Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.


8.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
365
8.3.2.3.2
Proton Release to the Cytoplasmic
Side of the Inner Mitochondrial Membrane
As redox systems are not known to be near the
cytoplasmic side of the inner mitochondrial
membrane, it is more difficult to consider oxi-
dation sites where formation of the positively
charged species, QH^^ and FMNH2^, could
occur. These are both species that, theoretically,
could release protons to the cytoplasmic side of
the membrane to complete proton transit in a
tightly coupled way across the inner mitochon-
drial membrane. These positively charged
species are expected to exhibit decreased
lipid solubility, although the intramolecular
ion pairing of negatively charged phosphate to
the positively charged isoalloxazine ring of
FMNH2^ might improve the possibility of FMN
use.
Obviously, our limited knowledge of the
molecular structure of Complex I does not
allow sufficient insight to make a specific
consihent-based proposal.
83.2.4 Dependence of Reduction Potential
on Hydrophobicity of Binding Site?
As was shown for N-methyl nicotinamide in
Figure 5.20C, the reduction potential of nicoti-
namide varies with hydrophobicity of binding
site.
This allows the possibility that ubiquinol
could be oxidized at two different sites at the
cytoplasmic side of the inner mitochondrial
membrane, but the question becomes oxidized
by what, as the redox centers have been con-
sidered to be on the matrix side of the mem-
brane. These problems with understanding
proton transport by Complex I are not present
with Complex III, as discussed in section 8.3.4.
8.3.3 Complex II:
Succinateiubiquinone Reductase—
Reduction of FAD by Succinate to
Produce FADH2, Which Ultimately
Results in Reduction of Ubiquinone
to Produce Ubiquinol for Subsequent
Reactions of the Electron
Transport Chain
Mammalian Complex II contains four subunits.
The larger two subunits are extramembrane on
the matrix side. They are a 79kDa flavoprotein
and a 29kDa iron-sulfur protein with three
iron sulfur centers: Fe2S2, Fe4S4, and Fe3S4. The
two membrane bound subunits bind a single
heme b and are reported to contain two or
more ubiquinone/ubiquinol binding sites.
8.3.3.1 Function of Succinate
Dehydrogenase, the E. coli Analogue of
Complex II (Succinate.'ubiquinone
Reductase)
The Singular Purpose of Succinate Dehydroge-
nase is to Provide Reduced Ubiquinone, That
is,
Ubiquinol, to the Inner Mitochondrial Mem-
brane as an Electron Carrier and as Chemical
Energy for Proton Transport. The overall reac-
tion is as follows:
OOCCH2CH2COO + Q = OOCCH =
CHCOO +QH2
Succinate + Ubiquinone =
Fumarate + Ubiquinol (8.12)
Equation (8.9) is a reversible reaction; it can
run either direction by changes in the relative
concentrations of succinate and fumarate. Even
so,
biology has different enzymes specializing in
producing fumarate from succinate under
aerobic conditions and for producing succinate
from fumarate under anaerobic conditions.
The two enzymes appear essentially identical
with the same prosthetic groups, that is, FAD
and the same three iron sulfur centers. There
is,
however, an important difference of side
products of reactive oxygen species (ROS)
that is greater in the reaction catalyzed by
fumarate reductase enzyme (see section
8.3.3.4
below) than in the reaction catalyzed by
succinate:ubiquinone reductase. It has to do
with a small difference in the accessibility of the
flavin prosthetic group to the aqueous phase.
The flavin appears better shielded from dis-
solved oxygen in succinate:ubiquinone reduc-
tase.^"^
Obviously, the electron transport chain
of the inner membrane of the mitochondrion
must function under aerobic conditions as the
terminal reaction due to Complex IV
(cytochrome c reductase) involves molecular
oxygen (O2) as a reactant, that is, the reduction
of oxygen to water.

366
8. Consilient Mechanisms for Protein-based Machines of Biology
83,3,2 Succinatembiquinone Reductase in
Three Distinct Reaction Steps
8.3.3.2.1
Step 1: Succinate Reduction of FAD
to Produce FADH2 and Fumarate
OOCCH2CH2COO +FAD
=
OOCCH
=
CHCOO
+ FADH2
(8.12a)
In this reaction, the protons and electrons
transfer together as hydrogen atoms.
8.3.3.2.2
Step 2: Oxidation of FADH2 to FAD
by Transfer of Electrons to FeS Centers with
Release of Protons to Matrix
FADH2
+ 2FeS =
FAD
+ 2FeS +
IW (8.12b)
This reaction of Complex II begins the separa-
tion of electrons from protons. However, why
does Complex II not contribute to proton
transport? If
FADH2
were oxidized on the cyto-
plasmic side of the lipid bilayer membrane, then
conceivably the two protons could be released
to the cytosol, as required for effective proton
transport.
8.3.3.2.3
Step
3:
Transfer of Electrons to
Ubiquininone (Coenzyme Q) from FeS
Centers to Produce Negatively Charged
Ubiquinone,
Q^~,
Which on Receipt of Protons
from Matrix Produces QH2
2FeS
+ Q + 2H
=
2FeS + QH2
(8.12c)
The reduction of ubiquinone by receipt of two
electrons to produce
Q^"
within the lipid bilayer
membrane, but yet on the matrix side, would
allow the pick up of two protons from the
matrix. The process would result from forma-
tion of the negatively charged Q^~ whereby
the competition for hydration due to the
apolar-polar repulsive free energy of hydra-
tion,
AGap,
would open an aqueous channel to
the matrix side of the membrane for entry of
two protons, 2H^. With the above-preferred
location for reaction (8.9b) combined with the
just noted location of reaction (8.9c), there
would be a net transport of two protons from
the matrix side to the cytoplasmic side of the
membrane. This would be in keeping with the
direction of proton transport for Complexes I,
III,
and
IV.
As shown below in Figures 8.8 and
8.9, however, transport could not occur by this
utilization of hypothesis 1 of the consilient
mechanism for proton pumping (see section
8.3.1.2), because the arrangement of the redox
centers is not as required to release two protons
to the cytosol. In keeping with this perspective,
of course. Complex II does not pump protons.
