Urry D.W. (Ed.) What Sustains Life? : Consilient Mechanisms for Protein-Based Machines and Materials
Подождите немного. Документ загружается.

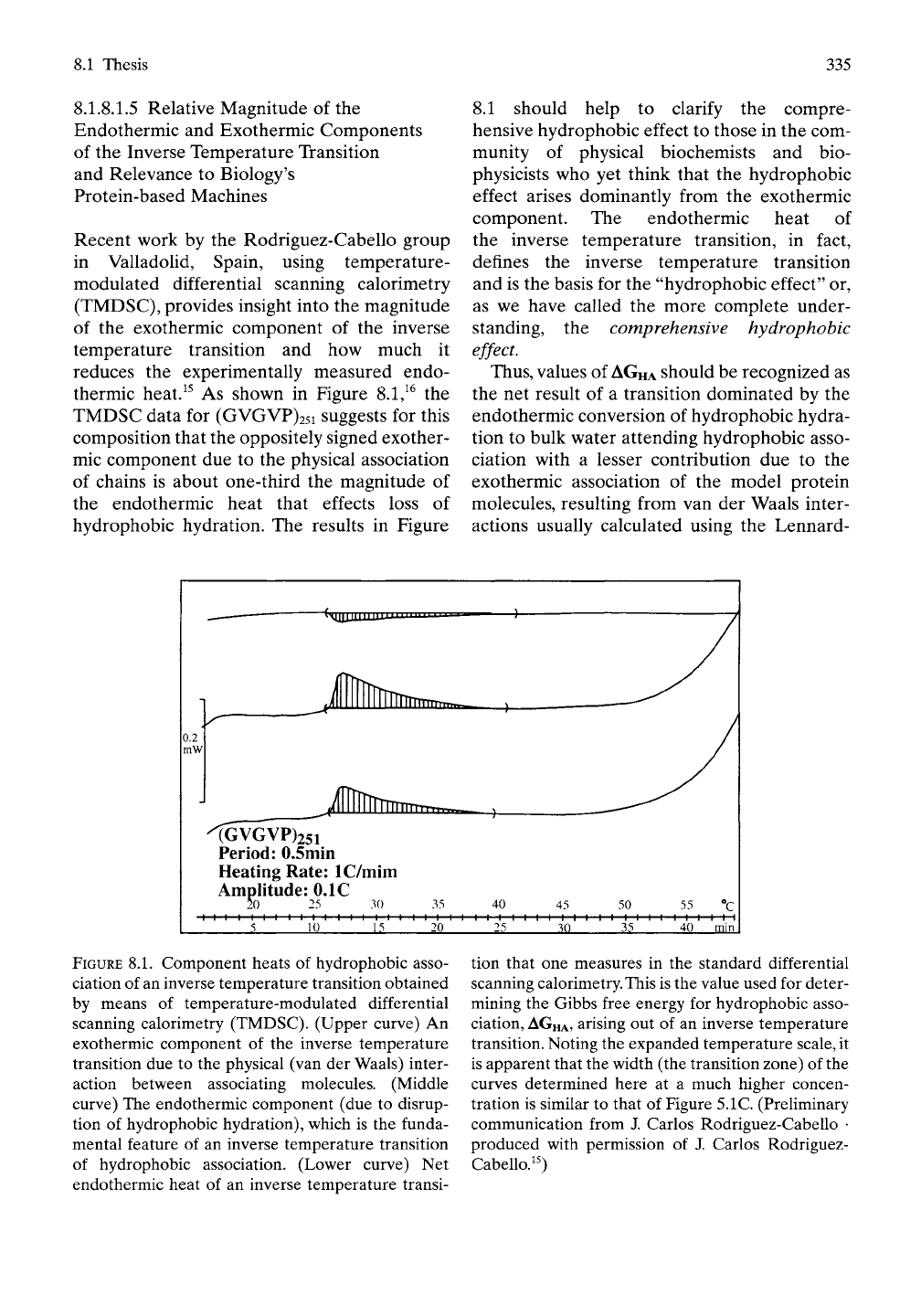
8.1 Thesis 335
8.1.8.1.5
Relative Magnitude of the
Endothermic and Exothermic Components
of the Inverse Temperature Transition
and Relevance to Biology's
Protein-based Machines
Recent work by the Rodriguez-Cabello group
in Valladolid, Spain, using temperature-
modulated differential scanning calorimetry
(TMDSC), provides insight into the magnitude
of the exothermic component of the inverse
temperature transition and how much it
reduces the experimentally measured endo-
thermic heat.^^ As shown in Figure 8.1/^ the
TMDSC data for (GVGVP)25i suggests for this
composition that the oppositely signed exother-
mic component due to the physical association
of chains is about one-third the magnitude of
the endothermic heat that effects loss of
hydrophobic hydration. The results in Figure
8.1 should help to clarify the compre-
hensive hydrophobic effect to those in the com-
munity of physical biochemists and bio-
physicists who yet think that the hydrophobic
effect arises dominantly from the exothermic
component. The endothermic heat of
the inverse temperature transition, in fact,
defines the inverse temperature transition
and is the basis for the "hydrophobic effect" or,
as we have called the more complete under-
standing, the comprehensive hydrophobic
effect.
