Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
395
Both protein machines of ATP synthase are
rotary motors, made possible by the mechani-
cal coupling of chemo-mechanical transduction
due to the Fo-motor to mechano-chemical
transduction of the Fi-motor. A single rotor
driven by the membranous Fo-motor in turn
drives the extramembranous Fi-motor. The
Fo-motor of yeast mitochondria, for example,
exhibits a 10-fold rotational symmetry, provid-
ing a 10-step rotary engine, whereas the Fi-
motor of all ATP synthases exhibits a threefold
rotational symmetry, resulting in a threefold
rotary engine.
8.4.1.1.2
Relevance of the Hydrophobic
Consilient Mechanism to the Fo-motor
The relevance of the Fo-motor of ATP synthase
to hydrophobicity is obvious in a v^ay that has
long been appreciated. It surprises no one that
protonation of the -COO" of aspartate or glu-
tamate to achieve the carboxyl -COOH would
facilitate solubility in a lipid bilayer. In fact,
our design for using the inverse temperature
transition in the first elastic-contractile model
protein-based machine, driven by proton chem-
ical energy, was based on the expectation that
-COOH would be more hydrophobic than
-COO".^^
To argue that the hydrophobic con-
silient mechanism is relevant to function of the
Fo-motor presents no strikingly new statement.
However, it does become possible to use values
for the Gibbs free energy of hydrophobic asso-
ciation obtained on analysis of the elastic-
contractile model proteins containing each of
the amino acid residues to provide reasonable
thermodynamic and efficiency insights for ATP
synthase.
8.4.1.1.3
Relevance of the Hydrophobic
Elastic ConsiUent Mechanism to the Fi-motor
Functioning as an ATPase: Analogy to the
Internal Combustion Rotary Engine
Analogy may be drawn between the Fi-motor
functioning in the ATPase mode, that
is,
a three-
fold chemo-mechanical rotary engine, and the
threefold rotary engine of the Mazda automo-
bile.
For the latter, localized energy (pressure-
volume) bursts produce motion by the
expansion resulting from conversion of liquid
to hot gasses on fuel ignition. Based on our view
of the hydrophobic consiHent mechanism and
specifically the apolar-polar repulsive free
energy of hydration, AGap, the analogy would
be that hydrolysis of ATP to form ADP and Pi
provides a burst of apolar-polar repulsion
directed at the most hydrophobic side of a
hydrophobically asymmetric rotor in a manner
that drives rotation.
Such an analogy could be cautioned by the
experimental finding that the ratio of ATP to
ADP
-I-
Pi at the catalytic site can be measured
as close to unity. If this were incorrectly viewed
as an equilibrium circumstance at the catalytic
site,
then one would consider the free energy
change on going from ATP to ADP -i- Pi to be
zero.
This is, however, erroneous. We know
under standard conditions that the hydrolytic
breakdown of ATP to ADP
-i-
Pi releases about
8kcal/mole of heat. Therefore, at the catalytic
site the energy resulting from the conversion of
ATP to ADP + Pi must raise the free energy of
the molecular structure. We propose that the
development of an increase of 8kcal/mole in
AGap, the apolar-polar repulsive free energy of
hydration, provides a rotational impulse by
structural deformation as required to drive the
y-rotor in the ATPase mode.
8.4.1.1.4 Relevance of the Hydrophobic
Elastic ConsiUent Mechanism to the Fi-motor
Functioning as an ATPase: Analogy to a
Three-pole DC Motor
Perhaps the better analogy, however, occurs
with a threefold symmetric electrical motor,
described by Kinosita et
al.^^
as a three-pole DC
motor. In their analogy "The rotor is a perma-
nent magnet, a static component." Rather than
being static, however, in our view the rotor and
the housing would have the capacity to store
elastic deformation. Instead of the repulsion
between like poles of a magnet, the push com-
ponent of force arises from the apolar-polar
repulsion between the most polar state attained
on formation of ADP
-H
Pi in the housing and
the most hydrophobic face of the y-rotor. Also,
instead of acting through space, the push com-
ponent of force requires an aqueous solvent
through which to function.

396
8. Consilient Mechanisms for Protein-based Machines of Biology
Thus the fundamental predictions of the
hydrophobic elastic consilient mechanism are
that the rotor would exhibit asymmetric
hydrophobicity, that different arrangements of
nucleotide analogues representing different
states of polarity at the catalytic sites would
orient the rotor, and that hydrolysis of ATP in
formation of the most polar state at a catalytic
site of the involved protein subunit(s) would
demonstrate a near-ideal elastic deformation
of the y-rotor and the protein subunit(s). Of
course, such a mechanism would exhibit high
efficiency and reversibility.
8,4.1.2 Predictions and the Demonstrated
Relevance of the Consilient Mechanisms
to the Fj-motor
8.4.1.2.1
Prediction of Hydrophobically
Asymmetric and Elastically Deformable
Rotor and Housing
Accordingly, in contrast to the obviousness of
the role of the hydrophobic consilient mecha-
nism to the Fo-motor of ATP synthase, the
relevance of the hydrophobic consilient
mechanism to the Fi-motor of ATP synthase
calls for presentation of new and otherwise
unexpected perspectives. For the hydrophobic
consilient mechanism to be a dominant factor
in the function of the Fi-motor, the primary
predictions focus on the rotor that mechani-
cally couples the two rotary engines. As noted
above, the first prediction is that the y-rotor
must be hydrophobically asymmetric within the
catalytic structure. A secondary prediction
becomes that rotor hydrophobic asymmetry
must be such that rotational orientation of the
rotor responds to the occupancy states of the
catalytic sites of the Fi-motor and that the rotor
and associated catalytic subunit(s) be capable
of nearly ideal reversible storage of deforma-
tion energy. Another important enabling pre-
diction, regarding the Fi-motor functioning as
an ATPase, is that elastic deformation arise out
of the sudden development of significant AGap
on hydrolysis of ATP to form ADP and Pi and
that this deforming force defines the direction
of rotation. Finally, the presence of the AGap on
formation of the most polar state would be
recognizable in the orientation and interac-
tions,
or lack
thereof,
of charged species
between rotor and the subunit containing the
most polar state.
