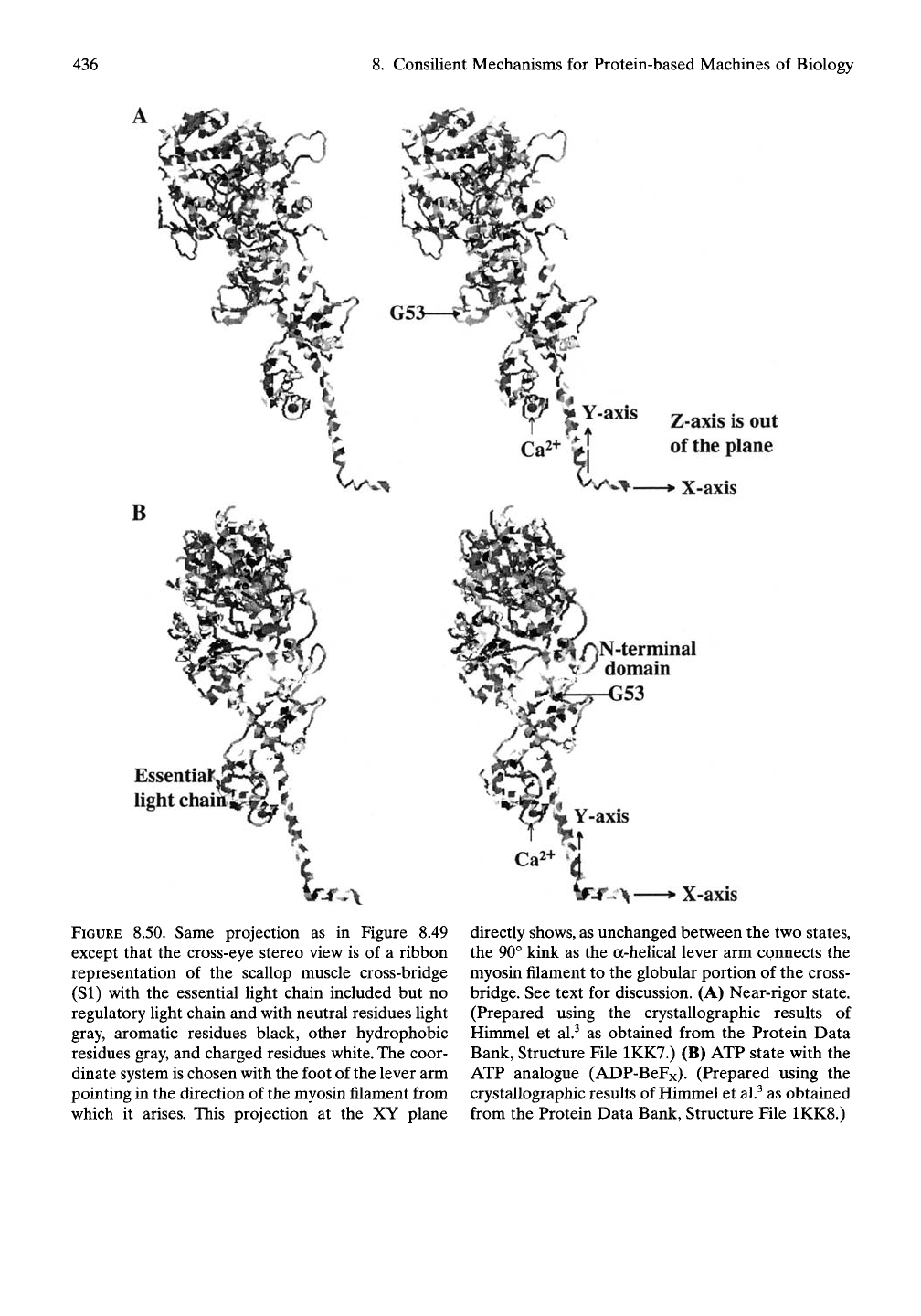
FIGURE 8.50. Same projection as in Figure 8.49
except that the cross-eye stereo view is of a ribbon
representation of the scallop muscle cross-bridge
(SI) with the essential Ught chain included but no
regulatory Ught chain and with neutral residues Ught
gray, aromatic residues black, other hydrophobic
residues gray, and charged residues
white.
The coor-
dinate system is chosen with the foot of the lever arm
pointing in the direction of the myosin filament from
which it arises. This projection at the XY plane
X-axis
directly shows, as unchanged between the two states,
the 90° kink as the a-heUcal lever arm connects the
myosin filament to the globular portion of the cross-
bridge. See text for discussion. (A) Near-rigor state.
(Prepared using the crystallographic results of
Himmel et al.^ as obtained from the Protein Data
Bank, Structure File 1KK7.) (B) ATP state with the
ATP analogue (ADP-BeFx). (Prepared using the
crystallographic results of Himmel et al.^ as obtained
from the Protein Data Bank, Structure File 1KK8.)
