Tsoulfanidis N. Measurement and detection of radiation
Подождите немного. Документ загружается.

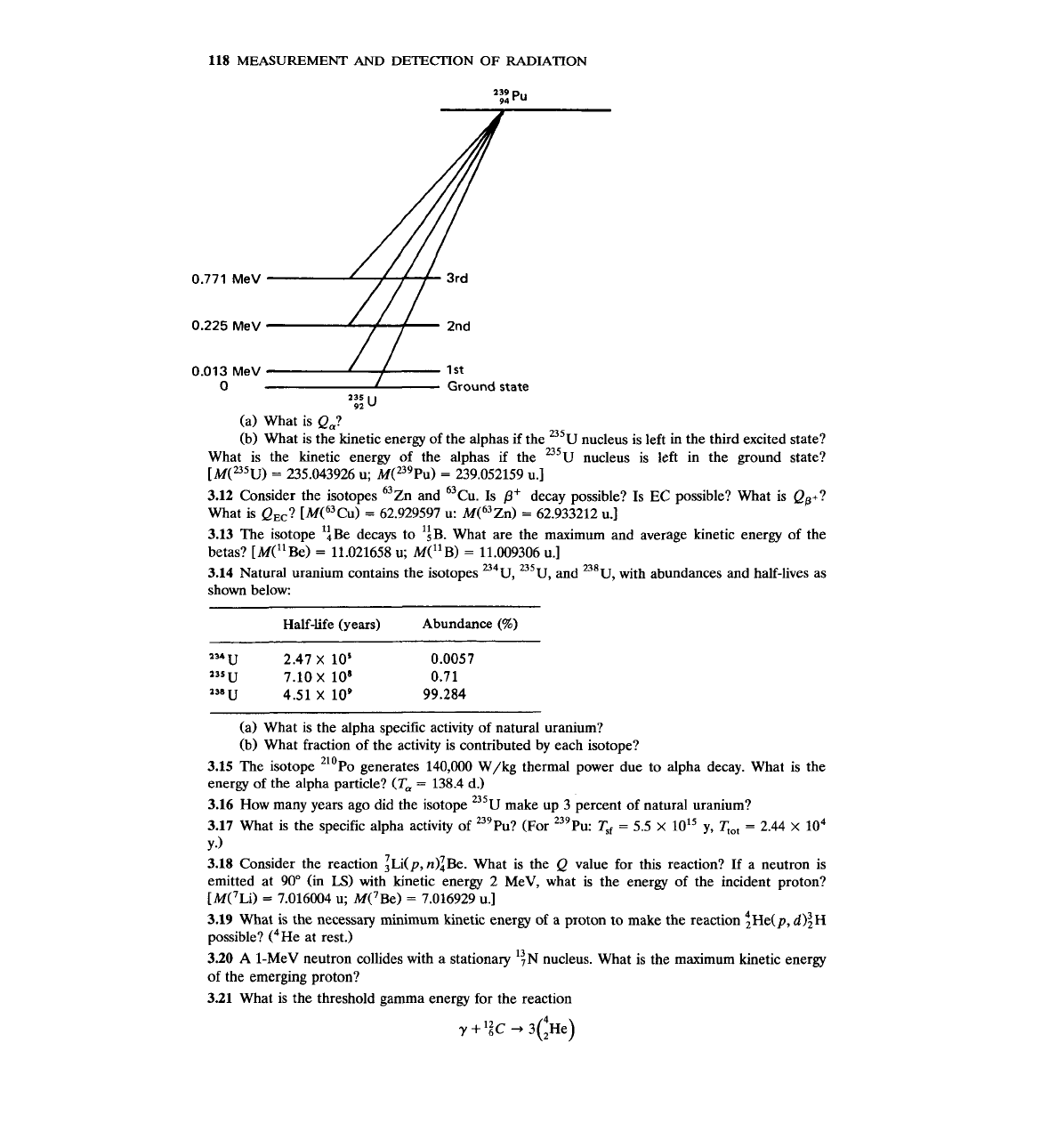
118
MEASUREMENT
AND
DETECTION
OF
RADIATION
=;:
Pu
MeV
//
1:
0
Ground
state
13s
"
92
(a) What is Q,?