Nonetheless, when ubiquinol is used by
Complex III, recall that the two protons that
added to ubiquinone to form ubiquinol came
from the matrix side.
8.3,3,3 Structure of Succinate
Dehydrogenase, the E. coli Analogue of
Complex II (Succinate-ubiquinone
Reductase)
The molecular structure of the analogue for
Complex II, succinate dehydrogenase from E.
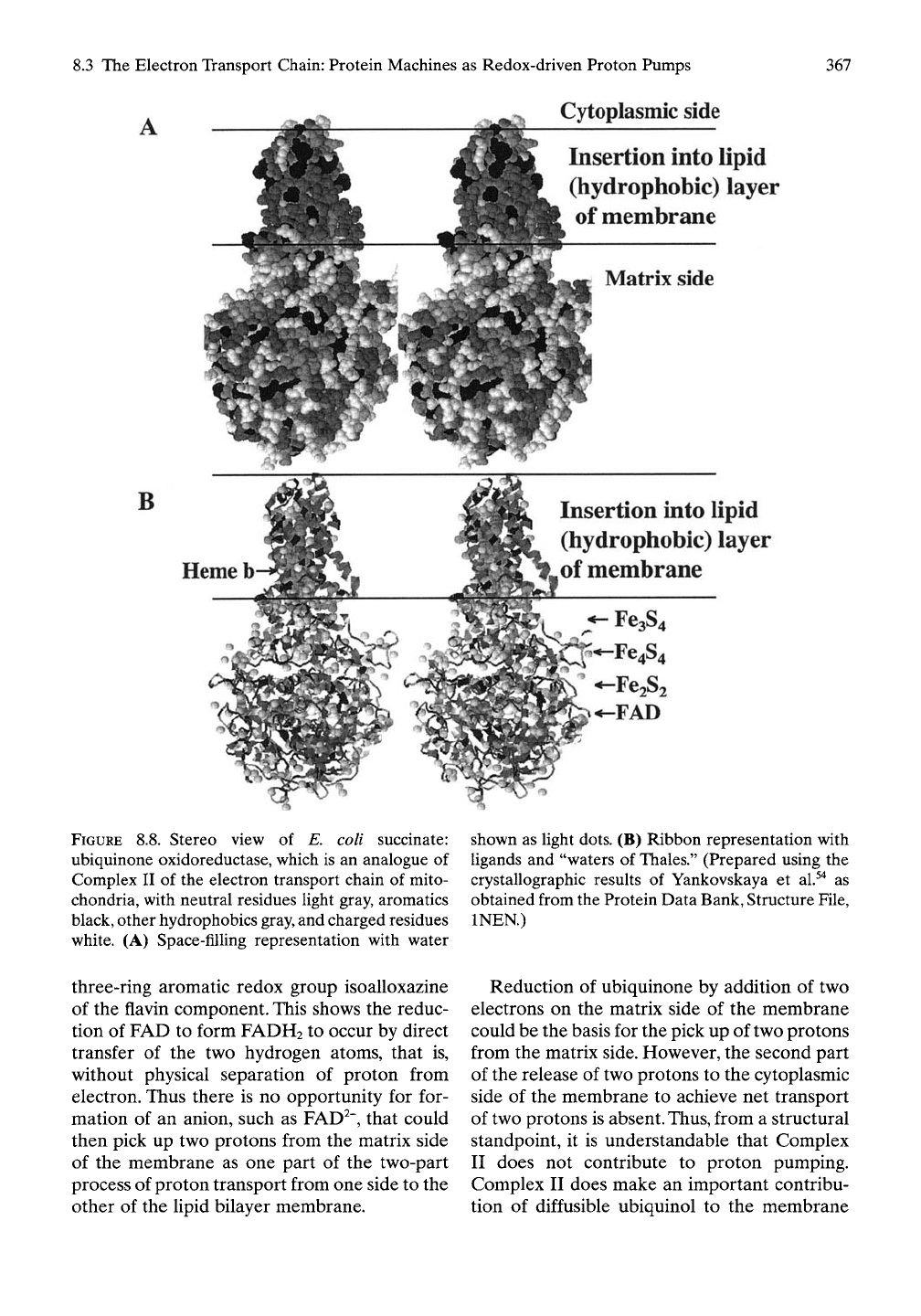
coli, is shown in Figure 8.8^"^ to exhibit the
general form shown in Figure 8.6. Figure 8.8A
uses a space-filling representation where
neutral residues are light gray, aromatic
residues are black, other hydrophobic residues
are gray, and charged residues are white. With
this means of representation, immediately
apparent is a hydrophobic tip for insertion into
the lipid layer of the membrane. Also apparent
is a larger globular, more hydrophilic com-
ponent that resides within the matrix side of
the membrane.
With the ribbon representation in Figure
8.8B, a more transparent view of the structure
allows visualization of the redox groups and the
distribution of water molecules, shown as light
dots.