8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
437
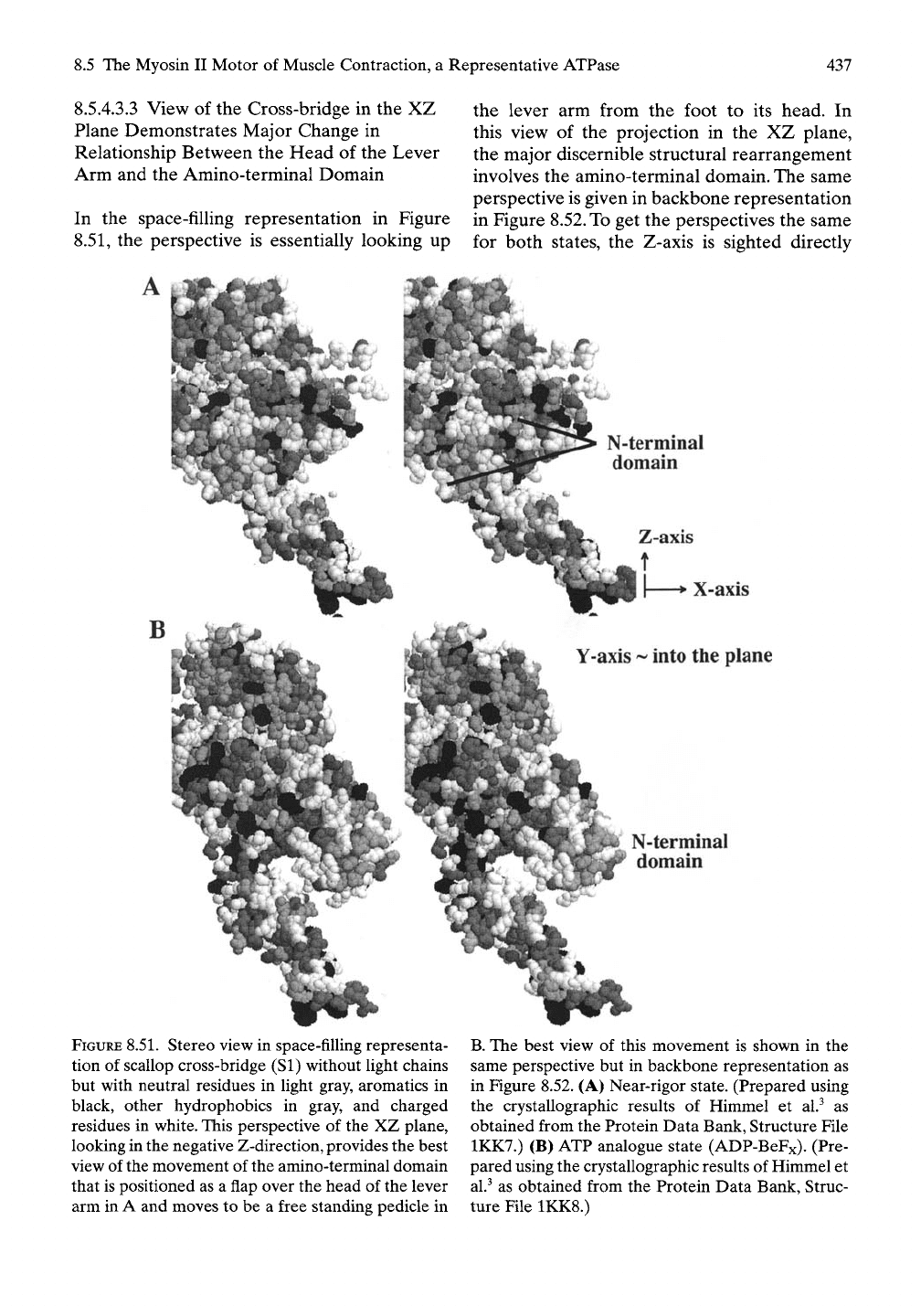
8.5.4.3.3
View of the Cross-bridge in the XZ
Plane Demonstrates Major Change in
Relationship Between the Head of the Lever
Arm and the Amino-terminal Domain
In the space-filling representation in Figure
8.51,
the perspective is essentially looking up
the lever arm from the foot to its head. In
this view of the projection in the XZ plane,
the major discernible structural rearrangement
involves the amino-terminal domain. The same
perspective is given in backbone representation
in Figure 8.52. To get the perspectives the same
for both states, the Z-axis is sighted directly
N-terminal
domain
X-axis
Y-axis'-'
into the plane
N-terminal
domain
FIGURE
8.51.
Stereo view in space-filling representa-
tion of scallop cross-bridge (SI) without light chains
but with neutral residues in light gray, aromatics in
black, other hydrophobics in gray, and charged
residues in white. This perspective of the XZ plane,
looking in the negative Z-direction, provides the best
view of the movement of the amino-terminal domain
that is positioned as a flap over the head of the lever
arm in A and moves to be a free standing pedicle in
B.
The best view of this movement is shown in the
same perspective but in backbone representation as
in Figure 8.52. (A) Near-rigor state. (Prepared using
the crystallographic results of Himmel et al.^ as
obtained from the Protein Data Bank, Structure File
1KK7.) (B) ATP analogue state (ADP-BeFx). (Pre-
pared using the crystallographic results of Himmel et
al.^
as obtained from the Protein Data Bank, Struc-
ture File 1KK8.)