(b) What is the kinetic energy of the alphas if the
235~
nucleus is left in the third excited state?
What is the kinetic energy of the alphas if the
U5~
nucleus is left in the ground state?
[M(=~u)
=
235.043926 u; M('~~Pu)
=
239.052159 u.]
3.12
Consider the isotopes 63~n and 63~~. IS
P+
decay possible? Is
EC
possible? What is
Qp+?
What is
Q,,?
[M(~~cu)
=
62.929597 u: M(63~n)
=
62.933212 u.]
3.13
The isotope ':Be decays to
':B.
What are the maximum and average kinetic energy of the
betas? [M(llBe)
=
11.021658 u; M("B)
=
11.009306 u.]
3.14
Natural uranium contains the isotopes 234~, u5~, and 238~, with abundances and half-lives as
shown below:
Half-life
(years)
Abundance
(%)
(a) What is the alpha specific activity of natural uranium?
(b) What fraction of the activity is contributed by each isotope?
3.15
The isotope 210~o generates 140,000 W/kg thermal power due to alpha decay. What is the
energy of the alpha particle?
(T,
=
138.4
d.)
3.16
How many years ago did the isotope
235~
make up 3 percent of natural uranium?
3.17
What is the specific alpha activity of 239~u? (For
239~~:
Cf
=
5.5
X
1015 y,
T,,,
=
2.44
X
lo4
YJ
3.18
Consider the reaction :Li(p, n);Be. What is the Q value for this reaction? If a neutron is
emitted at 90" (in
LS)
with kinetic energy 2 MeV, what is the energy of the incident proton?
[M('Li)
=
7.016004 u; M(7~e)
=
7.016929 u.]
3.19
What is the necessary minimum kinetic energy of a proton to make the reaction ;~e(p,
d):~
possible? (4~e at rest.)
3.20
A
1-MeV neutron collides with a stationary
';N
nucleus. What is the maximum kinetic energy
of the emerging proton?
3.21
What is the threshold gamma energy for the reaction
-y
+
':c
-
3(l~e)

REVIEW
OF
ATOMIC
AND
NUCLEAR PHYSICS
119
3.22
What is the energy expected to be released as a result of a thermal neutron induced fission in
23
9
Pu if the two fission fragments have masses
M,
=
142
u
and
M2
=
95
u?
BIBLIOGRAPHY
Born, M.,
Atomic Physics,
Blackie
&
Son Limited, London,
1962.
Evans,
R.
D.,
The Atomic Nucleus,
McGraw-Hill, New York,
1972.
Schiff, L.
I.,
Quantum Mechanics,
McGraw-Hill, New York,
1955.
Segre,
E.,
Nuclei and Particles,
Benjamin, New York,
1964.
REFERENCES
1.
Stamatelatos, M. G., and England,
T.
R.,
Nucl. Sci. Eng.
63:304 (1977).
2.
Bateman, H.,
Proc. Cambridge Philos. Soc.
15423 (1910).
3.
DiIorio, G.
J.,
"Direct Physical Measurement of Mass Yields in Thermal Fission of Uranium
235,"
Ph.D. thesis, University of Illinois at Urbana-Champaign,
1976.
4.
Lederer,
C.
M., and Shirley, V. S. (eds.),
Table of Isotopes,
7th ed., Wiley-Interscience, New York,
1978.

CHAPTER
FOUR
ENERGY LOSS
AND
PENETRATION OF
RADIATION THROUGH MATTER
4.1
INTRODUCTION
This chapter discusses the mechanisms by which ionizing radiation interacts and
loses energy as it moves through matter. The study of this subject is extremely
important for radiation measurements because the detection of radiation is
based on its interactions and the energy deposited in the material of which the
detector is made. Therefore, to be able to build detectors and interpret the
results of the measurement, we need to know how radiation interacts and what
the consequences are of the various interactions.
The topics presented here should be considered only an introduction to this
extensive subject. Emphasis is given to that material considered important for
radiation measurements. The range of energies considered is shown in Table
1.1.
For the discussion that follows, ionizing radiation is divided into three
groups:
1.
