Tsoulfanidis N. Measurement and detection of radiation
Подождите немного. Документ загружается.



PREFACE TO THE SECOND EDITION
For an author it is very gratifying to discover that a technical book is still
relevant more than ten years after it was first published. This is the case with
this book because it addresses the fundamentals of nuclear radiation counting,
which have not significantly changed during that period of time. Like the first
edition, this book is written for persons who have no prior knowledge of
radiation counting. These include undergraduate students in nuclear science
and engineering; first-year graduate students who enter this field from another
discipline; health physicists and health physics technicians; nuclear medicine
technical personnel; and scientists, engineers, and technicians in laboratories
where atomic and nuclear radiation are used. In addition, according to com-
ments from former students and colleagues, the book has proven to be an
excellent reference.
The second edition follows the same guidelines as the first-namely simplic-
ity in writing and use of many examples. The main structural change is the
elimination of Chap. 17 (Special Detectors and Spectrometers) and the reloca-
tion of the material in appropriate chapters. For example, rate meters and
gas-filled detectors are now discussed in Chap.
5.
Self-powered detectors are
now included in Chap. 14 along with other neutron detectors. Chapter
16
deals
with solid-state track recorders and thermoluminescent dosimeters.
As
should be expected, all chapters have been corrected for errors, revised
for clarification, and new examples have been added as needed. The more
substantive revisions were made in the following chapters: In Chap.
2
there is
now a better explanation of the
X2
procedure and the minimum detectable
activity
(MDA).
In Chap. 4, relative to the stopping power of charged particles,
there is a more detailed discussion and presentation of the latest formulas of
gamma-ray build-up factors. The Long Range Alpha Detector (LRAD), a clever
new counter of alpha radiation, is introduced in Chap.
5.
In Chap.
7,
pure
germanium detectors, which are prominent devices for the detection of gamma

xxii
PREFACE
TO
THE
SECOND EDITION
rays, are introduced. In Chap.
12
the latest information about Ge detectors is
presented. Magnetic and electrostatic spectrometers and the position-sensitive
detectors are included in Chap.
13.
In Chap.
14,
the LSL-M2 unfolding code is
introduced as well as compensated ion chambers and self-powered neutron
detectors. Chapter
16
is almost completely rewritten. There is an improved
presentation in the dose rate calculation, detailed discussion of the new protec-
tion guides and exposure limits, and an expanded list of dosimeters.
I am grateful to Dr. Eiji Sakai who translated the First Edition into
Japanese and in doing so discovered several typos and, more importantly,
offered many suggestions that are incorporated into the Second Edition and
make it better.
Nicholas Tsoulfanidis

CHAPTER
ONE
INTRODUCTION TO
RADIATION MEASUREMENTS
1.1
WHAT
IS
MEANT
BY
RADIATION?
The word
radiation
was used until about 1900 to describe electromagnetic
waves. Around the turn of the century, electrons, X-rays, and natural radioactiv-
ity were discovered and were also included under the umbrella of the term
radiation.
The
newly discovered radiation showed characteristics
of
particles, in
contrast to the electromagnetic radiation, which was treated as a wave. In the
1920s, DeBroglie developed his theory of the duality of matter, which was soon
afterward proved correct by electron diffraction experiments, and the distinction
between particles and waves ceased to
be
important. Today, radiation refers to
the whole electromagnetic spectrum as well as to all the atomic and subatomic
particles that have been discovered.
One of the many ways in which different types of radiation are grouped
together is in terms of ionizing and nonionizing radiation. The word
ionizing
refers to the ability of the radiation to ionize an atom or a molecule of the
medium it traverses.
Nonionizing radiation is electromagnetic radiation with wavelength
A
of
about 10 nm or longer. That part of the electromagnetic spectrum includes
radiowaves, microwaves, visible light
(A
=
770-390 nm), and ultraviolet light
(A
=
390-10 nm).
Ionizing radiation includes the rest of the electromagnetic spectrum (X-rays,
A
=
0.01-10 nm) and y-rays with wavelength shorter than that of X-rays. It also

2
MEASUREMENT
AND
DETECTION
OF
RADIATION
includes all the atomic and subatomic particles, such as electrons, positrons,
protons, alphas, neutrons, heavy ions, and mesons.
The material in this text refers only to ionizing radiation. Specifically, it
deals with detection instruments and methods, experimental techniques, and
analysis of results for radiation in the energy range shown in Table 1.1. Particles
with energies listed in Table
1.1
are encountered around nuclear reactors,
around installations involving production or use of natural or manufactured
radioisotopes, and also around low-energy accelerators. Not included in Table
1.1
are cosmic rays and particles produced by high-energy accelerators (GeV
energy range).