438
8. Consilient Mechanisms for Protein-based Machines of Biology
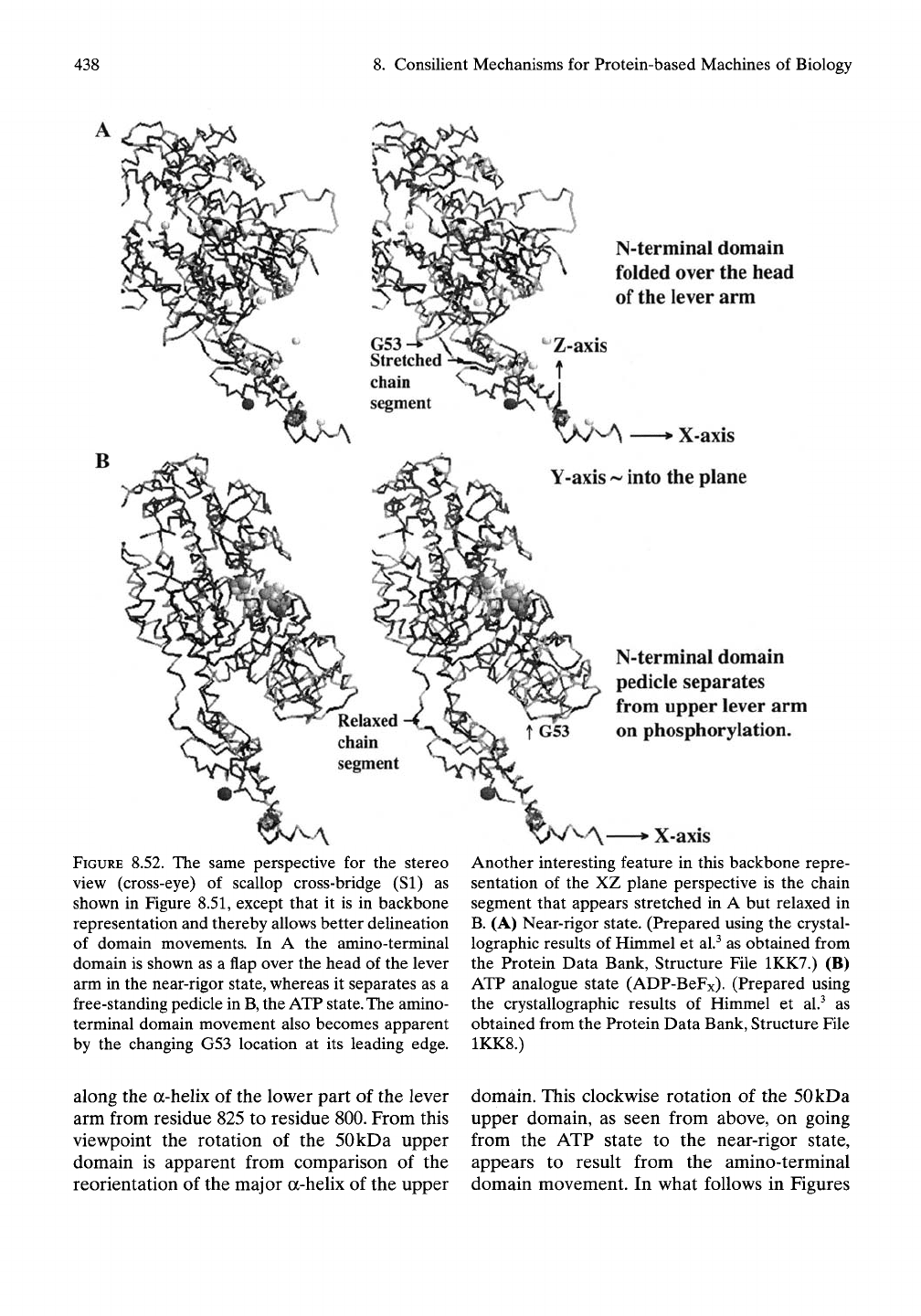
B
N-terminal domain
folded over the head
of
the
lever arm
—> X-axis
Y-axis ^ into the plane
FIGURE 8.52. The same perspective for the stereo
view (cross-eye) of scallop cross-bridge (SI) as
shown in Figure 8.51, except that it is in backbone
representation and thereby allows better delineation
of domain movements. In A the amino-terminal
domain is shown as a flap over the head of the lever
arm in the near-rigor state, whereas it separates as a
free-standing pedicle in
B,
the ATP
state.
The
amino-
terminal domain movement also becomes apparent
by the changing G53 location at its leading edge.
N-terminal domain
pedicle separates
from upper lever arm
on phosphorylation.
X-axis
Another interesting feature in this backbone repre-
sentation of the XZ plane perspective is the chain
segment that appears stretched in A but relaxed in
B.
(A) Near-rigor state. (Prepared using the crystal-
lographic results of Himmel et al.^ as obtained from
the Protein Data Bank, Structure File 1KK7.) (B)
ATP analogue state (ADP-BeFx). (Prepared using
the crystallographic results of Himmel et al.^ as
obtained from the Protein Data Bank, Structure File
1KK8.)
along the a-helix of the low^er part of the lever
arm from residue 825 to residue 800. From this
viewpoint the rotation of the 50kDa upper
domain is apparent from comparison of the
reorientation of the major a-helix of the upper
domain. This clockwise rotation of the 50kDa
upper domain, as seen from above, on going
from the ATP state to the near-rigor state,
appears to result from the amino-terminal
domain movement. In what follows in Figures

8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
439
8.53 and 8.54, the hydrophobic consiUent mech-
anism provides an explanation of the physical
basis for this movement.
Another feature of this perspective involves
the apparent stretching of a chain segment, as
labeled in Figure 8.52. The elastic force result-
ing from such an extension derives from the
elastic consihent mechanism. As will be argued
below, hydrophobic association gives rise to
the development of a functional elastic force.
Such a perspective may become a classic
demonstration in protein-based machines, as it
has been seen in Complex III of the electron
transport chain and in the repulsive aspect of
the comprehensive hydrophobic effect causing
an elastic deformation during functioning of
ATP synthase/Fi-ATPase.