8.4.1.2.2
Demonstration of an Asymmetrically
Hydrophobic Rotor by Calculation
of AGHA, Gibbs Free Energy of
Hydrophobic Association
Demonstrations of these predictions constitute
the message of this section 8.4, and its success
introduces the perspective of a conjoined
hydrophobic elastic consilient mechanism. With
the values in Table 5.3 and the crystal structure
with three different states of occupancy, empty,
ATP,
and ADP, the three sides of the rotor
can be identified and the respective Gibbs
free energies of hydrophobic association,
AGHA, have been estimated to be -20, 0, and
+9kcal/mole. The most hydrophobic face asso-
ciates with the empty site, the neutral face with
the ATP bound site, and the most polar face
with the ADP site which in the synthesis mode
would be in position to add Pj. As expected
from the magnitude of the resulting AGap for a
series of crystal structures wherein the least
polar occupancy state for the catalytic site
could be defined, the most hydrophobic side of
the rotor resides in apposition to the least polar
site.
8.4.1.2.3
Prediction and Demonstration That
the Most Hydrophobic Face of the Rotor
Hydrophobically Associates with the Most
Hydrophobic State of the Housing
On the basis of the hydrophobic consilient
mechanism, predictions of the relative
hydrophobicities of the housings at the location
of the changeable catalytic sites in order of
increasing hydrophobicity (decreasing polarity)
are ADP plus Pi, ATP, ADP, Pj when the latter
is not in position to have direct through-water
interaction with the rotor, and the empty cat-
alytic site. The empty catalytic site indeed
hydrophobically associates with the side of the
rotor found by calculation to have greatest
hydrophobicity. Furthermore, for several
dif-
ferent patterns of site occupancy available from
crystal structures, the side of the rotor deemed
most hydrophobic by the consilient hydropho-

8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
397
bic mechanism always aligns with the most
hydrophobic side of the rotor.
8.4.1.2.4 Prediction of the Direction of Rotor
Rotation for the ATPase and Synthesis Modes
Fortunately, a crystal structure has been
reported in which a stable analogue, ADP plus
SOf, of the most polar state, ADP plus HPO4"
, has been solved.^^ As expected for the most
potent configuration, the SOj' was nearly fully
exposed through an intervening aqueous
solvent to the y-rotor, and the very polar sulfate
was located just above the level at which the
rotor changed from a double-stranded a-helical
coiled coil at the amino-terminus of the y-chain
to a single-stranded a-helix that ultimately
ended at the carboxyl terminus of the y-chain
at the base of the Fi-motor. At this position
the apolar-polar repulsion occurring between
the polar SO4" and the hydrophobic side of the
rotor applies most directly to the amino-
terminal side of the double strand resulting in
a torque that would provide a counterclockwise
rotation for the y-rotor during function as an
ATPase. Synthesis would be achieved by a
reversal of the direction of rotation, wherein
the maximal apolar-polar repulsion would be
applied to the ADP plus Pi state and would be
relieved by formation of ATP.
8.4.1.2.5
Demonstration of an Elastically
Deformable Rotor and Housing
In the crystal structure with a (i-subunit con-
taining the sulfate analogue of the most polar
ADP plus Pi state, the y-rotor and the most polar
catalytic (i-subunit are found displaced from
each other by a mean distance of 2.9 A distance
and the y-rotor is twisted up to 20° when com-
pared with their relationship when the (3-
subunit is empty.^^ Quoting from Menz et al.,^^
"Note that interacting residues in the PE-
subunit and the y-subunit move in opposite
directions." This repulsion occurs for a configu-
ration in which the most hydrophobic side of
the y-rotor is in apposition to a slightly less polar
analogue, ADP^~ -\- S04~, of the most polar
natural occupancy state, ADP^" + HPO4", for a
catalytic (i-subunit. In our view, this displace-
ment results from a very large near-maximal
AGap available to the Fi-motor, that is, a near
maximal apolar-polar repulsive free energy of
hydration between the hydrophobic side of the
y-rotor and the very polar state, ADP
-H
SO4", of
a catalytic (3-subunit. This near-maximal repul-
sion provides an elastic deformation of rotor
and housing as required for efficient
function of the ATPase in its performance of
chemo-mechanical transduction.
8.4.1.2.6 Pattern of Charged Side Chain
Orientations Reflects Presence of a
Dominant AGap
An interesting orientation of side chains in
the housing, on the inner surface of the
(aP)3-
subunit structure, becomes rational once the
presence of an apolar-polar repulsion is recog-
nized. In particular, the negatively charged
aspartic acid residue, D315, is observed in the
crystal structure between the analogue of the
most repulsive state of the catalytic site and the
y-rotor. At this position the carboxylate of D315
is surrounded by water molecules and is bent
away from the hydrophobic side of the rotor
and toward the sulfate group with its two
negative charges yet with space for only a
few water molecules separating sulfate from
carboxylate.
Why, with the capacity to position itself at
greater distance, would the carboxylate of
D315 accept a location of higher charge-charge
repulsion? In our view, an apolar repulsion
emanating from the hydrophobic side of the y-
rotor causes it to reside in such a configuration
and as such the repulsion would effect an
element of elastic deformation in the housing
of the Fi-motor. On the other hand, residue
D316,
which is adjacent to the hydrophobic
rotor, is bent flat in the opposite direction
against the rotor where it exhibits ion pairing
and hydrogen bonding. These side chain orien-
tations reflect the AGap causing elastic defor-
mations due to the proximal hydrophobic side
of the y-rotor.