1.2
STATISTICAL NATURE OF RADIATION EMISSION
Radiation emission is nothing more than release of energy by a system as it
moves from one state to another. According to classical physics, exchange or
release of energy takes place on a continuous basis; i.e., any amount of energy,
no matter how small, may be exchanged as long as the exchange is consistent
with conservation laws. The fate of a system is exactly determined if initial
conditions and forces acting upon it are given. One may say that classical physics
prescribed a "deterministic" view of the world.
Quantum theory changed all that. According to quantum theory, energy can
be exchanged only in discrete amounts when a system moves from one state to
another. The fact that conservation laws are satisfied is a necessary but not
a sufficient condition for the change of a system. The fate of the system is
not determined exactly if initial conditions and forces are known. One can only
talk about the probability that the system will do something or do nothing.
Thus, with the introduction of quantum theory, the study of the physical world
changed from "deterministic" to "probabilistic."
The emission of atomic and nuclear radiation obeys the rules of quantum
theory.
As
a result of this, one can only talk about the probability that a reaction
will take place or that a particle will be emitted. If one attempts to measure the
number of particles emitted by a nuclear reaction, that number is not constant
in time; it has a statistical uncertainty because of the probabilistic nature of the
phenomenon under study.
Table
1.1
Maximum
Energy Considered
Particle Energy
(MeV)
a
20
P
10
Y
20
n
20
Heavy ions
100

INTRODUCTION TO RADIATION
MEASUREMENTS
3
Consider a radioactive source emitting electrons and assume that one
attempts to measure the number of electrons per unit time emitted by the
source. For
eveq atom of the source there is a probability, not a certainty, that
an electron will be emitted during the next unit of time. One can never measure
the "exact" number. The number of particles emitted per unit time is different
for successive units of time. Therefore, one can only determine the average
number of particles emitted. That average, like any average, carries with it an
uncertainty, an error. The determination of this error is an integral part of any
radiation measurement.
1.3
THE ERRORS
AND
ACCURACY
AND
PRECISION
OF MEASUREMENTS
A
measurement is an attempt to determine the value of a certain parameter or
quantity. Anyone attempting a measurement should keep in mind the following
two axioms regarding the result of the measurement:
Axiom
1
No measurement yields a result without an error.
Axiom
2
The result of a measurement is almost worthless unless the error
associated with that result is also reported.
The term
error
is used to define the following concept:
Error
=
(measured or computed value of quantity Q)
-
(true value of Q)
Error
=
estimated uncertainty of the measured or computed value of Q.
Related to the error of a measurement are the terms
accuracy
and
preci-
sion.
The dictionary gives essentially the same meaning for both accuracy and
precision, but in experimental work they have different meanings.
The accuracy of an experiment tells us how close the result of the measure-
ment is to the true value of the measured quantity. The precision of an
experiment is a measure of the exactness of the result. As an example, consider
the measurement of the speed of light, which is known, from measurements, to
be equal to 2.997930
X
lo8
m/s.
Assume that a measurement gave the result 2.9998
X
10' m/s. The differ-
ence between these two numbers is an estimate of the accuracy of the measure-
ment. On the other hand, the precision of the measurement is related to the
number of significant figurest representing the result. The number 2.9998
x
lo8
indicates that the result has been determined to be between 2.9997 and 2.9999
or, equivalently, that it is known to
1
part in 30,000 (1/29998).
'AS
an example of the number of significant figures, each of the following numbers has five
significant figures:
2.9998. 29998. 20009. ,0029998, 2.9880
x
10'.
4
MEASUREMENT
AND
DETECTION
OF
RADIATION
If
the measurement is repeated and the new result is 2.9999
x
lo8
m/s, the
accuracy has changed but not the precision. If, on the other hand, the result
of the measurement is 2.99985
x
lo8
m/s, both precision and accuracy have
changed.