The FAD resides nearly centered within
the globular component and is associated with
very few water molecules. Then, in a stepwise
manner, the three iron-sulfide centers fill in
between FAD and the lipid bilayer, and just
within the region of the lipid bilayer resides the
heme b group. Reduction of ubiquinol occurs
between the Fe3S4 center. On removal of the
protein, this distribution of redox centers
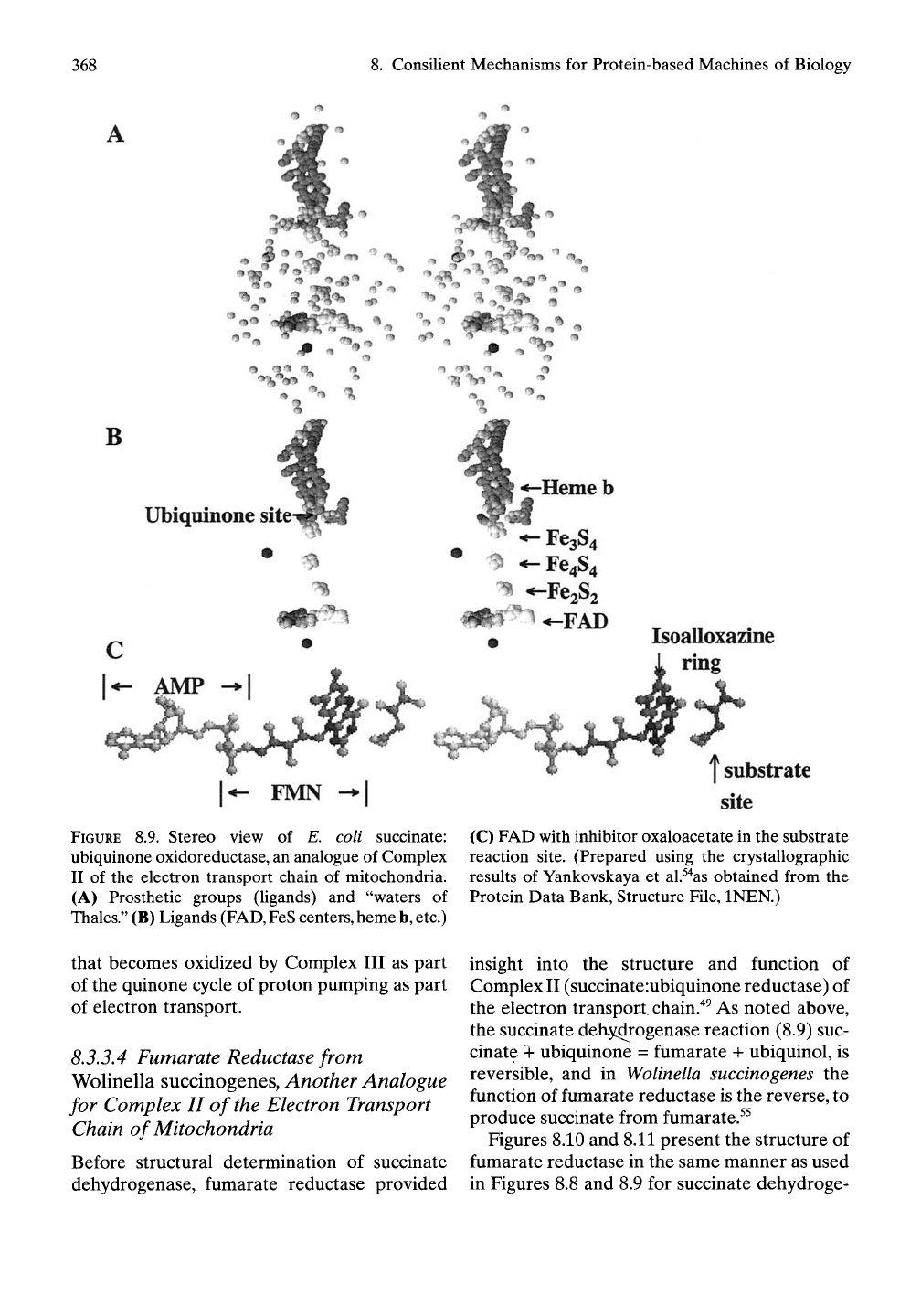
becomes more apparent in Figure 8.9A,B.
An expanded view of the FAD group is
shown in Figure 8.9C along with the inhibitor,
oxaloacetate, that resides in the substrate (suc-
cinate) site. FAD is shown to be the phosphate
to phosphate coupling of adenosine monophos-
phate (AMP) with FMN. The key point of
Figure 8.9C is the proximity of substrate to the
!.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
Cytoplasmic side
Insertion into lipid
(biydrophobic) layer
of membrane
367
Matrix side
B
Heme
b
Insertion into lipid
(hydrophobic) layer
of membrane
*- Fe3S4
VFe4S4
"^FejSj
-•-FAD
FIGURE 8.8. Stereo view of E. coli succinate:
ubiquinone oxidoreductase, which is an analogue of
Complex II of the electron transport chain of mito-
chondria, with neutral residues light gray, aromatics
black, other hydrophobics
gray,
and charged residues
white. (A) Space-filling representation with water
three-ring aromatic redox group isoalloxazine
of the flavin component. This shows the reduc-
tion of FAD to form FADH2 to occur by direct
transfer of the two hydrogen atoms, that is,
without physical separation of proton from
electron. Thus there is no opportunity for for-
mation of an anion, such as FAD^", that could
then pick up two protons from the matrix side
of the membrane as one part of the two-part
process of proton transport from one side to the
other of the lipid bilayer membrane.
shown as light dots. (B) Ribbon representation with
ligands and "waters of Thales." (Prepared using the
crystallographic results of Yankovskaya et al.^"* as
obtained from the Protein Data Bank, Structure File,
INEN.)
Reduction of ubiquinone by addition of two
electrons on the matrix side of the membrane
could be the basis for the pick up of two protons
from the matrix side. However, the second part
of the release of two protons to the cytoplasmic
side of the membrane to achieve net transport
of two protons is absent. Thus, from a structural
standpoint, it is understandable that Complex
II does not contribute to proton pumping.
Complex II does make an important contribu-
tion of diffusible ubiquinol to the membrane
368
8. Consilient Mechanisms for Protein-based Machines of Biology
B
Ubiquinone site
fl*r^*-FAD
.$*^^J^^
Isoalloxazine
ring
|-«-
FMN -•I
FIGURE 8.9. Stereo view of E. coli succinate:
ubiquinone oxidoreductase, an analogue of Complex
II of the electron transport chain of mitochondria.
(A) Prosthetic groups (ligands) and "waters of
Thales."
(B) Ligands
(FAD,
FeS
centers,
heme
b,
etc.)
that becomes oxidized by Complex III as part
of the quinone cycle of proton pumping as part
of electron transport.