8.5.4.4 Hydrophobic
Association/Dissociation of the Head of
the Lever Arm and the Amino-terminal
Domain
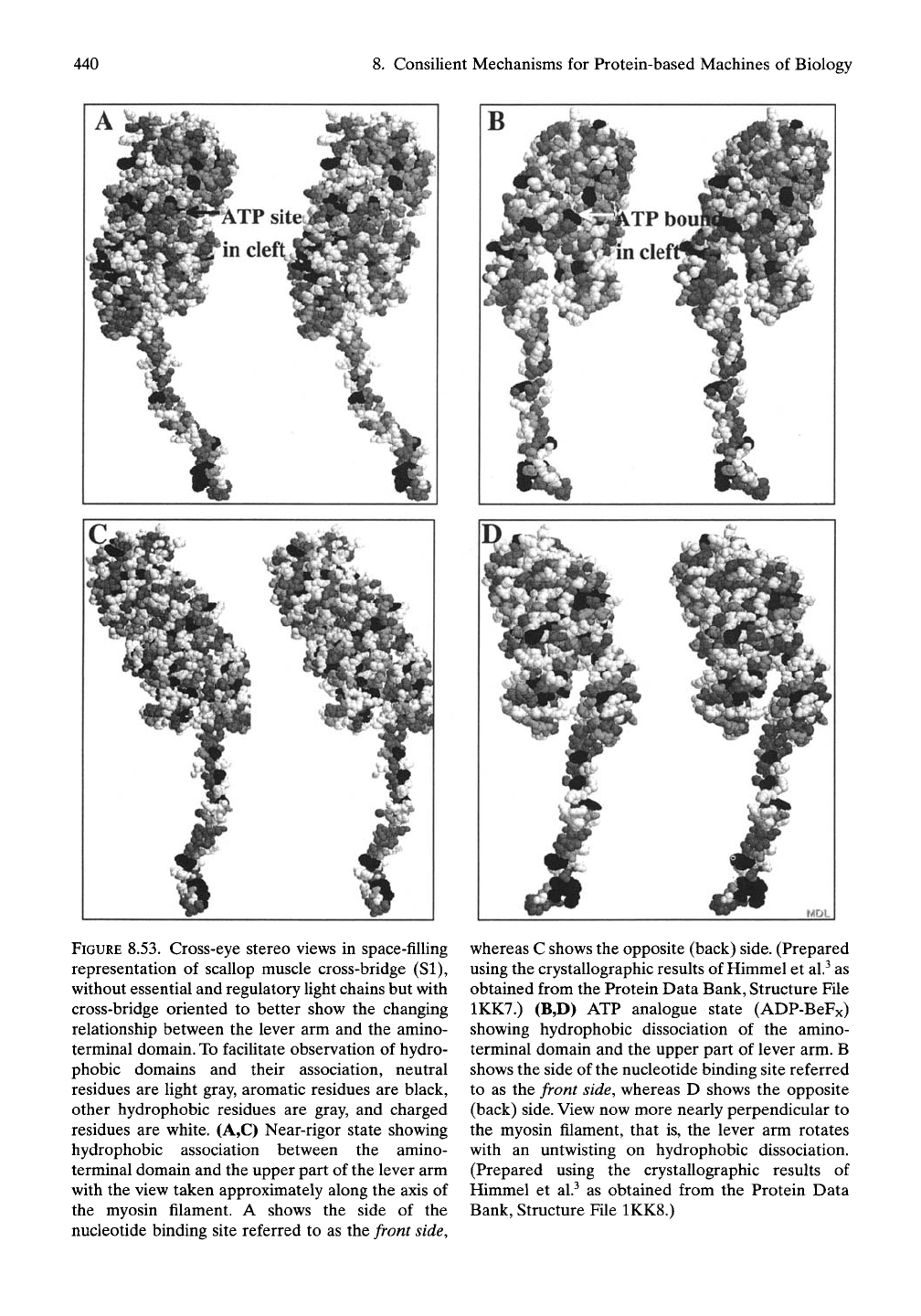
Figure 8.53 shows stereo views of the scallop
muscle cross-bridge, absent the Ught
chains,
that
were oriented to better characterize the nature
of the association (in the near-rigor state) and
the dissociation (in the ATP bound state)
involving the head of the lever arm and the
amino-terminal domain. The stereo views help
to show that the association in Figure 8.53A,C
is hydrophobic, whereas the proximal surfaces
of the head of the lever arm and amino-
terminal domain in Figure 8.53B,D are domi-
nated by charged residues.
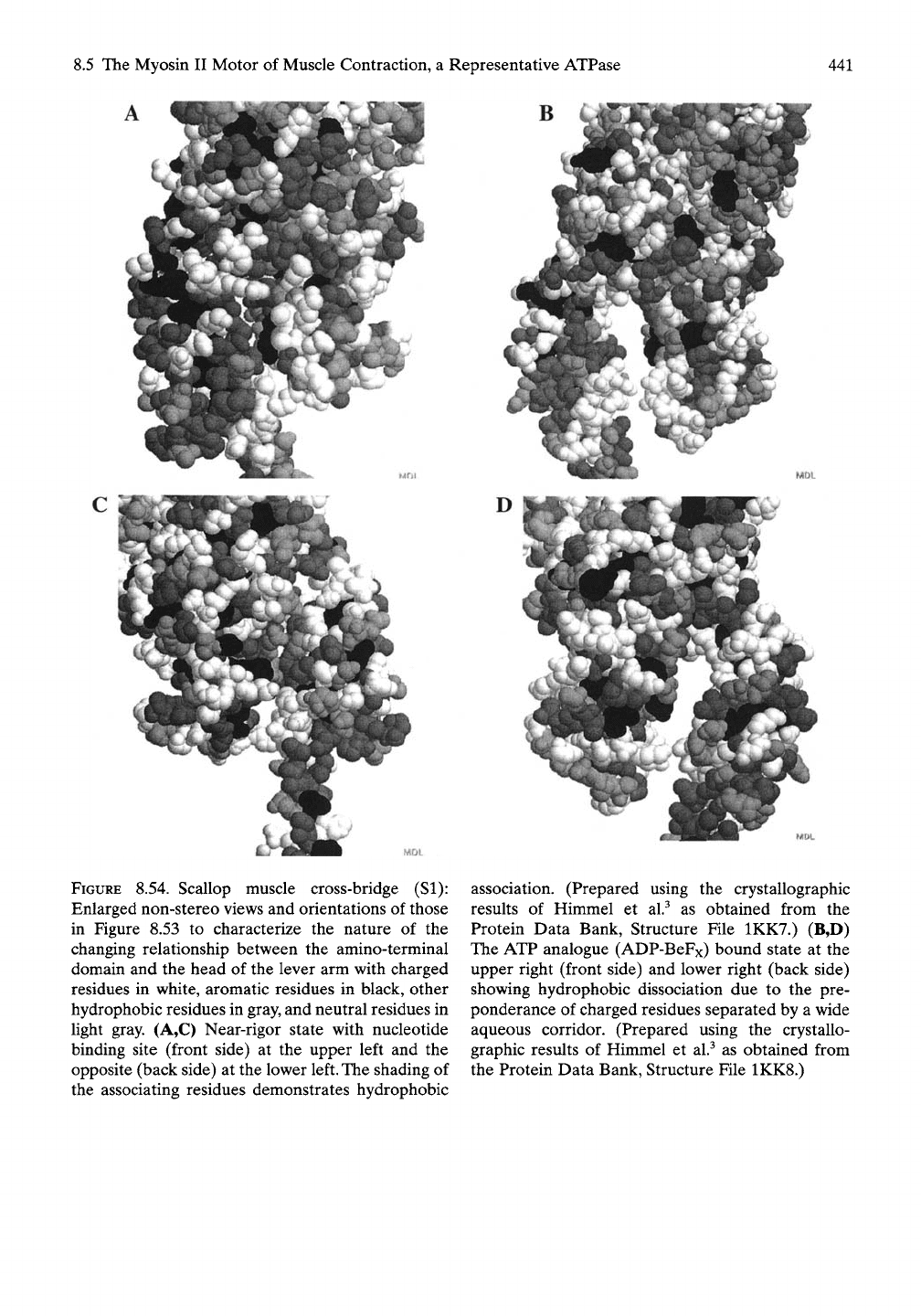
Enlarged views of the relationship between
the amino-terminal domain and the head of the
lever arm, shown in stereo in Figure 8.53, are
given in Figure 8.54 for the purpose of better
seeing the interacting residues in the associated
near-rigor state. On the left side of the figure
the association is hydrophobic, with, as seen in
the stereo view of Figures 8.53A, the lower
charged part between the domains having
twisted sUghtly out of
co-planarity.
On the other
hand, the ATP analogue state is dissociated
with a preponderance of charged (white)