Charges particles: electrons (e-), positrons (e+), protons
(P),
deuterons
(d),
alphas
(a),
heavy ions
(A
>
4)
2.
Photons: gammas
(Y)
or X-rays
3.
Neutrons
(n)
The division into three groups is convenient because each group has its own
characteristic properties and can be studied separately.

122
MEASUREMENT AND DETECTION OF RADIATION
A charged particle moving through a material interacts, primarily, through
Coulomb forces, with the negative electrons and the positive nuclei that consti-
tute the atoms of that material. As a result of these interactions, the charged
particle loses energy continuously and finally stops after traversing a finite
distance, called the
range.
The range depends on the type and energy of the
particle and on the material through which the particle moves. The probability
of a charged particle going through a piece of material without an interaction is
practically zero. This fact is very important for the operation of charged-particle
detectors.
Neutrons and gammas have no charge. They interact with matter in ways
that will be discussed below, but there is a finite nonzero probability that a
neutron or a y-ray may go through any thickness of any material without hav-
ing an interaction. As a result, no finite range can be defined for neutrons or
gammas.
4.2 MECHANISMS OF CHARGED-PARTICLE ENERGY LOSS
Charged particles traveling through matter lose energy in the following ways:
1. In Coulomb interactions with electrons and nuclei
2.
By emission of electromagnetic radiation (bremsstrahlung)
3.
In nuclear interactions
4. By emission of Cerenkov radiation
For charged particles with kinetic energies considered here, nuclear interac-
tions may be neglected, except for heavy ions
(A
>
4)
(see Sec.4.7).
Cerenkov radiation constitutes a very small fraction of the energy loss. It is
important only because it has a particle application in the operation of Cerenkov
counters (see Evans). Cerenkov radiation is visible electromagnetic radiation
emitted by particles traveling in a medium, with speed greater than the speed of
light in that medium.
4.2.1. Coulomb Interactions
Consider a charged particle traveling through a certain material, and consider
an atom of that material. As shown in Fig. 4.1, the fast charged particle may
interact with the atomic electrons or the nucleus of the atom. Since the radius of
the nucleus is approximately 10-l4 m and the radius of the atom is
lo-''