Thus,
values of
AGHA
should be recognized as
the net result of a transition dominated by the
endothermic conversion of hydrophobic hydra-
tion to bulk water attending hydrophobic asso-
ciation with a lesser contribution due to the
exothermic association of the model protein
molecules, resulting from van der Waals inter-
actions usually calculated using the Lennard-
f^mmnnimn:
|0.2
mWl
^lllllflTlTTTmTmrT^
__^/l11lTlTrTmTTTTT^^
)
"(GVGVP)25i
Period: O.Smin
Heating Rate: IC/mim
Amplitude: O.IC
20 25 30 35 40 45
H-H—I—I—I—t—I
I I I—I I I
5 10
I I I—t—HH—HH—I—+—I—I I I I
11 20 25
2IL
50 55 *c
I I I I I I I I
I—h-»-H
35 40 min
FIGURE
8.1.
Component heats of hydrophobic asso-
ciation of an inverse temperature transition obtained
by means of temperature-modulated differential
scanning calorimetry (TMDSC). (Upper curve) An
exothermic component of the inverse temperature
transition due to the physical (van der Waals) inter-
action between associating molecules. (Middle
curve) The endothermic component (due to disrup-
tion of hydrophobic hydration), which is the funda-
mental feature of an inverse temperature transition
of hydrophobic association. (Lower curve) Net
endothermic heat of an inverse temperature transi-
tion that one measures in the standard differential
scanning
calorimetry.
This
is the value used for deter-
mining the Gibbs free energy for hydrophobic asso-
ciation,
AGHA,
arising out of an inverse temperature
transition. Noting the expanded temperature
scale,
it
is apparent that the width (the transition zone) of the
curves determined here at a much higher concen-
tration is similar to that of Figure 5.1C (Preliminary
communication from J. Carlos Rodriguez-Cabello •
produced with permission of J. Carlos Rodriguez-
Cabello.^0

336
8. Consilient Mechanisms for Protein-based Machines of Biology
Jones 6-12 potential^^ or the Buckingham
potential functions. ^^ It will be interesting, in
future work, to determine the relative magni-
tude of the endothermic and exothermic com-
ponents for each of the amino acid residues and
for other biologically relevant chemical modifi-
cations, as they contribute as guest residues
to the inverse temperature transition of
(GVGVP)n and of other informative host
model proteins.
8.1.8.1.6 The "Waters of Thales" as a
Requirement of the ConsiUent Mechanisms
As regards biology's protein-based machines,
the comprehensive hydrophobic effect, domi-
nated as it is by changes in hydrophobic
hydration of the inverse temperature transi-
tion, would become less dominant as the pres-
ence of water in the more polar state of a
protein-based machine became more limited,
that is, as the "waters of Thales" decrease.^^'^^
The long-recognized idea of cold denaturation
of proteins (enzymes),^^ that is, the loss of
structure required for function on lowering the
temperature, constitutes the same phenomenon
as the formation of structure on raising the tem-
perature. On observation of the phenomenon
in elastic-contractile model proteins, we devel-
oped the more general term of inverse temper-
ature transition, as it can be observed under
circumstances not relevant to cold denatura-
tion, such as the dissolution of crystals of
hydrophobically associated cyclic model pro-
teins on lowering the temperature.^^ Of course,
the comprehensive hydrophobic effect derives
from the inverse temperature transition. The
fundamental change underlying the inverse
temperature transition, however considered, is
one of changing the amount and nature of
protein hydration.
One of the more challenging locations, there-
fore,
for consideration of the comprehensive
hydrophobic effect in the panoply of biological
energy conversions is the electron transport
chain embedded within the inner mito-
chondrial membrane. Essential parts of these
protein-based machines insert into and func-
tion in very hydrophobic lipid bilayers. Here
the ingress and egress of protons for develop-
ment of the proton concentration gradient
across the inner mitochondrial membrane
becomes the output energy of an electron
transfer process that is so crucial to living
organisms. A central player, the ubiquinone
coenzyme Q,^^ converts from very hydrophobic
lipid diffusible species on the one hand to a pos-
itively charged species on oxidation at one site
and on the other hand to a negatively charged
species on receiving electrons at another site.
Both occur as key elements of the function of
Complex III of the electron transport chain.
By the hydrophobic consilient mechanism
and specifically due to the apolar-polar repul-
sive free energy of hydration, AGap, formations
of these charged coenzyme Q species contain
the thermodynamic key to transforming
"buried water molecules"^"^'^^ observed in the
crystal structure into pathways for proton entry
into and exit from the inner mitochondrial
membrane that results in the development of
the proton concentration gradient. In particu-
lar, formation by oxidation of the positively
charged ubiquinol near one side of the mem-
brane would disrupt hydrophobic association,
allowing release of protons to that side of the
membrane, whereas formation of the nega-
tively charged ubiquinone by reduction near
the other side of the membrane would disrupt
hydrophobic association to open channels for
the uptake of proton from that side of the mem-
brane (see discussion below in section 8.4.4).
8.1.8.1.7
The Coupling of Hydrophobic
Association with Development of
Elastic Force
Complex III is an example of the consiUent
mechanism for elasticity that includes the cou-
pUng of hydrophobic association with develop-
ment of an elastic force. In particular, the
Rieske iron protein (RIP) of Complex III
resides on the cytoplasmic side and contains a
long hydrophobic a-helix that passes through
the lipid bilayer from the cytoplasmic side to
emerge on the matrix side with charged
residues that combine to anchor the iron
protein to the membrane. On the cytoplasmic
side,
a sequence of about 15 residues that is
continuous with the transmembrane anchor

8.1 Thesis
337
tethers the globular component containing the
FeS center.