residues separating the two surfaces.
The explanation for the structural transition
between the near-rigor and ATP bound states
is given in section
8.5.4.7
(with the assistance
of Figures 8.58 and 8.59). First, however, two
issues require discussion before this pair of
states can be considered models for producing
the motion of muscle contraction. One issue
involves a closer examination of the stretched/
relaxed chain segment of Figure 8.52 in order
to see if this chain segment can provide an
example of elastic force development that
results from hydrophobic association demon-
strated in Figures 8.53 and 8.54. The pair of
structures to be of greater significance should
provide an understanding of force develop-
ment, as required to explain isometric con-
traction and to understand energy-efficient
production of motion. The second issue is how
effectively the two structures demonstrate the
displacement required for the sliding filament
mechanism of muscle contraction.
8.5.4.5 Evidence for Elastic Force
Development Resulting from
Hydrophobic Association
8.5.4.5.1
Stretching the Relay Loop on
Hydrophobic Association of the Head of the
Lever Arm and the Amino-terminal Domain
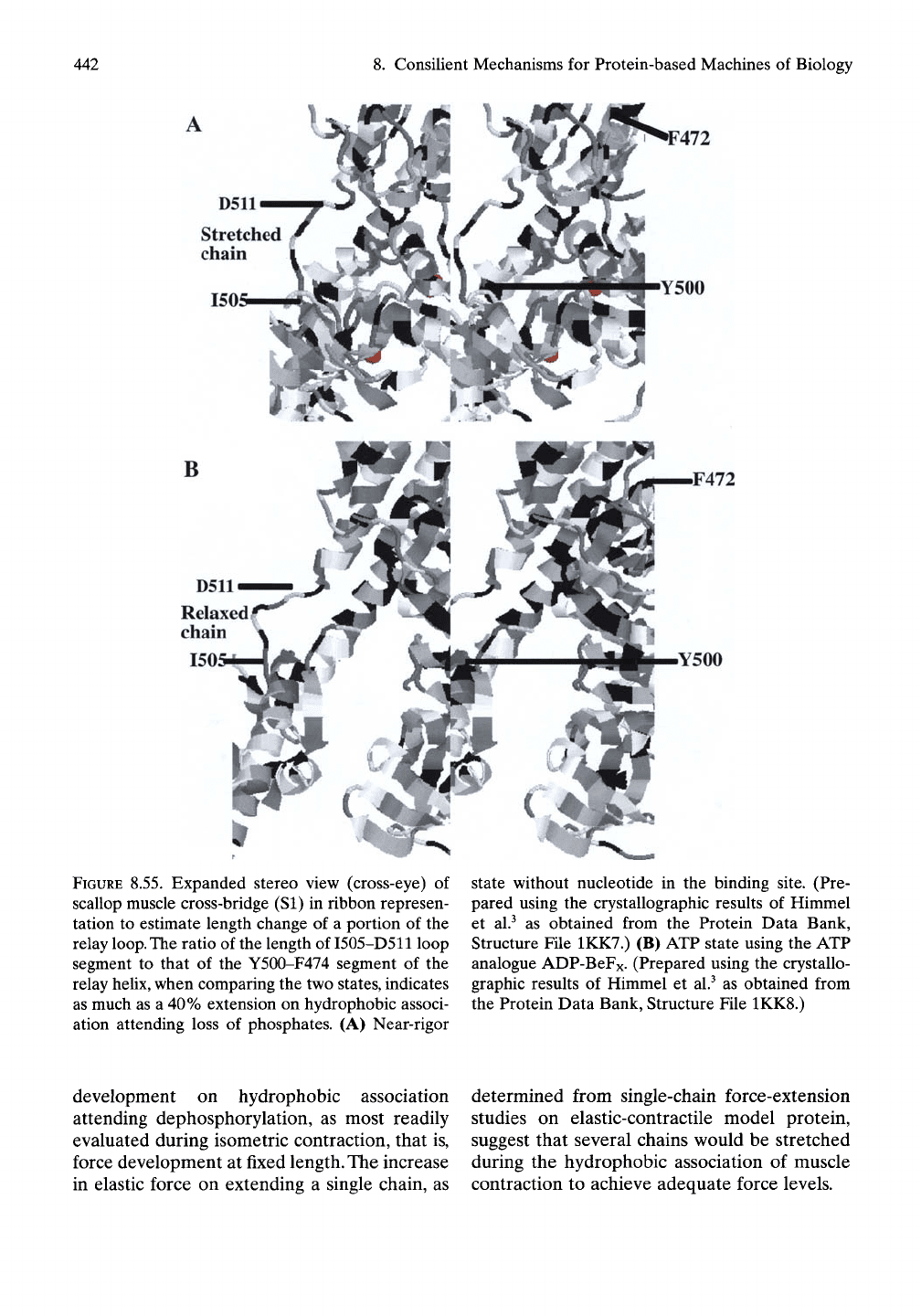
The suggestion of a stretched chain segment is
shown in Figure
8.52.