If we had not already derived AGap from
analysis of the data on elastic-contractile model
proteins, as reviewed in Chapter
5,
with the per-
spectives presented below we would have had
to invent such a repulsive force to explain the

398
8. Consilient Mechanisms for Protein-based Machines of Biology
structural information available for the Fi-
motor of ATP synthase. Of course, AGap pro-
vides the force required to adapt the three-
pole DC motor described in Figures 5, 7, and
8 of Kinosita et al7^ to those forces available
to protein-based machines functioning in an
aqueous milieu.
8.4.2 Schematic Representation
of ATP Synthase Structure:
A Chemo-chemical Transducer
8.4.2.1 Subunits Divided into Five
Structural Classes: Membranous Rotor,
Extramembranous Rotor, Motor Housings
for Each of the Two Rotors, and Stator
Interlocking Motor Housings
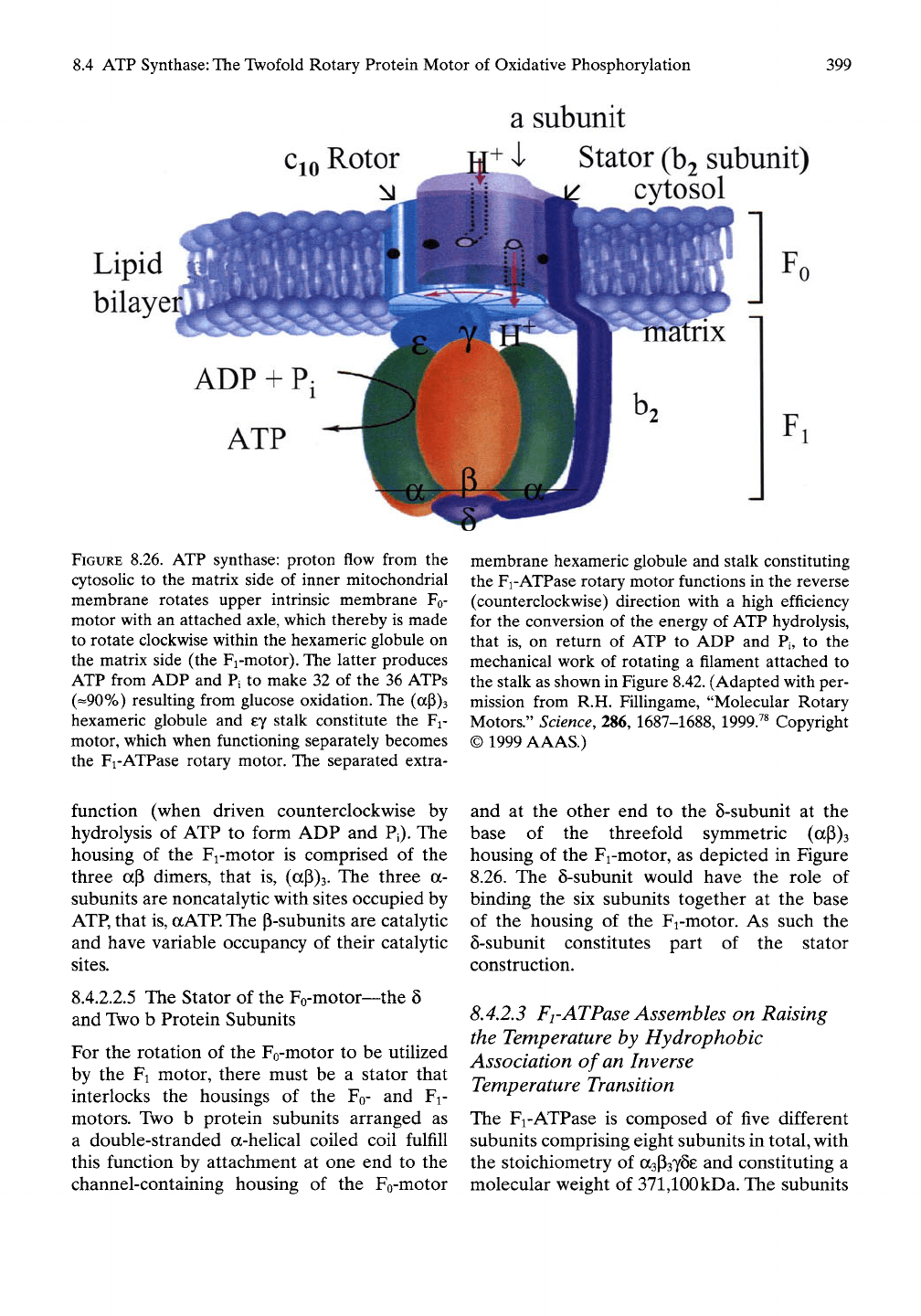
As represented schematically in Figure 8.26/^
ATP synthase contains a basic set of compo-
nents that are common throughout biology.
There are two rotary motors, the membranous
Fo-motor and the extramembranous Fi-motor.
An extramembranous rotor driven by the
membranous Fo-motor couples rotations in
each motor and drives ATP synthesis in the
extramembranous Fi-motor. The effective
housings of the two rotary motors are held
fixed, one with respect to the other, by a stator
element. In this way one complete rotation in
the Fo-motor accomplishes one complete rota-
tion in the Fi-motor.
Thus,
utilizing Figure 8.26, the initial descrip-
tion of structure becomes enumeration of the
constituent protein subunits and their number
of repetitions given as a subscript for each of
the four structural components: (1) The mem-
branous protein subunits are the 10 repeats of
protein subunit c, that is, Cio. (2) The motor
housing for the membranous rotor utilizes the
a protein subunit in combination with the lipid
bilayer. (3) The extramembranous rotor is com-
prised of the
Y
and 8 protein subunits. (4) The
motor housing for the extramembranous rotor
utilizes three a and three
(5
protein subunits. (5)
The stator component for interlocking the two
motor housings utilizes two b protein subunits
in combination with the 5 protein subunit.