By the hydrophobic and elastic consilient
mechanisms the physical processes would
proceed as follows: The most favorable
hydrophobic association available to RIP is the
hydrophobic association of the hydrophobic tip
containing the FeS center with the Qo site con-
taining the ubiquinol within cytochrome b. For
this most favorable hydrophobic association to
occur, the tether becomes stretched. On chang-
ing the charge by addition of the negative elec-
tron to the FeS center and passing a second
electron on to the heme of cytochrome bL, the
ubiquinol at the Qo oxidation site takes on two
positive charges that disrupt the hydrophobic
association of this site. Hydrophobic dissocia-
tion of the very hydrophobic tip of the FeS
center allows the stretched entropic elastic
segment of the tether to lift the globular com-
ponent of RIP using an aromatic side chain as
a fulcrum and to flip the tip to hydrophobically
associate at the site for reduction of
cytochrome Ci. This represents an interlinking
of hydrophobic association and elastic force
development as repeatedly demonstrated with
the elastic-contractile model proteins in
Chapter 5. On oxidation of the FeS center at
cytochrome Ci, the hydrophobic tip containing
the FeS center returns to its most favorable
hydrophobic association at the Qo site contain-
ing a new molecule of ubiquinol to be posi-
tioned for the next cycle.
Thus,
the challenge to the consilient mecha-
nisms posed by the electron transport chain
transforms into a showcase example that
includes the two distinct but interlinked physi-
cal processes of the development of entropic
elastic force during hydrophobic association to
bring both consilient mechanisms to bear.
8.1.8.1.8
ATt as a Simple On-Off Switch
Another point to note is the simplicity of
looking at hydrophobic association/dissociation
from the perspective of Tt. Should Tt be just
above the operating temperature, say, the phys-
iological temperature of 37°C, then reducing
the expression of charged species sufficient to
lower Tt the width of the temperature interval
for the transition (see Figure 5.5) drives essen-
tially complete hydrophobic association. Thus,
the change in Tt, the ATt, functions as a simple
sliding on-off switch for driving hydrophobic
association/dissociation. For the slope in Figure
5.10 resulting from charge formation, a ATt ade-
quate to turn on or off hydrophobic association
requires a relatively small
AGHA-
8.1.8.1.9
Coherence Between AGap and
AGHA
Calculated Using Different
Experimental Data Demonstrates Presence
and Dominance of the Consilient Mechanism
in Model Proteins
For Model Protein i in Table 5.5, which is
(GVGVP GVGVP GEGVP GVGVP GVGVP
GVGVP)36(GVGVP), abbreviated as E/OF, we
can write AGHA[E /OF -^
E70F],
where E stands
for the -COOH state and E" for the -COO"
state of the glutamic acid (Glu, E) residue.
From Figure 5.10 and Table 5.3, we have that
AGHA[E/OF -^ E70F] is 5.22kcal/mole-
(GEGVP). Using the data in Figure 5.34, the
pKa of Model Protein i is 4.5. Now the pKa of
a carboxyl group in glutamic acid (Glu, E) or
aspartic acid (Asp, D), absent significant elec-
trostatic interactions and hydrophobic compe-
tition for hydration, is 3.8 to 4.0. Therefore, the
pKa of Model Protein i is shifted by 0.5 to 0.7
pH units due to the presence of the vahne (Val,
V) residues from the situation without such
hydrophobic residues and without significant
proximity of other polymer charges.
From Equation (5.13) in Chapter 5, we
have that AGap = 2.3 RT ApKa, such that,
AGap for Model Protein i becomes 0.7 to
1.0kcal/mole(E); this is for one E in six pen-
tamers. As the value of AGHA[E/OF -^ E70F] is
5.22kcal/mole-(GEGVP) for one E in each
pentamer, 5.22kcal/mole-(GEGVP) should be
divided by six (5.22/6 = 0.87) for proper
comparison. As 0.87kcal/mole obtained from
AGHA[E/OF -^ E70F] is within the range of 0.7
to 1.0kcal/mole(E) obtained from AGap of acid-
base titration data, we have coherence between
the effect of ionization on change in the Gibbs
free energy for hydrophobic association, AGHA,
which is derived from calorimetric data and the
hydrophobic induced pKa shift, AGap, which is

338
8. Consilient Mechanisms for Protein-based Machines of Biology
derived from acid-base titration data. Thus, an
additional reason exists why AGHA[E/OF ->
E70F] should be interpreted as resulting from
an apolar-polar repulsive free energy of
hydration.
Accordingly, the comprehensive hydropho-
bic effect provides an explanation for both pKa
shifts and the control of hydrophobic associa-
tion by changes in the polarity of functional
groups such as quinones, flavins, nicotinamides,
and hemes of cytochromes. Furthermore, the
phosphorylation or dephosphorylation of a
serine (Ser, S), of a threonine (Thr, T), or of a
tyrosine (Tyr, Y) and the change from ADP to
ATP and the most polar state of the localized
presence of both ADP and inorganic phosphate
(Pi) represent the most dramatic changes in
polarity that occur routinely in biology. As dis-
cussed in section 8.1.11.2, a sound basis exists
for the view that the high energy released on
hydrolysis of phosphate compounds derives
from a limitation in the availability of adequate
hydration prior to hydrolysis. Therefore,
controlling the availability of hydration for
phosphate groups by varying the presence of
competing hydrophobic groups provides the
means whereby a protein can either energize a
phosphate or utilize the energy of phosphate
hydrolysis.
8.1.8.1.10 Does There Exist a Competition for
Hydration Between Hydrophobic and Polar
(e.g.. Charged) Groups as a Key Part of the
Functioning of Protein-based Machines
of Biology?
In our view, AGap provides the basis whereby
raising the free energy of ADP and
Pi,
by forced
apposition of the very hydrophobic side of the
y-rotor in ATP synthase, results in synthesis of
ATP.