An optimized perspective
for considering this potential source of elastic
force development is given in Figure 8.55. The
estimate of length change uses the change of
ratios of the relay loop segment I505-D511 to
the relay helix segment Y500-F472 that accom-
panies the change of states at the nucleotide
binding
site.
There appears to be an extension of
as much as
40%
accompanying the hydrophobic
association resulting from loss of phosphate (or
equivalent) from the nucleotide binding site.
8.5.4.5.2
Presence of a Number of Flexible
Loops for Potential Storage of Elastic Force
By the elastic consihent mechanism the
extension of single flexible loops causes an
increase in the elastic force. It appears that one
such loop has been identified in Figure 8.55.
Rayment et al.^^"^ note a number of flexible
loops in the myosin cross-bridge. Each of
these becomes a candidate for elastic force
440
8. Consilient Mechanisms for Protein-based Machines of Biology
1 -..-i
^B^-i-
«.'C«^
^TP site^
Pin clef^jJFte
riln^^^HII^^B-4k'
«>.
'i
Rli^^^BHki'"^
KrrZpk
i^T^^^jh
FIGURE 8.53. Cross-eye stereo views in space-filling
representation of scallop muscle cross-bridge (SI),
without essential and regulatory fight chains but with
cross-bridge oriented to better show the changing
relationship between the lever arm and the amino-
terminal domain.
To
facilitate observation of hydro-
phobic domains and their association, neutral
residues are fight gray, aromatic residues are black,
other hydrophobic residues are gray, and charged
residues are white. (A,C) Near-rigor state showing
hydrophobic association between the amino-
terminal domain and the upper part of the lever arm
with the view taken approximately along the axis of
the myosin filament. A shows the side of the
nucleotide binding site referred to as the front side,
whereas C shows the opposite (back) side. (Prepared
using the crystallographic results of Himmel et al.^ as
obtained from the Protein Data Bank, Structure Ffie
1KK7.) (B,D) ATP analogue state (ADP-BeFx)
showing hydrophobic dissociation of the amino-
terminal domain and the upper part of lever arm. B
shows the side of the nucleotide binding site referred
to as the front side, whereas D shows the opposite
(back)
side.
View now more nearly perpendicular to
the myosin filament, that is, the lever arm rotates
with an untwisting on hydrophobic dissociation.
(Prepared using the crystallographic results of
Himmel et al.^ as obtained from the Protein Data
Bank, Structure File 1KK8.)
8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase
441
FIGURE 8.54. Scallop muscle cross-bridge (SI):
Enlarged non-stereo views and orientations of those
in Figure 8.53 to characterize the nature of the
changing relationship between the amino-terminal
domain and the head of the lever arm with charged
residues in white, aromatic residues in black, other
hydrophobic residues in gray, and neutral residues in
light gray. (A,C) Near-rigor state with nucleotide
binding site (front side) at the upper left and the
opposite (back side) at the lower left. The shading of
the associating residues demonstrates hydrophobic
^1^
ll»^
t&A.^
L'-i»;
association. (Prepared using the crystallographic
results of Himmel et al.^ as obtained from the
Protein Data Bank, Structure File 1KK7.) (B,D)
The ATP analogue (ADP-BeFx) bound state at the
upper right (front side) and lower right (back side)
showing hydrophobic dissociation due to the pre-
ponderance of charged residues separated by a wide
aqueous corridor. (Prepared using the crystallo-
graphic results of Himmel et al.^ as obtained from
the Protein Data Bank, Structure File 1KK8.)
442
8. Consilient Mechanisms for Protein-based Machines of Biology
rF472
Stretched
chain
•Y500
.F472
¥500
FIGURE 8.55. Expanded stereo view (cross-eye) of
scallop muscle cross-bridge (SI) in ribbon represen-
tation to estimate length change of a portion of the
relay
loop.
The
ratio of the length of
I505-D511
loop
segment to that of the Y500-F474 segment of the
relay
heUx,
when comparing the two states, indicates
as much as a 40% extension on hydrophobic associ-
ation attending loss of phosphates. (A) Near-rigor
state without nucleotide in the binding site. (Pre-
pared using the crystallographic results of Himmel
et al.^ as obtained from the Protein Data Bank,
Structure File 1KK7.) (B) ATP state using the ATP
analogue ADP-BeFx. (Prepared using the crystallo-
graphic results of Himmel et al.^ as obtained from
the Protein Data Bank, Structure File 1KK8.)
development on hydrophobic association
attending dephosphorylation, as most readily
evaluated during isometric contraction, that is,
force development at fixed length. The increase
in elastic force on extending a single chain, as
determined from single-chain force-extension
studies on elastic-contractile model protein,
suggest that several chains would be stretched
during the hydrophobic association of muscle
contraction to achieve adequate force levels.