In addition, in animals mitochondrial ATP
synthase contains another eight types of sub-
units,
a number of which function in the cou-
pling of the Fo-rotor to the y-rotor.^^
8.4.2.2 Structural and Functional Roles of
Basic Protein Subunits in ATP Synthase
8.4.2.2.1
Rotor of the Fo-motor—The 10 c
Protein Subunits
The number of c protein subunits constituting
the Fo-motor depends on the species and has
been reported to vary from 9 to 14. In yeast,
it is 10, as in Figure 8.26, whereas in E. coli it
is 12. The c subunit occurs as an a-helix in a
hairpin configuration, with one side of the
hairpin forming the inner wall and the second
side of the a-helical hairpin interacting with the
housing of the Fo-motor.
8.4.2.2.2
Housing of the Fo-motor—the a
Protein Subunit and the Lipid Bilayer
As presently described, the housing of the Fo-
motor becomes the lipid bilayer and five trans-
membrane heUces of subunit a that combine to
form two half-proton channels.^^ As shown in
Figure 8.26, one half-channel spans from the
cytosol to the middle of the membrane, and the
second half-channel completes the transmem-
brane transit from middle of the lipid bilayer to
the matrix side of the membrane.
8.4.2.2.3
Rotor Driven by the Fo-motor
(Rotor of the Fi-motor)—the Single y and E
Protein Subunits
The rotor that is driven by the Fo-motor com-
prises a single y-subunit and a small e-subunit
attached to the y-subunit at a point proximal to
the base of the Fo-motor. This is called the y-
rotor. In the hydrophobic elastic consilient
mechanism, the interactions of a hydrophobi-
cally asymmetric y-rotor with the housing of the
Fi-motor with different occupancy states of the
catalytic sites constitute the basis for mechano-
chemical transduction of the Fi-motor.
8.4.2.2.4 Housing of the Fi-motor—the Three
a and Three
(3
Protein Subunits
The housing of the Fi-motor contains the heart
of the ATP synthase function (when the y-rotor
is driven clockwise by the Fo-motor) or ATPase
8.4 ATP
Synthase:
The Twofold Rotary Protein Motor of Oxidative Phosphorylation
399
Cjo
Rotor
a subunit
+jt Stator (bj subunit)
cytosol
Lipid I
bilayer
ADP + P
FIGURE 8.26. ATP synthase: proton flow from the
cytosoUc to the matrix side of inner mitochondrial
membrane rotates upper intrinsic membrane FQ-
motor with an attached axle, which thereby is made
to rotate clockwise within the hexameric globule on
the matrix side (the Fi-motor). The latter produces
ATP from ADP and Pi to make 32 of the 36 ATPs
(==90%) resulting from glucose oxidation. The (aP)3
hexameric globule and ey stalk constitute the Fi-
motor, which when functioning separately becomes
the Fi-ATPase rotary motor. The separated extra-
membrane hexameric globule and stalk constituting
the Fi-ATPase rotary motor functions in the reverse
(counterclockwise) direction with a high efficiency
for the conversion of the energy of ATP hydrolysis,
that is, on return of ATP to ADP and Pj, to the
mechanical work of rotating a filament attached to
the stalk as shown in Figure 8.42. (Adapted with per-
mission from R.H. Fillingame, "Molecular Rotary
Motors."
Science,
286, 1687-1688, 1999.^^ Copyright
© 1999 AAAS.)
function (when driven counterclockw^ise by
hydrolysis of ATP to form ADP and Pi). The
housing of the Fi-motor is comprised of the
three ap dimers, that is,
(aP)3.
The three a-
subunits are noncatalytic with sites occupied by
ATP,
that is, aATR The p-subunits are catalytic
and have variable occupancy of their catalytic
sites.
8.4.2.2.5
The Stator of the Fo-motor-
and Two b Protein Subunits
-the 5
For the rotation of the Fo-motor to be utilized
by the Fi motor, there must be a stator that
interlocks the housings of the FQ- and Fi-
motors. Two b protein subunits arranged as
a double-stranded a-helical coiled coil fulfill
this function by attachment at one end to the
channel-containing housing of the Fo-motor
and at the other end to the 5-subunit at the
base of the threefold symmetric (aP)3
housing of the Fi-motor, as depicted in Figure
8.26. The 8-subunit would have the role of
binding the six subunits together at the base
of the housing of the Fi-motor. As such the
5-subunit constitutes part of the stator
construction.
8,4.2,3 Fj-ATPase Assembles on Raising
the Temperature by Hydrophobic
Association of an Inverse
Temperature Transition
The Fi-ATPase is composed of five different
subunits comprising eight subunits in total, with
the stoichiometry of asPsySe and constituting a
molecular weight of 371,100 kDa. The subunits

400
8. Consilient Mechanisms for Protein-based Machines of Biology
of Fi-ATPase dissociate on lowering the
temperature to 0°C, that is, they exhibit cold
denaturation.^^'^^'^^
This,
of course, indicates that
under the appropriate conditions the subunits
would assemble on raising the temperature. For
enzymatic activity, this would necessarily
include the y-rotor with its double-stranded a-
helical coiled coil within the threefold symmet-
ric (aP)3 with approximate threefold symmetry
but which looks much like an orange with six
sections. Accordingly, the Fi-ATPase is of a
nature to exhibit an inverse temperature tran-
sition of hydrophobic association/dissociation
with a Tt-value somewhere between physiolog-
ical temperature and 4°C.