8.33.4
Fumarate Reductase from
Wolinella succinogenes, Another Analogue
for Complex II of the Electron Transport
Chain of Mitochondria
Before structural determination of succinate
dehydrogenase, fumarate reductase provided
f substrate
site
(C) FAD with inhibitor oxaloacetate in the substrate
reaction site. (Prepared using the crystallographic
results of Yankovskaya et al.^'^as obtained from the
Protein Data Bank, Structure File, INEN.)
insight into the structure and function of
Complex II (succinate.ubiquinone reductase) of
the electron transport chain."^^ As noted above,
the succinate dehydrogenase reaction (8.9) suc-
cinate + ubiquinone = fumarate + ubiquinol, is
reversible, and in Wolinella succinogenes the
function of fumarate reductase is the reverse, to
produce succinate from fumarate.^^
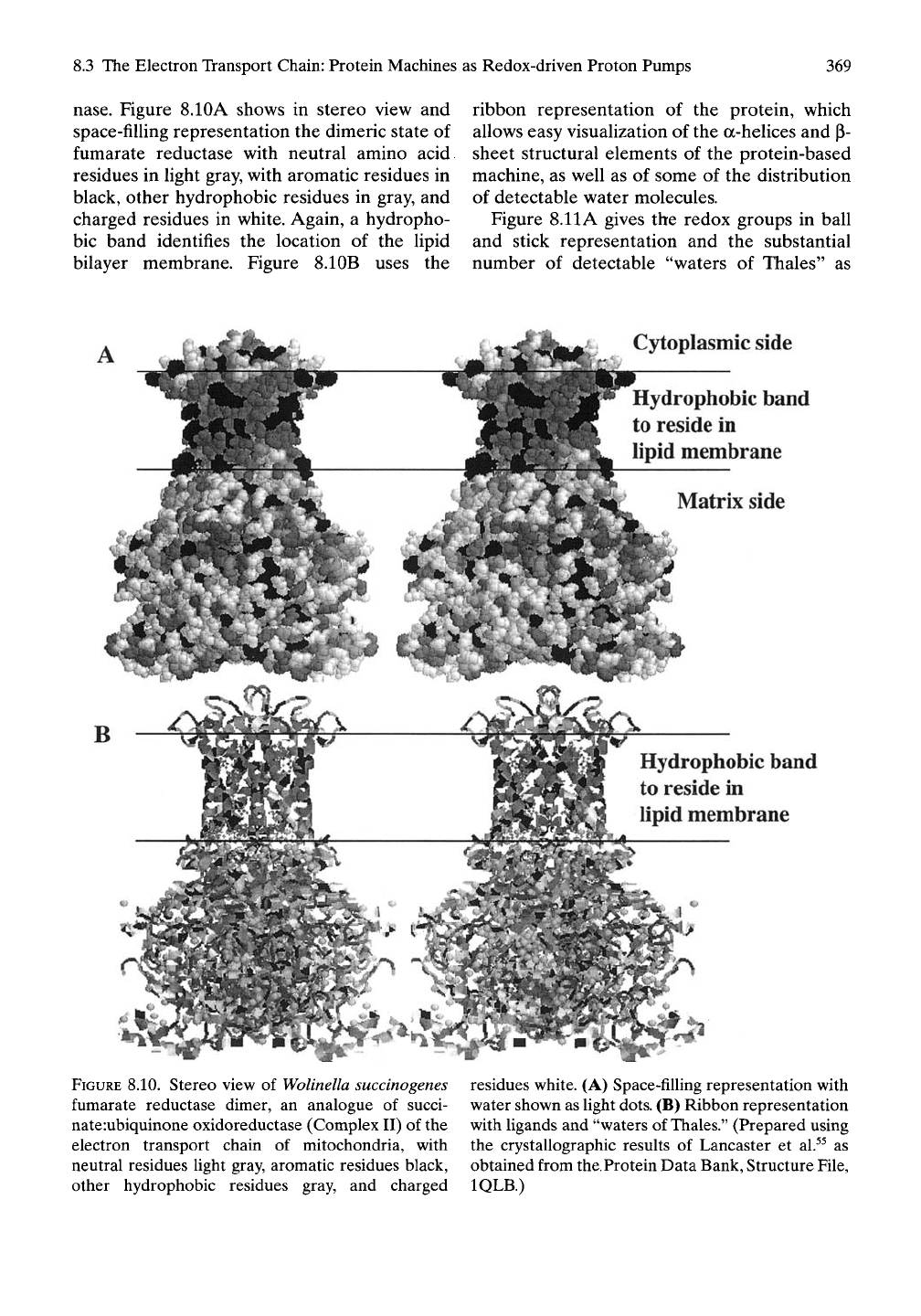
Figures 8.10 and 8.11 present the structure of
fumarate reductase in the same manner as used
in Figures 8.8 and 8.9 for succinate dehydroge-
8.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
369
nase.
Figure 8.10A shows in stereo view and
space-filling representation the dimeric state of
fumarate reductase with neutral amino acid
residues in light gray, with aromatic residues in
black, other hydrophobic residues in gray, and
charged residues in white. Again, a hydropho-
bic band identifies the location of the lipid
bilayer membrane. Figure 8.10B uses the
ribbon representation of the protein, which
allows easy visualization of the a-helices and p-
sheet structural elements of the protein-based
machine, as well as of some of the distribution
of detectable water molecules.