8.5 The Myosin II Motor of Muscle Contraction, a Representative ATPase 443
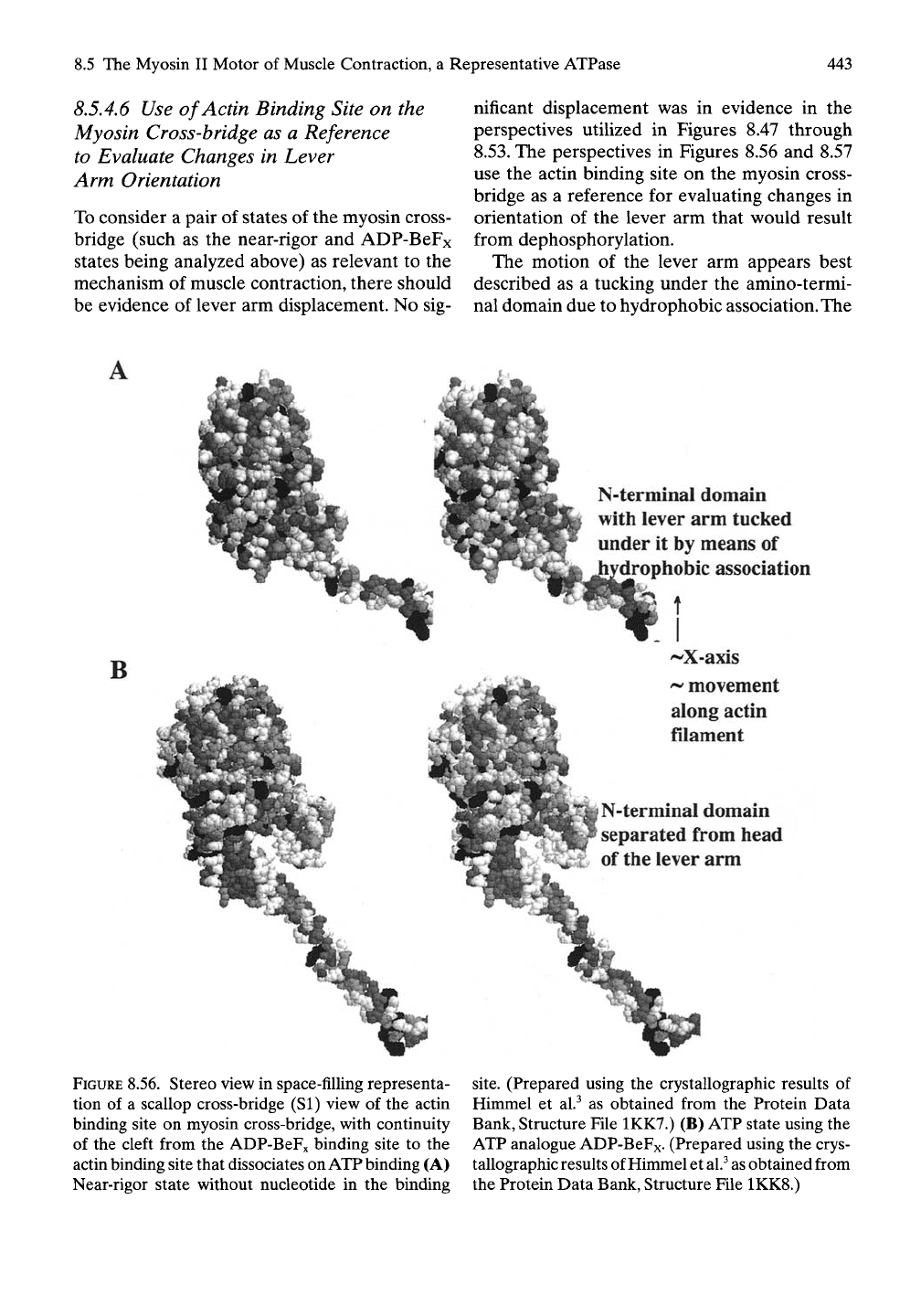
8.5.4.6
Use ofActin Binding Site on the
Myosin Cross-bridge as a Reference
to Evaluate Changes in Lever
Arm Orientation
To consider a pair of states of the myosin cross-
bridge (such as the near-rigor and ADP-BeFx
states being analyzed above) as relevant to the
mechanism of muscle contraction, there should
be evidence of lever arm displacement. No sig-
nificant displacement was in evidence in the
perspectives utilized in Figures 8.47 through
8.53.
The perspectives in Figures 8.56 and 8.57
use the actin binding site on the myosin cross-
bridge as a reference for evaluating changes in
orientation of the lever arm that w^ould result
from dephosphorylation.
The motion of the lever arm appears best
described as a tucking under the amino-termi-
nal domain due to hydrophobic association. The
B
N-terminal domain
with lever arm tucked
under it by means of
drophobic association
t
I
'^X-axis
^ movement
along actin
filament
N-terminal domain
separated from head
of the lever arm
FIGURE 8.56. Stereo view in space-filling representa-
tion of a scallop cross-bridge (SI) view of the actin
binding site on myosin cross-bridge, with continuity
of the cleft from the ADP-BeFx binding site to the
actin binding site that dissociates on ATP binding (A)
Near-rigor state without nucleotide in the binding
site.