Knowledge of the differential scanning
calorimetry data and the temperature of the
transition for assembly on raising the tempera-
ture would allow determination of the free
energy of hydrophobic association, the
AGHA-
It
would be of interest to obtain such data with
systematic increases in the number of sites occu-
pied by ATP. Based on the hydrophobic con-
silient mechanism and in analogy to forming the
more polar state by ionization of carboxyls to
form carboxylates in the elastic-contractile
model proteins, we would predict that the heat
of the inverse temperature transition would
decrease and the temperature of the transition
would rise as occupancy of the nucleotide-
binding sites progressed from being the most
hydrophobic state of empty to becoming the
most polar state with all sites containing ATP.
8.4.3 Initial Structural Information for
ATP Synthase from Electron Density
Maps and Thermodynamic Data
8.4.3.1 Overall Structure
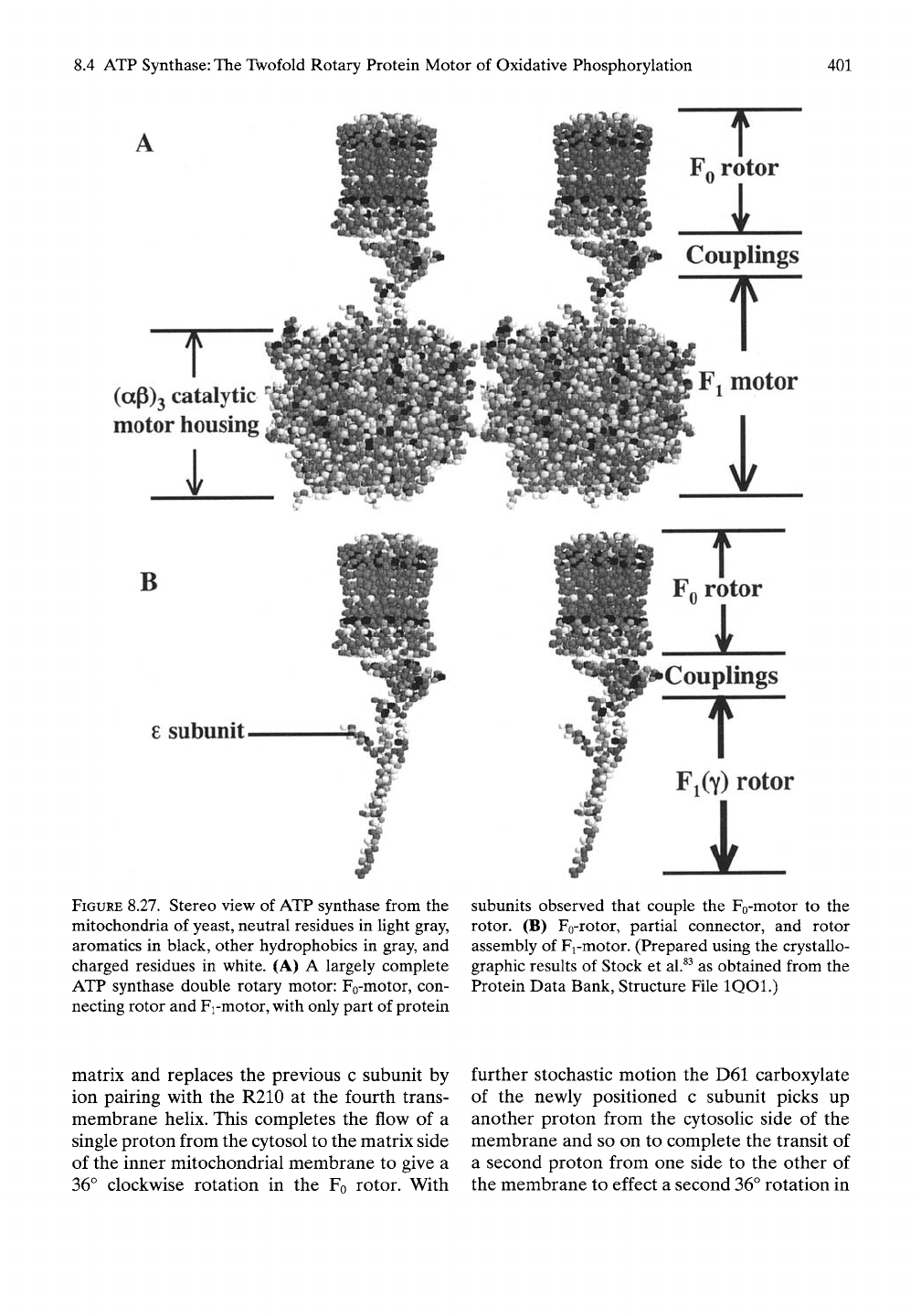
By means of electron density maps on crystals
of yeast mitochondrial ATP synthase. Stock et
al.^^
obtained the associated
FQ-
and Fi-motors,
as demonstrated in Figure 8.27A. The Fo-rotor
was obtained in sufficient detail to identify 10 c
subunits and to locate the critical D61 residue
that undergoes protonation/deprotonation of
its carboxylate functional group. The small
white spherical dots crossing the middle of the
Fo-rotor locate the D61 residue in each subunit.
Figure
8.27B
shows only the rotating ele-
ments of ATP synthase with 10 a-helical
hairpins making up the Fo-rotor and the
double-stranded a-helical coiled coil (upper
two-thirds) of y-subunit decorated at its upper
part with the 8-subunit shown jutting out to
constitute the Y"i"<^tor (the Fi-rotor). Also
shown is the coupling component, which is
incomplete.