Also, in myosin II motor AGap provides
the basis whereby this ATPase drives muscle
contraction. In particular, in broad-brush
strokes, ATP binds in a cleft directed in two
directions, (1) toward the hydrophobic associa-
tion of the cross-bridge to actin binding site
and (2) toward the hydrophobic association
between the head of the lever arm and the
amino-terminal domain of the cross-bridge.
Directing the ATP thirst for water in both
directions by means of the cleft causes the
cross-bridge to hydrophobically dissociate from
the actin binding site and the head of the lever
arm to hydrophobically dissociate from the
amino-terminal domain of the globular compo-
nent of the cross-bridge. Conversely, the split-
ting of ATP to form ADP plus Pi and release of
Pi results in a decrease in AGap with the conse-
quence of hydrophobic reattachment of
cross-bridge to actin site and hydrophobic
re-association of head of the lever arm to the
amino-terminal domain to provide the power-
stroke (the contraction) of the myosin II motor.
8.1.8.1.11 Positive Cooperativity Increases as
Charged Species Compete for Water with
Additional More-hydrophobic Residues
As developed in Chapter 5, positive coopera-
tivity arises due to competition for hydration
between apolar (hydrophobic) and polar (e.g.,
charged) residues constrained by location to
coexist along a protein sequence and ultimately
within the folded and assembled structure dic-
tated by the sequence. By way of description of
the molecular process, we begin with associated
hydrophobic domains with a proximal car-
boxyl, -COOH. During occasional fluctuations,
paired hydrophobic domains momentarily
begin to dissociate. As too much hydrophobic
hydration forms, re-association (insolubility of
hydrophobic groups) recurs, because the free
energy becomes unfavorable, that
is,
the (-TAS)
term for solubility of the hydrophobic groups
becomes too positive and overwhelms the
inherently negative AH term.
During the occasional fluctuation of
hydrophobic dissociation, a carboxyl ionizes to
form the charged carboxylate, -COO". To form,
the charged carboxylate, however, must achieve
its hydration by destructuring hydrophobic
hydration. This constitutes an increase in free
energy for the system, and reclosure occurs.
Should the pH be high enough to give the
opportunity for formation of a second car-
boxylate during an opening fluctuation, the
second carboxylate actually forms more readily
than the first, because it has less hydrophobic
hydration to disrupt. Because together the
carboxylates destructure more hydrophobic

8.1 Thesis 339
hydration due to overlapping hydration
spheres, the probability for remaining dissoci-
ated increases. The occurrence of two carboxy-
lates increases the chance for formation of a
third and fourth carboxylate, and so forth. As
with the second carboxylate, the third and
fourth carboxylates have less hydrophobic
hydration to destructure, and therefore they
have a greater probabiHty of formation; they
form with a lower pKa.
In fact, as seen in the Hill plots in Figure 5.31,
ionizations of subsequent carboxyls occur at
lower pKa values. As more valine (Val, V)
residues are stepwise replaced by more
hydrophobic phenylalanine (Phe, F) residues,
this trend continues with an increase in the
initial pKa value and greater decreases in the
pKa values for subsequent ionizations during
the hydrophobic dissociation process. By the
time five Val (V) residues have been replaced
by the more hydrophobic Phe (F) residues, as
in Model Protein v, (GVGVP GVGFP GEGFP
GVGVP GVGFP GFGFP)42(GVGVP) in
Figure 5.31A, the mean pKa has increased to
6.4. Furthermore, analysis of the Hill plot in
Figure 5.31 A shows that formation of the first
carboxylate occurs with a pKa of 7.0 and that
of the last carboxylate of the 42 repeating units
of six pentamers occurs with a much lower
pKa of 5.7.
8.1.8.1.12 Ionization of the Last Carboxyl at a
pKa Greater than 3.8 to 4.0 Requires That a
Residual Apolar-Polar Repulsive Free Energy
of Hydration Exists Even in the Completely
Unfolded, Fully Dissolved, Soluble State
Even when (GVGVP GVGFP GEGFP
GVGVP GVGFP GFGFP)42(GVGVP) is
completely unfolded with F residues spatially
distributed toward near-maximal distances
from E" residues, to the extent permitted by the
sequence, there remains an apolar-polar repul-
sive free energy of hydration. Even when the
carboxylates are as completely surrounded
by water as possible, they still cannot access
water completely undisturbed by hydrophobic
residues. The common explanation that pKa
shifts result from the carboxyl being forced by
some "conformational change" into a pocket of
low dielectric constant within a protein is not
tenable for the completely ionized, unfolded
state of this model protein.
Even if the two Phe (F) residues most prox-
imal in the sequence to the carboxylate are Val
(V) residues, as in (GVGVP GVGVP GEGVP
GVGVP GVGFP GFGFP)39(GVGVP), a
residual pKa shift for the hydrophobically dis-
sociated state remains after all carboxyls are
ionized, as shown in Figure 5.31B. These
find-
ings argue that treatments of the structure and
fiinction of protein-based machines are un-
satisfactory until proper inclusion of the
apolar-polar repulsive free energy of hydration
occurs.