m, one
might expect that
Number of interactions with electrons
(R')
atom (10-lo)'
- -
-
-
=
lo8
number of interactions with nuclei
(R~)
nucleus
14)'
This simplified argument indicates that collisions with atomic electrons are more
important than with nuclei. Nuclear collisions will not be considered here.
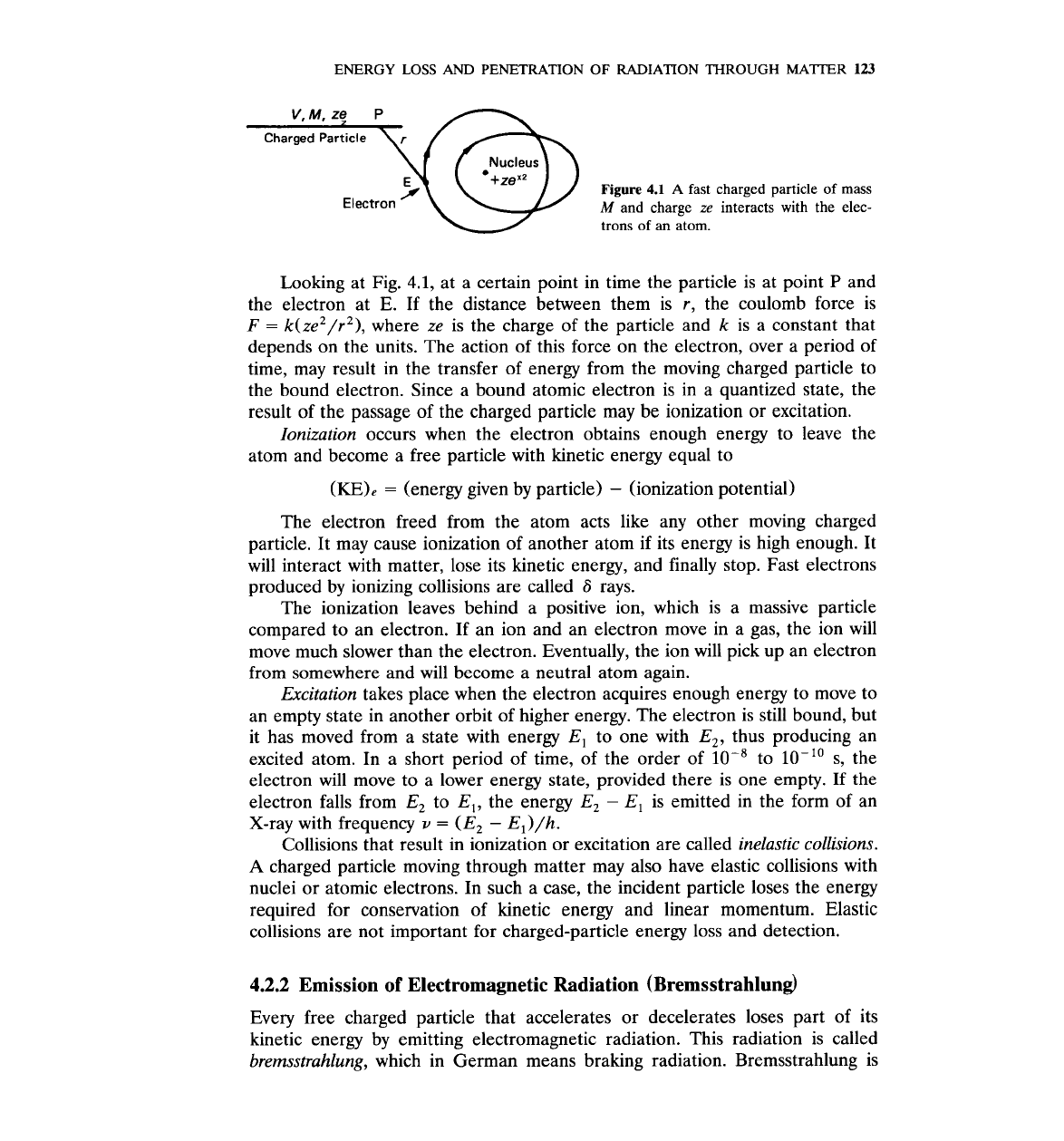
ENERGY
LOSS
AND
PENETRATION
OF
RADIATION
THROUGH
MATTER
123
Nucleus
+zex2
Figure
4.1
A
fast charged particle
of
mass
Electron
M
and charge
ze
interacts
with
the elec-
trons of an atom.
Looking at Fig.
4.1,
at a certain point in time the particle is at point
P
and
the electron at
E.
If the distance between them is r, the coulomb force is
F
=
k(ze2/r2), where ze is the charge of the particle and k is a constant that
depends on the units. The action of this force on the electron, over a period of
time, may result in the transfer of energy from the moving charged particle to
the bound electron. Since a bound atomic electron is in a quantized state, the
result of the passage of the charged particle may be ionization or excitation.
Ionization occurs when the electron obtains enough energy to leave the
atom and become a free particle with
kinetic energy equal to
(KE)e
=
(energy given by particle)
-
(ionization potential)
The electron freed from the atom acts like any other moving charged
particle. It may cause ionization of another atom if its energy is high enough. It
will interact with matter, lose its kinetic energy, and finally stop. Fast electrons
produced by ionizing collisions are called
6
rays.
The ionization leaves behind a positive ion, which is a massive particle
compared to an electron. If an ion and an electron move in a gas, the ion will
move much slower than the electron. Eventually, the ion will pick up an electron
from somewhere and will become a neutral atom again.
Excitation takes place when the electron acquires enough energy to move to
an empty state in another orbit of higher energy. The electron is still bound, but
it has moved from a state with energy
El
to one with
E2,
thus producing an
excited atom. In a short period of time, of the order of to
lo-''
s, the
electron will move to a lower energy state, provided there is one empty. If the
electron falls from
E,
to
E,,
the energy
E,
-
El
is emitted in the form of an
X-ray with frequency
v
=
(E2
-
E,)/h.
Collisions that result in ionization or excitation are called inelastic collisions.