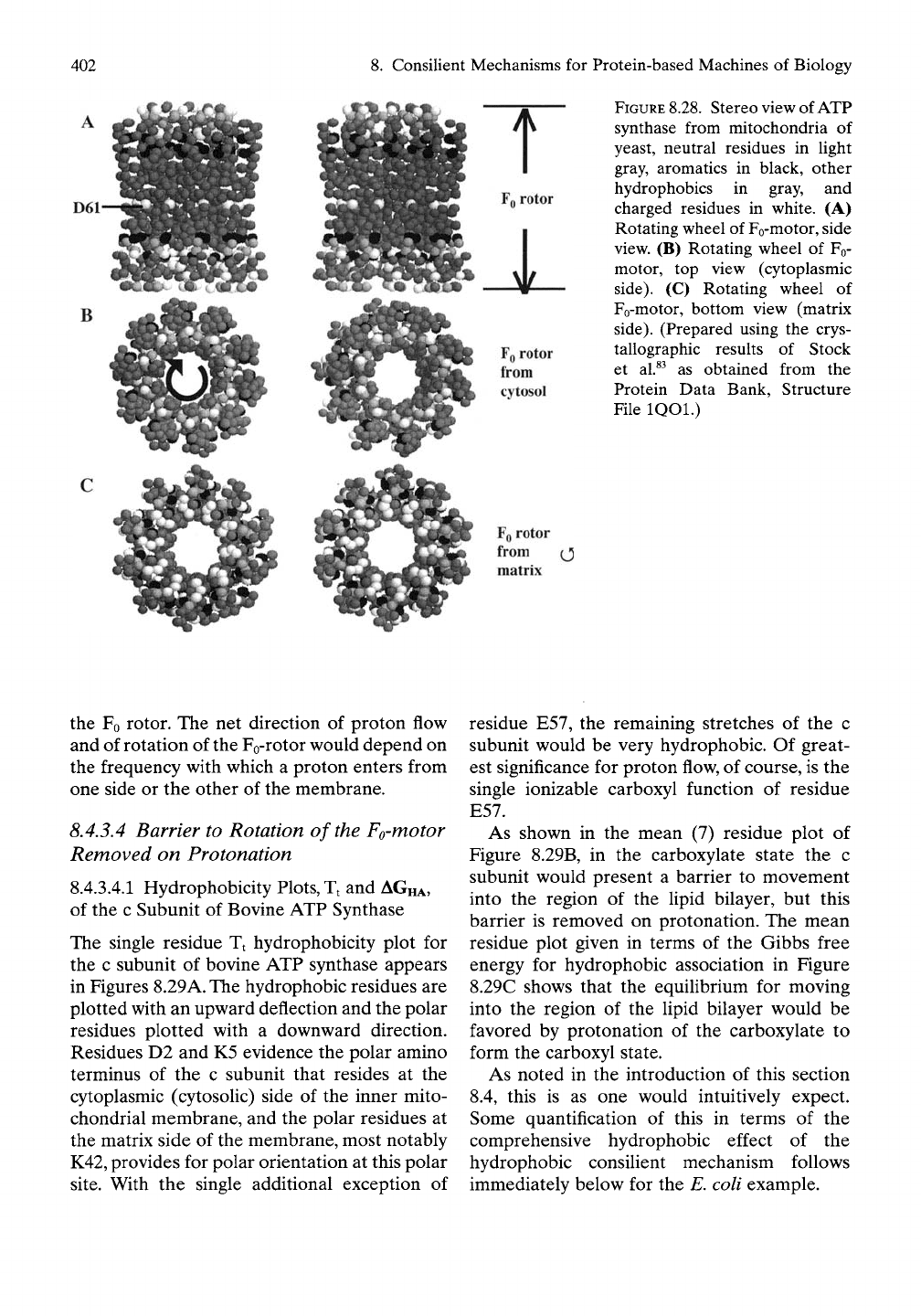
8.4.3.2 Three Perspectives of the Structure
of the Fo-rotor
Shown in cross-eye stereo side view in Figure
8.28 are three views of the Fo-rotor, that is, of
the rotating wheel of the Fo-motor. At this level
of resolution each residue is given as a sphere.
Furthermore, each of the 10 subunits of the
Fo-rotor is represented as a double-stranded
a-helical hairpin with the ends at the top
(cytosolic side) and the turn at the bottom
(matrix side) and with one side of the hairpin
in direct interaction with the lipid bilayer and
the other side forming the inner wall of the
rotating wheel. In Figure
8.28A
the aspartic
acid residue D61 is noted from outside. This
residue exists with its side chain as a carboxyl
(-COOH) when adjacent to the lipid bilayer,
but it releases its proton to form the carboxy-
late (-COO~) when it reaches the effective
channel when adjacent to the a-subunit.
8.4.3.3 Proton Flow (Cytosol to Matrix)
Drives Clockwise Rotation of the Fo-motor
A stereo view from the cytosolic (top) side of
the Fo-rotor is given in Figure 8.28B. From this
view the ends of the c subunits are seen. From
the matrix (bottom) side in Figure 8.28C, the
hairpin turn of the c subunits are seen. Briefly,
following FilUngame et al.,^^ a cytosolic proton
flows down a half-channel using the fourth of
five transmembrane helices of subunit a and
adds to the carboxylate of D61 that was ion
paired with arginine-210 (R210) of the fourth
transmembrane helix of subunit a. The more
hydrophobic newly protonated helix of the c
subunit rotates in a closkwise direction into the
lipid layer as the subsequent c subunit releases
its D61 proton by a second half-channel to the
8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
401
T
(ap)3 catalytic
motor housing
i
B
8 subunit
FIGURE 8.27. Stereo view of ATP synthase from the
mitochondria of yeast, neutral residues in light gray,
aromatics in black, other hydrophobics in gray, and
charged residues in white. (A) A largely complete
ATP synthase double rotary motor: Fo-motor, con-
necting rotor and Fi-motor, with only part of protein
subunits observed that couple the Fo-motor to the
rotor. (B) Fo-rotor, partial connector, and rotor
assembly of Fi-motor. (Prepared using the crystallo-
graphic results of Stock et al.^^ as obtained from the
Protein Data Bank, Structure File IQOl.)
matrix and replaces the previous c subunit by
ion pairing with the R210 at the fourth trans-
membrane helix. This completes the flow of a
single proton from the cytosol to the matrix side
of the inner mitochondrial membrane to give a
36° clockwise rotation in the FQ rotor. With
further stochastic motion the D61 carboxylate
of the newly positioned c subunit picks up
another proton from the cytosolic side of the
membrane and so on to complete the transit of
a second proton from one side to the other of
the membrane to effect a second 36° rotation in
402
8. Consilient Mechanisms for Protein-based Machines of Biology
D61
B
FQ
rotor
from (J
matrix
FIGURE
8.28.