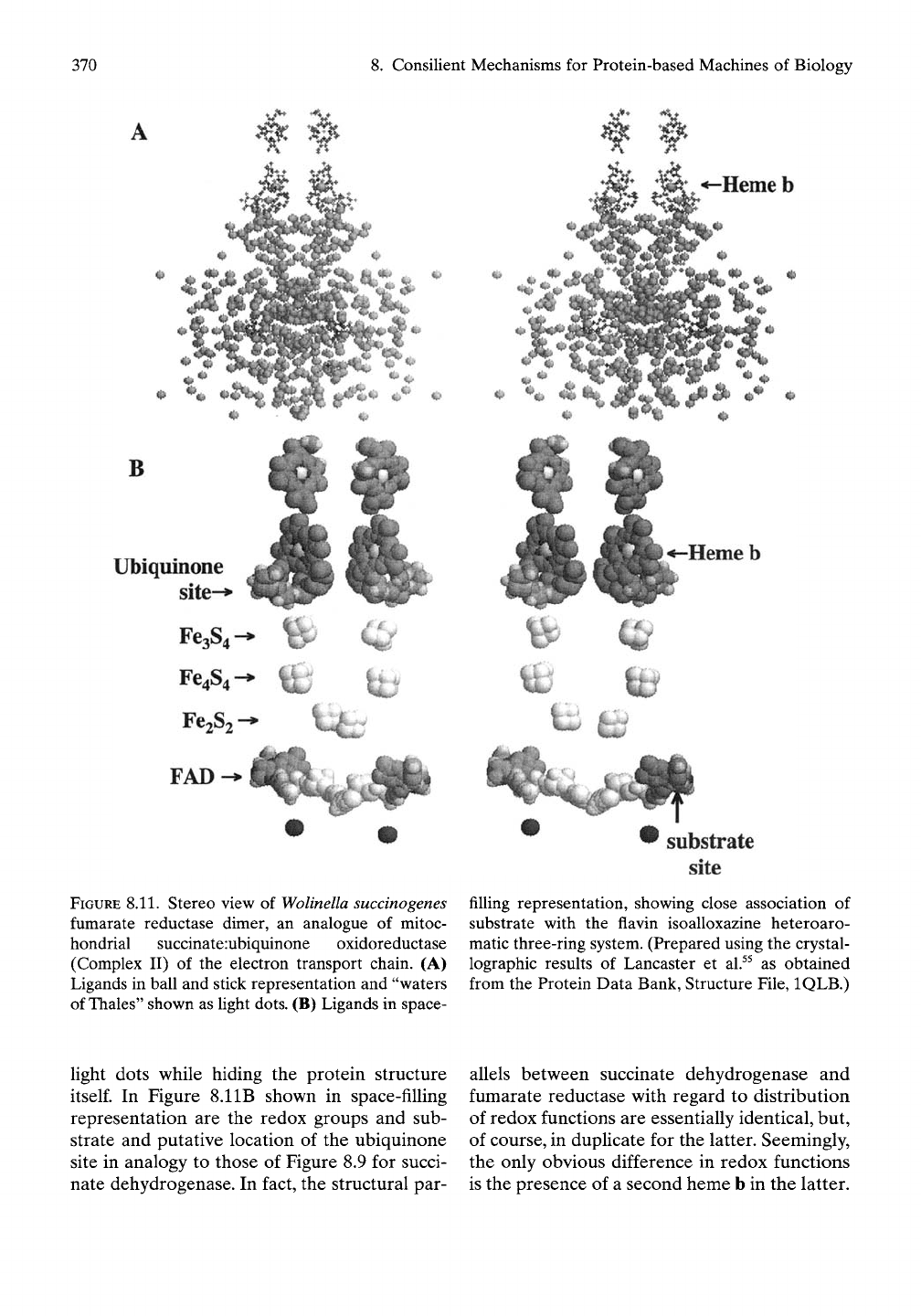
Figure 8.11 A gives the redox groups in ball
and stick representation and the substantial
number of detectable "waters of Thales" as
Cytoplasmic side
Hydropliobic band
to reside in
lipid membrane
Matrix side
Hydrophobic band
to reside in
lipid membrane
FIGURE 8.10. Stereo view of
Wolinella succinogenes
fumarate reductase dimer, an analogue of succi-
nateiubiquinone oxidoreductase (Complex II) of the
electron transport chain of mitochondria, with
neutral residues light gray, aromatic residues black,
other hydrophobic residues gray, and charged
residues white. (A) Space-filling representation with
water shown as Hght
dots.
(B) Ribbon representation
with ligands and "waters of
Thales."
(Prepared using
the crystallographic results of Lancaster et al.^^ as
obtained from the.Protein Data Bank, Structure File,
IQLB.)
370
8. Consilient Mechanisms for Protein-based Machines of Biology
^-Heme b
Ubiquinone
site-*
Fe3S4
Fe4S4'
FezSj
FAD-«
- fe^ <ir
-* SB ii)
J^^^ ^^^IK-Heme
b
%>
(J?
& ^
^"W**^ B^l^
^ • substrate
site
FIGURE 8.11. Stereo view of
Wolinella succinogenes
fumarate reductase dimer, an analogue of mitoc-
hondrial succinate :ubiquinone oxidoreductase
(Complex II) of the electron transport chain. (A)
Ligands in ball and stick representation and "waters
of
Thales"
shown as hght dots. (B) Ligands in space-
filling representation, showing close association of
substrate with the flavin isoalloxazine heteroaro-
matic three-ring system. (Prepared using the crystal-
lographic results of Lancaster et al.^^ as obtained
from the Protein Data Bank, Structure File, IQLB.)
light dots while hiding the protein structure
itself.
In Figure 8.11B shown in space-filling
representation are the redox groups and sub-
strate and putative location of the ubiquinone
site in analogy to those of Figure 8.9 for succi-
nate dehydrogenase. In fact, the structural par-
allels between succinate dehydrogenase and
fumarate reductase with regard to distribution
of redox functions are essentially identical, but,
of course, in duplicate for the latter. Seemingly,
the only obvious difference in redox functions
is the presence of a second heme b in the latter.

8.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
371
This,
however,
is
without known functional con-
sequence, as both proteins seem to function in
the absence of the heme groups."^^'^^ Also, very
apparent is the greater number of detectable
water molecules proximal to the flavin group.
Although this organism functions anaerobi-
cally, molecular oxygen, when present, is an
order of magnitude more likely to become
ROS.^^
The disarming of ROS and their dam-
aging effects is the reason that antioxidants are
so beneficial. Of
course,
by the consilient mech-
anism, the action of ROS on proteins destroys
function, because such polar additions to pro-
teins limit the hydrophobic associations essen-
tial to function (see section 7.7 of Chapter 7).