A
charged particle moving through matter may also have elastic collisions with
nuclei or atomic electrons. In such a case, the incident particle loses the energy
required for conservation of kinetic energy and linear momentum. Elastic
collisions are not important for charged-particle energy loss and detection.
4.2.2
Emission of Electromagnetic Radiation (Bremsstrahlung)
Every free charged particle that accelerates or decelerates loses part of its
kinetic energy by emitting electromagnetic radiation. This radiation is called
bremsstrahlung, which in German means braking radiation. Bremsstrahlung is

124
MEASUREMENT
AND
DETECTION
OF
RADIATION
not a monoenergetic radiation. It consists of photons with energies from zero up
to a maximum equal to the kinetic energy of the particle.
Emission of bremsstrahlung is predicted not only by quantum mechanics but
also by classical physics. Theory predicts that a charge that is accelerated
radiates energy with intensity proportional to the square of its acceleration.
Consider a charged particle with charge ze and mass
M
moving in a certain
material of atomic number Z. The Coulomb force between the particle and
a
nucleus of the material is
F
-
zeZe/r2, where r
=
distance between the two
charges. The acceleration of the incident charged particle is
a
=
F/M
-
zze2/M. Therefore the intensity of the emitted radiation
I
is
This expression indicates that
1.
For two particles traveling in the same medium, the lighter particle will emit
a much greater amount of bremsstrahlung than the heavier particle (other
things being equal).
2.
More bremsstrahlung is emitted if a particle travels in a medium with high
atomic number Z than in one with low atomic number.
For charged particles with energies considered here, the kinetic energy lost
as bremsstrahlung might be important for electrons only. Even for electrons, it
is important for high-Z materials like lead (Z
=
82).
For more detailed treat-
ment of the emission of bremsstrahlung, the reader should consult the refer-
ences listed at the end of the chapter.
4.3
STOPPING POWER DUE TO IONIZATION AND EXCITATION
A
charged particle moving through a material exerts Coulomb forces on many
atoms simultaneously. Every atom has many electrons with different ionization
and excitation potentials. As a result of this, the moving charged particle
interacts with a tremendous number of electrons-millions. Each interaction
has its own probability for occurrence and for a certain energy loss. It is
impossible to calculate the energy loss by studying individual collisions. Instead,
an average energy loss is calculated per unit distance traveled. The calculation is
slightly different for electrons or positrons than for heavier charged particles
like
p,
d,
and
a,
for the following reason.
It was mentioned earlier that most of the interactions of a charged particle
involve the particle and atomic electrons. If the mass of the electron is taken as
1,
then the masses of the other common heavyt charged particles are the
'1n this discussion, "heavy" particles are all charged particles except electrons and positrons.
ENERGY LOSS AND PENETRATION
OF
RADIATION
THROUGH
MATIER
125
following:
Electron mass
=
1
Proton mass
=
1840
Deuteron mass
=
2(1840)
Alpha mass
=
4(1840)
If the incoming charged particle is an electron or a positron, it may collide with
an atomic electron and lose all its energy in a single collision because the
collision involves two particles of the same mass. Hence, incident electrons or
positrons may lose a large fraction of their kinetic energy in one collision. They
may also be easily scattered to large angles, as a result of which their trajectory
is zig-zag (Fig.
4.2).
Heavy charged particles, on the other hand, behave differ-
ently. On the average, they lose smaller amounts of energy per collision. They
are hardly deflected by atomic electrons, and their trajectory is almost a straight
line.
Assuming that all the atoms and their atomic electrons act independently,
and considering only energy lost to excitation and ionization, the average energy
losst per unit distance traveled by the particle is given by Eqs. 4.2,
4.3,
and 4.4.
(For their derivation, see the chapter bibliography: Evans, Segr6, and Roy and
Reed.)
Stopping power due to ionization-excitation for
p,
d,
t,
a.
Stopping power due to ionization-excitation for electrons.
Stopping power due to ionization-excitation for positrons.
'since
E
=
T
+
Mc2
and
Mc2
=
constant,
dE/dx
=
dT/dx;
thus, Eqs.
4.2
to 4.4 express the
kinetic as well as the total energy loss per unit distance.
*1n SI units, the result would be
J/m;
1
MeV
=
1.602
X
lo-"
J.