Stereo view of ATP
synthase from mitochondria of
yeast, neutral residues in light
gray, aromatics in black, other
hydrophobics in gray, and
charged residues in white. (A)
Rotating wheel of Fo-motor, side
view. (B) Rotating wheel of Fo-
motor, top view (cytoplasmic
side).
(C) Rotating wheel of
Fo-motor, bottom view (matrix
side).
(Prepared using the crys-
tallographic results of Stock
et al.^^ as obtained from the
Protein Data Bank, Structure
File IQOl.)
the Fo rotor. The net direction of proton flow
and of rotation of the Fo-rotor would depend on
the frequency with which a proton enters from
one side or the other of the membrane.
8.4.3.4 Barrier to Rotation of the Fo-motor
Removed on Protonation
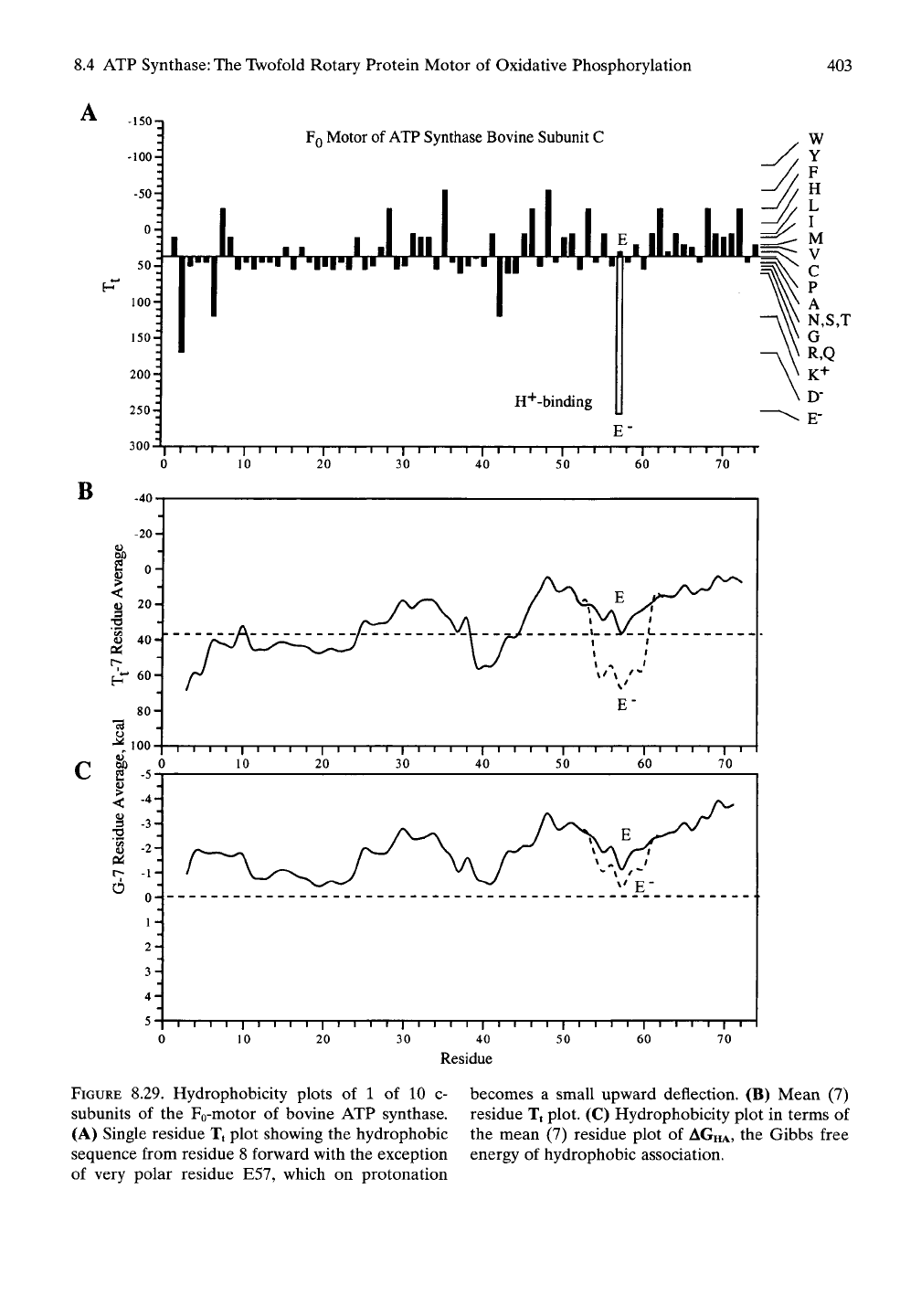
8.4.3.4.1
Hydrophobicity Plots, Tt and
AGHA,
of the c Subunit of Bovine ATP Synthase
The single residue Tt hydrophobicity plot for
the c subunit of bovine ATP synthase appears
in Figures 8.29A. The hydrophobic residues are
plotted with an upward deflection and the polar
residues plotted with a downward direction.
Residues D2 and K5 evidence the polar amino
terminus of the c subunit that resides at the
cytoplasmic (cytosolic) side of the inner mito-
chondrial membrane, and the polar residues at
the matrix side of the membrane, most notably
K42,
provides for polar orientation at this polar
site.
With the single additional exception of
residue E57, the remaining stretches of the c
subunit would be very hydrophobic. Of great-
est significance for proton flow, of course, is the
single ionizable carboxyl function of residue
E57.