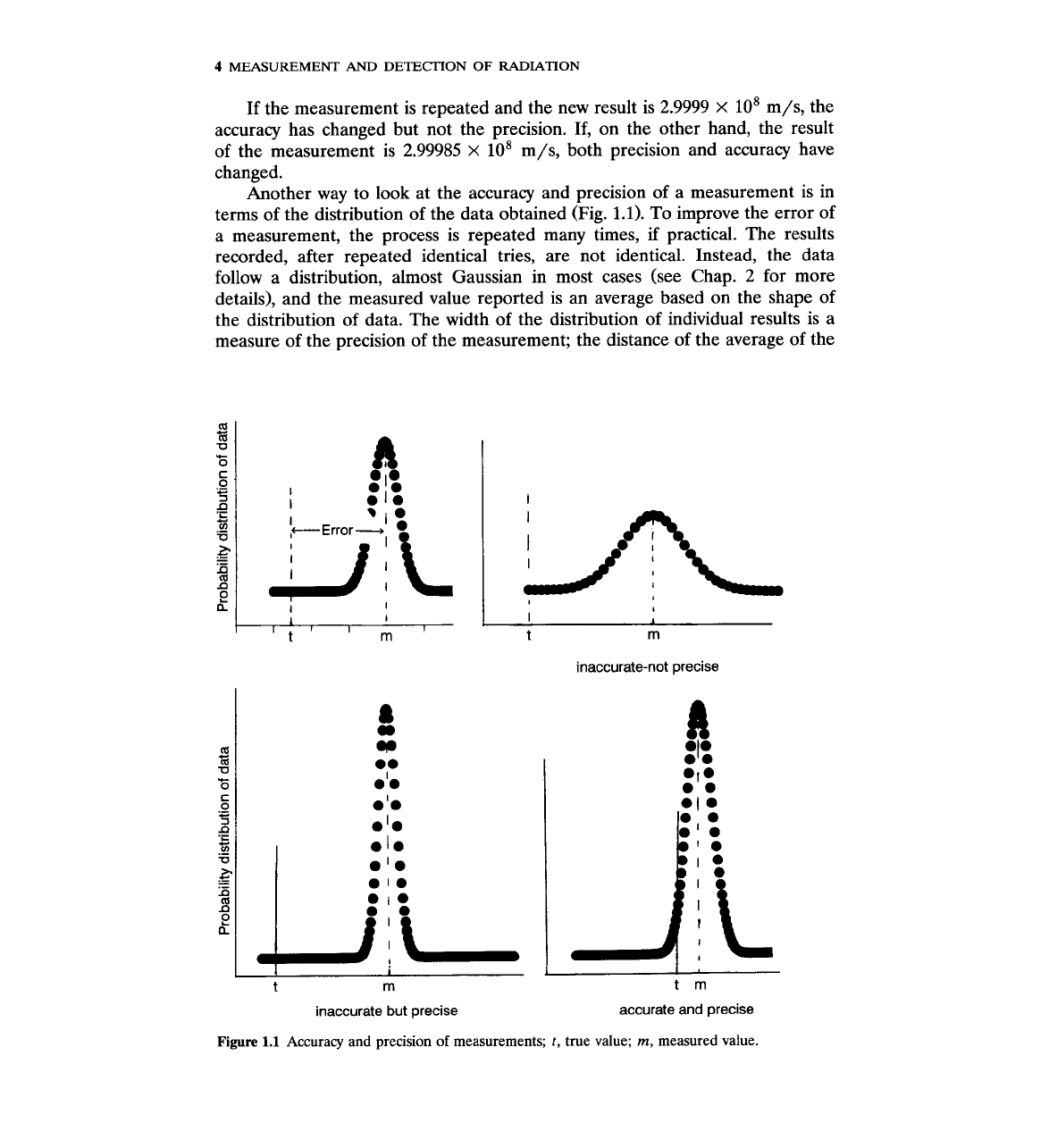
Another way to look at the accuracy and precision of a measurement is in
terms of the distribution of the data obtained (Fig. 1.1). To improve the error of
a measurement, the process is repeated many times, if practical. The results
recorded, after repeated identical tries, are not identical. Instead, the data
follow a distribution, almost Gaussian in most cases (see Chap. 2 for more
details), and the measured value reported is an average based on the shape of
the distribution of data. The width of the distribution of individual results is a
measure of the precision of the measurement; the distance of the average of the
inaccurate-not precise
inaccurate but precise
accurate and precise
Figure
1.1
Accuracy and precision
of
measurements;
t,
true value;
m,
measured value.

INTRODUCTION TO RADIATION MEASUREMENTS
5
distribution from the true value is a measure of the accuracy of the measure-
ment.
Every experimenter should consider accuracy and precision simultaneously.
It would be a waste of effort to try to improve the precision of a measurement if
it is known that the result is inaccurate. On the other hand, it is almost useless
to try to achieve very high accuracy if the precision of the measurement is low.
Limitations in the accuracy and precision of measurements result from
many causes. Among the most important are
1.