8.1.8.2 The Amount of Hydrophobic
Hydration on Potentially Complementary
Surfaces Determines Whether
Hydrophobic Association or
Dissociation Occurs
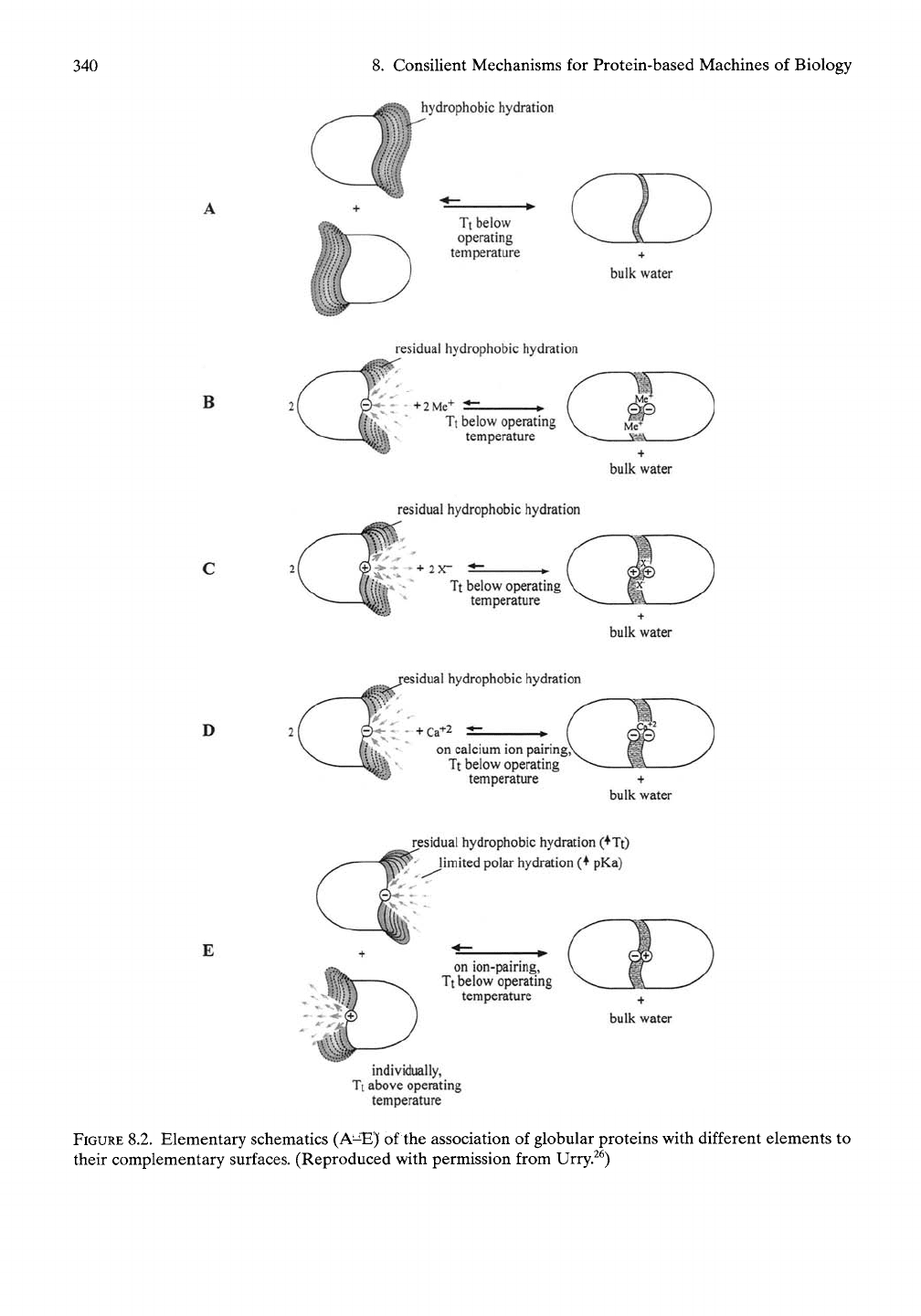
Figure 8.2 provides an elementary schematic of
the association of globular proteins with con-
toured surfaces for proper steric association,
but each having different elements whereby
complementarity could be achieved.^^ In part
the following paragraphs demonstrate the rele-
vance to globular proteins in the analyses of
Chapter 5 that utilized model proteins of trans-
lational symmetry undergoing phase transi-
tions.
This section is preliminary to the more
involved but parallel discussion of the
hydrophobic association of myosin cross-bridge
with actin (and of components within the cross-
bridge) in the absence of ATP that dissociate in
the presence of bound ATP, as schematically
shown in Figure 2.16.
8.1.8.2.1
Complementary Surfaces Associate
when They Have the Potential for Too
Much Hydrophobic Hydration at a
Given Temperature
Consider a situation with globular protein sub-
units that are soluble in solution at a sufficiently
low temperature and that have simple com-
plementary hydrophobic surfaces covered with
much hydrophobic hydration, as in Figure
8.2A.^^
Raising the temperature from below to
340
8. Consilient Mechanisms for Protein-based Machines of Biology
B
t3
hydrophobic hydration
Tt below
operating
temperature
residual hydrophobic hydration
+
2MC+
Tt below operating
temperature
residual hydrophobic hydration
+ 2X-
Tt below operating
temperature
z
bulk water
bulk water
bulk water
sidual hydrophobic hydration
+ Ca-^2
on calcium ion pairing)
Tt below operating
temperature
bulk water
£
residual hydrophobic hydration (•Tt)
limited polar hydration
(•
pKa)
on lon-painng,
Tt below operating
temperature
•**
'^JM^
J
bulk water
indivkkially,
Tt above operating
temperature
FIGURE
8.2. Elementary schematics (A^^)( of the association of globular proteins with different elements to
their complementary surfaces. (Reproduced with permission from Urry.^^)

8.1 Thesis 341
above the temperature for the onset of the
inverse temperature transition for hydrophobic
association initiates dimer formation. Now,
whether or not phase separation occurs
depends simply on whether or not the dimer is
soluble. If the dimer is soluble, there would be
no phase separation. If the dimer were itself
insoluble by means of extended association of
dimers, there could be fiber formation with
gelation and even phase separation. The former
applies to hemoglobin S and to actin filament
formation, where G(globular)-actin is soluble,
but, when ATP binding initiates association,
F(filamentous)-actin forms.