126
MEASUREMENT AND DETECTION OF RADIATION
Electron or positron
trajectory
Figure
4.2
Possible electron and
-I--
Heavy particle trajectory
heavy particle trajectories.
where r,
=
4.rrr;
=
mc2
=
Y=
M=
P
=
N=
N=
z
=
Z
=
I
=
e2/mc2
=
2.818
X
10-l5 m
=
classical electron radius
9.98
x
m2
.=
m2
=
cm2
rest mass energy of the electron
=
0.511 MeV
(T
+
MC~)/MC~
=
I/
4-
T
=
kinetic energy
=
(y
-
1)Mc2
rest mass of the particle
v/c
c
=
speed of light in vacuum
=
2.997930
X
lo8
m/s
=
3
x
lo8
m/s
number of atoms/m3 in the material through which the particle
moves
p(NA/A) NA
=
Avogadro's number
=
6.022
X
atoms/mol
A
=
atomic weight
atomic number of the material
charge of the incident particle
(z
=
1
for e-, e+,
p,
d;
z
=
2 for
a)
mean excitation potential of the material
An
approximate equation fo; I, which gives good results for
Z
>
12,' is
Table 4.1 gives values of
I
for many common elements.
Many different names have been used for the quantity
dE/&:
names like
energy loss, specific energy loss, differential energy loss, or stopping power. In
Table
4.1
Values of Mean Excitation Potentials for
Common Elements and Compoundst
Element
I
(eV) Element
I
(eV)
H
20.4 Fe 281*
He 38.5 Ni 303*
Li 57.2 Cu 321*
Be 65.2 Ge 280.6
B
70.3 Zr 380.9
C 73.8
I
491
N 97.8 Cs 488
0
115.7 Ag 469*
Na 149 Au 771
*
A1
160* Pb 818.8
Si
174.5
U
839*
'values of
I
with
*
are from experimental results
of
refs. 2
and 3. Others are from refs. 4 and
5.

ENERGY LOSS
AND
PENETRATION OF RADIATION THROUGH
MAlTER
127
this text, the term
stoppingpower
will be used for
dE/h
given by Eq. 4.2 to 4.4,
as well as for a similar equation for heavier charged particles presented in Sec.
4.7.2.
It should be noted that the stopping power
1.
Is independent of the mass of the particle
2.
Is proportional to
z2
[(chargeI2] of particle
3. Depends on the speed
u
of particle
4. Is proportional to the density of the material
(N)
For low kinetic energies,
dE/h
is almost proportional to
l/v2.
For relativistic
energies, the term in brackets predominates and
dE/h
increases with kinetic
energy. Figure 4.3 shows the general behavior of
dE/h
as a function of kinetic
energy. For all particles,
dE/h
exhibits a minimum that occurs approximately
at
y
=
3. For electrons,
y
=
3 corresponds to
T
=
1
MeV; for alphas,
y
=
3
corresponds to
T
-
7452 MeV; for protons,
y
=
3 corresponds to
T
z
1876
MeV. Therefore, for the energies considered here (see Table
1.1),
the
dE/h
for protons and alphas will always increase, as the kinetic energy of the particle
decreases (Fig. 4.3, always on the left of the curve minimum); for electrons,
depending on the initial kinetic energy,
dE/h
may increase or decrease as the
electron slows down.
Equations 4.3 and 4.4, giving the stogping power for electrons and positrons,
respectively, are essentially the same. Their difference is due to the second term
in the bracket, which is always much smaller than the logarithmic term. For an
electron and positron with the same kinetic energy, Eqs. 4.3 and 4.4 provide
results that are different by about
10
percent or less. For low kinetic energies,
dE/h
for positrons is larger than that for electrons; at about
2000
keV, the
energy loss is the same; for higher kinetic energies,
dE/h
for positron is less
than that for electrons.
As stated earlier, Eqs. 4.2 to 4.4 disregard the effect of forces between
atoms and atomic electrons of the attenuating medium.
A
correction for this
density
effect6,'
has been made, but it is small and it will be neglected here. The
density effect reduces the stopping power slightly.
Figure
4.3
Change of stopping power with the kinetic energy
of
the particle.