As shown in the mean (7) residue plot of
Figure 8.29B, in the carboxylate state the c
subunit would present a barrier to movement
into the region of the lipid bilayer, but this
barrier is removed on protonation. The mean
residue plot given in terms of the Gibbs free
energy for hydrophobic association in Figure
8.29C
shows that the equilibrium for moving
into the region of the lipid bilayer would be
favored by protonation of the carboxylate to
form the carboxyl state.
As noted in the introduction of this section
8.4, this is as one would intuitively expect.
Some quantification of this in terms of the
comprehensive hydrophobic effect of the
hydrophobic consiUent mechanism follows
immediately below for the E. coli example.
8.4 ATP Synthase: The Twofold Rotary Protein Motor of Oxidative Phosphorylation
403
-150-
-100-
-50-
OH
B
50H
100-
150-
200"
250-
300 •
FQ
Motor of ATP Synthase Bovine Subunit C
I U
I J III! I lllilll
-40-
-20-
CJ
0/J
2
>
<
D
3
•T3
(/3
OB^
t^
b^
0
20
40
60
80-
100-
2
<
I
O
-4H
-3-]
-2H
-1-1
OH
lA
2-1
3i
4
5
i-i"i I
-|i|-|
|i |i I •!• •
H"^-binding
UlUiJlIlL
I
I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I I
10 20 30 40 50 60 70
10
20
> I I
30
—I—T-
40
50
60
70
v/ E-
10
-I—I—
20
30 40
Residue
I
' I
50
I
I I
60
T-r-
70
FIGURE
8.29. Hydrophobicity plots of 1 of 10 c- becomes a small upward deflection. (B) Mean (7)
subunits of the Fo-motor of bovine ATP synthase, residue Tt plot. (C) Hydrophobicity plot in terms of
(A) Single residue Tt plot showing the hydrophobic the mean (7) residue plot of
AGHA,
the Gibbs free
sequence from residue 8 forward with the exception energy of hydrophobic association,
of very polar residue E57, which on protonation

404
8. Consilient Mechanisms for Protein-based Machines of Biology
8.4.3.4.2
Thermodynamics of Protonation
and Efficiency of Energy Conversion:
AGHA[D- -> D'] « -3.8kcal/mole
From Table 5.3 we find that the decrease in
Gibbs free energy for hydrophobic association
on protonation of the carboxylate (-COO")
of aspartate, D~, to produce the carboxyl
(-COOH) of aspartic acid,
D^,
is approximately
-3.8kcal/mole. The protonation of 10 aspartic
acid residues as required for one complete rota-
tion of the Fo-motor could be expected to
provide an energy of 38kcal/rotation, which
constitutes one complete rotation of the y-
rotor. Accordingly, by means of the Fi-motor,
one complete rotation of the y-rotor translates
into the production of 3 mole of ATP from
ADP plus Pi. Because the approximate heat
released on hydrolysis of
1
mol of ATP to ADP
plus Pi is 8kcal/mole, one complete rotation of
the y-rotor would produce 24kcal worth of ATP
per rotation. An energy input of 30kcal/mole
and an output energy of 24kcal per rotation
gives an efficiency maximum of about 63%.
Thus,
the value of AGHA[D- -^ D^] - -3.8
kcal/mole appears reasonable, even though it
was a value obtained from the experimental
determination of the temperature for onset of
the inverse temperature transition using the
sigmoid curve of Figure 5.10 rather than
directly measured by differential scanning
calorimetry using the difference in heat of the
inverse temperature transition for the two
states of the aspartic acid residue.
8.4.4 Crystal Structure and
Function of the Fi-motor of ATP
Synthase-Fi-ATPase
8.4.4,1 Boyer Caution About Crystal
Structure Not Being That of Active
Catalytic State
Our biochemical understanding of the mecha-
nism of ATP synthase^"^ comes in largest
measure from the pioneering work of
Boyer.^^"^^ Because of this extensive expertise
and insight, the Boyer perspective on crystal
structure data reflects the judicious critic of the
limitations of crystal structures when attempt-
ing to describe the dynamics of catalysis:
The contribution of Menz et al is welcomed as
providing essential information for the difficult
task still ahead of satisfactorily correlating structure
with events of substrate binding, covalent catalysis,
and product release. For this task, it needs to be
recognized that forms revealed by X-ray analysis
of static inhibited enzymes may not appear as
such during active catalysis, even though they
are likely representative of most intermediate
forms.^^
Even so, crystal structures provide the best
snapshots of forces in action. Crystal structures
provide an unparalleled opportunity to assess
relevance to the major protein-based machines
of biology of the free energy transduction so
dominantly displayed by elastic-contractile
model proteins (as developed in Chapter 5). If
the apolar-polar repulsive free energy of
hydration, AGap, the operative component of
the Gibbs free energy of hydrophobic associa-
tion, AGHA, is active in ATP synthase, then it
should become apparent in these snapshots.
8.4.4.2 Assembled Subunits, (ap)3 yeS, of
the Fj-ATPase
8.4.4.2.1
Structural Information
The Fi-ATPase contains five different polypep-
tide subunits, a, [3, y, 8 and e, with the stoi-
chiometry of 3:3:1:1:1, that is, aapsYiSiEi. From
crystal structure data the a- and P-subunits may
be described as the trimer,
(ap)3,
with approxi-
mate threefold symmetry, but looking much
like a peeled orange with six nearly identical
sections. A cross section of the (ap)3Y structure
at the level of the p,Y-phosphates is shown
in Figure 8.30, which, among other things,
provides visualization of the approximate
threefold symmetry despite the different occu-
pancy sites and the obviously asymmetric posi-
tion of the y-rotor. The y-subunit forms the core
of the structure residing close to the threefold
axis.
As shown in Figure 8.27B, the y-subunit
enters the upper two-thirds of the (aP)3 orange-