Again, the structure provides an understand-
ing of why this protein with all of the redox
functions to effect proton transport does not
pump
protons.
As shown below, the structure of
Complex III (ubiquinonexytochrome c oxi-
doreductase) provides remarkable insight into
the coupling of electron flow to proton
pumping, especially when viewed from the
vantage point of the consilient mechanism with
its functional element of an apolar-polar repul-
sive free energy of hydration, AGap.
8.3.4 Complex III:
Ubiquinonexytochrome c
Oxidoreductase (The Cytochrome
bci Complex)
With the exciting achievement of molecular
structures for Complex ni,^"^'^^'^^^^ combined
with extensive studies on kinetics, thermody-
namics, and stoichiometry,"^^ came the first clear
insight into the coupling of electron transport
to transmembrane proton pumping. This pro-
cess,
so essential to sustain Life, is no longer
shrouded in mystery. The following constitutes
the first report whereby the proposed consiUent
mechanism extends our understanding of
mechanistic detail for the function of Complex
III,
and both hydrophobic and elastic consilient
mechanisms come into play.^^
The hydrophobic and elastic consilient mech-
anisms, two distinct but interlinked physical
processes of hydrophobic association/dissocia-
tion and elastic force development/relaxation,
couple to achieve movement. In functional
homodimer of Complex III, hydrophobic asso-
ciation of the RIP FeS center at the Qo site in
one monomer occurs with elastic force devel-
opment in the tether that connects the globular
component of the RIP to its membrane anchor
in the other monomer. Formation of the posi-
tively charged ubiquinol, as electrons pass to
the FeS center and to heme bL, effects
hydrophobic dissociation and allows relaxation
of the elastic forces whereby the extended
tether moves the FeS center to heme Ci for
transfer of its electron.
Proton gating represents another demonstra-
tion of the consilient mechanism in Complex
III.
The operative element of the change in
Gibbs free energy for hydrophobic association,
the apolar-polar repulsive free energy of
hydrophobic hydration,
AGap,
uses the develop-
ment of negative charge at the Qi site to open
an aqueous channel for uptake of proton from
the matrix side of the membrane. As noted
above, the formation of positive charge at the
Qo site displaces the hydrophobically associ-
ated RIP and enables release of two protons to
the cytoplasmic side of the membrane. At both
Qo
and Qi sites the formation of charge disrupts
hydrophobic association by means of
AGap
and
opens the door to proton egress and ingress.
These roles of the consihent mechanism are
demonstrated with structures of Complex III
reported in the literature and are available for
development and analysis from the Protein
Data Bank using programs such as FrontDoor
to Protein Explorer by Eric Martz, which is
available at no cost at www.proteinexplorer.
org.
8.3A.1 General Structural Description of
Complex III (Ubiquinol: cytochrome c
Oxidoreductase)
8.3.4.1.1
Composition
Eleven protein subunits comprise the monomer
of Complex III, but only three of these subunits
contain the redox
centers.
They are cytochrome
b,
an integral membrane protein with two
hemes (bt and bn), the RIP on the cytoplasmic
side with a single a-helical membrane anchor,
and cytochrome Ci also located on the cyto-
plasmic side and also with a single a-hehcal
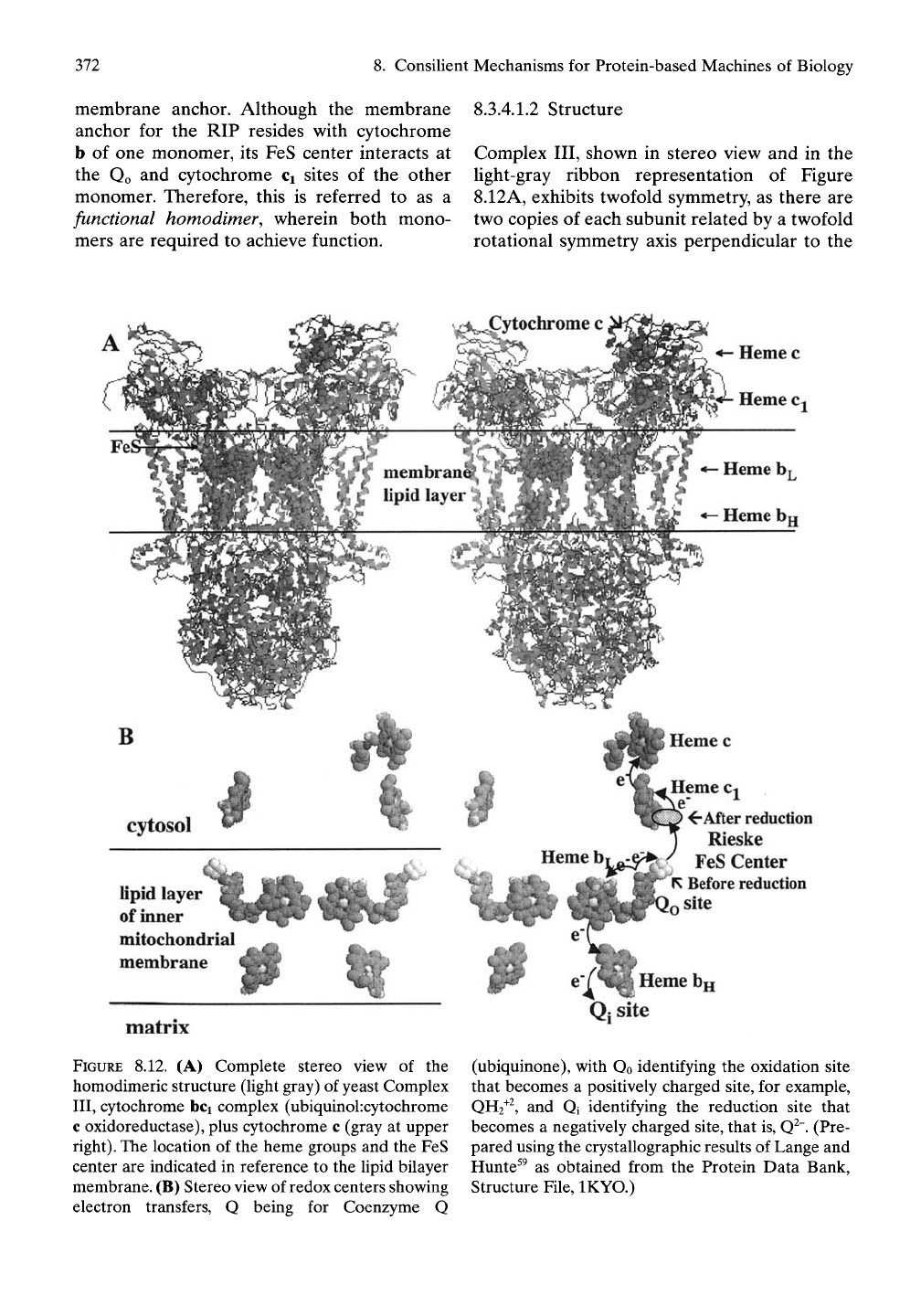
372
membrane anchor. Although the membrane
anchor for the RIP resides with cytochrome
b of one monomer, its FeS center interacts at
the Qo and cytochrome Ci sites of the other
monomer. Therefore, this is referred to as a
functional homodimer, wherein both mono-
mers are required to achieve function.