(Prepared using the crystallographic results of
Himmel et al.^ as obtained from the Protein Data
Bank, Structure File 1KK7.) (B) ATP state using the
ATP analogue ADP-BeFx- (Prepared using the crys-
tallographic results of Himmel et
al.^
as
obtained from
the Protein Data Bank, Structure File 1KK8.)
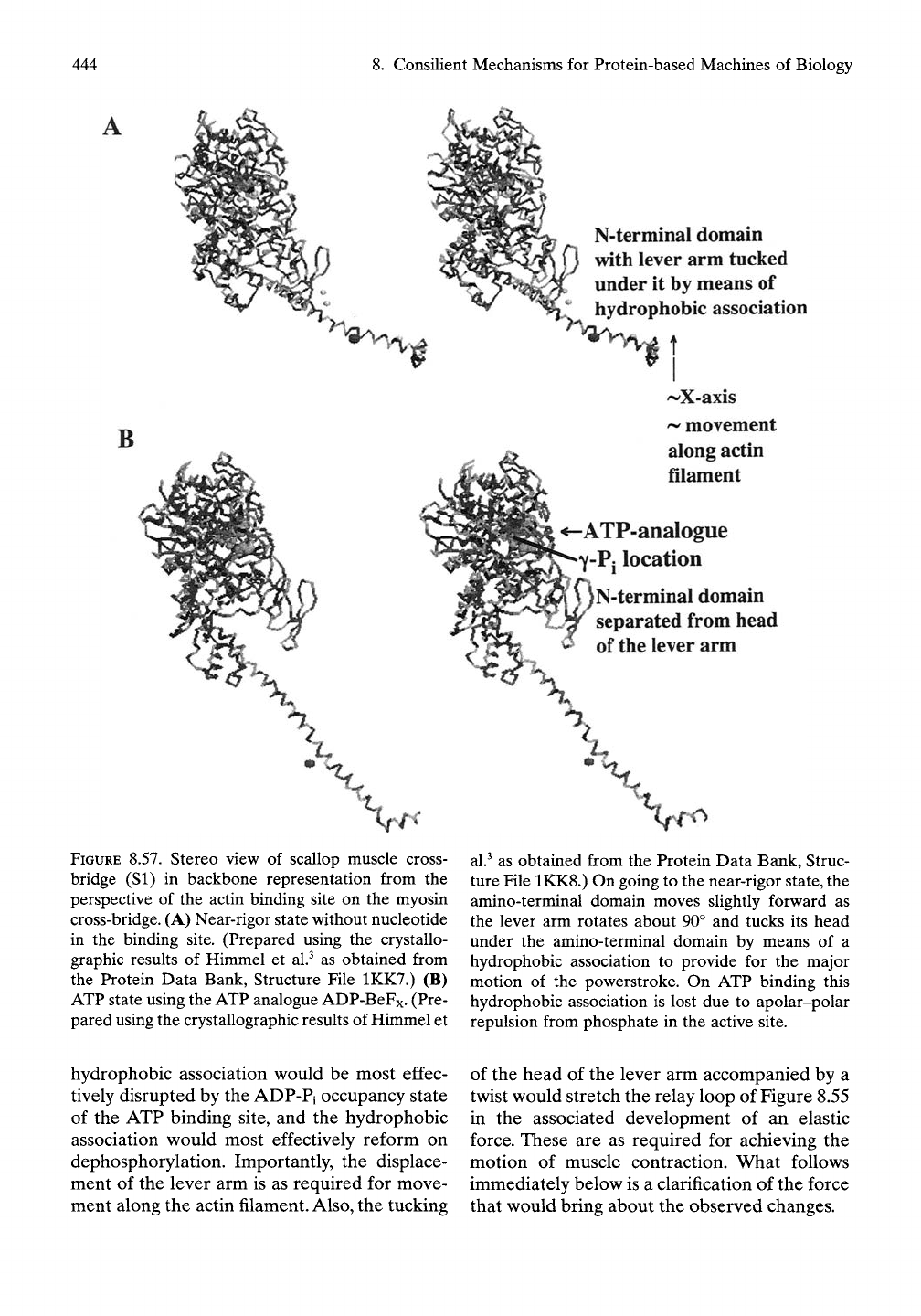
444
8. Consilient Mechanisms for Protein-based Machines of Biology
FIGURE 8.57. Stereo view of scallop muscle cross-
bridge (SI) in backbone representation from the
perspective of the actin binding site on the myosin
cross-bridge. (A) Near-rigor state without nucleotide
in the binding site. (Prepared using the crystallo-
graphic results of Himmel et al.^ as obtained from
the Protein Data Bank, Structure File 1KK7.) (B)
ATP state using the ATP analogue ADP-BeFx. (Pre-
pared using the crystallographic results of Himmel et
N-terminal domain
with lever arm tucked
under it by means of
hydropliobic association
t
I
'^X-axis
^ movement
along actin
filament
ATP-analogue
Y-Pj
location
N-terminal domain
separated from head
of
the
lever arm
al.^
as obtained from the Protein Data Bank, Struc-
ture File 1KK8.) On going to the near-rigor state, the
amino-terminal domain moves sUghtly forward as
the lever arm rotates about 90° and tucks its head
under the amino-terminal domain by means of a
hydrophobic association to provide for the major
motion of the powerstroke. On ATP binding this
hydrophobic association is lost due to apolar-polar
repulsion from phosphate in the active site.
hydrophobic association would be most effec-
tively disrupted by the ADP-Pi occupancy state
of the ATP binding site, and the hydrophobic
association would most effectively reform on
dephosphorylation. Importantly, the displace-
ment of the lever arm is as required for move-
ment along the actin filament. Also, the tucking
of the head of the lever arm accompanied by a
twist would stretch the relay loop of Figure 8.55
in the associated development of an elastic
force. These are as required for achieving the
motion of muscle contraction. What follows
immediately below is a clarification of the force
that would bring about the observed changes.