Incorrectly calibrated instruments.
2.
Algebraic or reading errors of the observer.
3.
Uncontrolled changes in environmental conditions, such as temperature,
pressure, and humidity.
4.
Inability to construct arbitrarily small measuring meter-sticks, rods, pointers,
clocks, apertures, lenses, etc.
5.
A
natural limit of sensitivity for any real measuring instrument detecting
individual effects of atoms, electrons, molecules, and protons.
6.
Imperfect method of measurement in most cases.
7.
Unknown exact initial state of the system. Or, even if the initial state is
known, it is impossible to follow the evolution of the system. For example, to
determine the state of a gas in a container, one should know the exact
position and velocity of every molecule at
t
=
0. Even if this is known, how
practical is it to follow
loz0
atoms or molecules moving in a box?
8.
Statistical nature of some processes, e.g., radioactive decay. There is a
probability that an atom of a radioactive isotope will decay in the next 10 s,
and this is as much information as one can report on this matter. The
probability can be calculated, but it is still a probability, never a certainty.
1.4
TYPES OF ERRORS
There are many types of errors, but they are usually grouped into two broad
categories: systematic and random.
Systematic (or determinate) errors are those that affect all the results in the
same way. Examples of systematic errors are
1.
Errors from badly calibrated instruments
2.
Personal errors (algebraic, wrong readings, etc.)
3.
Imperfect technique
Systematic errors introduce uncertainties that do not obey a particular law
and cannot be estimated by repeating the measurement. The experimenter
should make every reasonable effort to minimize or, better yet, eliminate
systematic errors. Once a systematic error is identified, all results are corrected
appropriately. For example, if a measurement of temperature is made and it is

6
MEASUREMENT
AND
DETECTION
OF
RADIATION
discovered that the thermocouple used overestimates the temperature by lo%,
all temperatures measured are decreased by 10%.
Random (or statistical) errors can either decrease or increase the results of
a measurement, but in a nonreproducible way. Most of the random errors
cannot be eliminated. They can be reduced, however, by either improving the
experimental apparatus, improving the technique, and/or repeating the experi-
ment many times. Examples of random errors are
Errors resulting from experimental apparatus (reading of instruments, elec-
tronic noise, etc.)
Errors from uncontrolled change in condition such as voltage, temperature,
or pressure
Probabilistic nature of the phenomenon under study
The determination of error associated with the measurement is a very
important task. It is probably as important as the measurement.
~echnical
journals and scientific reports never report results of experiments without the
error corresponding to these results.
A
measurement reported without an error
is almost worthless. For this reason, the study of errors is a topic of great
importance for scientists and engineers.
This text does not give a complete theory of error. Only the fundamentals
needed for a basic understanding of the statistical analysis of errors are
presented. The objective is to present methods that provide an estimate of the
error of a certain measurement or a series of measurements and procedures
that minimize the error.
Only random errors are discussed from here on. In every measurement,
systematic and random errors should be treated separately. Systematic and
random errors should never be combined using the methods discussed in Chap.
2.
Those methods apply to random errors only.
1.5
NUCLEAR INSTRUMENTATION
1.5.1
Introduction
This section is addressed to the person who has not seen or used radiation
instruments.+ Its purpose is to present a general description of the physical
appearance and operation of the basic components of a radiation counting
system. Every component is treated like a "black box,"
i.e., input and output are
discussed without any details about how the output is obtained. Details about
the construction and operation of individual units are given in later chapters.
t~he term
radiation imtmrnents
refers to instruments used for the detection of ionizing
radiation as explained in Sec.
1.1
INTRODUCTION TO RADIATION MEASUREMENTS
7
Detectors are discussed in Chaps.
5
through
7,
and the rest of the electronics is
discussed in Chap.
10.
Counting systems are classified into two types, according to the method of
operation:
1.
Pulse-type systems. The output consists of voltage pulses, one pulse per
particle detected.
2.
Current-type systems. The output is an average value, resulting from the
detection of many particles.