8. Consilient Mechanisms for Protein-based Machines of Biology
8.3.4.1.2
Structure
Complex III, shown in stereo view and in the
light-gray ribbon representation of Figure
8.12A,
exhibits twofold symmetry, as there are
two copies of each subunit related by a twofold
rotational symmetry axis perpendicular to the
•- Heme c
^ Heme Cj
membranS^^
lipid layer
>
B
cytosol
4
lipid layer
ofimier
mitochondrial
membrane
^After reduction
Rieske
FeS Center
1^
Before reduction
site
Heme bg
matrix
FIGURE 8.12. (A) Complete stereo view of the
homodimeric structure (light gray) of yeast Complex
III,
cytochrome bci complex (ubiquinolxytochrome
c oxidoreductase), plus cytochrome c (gray at upper
right).
The location of the heme groups and the FeS
center are indicated in reference to the lipid bilayer
membrane. (B) Stereo view of redox centers showing
electron transfers, Q being for Coenzyme Q
Qj site
(ubiquinone), with Qo identifying the oxidation site
that becomes a positively charged site, for example,
QH2^^,
and Qi identifying the reduction site that
becomes a negatively charged site, that is, Q^". (Pre-
pared using the crystallographic results of Lange and
Hunte^^ as obtained from the Protein Data Bank,
Structure File, IKYO.)

8.3 The Electron Transport Chain: Protein Machines as Redox-driven Proton Pumps
373
membrane plane. Also shown in a darker gray
ribbon representation in Figure 8.12A is the
presence of a single molecule of cytochrome c
at the top righthand side in position to receive
an electron from the heme of cytochrome Ci
and to leave that site on the cytosolic side as the
reduction product of the overall reaction. The
prosthetic groups are shown again in stereo
view and in twofold symmetry in Figure 8.12B
plus the single cytochrome c heme center at the
upper right.
The ribbon representation shows transmem-
brane a-helices traversing through the lipid
bilayer membrane, containing heme b pros-
thetic groups, the Qo and Qi sites, and the FeS
center at the tip of the globular component of
the RIP and associated with the Qo site.
8.3,4.2 Phenomenology of Ubiquinol
Oxidation to Reduce Cytochrome c with
Net Proton Transport
8.3.4.2.1
Overall Reaction
The overall equation for the reaction catalyzed
by Complex III follows is given above in Equa-
tion
(8.7).
At first glance this balanced equation
seems to have redundant reactants and prod-
ucts.
Why does the equation have two QH2
molecules as reactant and yet give one QH2 as
product? Inversely for Q, why is there one Q
molecule as a reactant and two as a product?
Also,
why does the reaction read two protons
from the matrix with four protons being
released to the cytosol, and yet this reaction is
generally credited with pumping four protons?
Taking the last question first, the additional two
protons came from the matrix by means of
reduction of ubiquinone by Complex II. Thus,
by supplying ubiquinol to the membrane pool.
Complex II in reality contributes to the net
transport of four protons by Complex III.
The answer to the first two questions is that
Equation (8.7), in addition to stoichiometry,
reveals elements of mechanism. A bifurcation
of electron flow on oxidation of a single QH2
molecule at the Qo site occurs with the first
electron going to the FeS center and the second
passing through hemes be and be to add an
electron to Q at the Qi site. Now a second QH2
molecule at the Qo site repeats the bifurcation
to complete the reduction of ubiquinone at the
Qi site, which
Q^"
picks up two protons from the
matrix side of the membrane to complete
the stoichiometry of two QH2 molecules used
at the Qo site with one recovered at the Qi site.
The net result is two QH2 molecules used giving
two Q molecules, but one molecule of Q was
reduced, thereby regaining one molecule of
QH2.
8.3.4.2.2
Electron Transfer Steps
Focusing on the electron transfer steps indi-
cated in Figure 8.12B, a single QH2 molecule at
the Qo site passes one electron to the FeS
center; the FeS center then moves to the heme
Ci and reduces it; the reduced heme Ci transfers
its electron to the heme of cytochrome
c^^.
The
result is the production of one molecule of
cytochrome c^^ and QH2^ at the Qo site. The
QH2^
molecule at the Qo site then transfers a
second electron that passes through the b
hemes to ubiquinone at the Qi site. A second
QH2 molecule is similarly oxidized with the
reduction of a second cytochrome c^"^ to result
in two molecules of cytochrome c^^. Although
the electron transfer phenomenology is well
recognized and even the mechanism of electron
tunneling over significant distances is an
accepted mechanism, there remain issues of the
mechanism whereby domains associate/dissoci-
ate and move where and when necessary. These
points are discussed in terms of the hydropho-
bic and elastic consilient mechanisms, the prin-
cipal message of this book, in section 8.3.4.3.