Independent of the question of dimer solu-
bility, the dimerization process is one of
hydrophobic association, and the change in
Gibbs free energy for dimer formation by
hydrophobic association, AGHA(dimerization) =
AHD - TASD, obtains. In the case of hydropho-
bic association, the transition is endothermic;
AHD
is positive. Therefore, as with the inverse
temperature transition, association occurs
because of a large negative (-TASD) term that
results from the large positive change in
ASD
as
more ordered hydrophobic hydration becomes
less ordered bulk water. Accordingly, dimer-
ization occurs due to a sufficiently large and
negative
AGHA,
wherein changes in solvent
entropy drive dimerization. Furthermore, the
process can be discussed in terms'of equilibria,
where the sign and magnitude of the equilib-
rium constant KD = exp(-AGHA/RT). As
written, the dimerizations in Figure 8.2 are
favorable with negative AGHA values as crudely
represented by the size and direction of the
arrows.
8.1.8.2.2
Surfaces with Too Little
Hydrophobic Hydration Do Not
Hydrophobically Associate
When too few hydrophobic residues or too
many charged species occur on the surface, too
little hydrophobic hydration results. Dimeriza-
tion will not occur, that is, the monomeric sub-
units retain their solubility in water. When the
charged species exhibit hydrophobically shifted
pKa values, then the surfaces are poised for
association, particularly if the charges on
opposing surfaces are of the opposite sign and
are arranged in a complementary way, as in
Figure 8.2E. A number of examples follow in
the next paragraph.
8.1.8.2.3
Charged Species Destructure
Hydrophobic Hydration But Experience
an Increase in Free Energy on Doing So
As shown in Figure 8.2B, C, even when suffi-
ciently hydrophobic surfaces have the same
charge, the oppositely signed ion in solution can
pair with the surface charge, and dimerization
by hydrophobic association can result.^^ This
was observed with elastic-contractile model
proteins in Figure 5.12. The more hydrophobic
poly[0.8(GVGIP),0.2(GEGIP)] associates at
physiological temperature in the presence of
0.3 N or more NaCl, whereas the less hydropho-
bic poly[0.8(GVGVP),0.2(GEGVP)] does not
(see Figure 5.12).
In Figure 8.2D calcium ion triggers
hydrophobic association of negatively charged
surfaces. As shown in Figures 5.15 and 5.27,
calcium ion is particularly effective in lowering
the value of Tj of Model Protein I, (GVGIP
GFGEP GEGFP GVGVP GFGFP GFGIP)4o
(GVGVP); 2E/5F/2I, and Model Protein ii:
(GVGVP GVGFP GEGFP GVGVP GVGVP
GVGVP)4o(GVGVP); E/2F When the model
protein is more hydrophobic and contains two
negative carboxylates in position for bidentate
interaction with the divalent calcium ion, the
decrease in Tt occurs at a much lower ion con-
centration for Model Protein I, which may be
considered a poor man's E-F hand. In particu-
lar. Figure 5.27 shows the decrease in Tt to
mirror the increase in hydrophobic hydration,
which ties directly to Figure 8.2D.
When the globular subunits have oppositely
signed charges on their surface, as in Figure
8.2E, each can exist alone as soluble monomers
in solution, but, when combined in a single solu-
tion, they associate to form dimers. The analogy
for the model proteins is shown in Figure 6.3.
In particular, the two model proteins, Model
Protein x': (GVGVP GVGVP GKGVP
GVGVP GVGVP GVGVP)22(GVGVP);

342
8. Consilient Mechanisms for Protein-based Machines of Biology
K/OF and Model Protein ii: (GVGVP GVGFP
GEGFP GVGVP GVGVP GVGVP)4o
(GVGVP); E/2F are each soluble in water at
physiological temperatures. When the two solu-
tions are combined, however, they hydrophobi-
cally associate as the temperature is raised
above 24°C (see Figure 6.3B).
The consilient mechanism depicted in the
elementary structures of Figure 8.2 are relevant
to protein function arising out of association of
globular components of molecular machines.
The principles arise in almost textbook fashion
in the function of the myosin II motor where
the key charged species are ATP, ADP, and Pi,
but in an important way also include the
charged side chains (see section 8.5.3).
8.1.8.3 ''Oil and Vinegar Don't Mix/' But,
When Oil-like and Vinegar-like Side
Chains of Proteins Are Forced to Coexist
by Virtue of Structure (Primary,
Secondary, Tertiary, and Quaternary), They
Interact in the Most Profound Way to
Achieve Function
Thus,
the old adage that "oil and vinegar don't
mix" remains relevant when the oil-like and
vinegar-like groups are constrained to coexist
along a protein sequence and in proximity to a
pair of hydrophobically associating/dissociating
surfaces. Although structure causes these dis-
parate groups to interact, they nonetheless
attempt to separate as oil from vinegar. In fact,
as substituents along a protein chain, oil-like
and vinegar-like side chains effectively repulse
each other as they each reach out for water
undisturbed by the other. Another important
element to recognize is that the apolar-polar
repulsion can propagate along a water-filled
cleft. Ion pairs, at a distance from the ATP
binding site within the hydrophobically fined
cleft, can be caused to separate by ATP binding,
and then the separated ion pairs, even by small
distances, assist to propagate polar dominance
to a more distant pair of hydrophobic faces.