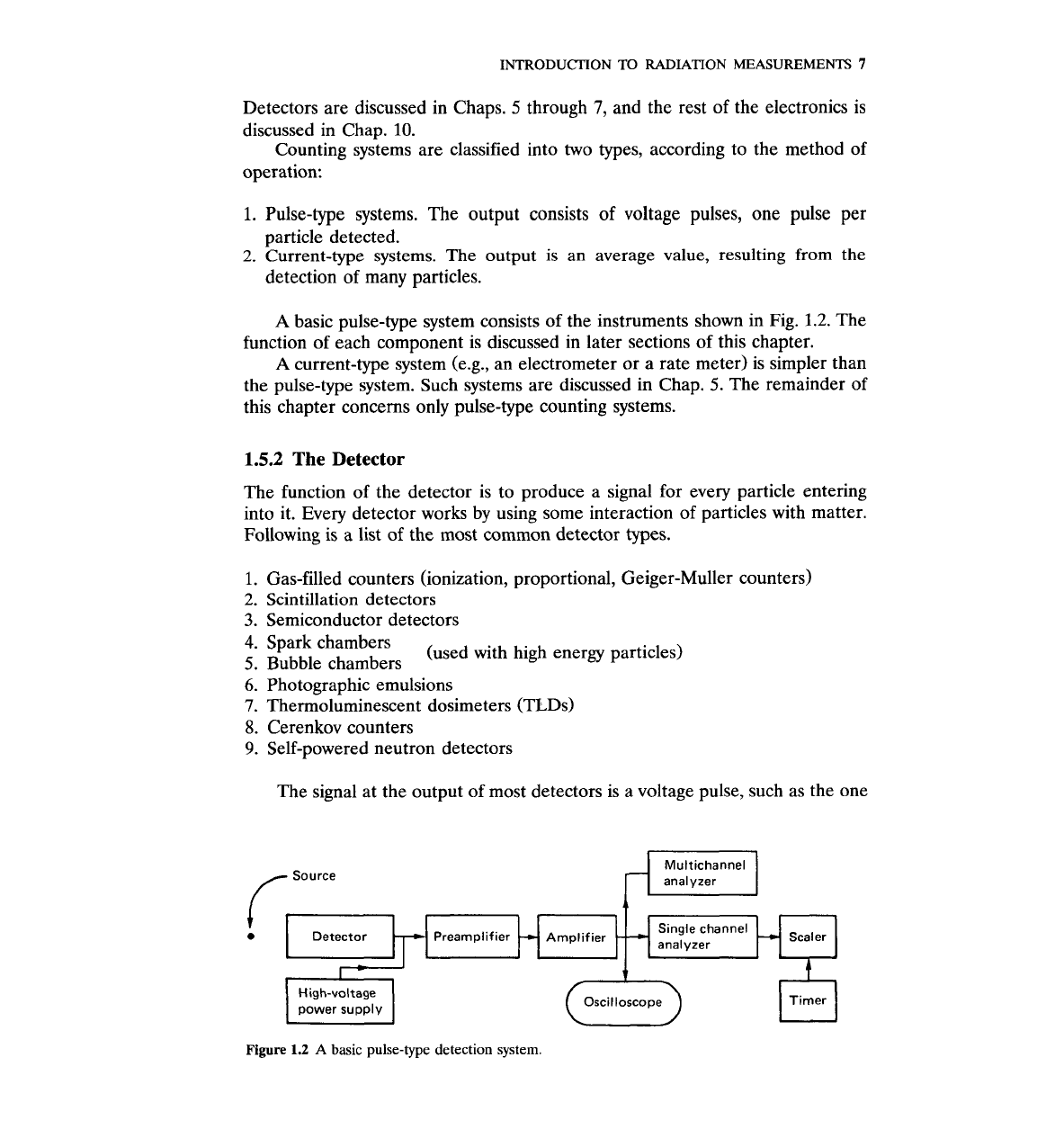
A
basic pulse-type system consists of the instruments shown in Fig.
1.2.
The
function of each component is discussed in later sections of this chapter.
A
current-type system (e.g., an electrometer or a rate meter) is simpler than
the pulse-type system. Such systems are discussed in Chap.
5.
The remainder of
this chapter concerns only pulse-type counting systems.
1.5.2
The Detector
The function of the detector is to produce a signal for every particle entering
into it. Every detector works by using some interaction of particles with matter.
Following is
a
list of the most common detector types.
1.
Gas-filled counters (ionization, proportional, Geiger-Muller counters)
2.
Scintillation detectors
3.
Semiconductor detectors
4.
Spark chambers
5.
Bubble chambers
(used with high energy particles)
6.
Photographic emulsions
7.
Thermoluminescent dosimeters (TLDs)
8.
Cerenkov counters
9.
Self-powered neutron detectors
The signal at the output of most detectors is a voltage pulse, such as the one
r
Source
Single channel
Detector
analyzer
power supply
Oscilloscope
0
Timer
cl
Figure
1.2
A
basic pulse-type detection system.