8.3.4.2.3
Proton Transport Sites
Occupied Qo and Qi sites involved in proton
transport are shown in stereo view in associa-
tion with the heme bt and bn ligands in Figure
8.13A in ball and stick representation and in
Figure 8.13B in space-filling representation.
This is a monomer subset shown in stereo of the
Ugands that were shown in the homodimer of
Complex III in Figure 8.12B, which contained
in additional the heme Ci and c ligands. The
added schematic representation of Figure
8.13A illustrates that the oxidation due to the
removal of two electrons from a molecule of
ubiquinol at the Qo site sets the stage for trans-
fer of two protons to the cytoplasmic side of
the inner mitochondrial membrane. Also, the
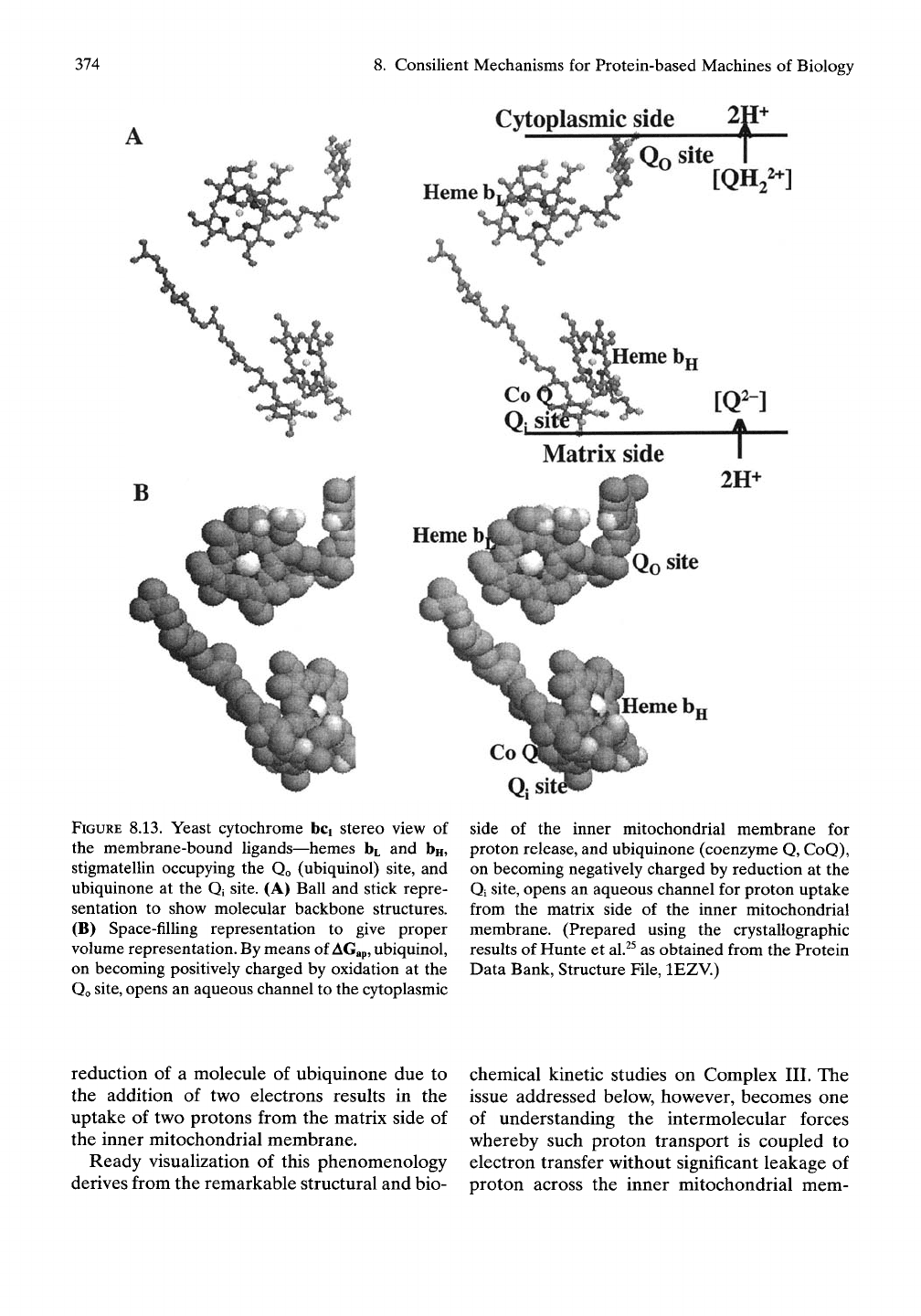
374
8. Consilient Mechanisms for Protein-based Machines of Biology
Cytoplasmic side 21
w
O:
sit^
FIGURE 8.13. Yeast cytochrome bci stereo view of
the membrane-bound Ugands—hemes bL and bn,
stigmatellin occupying the Qo (ubiquinol) site, and
ubiquinone at the Qi site. (A) Ball and stick repre-
sentation to show molecular backbone structures.
(B) Space-filling representation to give proper
volume representation. By means of
AGap,
ubiquinol,
on becoming positively charged by oxidation at the
Qo
site,
opens an aqueous channel to the cytoplasmic
side of the inner mitochondrial membrane for
proton release, and ubiquinone (coenzyme Q, CoQ),
on becoming negatively charged by reduction at the
Qi site, opens an aqueous channel for proton uptake
from the matrix side of the inner mitochondrial
membrane. (Prepared using the crystallographic
results of Hunte et
al.^^
as obtained from the Protein
Data Bank, Structure File, lEZV.)
reduction of a molecule of ubiquinone due to
the addition of two electrons results in the
uptake of two protons from the matrix side of
the inner mitochondrial membrane.
Ready visualization of this phenomenology
derives from the remarkable structural and bio-
chemical kinetic studies on Complex III. The
issue addressed below, however, becomes one
of understanding the intermolecular forces
whereby such proton transport is coupled to
electron transfer without significant leakage of
proton across the inner mitochondrial mem-