As briefly considered immediately below, this
driving force provides a more efficient mecha-
nism for energy conversion in an aqueous
medium than the more commonly considered
electrostatic mechanisms.
8.1.9 On Efficiencies of Energy
Conversion in the Aqueous Medium
of Biology
This section considers efficiency of energy con-
version by protein-based machines of biology
by noting two points. The points arise from
studies on elastic-contractile model proteins
and draw from relatively new concepts con-
cerned with control of hydrophobic association
and with the nature of elasticity. The first
point provides direct experimental evidence
for a dramatically greater efficiency of the
apolar-polar repulsion mechanism over the
classic electrostatic charge-charge repulsion
mechanism for chemomechanical transduction
by polymers in water. The first point goes on to
address electrochemical transduction efficiency
with relevance, in principle, to the energy con-
version of the electron transport chain.
The second point addresses the nature of
elastic force development in relation to under-
standing efficient energy conversion. If the
energy required for chain deformation during
elastic force development becomes lost to other
parts of the protein and to the surrounding
water, then so too is efficient energy conversion
lost. In other words, elastomeric force develop-
ment on deformation in a protein-based
machine followed by marked hysteresis on
relaxation necessarily denotes an inefficient
protein-based machine.
8.1.9.1 For Mechanochemical
Transduction in Water Apolar-Polar
Repulsion-based Is More Efficient than
Charge-Charge Repulsion-based
Molecular Machines
8.1.9.1.1
Simple Comparisons of the
Chemomechanical Transductional Efficiencies,
r| = fAl/AjixAn, of the Charge-Charge
Repulsion and the Apolar-Polar Repulsion,
AGap, Mechanisms
The two molecular systems to be compared are
PMA, poly(methacryUc acid), which is
[-CH2-(CH3)C(COOH)-]n, and Model Protein
iv in Table 5.5, namely, (GVGVP GVGFP
GEGFP GVGVP GVGFP GVGFP)^
(GVGVP), abbreviated as E/4F. The two poly-

8.1 Thesis
343
mers were cross-linked to achieve similar elastic
moduli, and both respond to changes in proton
chemical energy, AjiAn = (-2.3RTApH)An. In the
following paragraphs, visual comparisons will be
made with reference to Figures 5.34 (comparing
Hill coefficients) and 5.35 (comparing the area
of acid base titration curves).
The statement of efficiency, r|, for proton-
driven chemomechanical engines can be
written as r| = fAL/A|iiAn = fAL/(-2.3RT
ApH)An. Because the chemical energy, AjLiAn, is
proportional to the change in pH, the slope of
the acid-base titration curve provides an imme-
diate comparison, where an increase in the
magnitude of the Hill coefficient means a
proportionately greater efficiency. The Hill
coefficient for PMA is 0.5, whereas, as seen in
Figure 5.34, that for E/4F is 2.7, suggesting on
this basis an expectation of at least a fivefold
more efficient energy conversion by E/4F.
The chemical energy also contains the
number of moles of proton, Ann, consumed in
the process. To consider both
A|IH
and
AUH,
the
acid-base titration curve can be used. The y-
axis contains the amount of acid consumed on
protonating the carboxylates, and the x-axis
provides the ApH required to do so. Accord-
ingly, the area defined by the acid-base titration
curve gives a measure of the chemical energy.
Because PMA has one carboxyl for every two-
backbone atoms, whereas E/4F has only one
carboxyl for every 90-backbone atoms, many
more protons will be consumed on driving the
PMA chemomechanical engine, as is apparent
from Figure 5.35. Missing in this comparison,
however, is the amount of work performed by
each engine. As discussed below, this is directly
determined and compared in Figure 5.36.
8.1.9.1.2
Experimental Comparison Following
pH Dependence of Length at Fixed Force
Using Acid-Base Titration Curves,
That Is, During Actual Performance of
Mechanical Work
Figure 5.36 shows the change in length for the
constant load of 2 grams, where the changes in
length, despite very different consumptions of
energy, are quite similar. In the comparison in
Figure 5.36, the PMA engine required 6.86 x
10"^
mole of protons, whereas the E/4F engine
requires 6.12x 10"^ mole of protons. If compar-
ison is made for the best AL/ApH slopes for
each engine, as shown in Figure 5.36, the ratio
of efficiencies, r|ap/r|cc is of the order of 40.
Clearly, in the aqueous milieu of biology the
electrostatic mechanism of charge-charge repul-
sion is not a serious contender for the efficient
machines available to biology. As noted in
section 8.1.8.3, ionizable functional amino acids
and other polar prosthetic groups forced,
through the process of biosynthesis (see
Chapter 6), to coexist within a protein chain
with substantial hydrophobic residues provides
the ingredients for efficient protein function.
8.1.9.2 Efficiency of Electro-chemical
Transduction in Water
8.1.9.2.1
Experimental Data on
Electro-chemical Transduction Efficiency
The previous considerations of energy conver-
sion resulted in the performance of mechanical
work, and, for the considered model proteins,
there is the obvious realization that hydro-
phobic association is tantamount to contrac-
tion. The control of hydrophobic association,
however, can interconvert any two energies
that can individually drive hydrophobic as-
sociation. The example here draws from the
demonstration that reduction of a redox couple
can power contraction by driving hydrophobic
association and that protonation of a carboxyl
can do likewise. Both processes, protonation of
a carboxylate and reduction of a redox group,
effect a decrease in AGHA, and both functional
groups are affected by any
AGHA-
AS
shown in
Figures 5.20B, 5.23, 5.25, 5.29, 5.31, 5.32, 5.34,
and 5.35B, Chapter 5 is replete with hydropho-
bic-induced pKa shifts. Equivalent hydropho-
bic-induced shifts in reduction potential for
amino-methyl nicotinamide are shown in
Figure 5.20C. Because pKa values and reduc-
tion potential are both coupled to hydro-
phobicity, it is to be expected that a change in
redox state that changes hydrophobicity would
bring about a change in pKa and vice versa.
Here we demonstrate the efficiency of that con-
version for a modest level of host polymer
hydrophobicity.^^

344
8. Consilient Mechanisms
for
Protein-based Machines
of
Biology
Reduction-induced
pKa
shift
was
demon-
strated
in the
designed polytricosapeptide
in
Table
5.5,
that
is,
Polymer
i:
Poly(GDGFP
GVGVP GVGVP GFGVP GVGVP
GVGK{NMeN}P), abbreviated
as
DK{NMeN}/2K containing both redox
(LysNMeN)
and
carboxyl (Asp,
D)
functional
entities. From Table 5.2, the difference
in
AGHA
on going from the oxidized state, NMeN^, to
the
relevant reduced state,
6-OH
tetrahydro
NMeN,
is an
approximate -4kcal/mole-NMeN.
On the other hand,
for
the pKa shift, the change
in AGHA
=
AGap
=
2.3RTApKa
=
-2.3(582
cal/mole)(2.5)
=
-3.35kcal/mole. From
the
pre-
ceding information,
the
efficiency
can be
written
as r| =
(output energy/input energy)
100
=
{AGap/AGHA[LysNMeN^ (oxidized)
-^
LysNmethyl-6-OH-tetrahydronicotinamide
(reduced)]}100
=
(3.35/4)100
-
83%. Thus,
one
estimates
a
high efficiency when the coupling
of
functional groups occurs
by
means
of a
common dependency on
AGHA-
8.1.9.2.2
Increase
in
Hydrophobicity Directly
Shifts Reduction Potentials
and
Increases
Efficiency, That Is,
the
Hill Coefficient
and
Positive Cooperativity,
for
NMeN
Attaching NMeN
to the
appropriate designed
series
of
microbially prepared polymers gives
the following polymers from Table
5.5:
Model Protein
ii':
(GVGVP GVGFP
GK
{NMeN}GFP GVGVP GVGVP GVGVP)22
(GVGVP), K{NMeN}/2F
Model Protein
Hi':
(GVGVP GVGVP
GK
{NMeNjGVP GVGVP GVGFP GFGFP)22
(GVGVP), K{NMeN}/3F
Model Protein
iv':
(GVGVP GVGFP
GK
{NMeN}GFP GVGVP GVGFP GVGFP)2i
(GVGVP), K{NMeN}/4F
The indicated increases
in
hydrophobicity
on
replacing
Val (V) by Phe (F)
were found
to
cause systematic shifts
in the
reduction poten-
tial
and
corresponding increases
in
steepness
(positive cooperativity)
as
shown
in
Figure
5.20C.
In
particular,
the
reduction potentials
are K{NMeN}/OF (-145 mV), K{NMeN}/2F
(-127
mV),
K{NMeN}/3F (-107 mV),
and
K{NMeN}/4F (-90mV).'^ Thus,
for the
redox
couple NMeN/NMeN^
the
reduction potentials
and efficiency depend
on
hydrophobicity
in a
systematic
way,
analogous
to the
dependence
on hydrophobicity
of pKa and
efficiency
for a
chemical couple such
as
-COOH/-COO~.
On
this basis,
the
dependence
of
reduction poten-
tial
on the
hydrophobicity
of
surrounding
residues
for
players, such
as
heme
bn and
heme
bL,
in the
electron transport chain
can be
considered.^^
8.1.9.3 Further Consideration
of the
Relevance
of the
Nature
of
Elastic Force
to Efficient Mechanisms
for
Energy Conversion
8.1.9.3.1
The
Case
of
Isometric Contractions
The development
of
force under conditions
of
fixed length,
as in an
isometric contraction,
involves
the
elastic deformation
of a
chain
or
chains within
the
protein-based machine.
On
relaxation, ideal elastic elements return
the
total energy
of
deformation
to the
protein-
based machine
for the
performance
of
mechan-
ical work. Thus,
the
approach toward high
efficiency
for the
function
of a
protein-based
linear motor,
or
even
for
the RIP domain move-
ment
in
Complex
III,
depends
on how
nearly
the extension
of an
elastomeric chain segment
approaches ideal elasticity.
It
is
important
to
realize that
the
model
protein (GVGVP)25i
can
exhibit ideal elastic
behavior.^
It is
also significant
to
recognize that
the source
of
ideal elastic force resides
in the
internal motions,
the
internal chain dynamics,
of
the
elastic chain segment. To
the
extent that
the energy
of
deformation becomes dispersed
into molecules
or
chain segments
not
integral
to
the
extended chain segment, energy
is
lost
and efficiency decreases. Thus, the nature
of
the
elastomeric force limits
the
possible efficiency
of
a
protein-based machine.
For an
effective
protein-based machine, deformations
of
chains
should occur
in
such
a way
that
the
energies
involved
be
recoverable.
8.1.9.3.2
Relevance
of
Nonideal Elasticity
(Hysteresis)
to
Efficient Performance
of
Mechanical Work
As noted above
for an
ideal elastomer,
the
energy expended
in
deformation,
for
example.
